Abstract
Identification of genes contributing to mouse seizure susceptibility can reveal novel genes or pathways that provide insight into human epilepsy. Using mouse chromosome substitution strains and interval-specific congenic strains (ISCS), we previously identified an interval conferring pilocarpine-induced limbic seizure susceptibility on distal mouse Chromosome 10 (Ch10). We narrowed the region by generating subcongenics with smaller A/J Ch10 segments on a C57BL/6J (B6) background and tested them with pilocarpine. We also tested pilocarpine susceptible congenics for 6Hz ECT, another model of limbic seizure susceptibility, to determine whether the susceptibility locus might have a broad effect on neuronal hyperexcitability across more than one mode of limbic seizure induction. ISCS Line 1, which contained the distal 2.7 Mb segment from A/J (starting at rs29382217), was more susceptible to both pilocarpine and ECT. Line 2, which was a subcongenic of Line1 (starting at rs13480828), was not susceptible; thus defining a 1.0 Mb critical region that was unique to Line1. Bioinformatic approaches identified 52 human orthologues within the unique Line 1 susceptibility region, the majority syntenic to human Ch12. Applying an epilepsy network analysis of known and suspected excitability genes and examination of interstrain genomic and brain expression differences revealed novel candidates within the region. These include Stat2, which plays a role in hippocampal GABA receptor expression after status epilepticus, and novel candidates Pan2, Cdk2, Gls2, and Cs, which are involved in neural cell differentiation, cellular remodeling, and embryonic development. Our strategy may facilitate discovery of novel human epilepsy genes.
Introduction
Approximately two-thirds of epilepsy has no identified antecedent cause; genetic factors play a critical role in this subset of the epilepsies (Ottman et al., 1996, Dibbens et al., 2007, Helbig et al., 2008, Mulley and Dibbens, 2009, Ottman, 2005, Ottman et al., Reid et al., 2009). Identification of seizure susceptibility genes can provide insight into pathophysiology and lead to the development of new treatments based on newly elucidated mechanisms. The genetics of most common forms of epilepsy appears to be “complex”--resulting from multiple genes and gene variants of small to moderate effect. This complexity poses challenges for gene discovery.
Mouse models are ideal for studying genetically complex disease. They have a short generation time, many inbred strains have been fully sequenced, and numerous methods exist to manipulate the mouse genome. Seizure susceptibility varies dramatically among inbred strains, providing a framework to identify genetic polymorphisms influencing propensity to seizures (Ferraro et al., 1995, Ferraro et al., 1999, Ferraro et al., 1998, Kosobud and Crabbe, 1990, Neumann and Collins, 1991, Neumann and Collins, 1992, Gershenfeld et al., 1999, Frankel et al., 2001, Hain et al., 2000, Ferraro et al., 2002, McKhann et al., 2003, Ferraro et al., 2004, Chaix et al., 2007, Schauwecker, 2011b, Winawer et al., 2011, Winawer et al., 2007a, Winawer et al., 2007b). Finally, similarities between the genomes of mice and humans enables genetic discoveries in the mouse to be translated to human cohorts.
Using mouse chromosome substitution strains (CSS) and interval-specific congenic strains (ISCS), we previously identified a critical interval on distal mouse Chromosome 10 (Ch10) contributing to pilocarpine-induced limbic seizure susceptibility (Winawer et al., 2011). Here, we screened subcongenic strains to narrow the pilocarpine susceptibility region further. We also investigated whether the same region confers susceptibility to limbic seizures induced by electroconvulsive stimuli (6Hz ECT) to determine whether the Ch10 locus has a broad effect on neural hyperexcitability crossing different seizure induction models. Mapping of a single susceptibility phenotype or model, such as pilocarpine, is useful to identify promising susceptibility regions or genes. Use of additional models of seizure induction, including ECT, to validate the importance of these identified regions or genes is a critical next step that has led to identification of mouse genes with human orthologues that play a role in epilepsy. (Otto et al., 2006, Otto et al., 2004, Buono et al., 2004, Ferraro et al., 2004, Tokuda et al., 2011, Frankel et al., 2009, Basel-Vanagaite et al., 2013) In this case, the complementary use of an electrical model as well as a pharmacologic one to confirm the phenotype associated with a susceptibility locus also makes it unlikely that pharmacokinetic factors might underlie susceptibility.
Finally, we explored the implicated susceptibility genes in the region using in silico analyses to prioritize candidates and identify gene pathways and networks that may contribute to the susceptibility phenotype.
Materials and Methods
EXPERIMENTAL METHODS
Animals
A/J, C57BL/6J (B6), and C57BL/6J-Chr 10A/J/NaJ (Jax stock number 4388; CSS-10) breeder pairs were purchased from the Jackson Laboratories and colonies of each were bred in our laboratory. Mice were group housed in a temperature and humidity-controlled colony, with a 12-hour light/dark schedule (lights on at 7 A.M., lights off at 7 P.M.) and food and water available ad libitum. Only males were tested for seizure susceptibility to avoid estrus cycle effects. Animals were tested during daylight hours for susceptibility to either pilocarpine or ECT between 10 and 12 weeks of age. All methods were approved by the Institutional Animal Care and Use Committee of Columbia University and met the guidelines of the National Institutes of Health.
Breeding
We created Ch10 interval specific congenic strains (ISCS) by backcrossing to B6 and selectively breeding successive rounds of littermates based on genotype to isolate sub-regions of A/J Ch10 on a B6 background. We began by using genotyped CSS-10 x B6 F2 mice, selecting mice heterozygous for an A/J Ch10 segment of interest, and backcrossing them to B6. Heterozygous offspring from these crosses were then brother-sister mated to produce a first round of congenics. Heterozygote mating was always used, allowing us to obtain B6 homozygous littermate controls for comparison. Figure 1 illustrates the ISCS presented here.
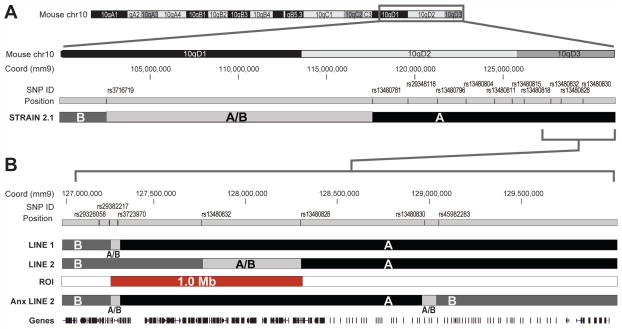
FIGURE 1. Schematic of the genomic region captured by selectively bred Interval Specific Congenic Strains (ISCS).
The cytogenetic band of murine Ch10 is shown with the base pair positions and SNP ID positions below. The grey rectangular outline superimposed on the cytogenetic band refers to the genomic region of interest mapped using ISCS. Genotyping markers through the region are provided (SNP ID) and their relative location and intermarker distance is shown. The genotypes for the three ISCS bred in house are shown immediately below the genotyping markers, where A=A/J at a locus, B= B6 at a locus, and A/B= undetermined genotype.
