Abstract
Differences in levels of environmentally induced memory T cells that cross-react with donor MHC molecules are postulated to account for the efficacy of allograft tolerance inducing strategies in rodents versus their failure in nonhuman primates and human transplant patients. Strategies to study the impact of donor-reactive memory T cells on allografts in rodents have relied on the pre-transplant induction of memory T cells cross-reactive with donor allogeneic MHC molecules through recipient viral infection, priming directly with donor antigen, or adoptive transfer of donor-antigen primed memory T cells. Each approach accelerates allograft rejection and confers resistance to tolerance induction, but also biases the T cell repertoire to strong donor-reactivity. The ability of endogenous memory T cells within unprimed mice to directly reject an allograft is unknown. Here we show a direct association between increased duration of cold ischemic allograft storage and numbers and enhanced functions of early graft infiltrating endogenous CD8 memory T cells. These T cells directly mediate rejection of allografts subjected to prolonged ischemia and this rejection is resistant to costimulatory blockade. These findings recapitulate the clinically significant impact of endogenous memory T cells with donor reactivity in a mouse transplant model in the absence of prior recipient priming.
Keywords: cardiac allograft rejection, heterologous immunity, ischemia-reperfusion injury, endogenous memory CD8 T cells
Introduction
High numbers of donor-reactive memory T cells in the peripheral blood of patients prior to transplant are associated with increased incidence of delayed graft function and acute rejection episodes (1, 2). These memory T cells are induced through prior infections and other environmental exposures and many exhibit heterologous immunity, cross-reactivity with unrelated pathogens and allogeneic MHC molecules. While strategies inducing tolerance and long-term allograft acceptance have been successfully implemented in rodent models, few if any have shown equal efficacy when translated to nonhuman primate (NHP) models and human transplant patients (3-6). A critical distinction between mice housed in pathogen-free conditions and NHPs or human patients is their acquired immune history. This difference in levels of heterologous immunity has been postulated to account for the failure of tolerance-inducing strategies in NHP models (7), as memory T cells, are rapidly activated and resistant to costimulatory blockade therapies (8-11).
Current strategies studying the impact of memory T cells on allograft outcomes in rodent models have relied primarily on the pre-transplant induction of memory T cells cross-reactive with donor allogeneic MHC molecules through recipient viral infection, priming directly with donor antigen, or the adoptive transfer of donor-antigen primed memory T cells (12-16). These approaches accelerate allograft rejection and undermine costimulatory blockade-induced tolerance strategies. However, even unprimed mice possess a repertoire of endogenous memory T cells, a proportion of which are alloreactive (16). We previously documented the infiltration of endogenous CD8+CD62Llow memory T cells into cardiac allografts of “naïve” unprimed recipients within hours of graft reperfusion and their activation by donor class I MHC to proliferate and produce IFN-γ (16). In keeping with prior observations, however, the large numbers of early infiltrating memory CD8 T cells within the allograft and their expression of effector mediators are insufficient to directly mediate graft rejection (17). These data suggest that the priming strategies currently used to generate and study costimulatory blockade resistant heterologous memory T cell responses in mice bias the T cell response to strong reactivity to donor antigens and raises questions about the robustness of endogenous memory T cell repertoires in unprimed mice. Whether naïve unmanipulated mice that have not been subjected to these priming strategies contain endogenous memory T cells capable of rejecting an allograft has not been previously investigated.
In seeking to understand why endogenous memory CD8 T cells within unprimed mice are unable to mediate cardiac allograft rejection, we realized that the donor grafts in these studies were subjected to minimal cold ischemic storage (0.5 h) prior to transplant, a protocol that is not only clinically unrealistic, but may minimize the activity of early graft infiltrating endogenous memory T cells and maximize the efficacy of tolerance-inducing strategies. Considering the critical role of ischemia-reperfusion injury (IRI) on allograft outcome (18-25), we tested the impact of increased duration of cold ischemic storage on early endogenous memory CD8 T cell infiltration and functions in cardiac allografts. Our results reveal a direct association between increased duration of cold ischemia and numbers of endogenous memory CD8 T cells in the graft within 48 h of reperfusion. The endogenous memory CD8 T cells are activated to directly mediate marked myocyte injury and the failure of allografts, but not isografts, subjected to prolonged ischemic storage. Inhibition of endogenous memory CD8 T cell graft infiltration attenuates this injury and prolongs graft survival equivalent to allografts subjected to minimal cold ischemia. These results bring the impact of endogenous alloreactive memory T cell responses observed in mouse models closer to those observed in NHP recipients and clinical transplant patients without prior biasing of the endogenous memory T cell repertoire to strong donor-reactivity.
Materials and Methods
Mice
Colonies of C57BL/6 (H-2b) and A/J (H-2a) mice were purchased from Charles River Laboratories (Wilmington, MA, USA). Male mice, 8–10 weeks of age, were used and all procedures involving animals were approved by the Institutional Animal Care and Use Committee at the Cleveland Clinic.
In vivo antibody treatments
Animals were treated on days–1 and 0 or on days +3 and +4 with 0.2 mg i.p. anti-LFA-1 mAb (rat IgG2b, clone FD441.8 from Bio X Cell, West Lebanon, NH, USA) or control rat IgG (Sigma-Aldrich, St. Louis, MO, USA). CD8 or CD4 T cell depletion was accomplished using a 1:1 cocktail of YTS169 and TIB105 or YTS191 and GK1.5 (Bio X Cell), respectively, 0.2mg given i.p. on days -3,-2,-1 and weekly following transplant. CD8 and CD4 T cell depletion was ≥ 98% in peripheral blood. A single dose of 0.5mg anti-TNFα mAb (XT-3.11, Bio X Cell) was given i.v. on day 0. Neutralizing IL-6 mAb (R&D Systems, Minneapolis, MN, USA) was administered i.p. 0.1 mg on days 0,+1. CTLA4Ig (Bio X Cell) was given at a daily dose of 0.25mg i.p. on days 0,+1.
Heterotopic cardiac transplantation and recovery
Standard methods of murine heterotopic intra-abdominal cardiac transplantation and recovery were adapted from the method of Corry et al. as previously reported by our laboratory (16, 26).
Flow cytometry analysis
Flow cytometric detection of graft-infiltrating cells was performed using a modification of the method published by Afanasyev and colleagues (27). Quantification of each leukocyte population was calculated as previously described (28). For intracellular staining tissue cells were ex vivo stimulated with PMA (10 ng/ml) and ionomycin (1mM) for 4 hrs in the presence of 2 mM monensin (Calbiochem, San Diego, CA) during the last 2 hrs. Cells were immediately fixed with 4% paraformaldehyde, permeabilized, and stained with fluorescence-conjugated Abs to IFN-γ, perforin, and granzyme B.
RNA extraction and quantitative analysis of chemokines
RNA was isolated from grafts using Fibrous Tissue kits (QIAGEN, Valencia, CA) and reverse transcribed using the High-Capacity cDNA Archive Kit (Applied Biosystems, Foster City, CA) as previously described (28). Duplicate runs of 3–4 RNA samples per group were tested and the data for each group are expressed as mean test cytokine expression level ± SEM.
ELISPOT
Donor-reactive T cells producing IL-2 and IFN-γ were enumerated by ELISPOT assay as previously detailed using whole splenocytes from cardiac graft recipients (29). After development with the chromagen, the total number of spots per well was quantified using an ImmunoSpot Series 2 Analyzer (Cellular Technology Ltd., Shaker Heights, OH, USA).
Immunohistochemistry
A midventricular portion of the cardiac graft was embedded in OCT compound (Sakura Finetek, Torrance, CA, USA) and immediately frozen in liquid nitrogen after recovery, and 6-μm-thick sections were prepared and stained as previously described (28). For some slides, methanol-fixed sections were stained with hematoxylin and eosin (H&E). Images were captured and analyzed with Image-Pro Plus (Media Cybernetics, Silver Springs, MD, USA).
