Abstract
Scope
Fish oil-derived n-3 PUFA may improve cardiometabolic health through modulation of innate immunity; however, findings in clinical studies are conflicting. We hypothesized that n-3 PUFA supplementation would dose-dependently reduce the systemic inflammatory response to experimental endotoxemia in healthy humans.
Methods and Results
The Fenofibrate and omega-3 Fatty Acid Modulation of Endotoxemia (FFAME) study was an 8-week randomized double-blind trial of placebo or n-3 PUFA supplementation (Lovaza 465mg EPA + 375mg DHA) at “low” (1/day, 900mg) or “high” (4/day, 3,600mg) dose in healthy individuals (N=60; age 18–45; BMI 18–30; 43% Female; 65% European-, 20% African-, 15% Asian-ancestry) before a low-dose endotoxin challenge (LPS 0.6ng/kg intravenous bolus). The endotoxemia-induced temperature increase was significantly reduced with high-dose (P =0.03) but not low-dose EPA+DHA compared to placebo. Although there was no statistically significant impact of EPA+DHA on individual inflammatory responses (TNFα, IL-6, MCP-1, IL-1RA, IL-10, CRP, SAA), there was a pattern of lower responses across all biomarkers with high-dose (9 of 9 observed), but not low-dose EPA+DHA.
Conclusions
EPA+DHA at 3,600mg/day, but not 900mg/day, reduced fever and had a pattern of attenuated LPS-induction of plasma inflammatory markers during endotoxemia. Clinically and nutritionally relevant long-chain n-3 PUFA regimens may have specific, dose-dependent, anti-inflammatory actions.
Keywords: endotoxemia, fish oil, inflammation, LPS, n-3 PUFA
1) INTRODUCTION
Complex cardiometabolic disorders including atherosclerosis and type 2 diabetes (T2DM) are characterized by activation of innate immunity in vascular, hepatic and adipose tissues and chronic low-grade systemic inflammation [1, 2]. Although innate immunity evolved in response to pathogenic stress, inappropriate or sustained triggering of inflammatory signaling in response to diet or lifestyle factors, microbiota, and genetics [3, 4] may accelerate and exacerbate chronic cardiometabolic disorders.
Both observational and interventional studies suggest that fish oil-derived long-chain omega-3 polyunsaturated fatty acids (n-3 PUFA) are protective against chronic inflammatory cardiometabolic diseases [5, 6] but findings of some large trials are conflicting [7–10]. Regular intake of dietary n-3 PUFA is thought to confer anti-inflammatory protection that may contribute to cardiometabolic benefits. However, the underlying mechanisms of action have not been fully elucidated, and optimal dosing for reported benefits is unknown. Differences in habitual diet and other environmental factors reduce the power of population-based-studies to detect effects of n-3 PUFA [11]. Clinical trials of fish oil supplementation have reported variable anti-inflammatory effects [12–14], perhaps due to modest action of fish oils coupled to heterogeneous dosing and application in non-ideal human settings. The effects of fish oil supplementation are expected to be subtle, when compared to anti-inflammatory pharmacologic intervention, and thus, may be difficult to detect in the resting physiological state.
While inflammation is commonly observed in human disease, this correlation does not establish causality. Precise models are required to establish directionality of association between inflammation, dietary interventions, and disease in humans. Induced inflammation, due to activation of toll like receptor-4 (TLR4) by lipopolysaccharide (LPS) during experimental endotoxemia, provides a model where activation of innate immunity and its metabolic consequences can be studied in humans in the absence of disease-related confounding or reverse causation [15–20]. In the Fenofibrate and omega-3 Fatty Acid Modulation of Endotoxemia (FFAME) study, we utilized an established low-dose endotoxemia model to examine the effect of n-3 PUFA on evoked systemic inflammation in healthy humans. Details of a separate fenofibrate arm of the study have been published [21]. In the FFAME n-3 PUFA study (N= 60) we investigated, relative to placebo, the dose-dependent anti-inflammatory actions of low and high doses of n-3 PUFA supplementation that are routinely used in clinic for prevention of heart disease (900 mg/day) or treatment of hypertriglyceridemia (3600 mg/day).