A. We previously reported mapping of pilocarpine-induced seizure susceptibility to STRAIN 2.1, which harbored an 11.4 Mb segment of the seizure-susceptible A/J strain (A) that extends to the telomeric end of Ch10, on a seizure-resistant B6 background (B).
B. Since our last publication, we created a smaller ICSC: LINE 1. LINE 1 congenics retained the seizure susceptibility phenotype, allowing us to narrow the susceptibility locus to a 2 Mb region of distal Ch10. This is shown expanded in 1B. The region captured by the Line 2 (seizure-resistant) subcongenic is also shown. The new rsSNPs in panel B not present in panel A are rs29326058 (127.238 Mb); rs29382217 (127.264 Mb); rs3723970 (127.277 Mb); rs13480830 (128.964 Mb) and rs45982283 (129.068 Mb). We used Sanger sequencing to define the proximal breakpoint of our LINE 1 congenic for the B6 allele at rs29326058 (127.238 Mb) and rs29382217 (127.264 Mb). We genotyped LINE 1 as A/J at SNP rs3723970 (127.277 Mb), rs13480832 (127.659 Mb), and rs13480830 (128.963 Mb). LINE 2 is a subcongenic of Line 1, genotyped as B6 at SNP rs13480832 (127.659 Mb), and A/J at rs13480828 (128.283) and rs13480830 (128.963 Mb). The A/J region unique to Line 1 (excluding the segment that is A/J in Line 2), labeled ROI, is 1.0 Mb in size (RED) extending from rs29382217 (127.264 Mb) to rs13480828 (128.283 Mb) and harbors 49 genes. ANX-LINE2 is a subcongenic of Ch10 generated separately for the study of anxiety –related phenotypes which overlaps our epilepsy interval of interest (Parker et al 2013 Line 2).
Pilocarpine Administration and Seizure Scoring
All seizure testing was performed between 12:00 p.m. and 5 p.m. to limit effects of diurnal variation. Animals were weighed immediately prior to testing. In order to limit peripheral side effects, 30 minutes before pilocarpine injection, all mice were given atropine methyl nitrate (1 mg/kg, i.p., TCI America, Portland OR), a competitive muscarinic acetylcholine receptor antagonist that does not cross the blood-brain barrier. Thirty minutes after the atropine injection mice were injected with pilocarpine hydrochloride (300 mg/kg i.p.; Sigma-Aldrich, St. Louis, MO), a muscarinic agonist.
Animals were observed by trained examiners for 2.5 hours following pilocarpine injection. Time of onset and offset for each seizure stage was recorded in minutes. From these data we extracted the highest seizure severity stage observed, proportion of animals having generalized seizures (reaching stage 4 or higher), duration of stages, and latency to each stage. We used a staging system adapted from established rodent seizure scales (Racine, 1972), described previously (Winawer et al., 2007b). We measured seizure severity, seizure duration, and whether animals reached stage 4 (generalized seizures) or higher. Stage 4 is easily identified in mice clearly delineated differences between resistant and susceptible strains. We examined these parameters in a newly created panel of Ch10 ISCS harboring smaller regions of A/J Ch10 on a B6 background, to further define the susceptibility locus.
6 Hz ECT Testing and Seizure Scoring
Testing was conducted with transcorneal electrodes and a Grass stimulator (Model S48) at 6 Hz, with 0.2-ms pulse width, 3.0-second shock duration, and constant voltage. Mice received a drop of an anesthetic solution (0.5% tetracaine in saline) on each eye prior to the stimulus. Each mouse was tested once, at a single current.
ECT severity was scored as: 0 = no seizure, 1 = stunned, 2 = partial seizure (jaw chomping, limb clonus, tail and/or body tremors), 3 = generalized seizure (hyperexcitability, rearing, falling on side). While the animals tested with ECT were genetically identical to those tested with pilocarpine, individual mice were tested either for ECT or for pilocarpine.
We examined dose-response curves over a range of currents to determine whether 6Hz ECT susceptibility paralleled pilocarpine susceptibility in in CSS10, A/J and B6 strains. We also sought to identify the best current for subsequent fine mapping studies in ISCS. In pilot studies, the CSS10 strain response to ECT paralleled the A/J response, and differed dramatically from that of B6 animals at 16mA current (see Results). We therefore characterized congenic strain ECT susceptibility at 16mA.
Genotyping
We used the Qiagen DNAeasy kit to extract DNA from mouse tail and spleen. Congenic animals were genotyped at the markers in Figure 1. Genotyping was performed at the University of Chicago using the ABI TaqMan Assay and a Step One PCR machine in accordance with the manufacturer’s instructions. In some cases we, we used primer3 to design primers, used PCR to amplify the SNPs and then sent the amplicons for Sanger sequencing to determine the genotypes. We defined the boundaries of the congenic regions; critical SNPs are listed in Figure 1. Line 1 harbors a ~2.7 Mb segment of A/J on distal mouse Ch10 and harbors a unique 1.0 Mb region of interest (ROI) not present in Line 2 (Figure 1).
Statistical Analysis of interval-specific congenic strains
For pilocarpine, we analyzed the highest stage reached, the proportion reaching stage 4 or higher, and the mean duration of stage 4 seizures for animals attaining stage 4. We compared seizure susceptibility to pilocarpine in the Line 1 ISCS with B6 littermate controls, and compared susceptibility in the Line 1 vs. Line 2 ISCS. For 6Hz ECT, we compared Line 1 congenic seizure severity with B6 littermate control severity, and Line 1 vs. Line 2 severity at 16 mA current.
Mann-Whitney tests were used for analysis of ordinal seizure score data (highest stage reached) for both pilocarpine and ECT. Fisher’s exact tests were used for the analysis of the proportion of animals reaching stage 4 (pilocarpine). T-tests were used for comparisons of the mean duration of stage 4 seizures (pilocarpine).
We used two-tailed tests for initial comparisons of Line 1 vs. B6 littermate controls’ response to pilocarpine. To test whether Line 1 animals were also more susceptible to 6Hz ECT than B6 littermate controls, we used one-sided tests. We also used one-sided tests to assess whether Line 1 congenics were more susceptible than Line 2 congenics for both pilocarpine and ECT.
BIOINFORMATICS METHODS
Candidate Genome Locus
The strain specific SNP markers (B6: rs29326058 and A/J: rs13480832) were landmarked to the ens37 (mm9) mouse reference genome establishing a maximum recombination interval from 127238197–127659071 bp on chromosome 10 for the Line 1 congenic and extending through the end of Ch10 genic coding region (Ch10: 127238197–129993255). Subsequent sequencing of SNP rs29382217 (127.264 Mb) and rs3723970 (127.277 Mb) identified the chromosomal recombination breakpoint within this region. Genotyping of Line 2 revealed a recombination interval from SNP rs13480832 (B6) to rs13480828 (A/J). The unique ROI present in Line 1 but not Line 2 was 1.0 Mb in length (Ch10: 127.264 –128. 283 Mb). Locus specific queries were performed using the UCSC Genome browser (www.ucscbrowser.com), the Gene Network browser (www.genenetwork.org) and the MGI Informatics database (http://www.informatics.jax.org/genes.shtml). A master candidate gene list for the ROI was compiled and curated for biological function, tissue expression and known interacting and/or regulatory molecules (Table S1). Human orthologues and their chromosomal coordinates were noted using the HUGO Gene Nomenclature Committee gene list (http://www.genenames.org/).