Statistics
Data analysis was performed using GraphPad Prism Pro (GraphPad Software Inc, San Diego CA). Cardiac allograft survival was plotted using Kaplan-Meier cumulative survival curves and differences in survival between groups determined using the Log-rank (Mantel-Cox) test. Differences between groups for cellular infiltration, RNA expression levels, and ELISPOT numbers were evaluated by unpaired Students' t-test to determine significance throughout; p < 0.05 was considered a significant difference. Error bars throughout indicate SEM.
Results
Prolonged cold ischemia promotes accelerated allograft rejection in anti-LFA-1 mAb treated recipients
Administration of anti-LFA-1 mAb to recipients of cardiac allografts on days 3 and 4 after transplantation does not affect early memory T cell infiltration and activation within the grafts, but delays primary effector T cell priming and their infiltration into the grafts. However, anti-LFA-1 mAb given to allograft recipients on days 3 and 4 post-transplant to test the impact of endogenous memory CD8 T cells on complete MHC-mismatched cardiac allograft survival indicated their early infiltration and proliferation in the grafts, but failed to indicate a role for these T cells in directly provoking rejection (17, 29). Since these allografts were subjected to minimal cold ischemic storage times before transplant (0.5 h), the impact of increased cold ischemic storage on endogenous memory T cell mediated allograft rejection was tested. A/J (H-2a) cardiac allografts were preserved in University of Wisconsin (UW) solution for 0.5 h (minimal) or 8 h (prolonged) at 4°C prior to transplantation to groups of C57BL/6 (H-2b) mice treated with control IgG or anti-LFA-1 mAb on days 3 and 4 post-transplant (Figure 1A). In grafts subjected to minimal ischemia, anti-LFA-1 mAb extended allograft survival to a mean survival time (MST) of 19 days, whereas the survival of allografts subjected to prolonged ischemia was significantly shortened (MST = day 11). Donor-reactive T cells producing IFN-γ and IL-2 were at low/undetectable numbers on day 9 post-transplant in spleens of anti-LFA-1 mAb-treated recipients rejecting allografts subjected to the prolonged ischemic storage, suggesting rejection was not mediated by primary effector T cells developing from donor-reactive precursor T cells in response to donor antigens (Figure 1B). This accelerated rejection in grafts subjected to prolonged cold ischemia was confirmed histologically by increased myocyte damage and leukocyte infiltration (Figure 1C). Importantly, isografts subjected to prolonged cold ischemic storage survived over 100 days.
Figure 1. Prolonged cold ischemia promotes accelerated allograft rejection in anti-LFA-1 mAb treated recipients.
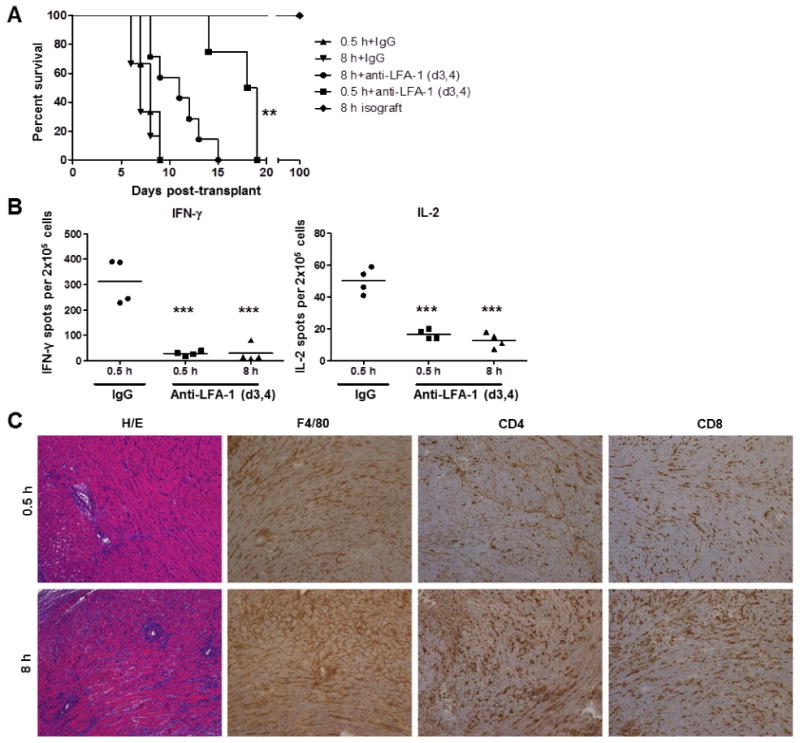
(A) Groups of 4-6 C57BL/6 recipient mice received syngeneic or complete MHC-mismatched A/J cardiac allografts subjected to minimal (0.5 h) or prolonged (8 h) cold ischemia and were treated with 200 μg control rat IgG or with anti-LFA-1 mAb on days 3 and 4 post-transplant. **p < 0.01 versus 8 h+anti-LFA-1 (d3,4), (B) Spleens were harvested from control and anti-LFA-1 mAb treated C57BL/6 allograft recipients on day 9 post-transplant. The number of donor-reactive splenocytes producing IFN-γ and IL-2 in each allograft recipient group was determined by ELISPOT assay. ***p < 0.001 (C) On day 12 post-transplant, cardiac allografts subjected to 0.5 or 8 h cold ischemia were harvested from C57BL/6 recipients treated with anti-LFA-1 mAb on days 3 and 4 post-transplant. Prepared sections were stained with hematoxylin-eosin, anti-F4/80 mAb to detect macrophages, anti-CD4 mAb, or with anti-CD8 mAb. Magnification, 100×.
Prolonged cold ischemia increases early infiltration of innate and adaptive leukocytes into allografts
The impact of increased cold ischemic storage of donor cardiac grafts on early cellular infiltration was tested by storing allografts or isografts for 0.5, 4, or 8 h prior to transplantation. The grafts were recovered 48 h after reperfusion and graft infiltrating neutrophils, macrophages, CD4, and CD8 T cells were quantified (Figure 2A). Compared to grafts subjected to minimal cold ischemia, neutrophil and macrophage infiltration increased 2-3 fold in both iso- and allografts subjected to 4 and 8 h of cold storage. While a small, but detectable population of CD4 and CD8 T lymphocytes was observed in iso- and allo-grafts subjected to minimal cold ischemia, a 2-4 fold step-wise increase in numbers was seen only in allografts subjected to prolonged cold storage and only a minor increase was observed in isografts. Further staining of graft infiltrating CD4 and CD8 T cells for markers of activation and memory revealed that the cells were of an activated effector/memory, CD44highCD62Llow, phenotype (data not shown). While NK cells and γδ T cells were detectable in cardiac allografts at this early time-point, their infiltration was not found to be significantly impacted by prolonged cold ischemic graft storage (Supplemental Figure 1).
Figure 2. Prolonged cold ischemia increases early infiltration of innate and adaptive leukocytes into cardiac allografts.
