Abstract
Hematopoietic stem cell transplantation is the only curative option for a number of malignant and non-malignant diseases. As the use of hematopoietic transplant has expanded, so too has the source of stem and progenitor cells. The predominate source of stem and progenitors today, particularly in settings of autologous transplantation, is mobilized peripheral blood. This review will highlight the historical advances which lead to the widespread use of peripheral blood stem cells for transplantation, with a look towards future enhancements to mobilization strategies.
Keywords: Hematopoietic stem cell (HSC), mobilization, granulocyte-colony stimulating factor (G-CSF), CXCR4, stem cell niche, AMD3100 (plerixafor)
Introduction
The first successful clinical cases utilizing peripheral blood stem cells (PBSC) for curative hematopoietic transplantation were reported over 26 years ago(1–5). Since then, PBSC have become the predominate source of hematopoietic stem cells (HSC) for transplantation. This review will highlight the history of stem cell research which planted the initial seeds for PBSC harvesting; the current state of clinical mobilization and what we have learned over the last two and half decades of practice; and the outlook for clinical enhancement in the future.
Sowing the Seeds: Early Mobilization Research
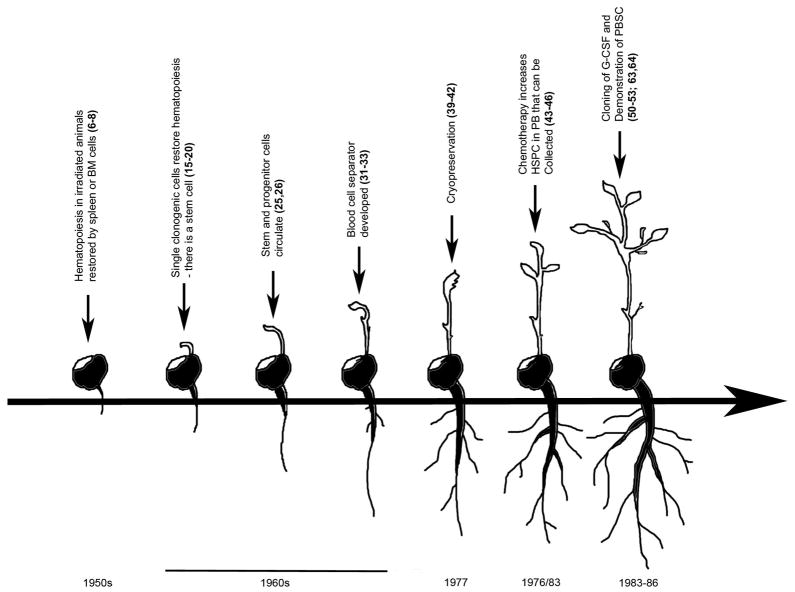
Early work establishing the foundation of hematopoietic mobilization began over 60 years ago (Figure 1). Restoration of hematopoiesis in irradiated animals by spleen and/or bone marrow (BM) derived cells was reported in the early 1950s (6–8). Shortly thereafter, it was demonstrated that allogeneic skin grafts were tolerated in mice who had received lethal irradiation followed by a hematopoietic transplant (9). This lead to the concept of chimerism, i.e., donor cells reconstituting the irradiated host, which was confirmed in later studies (10–14). In the 1960’s, Till and McCulloch and colleagues published hallmark studies showing that single clonogenic cells existed within the bone marrow that could self-renew and restore hematopoiesis (15–20), thus the hypothesis of the in vivo existence of a hematopoietic stem cells was germinated. These early assays utilized lethally irradiated mice that were injected with bone marrow cells and showed macroscopic nodules that formed on/in the spleens proportional to the number of marrow cells injected (17). They hypothesized that the spleen colonies (colony-forming units-spleen (CFU-S)) were derived from a single cell, which they later demonstrated by analysis of chromosomal markers (20). These studies laid the groundwork for clinical hematopoietic transplantation.
Figure 1.
Timeline of major advances leading to the use of peripheral blood stem cells for hematopoietic transplantation.
In the 1930’s, well prior to studies using isolated donor cells to recover hematopoiesis following irradiation, Woenckhaus performed experiments in which one rat, as part of a parabiotic pair, was lethally irradiated while the other rat was shielded with lead. One third of the pairs survived the procedure, suggesting a circulating radiation protection factor produced by the non-irradiated rat (21). A similar parabiotic experiment was also reported two decades later (22). As methods to assess DNA replication, and thus cell division, began to emerge, reports documented the presence of circulating, non-leukemic, blood cells capable of division outside of the bone marrow (23, 24). These experiments suggested that a large organ like the bone marrow was capable of exchanging cells through the peripheral blood system, providing a potential common pool of cells with proliferative capacity able to directly contribute to recovery after damage and maintain total system homeostasis.
In 1960, a report demonstrated the successful transplantation of cells with erythropoietic potential from normal circulating leukocytes (25). This was later expanded upon by Goodman and Hodgson to demonstrate the presence of a peripheral blood cell capable of hematopoietic reconstitution in lethally irradiated hosts (26). Later experiments utilizing CFU-S as a surrogate of HSC function suggested that peripheral blood leukocytes contained 1/100th of the repopulating ability of bone marrow leukocytes (27). The presence of peripheral blood hematopoietic repopulating cells was later confirmed in transplantation studies in dogs (28–30).
These early studies in rodents and dogs suggested that peripheral blood could be an alternative source to bone marrow of cells with hematopoietic reconstituting potential. However, Lewis and colleagues in 1968 suggested that the frequency of repopulating cells (estimated to be 1/100th that of marrow), was too low in peripheral blood and they concluded that “with present techniques, the use of blood leukocytes for effecting hematopoietic grafts in man may not be technically feasible. In terms of present day knowledge, it is difficult to envision that circulating stem cells will be found to be of any great value to man.” (27). Fortuitously, around the same time, researchers at the National Cancer Institute along with the International Business Machines Corporation jointly developed a continuous flow centrifuge (NCI-IBM Blood Cell Separator) as a means to isolate leukocytes for biopsy, or for subsequent infusion into granulocytic patients (31–33). This apheresis technique reduced one of the technical hurdles of acquiring enough peripheral blood repopulating units for transplantation.
In the early 1970’s, several reports confirmed the presence of clonogenic hematopoietic progenitors in the peripheral blood of man (34–36), one of which utilized apheresis (36), thus planting the initial seeds for the potential to harvest HSCs from blood for transplant. However, early attempts at repetitive white blood cell transfusions from healthy twin donors did not result in durable engraftment (37, 38), presumably still due to the relatively low HSC cell number in peripheral blood compared to bone marrow. To compensate for low HSC number, cryopreservation techniques were developed to allow for large pools of peripheral blood leukocytes to be collected (39), and transplants using cryopreserved peripheral blood cells were used to treat patients with chronic myeloid leukemia (CML), with some documented short term success (40–42). Several years later, many institutes began to report successful hematopoietic engraftment using cryopreserved cells acquired from multiple rounds of apheresis prior to transplantation (1–5).
While these early studies demonstrated some successes, multiple rounds of apheresis, over the course of many days and weeks, coupled with multiple cryopreservations and subsequent infusions of large volumes of cells made these early regimens impractical for wide clinical application. To expand the use of PBSC to a broader range of hematopoietic transplantation, more stem cells needed to be acquired in a shorter period of time. Several earlier reports assessing the bone marrow compartment following chemotherapy demonstrated an increase in hematopoietic progenitor activity (43–45). A report by Richman and colleagues further explored this phenomenon by assessing hematopoietic progenitors in the peripheral blood following administration of cyclophosphamide and adriamycin (46). These studies demonstrated that chemotherapy treatment could increase in hematopoietic progenitors in the blood by upwards of a 20 fold. Intriguingly, the authors proposed that chemotherapy could possibly be used as a means to facilitate acquisition of PBSC for transplantation. As a preliminary test of hypothesis they studied one patient in which they harvested bone marrow and apheresis products before and after chemotherapy. This single patient demonstrated that at baseline, they would need to perform 296 hours of apheresis to acquire the same number of hematopoietic progenitors as was obtained from the bone marrow harvest, but after chemotherapy, the amount of apheresis time would be reduced to only 11 hours (46). A similar conclusion was made several years later by Stiff and colleagues(47). These results suggested that another major hurdle for the clinical translation of PBSC as a viable alternative to bone marrow could be crossed; mobilization of HSC to the periphery with the use of chemotherapy to decrease the amount of apheresis procedures and transfusion volumes. This suggestion was confirmed in a number of subsequent studies (48, 49).