2) MATERIALS AND METHODS
Clinical Trial Design
Subjects
Healthy volunteers were recruited to the Clinical and Translational Research Center (CTRC) of the University of Pennsylvania (UPenn) between February 2010 and March 2011. Inclusion criteria included healthy men or healthy non-pregnant, non-lactating women, aged 18–45 years with a BMI of 18–30 kg/m2. Exclusions included inflammatory disease, cigarette smoking, medication, substance, or supplement use, and habitual intake of high omega-3 fish (tuna and other non-fried fish) > 3 to 4 servings per month. Physical exam, routine laboratory tests and electrocardiogram (ECG) were normal in all volunteers. The trial was conducted with the approval of the UPenn Institutional Review Board, and all participants provided written informed consent. The trial was approved by the FDA and registered at clinicaltrials.gov with the number NCT01048502.
Trial Design
An overview of the design of the FFAME trial is provided in Figure 1. This was an investigator-initiated, double-blind, placebo-controlled study. Participants were randomized to 1 of 4 treatment arms: placebo, fish-oil derived omega-3-acid ethyl ester eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) (EPA+DHA; Lovaza, GlaxoSmithKline; 465mg EPA + 375mg DHA) supplemented at either 1/day (900mg) or 4/day (3600mg), or fenofibrate (Tricor, Abbott Laboratories) 145 mg/day. Each EPA+DHA capsule contained α-tocopherol (4mg) as an antioxidant. The trial was designed to enroll 80 subjects to full completion of the inpatient endotoxin protocol across all study arms, with ~20 subjects per group. This report focuses on the EPA+DHA-versus-placebo aspect of the trial, which was pre-specified a priori as a distinct hypothesis from the recently published [21] fenofibrate trial. The EPA+DHA component of the study was designed to determine whether pretreatment (median duration 7 weeks) of healthy volunteers with prescription n-3 PUFA would result in dose-related attenuation of the inflammatory response to low-dose endotoxin, blocking the release of cytokines, chemokines, and acute phase reactants.
Figure 1. Design of the FFAME Study.
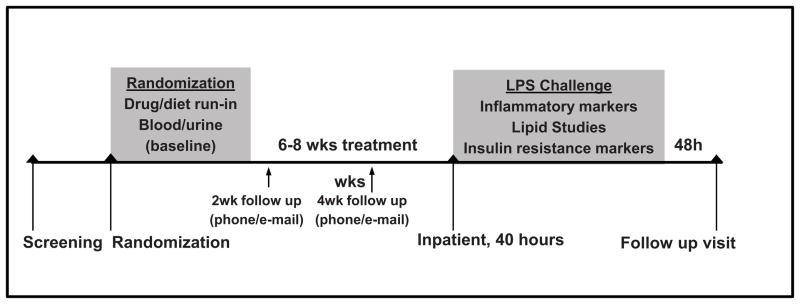
After screening, subjects were randomized to EPA+DHA or placebo for 6–8 weeks, followed by a lipopolysaccharide (LPS) challenge inpatient visit.
Interventions
Lovaza capsules and matching placebos were provided by GlaxoSmithKline Pharmaceuticals (Research Triangle Park, NC). Each 1 gram placebo contained corn oil (99.4%) with α-tocopherol (0.6%) as an antioxidant. Subjects assigned to the Lovaza groups were also assigned matching Tricor placebo (containing lactose in a gelatin capsule) while subjects assigned to the Tricor group were given matching Lovaza placebos. Subjects in the placebo group took both the Tricor and Lovaza placebos.
