Abstract
Numerous epidemiological studies have reported that the long-term use of nonsteroidal anti-inflammatory drugs (NSAIDs) is associated with a significant decrease in cancer incidence and delayed progression of malignant disease. The use of NSAIDs has also been linked with reduced risk from cancer-related mortality and distant metastasis. Certain prescription strength NSAIDs, such as sulindac, have been shown to cause regression of precancerous lesions. Unfortunately, the extended use of NSAIDs for chemoprevention results in potentially fatal side effects related to their cyclooxygenase (COX)-inhibitory activity and suppression of prostaglandin synthesis. While the basis for the tumor growth-inhibitory activity of NSAIDs likely involves multiple effects on tumor cells and their microenvironment, numerous investigators have concluded that the underlying mechanism is not completely explained by COX inhibition. It may therefore be possible to develop safer and more efficacious drugs by targeting such COX-independent mechanisms. NSAID derivatives or metabolites that lack COX-inhibitory activity, but retain or have improved anticancer activity support this possibility. Experimental studies suggest that apoptosis induction and suppression of β-catenin-dependent transcription are important aspects of their antineoplastic activity. Studies show that the latter involves phosphodiesterase inhibition and the elevation of intracellular cyclic GMP levels. Here, we review the evidence for COX-independent mechanisms and discuss progress towards identifying alternative targets and developing NSAID derivatives that lack COX-inhibitory activity but have improved antineoplastic properties.
Keywords: Chemoprevention, NSAIDs, sulindac, colorectal cancer
Introduction
Despite significant advances in early diagnosis and the development of molecularly targeted drugs, cancer remains the leading cause of mortality in the Western world (1). Chemoprevention using pharmaceuticals or by dietary intervention represents a well-accepted approach to inhibit disease progression in individuals with precancerous lesions, and in high-risk populations with genetic predispositions or long-term exposure to environmental carcinogens such as cigarette smoke. However, the implementation of chemoprevention strategies mandates exceptional safety and efficacy. Over the past three decades, epidemiological, clinical and experimental studies have established that nonsteroidal anti-inflammatory drugs (NSAIDs) inhibit carcinogenesis in various tissues and at different stages of progression. Despite the strong evidence of activity, the use of NSAIDs for cancer chemoprevention is not recommended because of potentially severe gastrointestinal, renal, and cardiovascular side effects that result from cyclooxygenase (COX) inhibition and the suppression of physiologically important prostaglandins. In addition, the chemopreventive efficacy of NSAIDs is incomplete, although it is unclear if this shortfall is due to dosage limitations or resistance factors.
The molecular and cellular mechanisms responsible for the cancer chemopreventive properties of NSAIDs are complex and likely involve multiple effects on cancer cells and their microenvironment. Inhibition of COX is generally thought to be the primary mechanism responsible for their antineoplastic activity, although numerous studies have concluded that alternative targets may be involved, as reviewed previously (2–4). Given that the use of NSAIDs for cancer chemoprevention is limited by COX-dependent toxicities, identifying the relevant targets that mediate their antitumor properties provides an opportunity to develop safer and more efficacious derivatives, or new chemical entities. In this review, we provide an overview of the chemopreventive effects of NSAIDs, highlight evidence that the mechanism involves COX-independent effects, and discuss progress towards identifying new targets and developing NSAID derivatives that lack COX-inhibitory activity.
Classification of NSAIDs
NSAIDs are a chemically diverse family of drugs available over-the-counter or by prescription and are commonly used for the treatment of inflammation, pain, or fever. Their anti-inflammatory activity is attributed to the inhibition of COX (5) enzymes that catalyze the conversion of arachidonic acid into prostaglandin H2, the precursor for the synthesis of prostaglandins (PGs), prostacyclin and thromboxane A2 – collectively referred to as eicosanoids. The three major PG products of COX activity, PGE2, PGD2 and PGF2α, promote inflammation, pain and fever. Vane and colleagues were the first to show that aspirin inhibits inflammation by suppressing PG synthesis (6), while COX inhibition was later shown to be responsible for this effect (7). Aside from their role in inflammation, eicosanoids are critically important for the homeostatic maintenance of the gastrointestinal (GI) mucosa, blood clotting, regulation of blood flow, and kidney function.
Two distinct isoforms of COX, COX-1 and COX-2, have been reported (8). COX-1 is constitutively expressed in most tissues, whereas COX-2 is induced by inflammatory stimuli, mitogens or growth factors, and is generally associated with pathological processes (9). Conventional NSAIDs, such as aspirin, ibuprofen, sulindac and indomethacin inhibit both COX-1 and -2, although aspirin has a unique mechanism involving irreversible acetylation of a serine residue in the catalytic domain of both enzymes (10). The recognition that COX-2 is the main mediator of inflammation led to the development of a new class of inhibitors with COX-2 selectivity (Coxibs) to circumvent GI and renal toxicities associated with nonselective NSAIDs. However, Coxibs were later found to increase the risk of heart attack and stroke (11, 12), which resulted in the recognition that all NSAIDs have risks of cardiovascular side effects.
Cancer Chemopreventive Properties of NSAIDs
Epidemiological and clinical evidence
Many population-based studies have concluded that long-term use of NSAIDs is associated with a lower risk of developing colonic adenomatous polyps and lower incidence of CRC (13, 14). Although fewer epidemiological studies have been conducted on cancers other than CRC, most have reported an inverse correlation between the long-term use of NSAIDs and incidence of tumors of the breast (15, 16), lung (17), prostate (18), bladder (19), ovary (20), esophagus (19) and stomach (19).
Clinical evidence of activity for the treatment of precancerous conditions was first reported in case studies by Waddell and Loughry in 1983, in which administration of sulindac (Clinoril®) reduced colonic adenomas in patients with familial adenomatous polyposis (FAP) (21). Later, three randomized clinical trials confirmed that sulindac at a daily dose of 300-400 mg reduced adenomas in FAP patients by an estimated 71% within 4-6 months of treatment (22). By comparison, the COX-2 selective inhibitor celecoxib (Celebrex®) at an 800 mg daily dose decreased rectal adenomas in FAP patients by only 23% after 6 months of treatment (23), which nonetheless led to the FDA approval of celecoxib for the treatment of FAP in 1999. The anticancer activity of COX-2 inhibitors also sparked considerable interest in the role of COX-2 in carcinogenesis. However, subsequent studies in patients with sporadic adenomas using another COX-2 inhibitor, rofecoxib, revealed unexpected cardiovascular toxicity (24) that caused it to be withdrawn from the market and essentially halted other clinical trials of Coxibs for cancer chemoprevention.
Several studies have also reported that NSAIDs reduce the risk of death in patients with advanced colon and breast cancers, and may prevent metastasis of primary tumors or reduce mortality after diagnosis of malignant disease (25, 26). One clinical study reported that indomethacin can significantly extend survival of patients with metastatic disease (27), which suggests that NSAIDs can inhibit biological processes associated with tumor cell invasion.
Evidence from experimental studies
The epidemiological evidence that NSAIDs reduce the risk of developing cancer is supported by an abundance of reports from experimental animal models, including carcinogen-induced or transgenic models of colorectal, breast and other types of cancer. Among the first reports of the anticancer activity of NSAIDs in rodent models are studies by Pollard et al. and Narisawa et al. that described the inhibitory effects of indomethacin on carcinogen-induced intestinal tumors (28, 29). Subsequent studies demonstrated antitumor efficacy for NSAIDs from different classes against colorectal carcinogenesis (30, 31). Many of these studies utilized the rodent azoxymethane (AOM) carcinogen model, which closely mimics human colorectal cancer with mutations in β-catenin and APC (32, 33). Consistent with their benefits for the treatment of FAP, NSAIDs and COX-2 inhibitors are also effective in the Min mouse, which harbors the same germline mutation in the APC gene (34, 35). Notably, NSAIDs were found to strongly inhibit the formation of aberrant crypt foci (ACF), the earliest detectable neoplastic lesions in the colorectum (36, 37). While most studies have reported that NSAIDs inhibit tumorigenesis if administered prior to AOM exposure, studies by Reddy and Rao established that NSAIDs are still highly effective when treatment is initiated later in tumor progression when ACF and adenomas already existed (38, 39). These observations are consistent with the ability of NSAIDs such as sulindac to cause the regression of existing lesions in FAP patients (40).