Enhancing the Harvest: Granulocyte-Colony Stimulating Factor
Today, the hematopoietic growth factor, granulocyte-colony stimulating factor (G-CSF) is widely used clinically to mobilize HSC for transplantation. Granulocyte colony-stimulating factor (G-CSF) was purified, cloned and produced recombinantly in bacteria between 1984 and 1986 (50–53). Although initially believed to be a pluripoietin as well an inducer of granulocyte differentiation, use of recombinant protein showed that G-CSF bound to a type 1 cytokine receptor (G-CSFR) to stimulate proliferation (54, 55) and differentiation (53) of several types of myeloid progenitor cells alone and in combination with other growth factors (56–58). The first clinical trials were performed in cancer patients receiving chemotherapy (59–62) leading to FDA approval in 1991. While G-CSF was being widely used successfully to treat neutropenia following chemotherapy, it was found that it increased the number of peripheral blood progenitor cells (63, 64). While early empiric regimens were variable, today, mobilization of HSC and HPC is accomplished with G-CSF administered at 5–10 ug/kg/day for 5–7 days in patients and normal donors, with one or more days of apheresis to achieve a minimum target dose ≥2×106 CD34+ cells/kg patient body weight. PBSC transplant with G-CSF mobilized HSC and HPC has been quite successful and has changed the normal transplant paradigm, making PBSC the predominate source of HSC for transplant. G-CSF mobilized PBSC have been associated with more rapid engraftment, shorter hospital stay (65–68), and in some circumstances, superior overall survival compared to bone marrow (69).
While successful, G-CSF regimens are often associated with morbidity in the form of bone pain, nausea, headache, and fatigue (70–73), which can be lifestyle disruptive in normal volunteers. In a small population of normal donors, G-CSF has also been associated with serious toxicity, including enlargement of the spleen (74, 75) and splenic rupture (76–79), and the pro-coagulant effects of G-CSF can increase the risk of myocardial infarction and cerebral ischemia in high-risk individuals (80, 81). Despite success, G-CSF induced mobilization regimens also have high failure rates, failing to mobilize sufficient HSC, particularly for autologous mobilization, necessitating additional mobilization attempts or precluding transplantation (82–86). Unsuccessful initial HSC mobilization often leads to expensive additional mobilization attempts and may preclude autologous transplant altogether (87–89). In one study assessing 1040 patients undergoing mobilization for autologous transplant, it was found that 47% failed to collect even the minimum of 2×106 CD34+ cells/kg in the first apheresis, and 22.5% did not reach this level even after 2 apheresis sessions (82). A recent study out of the Mayo Clinic demonstrated that 30% of multiple myeloma (MM) and 71% of non-hodgkin’s lymphoma (NHL) patients failed to reach the “optimum” level of CD34+ cell collection (>5×106 cells/kg) (90). A recent economic analysis at M.D. Anderson Cancer Center determined that reducing the apheresis by 1 day has the potential to decrease the medicals costs by $6,600 (91). Thus, improved/alternative regimens and mobilizers are needed.
The New Branches of HSC Mobilization
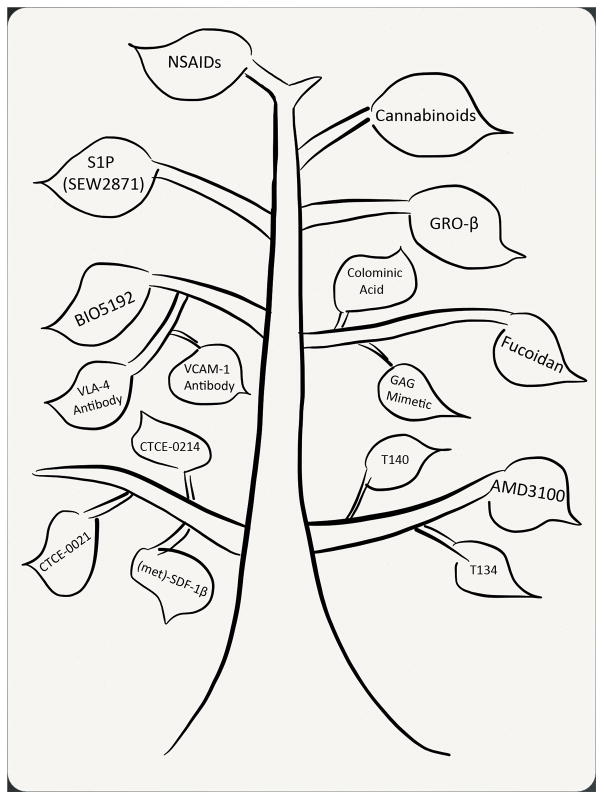
While the discovery of enhanced peripheral progenitors following chemotherapy, and then after growth factors such as G-CSF and GM-CSF, altered the paradigm of clinical transplantation, lack of initial understanding of the mechanism of action of these cytokines hampered further development in the field, which relied primarily upon empiric trial. Clues to the mechanism of action of G-CSF were not inherently obvious; however, early on it was appreciated that the mobilization mechanisms may be both HSPC intrinsic and extrinsic. A seminal paper published in 2002 (92) linked G-CSF mobilization to disruption of the SDF1/CXCR4 axis and led to a number of studies showing that altering this pathway by a variety of methods led to PBSC mobilization in varying degree. Numerous mobilization agents have sprouted from these early discoveries (Figure 2).
Figure 2.
The new branches of hematopoietic stem cell mobilization, showing alternative agents to G-CSF.
One of the most successful mobilizing agents to come along after G-CSF is the CXCR4 antagonist AMD3100 (Plerixafor; Mozobil™), which is capable of mobilizing HSC and HPC alone and in combination with G-CSF (93–98) and received FDA approval in 2008. Other CXCR4 antagonists such as T140 (99) and T134 (100) have also been reported to mobilize HSPC as well as CXCR4 partial agonists, including (met)-SDF-1β (101), CTCE-0214 (102), and CTCE-0021 (98). These agents are believed to initiate mobilization by antagonizing the CXCR4 receptor thus breaking the retentive bond between HSC and HPC and their SDF-1 producing stromal microenvironmental support. Betafectin (103, 104), sulfated polysaccharides (Fucoidan) (105–107), sulfated colominic acid (108), and the smaller glycosaminoglycan (GAG) mimetics (109), which appear to alter plasma SDF-1α levels (107–109), enhance matrix metalloproteinase-9 (MMP-9) production (103, 106, 109) and increase CXCR4 receptor function (108) among other mechanisms are also capable of enhancing HSPC mobilization.
While these mobilization strategies focused on disruption of the SDF-1α/CXCR4 interaction within the bone marrow microenvironment and/or alteration of the chemotactic gradient between blood and marrow, other mechanistic studies based upon knowledge of integrin adhesion interactions between hematopoietic cells and their stromal niches led to strategies designed to “untether” HSPC from the niche. Hematopoietic mobilization has been achieved by disrupting the VLA-4/VCAM-1 axis, with antibodies against VLA-4 (110, 111), antibodies against VCAM-1 (112, 113), or a small molecule inhibitor of VLA-4 (BIO5192) (114). Similarly, disruption of the Eph-ephrin A3 axis with a soluble EphA3-Fc fusion protein (115) or treatment with defibrotide(116), an adenosine receptor agonist which disrupts P-selectin (117) and intercellular adhesion molecule-1 (ICAM-1) (118), also enhance mobilization.
Interest in chemokines in the 1990’s spurred by the identification of multiple classes of chemotactic peptides that affect HSPCs led to the identification of an alternate chemokine pathway involved in mobilization (119, 120). The CXCR2 agonists IL8, GROβ and GROβΔ4 cause rapid HSPC mobilization within 15 minutes of treatment (121–123). In contrast to CXCR4, CXCR2 is not expressed on HSPC, and mechanistic studies identified that mobilization was mediated through induction of matrix metalloproteinase-9 disrupting hematopoietic retention in the niche (123, 124), and demonstrating that mobilization agents can target non-hematopoietic cells to illicit mobilization responses.
Recent evidence also suggests that disruption of fatty acid signaling can alter hematopoietic trafficking and be used as a pharmaceutical tool to enhance HSPC mobilization. Sphingosine-1-phosphate (S1P) can act on HSC and HPC through the S1P receptor S1P1 (125), and alter CXCR4/SDF-1α signaling and chemotaxis (125–127). S1P has been previously reported to direct trafficking of immature B cells (128) and trafficking of HSPC from blood, bone marrow and lymph tissues (129). G-CSF treatment results in an increase in peripheral S1P concentration directing HSPC chemoattraction to the periphery, resulting in mobilization (130). Additional reports have supported the hypothesis that a S1P signaling gradient can regulate HSPC trafficking and mobilization (131–134). Endocannabinoids, members of the arachidonic acid family of fatty acids, are also capable of altering HSPC trafficking and enhancing G-CSF mobilization through effects on cannabinoid receptors expressed on HSPC (135). Prostaglandin E2 (PGE2), another member of the arachidonic acid family, acts in a Yin and Yang relationship with G-CSF (135) and regulates CXCR4 expression on HSC and HPC and facilitates homing and engraftment during transplantation (136). Blocking PGE2 signaling in the bone marrow with FDA approved NSAIDs alters HSC and HPC retention in the stromal niche, resulting in enhanced HSPC trafficking and mobilization in combination with multiple mobilizing agents (137).