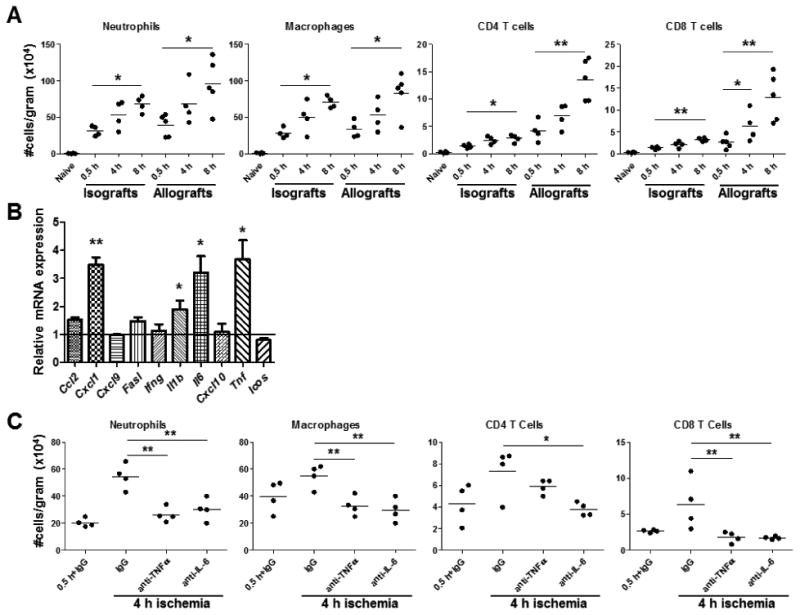
(A) Groups of 4-5 C57BL/6 recipient mice received syngeneic or complete MHC-mismatched A/J cardiac allografts subjected to 0.5, 4, or 8 h cold ischemia prior to transplantation. Grafts were recovered 48 h post-transplant, digested to prepare single cell suspensions, and graft infiltrating cells were analyzed and quantified by antibody staining and flow cytometry. *p < 0.05, **p < 0.01 (B) Groups of 4-5 C57BL/6 recipient mice received syngeneic isografts subjected to 0.5 or 4 h cold ischemic storage prior to transplantation. Grafts were recovered 48 h post-transplant, whole cell RNA was isolated from graft homogenates, and quantitative real-time PCR was used to measure expression levels of mRNA encoding the indicated cytokines and chemokines. Differences in mRNA are expressed relative to isografts subjected to 0.5 h ischemia. *p < 0.05, **p < 0.01 (C) Groups of 4-6 C57BL/6 recipient mice received complete MHC-mismatched A/J cardiac allografts subjected to 0.5 or 4 h cold ischemia and were treated with 200 μg control rat IgG or with 500 μg anti-TNFα mAb on day 0 or 100 μg anti-IL-6 mAb on days 0 and 1 post-transplant. Graft infiltrating cells were quantified 48 h post-transplant. *p < 0.05, **p < 0.01
Prolonged ischemia induces increased acute phase cytokine production in allografts
To test mechanisms induced by prolonged ischemia which may direct early CD8 memory T cell infiltration into cardiac grafts, isografts subjected to minimal or prolonged cold ischemia were transplanted and intra-graft mRNA levels of acute phase cytokines (TNFα, IL-1β, and IL-6), neutrophil (CXCL1/KC) and macrophage (CCL2/MCP-1) chemoattractants, and genes associated with memory CD8 T cell infiltration and activation (IFN-γ, CXCL9/MIG, CXCL10/IP-10, and ICOS) were quantified by qPCR (Figure 2B). Isografts were used as they allow study of the impact of ischemic duration on graft inflammation and injury in the absence of a donor-antigen reactive immune response. Isografts subjected to prolonged ischemia prior to transplantation had significantly higher expression of TNFα, IL-1β, IL-6, and CXCL1 at 48 h after reperfusion. When cardiac allograft recipients were treated with anti-TNFα or anti-IL-6 mAb at the time of reperfusion, the number of CD8 T cells, macrophages, and neutrophils infiltrating allografts subjected to prolonged ischemia was significantly decreased to the levels observed in allografts subjected to minimal cold ischemia when assessed at 48 h post-transplant (Figure 2C). Combining anti-TNFα and anti-IL-6 treatment at the time of reperfusion did not provide further synergistic dampening of early leukocyte infiltration (data not shown).
Prolonged cold ischemia increases the effector functions expressed by early infiltrating memory CD8 T cells
Memory CD8 T cells typically produce IFN-γ and TNFα and express perforin, granzyme B, and FasL, mediators of target cell apoptosis (30). To investigate the effects of early endogenous memory CD8 T cell infiltration on early graft inflammation, allografts subjected to prolonged ischemia were transplanted to WT mice treated with control IgG or CD8 T cell depleting antibody (Figure 3A). Compared to allografts subjected to minimal ischemic times, allografts subjected to prolonged ischemia had 40-50 fold increased mRNA levels of IFN-γ and perforin and heightened FasL and TNFα expression. The expression of these effector molecules derived predominantly from the activities of the early infiltrating memory CD8 T cells as recipients depleted of CD8 T cells had markedly reduced levels at this early time-point. To directly interrogate the capacity of endogenous memory CD8 T cells to express effector function, intracellular staining of early graft infiltrating memory CD8 T cells for IFN-γ, perforin, and granzyme B demonstrated that while memory CD8 T cells in grafts subjected to minimal 0.5 h ischemia had a basal level of effector molecule expression similar to CD8 T cells within the naïve spleen, memory CD8 T cells infiltrating grafts subjected to 8 h of ischemia had significantly elevated intracellular IFN-γ, perforin, and granzyme B (Figure 3B). The absence of early allograft infiltrating memory CD8 T cells also reduced acute phase cytokine expression (IL-1β, IL-6), and neutrophil (CXCL1/KC) and effector T cell chemoattractants (CCL3/MIP-1α, CCL4/MIP-1β, CCL5/RANTES) within allografts at 48 h post-transplant (Figure 3C), indicating that the effector functions expressed by these early memory CD8 T cells play a crucial role in propagating early tissue inflammation in grafts subjected to prolonged ischemia.
Figure 3. Prolonged cold ischemia increases the effector functions expressed by early infiltrating memory CD8 T cells.
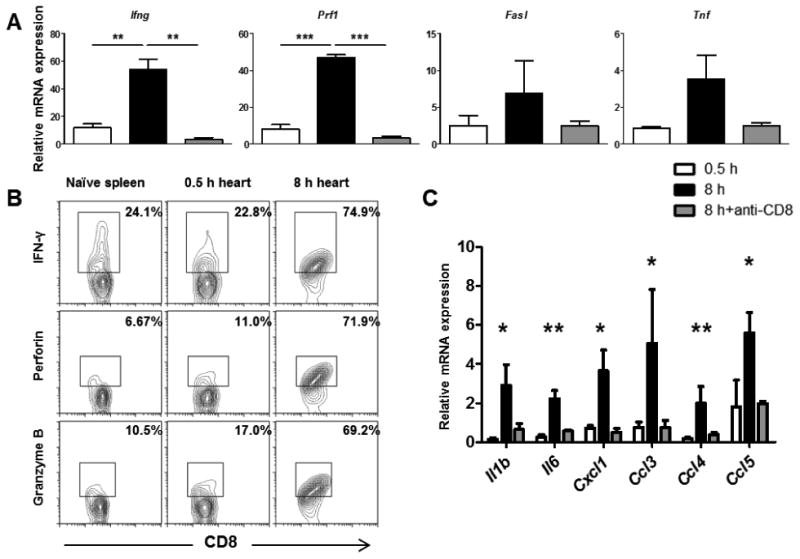
Groups of 4-5 C57BL/6 recipient mice received complete MHC-mismatched A/J cardiac allografts subjected to 0.5 or 8 h cold ischemia and were treated with 200 μg control rat IgG or with anti-CD8 mAb on days -3,-2,-1 prior to transplant. (A) Grafts were recovered 48 h post-transplant, whole cell RNA was isolated from graft homogenates, and quantitative real-time PCR was used to measure expression levels of mRNA encoding T cell effector molecules. Differences in mRNA levels are expressed relative to isografts subjected to 0.5 h ischemia. **p < 0.01, ***p < 0.001 (B) Grafts were recovered 48 h post-transplant, digested to prepare single cell suspensions, and intracellular staining of graft infiltrating memory CD8 T cells was performed using antibodies against IFN-γ, perforin, and granzyme B. (C) Quantitative real-time PCR was used to measure expression levels of mRNA encoding the indicated cytokines and chemokines from graft homogenates 48 h post-transplant. *p < 0.05, **p < 0.01
Endogenous memory CD8 T cells mediate severe allograft injury and rejection
To test the impact of increased endogenous memory CD8 T cell infiltration and activation on the viability of allografts subjected to prolonged cold ischemic storage, allografts subjected to minimal or prolonged ischemia were harvested on day 5 post-transplant for histopathologic evaluation (Figure 4). Compared to allografts subjected to 0.5 h of cold ischemia, allografts subjected to 8 h of cold ischemia had more intense inflammatory cell infiltrates, loss of myocyte nuclei, and prominent karyorrhexis (nuclear fragmentation), characteristic of heightened graft necrosis and apoptosis. Depletion of CD8 T cells from recipients of allografts subjected to prolonged ischemia resulted in greatly ameliorated graft histopathology with reductions in cellular infiltration and myocyte damage to levels lower than observed in grafts subjected to minimal ischemia. Treatment with anti-CD8 mAb did not deplete recipient NK or γδ T cells further implicating CD8 T cells as the major mechanism of acute graft damage at this time (Supplemental Figure 2).