COX-independent mechanisms of NSAID Chemoprevention
Observations that certain eicosanoids, such as PGE2, are elevated in various human tumor tissues (41) and can stimulate tumor cell proliferation (42), along with studies implicating COX-2 in tumor progression (43) and regulation of apoptosis (44), led to the widely accepted belief that COX-2 is an important target responsible for the chemopreventive effects of NSAIDs. However, numerous studies challenge this assumption by providing evidence that these effects can be exerted through a COX-independent mechanism. For example, in vitro studies have demonstrated that NSAIDs inhibit proliferation and/or induce apoptosis in multiple tumor cell lines of different origins irrespective of COX-1 or COX-2 expression (45, 46). In addition, the growth inhibitory activity of NSAIDs cannot be reversed by PG supplementation (47). There is also a discrepancy between the potency of a particular NSAID to inhibit COX-1 and/or COX-2 and its potency to inhibit tumor cell growth, whereby the concentration required to inhibit tumor cell proliferation is much higher than that required to inhibit COX activity, as illustrated in Table 1. This is an important consideration since experimental and clinical studies typically demonstrate chemopreventive efficacy of NSAIDs at doses appreciably higher than those necessary for anti-inflammatory effects. For example, celecoxib caused a significant reduction in colorectal polyp burden in FAP patients at a dose of 800 mg/day but not at the standard anti-inflammatory dose of 200 mg/day bid (23). The possibility that an off-target effect accounts for the chemopreventive activity of NSAIDs may therefore explain their incomplete efficacy in clinical trials involving standard anti-inflammatory dosages.
Table 1.
Potency of a panel of NSAIDs to inhibit colon tumor cell growth and cyclooxygenases.
| NSAID | Growth IC501 (µmol/L) |
COX-1 IC502 (µmol/L) |
COX-2 IC502 (µmol/L) |
Serum levels (µmol/L)3 |
|---|---|---|---|---|
| Celecoxib | 50 | >30 | 2.25 | 2 |
| Sulindac sulfide | 60 | 1.02 | 10.4 | 15 |
| Diclofenac | 160 | 0.14 | 0.05 | 6 |
| Indomethacin | 180 | 0.16 | 0.46 | 1.4 |
| Piroxicam | 900 | 0.76 | 8.9 | 17 |
| Ibuprofen | 975 | 4.75 | >30 | 40 |
| Flurbiprofen | 1800 | 0.44 | 6.42 | 53 |
| Aspirin | 5000 | 4.5 | 13.9 | 10 |
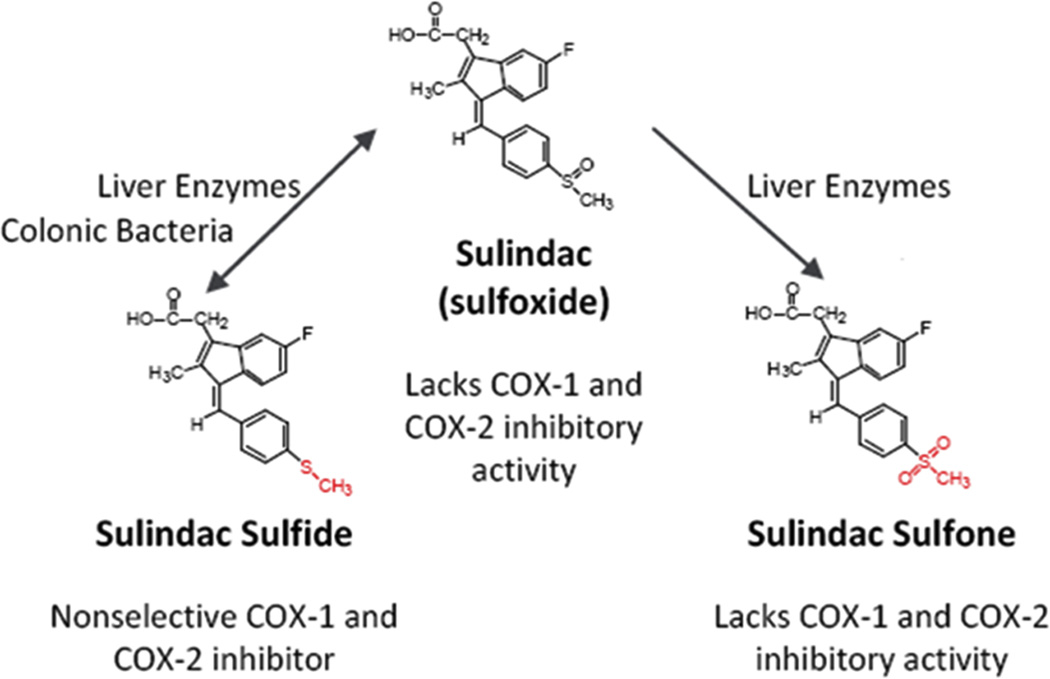
Perhaps the strongest evidence for a COX-independent mechanism comes from experimental studies showing that non-COX inhibitory metabolites (48), enantiomers (49) or derivatives (50) retain or have improved antitumor activity compared with the parent NSAID. Among these, the sulfone metabolite of sulindac, exisulind, is the most studied, for which there is an abundance of evidence of efficacy from various rodent models of carcinogenesis (51–53), as summarized in Table 2. Figure 1 illustrates the metabolism of sulindac into the active sulfide form and the non-COX-inhibitory sulfone. In addition, exisulind has been reported to inhibit tumor cell growth and induce apoptosis in multiple tumor types despite lacking COX-1 or COX-2 inhibitory activity (48). In studies involving the AOM model of rat colon tumorigenesis, exisulind inhibited tumor formation at dosages that did not reduce prostaglandin levels in the colon mucosa, and achieved plasma concentrations above those required to inhibit tumor cell growth and induce apoptosis in vitro (52). In clinical trials, exisulind displayed significant adenoma regression in patients with familial (54) or sporadic (55) adenomatous polyposis but did not receive FDA approval due to hepatotoxicity and because of inherent problems with disease variation among FAP patients that were encountered during the registration trial. Nonetheless, its strong chemopreventive activity in preclinical models supports the importance of COX-independent mechanisms and the rationale for developing other non-COX-inhibitory sulindac derivatives with improved potency and target selectivity.
Table 2.
Chemopreventive efficacy of sulindacsulfone (exisulind) in rodent models of carcinogenesis.
| Model | Species | Dosage | Efficacy | Reference |
|---|---|---|---|---|
| Colon | Rat | 1000 – 2000 ppm | 69–81% | (52) |
| Colon | Rat | 600–1200 ppm | 41–83% | (109) |
| Colon (ACF) | Rat | 20 mg/kg bid | 31% | (110) |
| Colon (ACF) | Rat | 1000–2000 ppm | 42–37% | (111) |
| Mammary | Rat | 300–600 ppm | 44–50% | (53) |
| Lung | Mouse | 250–750 ppm | 32–82% | (51) |
| Bladder | Rat | 800–1200 ppm | 36–64% | (112) |
| Prostate | Rat | 1000 ppm | 80% | (113) |
Figure 1.
Metabolism of sulindac. Prodrug sulindac undergoes reversible reduction into the active sulfide form through the action of liver enzymes and colonic bacteria. Sulindac sulfide is a non-selective COX inhibitor and is responsible for the anti-inflammatory properties of sulindac. The sulfone metabolite is generated by irreversible oxidation of the sulfoxide in the liver, and does not have anti-inflammatory activity. Figure adapted from Gurpinar et al., Frontiers in Oncology, 2013 (106).
Molecular Targets
While an NSAID may act upon a COX-independent target with relatively high specificity, it is generally recognized that a combinatorial action on multiple pathways through direct molecular targets as well as epigenetic and post-transcriptional mechanisms is responsible for the chemopreventive properties of NSAIDs. Some of the major pathways targeted by NSAIDs are discussed below and illustrated in Table 3.
Table 3.
Cyclooxygenase-independent molecular targets of NSAIDs and their metabolites.
| Sulindac | Sulindac sulfide | Sulindacsulfone | Celecoxib | Aspirin | Salicylate | Indomethacin | R-etodolac | References | |
|---|---|---|---|---|---|---|---|---|---|
| COX-1 | - | x | - | - | x | - | x | - | |
| COX-2 | - | x | - | x | x | - | x | - | |
| COX-independent targets | |||||||||
| cGMPPDE | - | x | x | x | - | - | - | - | (65, 71, 72, 114) |
| PPARγ | - | - | - | - | - | - | x | - | (78) |
| PPARδ | - | x | - | - | - | - | x | - | (80) |
| RXRα | - | x | - | - | - | - | - | x | (81, 82) |
| IKKβ | x | - | - | - | x | x | - | - | (74, 75) |
| SERCA | - | x | - | x | - | - | - | - | (74, 87) |
| CA IX/XII | - | - | - | x | - | - | - | - | (83, 115) |
| Sp1 | - | - | - | x | - | - | - | - | (91) |
| AMPK | - | - | - | - | x | x | - | - | (76, 77) |
| Gene expression | |||||||||
| NAG-1 | - | x | x | - | x | x | x | - | (95, 97) |
| 15-Lox-1 | - | - | x | - | - | - | - | - | (116) |
Table adapted from Gurpinar et al., Frontiers in Oncology, 2013 (106).