Intriguingly, G-CSF mobilization is reduced in chemically sympathectomized mice; mice treated with the β-blocker propanolol; or mice genetically deficient in the gene for dopamine β-hydroxylase (Dbh), an enzyme that converts dopamine into norepinephrine, demonstrating that mobilization requires peripheral β2-adrenergic signals (138). This study also demonstrated that G-CSF attenuated osteoblast function, via the sympathetic nervous system (SNS), resulting in osteoblasts having a marked flattened appearance. In addition to the bone marrow microenvironmental niche, human CD34+ cells also express β2-adrenergic and dopamine receptors that are upregulated after G-CSF treatment (139), and neurotransmitters have been demonstrated to serve as direct chemoattractants to hematopoietic cells (139) and increase CXCR4 expression (140). Epinephrine treatment also results in mobilization (139). These studies suggest that targeting of the SNS may serve as an adjunct therapy to enhance mobilization, though these strategies as of now are not specific in targets and would likely lead to many complications in patients and healthy donors.
Planting New Ground
At present, the wide array of agents that have been shown to mobilize HSC and HPC, leads us to conclude that we do not truly fully understand the biology of enhanced HSPC trafficking, and only a better understanding of this process can lead to better mobilizers and/or regimens. However, these new seeds of knowledge on mechanisms of action should not remain ungerminated in the quest for the “perfect” or “optimal” mobilizing agent. Taking cues from the fields of chemotherapy and microbiology, combinations of agents often provide additive/synergistic and unique actions. The small molecule CXCR4 antagonist Plerixafor (AMD3100), which was shown in preclinical combination studies with G-CSF to enhance mobilization compared to G-CSF alone (93, 141–144), is now approved by the FDA for mobilization of PBSC in patients with NHL and MM, however a significant portion of patients still fail to mobilize sufficient numbers even after plerixafor administration. The use of Plerixafor can also come at a cost of >$25,000 per patient in some settings compared to G-CSF (145), suggesting that preemptive use in all patients in not a sound pharmacoeconomic strategy. To save cost associated with the use of plerixafor, a number of centers have advocated a risk-adapted approach whereby plerixafor is added after 4 days of filgrastim only in those patients who show evidence of “suboptimal” mobilization based on assessment of peripheral blood CD34+ cell measurement (146–148) or alternatively on the basis of the CD34+ cell dose collected on the first day’s apheresis (148). However, as the target or “acceptable” CD34+ cell dose, as well as the “acceptable” number of aphereses in which this achieved, vary somewhat by institution, precise recommendations also vary; currently no standard algorithm exists. At Indiana University, we target for a minimum CD34+ cell dose of 10×106/kg for MM (for potentially tandem or second late transplantation), and 5×106/kg for NHL patients. To minimize utilization of apheresis resources, we also attempt to collect the target dose in only 1–2 aphereses if possible. Therefore, patients begin mobilization with G-CSF (10 μg/kg/day) for 4 days. If the first day’s collection on day 5 is less than half of the target, plerixafor is added on the evening of day 5 (G-CSF continued), and apheresis performed the next day. Thus, while Plerixafor plus G-CSF has clearly made an impact on the ability to mobilize patient populations known to be difficult to mobilize, the need to search for more effective, and less costly, mobilizing agents still remains.
As described earlier, NSAIDs alter HSC and HPC retention in the stromal niche, resulting in enhanced HSPC trafficking and when used with G-CSF results in a PBSC graft that restores neutrophils and platelets faster than observed with the PBSC grafts mobilized by G-CSF alone (137). This finding represents an exciting opportunity to utilize a highly effective but inexpensive FDA approved drug to enhance PBSC mobilization and may be more appropriate for preemptive strategies, eliminating the need for guesswork on “poor” versus “good mobilizers” to decide who receives treatment and who does not.
Another fertile area to cultivate may in fact be the use of rapid mobilizers alone. In the studies reported to date, it is clear that these agents, particularly chemokines and their receptors, differ dramatically in their mobilization mechanism compared to G-CSF. These agents mobilize in minutes to hours compared to days for G-CSF. They are, however, usually less active than G-CSF based upon numbers of hematopoietic progenitors mobilized. However, in preclinical studies, the PBSC graft mobilized by these agents appears to contain a population of more immature HSPC with inherent superior engraftment capacity compared to G-CSF alone (149–151). A combination that we have recently investigated utilizes the CXCR4 antagonist AMD3100 with the CXCR2 agonist GROβΔ4. When these 2 agents are administered either simultaneously or within 5 minutes of each other, a level of HSPC mobilization is reached within 15 minutes that is equal to that of G-CSF used alone for 4 days (150). Similarly, in primary competitive transplantation models, the graft mobilized by combination of AMD3100 plus GROβ showed equal or slightly better chimerism compared to a PBSC graft mobilized by a four day regimen of G-CSF. However, in secondary non-competitive transplant models transplant of equal numbers of bone marrow cells harvested at 6 months from mice who had received AMD3100 plus GROβ mobilized grafts showed significantly enhanced chimerism compared to mice receiving G-CSF mobilized PBSC, clearly suggesting superior engraftment and enhanced competitiveness of the primary graft compared to G-CSF. Translation of the rapid mobilizer strategy to clinical transplantation may in fact alter the transplant paradigm and allow for transplant without graft cryopreservation, and the inherent loss of stem and progenitor cells by freezing, thaw and wash. The level of mobilization seen in mice with this combination suggests that the need for multiple days of apheresis can be significantly reduce or even eliminated. Since recombinant GROβ has been administered in man, it will be interesting to see if preclinical findings can be translated.
Defining a successful harvest
The inherent enhanced reconstituting capacity of PBSC grafts mobilized in part through agonism of the CXCR4 and CXCR2 receptors, or use of NSAIDs with G-CSF raise the issue of defining an “optimal graft” as defined by CD34+ cells per kg body weight. Clearly, CD34+ cell number is a useful current guideline for transplant that is based on the paradigm of using G-CSF as a single agent mobilizer. Preclinical data with AMD3100 and GROβ have suggested that there is a qualitative difference in the PBSC mobilized by these agents (93, 121, 150, 152, 153). Similarly, in a trial exploring AMD3100 as a single agent without G-CSF, reduced CD34+ cell number with AMD3100 was seen compared to G-CSF, yet the transplanted patients showed rapid and durable trilineage hematopoiesis (154). It will remain to be determined if minimum CD34 count, i.e., 2×106/kg will be useful as a guideline for grafts mobilized by other agents.
Conclusion
G-CSF mobilized PBSC have had a significant impact on the expansion of hematopoietic transplantation as a curative option for numerous malignant and non malignant diseases. The flurry of mechanism based research studies that has followed the largely clinical trial based development of G-CSF based PBSC mobilization identified new agents and potential pathways for improvements to mobilization, and has led to FDA approval for Plerixafor in combination with G-CSF for hard to mobilize patients. Preclinical studies have increased our understanding of HSPC trafficking and although clearly incomplete, we have reached a time where these new seeds of knowledge can and should be germinated to develop improved varieties of PBSC mobilization that based upon preclinical data may yield stronger and more robust effects.
Acknowledgments
The authors would like to thank Tiffany Tate for artwork assistance. JH is supported by NIH grant HL087735.
Footnotes
Author Contributions:
Jonathan Hoggatt: Conception and design, collection and/or assembly of data, manuscript writing, and final approval of manuscript.
Jennifer M. Speth: Collection and/or assembly of data, final approval of manuscript
Louis M. Pelus: Conception and design, collection and/or assembly of data, manuscript writing, and final approval of manuscript.