Figure 4. Early infiltrating memory CD8 T cells mediate allograft injury.
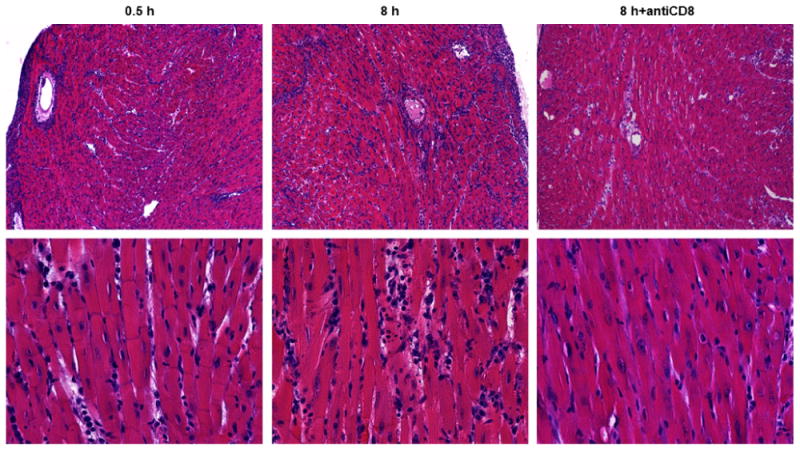
Groups of 4 C57BL/6 recipient mice received complete MHC-mismatched A/J cardiac allografts subjected to 0.5 or 8 h cold ischemia and were treated with 200 μg control rat IgG or anti-CD8 mAb on days -3,-2,-1 prior to transplant. Grafts were recovered 5 days post-transplant and prepared sections were stained with hematoxylin-eosin. Magnification: top panels 100×, bottom panels 400×.
Increasing the duration of ischemia did not result in a marked change in allograft survival of untreated C57BL/6 recipients (Figure 5A), most likely due to the strength of the alloimmune response to the complete MHC-mismatched grafts. To more accurately test the impact of endogenous CD8 memory T cells on allograft outcome, a strategy was needed to separate the activities of the endogenous memory CD8 T cells from the donor-reactive primary effector T cells that develop in the spleen. CTLA4-Ig inhibits the development of primary effector T cells but not the infiltration and activation of endogenous memory CD8 T cells in the allograft (31). While CTLA4-Ig was able to extend survival of grafts subjected to 0.5 h of ischemia to 40-60 days, grafts subjected to 8 h of cold ischemia all rejected by day 17 in CTLA4-Ig treated recipients (Figure 5A). This resistance to costimulatory blockade was alleviated when CD8 T cells were depleted from the recipient prior to and following the transplant. Donor-reactive primary effector T cell development in the spleens of recipients of allografts subjected to minimal or prolonged ischemic storage was absent at the time of rejection (Figure 5B), further implicating early infiltrating memory CD8 T cells as the primary mediators rejecting allografts subjected to prolonged cold ischemic storage in CTLA4-Ig treated recipients.
Figure 5. Early allograft infiltrating memory CD8 T cells detrimentally affect graft survival.
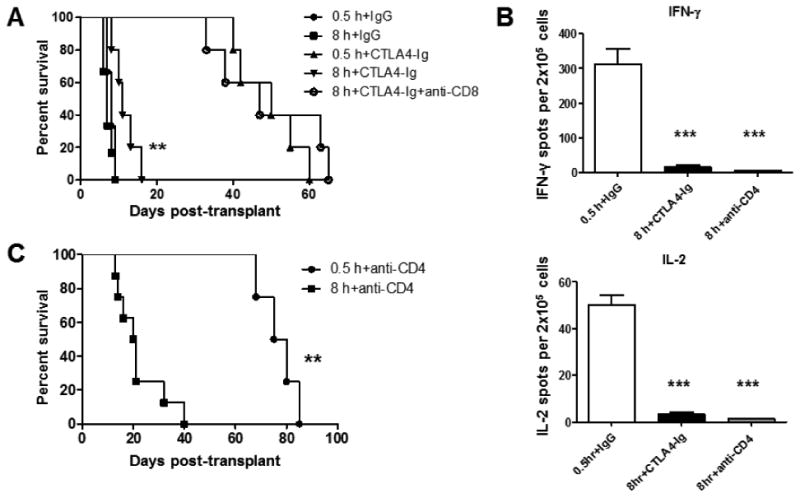
(A) Survival of complete MHC-mismatched A/J cardiac allografts (n = 4-6) subjected to 0.5 or 8 h cold ischemia in recipients treated with 200 μg control rat IgG or 250 μg CTLA4-Ig mAb on days 0 and 1 post-transplant. CD8 T cell depletion was performed by treating recipients with 200 μg anti-CD8 mAb on days -3,-2,-1 prior to transplant and weekly thereafter. **p < 0.01 versus 0.5 h+CTLA4-Ig (B) At the time of graft rejection, spleens were harvested from transplant recipients and the number of donor-reactive T cells producing IFN-γ and IL-2 in each allograft recipient group was determined by ELISPOT assay. ***p < 0.001 (C) Survival of cardiac allografts (n = 4-8) subjected to 0.5 or 8 h cold ischemia in recipients depleted of CD4 T cells by treatment with 200 μg anti-CD4 mAb on days -3,-2,-1 prior to transplant and weekly thereafter. **p < 0.01
Peri-transplant CD4 T cell depleting therapies prolong allograft survival in mouse models by inhibiting de novo primary effector CD8 T cell development, an observation reminiscent of the inability of endogenous memory CD8 T cells to provoke graft failure when the allograft is subjected to minimal cold ischemic storage prior to transplantation (32-34). The impact of prolonged cold ischemic storage was tested on the efficacy of CD4-depleting monoclonal antibody (mAb) treatment to achieve prolonged allograft survival. Anti-CD4 mAb given at the time of transplantation prolonged survival of allografts subjected to minimal ischemia past day 70 post-transplant (Figure 5C). In contrast, this strategy was largely ineffective for allografts subjected to prolonged ischemia with rejection of the cardiac allografts observed as early as day 14 post-transplant. Despite the rapid allograft rejection, anti-CD4 mAb treatment completely inhibited de novo primary effector T cell development in the recipient spleen when assessed at the time of graft rejection (Figure 5B). These data further indicate that early infiltrating endogenous CD8 memory T cells are able to directly reject cardiac allografts subjected to prolonged cold ischemia independent of either memory CD4 T cells or primary donor-reactive CD8 T cells which require CD4 T cell help for development into effector cells.