Induction of Apoptosis
NSAIDs have long been recognized to inhibit tumor cell growth in cell culture models with significantly different potencies across chemical families (56). The basis for this activity was first reported to involve apoptosis induction by two independent groups in 1995 (57, 58). The mechanism appeared to be unrelated to COX inhibition as evident by the ability of exisulind to also induce apoptosis. Apoptosis emerged as the major mechanism of NSAID chemoprevention following observations that treatment with sulindac can stimulate apoptosis in the normal rectal mucosa of FAP patients (59), normal intestinal mucosa of APCMin mice (60) and in the colorectal carcinomas of carcinogen-treated rats (61). In addition, exisulind was reported to induce apoptosis in rectal polyps of FAP patients but not in normal rectal mucosa, which implies an aspect of tumor selectivity (54). Consistent with these observations, studies using cell culture models demonstrate that NSAIDs, as well as their non-COX-inhibitory derivatives, can induce apoptosis in various cancer cell lines.
Effects on Wnt/β-catenin pathway
Dysregulation of Wnt signaling due to inactivating mutations in APC or activating mutations in β-catenin, is involved in the development of multiple types of cancer, especially CRC (62). The efficacy of NSAIDs to inhibit polyp formation in FAP patients and APCMin mice suggested that they may compensate for such mutations by inhibiting Wnt signaling. Studies have reported that sulindac can reduce nuclear β-catenin levels and induce β-catenin degradation, which could explain its antiproliferative and pro-apoptotic activity (63, 64). Similarly, both exisulind (65) and celecoxib (66) were reported to decrease β-catenin levels and inhibit the transcriptional activity of the β-catenin/TCF/Lef complex. NSAIDs may therefore inhibit tumor cell growth by suppressing oncogenic β-catenin signaling through a COX-independent mechanism. Notably, colonic polyps of FAP patients treated with sulindac show reduced nuclear accumulation of β-catenin (67). Moreover, a recent study by Qui et al. showed that sulindac can selectively eliminate intestinal stem cells with nuclear or phosphorylated β-catenin and aberrant Wnt signaling in APCMin mice and in human colonic polyps through the induction of apoptosis (68). These observations are corroborated by findings that sulindac downregulates β-catenin levels in hematopoietic progenitor cells which carry oncogenic fusion proteins, resulting in reduced stem cell capacity and increased differentiation potential (69). These studies suggest that removal of cancer stem cells through direct inhibitory effects on Wnt/β-catenin signaling and induction of apoptosis is an important mechanism that mediates the chemopreventive effects of sulindac.
Modulation of cGMP PDE signaling
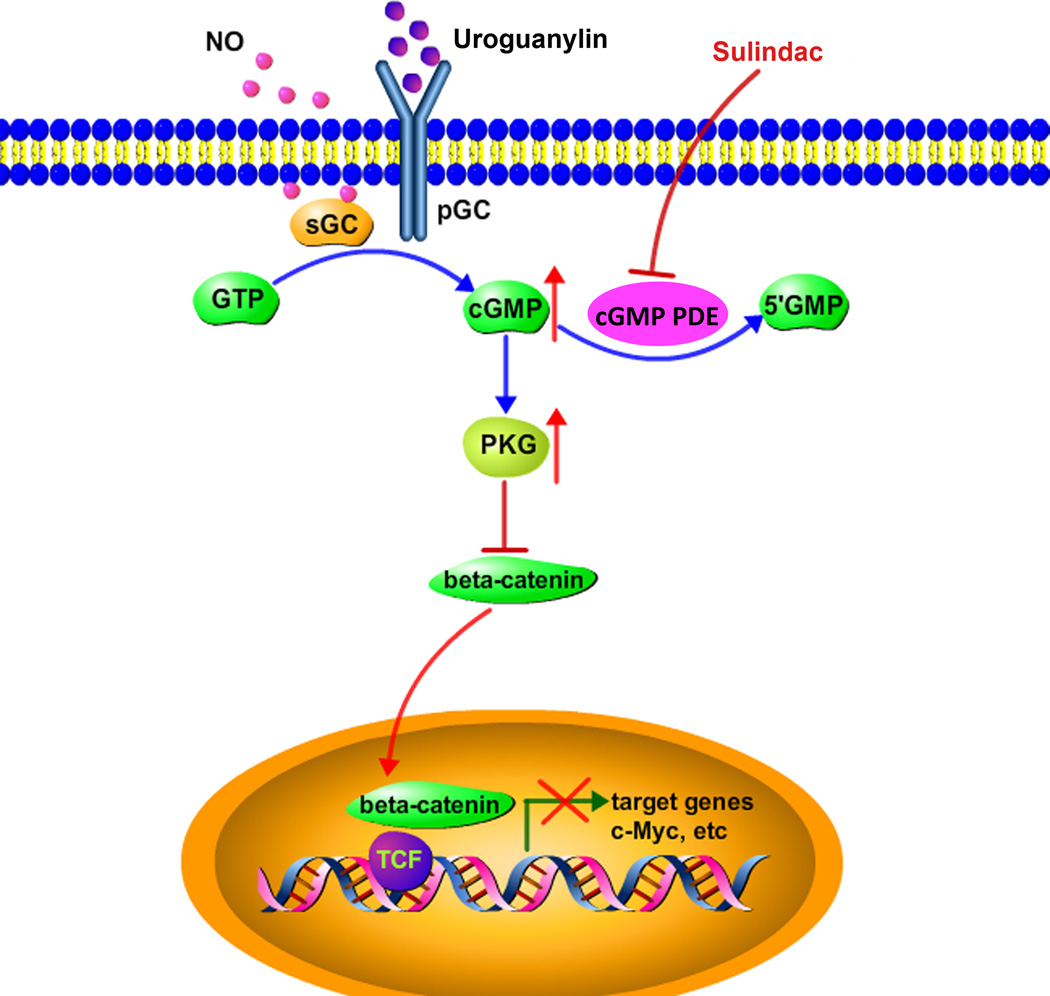
Previous studies with exisulind suggested that cyclic guanosine monophosphate phosphodiesterase (cGMP PDE) inhibition is an important COX-independent mechanism to suppress β-catenin signaling (65). In these studies, exisulind and several potent derivatives were found to inhibit cGMP PDE activity and reduce oncogenic levels of β-catenin by increasing intracellular cGMP levels and activating cGMP-dependent protein kinase (PKG). Although exisulind displayed modest potency to inhibit PDE and did not show evidence of selectivity for cGMP degrading isozymes, more recent studies with sulindac sulfide showed appreciably greater potency and selectivity to inhibit cGMP hydrolysis among several cGMP degrading isozymes, including PDE2, 3, 5, and 10 (70). Notably, studies showing an association between inhibition of the cGMP-specific PDE5 isozyme and the tumor cell growth inhibitory activity of sulindac reinforce the importance of cGMP signaling (71). Moreover, the ability of PDE5 siRNA to mimic the selective nature by which sulindac induces apoptosis provides strong evidence for a role of the cGMP/PKG pathway in suppressing oncogenic β-catenin signaling. Other NSAIDs also inhibit cGMP PDE activity, which in many cases matches their potency to suppress tumor cell growth (72). As such, the contribution of additional cGMP-hydrolyzing PDE isozymes cannot be excluded.
PKG is thought to be the main kinase responsible for the anti-proliferative and apoptosis inducing activity of cGMP signaling. PKG activation attenuates β-catenin mRNA levels by directly inhibiting transcription from the CTNNB1 gene (70) and by suppressing β-catenin nuclear translocation, possibly by inducing its sequestration by FOXO4 (73). These observations point to a mechanistic link between NSAID inhibition of cGMP PDE and the suppression of Wnt signaling that is independent of COX binding, as illustrated in Figure 2.
Figure 2.
A mechanistic model of the cGMP/PKG pathway and the antineoplastic properties of sulindac.
Other targets
Several additional molecules shown to be direct NSAID targets are particularly noteworthy. For example, studies provide evidence that aspirin and its deacetylated metabolite salicylate, as well as sulindac sulfide and exisulind can inhibit NF-κB signaling (74, 75). Aspirin and salicylate were found to be ATP-competitive inhibitors of IKKβ, the upstream positive regulator of NF-κB, suggesting that the antiapoptotic effects involve direct binding to IKKβ. A recent report by Hawley and colleagues showed that salicylate can also bind and inhibit AMPK, a key protein kinase involved in the regulation of cellular metabolism and proliferation (76). These findings are consistent with a concomitant report by Din et al. which showed that aspirin can activate AMPK in colon tumor cell lines and in the rectal mucosa of patients on a daily aspirin regimen (77) and suggest that AMPK may be an important target that mediates the chemopreventive effects of aspirin.
In addition, indomethacin, ibuprofen and sulindac sulfide have all been reported to induce PPARγ promoter activity, the loss of which is implicated in colorectal carcinogenesis (78, 79). On the other hand, indomethacin and sulindac sulfide both can bind and repress transcriptional activity of PPARδ, a growth-promoting protein activated by COX-2-derived prostacyclin (80). Furthermore, the R-enantiomer of etodolac, which lacks COX-inhibitory activity, has been shown to bind RXRα and selectively induce apoptosis in tumor cell lines (81). Sulindac sulfide was later demonstrated to specifically bind a truncated form of RXRα expressed in cancer cells and lead to apoptosis through suppression of Akt signaling (82). In the same study, a sulindac derivative devoid of COX-inhibitory activity but with improved potency to bind RXRα, K-80003, was shown to have significant antitumor activity in vitro and in vivo.