Strain dependent genetic variation
We utilized three publically released genomics datasets generated for the analysis of mouse diversity. The selected datasets were specifically collected and curated for strain specific differences and were generated by independent groups using different methodologies and platforms. While individual datasets are reported as raw unvalidated reports on genetic variation, we performed an integrated analysis where the identification of the same genetic variant in multiple (>1) datasets cross-validated the inter-strain differences (Table S2). The three bioinformatics servers used were 1) MGI Informatics Strain/SNP query (http://www.informatics.jax.org/strains_SNPs.shtml), 2) Gene Network variant query (http://www.genenetwork.org/webqtl/) and 3) Wellcome Sanger Institute Mouse Genome Project SNP query (http://www.sanger.ac.uk/sanger/Mouse_SnpViewer/rel-1303). Single nucleotide polymorphisms (SNPs) in the interval of interest that differed between strains were analyzed using for potential pathogenicity using established murine gene models to landmark variants to functional regions (e.g. promoter, exon, splice site, intron) and classify the variant by type (e.g. missense, nonsense, synonymous) (Table S3).
Comparing strain specific candidate gene expression
We performed all bioinformatics analysis of microarray expression datasets using the GenomeSpace (www.genomespace.org) interface. Three microarray expression datasets were selected for analysis where transcript levels in adult (7–11 week old) male mice of A/J and B6 strains, among others were analyzed simultaneously on Affymetrix expression arrays. Hippocampal transcript comparisons were performed using datasets GSE5429 (N= 6 animals) and GSE4734 (N= 2–5 animals) while whole brain transcript differences were compared in dataset GSE10744 (N=3 animals), Array data was analyzed in GenePattern (http://genepattern.broadinstitute.org) for expression differences (either upregulation or downregulation) in the candidate genes was different in A/J relative to the expression in B6.
Results
Fine Mapping of Pilocarpine and 6Hz ECT-induced Seizures using ISCS
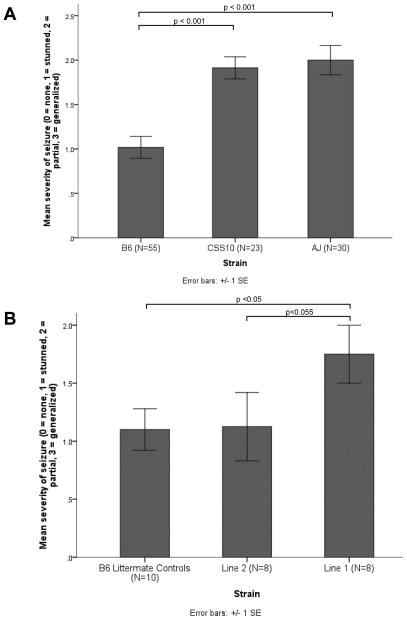
The Line 1 congenic (Figure 1A), which contains a 2.7 Mb region of AJ Ch10 on a B6 background, was more susceptible to pilocarpine than B6 littermate controls (Figure 2A, B, C). A greater proportion of line 1 animals reached stage 4 seizures or higher (60% vs. 33%; Fisher’s Exact p=0.05) and duration of stage 4 seizures was longer for Line 1 than controls (t(12) = 2.42 p < 0.001). Mean seizure severity scores were higher for Line 1 animals than B6 littermate controls (4.0 vs. 3.4), but the difference did not attain statistical significance (Mann-Whitney U, p < 0.06).
FIGURE 2. Response to pilocarpine injection in ICSCs and B6 littermate controls.
A. Mean highest seizure stage reached for ICSC Line 1, Line 2, and B6 littermate controls (Mann Whitney).
B. Proportion of animals reaching stage 4 (generalized) seizures by strain. (Fischer’s exact).
C. Duration of stage 4 (generalized) seizures for animals reaching stage 4 (t-test).
Line 2 animals, a subcongenic of Line 1 (Figure 1B), were similar to B6 littermate controls in the proportion reaching stage 4 seizures, highest stage reached, and duration of stage 4 seizures (Figure 2A, B, C); thus line 2 animals appeared not to have captured the susceptibility phenotype. Line 1 animals progressed to higher stages than Line 2 animals (Mann Whitney U; p<0.05), and duration of stage 4 seizures was longer for Line 1 than Line 2 animals (t(12)= 2.775; p<0.02). A greater proportion of Line 1 animals reached stage 4 compared to Line 2 animals, (60% vs. 22%; Fischer’s Exact p=0.07), though the results did not attain statistical significance.
Since Line 2 was less susceptible than Line 1, with a phenotype indistinguishable from B6 control animals, we limited our candidate gene analyses to the smaller, 1.0 Mb A/J region on Ch10 encompassed by Line 1 but not in Line 2.
In pilot ECT studies we determined that A/J and CSS10 animals were more susceptible to 6Hz ECT than B6; this difference was most pronounced at 16 mA current (A/J vs. B6 seizure severity, Mann-Whitney p < 0.001; CSS10 vs. B6, Mann-Whitney p < 0.001) (Figure 3A). We therefore compared strain responses to ECT at 16mA. Line 1 animals had greater ECT-induced seizure severity than B6 littermate controls at 16mA, and captured the CSS-10 and A/J susceptibility phenotype (Line 1 vs. B6 littermate controls, Mann-Whitney U. p<0.05) (Figure 3B). Line 1 also appeared to have greater ECT-induced seizure severity than Line 2 animals, though this difference did not achieve statistical significance (Mann-Whitney U., p < 0.055) (Figure 3B).
FIGURE 3. 6-Hertz Electroconvulsive threshold testing (6 Hz ECT) injection in chromosome substitution strains and B6 littermate controls.
A. Response of A/J, C57/Bl6 (B6), and C57BL/6J-Chr 10A/J/NaJ (CSS10) chromosome substitution strain mice to 6 Hz ECT at 16 mA current (A/J vs. B6, p < 0.001; CSS10 vs. B6, p < 0.001).
B. Response of interval specific congenic strains vs. B6 littermate controls to 6Hz ECT at 16 mA current (Line 1 vs. B6 littermate controls, p<0.05; Line 1 vs. Line 2, p<0.055).
