Coagulation factor IX (FIX) a plasma protein whose absence results in hemophilia B, a disease ameliorated by injection of FIX. The pharmacokinetics of infused FIX are complicated. Fifty-80% of injected FIX disappears within 5 minutes. [1–3] Furthermore, Briet has observed that FIX injected into hemophilia B patients lacking antigen disappears more rapidly than when injected into patients with normal levels of inactive FIX. [4] Furthermore,, in hemophilia B patients that receive continuous infusion of FIX for surgery, the amount of FIX required to maintain a level of 100% decreases with time—as though one were saturating binding sites. [5] These data suggest the existence of a significant extravascular FIX compartment.
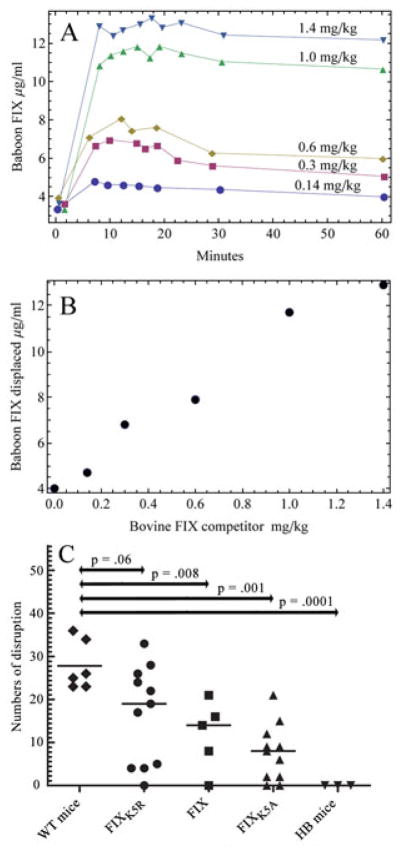
In relevant work, Stern et al. injected increasing amounts of bovine FIX into baboons and measured the concentration of baboon FIX released into the circulation using an antibody specific for baboon FIX. [6] Their Figure 1B showed a rapid, reversible equilibrium between blood and extravascular FIX. Figure 1A is a reproduction of Stern’s figure 1B. A plot of the maximum amount of displaced baboon FIX (10 minute time point) versus the amount of bovine FIX competitor injected is shown in our Figure 1B. The amount of baboon FIX in circulation shows no sign of leveling off at the highest concentration of injected bovine FIX; this indicates that the extravascular compartment is not saturated and contains at least three–fold more FIX than there is in the circulation.
Figure 1.
(A) Figure 1A is from Stem DM, Knitter G, Kisiel W, Nawroth PP, In vivo evidence of intravascular binding sites coagulation factor IX, B J Haematology 1987;66:227–32. It is reproduced here with permission. It shows the amount of baboon FIX released into circulation following injection of bovine FIX at varying concentrations. (B) A replot of Figure 1A of the increase in baboon FIX in circulation at 10 minutes versus the amount of injected bovine FIX. (C) Haemophilia B mice received a bolus injection (0.9 mg/kg) of: FIXK5R, which binds tighter than wild-type to collagen IV, FIXWT, and FIXK5A which binds weaker than wild-type to collagen IV. Seven days after injection the ability of these molecules to promote haemostasis in a saphenous vein bleeding test [14] were compared to wild type and hemophilia B mice. The P-values are from a one-sided Mann-Whitney model. The P Value for FIXK5R vs FIXK5A was 0.003.
FIX has been shown to bind tightly (~5 nM), specifically and reversibly to endothelial cells. [7–9] The binding is not competed by prothrombin, factor X, factor VII, or a FIX molecule whose Gla domain has been replaced with that of factor VII. [1, 2, 6, 10, 11] We previously demonstrated that the binding of FIX involved the omega loop of FIX’s Gla domain and that the endothelial cell binding site is type IV collagen. [11, 12] Changing lysine 5 to arginine or alanine increases or decreases, respectively, the affinity of FIX for collagen IV compared to FIXWT. These observations strongly suggest that the rapid initial loss of FIX from circulation is related to its affinity for collagen IV. [2] Consistent with this notion, Herzog et al. [13] found that FIX expressed in muscle was poorly released into the circulation and was bound at a position around that co-localized with collagen IV.