Several carbonic anhydrases (CAs I, II, IV, IX, XII) are inhibited by celecoxib in the low nanomolar range, at values significantly lower than its IC50 for COX-2 inhibition (83). CAs are enzymes that regulate acid-base balance in tissues and are crucial for hypoxic adaptation in tumor cells. Their expression levels correlate with tumor aggressiveness and a poor prognosis (84). Another direct target of celecoxib is the sarcoplasmic/ER Ca+2 ATPase (SERCA) that maintains the Ca+2 gradient between the cytosol and the ER. Binding of celecoxib, as well as its non-COX-inhibitory derivative dimethylcelecoxib (DMC), leads to rapid release of calcium from the ER, followed by activation of ER stress response (ESR) and induction of apoptosis (85, 86). A more recent study has shown that sulindac sulfide can also bind SERCA in a similar fashion albeit with low potency (87).
Inhibition of Angiogenesis and Metastasis
NSAIDs, such as sulindac sulfide (88), exisulind (89) and celecoxib (90) have been shown to also inhibit angiogenesis and tumor cell invasion, although these observations are largely limited to the preclinical setting. It is plausible to suggest that the antiangiogenic properties of NSAIDs result from direct effects on endothelial cell survival and proliferation via the aforementioned targets, such as cGMP PDEs, IKKβ or SERCA. However, several other molecules involved in angiogenesis regulation have also been proposed to mediate these effects. For example, celecoxib can directly inhibit the DNA-binding activity of Sp1 transcription factor, a crucial driver of VEGF overexpression in cancer cells (91). In addition, sulindac sulfide, exisulind and celecoxib have all been shown to inhibit invasion through downregulation of matrix metalloproteins (MMPs) 2 and 9 (92). These are the principal enzymes involved in degrading type IV collagen of the basement membrane enabling endothelial cells to reach hypoxic tumors and cancer cells to invade adjacent tissue leading to metastasis (93). Furthermore, a recent report provides evidence that sulindac sulfide can inhibit tumor cell invasion by suppressing Nf-κB-mediated transcription of microRNAs in human colon and breast cancer cell lines (94). Overall, these reports demonstrate that NSAIDs can attenuate angiogenesis and invasion through COX-independent pathways.
Effects on gene expression
NSAIDs have been reported to modulate the expression of various genes involved in the regulation of cell survival and proliferation. Multiple NSAIDs, including indomethacin, aspirin and sulindac sulfide, were found to induce the expression of NSAID-activated gene (NAG-1/GDF-15) independent of COX inhibition in colorectal cancer cell lines (95). Although the precise biological functions of NAG-1 are poorly understood, it is a member of the TGF-β superfamily that exhibits pro-apoptotic and anti-tumorigenic activity in animal and cell culture models (96). A recent study by Wang and colleagues found that NAG-1 is strongly induced in the liver of Min mice after sulindac treatment suggesting that NAG-1 induction may contribute to the tumor inhibitory effects of sulindac (97).
Novel NSAID derivatives
Several groups have synthesized derivatives using various NSAID scaffolds to reduce their COX inhibitory activity, while improving potency to inhibit tumor cell growth. Our group developed a rational drug design approach to selectively block COX binding by substituting the negatively charged carboxylic acid moiety of sulindac sulfide, which is common to most NSAIDs and essential for COX binding via its interaction with positively charged moieties in the active site. One such derivative, referred to as sulindac sulfide amide (SSA), was found to have significantly higher potency to inhibit colon tumor growth compared with sulindac sulfide, despite lacking COX-1 or -2 inhibitory activity (98). With promising drug-like properties, SSA was shown to be highly effective in a colon tumor xenograft model alone and in combination with camptothecin. Other investigators have shown the ability of SSA to inhibit tumor formation in the TRAMP model of prostate cancer (99). Recent studies have shown that SSA inhibits tumor cell growth primarily through the induction of autophagy via suppression of Akt/mTOR signaling (100). Sulindac sulfide mimicked these effects on Akt signaling and induced autophagy, but only at concentrations higher than those required to inhibit tumor cell growth, whereas apoptosis appeared to be the primary mechanism of cell death. Additional sulindac derivatives have since been developed, for example, that selectively inhibit PDE5 and have antitumor activity without inhibiting COX-1 or COX-2 (50). Recent efforts to develop improved chemopreventive agents also include the synthesis of phospho-derivatives that lack COX-inhibitory activity, such as phospho-sulindac and phospho-aspirin, but display high safety and efficacy in preclinical models of various cancer types (101, 102). Furthermore, the sulindac derivative K-80003 that selectively targets RXRα (82) and celecoxib derivatives OSU-03012 (103) and dimethyl-celecoxib (104) that inhibit PDK-1 without COX inhibition, represent other examples of separating COX-inhibitory activity and antitumor efficacy. These experimental agents demonstrate the feasibility of developing safer and more efficacious drugs for chemoprevention by chemically designing out COX-binding while improving target selectivity. Moreover, they highlight the utility of NSAIDs as pharmacological probes for target discovery, which could result in the development of new chemical entities with the potential for greater tumor selectivity.
Summary
Traditional NSAIDs and selective COX-2 inhibitors represent some of the most extensively studied agents with known chemopreventive activity. However, toxicities resulting from COX inhibition and incomplete efficacy limit their use for cancer chemoprevention. Currently, there are no approved therapies for the primary chemoprevention of FAP and preventive options are severely limited for high-risk individuals with precancerous lesions. A safe and efficacious chemopreventive drug can serve as an adjunct to surgery and prevent the formation of new lesions while reducing the overall risk of disease progression. However, further progress depends on increased understanding of the molecular mechanisms underlying the antineoplastic activity of NSAIDs. As summarized above, the inhibition of COX cannot explain all the observed chemopreventive effects of these drugs. Elucidating the involved targets and signaling pathways provides the opportunity to specifically target key molecules, select patient populations that are most likely to benefit from chemoprevention, and explain the underlying mechanisms of resistance. These studies will likely contribute to future chemopreventive strategies by enabling the identification of novel agents or guiding the modification of existing ones. Finally, using NSAIDs in combination with another chemopreventive or therapeutic agent represents an attractive strategy to increase efficacy and reduce toxicity. As established by a landmark phase III clinical study (105), sulindac is highly effective in combination with difluoromethylornithine (DFMO) for the prevention of sporadic colorectal adenomas in patients with a history of resected adenomas. Results from similar combination therapy trials can be put to immediate use given that NSAIDs are FDA approved and have a strong record of chemopreventive activity.
Acknowledgments
Grant Support: This work was supported by NIH grants, NCI 1R01CA131378 and 1R01CA148817-01A1 to G.A.P.
Abbreviations
- RXRα
retinoid X receptor alpha
- PDK-1
3-phosphoinositide-dependent kinase-1
- FAP
familial adenomatous polyposis
- APC
adenomatous polyposis coli
- IKKβ
IκB kinase
- AMPK
AMP-activated protein kinase
- PPAR
peroxisome proliferator activated-receptor
- RXR-α
retinoid X receptor alpha
- NF-κB
nuclear factor kappa-light-chain-enhancer of activated B cells
- TGF-β
transforming growth factor beta
Footnotes
Disclosure of Potential Conflicts of Interest: The authors have no disclosures.