Bioinformatics
Congenic region is syntenic to human chromosome 12 (Ch12)
In order to define the boundaries of the susceptibility locus, we performed genotyping using TaqMan custom assays and Sanger sequencing across specific single nucleotide polymorphisms (SNPs) that are polymorphic between B6 and A/J. The recombination interval between B6 and A/J spanned from Ch10: 127.264–127.277. Inclusion of the A/J genotyped region and the tip of Ch10 beyond the final A/J SNP marker (rs13480830) yielded a 2.7 Mb region (Ch10: 127263815–129993255) of interest harboring 119 genes (Table S1). Restriction to the Line 1 minus Line 2 region yielded a 1.0 Mb region of interest (ROI; Ch10: 127263815–128283020) harboring 49 genes. The excluded genes in Line 2 include 59 olfactory receptor genes and 4 vomeronasal receptor genes which due to their biological function are unlikely to contribute to seizure susceptibility and were not included in subsequent analysis, further supporting exclusion of the Line 2 segment from the ROI (Table S1). No monogenic human or mouse previously identified epilepsy genes were present in the 1.0 Mb ROI.
Human orthologues were identified for 52 of the genes on mouse Ch10 (Table S1). The majority (96%) of the candidate genes are syntenic to human Ch 12q12-15, while two genes ATP5B and NACA localize to other regions of human Ch12, 12p13.3 and 12q23-12q24.1 respectively. 45/49 of the genes containing A/J alleles in the 1.0 Mb region present in Line 1 but not Line 2 have human orthologues and 100% of these genes map to human Ch12. Human genomic aberrations in the 12q13 region have been reported for several human epilepsy phenotypes mapping to Ch12p 11–13 (Grosso et al., 2004, Margari et al., 2012, Morar et al., 2011, Vaglio et al., 2007); the causative genes were not identified.
Bioinformatics identified all variants that differed between A/J and B6 strains in the ROI yielding 930 SNPs for analysis (Table S2) The comprehensive data set from the Wellcome Trust Mouse Genomes Project (Keane et al., 2011, Yalcin et al., 2011) identified 8 nonsynonymous polymorphisms (nsSNPs) encoding an amino acid substitution in 5 genes (Table S3) of which 4/8 nsSNPs were also present in both the Jax and Gene Network Databases (Table S2). Apon, Gls2, Cs and Pan2 each have a single missense mutation while Stat2 had four missense mutations and gained a start codon in strain A/J. Only 1 of the 4 nsSNPs in Stat2 was also identified in the Gene Network mouse diversity dataset, however this single cross-validated nsSNP was sufficient to nominate Stat2 to candidate gene status given its role as a STAT transcription factor that functions in the JAK-STAT pathway. This pathway regulates GABA receptor expression in the hippocampus following status epilepticus (Brooks-Kayal et al., 2009) making it a good candidate for influencing excitability thresholds. All nsSNP-containing candidate genes are located in the A/J region present in Line 1 but not in line 2.
In silico expression profiling of candidate genes in the brains of A/J mice compared with B6 mice reveals novel gene candidates
In addition to coding differences we considered that the QTL could be caused by an expression difference. While five candidate genes were nominated from analysis of potentially pathogenic genetic variants, we noted extensive genetic variation in the untranslated and regulatory regions of the gene models (Table S3). We used available microarray expression datasets where untreated wildtype A/J and B6 mice were analyzed simultaneously in the same experiment to interrogate relative transcript expression of candidate genes in brain. We found that Rdh7, Rdh16, Mip and Cdk2, were differentially expressed between B6 and A/J; none of these genes has been previously implicated in seizure susceptibility. We also identified two additional genes with expression differences, Timeless and Rnf41, implicated in neurobehavioral disorders in humans (Mansour et al., 2006) and mice (Kim et al., 2009). Rnf41 was previously identified as a candidate gene for seizure susceptibility and anxiety through targeted expression, but without identification of potential pathogenic variants (Kim et al., 2009).
Epilepsy network analysis identifies new candidate genes
With a shortened list of 11 seizure susceptibility candidate genes identified through a combination of differential gene variants and transcript expression analysis (Figure 4) we employed a series of network and GO analyses to identify connected pathways involved in brain excitability. Of those genes with nsSNPs, Cs, Gls2, and Apon are involved in cell membrane lipid synthesis during neuron development (Figure 4). Pan2, another missense mutation containing gene, polarizes the cell axis during development. Given the function of these genes in the developing brain, these variants could act during development to alter excitability thresholds and play a role in seizure susceptibility. Cdk2, a differentially expressed gene, also plays a functional role in cell development. Defects in Cdk2 can cause aberrant neuronal proliferation and development (Lim and Kaldis, 2012), it has been shown that pharmacological inhibition of the Cdk2 kinase by roscovitine or retinoic acid can reduce epileptic hyperexcitability (Jansen et al., 2005). This analysis of the locus of interest in conjunction with in vivo increased susceptibility in Line1 suggests several high-priority novel candidate genes on the distal end of murine chromosome 10.
FIGURE 4. Flowchart detailing integrated bioinformatics for the evaluation and nomination of candidate genes.
The 2.7 Mb region of interest in the seizure susceptible Line 1 congenic yielded a total of 119 genes while Line 2 shared 70 of these genes on the distal end of chromosome 10. The Line 2 congenic which was less susceptible to seizure induction harbored seven (7) human orthologues, 55 olfactory receptor genes and 4 vomeronasal genes all of which were excluded from analysis. The 45 genes in Line 1 with human orthologues and the four (4) mouse specific (grey genes) were analyzed. This set of 49 genes was used for strain specific genetic analysis yielding 5 genes with a possible pathogenic variant (nsSNP). All five genes had a nsSNP that was identified in >1 independent mouse diversity genome datasets. The Wellcome Trust Mouse Genomes Project identified Stat2 (black circle) as potentially harboring compound missense mutations, having 4 total nsSNPs and a new start codon. Relative gene expression analysis revealed 6 candidate genes with differential expression in strain A/J vs. B6. These 11 candidate genes for seizure susceptibility were then subjected to network and pathway analysis which identified five (5) genes as having a role in cell and neural development and differentiation, nominating these as preeminent candidates for future study.
Discussion
Using two limbic seizure models, we identified a 1.0 Mb region on mouse Ch10 influencing response to both pilocarpine and 6Hz ECT. We then used bioinformatic strategies to prioritize a number of promising candidate genes in the region. Pilocarpine, a muscarinic cholinergic agonist, induces continuous limbic seizures in rodents, followed by cell loss similar to that observed in human temporal lobe epilepsy (TLE), and delayed chronic spontaneous limbic seizures (Turski et al., 1989, Turski, 2000, Borges et al., 2003, Leite et al., 2002). ECT is one of the most commonly employed models for screening and identifying new anticonvulsant drugs (White et al., 1995), and has been shown to be useful for identifying genetic contributions to seizure susceptibility in rodent models (Engstrom and Woodbury, 1988, Ferraro et al., 1998, Frankel et al., 2001, Otto et al., 2004, Yang et al., 2003). ECT has also been used to identify a murine candidate gene that was subsequently demonstrated to be relevant to human epilepsy (Buono et al., 2004, Ferraro et al., 2001, Ferraro et al., 2004).