References
- 1.Bell AJ, Figes A, Oscier DG, et al. Peripheral blood stem cell autografts in the treatment of lymphoid malignancies: initial experience in three patients. BR J HAEMATOL. 1987;66(1):63–68. doi: 10.1111/j.1365-2141.1987.tb06891.x. [DOI] [PubMed] [Google Scholar]
- 2.To LB, Dyson PG, Branford AL, et al. Peripheral blood stem cells collected in very early remission produce rapid and sustained autologous haemopoietic reconstitution in acute non-lymphoblastic leukaemia. BONE MARROW TRANSPLANT. 1987;2(1):103–108. [PubMed] [Google Scholar]
- 3.Reiffers J, Bernard P, David B, et al. Successful autologous transplantation with peripheral blood hemopoietic cells in a patient with acute leukemia. EXP HEMATOL. 1986;14(4):312–315. [PubMed] [Google Scholar]
- 4.Kessinger A, Armitage JO, Landmark JD, et al. Reconstitution of human hematopoietic function with autologous cryopreserved circulating stem cells. EXP HEMATOL. 1986;14(3):192–196. [PubMed] [Google Scholar]
- 5.Korbling M, Dorken B, Ho AD, et al. Autologous transplantation of blood-derived hemopoietic stem cells after myeloablative therapy in a patient with Burkitt’s lymphoma. BLOOD. 1986;67(2):529–532. [PubMed] [Google Scholar]
- 6.Lorenz E, Uphoff D, REID TR, et al. Modification of irradiation injury in mice and guinea pigs by bone marrow injections. J NATL CANCER INST. 1951;12(1):197–201. [PubMed] [Google Scholar]
- 7.Jacobson LO, Simmons EL, Marks EK, et al. Recovery from radiation injury. SCIENCE. 1951;113(2940):510–511. doi: 10.1126/science.113.2940.510. [DOI] [PubMed] [Google Scholar]
- 8.Jacobson LO, Simmons EL, Marks EK, et al. The role of the spleen in radiation injury and recovery. J LAB CLIN MED. 1950;35(5):746–770. [PubMed] [Google Scholar]
- 9.Main JM, Prehn RT. Successful skin homografts after the administration of high dosage X radiation and homologous bone marrow. J NATL CANCER INST. 1955;15(4):1023–1029. [PubMed] [Google Scholar]
- 10.Ford CE, Hamerton JL, Barnes DW, et al. Cytological identification of radiation-chimaeras. NATURE. 1956;177(4506):452–454. doi: 10.1038/177452a0. [DOI] [PubMed] [Google Scholar]
- 11.MAKINODAN T. Circulating rat cells in lethally irradiated mice protected with rat bone marrow. PROC SOC EXP BIOL MED. 1956;92(1):174–179. doi: 10.3181/00379727-92-22421. [DOI] [PubMed] [Google Scholar]
- 12.Nowell PC, COLE LJ, Habermeyer JG, et al. Growth and continued function of rat marrow cells in x-radiated mice. CANCER RES. 1956;16(3):258–261. [PubMed] [Google Scholar]
- 13.Trentin JJ. Mortality and skin transplantability in x-irradiated mice receiving isologous, homologous or heterologous bone marrow. PROC SOC EXP BIOL MED. 1956;92(4):688–693. doi: 10.3181/00379727-92-22582. [DOI] [PubMed] [Google Scholar]
- 14.Vos O, DAVIDS JA, WEYZEN WW, et al. Evidence for the cellular hypothesis in radiation protection by bone marrow cells. ACTA PHYSIOL PHARMACOL NEERL. 1956;4(4):482–486. [PubMed] [Google Scholar]
- 15.Wu AM, Till JE, Siminovitch L, et al. A cytological study of the capacity for differentiation of normal hemopoietic colony-forming cells. J CELL PHYSIOL. 1967;69(2):177–184. doi: 10.1002/jcp.1040690208. [DOI] [PubMed] [Google Scholar]
- 16.Wu AM, Till JE, Siminovitch L, et al. Cytological evidence for a relationship between normal hemotopoietic colony-forming cells and cells of the lymphoid system. J EXP MED. 1968;127(3):455–464. doi: 10.1084/jem.127.3.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Till JE, McCulloch EA. A direct measurement of the radiation sensitivity of normal mouse bone marrow cells. RADIAT RES. 1961;14:213–222. [PubMed] [Google Scholar]
- 18.Siminovitch L, McCulloch EA, Till JE. The Distibution of Colony-forming Cells Among Spleen Colonies. J CELL PHYSIOL. 1963;62:327–336. doi: 10.1002/jcp.1030620313. [DOI] [PubMed] [Google Scholar]
- 19.Leung CG, Xu Y, Mularski B, et al. Requirements for survivin in terminal differentiation of erythroid cells and maintenance of hematopoietic stem and progenitor cells. J EXP MED. 2007;204(7):1603–1611. doi: 10.1084/jem.20062395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Becker AJ, McCulloch EA, Till JE. Cytological demonstration of the clonal nature of spleen colonies derived from transplanted mouse marrow cells. NATURE. 1963;197:452–454. doi: 10.1038/197452a0. [DOI] [PubMed] [Google Scholar]
- 21.Woenckhaus E. Beitrag zur Allgemeinwirkung der Roentgenstrahlen. ARCH F EXP PATH UND PHARM. 1930;150:182. [Google Scholar]
- 22.Brecher G, CRONKITE EP. Post-radiation parabiosis and survival in rats. PROC SOC EXP BIOL MED. 1951;77(2):292–294. doi: 10.3181/00379727-77-18754. [DOI] [PubMed] [Google Scholar]
- 23.BOND VP, Fliedner TM, CRONKITE EP, et al. Proliferative potentials of bone marrow and blood cells studied by in vitro uptake of H3-thymidine. ACTA HAEMATOL. 1959;21(1):1–15. doi: 10.1159/000205651. [DOI] [PubMed] [Google Scholar]
- 24.BOND VP, CRONKITE EP, Fliedner TM, et al. Deoxyribonucleic acid synthesizing cells in peripheral blood of normal human beings. SCIENCE. 1958;128(3317):202–203. doi: 10.1126/science.128.3317.202. [DOI] [PubMed] [Google Scholar]
- 25.POPP RA. Erythrocyte repopulation in x-irradiated recipients of nucleated, peripheral blood cells of normal mice. PROC SOC EXP BIOL MED. 1960;104:722–724. doi: 10.3181/00379727-104-25966. [DOI] [PubMed] [Google Scholar]
- 26.Goodman JW, Hodgson GS. Evidence for stem cells in the peripheral blood of mice. BLOOD. 1962;19:702–714. [PubMed] [Google Scholar]
- 27.Lewis JP, Passovoy M, Freeman M, et al. The repopulating potential and differentiation capacity of hematopoietic stem cells from the blood and bone marrow of normal mice. J CELL PHYSIOL. 1968;71(2):121–132. doi: 10.1002/jcp.1040710203. [DOI] [PubMed] [Google Scholar]
- 28.Fliedner TM, Flad HD, Bruch C, et al. Treatment of aplastic anemia by blood stem cell transfusion: a canine model. HAEMATOLOGICA. 1976;61(2):141–156. [PubMed] [Google Scholar]
- 29.Storb R, Epstein RB, Ragde H, et al. Marrow engraftment by allogeneic leukocytes in lethally irradiated dogs. BLOOD. 1967;30(6):805–811. [PubMed] [Google Scholar]
- 30.CAVINS JA, SCHEER SC, THOMAS ED, et al. THE RECOVERY OF LETHALLY IRRADIATED DOGS GIVEN INFUSIONS OF AUTOLOGOUS LEUKOCYTES PRESERVED AT-80 C. BLOOD. 1964;23:38–42. [PubMed] [Google Scholar]
- 31.Buckner D, Graw RG, Jr, Eisel RJ, et al. Leukapheresis by continuous flow centrifugation (CFC) in patients with chronic myelocytic leukemia (CML) BLOOD. 1969;33(2):353–369. [PubMed] [Google Scholar]
- 32.Buckner D, Eisel R, Perry S. Blood cell separation in the dog by continuous flow centrifugation. BLOOD. 1968;31(5):653–672. [PubMed] [Google Scholar]
- 33.Freireich EJ, Judson G, Levin RH. Separation and collection of leukocytes. CANCER RES. 1965;25(9):1516–1520. [PubMed] [Google Scholar]
- 34.Chervenick PA, Boggs DR. In vitro growth of granulocytic and mononuclear cell colonies from blood of normal individuals. BLOOD. 1971;37(2):131–135. [PubMed] [Google Scholar]
- 35.Kurnick JE, Robison WA. Colony growth of human peripheral white blood cells in vitro. BLOOD. 1971;37(2):136–141. [PubMed] [Google Scholar]
- 36.McCredie KB, Hersh EM, Freireich EJ. Cells capable of colony formation in the peripheral blood of man. SCIENCE. 1971;171(968):293–294. doi: 10.1126/science.171.3968.293. [DOI] [PubMed] [Google Scholar]
- 37.Abrams RA, Glaubiger D, Appelbaum FR, et al. Result of attempted hematopoietic reconstitution using isologous, peripheral blood mononuclear cells: a case report. BLOOD. 1980;56(3):516–520. [PubMed] [Google Scholar]
- 38.Hershko C, Gale RP, Ho WG, et al. Cure of aplastic anaemia in paroxysmal nocturnal haemoglobinuria by marrow transfusion from identical twin: Failure of peripheral-leucocyte transfusion to correct marrow aplasia. LANCET. 1979;1(8123):945–947. doi: 10.1016/s0140-6736(79)91720-3. [DOI] [PubMed] [Google Scholar]