Author Contributions: Conception and Design: E.G., W.E.G., G.A.P.; Writing, review and/or revision of the manuscript: E.G., W.E.G., G.A.P.; Administrative, Technical and or Material Support: E.G., W.E.G., G.A.P.; Study Supervision: W.E.G., G.A.P.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA: a cancer journal for clinicians. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Alberts DS, Hixson L, Ahnen D, Bogert C, Einspahr J, Paranka N, et al. Do NSAIDs exert their colon cancer chemoprevention activities through the inhibition of mucosal prostaglandin synthetase? Journal of cellular biochemistry Supplement. 1995;22:18–23. doi: 10.1002/jcb.240590804. [DOI] [PubMed] [Google Scholar]
- 3.Ahnen DJ. Colon cancer prevention by NSAIDs: what is the mechanism of action? The European journal of surgery Supplement : = Acta chirurgica Supplement. 1998;(582):111–114. doi: 10.1080/11024159850191544. [DOI] [PubMed] [Google Scholar]
- 4.Wang X, Baek SJ, Eling T. COX inhibitors directly alter gene expression: role in cancer prevention? Cancer metastasis reviews. 2011;30:641–657. doi: 10.1007/s10555-011-9301-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vane JR, Botting RM. Mechanism of action of antiinflammatory drugs. International journal of tissue reactions. 1998;20:3–15. [PubMed] [Google Scholar]
- 6.Vane JR. Inhibition of prostaglandin synthesis as a mechanism of action for aspirin-like drugs. Nature: New biology. 1971;231:232–235. doi: 10.1038/newbio231232a0. [DOI] [PubMed] [Google Scholar]
- 7.Vane JR, Bakhle YS, Botting RM. Cyclooxygenases 1 and 2. Annual review of pharmacology and toxicology. 1998;38:97–120. doi: 10.1146/annurev.pharmtox.38.1.97. [DOI] [PubMed] [Google Scholar]
- 8.Smith WL, Garavito RM, DeWitt DL. Prostaglandin endoperoxide H synthases (cyclooxygenases)-1 and-2. The Journal of biological chemistry. 1996;271:33157–33160. doi: 10.1074/jbc.271.52.33157. [DOI] [PubMed] [Google Scholar]
- 9.Williams CS, DuBois RN. Prostaglandin endoperoxide synthase: why two isoforms? The American journal of physiology. 1996;270(3 Pt 1):G393–G400. doi: 10.1152/ajpgi.1996.270.3.G393. [DOI] [PubMed] [Google Scholar]
- 10.Berg J, Christoph T, Widerna M, Bodenteich A. Isoenzyme-specific cyclooxygenase inhibitors: a whole cell assay system using the human erythroleukemic cell line HEL and the human monocytic cell line Mono Mac 6. Journal of pharmacological and toxicological methods. 1997;37:179–186. doi: 10.1016/s1056-8719(97)00016-6. [DOI] [PubMed] [Google Scholar]
- 11.Mukherjee D, Nissen SE, Topol EJ. Risk of cardiovascular events associated with selective COX-2 inhibitors. JAMA : the journal of the American Medical Association. 2001;286:954–959. doi: 10.1001/jama.286.8.954. [DOI] [PubMed] [Google Scholar]
- 12.Solomon SD, Wittes J, Finn PV, Fowler R, Viner J, Bertagnolli MM, et al. Cardiovascular risk of celecoxib in 6 randomized placebo-controlled trials: the cross trial safety analysis. Circulation. 2008;117:2104–2113. doi: 10.1161/CIRCULATIONAHA.108.764530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thun MJ, Henley SJ, Patrono C. Nonsteroidal anti-inflammatory drugs as anticancer agents: mechanistic, pharmacologic, and clinical issues. Journal of the National Cancer Institute. 2002;94:252–266. doi: 10.1093/jnci/94.4.252. [DOI] [PubMed] [Google Scholar]
- 14.Ruder EH, Laiyemo AO, Graubard BI, Hollenbeck AR, Schatzkin A, Cross AJ. Non-steroidal anti-inflammatory drugs and colorectal cancer risk in a large, prospective cohort. The American journal of gastroenterology. 2011;106:1340–1350. doi: 10.1038/ajg.2011.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sharpe CR, Collet JP, McNutt M, Belzile E, Boivin JF, Hanley JA. Nested casecontrol study of the effects of non-steroidal anti-inflammatory drugs on breast cancer risk and stage. British journal of cancer. 2000;83:112–120. doi: 10.1054/bjoc.2000.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khuder SA, Mutgi AB. Breast cancer and NSAID use: a meta-analysis. British journal of cancer. 2001;84:1188–1192. doi: 10.1054/bjoc.2000.1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Muscat JE, Chen SQ, Richie JP, Jr, Altorki NK, Citron M, Olson S, et al. Risk of lung carcinoma among users of nonsteroidal antiinflammatory drugs. Cancer. 2003;97:1732–1736. doi: 10.1002/cncr.11242. [DOI] [PubMed] [Google Scholar]
- 18.Norrish AE, Jackson RT, McRae CU. Non-steroidal anti-inflammatory drugs and prostate cancer progression. International journal of cancer Journal international du cancer. 1998;77:511–515. doi: 10.1002/(sici)1097-0215(19980812)77:4<511::aid-ijc6>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 19.Thun MJ, Namboodiri MM, Calle EE, Flanders WD, Heath CW., Jr Aspirin use and risk of fatal cancer. Cancer research. 1993;53:1322–1327. [PubMed] [Google Scholar]
- 20.Cramer DW, Harlow BL, Titus-Ernstoff L, Bohlke K, Welch WR, Greenberg ER. Over-the-counter analgesics and risk of ovarian cancer. Lancet. 1998;351:104–107. doi: 10.1016/S0140-6736(97)08064-1. [DOI] [PubMed] [Google Scholar]
- 21.Waddell WR, Loughry RW. Sulindac for polyposis of the colon. Journal of surgical oncology. 1983;24:83–87. doi: 10.1002/jso.2930240119. [DOI] [PubMed] [Google Scholar]
- 22.Keller JJ, Giardiello FM. Chemoprevention strategies using NSAIDs and COX-2 inhibitors. Cancer biology & therapy. 2003;2(4 Suppl 1):S140–S149. [PubMed] [Google Scholar]
- 23.Steinbach G, Lynch PM, Phillips RK, Wallace MH, Hawk E, Gordon GB, et al. The effect of celecoxib, a cyclooxygenase-2 inhibitor, in familial adenomatous polyposis. The New England journal of medicine. 2000;342:1946–1952. doi: 10.1056/NEJM200006293422603. [DOI] [PubMed] [Google Scholar]
- 24.Bresalier RS, Sandler RS, Quan H, Bolognese JA, Oxenius B, Horgan K, et al. Cardiovascular events associated with rofecoxib in a colorectal adenoma chemoprevention trial. The New England journal of medicine. 2005;352:1092–1102. doi: 10.1056/NEJMoa050493. [DOI] [PubMed] [Google Scholar]
- 25.Holmes MD, Chen WY, Li L, Hertzmark E, Spiegelman D, Hankinson SE. Aspirin intake and survival after breast cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2010;28:1467–1472. doi: 10.1200/JCO.2009.22.7918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chan AT, Ogino S, Fuchs CS. Aspirin use and survival after diagnosis of colorectal cancer. JAMA : the journal of the American Medical Association. 2009;302:649–658. doi: 10.1001/jama.2009.1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lundholm K, Gelin J, Hyltander A, Lonnroth C, Sandstrom R, Svaninger G, et al. Anti-inflammatory treatment may prolong survival in undernourished patients with metastatic solid tumors. Cancer research. 1994;54:5602–5606. [PubMed] [Google Scholar]
- 28.Pollard M, Luckert PH. Indomethacin treatment of rats with dimethylhydrazine-induced intestinal tumors. Cancer treatment reports. 1980;64:1323–1327. [PubMed] [Google Scholar]
- 29.Narisawa T, Sato M, Tani M, Kudo T, Takahashi T, Goto A. Inhibition of development of methylnitrosourea-induced rat colon tumors by indomethacin treatment. Cancer research. 1981;41:1954–1957. [PubMed] [Google Scholar]
- 30.Rao CV, Rivenson A, Simi B, Zang E, Kelloff G, Steele V, et al. Chemoprevention of colon carcinogenesis by sulindac, a nonsteroidal anti-inflammatory agent. Cancer research. 1995;55:1464–1472. [PubMed] [Google Scholar]
- 31.Kawamori T, Rao CV, Seibert K, Reddy BS. Chemopreventive activity of celecoxib, a specific cyclooxygenase-2 inhibitor, against colon carcinogenesis. Cancer research. 1998;58:409–412. [PubMed] [Google Scholar]
- 32.Takahashi M, Fukuda K, Sugimura T, Wakabayashi K. Beta-catenin is frequently mutated and demonstrates altered cellular location in azoxymethaneinduced rat colon tumors. Cancer research. 1998;58:42–46. [PubMed] [Google Scholar]
- 33.De Filippo C, Caderni G, Bazzicalupo M, Briani C, Giannini A, Fazi M, et al. Mutations of the Apc gene in experimental colorectal carcinogenesis induced by azoxymethane in F344 rats. British journal of cancer. 1998;77:2148–2151. doi: 10.1038/bjc.1998.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Beazer-Barclay Y, Levy DB, Moser AR, Dove WF, Hamilton SR, Vogelstein B, et al. Sulindac suppresses tumorigenesis in the Min mouse. Carcinogenesis. 1996;17:1757–1760. doi: 10.1093/carcin/17.8.1757. [DOI] [PubMed] [Google Scholar]