To address whether targeting the early infiltration of these memory T cells could therapeutically prolong allograft survival we took advantage of the ability of peri-transplant anti-LFA-1 antibody treatment to inhibit early T cell graft infiltration. Anti-LFA-1 antibody given on days -1 and 0 prolonged survival of allografts subjected to minimal or prolonged ischemia nearly identically, with a MST of 33 days (Figure 6A). This prolongation in allograft survival was accompanied by the ability of anti-LFA-1 mAb to inhibit endogenous memory CD8 T cell infiltration into allografts subjected to 8 h prolonged cold ischemic storage (Figure 6B).
Figure 6. Pre-transplant treatment with anti-LFA-1 mAb eliminates the survival deficit in grafts subjected to prolonged ischemia.
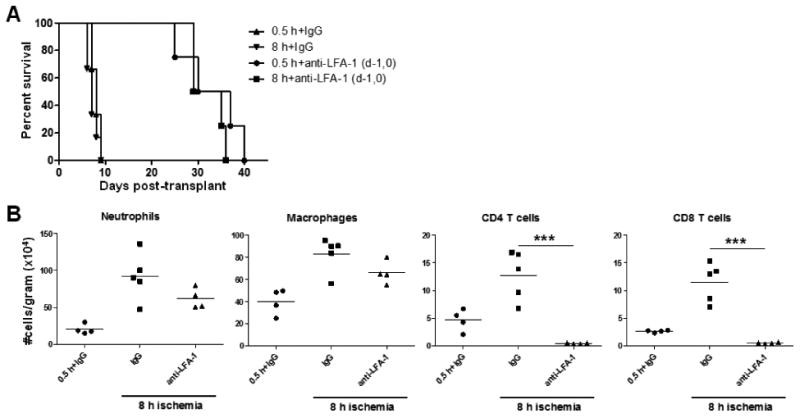
(A) Survival of complete MHC-mismatched A/J cardiac allografts (n = 4-5) subjected to 0.5 or 8 h cold ischemia in recipients treated with 200 μg control rat IgG or anti-LFA-1 mAb on days -1 and 0. (B) Grafts were recovered 48 h post-transplant, digested, and graft infiltrating cells were analyzed and quantified by antibody staining and flow cytometry. **p < 0.01
Discussion
While memory T cells are highly specific for their priming antigen, they maintain a diverse T cell receptor repertoire and can be degenerate in the number of antigens that they can recognize (35). Many memory T cells generated in response to viruses and bacteria exhibit heterologous immunity, cross-reactivity with unrelated pathogens and allogeneic MHC molecules, and have a detrimental effect on allograft outcome (1, 2, 12, 36-38). Despite their recognition as a major barrier to successful transplantation and tolerance induction, however, the extent of graft injury directly mediated by endogenous memory T cells with reactivity to allograft antigens remains poorly delineated. Current strategies relying on induction of memory T cells cross-reactive with donor allogeneic MHC molecules through recipient viral infection, pre-sensitization directly with donor antigen, or the adoptive transfer of donor-antigen primed memory T cells render tolerance induction protocols with donor-specific transfusion and/or costimulation blockade ineffective (12-16). Such studies have demonstrated that a critical threshold of prior viral infection(s) and/or donor-specific memory T cells is required to resist tolerance induction.
We have previously documented the substantial presence of endogenous CD8 memory T cells within naïve unprimed mice which distinguish allogeneic from syngeneic grafts, react with donor class I MHC, and rapidly infiltrate cardiac allografts within hours of graft reperfusion (16, 39). Despite proliferating extensively within the graft and producing IFN-γ, these endogenous CD8 memory T cells from unmanipulated recipients exhibited insufficient effector functions to directly mediate allograft rejection (17). These findings suggest that the priming strategies currently used to generate donor-reactive memory responses in rodents disproportionately biases the T cell response to strong reactivity to donor antigens. While such approaches yield accelerated allograft rejection and resistance to costimulatory blockade induced tolerance, the degree to which the endogenous memory T cell repertoire has been skewed in such rodent models is rarely seen naturally in NHPs and human patients.
IRI is also an inherent component and pathological challenge of transplantation detrimentally impacting graft outcome. Organs from unrelated living donors have superior graft function and survival compared to grafts from deceased donors and longer periods of ischemia imposed on grafts correlate with poorer function and survival (18-25). The inflammation associated with reperfusion injury is largely initiated by oxygen radical generation, complement activation, and endothelial cell dysfunction and these deleterious effects are exacerbated following prolonged ischemia (40-42). While these and many other key biochemical mediators of IRI are now identified, cellular mediators of the inflammation within grafts subjected to increased ischemia remain poorly defined. Importantly, the impact of increased ischemic time on the infiltration and effector functions of donor-reactive memory T cells has not been previously investigated.
This study connects the detrimental graft outcomes associated with prolonged graft ischemia and endogenous heterologous memory T cell responses. We report that endogenous donor-reactive memory CD8 T cells of “naïve” unprimed mice are activated to directly mediate cardiac allograft rejection following prolonged cold ischemic graft storage. Prolonged cold ischemic allograft storage resulted in heightened early cellular infiltration that appears to be mediated, at least in part, by increased production of acute phase cytokines including IL-1β, IL-6, and TNFα as cytokine neutralization markedly attenuates leukocyte infiltration into grafts subjected to prolonged cold ischemia. Interestingly, recipient CD8 T cell depletion prior to transplant markedly decreases early acute phase cytokine and chemokine expression within grafts subjected to prolonged ischemia. The heightened myocyte inflammation and graft damage associated with prolonged ischemic storage was alleviated by recipient CD8 T cell depletion prior to transplant, implicating these T cells in the early inflammation and directly or indirectly mediating this graft injury. Allografts subjected to prolonged ischemia were resistant to the effects of CTLA4-Ig therapy, which significantly prolonged survival of allografts subjected to minimal cold ischemia. Renal transplant patients treated with Belatacept, a second generation CTLA4-Ig recently approved for use in human transplantation, experienced increased incidence and severity of acute rejection episodes despite superior renal function when compared to cyclosporine treatment (43) and one factor postulated to contribute to this increased acute rejection is the activity of endogenous memory T cells resistant to costimulatory blockade. Consistent with these clinical observations, depletion of CD8 T cells prior to transplantation alleviated costimulatory blockade resistance in murine recipients of prolonged ischemic allografts. Alternatively, depletion of CD4 T cells had minimal impact on early graft inflammation or rejection of allografts subjected to prolonged ischemic storage, indicating that the role of endogenous memory CD4 T cells in the acute rejection of the allografts is largely dispensable. It should be noted that in recipients depleted of CD8 T cells alone, primary effector CD4 T cells are fully capable of being primed and mediating cardiac allograft rejection as previously published (Supplemental Figure 3) (33, 34, 44).
Our previous studies demonstrated that the early endogenous memory CD8 T cells infiltrating cardiac allografts subjected to minimal ischemic times react specifically against donor class I MHC, which induces their proliferation and production of IFN-γ (16). To directly investigate the antigen-specificity of this early endogenous memory CD8 T cell response, we previously took advantage of available TCR-transgenic systems and found that adoptive transfer of Ld-reactive TCR-transgenic memory CD8 T cells only induced IFN-γ and subsequent CXCL9 production in Ld-expressing grafts and not Ld-null grafts, evidence supporting a donor-specific response in this early inflammation. Similarly, adoptive transfer of polyclonal donor-antigen primed memory CD8 T cells preferentially infiltrated and induced IFN-γ and CXCL9 in donor-specific grafts rather than third-party allografts (16). The findings presented in this paper report that the infiltration of endogenous memory CD8 T cells is markedly increased in cardiac allografts versus isografts subjected to prolonged cold storage (Figure 2) and is in keeping with a donor-specific response. Moreover, intragraft elevations in expression of CD8 T cell effector molecules IFN-γ, FasL, perforin, and granzyme were seen in allografts subjected to prolonged cold ischemic storage, but not in isografts (Figure 2B, Figure 3A). While cardiac allografts subjected to prolonged ischemia are acutely rejected, isografts survive long-term following prolonged cold storage, indicating that recognition of allogeneic MHC is a critical determinant of the inflammation observed in this model. Our intent in the current studies was to avoid using donor-reactive T cells that were biased to strong allograft reactivity, precluding us from using TCR-transgenic animals or transferred memory T cells from donor antigen primed recipients. It is important to consider the potential differences in alloreactivities of the endogenous memory CD8 T cells investigated in our studies, which are likely to have reactivity to multiple allogeneic class I MHC alleles, and those biased to strong donor reactivity, such as transgenic T cells, that are less likely to express such poly-alloreactivities. Although the data in this report are consistent with an antigen-specific endogenous memory CD8 T cell response, our current studies do not formally distinguish the specificity of T cells in the allograft. We are in the process of more rigorous experiments to test the donor-reactivities and functions of these endogenous memory CD8 T cells in grafts subjected to prolonged cold ischemic storage and are interested in comparing these T cells to memory CD8 T cells generated through prior donor antigen priming.