The 6Hz ECT stimulus evokes seizures consisting of rhythmic face and jaw movements, forelimb clonus, neck extension, rearing/falling, and transient unsteady gait. 6Hz frequency stimulation can model limbic seizures, or factors influencing secondary generalization (Swinyard, 1972b). ECT is a well-established test of seizure susceptibility that is sensitive to genetic differences (Swinyard, 1972a, White et al., 1995, Ferraro et al., 1998, Frankel et al., 2001, Otto et al., 2004, Yang et al., 2003, Ferraro et al., 2001). Our results point to a gene or genes on mouse Ch10 that contributes to susceptibility to both pilocarpine and ECT-induced limbic seizures. This suggests we are targeting genes with a broad effect on seizure susceptibility, and also makes it unlikely that artifacts, such as those due to pharmacologic variables, might have contributed to our pilocarpine finding.
One of our goals is to use mouse models to identify candidate susceptibility genes that should be examined in humans. We have therefore identified all orthologous human genes in the congenic region. None has been previously identified as playing a role in human epilepsy. Our data suggest that either common or rare variants in these high priority candidates should be more closely examined in an effort to identify novel human epilepsy genes.
In the course of parallel experiments using chromosome substitution strains and ISCS in our laboratories, we previously mapped a behavioral phenotype modeling anxiety to a 6.7Mb region (122.4–129.1 Mb) on Ch10 (Parker et al., 2013). Our pilocarpine/ECT trait and anxiety traits mapped to the same narrow region on distal Ch10. The co-registration of the two traits led us to test the independently generated anxiety-prone strains for seizure susceptibility. The results of these experiments are described in the Supplemental Materials (S4) provided with this submission.
Work from others also supports the presence of a region on distal Ch10 contributing to seizure susceptibility and differences in locomotor behaviors likened to an anxiety phenotype to β-CCM (a GABAA receptor inverse agonist) on distal Ch10 in A/J vs. B6 mice (Kim et al., 2009, Zhang et al., 2005). This group nominated a candidate gene, Rnf41, as underlying the combined seizure and anxiety phenotype. Rnf41 is one of the candidate genes in our current region, but the bioinformatics candidate gene analysis that we present here, which integrates sequence, expression, and pathway analysis data, suggests several stronger candidates. In addition, separate work that we have performed regarding anxiety-related behavioral phenotypes (Parker et al., 2013), which maps to this same region and may be due to the same allele that influences seizure sensitivity, suggests Rnf41 is outside the critical region.
Approximately one third of patients with epilepsy have seizures that are not controlled by current therapies (Kwan and Brodie, 2000). Thus, there is a pressing need for new strategies to treat epilepsy; and genetic discovery may open up new avenues for treatment. One approach to identifying new epilepsy genes is prioritization of candidates using what is known about existing epilepsy genes, such as the importance of genes influencing ion channel function. Although this approach has a strong foundation, it may not identify innovative pathways generate new treatments. The approach we describe has allowed us to identify several high priority novel candidate genes by identifying genes influencing seizure susceptibility in mice. This approach has proven to be successful in the identification genes that play a role in human epilepsy arising from identification of a mouse seizure susceptibility locus (Bockenhauer et al., 2009, Buono et al., 2004, Ferraro et al., 2001, Ferraro et al., 2004, Sala-Rabanal et al., 2010, Otto et al., 2004, Yang et al., 2003, Tokuda et al., 2011). Mapping studies in mice have also provided insight into genes controlling seizure induced cell death (Kong et al., 2008, Lorenzana et al., 2007, Schauwecker, 2002, Schauwecker, 2011a, Schauwecker et al., 2004) and modifier genes in epilepsy (Hawkins and Kearney, 2012, Bergren et al., 2009, Kearney et al., 2002, Martin et al., 2007, Sprunger et al., 1999).
The strategies used here can serve as a foundation for the discovery of genes that underlie genetically complex human disorders like epilepsy, improving understanding of pathophysiology and informing novel strategies for treatment.
Supplementary Material
Acknowledgments
Supported by NINDS R01 NS061991
Footnotes
None of the authors has any conflicts of interest to disclose.
References
- BASEL-VANAGAITE L, HERSHKOVITZ T, HEYMAN E, RASPALL-CHAURE M, KAKAR N, SMIRIN-YOSEF P, VILA-PUEYO M, KORNREICH L, THIELE H, BODE H, LAGOVSKY I, DAHARY D, HAVIV A, HUBSHMAN MW, PASMANIK-CHOR M, NURNBERG P, GOTHELF D, KUBISCH C, SHOHAT M, MACAYA A, BORCK G. Biallelic SZT2 mutations cause infantile encephalopathy with epilepsy and dysmorphic corpus callosum. Am J Hum Genet. 2013;93:524–9. doi: 10.1016/j.ajhg.2013.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BERGREN SK, RUTTER ED, KEARNEY JA. Fine mapping of an epilepsy modifier gene on mouse Chromosome 19. Mamm Genome. 2009;20:359–66. doi: 10.1007/s00335-009-9193-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOCKENHAUER D, FEATHER S, STANESCU HC, BANDULIK S, ZDEBIK AA, REICHOLD M, TOBIN J, LIEBERER E, STERNER C, LANDOURE G, ARORA R, SIRIMANNA T, THOMPSON D, CROSS JH, VAN’T HOFF W, AL MASRI O, TULLUS K, YEUNG S, ANIKSTER Y, KLOOTWIJK E, HUBANK M, DILLON MJ, HEITZMANN D, ARCOS-BURGOS M, KNEPPER MA, DOBBIE A, GAHL WA, WARTH R, SHERIDAN E, KLETA R. Epilepsy, ataxia, sensorineural deafness, tubulopathy, and KCNJ10 mutations. N Engl J Med. 2009;360:1960–70. doi: 10.1056/NEJMoa0810276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BORGES K, GEARING M, MCDERMOTT DL, SMITH AB, ALMONTE AG, WAINER BH, DINGLEDINE R. Neuronal and glial pathological changes during epileptogenesis in the mouse pilocarpine model. Exp Neurol. 2003;182:21–34. doi: 10.1016/s0014-4886(03)00086-4. [DOI] [PubMed] [Google Scholar]