- 35.Jacoby RF, Seibert K, Cole CE, Kelloff G, Lubet RA. The cyclooxygenase-2 inhibitor celecoxib is a potent preventive and therapeutic agent in the min mouse model of adenomatous polyposis. Cancer research. 2000;60:5040–5044. [PubMed] [Google Scholar]
- 36.Takahashi M, Fukutake M, Yokota S, Ishida K, Wakabayashi K, Sugimura T. Suppression of azoxymethane-induced aberrant crypt foci in rat colon by nimesulide, a selective inhibitor of cyclooxygenase 2. Journal of cancer research and clinical oncology. 1996;122:219–222. doi: 10.1007/BF01209649. [DOI] [PubMed] [Google Scholar]
- 37.Steele VE, Rao CV, Zhang Y, Patlolla J, Boring D, Kopelovich L, et al. Chemopreventive efficacy of naproxen and nitric oxide-naproxen in rodent models of colon, urinary bladder, and mammary cancers. Cancer Prev Res (Phila) 2009;2:951–956. doi: 10.1158/1940-6207.CAPR-09-0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reddy BS, Maruyama H, Kelloff G. Dose-related inhibition of colon carcinogenesis by dietary piroxicam, a nonsteroidal antiinflammatory drug, during different stages of rat colon tumor development. Cancer research. 1987;47:5340–5346. [PubMed] [Google Scholar]
- 39.Reddy BS, Hirose Y, Lubet R, Steele V, Kelloff G, Paulson S, et al. Chemoprevention of colon cancer by specific cyclooxygenase-2 inhibitor, celecoxib, administered during different stages of carcinogenesis. Cancer research. 2000;60:293–297. [PubMed] [Google Scholar]
- 40.Giardiello FM, Hamilton SR, Krush AJ, Piantadosi S, Hylind LM, Celano P, et al. Treatment of colonic and rectal adenomas with sulindac in familial adenomatous polyposis. The New England journal of medicine. 1993;328:1313–1316. doi: 10.1056/NEJM199305063281805. [DOI] [PubMed] [Google Scholar]
- 41.Bennett A, Del Tacca M. Proceedings: Prostaglandins in human colonic carcinoma. Gut. 1975;16:409. [PubMed] [Google Scholar]
- 42.Qiao L, Kozoni V, Tsioulias GJ, Koutsos MI, Hanif R, Shiff SJ, et al. Selected eicosanoids increase the proliferation rate of human colon carcinoma cell lines and mouse colonocytes in vivo. Biochimica et biophysica acta. 1995;1258:215–223. doi: 10.1016/0005-2760(95)00100-q. [DOI] [PubMed] [Google Scholar]
- 43.Oshima M, Dinchuk JE, Kargman SL, Oshima H, Hancock B, Kwong E, et al. Suppression of intestinal polyposis in Apc delta716 knockout mice by inhibition of cyclooxygenase 2 (COX-2) Cell. 1996;87:803–809. doi: 10.1016/s0092-8674(00)81988-1. [DOI] [PubMed] [Google Scholar]
- 44.Tsujii M, DuBois RN. Alterations in cellular adhesion and apoptosis in epithelial cells overexpressing prostaglandin endoperoxide synthase 2. Cell. 1995;83:493–501. doi: 10.1016/0092-8674(95)90127-2. [DOI] [PubMed] [Google Scholar]
- 45.Elder DJ, Halton DE, Hague A, Paraskeva C. Induction of apoptotic cell death in human colorectal carcinoma cell lines by a cyclooxygenase-2 (COX-2)-selective nonsteroidal anti-inflammatory drug: independence from COX-2 protein expression. Clinical cancer research : an official journal of the American Association for Cancer Research. 1997;3:1679–1683. [PubMed] [Google Scholar]
- 46.Vogt T, McClelland M, Jung B, Popova S, Bogenrieder T, Becker B, et al. Progression and NSAID-induced apoptosis in malignant melanomas are independent of cyclooxygenase. II. Melanoma research. 2001;11:587–599. doi: 10.1097/00008390-200112000-00005. [DOI] [PubMed] [Google Scholar]
- 47.Hanif R, Pittas A, Feng Y, Koutsos MI, Qiao L, Staiano-Coico L, et al. Effects of nonsteroidal anti-inflammatory drugs on proliferation and on induction of apoptosis in colon cancer cells by a prostaglandin-independent pathway. Biochemical pharmacology. 1996;52:237–245. doi: 10.1016/0006-2952(96)00181-5. [DOI] [PubMed] [Google Scholar]
- 48.Piazza GA, Rahm AK, Finn TS, Fryer BH, Li H, Stoumen AL, et al. Apoptosis primarily accounts for the growth-inhibitory properties of sulindac metabolites and involves a mechanism that is independent of cyclooxygenase inhibition, cell cycle arrest, and p53 induction. Cancer research. 1997;57:2452–2459. [PubMed] [Google Scholar]
- 49.Janssen A, Maier TJ, Schiffmann S, Coste O, Seegel M, Geisslinger G, et al. Evidence of COX-2 independent induction of apoptosis and cell cycle block in human colon carcinoma cells after S- or R-ibuprofen treatment. European journal of pharmacology. 2006;540:24–33. doi: 10.1016/j.ejphar.2006.04.030. [DOI] [PubMed] [Google Scholar]
- 50.Whitt JD, Li N, Tinsley HN, Chen X, Zhang W, Li Y, et al. A novel sulindac derivative that potently suppresses colon tumor cell growth by inhibiting cGMP phosphodiesterase and beta-catenin transcriptional activity. Cancer Prev Res (Phila) 2012;5:822–833. doi: 10.1158/1940-6207.CAPR-11-0559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Malkinson AM, Koski KM, Dwyer-Nield LD, Rice PL, Rioux N, Castonguay A, et al. Inhibition of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone-induced mouse lung tumor formation by FGN-1 (sulindac sulfone) Carcinogenesis. 1998;19:1353–1356. doi: 10.1093/carcin/19.8.1353. [DOI] [PubMed] [Google Scholar]
- 52.Piazza GA, Alberts DS, Hixson LJ, Paranka NS, Li H, Finn T, et al. Sulindac sulfone inhibits azoxymethane-induced colon carcinogenesis in rats without reducing prostaglandin levels. Cancer research. 1997;57:2909–2915. [PubMed] [Google Scholar]
- 53.Thompson HJ, Jiang C, Lu J, Mehta RG, Piazza GA, Paranka NS, et al. Sulfone metabolite of sulindac inhibits mammary carcinogenesis. Cancer research. 1997;57:267–271. [PubMed] [Google Scholar]
- 54.Stoner GD, Budd GT, Ganapathi R, DeYoung B, Kresty LA, Nitert M, et al. Sulindac sulfone induced regression of rectal polyps in patients with familial adenomatous polyposis. Advances in experimental medicine and biology. 1999;470:45–53. doi: 10.1007/978-1-4615-4149-3_5. [DOI] [PubMed] [Google Scholar]
- 55.Arber N, Kuwada S, Leshno M, Sjodahl R, Hultcrantz R, Rex D. Sporadic adenomatous polyp regression with exisulind is effective but toxic: a randomised, double blind, placebo controlled, dose-response study. Gut. 2006;55:367–373. doi: 10.1136/gut.2004.061432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hixson LJ, Alberts DS, Krutzsch M, Einsphar J, Brendel K, Gross PH, et al. Antiproliferative effect of nonsteroidal antiinflammatory drugs against human colon cancer cells. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 1994;3:433–438. [PubMed] [Google Scholar]
- 57.Piazza GA, Rahm AL, Krutzsch M, Sperl G, Paranka NS, Gross PH, et al. Antineoplastic drugs sulindac sulfide and sulfone inhibit cell growth by inducing apoptosis. Cancer research. 1995;55:3110–3116. [PubMed] [Google Scholar]
- 58.Shiff SJ, Qiao L, Tsai LL, Rigas B. Sulindac sulfide, an aspirin-like compound, inhibits proliferation, causes cell cycle quiescence, and induces apoptosis in HT-29 colon adenocarcinoma cells. The Journal of clinical investigation. 1995;96:491–503. doi: 10.1172/JCI118060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Keller JJ, Offerhaus GJ, Polak M, Goodman SN, Zahurak ML, Hylind LM, et al. Rectal epithelial apoptosis in familial adenomatous polyposis patients treated with sulindac. Gut. 1999;45:822–828. doi: 10.1136/gut.45.6.822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mahmoud NN, Boolbol SK, Bilinski RT, Martucci C, Chadburn A, Bertagnolli MM. Apc gene mutation is associated with a dominant-negative effect upon intestinal cell migration. Cancer research. 1997;57:5045–5050. [PubMed] [Google Scholar]
- 61.Brown WA, Skinner SA, Malcontenti-Wilson C, Vogiagis D, O'Brien PE. Nonsteroidal anti-inflammatory drugs with activity against either cyclooxygenase 1 or cyclooxygenase 2 inhibit colorectal cancer in a DMH rodent model by inducing apoptosis and inhibiting cell proliferation. Gut. 2001;48:660–666. doi: 10.1136/gut.48.5.660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kolligs FT, Bommer G, Goke B. Wnt/beta-catenin/tcf signaling: a critical pathway in gastrointestinal tumorigenesis. Digestion. 2002;66:131–144. doi: 10.1159/000066755. [DOI] [PubMed] [Google Scholar]