These observations led us to test whether infused FIX might protect hemophilia B mice from bleeding longer than expected based on half-life and whether FIXK5R protects better than FIXK5A. Figure 1C reveals that infused FIX protects much longer than would be predicted by its’ half-life; thus, there is good protection (demonstrated by a saphenous vein bleeding model [14]) 7 days after injection--even though the plasma levels of all of the infused FIX molecules were below one percent by day 3 after infusion. These results demonstrate that extravascular, collagen IV-bound FIX provides an important coagulant function in the absence of circulating FIX. Moreover, there is a clear gradient of protection which correlates to the affinity of the molecules for collagen IV. The effect of extravascular FIX at higher plasma FIX concentrations or whether plasma and extravascular FIX play different physiologic roles is not known.
For FIX, the terminal half-life (β) is usually considered the relevant parameter, while the distribution half-life (α) is ignored. The goal of prophylaxis in patients with severe hemophilia B is to maintain trough levels of FIX activity in the circulation above 1%. [15, 16] Therefore FIX products with extended circulation times are being developed. [17–19] The increased size of these forms of FIX, however, appears to change their distribution into the extravascular compartment with initial recoveries in plasma following infusion that are 20–94% greater than that of FIXWT. Therefore, it will be interesting to compare the hemostatic effectiveness of the new, longer lasting, factor IX molecules to that of a molecule with enhanced collagen IV binding such as FIXK5R. [17–19] It is conceivable that FIXK5R may be as effective in its hemostatic properties as the molecules that were designed to have an increased terminal half-life and reduced clearance. If this is the case, moreover, it seems likely that additional alterations in FIX may be found that increase its’ collagen IV affinity more than the approximately three fold observed for FIXK5R.
In conclusion, we present evidence that FIX effectively prevents bleeding even after its blood level has been well below one percent for several days. In addition, seven days after a bolus infusion, a FIX variant that binds tighter to collagen IV provides significantly better hemostatic protection in hemophilia B mice than a FIX molecule with lower affinity for collagen IV. This demonstrates that collagen IV-binding by FIX provides a longer lasting extravascular reservoir of FIX at a hemostatically functional location. Furthermore, our results suggest that a therapeutic focus limited to increasing the terminal plasma half-life of FIX alone at the expense of its’ tissue distribution may not be the optimal approach for the treatment of hemophilia B.
Acknowledgments
Our study was supported by the National Institutes of Health (D W Stafford: HL006350, G J Broze: HL077193).
Footnotes
Addendum
DWS.--study design. All authors analyzed and interpreted data.
DF, KAS, DWS prepared figures. All authors wrote and edited the report.
All procedures were approved by the IACUC
Disclosure of Conflicts of Interest
Dr. Stafford and the University of North Carolina have applied for a patent for the use of a FIX that binds tighter to collagen IV as a means of increasing the hemostatic effect of recombinant FIX molecules.
References
- 1.Fuchs HE, Trapp HG, Griffith MJ, Roberts HR, Pizzo SV. Regulation of factor IXa in vitro in human and mouse plasma and in vivo in the mouse. Role of the endothelium and the plasma proteinase inhibitors. J Clin Invest. 1984;73:1696–703. doi: 10.1172/JCI111377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gui T, Lin HF, Jin DY, Hoffman M, Straight DL, Roberts HR, Stafford DW. Circulating and binding characteristics of wild-type factor IX and certain Gla domain mutants in vivo. Blood. 2002;100:153–8. doi: 10.1182/blood.v100.1.153. [DOI] [PubMed] [Google Scholar]
- 3.Roth DA, Kessler CM, Pasi KJ, Rup B, Courter SG, Tubridy KL. Recombinant Factor IXSG. Human recombinant factor IX: safety and efficacy studies in hemophilia B patients previously treated with plasma-derived factor IX concentrates. Blood. 2001;98:3600–6. doi: 10.1182/blood.v98.13.3600. [DOI] [PubMed] [Google Scholar]
- 4.Briet E. PhD Thesis. 1977. Three problems of haemophilia B. [Google Scholar]