In light of the impact of endogenous memory CD8 T cells in clinical transplantation and the results in the current studies demonstrating their ability to directly mediate marked allograft injury and graft failure, strategies to interfere with their graft infiltration should improve transplant outcomes. Our previous studies have demonstrated the ability to inhibit infiltration of endogenous memory CD8 T cells into allografts subjected to minimal ischemia by neutralization of acute phase cytokines and by integrin blockade at the time of reperfusion. Peri-transplant neutralization of TNFα markedly attenuates the early inflammatory response in allografts by decreasing early leukocyte graft infiltration, inhibiting alloreactive T cell-priming, and promoting long-term allograft survival when combined with costimulatory blockade therapy (28). LFA-1 antagonism is very effective in inhibiting acute rejection and prolonging allograft survival in rodents (29, 45-48). Clinically, anti-TNFα mAb and TNFR-binding proteins are currently being used to treat many inflammatory diseases and our studies suggest these reagents may have some efficacy in diminishing early IRI-induced graft inflammation (49-51). Although anti-LFA-1 antibody is not currently available for clinical use, targeting other trafficking molecules on endogenous memory CD8 T cells may serve the same purpose and warrants further investigation.
In summary, these studies implicate an important role for endogenous memory CD8 T cells in directly mediating allograft injury and demonstrate that these T cells can directly provoke graft failure in mice without prior recipient priming to bias the endogenous memory T cell repertoire to strong donor reactivity. This recapitulates the impact of endogenous heterologous immunity seen in clinical transplantation. Studies using this clinically relevant strategy should provide a more accurate basis for translating experimental strategies to NHP models and clinical transplant patients.
Supplementary Material
Supplemental Figure 1. Prolonged cold ischemic storage minimally impacts early NK and gamma-delta T cell allograft infiltration. Groups of 4 C57BL/6 recipient mice received complete MHC-mismatched A/J cardiac allografts subjected to minimal (0.5 h) or prolonged (8 h) cold ischemia prior to transplantation. Grafts were recovered 48 h post-transplant, digested to prepare single cell suspensions, and graft infiltrating cells were analyzed by flow cytometry using antibodies against (A) NK and (B) gamma-delta T cell surface markers.
Supplemental Figure 2. Anti-CD8 mAb effectively depletes CD8 T cells while minimally affecting NK or gamma-delta T cells. Groups of 4 C57BL/6 recipient were treated with 200 μg control rat IgG or anti-CD8 mAb on days -3, -2, -1 prior to analysis. On day 0, animals were sacrificed and blood and spleen samples were collected and analyzed by flow cytometry using antibodies against (A) NK and (B) gamma-delta T cell surface markers.
Supplemental Figure 3. Primary effector CD4 T cells mediate cardiac allograft rejection in the absence of CD8 T cells. (A) Groups of 5 C57BL/6 recipient mice received complete MHCmismatched A/J cardiac allografts subjected to minimal (0.5 h) or prolonged (8 h) cold ischemia prior to transplantation. Recipients were treated with 200 μg control rat IgG or anti-CD8 mAb on days -3, -2, -1 prior to transplant and on day 5 post-transplant. (B) On day 8 posttransplant, spleens and grafts were harvested from transplant recipients and analyzed and quantified by antibody staining and flow cytometry.
Acknowledgments
This work was supported by NIH RO1-AI40459 and PO1-AI087586 (R.F.). C.S was supported in part by NIH TL1-24991, T32-AI089474, and the Case Western University School of Medicine MSTP.
Abbreviations
- CTLA4-Ig
cytotoxic T lymphocyte antigen 4 immunoglobulin
- LFA-1
lymphocyte function associated antigen-1
- IFN
interferon
- IRI
ischemia-reperfusion injury
- NHP
non-human primate
- TNF-alpha
tumor necrosis factor alpha
Footnotes
DISCLOSURE: The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
Supporting Information: Additional Supporting Information may be found in the online version of this article.
References
- 1.Augustine JJ, Siu DS, Clemente MJ, Schulak JA, Heeger PS, Hricik DE. Pre-transplant IFN-gamma ELISPOTs are associated with post-transplant renal function in African American renal transplant recipients. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2005;5(8):1971–5. doi: 10.1111/j.1600-6143.2005.00958.x. Epub 2005/07/06. [DOI] [PubMed] [Google Scholar]
- 2.Heeger PS, Greenspan NS, Kuhlenschmidt S, Dejelo C, Hricik DE, Schulak JA, et al. Pretransplant frequency of donor-specific, IFN-gamma-producing lymphocytes is a manifestation of immunologic memory and correlates with the risk of posttransplant rejection episodes. J Immunol. 1999;163(4):2267–75. Epub 1999/08/10. [PubMed] [Google Scholar]
- 3.Lenschow DJ, Zeng Y, Thistlethwaite JR, Montag A, Brady W, Gibson MG, et al. Long-term survival of xenogeneic pancreatic islet grafts induced by CTLA4lg. Science. 1992;257(5071):789–92. doi: 10.1126/science.1323143. [DOI] [PubMed] [Google Scholar]
- 4.Larsen CP, Elwood ET, Alexander DZ, Ritchie SC, Hendrix R, Tucker-Burden C, et al. Long-term acceptance of skin and cardiac allografts after blocking CD40 and CD28 pathways. Nature. 1996;381(6581):434–8. doi: 10.1038/381434a0. [DOI] [PubMed] [Google Scholar]
- 5.Levisetti MG, Padrid PA, Szot GL, Mittal N, Meehan SM, Wardrip CL, et al. Immunosuppressive effects of human CTLA4Ig in a non-human primate model of allogeneic pancreatic islet transplantation. Journal of immunology. 1997;159(11):5187–91. [PubMed] [Google Scholar]
- 6.Kirk AD, Harlan DM, Armstrong NN, Davis TA, Dong Y, Gray GS, et al. CTLA4-Ig and anti-CD40 ligand prevent renal allograft rejection in primates. Proceedings of the National Academy of Sciences of the United States of America. 1997;94(16):8789–94. doi: 10.1073/pnas.94.16.8789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vincenti F. Costimulation blockade--what will the future bring? Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2007;22(5):1293–6. doi: 10.1093/ndt/gfl830. [DOI] [PubMed] [Google Scholar]
- 8.London CA, Lodge MP, Abbas AK. Functional responses and costimulator dependence of memory CD4+ T cells. J Immunol. 2000;164(1):265–72. doi: 10.4049/jimmunol.164.1.265. Epub 1999/12/22. [DOI] [PubMed] [Google Scholar]
- 9.Suresh M, Whitmire JK, Harrington LE, Larsen CP, Pearson TC, Altman JD, et al. Role of CD28-B7 interactions in generation and maintenance of CD8 T cell memory. J Immunol. 2001;167(10):5565–73. doi: 10.4049/jimmunol.167.10.5565. Epub 2001/11/08. [DOI] [PubMed] [Google Scholar]
- 10.Valujskikh A, Pantenburg B, Heeger PS. Primed allospecific T cells prevent the effects of costimulatory blockade on prolonged cardiac allograft survival in mice. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2002;2(6):501–9. doi: 10.1034/j.1600-6143.2002.20603.x. Epub 2002/07/18. [DOI] [PubMed] [Google Scholar]