- 39.Fliedner TM, Korbling M, Calvo W, et al. Cryopreservation of blood mononuclear leukocytes and stem cells suspended in a large fluid volume. A preclinical model for a blood stem cell bank. BLUT. 1977;35(3):195–202. doi: 10.1007/BF00999460. [DOI] [PubMed] [Google Scholar]
- 40.Goldman JM, Johnson SA, Catovsky D, et al. Autografting for chronic granulocytic leukemia. N ENGL J MED. 1981;305(12):700. doi: 10.1056/NEJM198109173051216. [DOI] [PubMed] [Google Scholar]
- 41.Korbling M, Burke P, Braine H, et al. Successful engraftment of blood derived normal hemopoietic stem cells in chronic myelogenous leukemia. EXP HEMATOL. 1981;9(6):684–690. [PubMed] [Google Scholar]
- 42.Goldman JM. Autografting cryopreserved buffy coat cells for chronic granulocytic leukaemia in transformation. EXP HEMATOL. 1979;7 (Suppl 5):389–397. [PubMed] [Google Scholar]
- 43.Greenberg P, Bax I, Mara B, et al. Alterations of granulopoiesis following chemotherapy. BLOOD. 1974;44(3):375–383. [PubMed] [Google Scholar]
- 44.Bull JM, DeVita VT, Carbone PP. In vitro granulocyte production in patients with Hodgkin’s disease and lymphocytic, histiocytic, and mixed lymphomas. BLOOD. 1975;45(6):833–842. [PubMed] [Google Scholar]
- 45.Senn JS, McCulloch EA. Kinetics of regeneration after cyclophosphamide in human marrow assessed by a cell culture method. EXP HEMATOL. 1970;20:8–9. [Google Scholar]
- 46.Richman CM, Weiner RS, Yankee RA. Increase in circulating stem cells following chemotherapy in man. BLOOD. 1976;47(6):1031–1039. [PubMed] [Google Scholar]
- 47.Stiff PJ, Murgo AJ, Wittes RE, et al. Quantification of the peripheral blood colony forming unit-culture rise following chemotherapy. Could leukocytaphereses replace bone marrow for autologous transplantation? TRANSFUSION. 1983;23(6):500–503. doi: 10.1046/j.1537-2995.1983.23684074271.x. [DOI] [PubMed] [Google Scholar]
- 48.To LB, Shepperd KM, Haylock DN, et al. Single high doses of cyclophosphamide enable the collection of high numbers of hemopoietic stem cells from the peripheral blood. EXP HEMATOL. 1990;18(5):442–447. [PubMed] [Google Scholar]
- 49.To LB, Haylock DN, Kimber RJ, et al. High levels of circulating haemopoietic stem cells in very early remission from acute non-lymphoblastic leukaemia and their collection and cryopreservation. BR J HAEMATOL. 1984;58(3):399–410. doi: 10.1111/j.1365-2141.1984.tb03987.x. [DOI] [PubMed] [Google Scholar]
- 50.Welte K, Platzer E, Lu L, et al. Purification and biochemical characterization of human pluripotent hematopoietic colony-stimulating factor. PROC NATL ACAD SCI U S A. 1985;82(5):1526–1530. doi: 10.1073/pnas.82.5.1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Platzer E, Oez S, Welte K, et al. Human pluripotent hemopoietic colony stimulating factor: activities on human and murine cells. IMMUNOBIOLOGY. 1986;172(3–5):185–193. doi: 10.1016/S0171-2985(86)80098-5. [DOI] [PubMed] [Google Scholar]
- 52.Nagata S, Tsuchiya M, Asano S, et al. The chromosomal gene structure and two mRNAs for human granulocyte colony-stimulating factor. EMBO J. 1986;5(3):575–581. doi: 10.1002/j.1460-2075.1986.tb04249.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Souza LM, Boone TC, Gabrilove J, et al. Recombinant human granulocyte colony-stimulating factor: effects on normal and leukemic myeloid cells. SCIENCE. 1986;232(4746):61–65. doi: 10.1126/science.2420009. [DOI] [PubMed] [Google Scholar]
- 54.Welte K, Bonilla MA, Gillio AP, et al. Recombinant human granulocyte colony-stimulating factor. Effects on hematopoiesis in normal and cyclophosphamide-treated primates. J EXP MED. 1987;165(4):941–948. doi: 10.1084/jem.165.4.941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Duhrsen U, Villeval JL, Boyd J, et al. Effects of recombinant human granulocyte colony-stimulating factor on hematopoietic progenitor cells in cancer patients. BLOOD. 1988;72(6):2074–2081. [PubMed] [Google Scholar]
- 56.Ikebuchi K, Ihle JN, Hirai Y, et al. Synergistic factors for stem cell proliferation: further studies of the target stem cells and the mechanism of stimulation by interleukin-1, interleukin-6, and granulocyte colony-stimulating factor. BLOOD. 1988;72(6):2007–2014. [PubMed] [Google Scholar]
- 57.Metcalf D, Nicola NA. Proliferative effects of purified granulocyte colony-stimulating factor (G-CSF) on normal mouse hemopoietic cells. J CELL PHYSIOL. 1983;116(2):198–206. doi: 10.1002/jcp.1041160211. [DOI] [PubMed] [Google Scholar]
- 58.Ikebuchi K, Clark SC, Ihle JN, et al. Granulocyte colony-stimulating factor enhances interleukin 3-dependent proliferation of multipotential hemopoietic progenitors. PROC NATL ACAD SCI U S A. 1988;85(10):3445–3449. doi: 10.1073/pnas.85.10.3445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gabrilove JL, Jakubowski A, Fain K, et al. Phase I study of granulocyte colony-stimulating factor in patients with transitional cell carcinoma of the urothelium. J CLIN INVEST. 1988;82(4):1454–1461. doi: 10.1172/JCI113751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gabrilove JL, Jakubowski A, Scher H, et al. Effect of granulocyte colony-stimulating factor on neutropenia and associated morbidity due to chemotherapy for transitional-cell carcinoma of the urothelium. N ENGL J MED. 1988;318(22):1414–1422. doi: 10.1056/NEJM198806023182202. [DOI] [PubMed] [Google Scholar]
- 61.Bronchud MH, Scarffe JH, Thatcher N, et al. Phase I/II study of recombinant human granulocyte colony-stimulating factor in patients receiving intensive chemotherapy for small cell lung cancer. BR J CANCER. 1987;56(6):809–813. doi: 10.1038/bjc.1987.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Morstyn G, Campbell L, Souza LM, et al. Effect of granulocyte colony stimulating factor on neutropenia induced by cytotoxic chemotherapy. LANCET. 1988;1(8587):667–672. doi: 10.1016/s0140-6736(88)91475-4. [DOI] [PubMed] [Google Scholar]
- 63.Duhrsen U, Villeval JL, Boyd J, et al. Effects of recombinant human granulocyte colony-stimulating factor on hematopoietic progenitor cells in cancer patients. BLOOD. 1988;72(6):2074–2081. [PubMed] [Google Scholar]
- 64.Tamura M, Hattori K, Nomura H, et al. Induction of neutrophilic granulocytosis in mice by administration of purified human native granulocyte colony-stimulating factor (G-CSF) BIOCHEM BIOPHYS RES COMMUN. 1987;142(2):454–460. doi: 10.1016/0006-291x(87)90296-8. [DOI] [PubMed] [Google Scholar]
- 65.Kennedy MJ, Davis J, Passos-Coelho J, et al. Administration of human recombinant granulocyte colony-stimulating factor (filgrastim) accelerates granulocyte recovery following high-dose chemotherapy and autologous marrow transplantation with 4-hydroperoxycyclophosphamide-purged marrow in women with metastatic breast cancer. CANCER RES. 1993;53(22):5424–5428. [PubMed] [Google Scholar]
- 66.McQuaker IG, Hunter AE, Pacey S, et al. Low-dose filgrastim significantly enhances neutrophil recovery following autologous peripheral-blood stem-cell transplantation in patients with lymphoproliferative disorders: evidence for clinical and economic benefit. J CLIN ONCOL. 1997;15(2):451–457. doi: 10.1200/JCO.1997.15.2.451. [DOI] [PubMed] [Google Scholar]
- 67.Jansen J, Thompson EM, Hanks S, et al. Hematopoietic growth factor after autologous peripheral blood transplantation: comparison of G-CSF and GM-CSF. BONE MARROW TRANSPLANT. 1999;23(12):1251–1256. doi: 10.1038/sj.bmt.1701806. [DOI] [PubMed] [Google Scholar]
- 68.Nemunaitis J, Rosenfeld CS, Ash R, et al. Phase III randomized, double-blind placebo-controlled trial of rhGM-CSF following allogeneic bone marrow transplantation. BONE MARROW TRANSPLANT. 1995;15(6):949–954. [PubMed] [Google Scholar]
