Abstract
The origins of pelvic high grade serous cancer (HGSC) have become a subject of intense scrutiny in view of proposals to reduce the incidence of the disease via opportunistic salpingectomy in healthy women. Accumulated data implicates the fimbria as a site of origin and descriptive molecular pathology and experimental evidence strongly support a serous carcinogenic sequence in the fallopian tube. Both direct and indirect ("surrogate") precursors suggest the benign tube undergoes important biologic changes after menopause, acquiring abnormalities in gene expression that are shared with malignancy. However, the tube can be linked to only some HGSCs, recharging arguments that nearby peritoneum/ovarian surface epithelium (POSE) also hosts progenitors to this malignancy. A major sticking point is the difference in immunophenotype between POSE and Müllerian epithelium, essentially requiring mesothelial to Müllerian differentiation prior to or during malignant transformation to HGSC. However, there is emerging evidence that an embryonic or progenitor phenotype exists in the adult female genital tract with the capacity to differentiate, normally or during neoplastic transformation. Recently, a putative cell of origin to cervical cancer has been identified in the squamo-columnar (SC) junction, projecting a model whereby embryonic progenitors give rise to immuno-phenotypically distinct neoplastic progeny under stromal influences via "top down" differentiation. A similar pattern of differentiation is implied in the endometrium and the juxtaposition of disparate epithelial immuno-phenotypes (POSE and underlying Müllerian inclusions) recapitulates this in the ovary. While a sudden mesothelial-Mullerian transition remains to be proven, it would explain the rapid evolution, short asymptomatic interval, and absence of a defined epithelial starting point in many HGSCs. Resolving this question will be critical to both expectations from prophylactic salpingectomy and future approaches to pelvic serous cancer prevention.
Introduction
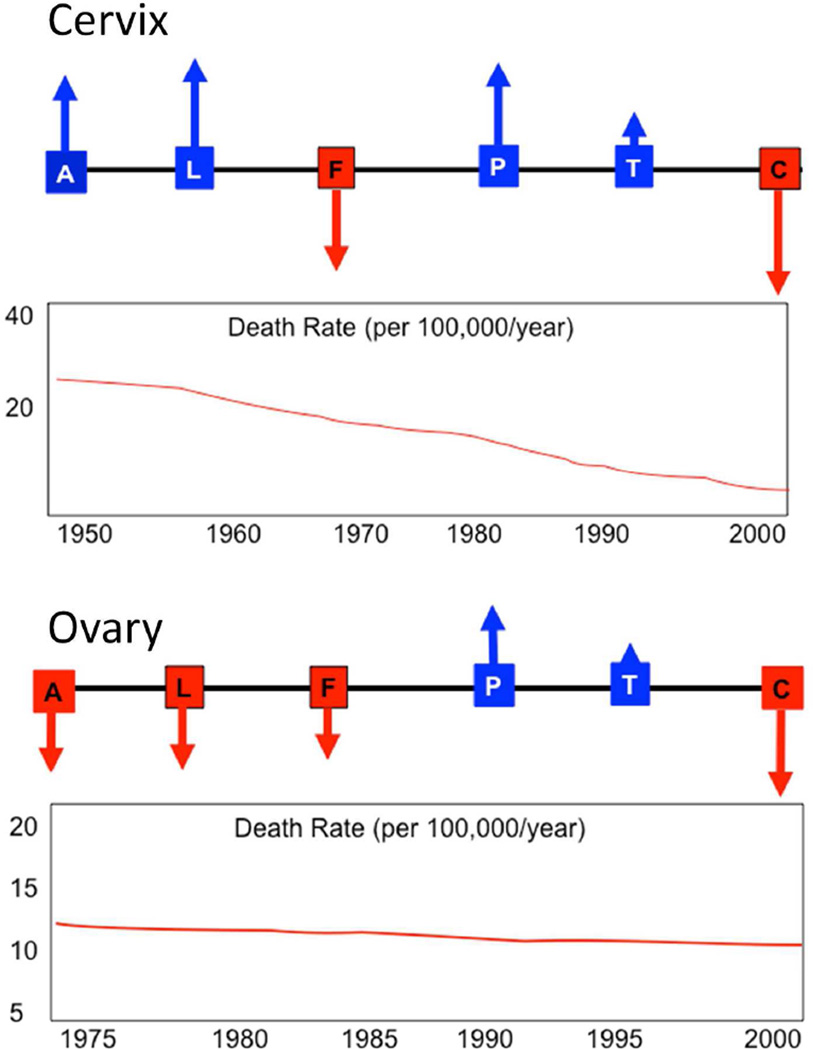
Progress towards the elimination of gynecologic cancer continues to evolve and the direction of success in achieving this goal will by definition be "ascending". Squamous cell carcinomas affect the vulva, vagina and cervix, and a high percentage of these tumors are linked to HPV infection. The conceptual advances and development of an effective vaccine in the past twenty-five years have made it possible to envision a long-term reduction in mortality and morbidity for HPV related tumors of the lower female genital tract [1]. This advance effectively promises to unshackle the medical profession and its patients from the incessantly expensive and involved process of precursor detection, precursor over-diagnosis and over management, reliance on an imperfect and emotionally charged diagnostic test for HPV, and the frustration of encountering cancers that arise despite increasingly sophisticated surveillance. Nevertheless, it is important to emphasize that the imperfect system for reducing the rate of cervical cancer has worked. This is because of at least six major variables that influence success in reducing mortality, the cervix fulfills four (Figure 1). 1) It is accessible, 2) there is significant lead time permitting precursor removal, and 3) removal of the precursor and its target, the squamocolumnar junction, seems to, in most cases, permanently protect the patient from cervical cancer. 4) Moreover, limited malignancies are treatable. Two negatives, 5) over-treatment of false leads (lesions that would never become malignant) and the 6) cost it entails are accepted downsides in a resource-rich society.
Figure 1.
Schematic view of variables influencing prevention of death by a malignancy (and its consequences), including accessibility (A), lead time for detection provided by a precursor (L), false leads (F) expending resources or leading to over treatment, long term protection (P) of a single intervention, Treatability (T) of existing malignancy, and economic and human costs (C). Up arrows (blue) confer a positive influence, down arrows (red) a negative influence. Approximate incidence rates over time are displayed below.
High grade serous cancer (HGSC) or its generic form pelvic serous cancer represents an entirely different challenge relative to cervix for several reasons. First, the fallopian tube and ovary are not practically accessible for the purposes of screening for microscopic disease, although molecular screening of lower genital tract fluids holds some promise. Second, there appears to be less lead time in the form of a long-standing precursor, as evidenced by both the apparently short symptom free interval from neoplastic transformation to discovery of advanced disease, cutting into the effectiveness of screening [2]. False leads are common and the disease is difficult to treat once spread has occurred. An upside is the fact that removal of the tubes and ovaries markedly reduces the risk of disease [3;4]. However, the costs incurred in terms of what would be required in a screening program plus morbidity and mortality incurred in removing the ovaries prior to menopause, are substantial.
Despite the above challenges, attempts to reduce the death rate from HGSC by screening continue. Detecting a cancer before it becomes lethal is the "holy grail" of screening, best exemplified by the successes of cervical cancer prevention. However, this strategy is not working for ovarian cancer and there are two potential explanations. The first is that precursors in the tube, which likely remain localized for months to a few years, cannot be detected by current technology. The second is a more ominous scenario. If a subset of tumors emerge de novo from the ovarian or peritoneal surfaces, they may never be preventable by screening. Identifying women more likely to get the disease, specifically those with BRCA mutations (BRCA+), has an obvious and profound impact on cancer risk for patients and relatives, reducing it from as high as 50% in the untreated to under 5 per cent following risk reducing salpingo-oophorectomy. However, 85 to 90 percent of future ovarian cancer patients cannot be identified in advance by a genetic risk factor. Other approaches such as aggressive early intervention, conventional, neoadjuvant and targeted therapy are being extensively investigated and have been shown to produce incremental improvements [5]. Still, sixty percent of women with HGSC will be dead within 5 years [6;7].