- 11.Ensminger SM, Witzke O, Spriewald BM, Morrison K, Morris PJ, Rose ML, et al. CD8+ T cells contribute to the development of transplant arteriosclerosis despite CD154 blockade. Transplantation. 2000;69(12):2609–12. doi: 10.1097/00007890-200006270-00022. Epub 2000/07/26. [DOI] [PubMed] [Google Scholar]
- 12.Adams AB, Williams MA, Jones TR, Shirasugi N, Durham MM, Kaech SM, et al. Heterologous immunity provides a potent barrier to transplantation tolerance. The Journal of clinical investigation. 2003;111(12):1887–95. doi: 10.1172/JCI17477. Epub 2003/06/19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Welsh RM, Markees TG, Woda BA, Daniels KA, Brehm MA, Mordes JP, et al. Virus-induced abrogation of transplantation tolerance induced by donor-specific transfusion and anti-CD154 antibody. Journal of virology. 2000;74(5):2210–8. doi: 10.1128/jvi.74.5.2210-2218.2000. Epub 2000/02/09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kitchens WH, Haridas D, Wagener ME, Song M, Ford ML. Combined costimulatory and leukocyte functional antigen-1 blockade prevents transplant rejection mediated by heterologous immune memory alloresponses. Transplantation. 2012;93(10):997–1005. doi: 10.1097/TP.0b013e31824e75d7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kitchens WH, Haridas D, Wagener ME, Song M, Kirk AD, Larsen CP, et al. Integrin antagonists prevent costimulatory blockade-resistant transplant rejection by CD8(+) memory T cells. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2012;12(1):69–80. doi: 10.1111/j.1600-6143.2011.03762.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schenk AD, Nozaki T, Rabant M, Valujskikh A, Fairchild RL. Donor-reactive CD8 memory T cells infiltrate cardiac allografts within 24-h posttransplant in naive recipients. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2008;8(8):1652–61. doi: 10.1111/j.1600-6143.2008.02302.x. Epub 2008/06/19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Setoguchi K, Hattori Y, Iida S, Baldwin WM, 3rd, Fairchild RL. Endogenous memory CD8 T Cells are activated within cardiac allografts without mediating rejection. American Journal of Transplantation. 2013;13 doi: 10.1111/ajt.12372. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Terasaki PI, Cecka JM, Gjertson DW, Takemoto S. High survival rates of kidney transplants from spousal and living unrelated donors. The New England journal of medicine. 1995;333(6):333–6. doi: 10.1056/NEJM199508103330601. Epub 1995/08/10. [DOI] [PubMed] [Google Scholar]
- 19.Jassem W, Koo DD, Cerundolo L, Rela M, Heaton ND, Fuggle SV. Cadaveric versus living-donor livers: differences in inflammatory markers after transplantation. Transplantation. 2003;76(11):1599–603. doi: 10.1097/01.TP.0000100400.82135.DC. Epub 2004/01/02. [DOI] [PubMed] [Google Scholar]
- 20.Hauptman PJ, Aranki S, Mudge GH, Jr, Couper GS, Loh E. Early cardiac allograft failure after orthotopic heart transplantation. American heart journal. 1994;127(1):179–86. doi: 10.1016/0002-8703(94)90523-1. Epub 1994/01/01. [DOI] [PubMed] [Google Scholar]
- 21.Ojo AO, Wolfe RA, Held PJ, Port FK, Schmouder RL. Delayed graft function: risk factors and implications for renal allograft survival. Transplantation. 1997;63(7):968–74. doi: 10.1097/00007890-199704150-00011. Epub 1997/04/15. [DOI] [PubMed] [Google Scholar]
- 22.Shoskes DA, Halloran PF. Delayed graft function in renal transplantation: etiology, management and long-term significance. The Journal of urology. 1996;155(6):1831–40. doi: 10.1016/s0022-5347(01)66023-3. Epub 1996/06/01. [DOI] [PubMed] [Google Scholar]
- 23.Strasberg SM, Howard TK, Molmenti EP, Hertl M. Selecting the donor liver: risk factors for poor function after orthotopic liver transplantation. Hepatology. 1994;20(4 Pt 1):829–38. doi: 10.1002/hep.1840200410. Epub 1994/10/01. [DOI] [PubMed] [Google Scholar]
- 24.Banner NR, Thomas HL, Curnow E, Hussey JC, Rogers CA, Bonser RS. The importance of cold and warm cardiac ischemia for survival after heart transplantation. Transplantation. 2008;86(4):542–7. doi: 10.1097/TP.0b013e31818149b9. Epub 2008/08/30. [DOI] [PubMed] [Google Scholar]
- 25.Russo MJ, Chen JM, Sorabella RA, Martens TP, Garrido M, Davies RR, et al. The effect of ischemic time on survival after heart transplantation varies by donor age: an analysis of the United Network for Organ Sharing database. The Journal of thoracic and cardiovascular surgery. 2007;133(2):554–9. doi: 10.1016/j.jtcvs.2006.09.019. Epub 2007/01/30. [DOI] [PubMed] [Google Scholar]
- 26.Corry RJ, Winn HJ, Russell PS. Primarily vascularized allografts of hearts in mice. The role of H-2D, H-2K, and non-H-2 antigens in rejection. Transplantation. 1973;16(4):343–50. doi: 10.1097/00007890-197310000-00010. Epub 1973/10/01. [DOI] [PubMed] [Google Scholar]
- 27.Afanasyeva M, Georgakopoulos D, Belardi DF, Ramsundar AC, Barin JG, Kass DA, et al. Quantitative analysis of myocardial inflammation by flow cytometry in murine autoimmune myocarditis: correlation with cardiac function. The American journal of pathology. 2004;164(3):807–15. doi: 10.1016/S0002-9440(10)63169-0. Epub 2004/02/26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ishii D, Schenk AD, Baba S, Fairchild RL. Role of TNFalpha in early chemokine production and leukocyte infiltration into heart allografts. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2010;10(1):59–68. doi: 10.1111/j.1600-6143.2009.02921.x. Epub 2009/12/05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Setoguchi K, Schenk AD, Ishii D, Hattori Y, Baldwin WM, 3rd, Tanabe K, et al. LFA-1 antagonism inhibits early infiltration of endogenous memory CD8 T cells into cardiac allografts and donor-reactive T cell priming. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2011;11(5):923–35. doi: 10.1111/j.1600-6143.2011.03492.x. Epub 2011/04/07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lowin B, Hahne M, Mattmann C, Tschopp J. Cytolytic T-cell cytotoxicity is mediated through perforin and Fas lytic pathways. Nature. 1994;370(6491):650–2. doi: 10.1038/370650a0. Epub 1994/08/25. [DOI] [PubMed] [Google Scholar]
- 31.Alegre ML, Fallarino F. Mechanisms of CTLA-4-Ig in tolerance induction. Current pharmaceutical design. 2006;12(2):149–60. doi: 10.2174/138161206775193046. [DOI] [PubMed] [Google Scholar]