- 69.Stem Cell Trialists’ Group. Allogeneic peripheral blood stem-cell compared with bone marrow transplantation in the management of hematologic malignancies: an individual patient data meta-analysis of nine randomized trials. J CLIN ONCOL. 2005;23(22):5074–5087. doi: 10.1200/JCO.2005.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Anderlini P, Rizzo JD, Nugent ML, et al. Peripheral blood stem cell donation: an analysis from the International Bone Marrow Transplant Registry (IBMTR) and European Group for Blood and Marrow Transplant (EBMT) databases. BONE MARROW TRANSPLANT. 2001;27(7):689–692. doi: 10.1038/sj.bmt.1702875. [DOI] [PubMed] [Google Scholar]
- 71.Anderlini P, Przepiorka D, Korbling M, et al. Blood stem cell procurement: donor safety issues. BONE MARROW TRANSPLANT. 1998;21 (Suppl 3):S35–S39. [PubMed] [Google Scholar]
- 72.Rowley SD, Donaldson G, Lilleby K, et al. Experiences of donors enrolled in a randomized study of allogeneic bone marrow or peripheral blood stem cell transplantation. BLOOD. 2001;97(9):2541–2548. doi: 10.1182/blood.v97.9.2541. [DOI] [PubMed] [Google Scholar]
- 73.Fortanier C, Kuentz M, Sutton L, et al. Healthy sibling donor anxiety and pain during bone marrow or peripheral blood stem cell harvesting for allogeneic transplantation: results of a randomised study. BONE MARROW TRANSPLANT. 2002;29(2):145–149. doi: 10.1038/sj.bmt.1703338. [DOI] [PubMed] [Google Scholar]
- 74.Platzbecker U, Prange-Krex G, Bornhauser M, et al. Spleen enlargement in healthy donors during G-CSF mobilization of PBPCs. TRANSFUSION. 2001;41(2):184–189. doi: 10.1046/j.1537-2995.2001.41020184.x. [DOI] [PubMed] [Google Scholar]
- 75.Stroncek D, Shawker T, Follmann D, et al. G-CSF-induced spleen size changes in peripheral blood progenitor cell donors. TRANSFUSION. 2003;43(5):609–613. doi: 10.1046/j.1537-2995.2003.00384.x. [DOI] [PubMed] [Google Scholar]
- 76.Falzetti F, Aversa F, Minelli O, et al. Spontaneous rupture of spleen during peripheral blood stem-cell mobilisation in a healthy donor. LANCET. 1999;353(9152):555. doi: 10.1016/S0140-6736(99)00268-8. [DOI] [PubMed] [Google Scholar]
- 77.Becker PS, Wagle M, Matous S, et al. Spontaneous splenic rupture following administration of granulocyte colony-stimulating factor (G-CSF): occurrence in an allogeneic donor of peripheral blood stem cells. BIOL BLOOD MARROW TRANSPLANT. 1997;3(1):45–49. [PubMed] [Google Scholar]
- 78.Balaguer H, Galmes A, Ventayol G, et al. Splenic rupture after granulocyte-colony-stimulating factor mobilization in a peripheral blood progenitor cell donor. TRANSFUSION. 2004;44(8):1260–1261. doi: 10.1111/j.1537-2995.2004.00413.x. [DOI] [PubMed] [Google Scholar]
- 79.Kroger N, Renges H, Sonnenberg S, et al. Stem cell mobilisation with 16 microg/kg vs 10 microg/kg of G-CSF for allogeneic transplantation in healthy donors. BONE MARROW TRANSPLANT. 2002;29(9):727–730. doi: 10.1038/sj.bmt.1703509. [DOI] [PubMed] [Google Scholar]
- 80.Hill JM, Syed MA, Arai AE, et al. Outcomes and risks of granulocyte colony-stimulating factor in patients with coronary artery disease. J AM COLL CARDIOL. 2005;46(9):1643–1648. doi: 10.1016/j.jacc.2005.01.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lindemann A, Rumberger B. Vascular complications in patients treated with granulocyte colony-stimulating factor (G-CSF) EUR J CANCER. 1993;29A(16):2338–2339. doi: 10.1016/0959-8049(93)90236-9. [DOI] [PubMed] [Google Scholar]
- 82.Pusic I, Jiang SY, Landua S, et al. Impact of mobilization and remobilization strategies on achieving sufficient stem cell yields for autologous transplantation. BIOL BLOOD MARROW TRANSPLANT. 2008;14(9):1045–1056. doi: 10.1016/j.bbmt.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 83.Pavone V, Gaudio F, Console G, et al. Poor mobilization is an independent prognostic factor in patients with malignant lymphomas treated by peripheral blood stem cell transplantation. BONE MARROW TRANSPLANT. 2006;37(8):719–724. doi: 10.1038/sj.bmt.1705298. [DOI] [PubMed] [Google Scholar]
- 84.Hosing C, Saliba RM, Ahlawat S, et al. Poor hematopoietic stem cell mobilizers: a single institution study of incidence and risk factors in patients with recurrent or relapsed lymphoma. AM J HEMATOL. 2009;84(6):335–337. doi: 10.1002/ajh.21400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gordan LN, Sugrue MW, Lynch JW, et al. Poor mobilization of peripheral blood stem cells is a risk factor for worse outcome in lymphoma patients undergoing autologous stem cell transplantation. LEUK LYMPHOMA. 2003;44(5):815–820. doi: 10.1080/1042819031000067585. [DOI] [PubMed] [Google Scholar]
- 86.Akhtar S, Weshi AE, Rahal M, et al. Factors affecting autologous peripheral blood stem cell collection in patients with relapsed or refractory diffuse large cell lymphoma and Hodgkin lymphoma: a single institution result of 168 patients. LEUK LYMPHOMA. 2008;49(4):769–778. doi: 10.1080/10428190701843213. [DOI] [PubMed] [Google Scholar]
- 87.Boeve S, Strupeck J, Creech S, et al. Analysis of remobilization success in patients undergoing autologous stem cell transplants who fail an initial mobilization: risk factors, cytokine use and cost. BONE MARROW TRANSPLANT. 2004;33(10):997–1003. doi: 10.1038/sj.bmt.1704486. [DOI] [PubMed] [Google Scholar]
- 88.Sugrue MW, Williams K, Pollock BH, et al. Characterization and outcome of “hard to mobilize”’ lymphoma patients undergoing autologous stem cell transplantation. LEUK LYMPHOMA. 2000;39(5–6):509–519. doi: 10.3109/10428190009113381. [DOI] [PubMed] [Google Scholar]
- 89.Weaver CH, Hazelton B, Birch R, et al. An analysis of engraftment kinetics as a function of the CD34 content of peripheral blood progenitor cell collections in 692 patients after the administration of myeloablative chemotherapy. BLOOD. 1995;86(10):3961–3969. [PubMed] [Google Scholar]
- 90.Gertz MA, Wolf RC, Micallef IN, et al. Clinical impact and resource utilization after stem cell mobilization failure in patients with multiple myeloma and lymphoma. BONE MARROW TRANSPLANT. 2010;45(9):1396–1403. doi: 10.1038/bmt.2009.370. [DOI] [PubMed] [Google Scholar]
- 91.Hosing C, Smith V, Rhodes B, et al. Assessing the charges associated with hematopoietic stem cell mobilization and remobilization in patients with lymphoma and multiple myeloma undergoing autologous hematopoietic peripheral blood stem cell transplantation. TRANSFUSION. 2011;51(6):1300–1313. doi: 10.1111/j.1537-2995.2011.03176.x. [DOI] [PubMed] [Google Scholar]
- 92.Petit I, Szyper-Kravitz M, Nagler A, et al. G-CSF induces stem cell mobilization by decreasing bone marrow SDF-1 and up-regulating CXCR4. NATURE IMMUNOLOGY. 2002;3:687–694. doi: 10.1038/ni813. [DOI] [PubMed] [Google Scholar]
- 93.Broxmeyer HE, Orschell CM, Clapp DW, et al. Rapid mobilization of murine and human hematopoietic stem and progenitor cells with AMD3100, a CXCR4 antagonist. J EXP MED. 2005;201(8):1307–1318. doi: 10.1084/jem.20041385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Broxmeyer HE, Hangoc G, Cooper S, et al. AMD3100 and CD26 modulate mobilization, engraftment, and survival of hematopoietic stem and progenitor cells mediated by the SDF-1/CXCL12-CXCR4 axis. ANN N Y ACAD SCI. 2007;1106:1–19. doi: 10.1196/annals.1392.013. [DOI] [PubMed] [Google Scholar]
- 95.Devine SM, Vij R, Rettig M, et al. Rapid mobilization of functional donor hematopoietic cells without G-CSF using AMD3100, an antagonist of the CXCR4/SDF-1 interaction. BLOOD. 2008;112(4):990–998. doi: 10.1182/blood-2007-12-130179. [DOI] [PubMed] [Google Scholar]
- 96.Liles WC, Broxmeyer HE, Rodger E, et al. Mobilization of hematopoietic progenitor cells in healthy volunteers by AMD3100, a CXCR4 antagonist. BLOOD. 2003;102(8):2728–2730. doi: 10.1182/blood-2003-02-0663. [DOI] [PubMed] [Google Scholar]
- 97.Liles WC, Rodger E, Broxmeyer HE, et al. Augmented mobilization and collection of CD34+ hematopoietic cells from normal human volunteers stimulated with granulocyte-colony-stimulating factor by single-dose administration of AMD3100, a CXCR4 antagonist. TRANSFUSION. 2005;45(3):295–300. doi: 10.1111/j.1537-2995.2005.04222.x. [DOI] [PubMed] [Google Scholar]