Two options for ovarian cancer death reduction are screening for tumor with a biomarker and screening for increased risk by germ line DNA analysis. An elevated biomarker such as CA125 may increase the risk of current ovarian cancer many fold, but cannot guarantee survival. In contrast, detection of a germ line BRCA1 or BRCA2 mutation reveals a similar fold-increase in future cancer risk. The difference between these two forms of information lies in the distinction between current and future. The latter approach (RRSO) offers a strategy to prevent death by preventing disease. Currently the prevention of cervical cancer is not efficient and requires a wholesale screening and vaccination of the entire population. Yet, it imposes minimal morbidity. Strategies to prevent HGSC can identify women at greatest risk (BRCA+) and maximize the benefit in this subgroup. However for the general population, a wholesale approach using opportunistic salpingectomy would be needed and is under study [8]. It is based on recent studies that have identified the distal tube as the source of many HGSCs and the prospect that prophylactic salpingectomy will significantly reduce the incidence of subsequent risk HGSC. In this instance the impact of this strategy on the death rate from HGSC will depend heavily on site of origin for this disease.
The fallopian tube and HGSC
The association between the fallopian tube and HGSC can be viewed from three perspectives. The first is the precursor perspective, in which a serous carcinogenic sequence can be outlined within the oviductal epithelium. Piek and colleagues were among the first to seriously consider the fallopian tube as a source for a significant proportion of HGSCs, based principally on the identification of "dysplastic" epithelial changes in the fallopian tubes of women with BRCA1 or BRCA2 mutations [9]. Second, with the advent of the SEE-FIM protocol and routine examination of the fimbria, this concept evolved to where the fimbria is assumed to be the site of origin for many HGSCs, evidenced by the identification of serous tubal intraepithelial carcinomas (STICs) in risk-reducing salpingo-oophorectomies (RRSOs) [10]. The finding of STICs in from 20–60 percent of HGSCs in the general tumor population has established STIC and the distal tube as an important source of these tumors [11]. Third, with the description of p53 signatures - bland-appearing cell outgrowths with p53 mutations - in the tube and their genetic similarity to STICs, a morphologically, genetically and histochemically defined serous carcinogenic sequence has emerged in this organ [12–14]. Experimental data from cell culture and animal models have established further tumorigenesis in the tube (Table 1) [15;16].
Table I.
Evidence supporting tubal and Peritoneal-Ovarian Surface Epithelial (POSE) origins to high grade serous cancer (HGSC)
| Fallopian tube |
Pro
|
| Con |
| Peritoneal-ovarian surface epithelium (POSE) |
Pro
|
Con
|
Defining precursors: premalignant, latent and surrogate
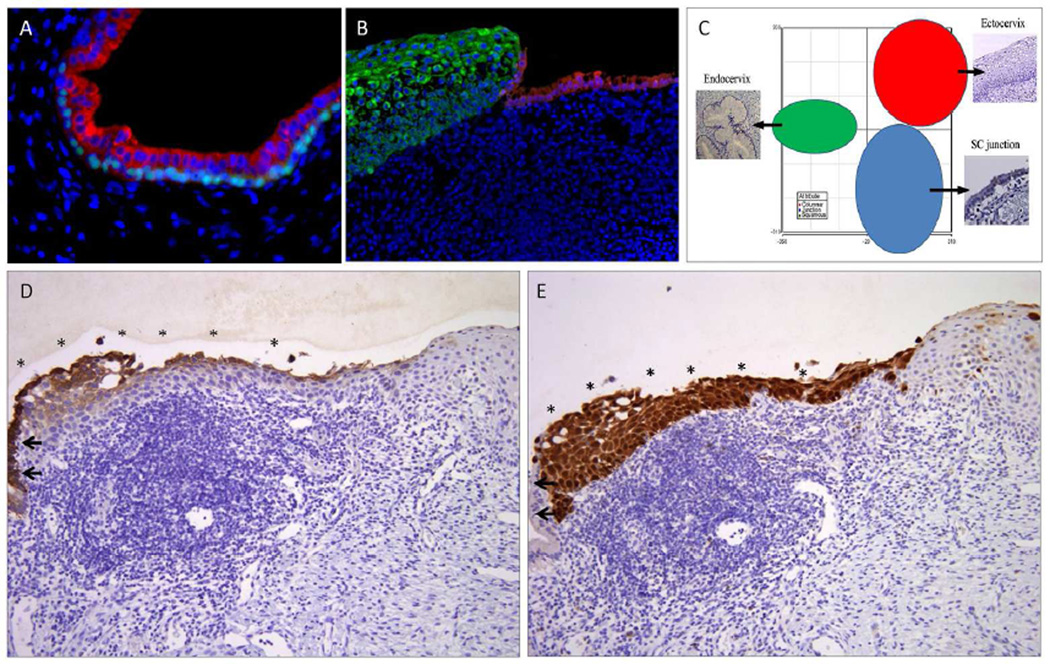
Precursors can be defined according to whether they are premalignant intraepithelial neoplasms, with the potential for invasion or spread or latent, in which they are non-neoplastic. The latter can be direct precursors, with the potential, albeit small, to progress to an intraepithelial neoplasm, or indirect, in which they share features of malignancy but appear to be a "dead end" i.e. do not manifest with the critical genetic changes. Intraepithelial neoplasms include STICs, which are morphologically malignant and presumed to confer some risk of metastatic disease. They are at the end of a narrow spectrum that includes atypias that have not yet invaded or acquired to capacity to spread directly to the peritoneal surfaces (Figure 2A&2B) [9,10,13,14]. Earlier precursor-defining events may occur within seemingly benign genital epithelium. Studies of p53 status, clonality, microsatellite instability and allelic imbalance uncovered fundamental genomic alterations in vulvar mucosa prior to the onset of nuclear atypias [17–20]. Beginning in 1998, PTEN, a tumor suppressor, was shown to be inactivated or mutated in endometrial cancer precursors [21;22]. Moreover, on further study, loss of PTEN could be identified in occasional endometrial glands in over 50 per cent of women with no significant pathologic abnormality [23]. The PTEN-null genotype in some cases could be traced from these normal glands to carcinoma, either in the same or follow-up specimens. Based on this, the term “latent precursor” was coined to define this entity and it took its place as an early event in the carcinogenic spectrum [24;25].
Figure 2.
Embryonic cells at the squamocolumnar junction and cervical neoplasia. A) During fetal life and postnatal, embryonic cells (Krt7, red) are progressively undermined by basal/reserve cells (p63, bright blue-green). B) In the adult, a small number of embryonic cells (Krt7, red) remain at the squamo columnar junction just proximal to the squamous epithelium (green). C) PCA map of expression data distinguishes these SC junction cells (blue) from the ectocervix (red) and endocervix (green). D) In this squamous intraepithelial lesion, the Krt7-positive SC junction cells (left, arrows) are continuous with similarly staining cells overlying the lesion (*). Note the Krt7 staining persists into the upper layers of the neoplastic epithelium. E) The latter cells, like the lesion beneath are strongly p16ink4 positive. This suggests a scenario whereby SC junction cells are infected and transformed, then undergo squamous differentiation from the “top down”.