- BROOKS-KAYAL AR, RAOL YH, RUSSEK SJ. Alteration of epileptogenesis genes. Neurotherapeutics. 2009;6:312–8. doi: 10.1016/j.nurt.2009.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BUONO RJ, LOHOFF FW, SANDER T, SPERLING MR, O’CONNOR MJ, DLUGOS DJ, RYAN SG, GOLDEN GT, ZHAO H, SCATTERGOOD TM, BERRETTINI WH, FERRARO TN. Association between variation in the human KCNJ10 potassium ion channel gene and seizure susceptibility. Epilepsy Res. 2004;58:175–83. doi: 10.1016/j.eplepsyres.2004.02.003. [DOI] [PubMed] [Google Scholar]
- CHAIX Y, FERRARO TN, LAPOUBLE E, MARTIN B. Chemoconvulsant-induced seizure susceptibility: toward a common genetic basis? Epilepsia. 2007;48(Suppl 5):48–52. doi: 10.1111/j.1528-1167.2007.01289.x. [DOI] [PubMed] [Google Scholar]
- DIBBENS LM, HERON SE, MULLEY JC. A polygenic heterogeneity model for common epilepsies with complex genetics. Genes Brain Behav. 2007;6:593–7. doi: 10.1111/j.1601-183X.2007.00333.x. [DOI] [PubMed] [Google Scholar]
- ENGSTROM FL, WOODBURY DM. Seizure susceptibility in DBA and C57 mice: the effects of various convulsants. Epilepsia. 1988;29:389–95. doi: 10.1111/j.1528-1157.1988.tb03736.x. [DOI] [PubMed] [Google Scholar]
- FERRARO TN, GOLDEN GT, SMITH GG, BERRETTINI WH. Differential susceptibility to seizures induced by systemic kainic acid treatment in mature DBA/2J and C57BL/6J mice. Epilepsia. 1995;36:301–7. doi: 10.1111/j.1528-1157.1995.tb00999.x. [DOI] [PubMed] [Google Scholar]
- FERRARO TN, GOLDEN GT, SMITH GG, DEMUTH D, BUONO RJ, BERRETTINI WH. Mouse strain variation in maximal electroshock seizure threshold. Brain Res. 2002;936:82–6. doi: 10.1016/s0006-8993(02)02565-9. [DOI] [PubMed] [Google Scholar]
- FERRARO TN, GOLDEN GT, SMITH GG, LONGMAN RL, SNYDER RL, DEMUTH D, SZPILZAK I, MULHOLLAND N, ENG E, LOHOFF FW, BUONO RJ, BERRETTINI WH. Quantitative genetic study of maximal electroshock seizure threshold in mice: evidence for a major seizure susceptibility locus on distal chromosome 1. Genomics. 2001;75:35–42. doi: 10.1006/geno.2001.6577. [DOI] [PubMed] [Google Scholar]
- FERRARO TN, GOLDEN GT, SMITH GG, MARTIN JF, LOHOFF FW, GIERINGER TA, ZAMBONI D, SCHWEBEL CL, PRESS DM, KRATZER SO, ZHAO H, BERRETTINI WH, BUONO RJ. Fine mapping of a seizure susceptibility locus on mouse Chromosome 1: nomination of Kcnj10 as a causative gene. Mamm Genome. 2004;15:239–51. doi: 10.1007/s00335-003-2270-3. [DOI] [PubMed] [Google Scholar]
- FERRARO TN, GOLDEN GT, SMITH GG, ST JEAN P, SCHORK NJ, MULHOLLAND N, BALLAS C, SCHILL J, BUONO RJ, BERRETTINI WH. Mapping loci for pentylenetetrazol-induced seizure susceptibility in mice. J Neurosci. 1999;19:6733–9. doi: 10.1523/JNEUROSCI.19-16-06733.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FERRARO TN, GOLDEN GT, SNYDER R, LAIBINIS M, SMITH GG, BUONO RJ, BERRETTINI WH. Genetic influences on electrical seizure threshold. Brain Res. 1998;813:207–10. doi: 10.1016/s0006-8993(98)01013-0. [DOI] [PubMed] [Google Scholar]
- FRANKEL WN, TAYLOR L, BEYER B, TEMPEL BL, WHITE HS. Electroconvulsive thresholds of inbred mouse strains. Genomics. 2001;74:306–12. doi: 10.1006/geno.2001.6564. [DOI] [PubMed] [Google Scholar]
- FRANKEL WN, YANG Y, MAHAFFEY CL, BEYER BJ, O’BRIEN TP. Szt2, a novel gene for seizure threshold in mice. Genes Brain Behav. 2009;8:568–76. doi: 10.1111/j.1601-183X.2009.00509.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GERSHENFELD HK, NEUMANN PE, LI X, ST JEAN PL, PAUL SM. Mapping quantitative trait loci for seizure response to a GABAA receptor inverse agonist in mice. J Neurosci. 1999;19:3731–8. doi: 10.1523/JNEUROSCI.19-10-03731.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GROSSO S, PUCCI L, FARNETANI M, DI BARTOLO RM, GALIMBERTI D, MOSTARDINI R, ANICHINI C, BALESTRI M, MORGESE G, BALESTRI P. Epilepsy and electroencephalographic findings in pericentric inversion of chromosome 12. J Child Neurol. 2004;19:604–8. doi: 10.1177/088307380401900807. [DOI] [PubMed] [Google Scholar]
- HAIN HS, CRABBE JC, BERGESON SE, BELKNAP JK. Cocaine-induced seizure thresholds: quantitative trait loci detection and mapping in two populations derived from the C57BL/6 and DBA/2 mouse strains. J Pharmacol Exp Ther. 2000;293:180–7. [PubMed] [Google Scholar]
- HAWKINS NA, KEARNEY JA. Confirmation of an epilepsy modifier locus on mouse chromosome 11 and candidate gene analysis by RNA-Seq. Genes Brain Behav. 2012;11:452–60. doi: 10.1111/j.1601-183X.2012.00790.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HELBIG I, SCHEFFER IE, MULLEY JC, BERKOVIC SF. Navigating the channels and beyond: unravelling the genetics of the epilepsies. Lancet Neurol. 2008;7:231–45. doi: 10.1016/S1474-4422(08)70039-5. [DOI] [PubMed] [Google Scholar]
- JANSEN LA, UHLMANN EJ, CRINO PB, GUTMANN DH, WONG M. Epileptogenesis and reduced inward rectifier potassium current in tuberous sclerosis complex-1-deficient astrocytes. Epilepsia. 2005;46:1871–80. doi: 10.1111/j.1528-1167.2005.00289.x. [DOI] [PubMed] [Google Scholar]