- 63.Tinsley HN, Gary BD, Keeton AB, Lu W, Li Y, Piazza GA. Inhibition of PDE5 by sulindac sulfide selectively induces apoptosis and attenuates oncogenic Wnt/betacatenin- mediated transcription in human breast tumor cells. Cancer Prev Res (Phila) 2011;4:1275–1284. doi: 10.1158/1940-6207.CAPR-11-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rice PL, Kelloff J, Sullivan H, Driggers LJ, Beard KS, Kuwada S, et al. Sulindac metabolites induce caspase- and proteasome-dependent degradation of betacatenin protein in human colon cancer cells. Molecular cancer therapeutics. 2003;2:885–892. [PubMed] [Google Scholar]
- 65.Thompson WJ, Piazza GA, Li H, Liu L, Fetter J, Zhu B, et al. Exisulind induction of apoptosis involves guanosine 3',5'-cyclic monophosphate phosphodiesterase inhibition, protein kinase G activation, and attenuated beta-catenin. Cancer research. 2000;60:3338–3342. [PubMed] [Google Scholar]
- 66.Maier TJ, Janssen A, Schmidt R, Geisslinger G, Grosch S. Targeting the betacatenin/ APC pathway: a novel mechanism to explain the cyclooxygenase-2- independent anticarcinogenic effects of celecoxib in human colon carcinoma cells. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2005;19:1353–1355. doi: 10.1096/fj.04-3274fje. [DOI] [PubMed] [Google Scholar]
- 67.Boon EM, Keller JJ, Wormhoudt TA, Giardiello FM, Offerhaus GJ, van der Neut R, et al. Sulindac targets nuclear beta-catenin accumulation and Wnt signalling in adenomas of patients with familial adenomatous polyposis and in human colorectal cancer cell lines. British journal of cancer. 2004;90:224–229. doi: 10.1038/sj.bjc.6601505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Qiu W, Wang X, Leibowitz B, Liu H, Barker N, Okada H, et al. Chemoprevention by nonsteroidal anti-inflammatory drugs eliminates oncogenic intestinal stem cells via SMAC-dependent apoptosis. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:20027–20032. doi: 10.1073/pnas.1010430107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Steinert G, Oancea C, Roos J, Hagemeyer H, Maier T, Ruthardt M, et al. Sulindac sulfide reverses aberrant self-renewal of progenitor cells induced by the AML-associated fusion proteins PML/RARalpha and PLZF/RARalpha. PloS one. 2011;6:e22540. doi: 10.1371/journal.pone.0022540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Li N, Xi Y, Tinsley HN, Gurpinar E, Gary BD, Zhu B, et al. Sulindac Selectively Inhibits Colon Tumor Cell Growth by Activating the cGMP/PKG Pathway to Suppress Wnt/beta-Catenin Signaling. Molecular cancer therapeutics. 2013;12:1848–1859. doi: 10.1158/1535-7163.MCT-13-0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tinsley HN, Gary BD, Keeton AB, Zhang W, Abadi AH, Reynolds RC, et al. Sulindac sulfide selectively inhibits growth and induces apoptosis of human breast tumor cells by phosphodiesterase 5 inhibition, elevation of cyclic GMP, and activation of protein kinase G. Molecular cancer therapeutics. 2009;8:3331–3340. doi: 10.1158/1535-7163.MCT-09-0758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tinsley HN, Gary BD, Thaiparambil J, Li N, Lu W, Li Y, et al. Colon tumor cell growth-inhibitory activity of sulindac sulfide and other nonsteroidal antiinflammatory drugs is associated with phosphodiesterase 5 inhibition. Cancer Prev Res (Phila) 2010;3:1303–1313. doi: 10.1158/1940-6207.CAPR-10-0030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kwon IK, Wang R, Thangaraju M, Shuang H, Liu K, Dashwood R, et al. PKG inhibits TCF signaling in colon cancer cells by blocking beta-catenin expression and activating FOXO4. Oncogene. 2010;29:3423–3434. doi: 10.1038/onc.2010.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yin MJ, Yamamoto Y, Gaynor RB. The anti-inflammatory agents aspirin and salicylate inhibit the activity of I(kappa)B kinase-beta. Nature. 1998;396:77–80. doi: 10.1038/23948. [DOI] [PubMed] [Google Scholar]
- 75.Yamamoto Y, Yin MJ, Lin KM, Gaynor RB. Sulindac inhibits activation of the NF-kappaB pathway. The Journal of biological chemistry. 1999;274:27307–27314. doi: 10.1074/jbc.274.38.27307. [DOI] [PubMed] [Google Scholar]
- 76.Hawley SA, Fullerton MD, Ross FA, Schertzer JD, Chevtzoff C, Walker KJ, et al. The ancient drug salicylate directly activates AMP-activated protein kinase. Science. 2012;336:918–922. doi: 10.1126/science.1215327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Din FV, Valanciute A, Houde VP, Zibrova D, Green KA, Sakamoto K, et al. Aspirin inhibits mTOR signaling, activates AMP-activated protein kinase, and induces autophagy in colorectal cancer cells. Gastroenterology. 2012;142:1504–1515. e3. doi: 10.1053/j.gastro.2012.02.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lehmann JM, Lenhard JM, Oliver BB, Ringold GM, Kliewer SA. Peroxisome proliferator-activated receptors alpha and gamma are activated by indomethacin and other non-steroidal anti-inflammatory drugs. The Journal of biological chemistry. 1997;272:3406–3410. doi: 10.1074/jbc.272.6.3406. [DOI] [PubMed] [Google Scholar]
- 79.Wick M, Hurteau G, Dessev C, Chan D, Geraci MW, Winn RA, et al. Peroxisome proliferator-activated receptor-gamma is a target of nonsteroidal anti-inflammatory drugs mediating cyclooxygenase-independent inhibition of lung cancer cell growth. Molecular pharmacology. 2002;62:1207–1214. doi: 10.1124/mol.62.5.1207. [DOI] [PubMed] [Google Scholar]
- 80.He TC, Chan TA, Vogelstein B, Kinzler KW. PPARdelta is an APC-regulated target of nonsteroidal anti-inflammatory drugs. Cell. 1999;99:335–345. doi: 10.1016/s0092-8674(00)81664-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kolluri SK, Corr M, James SY, Bernasconi M, Lu D, Liu W, et al. The Renantiomer of the nonsteroidal antiinflammatory drug etodolac binds retinoid X receptor and induces tumor-selective apoptosis. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:2525–2530. doi: 10.1073/pnas.0409721102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhou H, Liu W, Su Y, Wei Z, Liu J, Kolluri SK, et al. NSAID sulindac and its analog bind RXRalpha and inhibit RXRalpha-dependent AKT signaling. Cancer cell. 2010;17:560–573. doi: 10.1016/j.ccr.2010.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Weber A, Casini A, Heine A, Kuhn D, Supuran CT, Scozzafava A, et al. Unexpected nanomolar inhibition of carbonic anhydrase by COX-2-selective celecoxib: new pharmacological opportunities due to related binding site recognition. Journal of medicinal chemistry. 2004;47:550–557. doi: 10.1021/jm030912m. [DOI] [PubMed] [Google Scholar]
- 84.Driessen A, Landuyt W, Pastorekova S, Moons J, Goethals L, Haustermans K, et al. Expression of carbonic anhydrase IX (CA IX), a hypoxia-related protein, rather than vascular-endothelial growth factor (VEGF), a pro-angiogenic factor, correlates with an extremely poor prognosis in esophageal and gastric adenocarcinomas. Annals of surgery. 2006;243:334–340. doi: 10.1097/01.sla.0000201452.09591.f3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tanaka K, Tomisato W, Hoshino T, Ishihara T, Namba T, Aburaya M, et al. Involvement of intracellular Ca2+ levels in nonsteroidal anti-inflammatory druginduced apoptosis. The Journal of biological chemistry. 2005;280:31059–31067. doi: 10.1074/jbc.M502956200. [DOI] [PubMed] [Google Scholar]
- 86.Pyrko P, Kardosh A, Liu YT, Soriano N, Xiong W, Chow RH, et al. Calciumactivated endoplasmic reticulum stress as a major component of tumor cell death induced by 2,5-dimethyl-celecoxib, a non-coxib analogue of celecoxib. Molecular cancer therapeutics. 2007;6:1262–1275. doi: 10.1158/1535-7163.MCT-06-0629. [DOI] [PubMed] [Google Scholar]
- 87.White MC, Johnson GG, Zhang W, Hobrath JV, Piazza GA, Grimaldi M. Sulindac sulfide inhibits sarcoendoplasmic reticulum Ca2+ ATPase, induces endoplasmic reticulum stress response, and exerts toxicity in glioma cells: relevant similarities to and important differences from celecoxib. Journal of neuroscience research. 2013;91:393–406. doi: 10.1002/jnr.23169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Elwich-Flis S, Soltysiak-Pawluczuk D, Splawinski J. Anti-angiogenic and apoptotic effects of metabolites of sulindac on chick embryo chorioallantoic membrane. Hybridoma and hybridomics. 2003;22:55–60. doi: 10.1089/153685903321538099. [DOI] [PubMed] [Google Scholar]