The p53 signature fulfilled the criteria for a latent precursor in the fallopian tube. Like the PTEN-null foci in the endometrium, it heralded the common occurrence of a limited number of genetic perturbations, specifically DNA damage and p53 mutation [14] (Figure 2C). The number of genetically altered epithelial cells was limited in most to a few cells or part of a plica, suggesting limited loss of growth control. Predictably, some foci exhibited expansion of the p53-positive cell population to involve one or more plicae, with nuclear atypia and subtle derangements in epithelial polarity. Such lesions were initially termed serous tubal intraepithelial lesions in transition (TILTs), synonymous with premalignant atypias (Figure 2B) [26]. Their resemblance to STICs has further anchored serous carcinogenesis to a spectrum of intraepithelial neoplasia in the distal tube [27;28].
An unresolved question is how serous tubal intraepithelial neoplasms should be classified. STICs seen in association with metastatic disease clearly merit this diagnosis; the lesion is either pre-metastatic carcinoma or is an intramucosal metastasis. In contrast, most "STICs" encountered in isolation are not followed by pelvic cancer and the criteria for these lesions overlap with that of lower grade, albeit neoplastic, entities termed TILTs or tubal intraepithelial lesions [27;28]. A recent National Cancer Institute meeting addressed specifically the overuse of "malignant" terms (such as ductal carcinoma in situ) for entities that had a low risk of being followed by invasive or metastatic disease [28a]. In view of this change in emphasis, a more appropriate term for STICs or TILTs encountered incidentally or following RRSOs would be serous tubal intraepithelial neoplasia. Subdivision into grades that can be made reproducibly and with prognostic impact awaits further studies [28b].
On closer examination of the fallopian tube, a second entity has emerged consisting of a cellular outgrowths which differ from p53 signatures in two respects: 1) they are not as regionally restricted, occurring in proximal and distal segments and 2) they do not exhibit p53 mutations [29]. These so-called secretory (stem?) cell outgrowths or SCOUTs contain a number of perturbations similar to p53 signatures; they are typically PAX2-null with variable expression of ALDH1 and several other markers also dys-regulated in neoplasia (Figure 2D). Interestingly, they or similarly described variants appear to be more frequent in older women and in women with a HGSC [30;31] (Zheng W, personal communication). This paradox of association with HGSC despite the absence of p53 mutations suggests that SCOUTs are associated with some risk factors for HGSC but do not participate directly in the carcinogenic sequence. Based on this they are best viewed as "surrogate precursors" [32]. They speak to stem cell outgrowths that share some common pathogenesis if not identical risks of progressing to cancer (Table 2). A striking aspect of SCOUTs is they provide a novel window into a "parallel universe" in the fallopian tube whereby abnormal stem cell outgrowth develops. Conceivably, both direct and indirect (surrogate) precursors share similar origins. This is particularly interesting considering the fact that cancers of serous and endometrioid origin cannot always be readily separated and may share certain risk factors (Conners, et al USCAP, Yang et al USCAP).
Table 2.
Precursor definition
| Type | Definition | Example(s) |
|---|---|---|
| Direct precursor | A cancer precursor at any stage with the potential to become malignant, in contrast to a surrogate precursor, which does not | Any entity other than a surrogate precursor SC junction HSIL PTEN-null endometrial gland P53 signature TILT STIC |
| Indirect (surrogate) precursor | Shares risk factors and genomic disturbances with cancer but is either not a direct precursor or carries a negligible or unproven risk of malignant outcome | Non-SC junction LSILs in the cervix Stem cell outgrowths in the fallopian tube with intact p53 function |
| Latent precursor | Benign epithelium sharing risk factors and genomic disturbances with cancer and can be seen in continuity with cancer; viewed as a direct precursor, albeit at low risk | pTEN-null glands in benign endometrium p53 signatures in the fallopian tube |
| Premalignant (low grade)* | An intraepithelial neoplasm not considered malignant but has the potential to either invade or become an intraepithelial carcinoma | HSIL of the cervix Endometrial intraepithelial neoplasia (atypical hyperplasia) of the endometrium. Endometrial glandular dysplasia Tubal intraepithelial lesion in transition (TILT) or low grade serous tubal intraepithelial neoplasia |
| Premalignant (high grade)* | An intraepithelial neoplasm at increased risk for metastasis. Viewed as potentially malignant. | Serous endometrial intraepithelial carcinoma (SEIC); serous tubal intraepithelial carcinoma (STIC) or high grade serous tubal intraepithelial neoplasia |
| Malignant | A non-invasive neoplasm with known ability to invade and metastasize | STIC encountered in the setting of invasive or metastatic disease |
These diagnoses pertain to lesions encountered incidentally or in risk reduction salpingo oophorectomies. STICs encountered in the setting of metastatic disease are presumed to be malignant.
Embryonic cells and differentiation
The most thoroughly studied neoplasm in the female genital tract is cervical cancer, for which a vaccine has become available that promises to significantly reduce the death rate. Two unanswered conundrums have been difficulty in predicting which cervical cancer precursors will progress and why cervical cancer originates at the squamocolumnar (SC) junction. In 2011, Wang et al, studying the origins of Barrett's metaplasia, uncovered a unique cell population at the SC junction of the esophagus that could be traced to residual embryonic cells [33]. These cells remained following induction and replacement of the esophago-gastric junctional epithelium. The remaining embryonic SC junction cells were postulated to be multipotential and capable of generating Barrett's metaplasia [34;35]. Recently, studies examined mice and human cervical SC junction for similar cells [36;37]. Similarly, Keratin (Krt) 7 expressing embryonic Müllerian epithelium was systematically replaced by basal p63/Krt5 positive basal/reserve cells leading to squamous differentiation (Figure 3A). Remarkably, a subset of these Krt7 positive cells persisted at the cervical SC junction (Figure 3B).
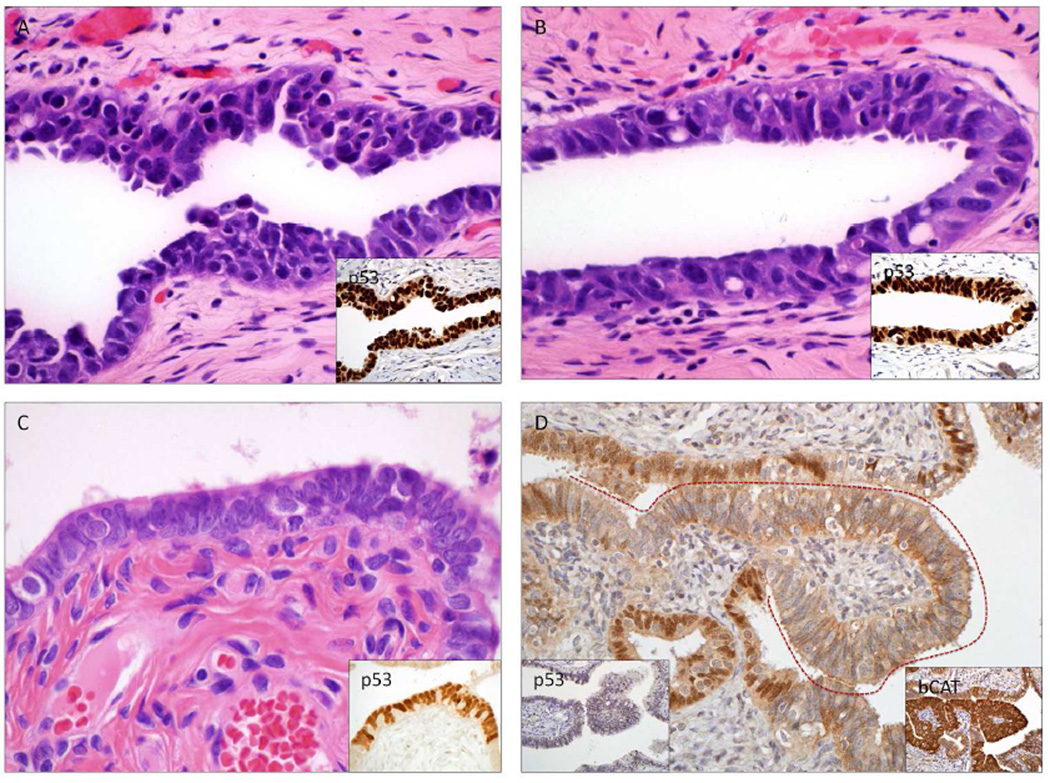
Figure 3.