- KEANE TM, GOODSTADT L, DANECEK P, WHITE MA, WONG K, YALCIN B, HEGER A, AGAM A, SLATER G, GOODSON M, FURLOTTE NA, ESKIN E, NELLAKER C, WHITLEY H, CLEAK J, JANOWITZ D, HERNANDEZ-PLIEGO P, EDWARDS A, BELGARD TG, OLIVER PL, MCINTYRE RE, BHOMRA A, NICOD J, GAN X, YUAN W, VAN DER WEYDEN L, STEWARD CA, BALA S, STALKER J, MOTT R, DURBIN R, JACKSON IJ, CZECHANSKI A, GUERRA-ASSUNCAO JA, DONAHUE LR, REINHOLDT LG, PAYSEUR BA, PONTING CP, BIRNEY E, FLINT J, ADAMS DJ. Mouse genomic variation and its effect on phenotypes and gene regulation. Nature. 2011;477:289–94. doi: 10.1038/nature10413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KEARNEY JA, BUCHNER DA, DE HAAN G, ADAMSKA M, LEVIN SI, FURAY AR, ALBIN RL, JONES JM, MONTAL M, STEVENS MJ, SPRUNGER LK, MEISLER MH. Molecular and pathological effects of a modifier gene on deficiency of the sodium channel Scn8a (Na(v)1.6) Hum Mol Genet. 2002;11:2765–75. doi: 10.1093/hmg/11.22.2765. [DOI] [PubMed] [Google Scholar]
- KIM S, ZHANG S, CHOI KH, REISTER R, DO C, BAYKIZ AF, GERSHENFELD HK. An E3 ubiquitin ligase, Really Interesting New Gene (RING) Finger 41, is a candidate gene for anxiety-like behavior and beta-carboline-induced seizures. Biol Psychiatry. 2009;65:425–31. doi: 10.1016/j.biopsych.2008.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KONG S, LORENZANA A, DENG Q, MCNEILL TH, SCHAUWECKER PE. Variation in Galr1 expression determines susceptibility to exocitotoxin-induced cell death in mice. Genes Brain Behav. 2008;7:587–98. doi: 10.1111/j.1601-183X.2008.00395.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOSOBUD AE, CRABBE JC. Genetic correlations among inbred strain sensitivities to convulsions induced by 9 convulsant drugs. Brain Res. 1990;526:8–16. doi: 10.1016/0006-8993(90)90243-5. [DOI] [PubMed] [Google Scholar]
- KWAN P, BRODIE MJ. Early identification of refractory epilepsy. N Engl J Med. 2000;342:314–9. doi: 10.1056/NEJM200002033420503. [DOI] [PubMed] [Google Scholar]
- LEITE JP, GARCIA-CAIRASCO N, CAVALHEIRO EA. New insights from the use of pilocarpine and kainate models. Epilepsy Res. 2002;50:93–103. doi: 10.1016/s0920-1211(02)00072-4. [DOI] [PubMed] [Google Scholar]
- LIM S, KALDIS P. Loss of Cdk2 and Cdk4 induces a switch from proliferation to differentiation in neural stem cells. Stem Cells. 2012;30:1509–20. doi: 10.1002/stem.1114. [DOI] [PubMed] [Google Scholar]
- LORENZANA A, CHANCER Z, SCHAUWECKER PE. A quantitative trait locus on chromosome 18 is a critical determinant of excitotoxic cell death susceptibility. Eur J Neurosci. 2007;25:1998–2008. doi: 10.1111/j.1460-9568.2007.05443.x. [DOI] [PubMed] [Google Scholar]
- MANSOUR HA, WOOD J, LOGUE T, CHOWDARI KV, DAYAL M, KUPFER DJ, MONK TH, DEVLIN B, NIMGAONKAR VL. Association study of eight circadian genes with bipolar I disorder, schizoaffective disorder and schizophrenia. Genes Brain Behav. 2006;5:150–7. doi: 10.1111/j.1601-183X.2005.00147.x. [DOI] [PubMed] [Google Scholar]
- MARGARI L, DI COSOLA ML, BUTTIGLIONE M, PANSINI A, BUONADONNA AL, CRAIG F, CARIOLA F, PETRUZZELLI MG, GENTILE M. Molecular cytogenetic characterization and genotype/phenotype analysis in a patient with a de novo 8p23.2p23.3 deletion/12p13.31p13.33 duplication. Am J Med Genet A. 2012;158A:1713–8. doi: 10.1002/ajmg.a.35400. [DOI] [PubMed] [Google Scholar]
- MARTIN MS, TANG B, PAPALE LA, YU FH, CATTERALL WA, ESCAYG A. The voltage-gated sodium channel Scn8a is a genetic modifier of severe myoclonic epilepsy of infancy. Hum Mol Genet. 2007;16:2892–9. doi: 10.1093/hmg/ddm248. [DOI] [PubMed] [Google Scholar]
- MCKHANN GM, 2ND, WENZEL HJ, ROBBINS CA, SOSUNOV AA, SCHWARTZKROIN PA. Mouse strain differences in kainic acid sensitivity, seizure behavior, mortality, and hippocampal pathology. Neuroscience. 2003;122:551–61. doi: 10.1016/s0306-4522(03)00562-1. [DOI] [PubMed] [Google Scholar]
- MORAR B, ZHELYAZKOVA S, AZMANOV DN, RADIONOVA M, ANGELICHEVA D, GUERGUELTCHEVA V, KANEVA R, SCHEFFER IE, TOURNEV I, KALAYDJIEVA L, SANDER JW. A novel GEFS+ locus on 12p13.33 in a large Roma family. Epilepsy Res. 2011;97:198–207. doi: 10.1016/j.eplepsyres.2011.08.009. [DOI] [PubMed] [Google Scholar]
- MULLEY JC, DIBBENS LM. Chipping away at the common epilepsies with complex genetics: the 15q13.3 microdeletion shows the way. Genome Med. 2009;1:33. doi: 10.1186/gm33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NEUMANN PE, COLLINS RL. Genetic dissection of susceptibility to audiogenic seizures in inbred mice. Proc Natl Acad Sci U S A. 1991;88:5408–12. doi: 10.1073/pnas.88.12.5408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NEUMANN PE, COLLINS RL. Confirmation of the influence of a chromosome 7 locus on susceptibility to audiogenic seizures. Mamm Genome. 1992;3:250–3. doi: 10.1007/BF00292152. [DOI] [PubMed] [Google Scholar]
- OTTMAN R. Analysis of genetically complex epilepsies. Epilepsia. 2005;46(Suppl 10):7–14. doi: 10.1111/j.1528-1167.2005.00350.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OTTMAN R, ANNEGERS JF, RISCH N, HAUSER WA, SUSSER M. Relations of genetic and environmental factors in the etiology of epilepsy. Ann Neurol. 1996;39:442–9. doi: 10.1002/ana.410390406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OTTMAN R, HIROSE S, JAIN S, LERCHE H, LOPES-CENDES I, NOEBELS JL, SERRATOSA J, ZARA F, SCHEFFER IE. Genetic testing in the epilepsies--report of the ILAE Genetics Commission. Epilepsia. 51:655–70. doi: 10.1111/j.1528-1167.2009.02429.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OTTO JF, YANG Y, FRANKEL WN, WHITE HS, WILCOX KS. A spontaneous mutation involving Kcnq2 (Kv7.2) reduces M-current density and spike frequency adaptation in mouse CA1 neurons. J Neurosci. 2006;26:2053–9. doi: 10.1523/JNEUROSCI.1575-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OTTO JF, YANG Y, FRANKEL WN, WILCOX KS, WHITE HS. Mice carrying the szt1 mutation exhibit increased seizure susceptibility and altered sensitivity to compounds acting at the m-channel. Epilepsia. 2004;45:1009–16. doi: 10.1111/j.0013-9580.2004.65703.x. [DOI] [PubMed] [Google Scholar]