- 5.Uprichard J, Adamidou D, Goddard NJ, Mann HA, Yee TT. Factor IX replacement to cover total knee replacement surgery in haemophilia B: a single-centre experience, 2000–2010. Haemophilia. 2012;18:46–9. doi: 10.1111/j.1365-2516.2011.02552.x. [DOI] [PubMed] [Google Scholar]
- 6.Stern DM, Knitter G, Kisiel W, Nawroth PP. In vivo evidence of intravascular binding sites for coagulation factor IX. British journal of haematology. 1987;66:227–32. doi: 10.1111/j.1365-2141.1987.tb01303.x. [DOI] [PubMed] [Google Scholar]
- 7.Heimark RL, Schwartz SM. Binding of coagulation factors IX and X to the endothelial cell surface. Biochemical and biophysical research communications. 1983;111:723–31. doi: 10.1016/0006-291x(83)90365-0. [DOI] [PubMed] [Google Scholar]
- 8.Stern DM, Drillings M, Nossel HL, Hurlet-Jensen A, LaGamma KS, Owen J. Binding of factors IX and IXa to cultured vascular endothelial cells. Proc Natl Acad Sci U S A. 1983;80:4119–23. doi: 10.1073/pnas.80.13.4119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stern DM, Nawroth PP, Kisiel W, Vehar G, Esmon CT. The binding of factor IXa to cultured bovine aortic endothelial cells. Induction of a specific site in the presence of factors VIII and X. J Biol Chem. 1985;260:6717–22. [PubMed] [Google Scholar]
- 10.Toomey JR, Smith KJ, Roberts HR, Stafford DW. The endothelial cell binding determinant of human factor IX resides in the gamma-carboxyglutamic acid domain. Biochemistry. 1992;31:1806–8. doi: 10.1021/bi00121a031. [DOI] [PubMed] [Google Scholar]
- 11.Cheung WF, van den Born J, Kuhn K, Kjellen L, Hudson BG, Stafford DW. Identification of the endothelial cell binding site for factor IX. Proc Natl Acad Sci U S A. 1996;93:11068–73. doi: 10.1073/pnas.93.20.11068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wolberg AS, Stafford DW, Erie DA. Human factor IX binds to specific sites on the collagenous domain of collagen IV. J Biol Chem. 1997;272:16717–20. doi: 10.1074/jbc.272.27.16717. [DOI] [PubMed] [Google Scholar]
- 13.Herzog RW, Hagstrom JN, Kung SH, Tai SJ, Wilson JM, Fisher KJ, High KA. Stable gene transfer and expression of human blood coagulation factor IX after intramuscular injection of recombinant adeno-associated virus. Proc Natl Acad Sci U S A. 1997;94:5804–9. doi: 10.1073/pnas.94.11.5804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Buyue Y, Whinna HC, Sheehan JP. The heparin-binding exosite of factor IXa is a critical regulator of plasma thrombin generation and venous thrombosis. Blood. 2008;112:3234–41. doi: 10.1182/blood-2008-01-136820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Collins PW, Fischer K, Morfini M, Blanchette VS, Bjorkman S. Implications of coagulation factor VIII and IX pharmacokinetics in the prophylactic treatment of haemophilia. Haemophilia. 2011;17:2–10. doi: 10.1111/j.1365-2516.2010.02370.x. [DOI] [PubMed] [Google Scholar]
- 16.Bjorkman S. A commentary on the differences in pharmacokinetics between recombinant and plasma-derived factor IX and their implications for dosing. Haemophilia. 2011;17:179–84. doi: 10.1111/j.1365-2516.2010.02431.x. [DOI] [PubMed] [Google Scholar]
- 17.Negrier C, Knobe K, Tiede A, Giangrande P, Moss J. Enhanced pharmacokinetic properties of a glycoPEGylated recombinant factor IX: a first human dose trial in patients with hemophilia B. Blood. 2011;118:2695–701. doi: 10.1182/blood-2011-02-335596. [DOI] [PubMed] [Google Scholar]
- 18.Ostergaard H, Bjelke JR, Hansen L, Petersen LC, Pedersen AA, Elm T, Moller F, Hermit MB, Holm PK, Krogh TN, Petersen JM, Ezban M, Sorensen BB, Andersen MD, Agerso H, Ahmadian H, Balling KW, Christiansen ML, Knobe K, Nichols TC, Bjorn SE, Tranholm M. Prolonged half-life and preserved enzymatic properties of factor IX selectively PEGylated on native N-glycans in the activation peptide. Blood. 2011;118:2333–41. doi: 10.1182/blood-2011-02-336172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shapiro AD, Ragni MV, Valentino LA, Key NS, Josephson NC, Powell JS, Cheng G, Thompson AR, Goyal J, Tubridy KL, Peters RT, Dumont JA, Euwart D, Li L, Hallen B, Gozzi P, Bitonti AJ, Jiang H, Luk A, Pierce GF. Recombinant factor IX-Fc fusion protein (rFIXFc) demonstrates safety and prolonged activity in a phase 1/2a study in hemophilia B patients. Blood. 2012;119:666–72. doi: 10.1182/blood-2011-07-367003. [DOI] [PMC free article] [PubMed] [Google Scholar]