Pre-metastatic, premalignant, latent and surrogate precursors in the uterus and fallopian tube (See Table 2). A) Serous tubal intraepithelial carcinoma, B) premalignant atypia (TILT), C) p53 mutations in benign-appearing salpingeal epithelium (p53 signatures), E) Stem cell outgrowths in the fallopian tube (SCOUTs), which are typically PAX2-null like serous cancer precursors and often express other genes abnormally such as b-catenin (B-cat).
The above cells demonstrate several novel properties. First, their transcription is distinct from squamous and columnar cells in the cervix (Figure 3C) and they share a similar immunophenotype with cervical epithelial malignancies and their precursors, being present in over 90% of HSIL and cancer (Figure 3D&E). Of interest, an earlier report noted the initiation of HPV infection in cells that resemble the SC junction cells [38]. A recent report identifies the Krt7 population as the one most vulnerable to HPV infection [39]. Indeed, by increasing HPV E7 mRNA stability, the 6-mer peptide SEQIKA present in krt7 protein may be important during viral latency, activation and concomitant cervical (pre)cancer development.
Interestingly, the SC junction immunophenotype is lost in a majority of LSILs and these lesions were rarely followed by a histologic diagnosis of HSIL (Herfs et al. Am J Surg Path accepted). This implies that when SC junction cells are infected directly by high risk HPVs, they are more likely to generate a high grade precursor (HSIL or adenocarcinoma in situ). Conversely, their metaplastic squamous progeny and ectocervical squamous cells are less prone to infection and progression to malignancy. The former can be viewed as true precursors and the latter as "surrogate" or low risk precursors, differing by their immunophenotype. In essence, the latter differ solely by the fact that the site of infection was the ectocervix rather than the SC junction (Herfs et al. Am J Surg Path accepted). A final observation of interest is the residual embryonic SC junction cells do not appear to regenerate following excision. This suggests that preemptive ablation of the SC junction could reduce the risk of cervical cancer, a concept supported by anecdotal and indirect evidence [36;40;41].
The knowledge gap
The association between the fallopian tube and HGSC is indisputable. Skeptics who would claim an absence of supporting data will find all of the necessary pieces that would match the cervix model: an initial molecular event (p53 mutation), a vulnerable region (fimbria), a precursor spectrum (p53 signatures, TILTs, and STICs) with an increasing volume of molecular data linking the tube to HGSC, and physical continuity between the precursor and its malignancy [9;10;14; 66]. What is missing is a consistent association between STIC and HGSC. Based on frequency of a co-existing STIC, the tube is implicated in HGSC – including ovarian and peritoneal – in from 19 to 59 per cent of cases [42–44]. An informative if imperfect parameter, the presence or absence of a dominant ovarian mass in a high grade Müllerian carcinoma (defined as 2× greater in diameter relative to its opposite and implying an ovarian origin) is inversely related to the presence of STIC, as is endometrioid histology [45;46]. Combined with observations of serous carcinomas associated with either adenofibromas or endometriosis, the ovary is accepted by most as a source of a percentage of HGSCs. However, these aforementioned pre-existing conditions are often missing and the demonstration of conspicuous atypia in non-malignant CICs is vanishingly rare. This leaves a well-defined subset of HGSCs demonstrates nothing more than ovarian tubal and peritoneal surface involvement. This begs the age-old question of whether the peritoneum/ovarian surface epithelium (POSE) – a covering that is largely mesothelial in is makeup - is capable of generating a high grade serous carcinoma [47].
Two issues are important in addressing the above question in the context of an ovarian source for HGSC. First the ovarian surface and Müllerian cortical inclusion cysts (CIC) have been presumed to give rise to borderline tumors, low and high grade serous carcinomas [48]. Precisely where mucinous and endometrioid tumors ultimately come from is controversial, but either inclusion cysts or endometriosis (be it originating from a CIC or transported endometrial tissue) are the presumed progenitors and these tumors are most commonly ovarian in location. It is the CIC that is implicated in many of these tumors and whether it comes from OSE or transported fallopian tube epithelial cells is a question that has not been fully answered. However, the strong predilection of these so-called “type I” tumors for a CIC or CIC-like origin contrasts with the relative lack of evidence that CICs commonly undergo transformation into HGSC and explain why the carcinogenic sequence in the tube is so popular [49–51]. It begins and ends in a defined Müllerian epithelium. It is the lack of a defined Müllerian epithelium on the ovarian surface that has stalled the ovarian theory and promoted the tubal theory for HGSC. The second issue is the apparently short symptomatic interval prior to the discovery of HGSC, which typically presents in high stage. This is the scenario even when no STIC can be found and is a stark contrast to the observation that when early carcinomas are discovered, either in RRSOs or incidentally, STIC is often present and the origin is usually assigned to the fallopian tube. So why are most early HGSCs connected to the fallopian tube via a STIC while most advanced HGSCs often have no STIC? This could be explained by the fact that most HGSCs originate in POSE and the site of origin is unappreciated or obscured by advanced carcinoma. An alternate theory however, would explain this phenomenon in the context of two origins, one in the fallopian tube and another in the POSE. The latter pathway, if particularly rapid in its evolution (not unexpected for HGSC), would rarely be detected early on and would explain why the majority of HGSCs detected have already spread. This would fit a scenario wherein the tumor arose from either the tube or the ovarian or POSE. However, to accept this one must envision a pathway where transformation and trans-differentiation (from mesothelial to epithelial) occur simultaneously.