- 98.Pelus LM, Bian H, Fukuda S, et al. The CXCR4 agonist peptide, CTCE-0021, rapidly mobilizes polymorphonuclear neutrophils and hematopoietic progenitor cells into peripheral blood and synergizes with granulocyte colony-stimulating factor. EXP HEMATOL. 2005;33(3):295–307. doi: 10.1016/j.exphem.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 99.Abraham M, Biyder K, Begin M, et al. Enhanced unique pattern of hematopoietic cell mobilization induced by the CXCR4 antagonist 4F-benzoyl-TN14003. STEM CELLS. 2007;25(9):2158–2166. doi: 10.1634/stemcells.2007-0161. [DOI] [PubMed] [Google Scholar]
- 100.Iyer CV, Evans RJ, Lou Q, et al. Rapid and recurrent neutrophil mobilization regulated by T134, a CXCR4 peptide antagonist. EXP HEMATOL. 2008;36(9):1098–1109. doi: 10.1016/j.exphem.2008.03.021. [DOI] [PubMed] [Google Scholar]
- 101.Shen H, Cheng T, Olszak I, et al. CXCR-4 desensitization is associated with tissue localization of hemopoietic progenitor cells. J IMMUNOL. 2001;166(8):5027–5033. doi: 10.4049/jimmunol.166.8.5027. [DOI] [PubMed] [Google Scholar]
- 102.Zhong R, Law P, Wong D, et al. Small peptide analogs to stromal derived factor-1 enhance chemotactic migration of human and mouse hematopoietic cells. EXP HEMATOL. 2004;32(5):470–475. doi: 10.1016/j.exphem.2004.01.011. [DOI] [PubMed] [Google Scholar]
- 103.Cramer DE, Wagner S, Li B, et al. Mobilization of hematopoietic progenitor cells by yeast-derived beta-glucan requires activation of matrix metalloproteinase-9. STEM CELLS. 2008;26(5):1231–1240. doi: 10.1634/stemcells.2007-0712. [DOI] [PubMed] [Google Scholar]
- 104.Patchen ML, Liang J, Vaudrain T, et al. Mobilization of peripheral blood progenitor cells by Betafectin PGG-Glucan alone and in combination with granulocyte colony-stimulating factor. STEM CELLS. 1998;16(3):208–217. doi: 10.1002/stem.160208. [DOI] [PubMed] [Google Scholar]
- 105.Frenette PS, Weiss L. Sulfated glycans induce rapid hematopoietic progenitor cell mobilization: evidence for selectin-dependent and independent mechanisms. BLOOD. 2000;96(7):2460–2468. [PubMed] [Google Scholar]
- 106.Sweeney EA, Priestley GV, Nakamoto B, et al. Mobilization of stem/progenitor cells by sulfated polysaccharides does not require selectin presence. PROC NATL ACAD SCI U S A. 2000;97(12):6544–6549. doi: 10.1073/pnas.97.12.6544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sweeney EA, Lortat-Jacob H, Priestley GV, et al. Sulfated polysaccharides increase plasma levels of SDF-1 in monkeys and mice: involvement in mobilization of stem/progenitor cells. BLOOD. 2002;99(1):44–51. doi: 10.1182/blood.v99.1.44. [DOI] [PubMed] [Google Scholar]
- 108.Kubonishi S, Kikuchi T, Yamaguchi S, et al. Rapid hematopoietic progenitor mobilization by sulfated colominic acid. BIOCHEM BIOPHYS RES COMMUN. 2007;355(4):970–975. doi: 10.1016/j.bbrc.2007.02.069. [DOI] [PubMed] [Google Scholar]
- 109.Albanese P, Caruelle D, Frescaline G, et al. Glycosaminoglycan mimetics-induced mobilization of hematopoietic progenitors and stem cells into mouse peripheral blood: structure/function insights. EXP HEMATOL. 2009;37(9):1072–1083. doi: 10.1016/j.exphem.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 110.Craddock CF, Nakamoto B, Andrews RG, et al. Antibodies to VLA4 integrin mobilize long-term repopulating cells and augment cytokine-induced mobilization in primates and mice. BLOOD. 1997;90(12):4779–4788. [PubMed] [Google Scholar]
- 111.Papayannopoulou T, Priestley GV, Nakamoto B, et al. Molecular pathways in bone marrow homing: dominant role of alpha(4)beta(1) over beta(2)-integrins and selectins. BLOOD. 2001;98(8):2403–2411. doi: 10.1182/blood.v98.8.2403. [DOI] [PubMed] [Google Scholar]
- 112.Kikuta T, Shimazaki C, Ashihara E, et al. Mobilization of hematopoietic primitive and committed progenitor cells into blood in mice by anti-vascular adhesion molecule-1 antibody alone or in combination with granulocyte colony-stimulating factor. EXP HEMATOL. 2000;28(3):311–317. doi: 10.1016/s0301-472x(99)00151-4. [DOI] [PubMed] [Google Scholar]
- 113.Papayannopoulou T, Priestley GV, Nakamoto B. Anti-VLA4/VCAM-1-induced mobilization requires cooperative signaling through the kit/mkit ligand pathway. BLOOD. 1998;91(7):2231–2239. [PubMed] [Google Scholar]
- 114.Ramirez P, Rettig MP, Uy GL, et al. BIO5192, a small molecule inhibitor of VLA-4, mobilizes hematopoietic stem and progenitor cells. BLOOD. 2009;114(7):1340–1343. doi: 10.1182/blood-2008-10-184721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ting M, Day B, Spanevello M, et al. Activation of ephrin A proteins influences hematopoietic stem cell adhesion and trafficking patterns. EXP HEMATOL. 2010 doi: 10.1016/j.exphem.2010.07.007. epub 07/23/10. [DOI] [PubMed] [Google Scholar]
- 116.Carlo-Stella C, Di NM, Magni M, et al. Defibrotide in combination with granulocyte colony-stimulating factor significantly enhances the mobilization of primitive and committed peripheral blood progenitor cells in mice. CANCER RES. 2002;62(21):6152–6157. [PubMed] [Google Scholar]
- 117.Scalia R, Kochilas L, Campbell B, et al. Effects of defibrotide on leukocyte-endothelial cell interaction in the rat mesenteric vascular bed: role of P-selectin. METHODS FIND EXP CLIN PHARMACOL. 1996;18(10):669–676. [PubMed] [Google Scholar]
- 118.Pellegatta F, Lu Y, Radaelli A, et al. Drug-induced in vitro inhibition of neutrophil-endothelial cell adhesion. BR J PHARMACOL. 1996;118(3):471–476. doi: 10.1111/j.1476-5381.1996.tb15427.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Fibbe WE, Pruijt JF, van KY, et al. The role of metalloproteinases and adhesion molecules in interleukin-8-induced stem-cell mobilization. SEMIN HEMATOL. 2000;37(1 Suppl 2):19–24. doi: 10.1016/s0037-1963(00)90085-4. [DOI] [PubMed] [Google Scholar]
- 120.Pelus LM, Horowitz D, Cooper SC, et al. Peripheral blood stem cell mobilization. A role for CXC chemokines. CRIT REV ONCOL HEMATOL. 2002;43(3):257–275. doi: 10.1016/s1040-8428(01)00202-5. [DOI] [PubMed] [Google Scholar]
- 121.Fukuda S, Bian H, King AG, et al. The chemokine GRObeta mobilizes early hematopoietic stem cells characterized by enhanced homing and engraftment. BLOOD. 2007;110(3):860–869. doi: 10.1182/blood-2006-06-031401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.King AG, Horowitz D, Dillon SB, et al. Rapid mobilization of murine hematopoietic stem cells with enhanced engraftment properties and evaluation of hematopoietic progenitor cell mobilization in rhesus monkeys by a single injection of SB-251353, a specific truncated form of the human CXC chemokine GRObeta. BLOOD. 2001;97(6):1534–1542. doi: 10.1182/blood.v97.6.1534. [DOI] [PubMed] [Google Scholar]
- 123.Pelus LM, Bian H, King AG, et al. Neutrophil-derived MMP-9 mediates synergistic mobilization of hematopoietic stem and progenitor cells by the combination of G-CSF and the chemokines GRObeta/CXCL2 and GRObetaT/CXCL2delta4. BLOOD. 2004;103(1):110–119. doi: 10.1182/blood-2003-04-1115. [DOI] [PubMed] [Google Scholar]
- 124.Pruijt JF, Verzaal P, van OR, et al. Neutrophils are indispensable for hematopoietic stem cell mobilization induced by interleukin-8 in mice. PROC NATL ACAD SCI U S A. 2002;99(9):6228–6233. doi: 10.1073/pnas.092112999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kimura T, Boehmler AM, Seitz G, et al. The sphingosine 1-phosphate receptor agonist FTY720 supports CXCR4-dependent migration and bone marrow homing of human CD34+ progenitor cells. BLOOD. 2004;103(12):4478–4486. doi: 10.1182/blood-2003-03-0875. [DOI] [PubMed] [Google Scholar]
- 126.Ryser MF, Ugarte F, Lehmann R, et al. S1P(1) overexpression stimulates S1P-dependent chemotaxis of human CD34+ hematopoietic progenitor cells but strongly inhibits SDF-1/CXCR4-dependent migration and in vivo homing. MOL IMMUNOL. 2008;46(1):166–171. doi: 10.1016/j.molimm.2008.07.016. [DOI] [PubMed] [Google Scholar]