- PARKER CC, SOKOLOFF G, LEUNG E, KIRKPATRICK SL, PALMER AA. A large QTL for fear and anxiety mapped using an F cross can be dissected into multiple smaller QTLs. Genes Brain Behav. 2013;12 (7):714–722. doi: 10.1111/gbb.12064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RACINE RJ. Modification of seizure activity by electrical stimulation. II. Motor seizure. Electroencephalogr Clin Neurophysiol. 1972;32:281–94. doi: 10.1016/0013-4694(72)90177-0. [DOI] [PubMed] [Google Scholar]
- REID CA, BERKOVIC SF, PETROU S. Mechanisms of human inherited epilepsies. Prog Neurobiol. 2009;87:41–57. doi: 10.1016/j.pneurobio.2008.09.016. [DOI] [PubMed] [Google Scholar]
- SALA-RABANAL M, KUCHERYAVYKH LY, SKATCHKOV SN, EATON MJ, NICHOLS CG. Molecular mechanisms of EAST/SeSAME syndrome mutations in Kir4.1 (KCNJ10) J Biol Chem. 2010;285:36040–8. doi: 10.1074/jbc.M110.163170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHAUWECKER PE. Modulation of cell death by mouse genotype: differential vulnerability to excitatory amino acid-induced lesions. Exp Neurol. 2002;178:219–35. doi: 10.1006/exnr.2002.8038. [DOI] [PubMed] [Google Scholar]
- SCHAUWECKER PE. Congenic strains provide evidence that a mapped locus on chromosome 15 influences excitotoxic cell death. Genes Brain Behav. 2011a;10:100–10. doi: 10.1111/j.1601-183X.2010.00644.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHAUWECKER PE. The relevance of individual genetic background and its role in animal models of epilepsy. Epilepsy Res. 2011b;97:1–11. doi: 10.1016/j.eplepsyres.2011.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHAUWECKER PE, WILLIAMS RW, SANTOS JB. Genetic control of sensitivity to hippocampal cell death induced by kainic acid: a quantitative trait loci analysis. J Comp Neurol. 2004;477:96–107. doi: 10.1002/cne.20245. [DOI] [PubMed] [Google Scholar]
- SPRUNGER LK, ESCAYG A, TALLAKSEN-GREENE S, ALBIN RL, MEISLER MH. Dystonia associated with mutation of the neuronal sodium channel Scn8a and identification of the modifier locus Scnm1 on mouse chromosome 3. Hum Mol Genet. 1999;8:471–9. doi: 10.1093/hmg/8.3.471. [DOI] [PubMed] [Google Scholar]
- SWINYARD E. Electrically induced convulsions. New York: Raven Press; 1972a. [Google Scholar]
- SWINYARD EA. Electrically induced convulsions. In: PURPURA D, PENRY J, TOWER D, editors. Expreimental Models of Epilepsy--A Manual for the Laboratory Worker. New York: Raven Press; 1972b. [Google Scholar]
- TOKUDA S, MAHAFFEY CL, MONKS B, FAULKNER CR, BIRNBAUM MJ, DANZER SC, FRANKEL WN. A novel Akt3 mutation associated with enhanced kinase activity and seizure susceptibility in mice. Hum Mol Genet. 2011;20:988–99. doi: 10.1093/hmg/ddq544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TURSKI L, IKONOMIDOU C, TURSKI WA, BORTOLOTTO ZA, CAVALHEIRO EA. Review: cholinergic mechanisms and epileptogenesis. The seizures induced by pilocarpine: a novel experimental model of intractable epilepsy. Synapse. 1989;3:154–71. doi: 10.1002/syn.890030207. [DOI] [PubMed] [Google Scholar]
- TURSKI WA. Pilocarpine-induced seizures in rodents--17 years on. Pol J Pharmacol. 2000;52:63–5. [PubMed] [Google Scholar]
- VAGLIO A, MILUNSKY A, HUANG XL, QUADRELLI A, MECHOSO B, QUADRELLI R. A fourteen years follow-up of a case of partial trisomy 12q and monosomy 12p recombinants of a familial pericentric inversion of chromosome 12: clinical, cytogenetic and molecular observations. Eur J Med Genet. 2007;50:224–32. doi: 10.1016/j.ejmg.2007.01.001. [DOI] [PubMed] [Google Scholar]
- WHITE HS, JOHNSON M, WOLF HH, KUPFERBERG HJ. The early identification of anticonvulsant activity: role of the maximal electroshock and subcutaneous pentylenetetrazol seizure models. Ital J Neurol Sci. 1995;16:73–7. doi: 10.1007/BF02229077. [DOI] [PubMed] [Google Scholar]
- WINAWER MR, GILDERSLEEVE SS, PHILLIPS AG, RABINOWITZ D, PALMER AA. Mapping a mouse limbic seizure susceptibility locus on chromosome 10. Epilepsia. 2011;52:2076–83. doi: 10.1111/j.1528-1167.2011.03256.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WINAWER MR, KUPERMAN R, NIETHAMMER M, SHERMAN S, RABINOWITZ D, GUELL IP, PONDER CA, PALMER AA. Use of chromosome substitution strains to identify seizure susceptibility loci in mice. Mamm Genome. 2007a;18:23–31. doi: 10.1007/s00335-006-0087-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WINAWER MR, MAKARENKO N, MCCLOSKEY DP, HINTZ TM, NAIR N, PALMER AA, SCHARFMAN HE. Acute and chronic responses to the convulsant pilocarpine in DBA/2J and A/J mice. Neuroscience. 2007b;149:465–75. doi: 10.1016/j.neuroscience.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YALCIN B, WONG K, AGAM A, GOODSON M, KEANE TM, GAN X, NELLAKER C, GOODSTADT L, NICOD J, BHOMRA A, HERNANDEZ-PLIEGO P, WHITLEY H, CLEAK J, DUTTON R, JANOWITZ D, MOTT R, ADAMS DJ, FLINT J. Sequence-based characterization of structural variation in the mouse genome. Nature. 2011;477:326–9. doi: 10.1038/nature10432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YANG Y, BEYER BJ, OTTO JF, O’BRIEN TP, LETTS VA, WHITE HS, FRANKEL WN. Spontaneous deletion of epilepsy gene orthologs in a mutant mouse with a low electroconvulsive threshold. Hum Mol Genet. 2003;12:975–84. doi: 10.1093/hmg/ddg118. [DOI] [PubMed] [Google Scholar]
- ZHANG S, LOU Y, AMSTEIN TM, ANYANGO M, MOHIBULLAH N, OSOTI A, STANCLIFFE D, KING R, IRAQI F, GERSHENFELD HK. Fine mapping of a major locus on chromosome 10 for exploratory and fear-like behavior in mice. Mamm Genome. 2005;16:306–18. doi: 10.1007/s00335-004-2427-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.