Progenitors, progeny and female genital tract neoplasia
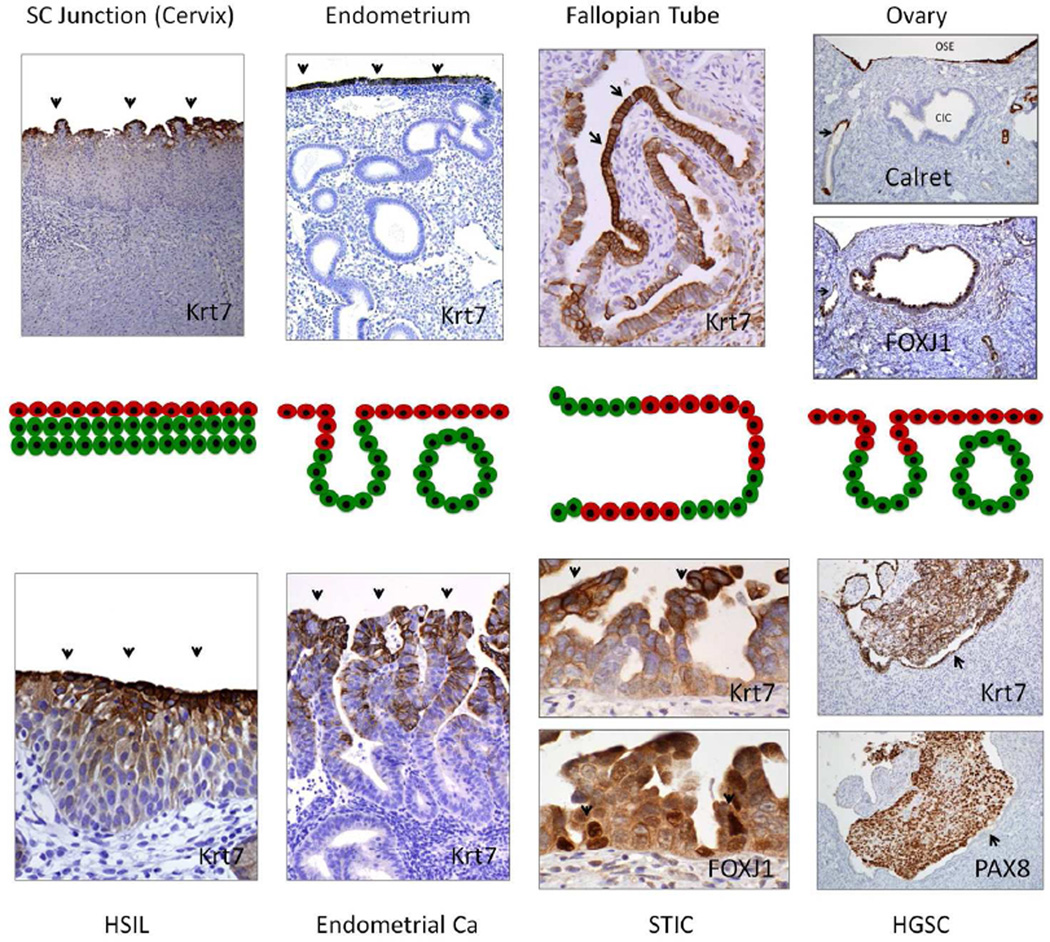
The cervix and esophageal SC junction models, in which residual multipotential embryonic epithelial cells can be transformed and differentiate into either a squamous or columnar neoplasms, are powerful examples of how a benign or neoplastic proliferation can emerge de novo from an immuno-phenotypically different progenitor cell [33–36]. This begs the obvious question of the fate of the Krt7 positive embryonic cells lining the remainder of the female genital tract. Similar albeit spatially different patterns can be appreciated and the recurring theme is one where the cells on the surface retain some of these embryonic markers whereas those extending from the surface into the stroma progressively lose Krt7 expression, signifying differentiation. In the postnatal cervix, Krt7 is lost in the advancing edges of the crypts. In the endometrium of the adult, Krt7 expression is most prominent on the surface, disappearing in the underlying glands (Figure 4) (Howitt and Crum, unpublished). A similar, albeit less reproducible pattern of Krt7 expression occurs in the tube in the adult, often seen most intense near the tips or sides of the plicae centrally and diminishing peripherally in poorly formed crypts (Figure 4).
Figure 4.
Evidence supporting linked progenitors and progeny in the female genital tract. In this model (middle panels), Krt7 positive progenitor cells (red) give rise to progeny (green) under physiologic conditions (above), and this transition is mirrored in the neoplasms that arise in these sites (below). Upper panels (left to right) highlight putative Krt7+ progenitor cells overlying or adjacent to squamous metaplasia (cervix), endometrial glands, and tubal epithelium. In the ovarian cortex putative progenitors in the OSE (Calret+/Krt7+) would trans-differentiate to ciliated Müllerian inclusions (FOXJ1+/Krt7+) in the ovarian cortex. In neoplasia (HSIL, endometrial cancer and STIC), putative Krt7+ tumor cell progenitors (arrows) may remain undifferentiated or differentiate in a "top down" pattern in which the Kr7− progeny are below rather than above their ancestors (HSIL, endometrial adenocarcinoma, STIC (arrows)). In the ovary, both tumor and OSE (arrow) are Krt7+, but are distinguished by differences in PAX8 staining. The presence of a potential progenitor (OSE) population in the ovary begs the question of its relationship to Mullerian differentiation and its potential role in pelvic serous cancer.
This topographically distinct pattern of differentiation raises three additional questions. The most obvious is the possibility that the Krt7 positive surface cells retain some stem-like capacity as shown in the cervix. In the endometrium these cells could participate in epithelial regeneration following menses, an observation made by Ferenczy in his classic ultra-structural studies nearly 40 years ago [52;53]. In those studies, he implied that regeneration emerged from the surface cells rather than the crypts. The second is whether vulnerability to carcinogenesis is influenced by the location of the target cells. Many endometrial intraepithelial neoplasms (EIN or atypical hyperplasia) and endometrioid adenocarcinomas involve glands and are Krt7 negative. In contrast uterine serous carcinomas are often strongly positive and the early lesions are usually discovered on the surface (Howitt unpublished). The third is whether this theme applies to ovarian cancer.
Serous carcinogenesis in the ovary: transportation or (trans) differentiation?
For the most part, Krt7 expression extends from the fallopian tube to the extra tubal mesothelium and ovarian surface epithelium/mesothelium. Near the tubal mesothelial junction, the Krt7+ cells are capable of undergoing differentiation to squamous metaplasia, producing Walthard cell rests, identical to what occurs in the cervix (Ning, unpublished) [54]. In addition, cell culture studies imply that the Krt7 population identifies a putative stem cell (Xian et al, unpublished). A number of studies have implicated the ovarian surface epithelium (OSE) in ovarian carcinogenesis, histochemically, in vitro and in animal models [55;56]. The distal fallopian tube and ovary are connected embryologically. Müllerian agenesis spares both the fimbria and ovary, suggesting a common embryonic link between the salpinx and ovarian surface epithelium [57;58]. A recent paper suggested a stem cell niche in the mesothelium that lies between the tube and ovary that is susceptible to tumorigenesis [59]. One problem with this more recent proposal is few HGSCs are encountered in this area alone as opposed to the fimbria or ovarian surface. Nevertheless, this concept is still compatible with serous carcinogenesis developing in OSE or related cells. It is supported by in vitro data as well as observations that transformation of OSE is facilitated by the underlying ovarian stroma [60–62].
At the core of the argument concerning the OSE and HGSC is the relationship between OSE and Müllerian cortical inclusion cysts (CICs); if OSE can undergo benign Müllerian differentiation there is no reason why it cannot do the same under a neoplastic stimulus, leading to HGSC. This would parallel the scenario in the SC junction. It is here that Figure 4 provokes the critical question of whether CICs are the progeny of OSE. Similar to the uterus and cervix, a surface epithelium, albeit mesothelial in immunophenotype (Calretinin+) is contiguous with horizontally spaced gland-like structures (CICs) with Müllerian (ciliated) differentiation (FOXJ1+). Both are Krt7 positive; the transition from calretinin positive to negative occurs within the ovarian stroma. This is similar to that seen in the endocervix and uterus (Figure 4). It implies a progenitor cell (OSE) that differentiates (CIC) under the influence of the adjacent stroma. In this model, as in the other sites in the female genital tract, the two populations harbor different vulnerabilities or exposures to carcinogenic stimuli, possibly explaining the multiplicity of tumor types seen in the tube, ovarian surface and ovarian parenchyma. The existence of a dual population of linked epithelia each with their respective tumor susceptibilities, unifies the female genital tract epithelium and would explain not only disparate tumor types emerging from the ovary but also the brutally short interval from no symptoms to advanced HGSC, as well as the mysterious absence of a tubal precursor in many HGSCs. At this point there is relatively little data supporting a direct transition from normal mesothelium to HGSC. However, we have encountered rare examples of surface neoplasms involving the ovary with both mesothelial and Mullerian differentiation. Given the rarity of of p53-positive CICs [63;64], the possibility that HGSC emerges rapidly and immediately following p53 inactivation merits further study [65].