- 89.Skopinska-Rozewska E, Piazza GA, Sommer E, Pamukcu R, Barcz E, Filewska M, et al. Inhibition of angiogenesis by sulindac and its sulfone metabolite (FGN-1): a potential mechanism for their antineoplastic properties. International journal of tissue reactions. 1998;20:85–89. [PubMed] [Google Scholar]
- 90.Lin HP, Kulp SK, Tseng PH, Yang YT, Yang CC, Chen CS, et al. Growth inhibitory effects of celecoxib in human umbilical vein endothelial cells are mediated through G1 arrest via multiple signaling mechanisms. Molecular cancer therapeutics. 2004;3:1671–1680. [PubMed] [Google Scholar]
- 91.Wei D, Wang L, He Y, Xiong HQ, Abbruzzese JL, Xie K. Celecoxib inhibits vascular endothelial growth factor expression in and reduces angiogenesis and metastasis of human pancreatic cancer via suppression of Sp1 transcription factor activity. Cancer research. 2004;64:2030–2038. doi: 10.1158/0008-5472.can-03-1945. [DOI] [PubMed] [Google Scholar]
- 92.Lee HC, Park IC, Park MJ, An S, Woo SH, Jin HO, et al. Sulindac and its metabolites inhibit invasion of glioblastoma cells via down-regulation of Akt/PKB and MMP-2. Journal of cellular biochemistry. 2005;94:597–610. doi: 10.1002/jcb.20312. [DOI] [PubMed] [Google Scholar]
- 93.Westermarck J, Kahari VM. Regulation of matrix metalloproteinase expression in tumor invasion. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 1999;13:781–792. [PubMed] [Google Scholar]
- 94.Li X, Gao L, Cui Q, Gary BD, Dyess DL, Taylor W, et al. Sulindac inhibits tumor cell invasion by suppressing NF-kappaB-mediated transcription of microRNAs. Oncogene. 2012;31:4979–4986. doi: 10.1038/onc.2011.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Baek SJ, Kim KS, Nixon JB, Wilson LC, Eling TE. Cyclooxygenase inhibitors regulate the expression of a TGF-beta superfamily member that has proapoptotic and antitumorigenic activities. Molecular pharmacology. 2001;59:901–908. [PubMed] [Google Scholar]
- 96.Eling TE, Baek SJ, Shim M, Lee CH. NSAID activated gene (NAG-1), a modulator of tumorigenesis. Journal of biochemistry and molecular biology. 2006;39:649–655. doi: 10.5483/bmbrep.2006.39.6.649. [DOI] [PubMed] [Google Scholar]
- 97.Wang X, Kingsley PJ, Marnett LJ, Eling TE. The role of NAG-1/GDF15 in the inhibition of intestinal polyps in APC/Min mice by sulindac. Cancer Prev Res (Phila) 2011;4:150–160. doi: 10.1158/1940-6207.CAPR-10-0196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Piazza GA, Keeton AB, Tinsley HN, Gary BD, Whitt JD, Mathew B, et al. A novel sulindac derivative that does not inhibit cyclooxygenases but potently inhibits colon tumor cell growth and induces apoptosis with antitumor activity. Cancer Prev Res (Phila) 2009;2:572–580. doi: 10.1158/1940-6207.CAPR-09-0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zhang Y, Zhang J, Wang L, Quealy E, Gary BD, Reynolds RC, et al. A novel sulindac derivative lacking cyclooxygenase-inhibitory activities suppresses carcinogenesis in the transgenic adenocarcinoma of mouse prostate model. Cancer Prev Res (Phila) 2010;3:885–895. doi: 10.1158/1940-6207.CAPR-09-0273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Gurpinar E, Grizzle WE, Shacka JJ, Mader BJ, Li N, Piazza NA, et al. A novel sulindac derivative inhibits lung adenocarcinoma cell growth through suppression of Akt/mTOR signaling and induction of autophagy. Molecular cancer therapeutics. 2013;12:663–674. doi: 10.1158/1535-7163.MCT-12-0785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Cheng KW, Wong CC, Alston N, Mackenzie GG, Huang L, Ouyang N, et al. Aerosol administration of phospho-sulindac inhibits lung tumorigenesis. Molecular cancer therapeutics. 2013;12:1417–1428. doi: 10.1158/1535-7163.MCT-13-0006-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Huang L, Mackenzie GG, Sun Y, Ouyang N, Xie G, Vrankova K, et al. Chemotherapeutic properties of phospho-nonsteroidal anti-inflammatory drugs, a new class of anticancer compounds. Cancer research. 2011;71:7617–7627. doi: 10.1158/0008-5472.CAN-11-2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zhu J, Huang JW, Tseng PH, Yang YT, Fowble J, Shiau CW, et al. From the cyclooxygenase-2 inhibitor celecoxib to a novel class of 3-phosphoinositidedependent protein kinase-1 inhibitors. Cancer research. 2004;64:4309–4318. doi: 10.1158/0008-5472.CAN-03-4063. [DOI] [PubMed] [Google Scholar]
- 104.Schonthal AH. Antitumor properties of dimethyl-celecoxib, a derivative of celecoxib that does not inhibit cyclooxygenase-2: implications for glioma therapy. Neurosurgical focus. 2006;20(4):E21. doi: 10.3171/foc.2006.20.4.14. [DOI] [PubMed] [Google Scholar]
- 105.Meyskens FL, Jr, McLaren CE, Pelot D, Fujikawa-Brooks S, Carpenter PM, Hawk E, et al. Difluoromethylornithine plus sulindac for the prevention of sporadic colorectal adenomas: a randomized placebo-controlled, double-blind trial. Cancer Prev Res (Phila) 2008;1:32–38. doi: 10.1158/1940-6207.CAPR-08-0042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Gurpinar E, Grizzle WE, Piazza GA. COX-Independent Mechanisms of Cancer Chemoprevention by Anti-Inflammatory Drugs. Frontiers in oncology. 2013;3:181. doi: 10.3389/fonc.2013.00181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Brideau C, Kargman S, Liu S, Dallob AL, Ehrich EW, Rodger IW, et al. A human whole blood assay for clinical evaluation of biochemical efficacy of cyclooxygenase inhibitors. Inflammation research : official journal of the European Histamine Research Society [et al] 1996;45:68–74. doi: 10.1007/BF02265118. [DOI] [PubMed] [Google Scholar]
- 108.Cryer B, Feldman M. Cyclooxygenase-1 and cyclooxygenase-2 selectivity of widely used nonsteroidal anti-inflammatory drugs. The American journal of medicine. 1998;104:413–421. doi: 10.1016/s0002-9343(98)00091-6. [DOI] [PubMed] [Google Scholar]
- 109.Reddy BS, Kawamori T, Lubet RA, Steele VE, Kelloff GJ, Rao CV. Chemopreventive efficacy of sulindac sulfone against colon cancer depends on time of administration during carcinogenic process. Cancer research. 1999;59:3387–3391. [PubMed] [Google Scholar]
- 110.Charalambous D, O'Brien PE. Inhibition of colon cancer precursors in the rat by sulindac sulphone is not dependent on inhibition of prostaglandin synthesis. Journal of gastroenterology and hepatology. 1996;11:307–310. doi: 10.1111/j.1440-1746.1996.tb01376.x. [DOI] [PubMed] [Google Scholar]
- 111.Wargovich MJ, Jimenez A, McKee K, Steele VE, Velasco M, Woods J, et al. Efficacy of potential chemopreventive agents on rat colon aberrant crypt formation and progression. Carcinogenesis. 2000;21:1149–1155. [PubMed] [Google Scholar]
- 112.Piazza GA, Thompson WJ, Pamukcu R, Alila HW, Whitehead CM, Liu L, et al. Exisulind, a novel proapoptotic drug, inhibits rat urinary bladder tumorigenesis. Cancer research. 2001;61:3961–3968. [PubMed] [Google Scholar]
- 113.Narayanan BA, Reddy BS, Bosland MC, Nargi D, Horton L, Randolph C, et al. Exisulind in combination with celecoxib modulates epidermal growth factor receptor, cyclooxygenase-2, and cyclin D1 against prostate carcinogenesis: in vivo evidence. Clinical cancer research : an official journal of the American Association for Cancer Research. 2007;13:5965–5973. doi: 10.1158/1078-0432.CCR-07-0744. [DOI] [PubMed] [Google Scholar]
- 114.Klein T, Eltze M, Grebe T, Hatzelmann A, Komhoff M. Celecoxib dilates guineapig coronaries and rat aortic rings and amplifies NO/cGMP signaling by PDE5 inhibition. Cardiovascular research. 2007;75:390–397. doi: 10.1016/j.cardiores.2007.02.026. [DOI] [PubMed] [Google Scholar]
- 115.Di Fiore A, Pedone C, D'Ambrosio K, Scozzafava A, De Simone G, Supuran CT. Carbonic anhydrase inhibitors: Valdecoxib binds to a different active site region of the human isoform II as compared to the structurally related cyclooxygenase II "selective" inhibitor celecoxib. Bioorganic & medicinal chemistry letters. 2006;16:437–442. doi: 10.1016/j.bmcl.2005.09.040. [DOI] [PubMed] [Google Scholar]
- 116.Shureiqi I, Chen D, Lotan R, Yang P, Newman RA, Fischer SM, et al. 15- Lipoxygenase-1 mediates nonsteroidal anti-inflammatory drug-induced apoptosis independently of cyclooxygenase-2 in colon cancer cells. Cancer research. 2000;60:6846–6850. [PubMed] [Google Scholar]