- 32.Vieyra M, Leisman S, Raedler H, Kwan WH, Yang M, Strainic MG, et al. Complement regulates CD4 T-cell help to CD8 T cells required for murine allograft rejection. The American journal of pathology. 2011;179(2):766–74. doi: 10.1016/j.ajpath.2011.04.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nozaki T, Rosenblum JM, Ishii D, Tanabe K, Fairchild RL. CD4 T cell-mediated rejection of cardiac allografts in B cell-deficient mice. J Immunol. 2008;181(8):5257–63. doi: 10.4049/jimmunol.181.8.5257. Epub 2008/10/04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Krieger NR, Yin DP, Fathman CG. CD4+ but not CD8+ cells are essential for allorejection. The Journal of experimental medicine. 1996;184(5):2013–8. doi: 10.1084/jem.184.5.2013. Epub 1996/11/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Selin LK, Cornberg M, Brehm MA, Kim SK, Calcagno C, Ghersi D, et al. CD8 memory T cells: cross-reactivity and heterologous immunity. Seminars in immunology. 2004;16(5):335–47. doi: 10.1016/j.smim.2004.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brehm MA, Markees TG, Daniels KA, Greiner DL, Rossini AA, Welsh RM. Direct visualization of cross-reactive effector and memory allo-specific CD8 T cells generated in response to viral infections. J Immunol. 2003;170(8):4077–86. doi: 10.4049/jimmunol.170.8.4077. Epub 2003/04/12. [DOI] [PubMed] [Google Scholar]
- 37.Selin LK, Brehm MA, Naumov YN, Cornberg M, Kim SK, Clute SC, et al. Memory of mice and men: CD8+ T-cell cross-reactivity and heterologous immunity. Immunological reviews. 2006;211:164–81. doi: 10.1111/j.0105-2896.2006.00394.x. Epub 2006/07/11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Burrows SR, Khanna R, Burrows JM, Moss DJ. An alloresponse in humans is dominated by cytotoxic T lymphocytes (CTL) cross-reactive with a single Epstein-Barr virus CTL epitope: implications for graft-versus-host disease. The Journal of experimental medicine. 1994;179(4):1155–61. doi: 10.1084/jem.179.4.1155. Epub 1994/04/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schenk AD, Gorbacheva V, Rabant M, Fairchild RL, Valujskikh A. Effector functions of donor-reactive CD8 memory T cells are dependent on ICOS induced during division in cardiac grafts. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2009;9(1):64–73. doi: 10.1111/j.1600-6143.2008.02460.x. Epub 2008/11/04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Boros P, Bromberg JS. New cellular and molecular immune pathways in ischemia/reperfusion injury. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2006;6(4):652–8. doi: 10.1111/j.1600-6143.2005.01228.x. [DOI] [PubMed] [Google Scholar]
- 41.Carden DL, Granger DN. Pathophysiology of ischaemia-reperfusion injury. The Journal of pathology. 2000;190(3):255–66. doi: 10.1002/(SICI)1096-9896(200002)190:3<255::AID-PATH526>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 42.Eltzschig HK, Eckle T. Ischemia and reperfusion-from mechanism to translation. Nature medicine. 2011;17(11):1391–401. doi: 10.1038/nm.2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vincenti F, Charpentier B, Vanrenterghem Y, Rostaing L, Bresnahan B, Darji P, et al. A phase III study of belatacept-based immunosuppression regimens versus cyclosporine in renal transplant recipients (BENEFIT study) American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2010;10(3):535–46. doi: 10.1111/j.1600-6143.2009.03005.x. Epub 2010/04/27. [DOI] [PubMed] [Google Scholar]
- 44.Bishop DK, Chan S, Li W, Ensley RD, Xu S, Eichwald EJ. CD4-positive helper T lymphocytes mediate mouse cardiac allograft rejection independent of donor alloantigen specific cytotoxic T lymphocytes. Transplantation. 1993;56(4):892–7. doi: 10.1097/00007890-199310000-00023. Epub 1993/10/01. [DOI] [PubMed] [Google Scholar]
- 45.Isobe M, Yagita H, Okumura K, Ihara A. Specific acceptance of cardiac allograft after treatment with antibodies to ICAM-1 and LFA-1. Science. 1992;255(5048):1125–7. doi: 10.1126/science.1347662. [DOI] [PubMed] [Google Scholar]
- 46.Lunsford KE, Koester MA, Eiring AM, Horne PH, Gao D, Bumgardner GL. Targeting LFA-1 and cd154 suppresses the in vivo activation and development of cytolytic (cd4-Independent) CD8+ T cells. Journal of immunology. 2005;175(12):7855–66. doi: 10.4049/jimmunol.175.12.7855. [DOI] [PubMed] [Google Scholar]
- 47.Murakawa T, Kerklo MM, Zamora MR, Wei Y, Gill RG, Henson PM, et al. Simultaneous LFA-1 and CD40 ligand antagonism prevents airway remodeling in orthotopic airway transplantation: implications for the role of respiratory epithelium as a modulator of fibrosis. Journal of immunology. 2005;174(7):3869–79. doi: 10.4049/jimmunol.174.7.3869. [DOI] [PubMed] [Google Scholar]
- 48.Nicolls MR, Coulombe M, Yang H, Bolwerk A, Gill RG. Anti-LFA-1 therapy induces long-term islet allograft acceptance in the absence of IFN-gamma or IL-4. Journal of immunology. 2000;164(7):3627–34. doi: 10.4049/jimmunol.164.7.3627. [DOI] [PubMed] [Google Scholar]
- 49.Present DH, Rutgeerts P, Targan S, Hanauer SB, Mayer L, van Hogezand RA, et al. Infliximab for the treatment of fistulas in patients with Crohn's disease. The New England journal of medicine. 1999;340(18):1398–405. doi: 10.1056/NEJM199905063401804. [DOI] [PubMed] [Google Scholar]
- 50.Charles P, Elliott MJ, Davis D, Potter A, Kalden JR, Antoni C, et al. Regulation of cytokines, cytokine inhibitors, and acute-phase proteins following anti-TNF-alpha therapy in rheumatoid arthritis. Journal of immunology. 1999;163(3):1521–8. [PubMed] [Google Scholar]
- 51.Chaudhari U, Romano P, Mulcahy LD, Dooley LT, Baker DG, Gottlieb AB. Efficacy and safety of infliximab monotherapy for plaque-type psoriasis: a randomised trial. Lancet. 2001;357(9271):1842–7. doi: 10.1016/s0140-6736(00)04954-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Prolonged cold ischemic storage minimally impacts early NK and gamma-delta T cell allograft infiltration. Groups of 4 C57BL/6 recipient mice received complete MHC-mismatched A/J cardiac allografts subjected to minimal (0.5 h) or prolonged (8 h) cold ischemia prior to transplantation. Grafts were recovered 48 h post-transplant, digested to prepare single cell suspensions, and graft infiltrating cells were analyzed by flow cytometry using antibodies against (A) NK and (B) gamma-delta T cell surface markers.
Supplemental Figure 2. Anti-CD8 mAb effectively depletes CD8 T cells while minimally affecting NK or gamma-delta T cells. Groups of 4 C57BL/6 recipient were treated with 200 μg control rat IgG or anti-CD8 mAb on days -3, -2, -1 prior to analysis. On day 0, animals were sacrificed and blood and spleen samples were collected and analyzed by flow cytometry using antibodies against (A) NK and (B) gamma-delta T cell surface markers.
Supplemental Figure 3. Primary effector CD4 T cells mediate cardiac allograft rejection in the absence of CD8 T cells. (A) Groups of 5 C57BL/6 recipient mice received complete MHCmismatched A/J cardiac allografts subjected to minimal (0.5 h) or prolonged (8 h) cold ischemia prior to transplantation. Recipients were treated with 200 μg control rat IgG or anti-CD8 mAb on days -3, -2, -1 prior to transplant and on day 5 post-transplant. (B) On day 8 posttransplant, spleens and grafts were harvested from transplant recipients and analyzed and quantified by antibody staining and flow cytometry.