Proof of principle
The success of a specific cancer prevention strategy is influenced by both age of onset and the lag time from precursor to clinical cancer. Because of the long latency period from precursor to cervical cancer, the benefit of childhood HPV vaccination will take decades to realize. Similarly, the benefit of removing fallopian tubes 20 years prior to the median age for malignancy will not be known for years. Recently proposed trials of salpingectomy in BRCA+ women might shorten the waiting period given the earlier onset of HGSC in this population. While this is under way, a reliable genomic strategy confirming or refuting the link between POSE and HGSC is needed. Critical morphologic clues to the pathogenesis of vulvar, cervix, endometrial and now pelvic serous cancer have now been discovered, unearthed by new hypotheses, serendipity, or ancillary techniques that suddenly brought them to light. Ultimately an alternate pathway to HGSC, if it exists, must be confirmed "through the glass". While the model of fallopian tube carcinogenesis may not encompass all HGSCs, it will more sharply focus the search for alternate pathways.
Illustrations
Schematic view of variables influencing prevention of death by a malignancy (and its consequences), including accessibility (A), lead time for detection provided by a precursor (L), false leads (F) expending resources or leading to over treatment, long term protection (P) of a single intervention, Treatability (T) of existing malignancy, and economic and human costs (C). Up arrows (blue) confer a positive influence, down arrows (red) a negative influence. Approximate incidence rates over time are displayed below.
Embryonic cells at the squamocolumnar junction and cervical neoplasia. A) During fetal life and postnatal, embryonic cells (Krt7, red) are progressively undermined by basal/reserve cells (p63, bright blue-green). B) In the adult, a small number of embryonic cells (Krt7, red) remain at the squamo columnar junction just proximal to the squamous epithelium (green). C) PCA map of expression data distinguishes these SC junction cells (blue) from the ectocervix (red) and endocervix (green). D) In this squamous intraepithelial lesion, the Krt7-positive SC junction cells (left, arrows) are continuous with similarly staining cells overlying the lesion (*). Note the Krt7 staining persists into the upper layers of the neoplastic epithelium. E) The latter cells, like the lesion beneath are strongly p16ink4 positive. This suggests a scenario whereby SC junction cells are infected and transformed, then undergo squamous differentiation from the “top down”.
Pre-metastatic, premalignant, latent and surrogate precursors in the uterus and fallopian tube (See Table 2). A) Serous tubal intraepithelial carcinoma, B) premalignant atypia (TILT), C) p53 mutations in benign-appearing salpingeal epithelium (p53 signatures), E) Stem cell outgrowths in the fallopian tube (SCOUTs), which are typically PAX2-null like serous cancer precursors and often express other genes abnormally such as b-catenin (B-cat).
Evidence supporting linked progenitors and progeny in the female genital tract. In this model (middle panels), Krt7 positive progenitor cells (red) give rise to progeny (green) under physiologic conditions (above), and this transition is mirrored in the neoplasms that arise in these sites (below). Upper panels (left to right) highlight putative Krt7+ progenitor cells overlying or adjacent to squamous metaplasia (cervix), endometrial glands, and tubal epithelium. In the ovarian cortex putative progenitors in the OSE (Calret+/Krt7+) would trans-differentiate to ciliated Müllerian inclusions (FOXJ1+/Krt7+) in the ovarian cortex. In neoplasia (HSIL, endometrial cancer and STIC), putative Krt7+ tumor cell progenitors (arrows) may remain undifferentiated or differentiate in a "top down" pattern in which the Kr7- progeny are below rather than above their ancestors (HSIL, endometrial adenocarcinoma, STIC (arrows)). In the ovary, both tumor and OSE (arrow) are Krt7+, but are distinguished by differences in PAX8 staining. The presence of a potential progenitor (OSE) population in the ovary begs the question of its relationship to Mullerian differentiation and its potential role in pelvic serous cancer.
Footnotes
Presented in part in the Nathan Kaufman Timely Topics Lecture at the United States Canadian Pathology Meeting, Baltimore, March, 2013.
This manuscript has not been published previously.
The authors declare that there are no conflicts of interest.
All experiments conducted in this study were with the approval of the Brigham and Women's Hospital Institutional Board.
Authors contributions are as follows:
Wrote and edited the manuscript: Crum, Herfs, Xian, McKeon
Performed experiments cited or illustrated in the manuscript: Herfs,
Hanamornroongruang, Howitt, Jimenez, Ning, Bijron
Provided conceptual input: Crum, Herfs, Xian, McKeon, Bijron
Assembled figures: Crum, Herfs, Bijron
Reference List
- 1.Wigle J, Coast E, Watson-Jones D. Human papillomavirus (HPV) vaccine implementation in low and middle-income countries (LMICs): Health system experiences and prospects. Vaccine. 2013 doi: 10.1016/j.vaccine.2013.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson GL, McIntosh M, Wu L, et al. Assessing lead time of selected ovarian cancer biomarkers: a nested case-control study. J Natl Cancer Inst. 2010;102:26–38. doi: 10.1093/jnci/djp438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Perez SP, Jimenez P, Olivera H, et al. Risk-reduction surgery in BRCA mutation carriers in a Spanish population: adherence and results. Clin Transl Oncol. 2008;10:660–664. doi: 10.1007/s12094-008-0267-9. [DOI] [PubMed] [Google Scholar]
- 4.Sigal BM, Munoz DF, Kurian AW, et al. A simulation model to predict the impact of prophylactic surgery and screening on the life expectancy of BRCA1 and BRCA2 mutation carriers. Cancer Epidemiol Biomarkers Prev. 2012;21:1066–1077. doi: 10.1158/1055-9965.EPI-12-0149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gilbert L, Basso O, Sampalis J, et al. Assessment of symptomatic women for early diagnosis of ovarian cancer: results from the prospective DOvE pilot project. Lancet Oncol. 2012;13:285–291. doi: 10.1016/S1470-2045(11)70333-3. [DOI] [PubMed] [Google Scholar]
- 6.Jemal A, Siegel R, Xu J, et al. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 7.Coleman MP, Forman D, Bryant H, et al. Cancer survival in Australia, Canada, Denmark, Norway, Sweden, and the UK, 1995–2007 (the International Cancer Benchmarking Partnership): an analysis of population-based cancer registry data. Lancet. 2011;377:127–138. doi: 10.1016/S0140-6736(10)62231-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morelli M, Venturella R, Mocciaro R, et al. Prophylactic salpingectomy in premenopausal low-risk women for ovarian cancer: primum non nocere. Gynecol Oncol. 2013;129:448–451. doi: 10.1016/j.ygyno.2013.03.023. [DOI] [PubMed] [Google Scholar]
- 9.Piek JM, Verheijen RH, Kenemans P, et al. BRCA1/2-related ovarian cancers are of tubal origin: a hypothesis. Gynecol Oncol. 2003;90:491. doi: 10.1016/s0090-8258(03)00365-2. [DOI] [PubMed] [Google Scholar]