- 127.Seitz G, Boehmler AM, Kanz L, et al. The role of sphingosine 1-phosphate receptors in the trafficking of hematopoietic progenitor cells. ANN N Y ACAD SCI. 2005;1044:84–89. doi: 10.1196/annals.1349.011. [DOI] [PubMed] [Google Scholar]
- 128.Allende ML, Tuymetova G, Lee BG, et al. S1P1 receptor directs the release of immature B cells from bone marrow into blood. J EXP MED. 2010;207(5):1113–1124. doi: 10.1084/jem.20092210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Massberg S, Schaerli P, Knezevic-Maramica I, et al. Immunosurveillance by hematopoietic progenitor cells trafficking through blood, lymph, and peripheral tissues. CELL. 2007;131(5):994–1008. doi: 10.1016/j.cell.2007.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Ratajczak MZ, Lee H, Wysoczynski M, et al. Novel insight into stem cell mobilization-plasma sphingosine-1-phosphate is a major chemoattractant that directs the egress of hematopoietic stem progenitor cells from the bone marrow and its level in peripheral blood increases during mobilization due to activation of complement cascade/membrane attack complex. LEUKEMIA. 2010;24(5):976–985. doi: 10.1038/leu.2010.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Golan K, Vagima Y, Ludin A, et al. S1P promotes murine progenitor cell egress and mobilization via S1P1-mediated ROS signaling and SDF-1 release. BLOOD. 2012;119(11):2478–2488. doi: 10.1182/blood-2011-06-358614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Juarez JG, Harun N, Thien M, et al. Sphingosine-1-phosphate facilitates trafficking of hematopoietic stem cells and their mobilization by CXCR4 antagonists in mice. BLOOD. 2012;119(3):707–716. doi: 10.1182/blood-2011-04-348904. [DOI] [PubMed] [Google Scholar]
- 133.Ratajczak J, Kucia M, Mierzejewska K, et al. A novel view of paroxysmal nocturnal hemoglobinuria pathogenesis: more motile PNH hematopoietic stem/progenitor cells displace normal HSPCs from their niches in bone marrow due to defective adhesion, enhanced migration and mobilization in response to erythrocyte-released sphingosine-1 phosphate gradient. LEUKEMIA. 2012;26(7):1722–1725. doi: 10.1038/leu.2012.46. [DOI] [PubMed] [Google Scholar]
- 134.Ratajczak MZ, Borkowska S, Ratajczak J. An emerging link in stem cell mobilization between activation of the complement cascade and the chemotactic gradient of sphingosine-1-phosphate. PROSTAGLANDINS OTHER LIPID MEDIAT. 2012 doi: 10.1016/j.prostaglandins.2012.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Hoggatt J, Pelus LM. Eicosanoid regulation of hematopoiesis and hematopoietic stem and progenitor trafficking. LEUKEMIA. 2010;24(12):1993–2002. doi: 10.1038/leu.2010.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Hoggatt J, Singh P, Sampath J, et al. Prostaglandin E2 enhances hematopoietic stem cell homing, survival, and proliferation. BLOOD. 2009;113(22):5444–5455. doi: 10.1182/blood-2009-01-201335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Hoggatt J, Mohammad KS, Singh P, et al. Differential stem- and progenitor- cell trafficking by prostaglandin E2. NATURE. 2013;494 (7441):365–369. doi: 10.1038/nature11929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Katayama Y, Battista M, Kao WM, et al. Signals from the sympathetic nervous system regulate hematopoietic stem cell egress from bone marrow. CELL. 2006;124(2):407–421. doi: 10.1016/j.cell.2005.10.041. [DOI] [PubMed] [Google Scholar]
- 139.Spiegel A, Shivtiel S, Kalinkovich A, et al. Catecholaminergic neurotransmitters regulate migration and repopulation of immature human CD34+ cells through Wnt signaling. NAT IMMUNOL. 2007;8(10):1123–1131. doi: 10.1038/ni1509. [DOI] [PubMed] [Google Scholar]
- 140.Gruber-Olipitz M, Stevenson R, Olipitz W, et al. Transcriptional pattern analysis of adrenergic immunoregulation in mice. Twelve hours norepinephrine treatment alters the expression of a set of genes involved in monocyte activation and leukocyte trafficking. J NEUROIMMUNOL. 2004;155(1–2):136–142. doi: 10.1016/j.jneuroim.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 141.DiPersio JF, Stadtmauer AP, Nademanee AP, et al. A phase III, multicenter, randomized, double bnlind, placebo-controlled, comparative trial of AMD3100 (Perixafor) + G-CSF vs. G-CSF + placebo for mobilization in multiple myeloma (MM) patients for autologous hematopoietic stem cell (aHSC) transplantation [abstract] BLOOD. 2007;110:137a. [Google Scholar]
- 142.DiPersio JF, Stadtmauer EA, Nademanee A, et al. Plerixafor and G-CSF versus placebo and G-CSF to mobilize hematopoietic stem cells for autologous stem cell transplantation in patients with multiple myeloma. BLOOD. 2009;113(23):5720–5726. doi: 10.1182/blood-2008-08-174946. [DOI] [PubMed] [Google Scholar]
- 143.DiPersio JF, Micallef IN, Stiff PJ, et al. Phase III prospective randomized double-blind placebo-controlled trial of plerixafor plus granulocyte colony-stimulating factor compared with placebo plus granulocyte colony-stimulating factor for autologous stem-cell mobilization and transplantation for patients with non-Hodgkin’s lymphoma. J CLIN ONCOL. 2009;27(28):4767–4773. doi: 10.1200/JCO.2008.20.7209. [DOI] [PubMed] [Google Scholar]
- 144.Pelus LM. Peripheral blood stem cell mobilization: new regimens, new cells, where do we stand. CURR OPIN HEMATOL. 2008;15(4):285–292. doi: 10.1097/MOH.0b013e328302f43a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Kymes SM, Pusic I, Lambert DL, et al. Economic evaluation of plerixafor for stem cell mobilization. AM J MANAG CARE. 2012;18(1):33–41. [PMC free article] [PubMed] [Google Scholar]
- 146.Chen AI, Bains T, Murray S, et al. Clinical experience with a simple algorithm for plerixafor utilization in autologous stem cell mobilization. BONE MARROW TRANSPLANT. 2012;47(12):1526–1529. doi: 10.1038/bmt.2012.74. [DOI] [PubMed] [Google Scholar]
- 147.Costa LJ, Alexander ET, Hogan KR, et al. Development and validation of a decision-making algorithm to guide the use of plerixafor for autologous hematopoietic stem cell mobilization. BONE MARROW TRANSPLANT. 2011;46(1):64–69. doi: 10.1038/bmt.2010.78. [DOI] [PubMed] [Google Scholar]
- 148.Micallef IN, Sinha S, Gastineau DA, et al. Cost-effectiveness analysis of a risk-adapted algorithm of plerixafor use for autologous peripheral blood stem cell mobilization. BIOL BLOOD MARROW TRANSPLANT. 2013;19(1):87–93. doi: 10.1016/j.bbmt.2012.08.010. [DOI] [PubMed] [Google Scholar]
- 149.Fruehauf S, Veldwijk MR, Seeger T, et al. A combination of granulocyte-colony-stimulating factor (G-CSF) and plerixafor mobilizes more primitive peripheral blood progenitor cells than G-CSF alone: results of a European phase II study. CYTOTHERAPY. 2009;11(8):992–1001. doi: 10.3109/14653240903121245. [DOI] [PubMed] [Google Scholar]
- 150.Hoggatt J, Pelus LM. Hematopoietic stem cell mobilization with agents other than G-CSF. METHODS MOL BIOL. 2012;904:49–67. doi: 10.1007/978-1-61779-943-3_4. [DOI] [PubMed] [Google Scholar]
- 151.Pelus LM, Fukuda S. Peripheral blood stem cell mobilization: the CXCR2 ligand GRObeta rapidly mobilizes hematopoietic stem cells with enhanced engraftment properties. EXP HEMATOL. 2006;34(8):1010–1020. doi: 10.1016/j.exphem.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 152.Hill JM, Zalos G, Halcox JP, et al. Circulating endothelial progenitor cells, vascular function, and cardiovascular risk. N ENGL J MED. 2003;348(7):593–600. doi: 10.1056/NEJMoa022287. [DOI] [PubMed] [Google Scholar]
- 153.Larochelle A, Krouse A, Metzger M, et al. AMD3100 mobilizes hematopoietic stem cells with long-term repopulating capacity in nonhuman primates. BLOOD. 2006;107(9):3772–3778. doi: 10.1182/blood-2005-09-3592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Devine SM, Vij R, Rettig M, et al. Rapid mobilization of functional donor hematopoietic cells without G-CSF using AMD3100, an antagonist of the CXCR4/SDF-1 interaction. BLOOD. 2008;112(4):990–998. doi: 10.1182/blood-2007-12-130179. [DOI] [PubMed] [Google Scholar]