- 10.Medeiros F, Muto MG, Lee Y, et al. The tubal fimbria is a preferred site for early adenocarcinoma in women with familial ovarian cancer syndrome. Am J Surg Pathol. 2006;30:230–236. doi: 10.1097/01.pas.0000180854.28831.77. [DOI] [PubMed] [Google Scholar]
- 11.Kindelberger DW, Lee Y, Miron A, et al. Intraepithelial carcinoma of the fimbria and pelvic serous carcinoma: Evidence for a causal relationship. Am J Surg Pathol. 2007;31:161–169. doi: 10.1097/01.pas.0000213335.40358.47. [DOI] [PubMed] [Google Scholar]
- 12.Xian W, Miron A, Roh M, et al. The Li-Fraumeni syndrome (LFS): a model for the initiation of p53 signatures in the distal Fallopian tube. J Pathol. 2010;220:17–23. doi: 10.1002/path.2624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Salvador S, Rempel A, Soslow RA, et al. Chromosomal instability in fallopian tube precursor lesions of serous carcinoma and frequent monoclonality of synchronous ovarian and fallopian tube mucosal serous carcinoma. Gynecol Oncol. 2008;110:408–417. doi: 10.1016/j.ygyno.2008.05.010. [DOI] [PubMed] [Google Scholar]
- 14.Lee Y, Miron A, Drapkin R, et al. A candidate precursor to serous carcinoma that originates in the distal fallopian tube. J Pathol. 2007;211:26–35. doi: 10.1002/path.2091. [DOI] [PubMed] [Google Scholar]
- 15.Levanon K, Ng V, Piao HY, et al. Primary ex vivo cultures of human fallopian tube epithelium as a model for serous ovarian carcinogenesis. Oncogene. 2010;29:1103–1113. doi: 10.1038/onc.2009.402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim J, Coffey DM, Creighton CJ, et al. High-grade serous ovarian cancer arises from fallopian tube in a mouse model. Proc Natl Acad Sci U S A. 2012;109:3921–3926. doi: 10.1073/pnas.1117135109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pinto AP, Miron A, Yassin Y, et al. Differentiated vulvar intraepithelial neoplasia contains Tp53 mutations and is genetically linked to vulvar squamous cell carcinoma. Mod Pathol. 2010;23:404–412. doi: 10.1038/modpathol.2009.179. [DOI] [PubMed] [Google Scholar]
- 18.Pinto AP, Lin MC, Sheets EE, et al. Allelic imbalance in lichen sclerosus, hyperplasia, and intraepithelial neoplasia of the vulva. Gynecol Oncol. 2000;77:171–176. doi: 10.1006/gyno.2000.5739. [DOI] [PubMed] [Google Scholar]
- 19.Pinto AP, Lin MC, Mutter GL, et al. Allelic loss in human papillomavirus-positive and -negative vulvar squamous cell carcinomas. Am J Pathol. 1999;154:1009–1015. doi: 10.1016/S0002-9440(10)65353-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lin MC, Mutter GL, Trivijisilp P, et al. Patterns of allelic loss (LOH) in vulvar squamous carcinomas and adjacent noninvasive epithelia. Am J Pathol. 1998;152:1313–1318. [PMC free article] [PubMed] [Google Scholar]
- 21.Mutter GL, Lin MC, Fitzgerald JT, et al. Altered PTEN expression as a diagnostic marker for the earliest endometrial precancers. J Natl Cancer Inst. 2000;92:924–930. doi: 10.1093/jnci/92.11.924. [DOI] [PubMed] [Google Scholar]
- 22.Levine RL, Cargile CB, Blazes MS, et al. PTEN mutations and microsatellite instability in complex atypical hyperplasia, a precursor lesion to uterine endometrioid carcinoma. Cancer Res. 1998;58:3254–3258. [PubMed] [Google Scholar]
- 23.Mutter GL, Ince TA, Baak JP, et al. Molecular identification of latent precancers in histologically normal endometrium. Cancer Res. 2001;61:4311–4314. [PubMed] [Google Scholar]
- 24.Lacey JV, Jr, Mutter GL, Ronnett BM, et al. PTEN expression in endometrial biopsies as a marker of progression to endometrial carcinoma. Cancer Res. 2008;68:6014–6020. doi: 10.1158/0008-5472.CAN-08-1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baak JP, Van DB, Steinbakk A, et al. Lack of PTEN expression in endometrial intraepithelial neoplasia is correlated with cancer progression. Hum Pathol. 2005;36:555–561. doi: 10.1016/j.humpath.2005.02.018. [DOI] [PubMed] [Google Scholar]
- 26.Gross AL, Kurman RJ, Vang R, et al. Precursor lesions of high-grade serous ovarian carcinoma: morphological and molecular characteristics. J Oncol. 2010;2010:126295. doi: 10.1155/2010/126295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carlson JW, Miron A, Jarboe EA, et al. Serous tubal intraepithelial carcinoma: its potential role in primary peritoneal serous carcinoma and serous cancer prevention. J Clin Oncol. 2008;26:4160–4165. doi: 10.1200/JCO.2008.16.4814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jarboe E, Folkins A, Nucci MR, et al. Serous carcinogenesis in the fallopian tube: a descriptive classification. Int J Gynecol Pathol. 2008;27:1–9. doi: 10.1097/pgp.0b013e31814b191f. [DOI] [PubMed] [Google Scholar]
- 28a.Esserman LJ, Thompson IM, Reid B. Overdiagnosis and Overtreatment in Cancer: An Opportunity for Improvement. JAMA. 2013:E1–E2. [Google Scholar]
- 28b.Ning G, Bijron JG, Yuan J, et al. Differential expression of p-ERM, a marker of cell polarity, in benign and neoplastic oviductal epithelium. Int J Gynecol Pathol. 2013;32:345–352. doi: 10.1097/PGP.0b013e31826feee2. [DOI] [PubMed] [Google Scholar]
- 29.Chen EY, Mehra K, Mehrad M, et al. Secretory cell outgrowth, PAX2 and serous carcinogenesis in the Fallopian tube. J Pathol. 2010;222:110–116. doi: 10.1002/path.2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bijron JG, Ning G, Laury AR, et al. Digital quantification of precursor frequency in the fallopian tube and its significance. Mod Pathol. 2012;25:1654–1661. doi: 10.1038/modpathol.2012.100. [DOI] [PubMed] [Google Scholar]
- 31.Quick CM, Ning G, Bijron J, et al. PAX2-null secretory cell outgrowths in the oviduct and their relationship to pelvic serous cancer. Mod Pathol. 2012;25:449–455. doi: 10.1038/modpathol.2011.175. [DOI] [PubMed] [Google Scholar]
- 32.Crum CP, McKeon FD, Xian W. The oviduct and ovarian cancer: causality, clinical implications, and "targeted prevention". Clin Obstet Gynecol. 2012;55:24–35. doi: 10.1097/GRF.0b013e31824b1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang X, Ouyang H, Yamamoto Y, et al. Residual embryonic cells as precursors of a Barrett's-like metaplasia. Cell. 2011;145:1023–1035. doi: 10.1016/j.cell.2011.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang X, Ouyang H, Yamamoto Y, et al. Residual embryonic cells as precursors of a Barrett's-like metaplasia. Cell. 2011;145:1023–1035. doi: 10.1016/j.cell.2011.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xian W, Ho KY, Crum CP, et al. Cellular origin of Barrett's esophagus: controversy and therapeutic implications. Gastroenterology. 2012;142:1424–1430. doi: 10.1053/j.gastro.2012.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Herfs M, Yamamoto Y, Laury A, et al. A discrete population of squamocolumnar junction cells implicated in the pathogenesis of cervical cancer. Proc Natl Acad Sci U S A. 2012;109:10516–10521. doi: 10.1073/pnas.1202684109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Herfs M, Vargas SO, Yamamoto Y, et al. A novel blueprint for 'top down' differentiation defines the cervical squamocolumnar junction during development, reproductive life, and neoplasia. J Pathol. 2013;229:460–468. doi: 10.1002/path.4110. [DOI] [PubMed] [Google Scholar]
- 38.Xue Y, Bellanger S, Zhang W, et al. HPV16 E2 is an immediate early marker of viral infection, preceding E7 expression in precursor structures of cervical carcinoma. Cancer Res. 2010;70:5316–5325. doi: 10.1158/0008-5472.CAN-09-3789. [DOI] [PubMed] [Google Scholar]
- 39.Kanduc D. Translational regulation of human papillomavirus type 16 E7 mRNA by the peptide SEQIKA, shared by rabbit alpha(1)-globin and human cytokeratin 7. J Virol. 2002;76:7040–7048. doi: 10.1128/JVI.76.14.7040-7048.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Taylor S, Wang C, Wright TC, et al. A comparison of human papillomavirus testing of clinician-collected and self-collected samples during follow-up after screen-and-treat. Int J Cancer. 2011;129:879–886. doi: 10.1002/ijc.25731. [DOI] [PubMed] [Google Scholar]
- 41.YOUNGE PA. Cancer of the uterine cervix; a preventable disease. Obstet Gynecol. 1957;10:469–481. [PubMed] [Google Scholar]
- 42.Tang S, Onuma K, Deb P, et al. Frequency of serous tubal intraepithelial carcinoma in various gynecologic malignancies: a study of 300 consecutive cases. Int J Gynecol Pathol. 2012;31:103–110. doi: 10.1097/PGP.0b013e31822ea955. [DOI] [PubMed] [Google Scholar]
- 43.Carlson JW, Miron A, Jarboe EA, et al. Serous tubal intraepithelial carcinoma: its potential role in primary peritoneal serous carcinoma and serous cancer prevention. J Clin Oncol. 2008;26:4160–4165. doi: 10.1200/JCO.2008.16.4814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Przybycin CG, Kurman RJ, Ronnett BM, et al. Are all pelvic (nonuterine) serous carcinomas of tubal origin? Am J Surg Pathol. 2010;34:1407–1416. doi: 10.1097/PAS.0b013e3181ef7b16. [DOI] [PubMed] [Google Scholar]
- 45.Roh MH, Kindelberger D, Crum CP. Serous tubal intraepithelial carcinoma and the dominant ovarian mass: clues to serous tumor origin? Am J Surg Pathol. 2009;33:376–383. doi: 10.1097/PAS.0b013e3181868904. [DOI] [PubMed] [Google Scholar]
- 46.Kotsopoulos J, Terry KL, Poole EM, et al. Ovarian cancer risk factors by tumor dominance, a surrogate for cell of origin. Int J Cancer. 2013;133:730–739. doi: 10.1002/ijc.28064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Genadry R, Poliakoff S, Rotmensch J, et al. Primary, papillary peritoneal neoplasia. Obstet Gynecol. 1981;58:730–734. [PubMed] [Google Scholar]
- 48.Kurman RJ, Shih I. The origin and pathogenesis of epithelial ovarian cancer: a proposed unifying theory. Am J Surg Pathol. 2010;34:433–443. doi: 10.1097/PAS.0b013e3181cf3d79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pothuri B, Leitao MM, Levine DA, et al. Genetic analysis of the early natural history of epithelial ovarian carcinoma. PLoS One. 2010;5:e10358. doi: 10.1371/journal.pone.0010358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gusberg SB, Deligdisch L. Ovarian dysplasia. A study of identical twins. Cancer. 1984;54:1–4. doi: 10.1002/1097-0142(19840701)54:1<1::aid-cncr2820540102>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 51.Hutson R, Ramsdale J, Wells M. p53 protein expression in putative precursor lesions of epithelial ovarian cancer. Histopathology. 1995;27:367–371. doi: 10.1111/j.1365-2559.1995.tb01528.x. [DOI] [PubMed] [Google Scholar]
- 52.Ferenczy A, Bertrand G, Gelfand MM. Proliferation kinetics of human endometrium during the normal menstrual cycle. Am J Obstet Gynecol. 1979;133:859–867. doi: 10.1016/0002-9378(79)90302-8. [DOI] [PubMed] [Google Scholar]
- 53.Ferenczy A, Richart RM. Scanning and transmission electron microscopy of the human endometrial surface epithelium. J Clin Endocrinol Metab. 1973;36:99–1008. doi: 10.1210/jcem-36-5-999. [DOI] [PubMed] [Google Scholar]
- 54.Seidman JD, Khedmati F. Exploring the histogenesis of ovarian mucinous and transitional cell (Brenner) neoplasms and their relationship with Walthard cell nests: a study of 120 tumors. Arch Pathol Lab Med. 2008;132:1753–1760. doi: 10.5858/132.11.1753. [DOI] [PubMed] [Google Scholar]
- 55.Mullany LK, Fan HY, Liu Z, et al. Molecular and functional characteristics of ovarian surface epithelial cells transformed by KrasG12D and loss of Pten in a mouse model in vivo. Oncogene. 2011;30:3522–3536. doi: 10.1038/onc.2011.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Deligdisch L, Gil J, Kerner H, et al. Ovarian dysplasia in prophylactic oophorectomy specimens: cytogenetic and morphometric correlations. Cancer. 1999;86:1544–1550. [PubMed] [Google Scholar]
- 57.Auersperg N. Ovarian surface epithelium as a source of ovarian cancers: Unwarranted speculation or evidence-based hypothesis? Gynecol Oncol. 2013;130:246–251. doi: 10.1016/j.ygyno.2013.03.021. [DOI] [PubMed] [Google Scholar]
- 58.Garrett LA, Vargas SO, Drapkin R, et al. Does the fimbria have an embryologic origin distinct from that of the rest of the fallopian tube? Fertil Steril. 2008;90:2008. doi: 10.1016/j.fertnstert.2008.01.071. [DOI] [PubMed] [Google Scholar]
- 59.Flesken-Nikitin A, Hwang CI, Cheng CY, et al. Ovarian surface epithelium at the junction area contains a cancer-prone stem cell niche. Nature. 2013;495:241–245. doi: 10.1038/nature11979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sasaki R, Narisawa-Saito M, Yugawa T, et al. Oncogenic transformation of human ovarian surface epithelial cells with defined cellular oncogenes. Carcinogenesis. 2009;30:423–431. doi: 10.1093/carcin/bgp007. [DOI] [PubMed] [Google Scholar]
- 61.Ong A, Maines-Bandiera SL, Roskelley CD, et al. An ovarian adenocarcinoma line derived from SV40/E-cadherin-transfected normal human ovarian surface epithelium. Int J Cancer. 2000;85:430–437. [PubMed] [Google Scholar]
- 62.King SM, Quartuccio SM, Vanderhyden BC, et al. Early transformative changes in normal ovarian surface epithelium induced by oxidative stress require Akt upregulation, DNA damage and epithelial-stromal interaction. Carcinogenesis. 2013;34:1125–1133. doi: 10.1093/carcin/bgt003. [DOI] [PubMed] [Google Scholar]
- 63.Barakat RR, Federici MG, Saigo PE, et al. Absence of premalignant histologic, molecular, or cell biologic alterations in prophylactic oophorectomy specimens from BRCA1 heterozygotes. Cancer. 2000;89:383–390. doi: 10.1002/1097-0142(20000715)89:2<383::aid-cncr25>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 64.Folkins AK, Jarboe EA, Saleemuddin A, et al. A candidate precursor to pelvic serous cancer (p53 signature) and its prevalence in ovaries and fallopian tubes from women with BRCA mutations. Gynecol Oncol. 2008;109:168–173. doi: 10.1016/j.ygyno.2008.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cai KQ, Wu H, Klein-Szanto AJ, et al. Acquisition of a second mutation of the Tp53 alleles immediately precedes epithelial morphological transformation in ovarian tumorigenicity. Gynecol Oncol. 2009;114:18–25. doi: 10.1016/j.ygyno.2009.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Marquez RT, Baggerly KA, Patterson AP, et al. Patterns of gene expression in different histotypes of epithelial ovarian cancer correlate with those in normal fallopian tube, endometrium, and colon. Clin Cancer Res. 2005;11:6116–6126. doi: 10.1158/1078-0432.CCR-04-2509. [DOI] [PubMed] [Google Scholar]