Endotoxemia study protocol
Participants attended the CTRC for 4 trial visits: visit one for screening; visit two after a 12-hour fast for randomization and collection of baseline labs; visit three, 6 to 8 weeks after randomization, for a ~40-hour inpatient stay consisting of an overnight fasting acclimatization phase and a post-LPS study phase; and visit four, 48–72 hours after completion of the LPS challenge, for follow-up blood draws. Serial whole blood samples, for separation of plasma and serum were collected before and 1, 2, 4, 6, 12, and 24 hours after intravenous bolus of 0.6 ng/kg (low-dose) U.S. standard reference endotoxin (lipopolysaccharide [LPS]; lot No. CCRE-LOT-1 +2; Clinical Center, Pharmacy Department at the National Institutes of Health, Bethesda, MD). Urine was collected serially throughout the inpatient visit. Temperature was measured every 30 minutes for the first 12 hours and then hourly for the remaining 16 hour inpatient stay. Heart rate was measured hourly for the first 8 hours post-LPS, followed by measurements at 12, 16, and 24 hours. Blood pressure was recorded every 15 minutes for 8 hours after LPS injection and then hourly for the remaining 16 hours.
Laboratory Methods
Lipidomic Analyses
Red blood cells (RBC) obtained from whole blood were lysed using ammonium chloride solution (0.8% NH4Cl, Stemcell Technologies), and membrane fatty acids extracted as described [22]. Briefly, fatty acids were hydrolyzed using KOH, extracted using acetonitrile on a StrataX cartridge (Phenomenex, Torrance, CA), dried using a SpeedVac centrifugal evaporator (Savant Inc.) and stored at −80°C prior to analysis. The composition of arachidonic acid (AA), eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) was determined by mass spectrometry as previously described [23].
In order to provide insight into the impact of high-dose EPA+DHA treatment on fatty acids on downstream modulation of systemic inflammatory parameters, urinary isoprostanes 8,12-iso-iPF2α-VI and 8,12-iso-iPF3α-VI were determined in the high-dose and placebo groups (N=16 placebo, N=21 Lovaza 4/day) by LC/MS/MS as described [23, 24]. At randomization, a morning spot urine sample was collected, while the post-treatment sample was collected over a 6-hour period (midnight - 6am). Briefly, the eicosanoids were extracted from urine by SPE (StrataX cartridge, Phenomenex, Torrance, CA), dried under a gentle stream of nitrogen, and stored at −80°C prior to analysis.
To further explore lipidomic changes with high-dose EPA+DHA, plasma lipidomics were carried out in a subset of high-dose and placebo (N=9 placebo, N=7 Lovaza 4/day) at the MRC Human Nutrition Research laboratory, Cambridge, UK. Plasma samples (15μl) were extracted using an automated Flexus sample preparation unit and lipidomics performed on the extract using chip-based nanoelectrospray with an Advion TriVersa Nanomate interfaced to the Thermo Exactive Orbitrap (Thermo Scientific). A mass acquisition window from 200 to 2000 m/z was used with acquisition in positive and negative mode. Acquired spectral raw data was processed using CALDERA, an in-house bioinformatics platform, performing sample specific mass recalibration using predefined sets of internal standards and the removal of commonly present contaminant ions. Automated compound annotation was carried out using exact mass-search in compound libraries and applying the referenced Kendrick mass defect approach. Features of interest were subsequently confirmed using fragmentation experiments on a Thermo Velos Orbitrap mass analyser.
Plasma inflammatory and metabolic markers
Serum amyloid A (SAA) and high-sensitivity C-reactive protein (CRP) were measured by latex particle-enhanced immunonephelometry on a Behring Nephelometer II Analyzer (Siemens Diagnostics; Munich, Germany). Plasma levels of tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6), interleukin-10 (IL-10), interleukin-1 receptor agonist (IL-1RA) and monocyte chemotactic protein-1 (MCP-1/CCL2) were measured using sandwich ELISAs according to the manufacturer’s instructions (Quantikine, R&D Systems; Minneapolis, MN). The intra- and inter-assay coefficients of variation across all plates were TNF-α, 7.5% and 14.8%; IL-6, 5.9% and 14.3%; IL-10, 6.6% and 8.0%; IL-1RA 2.3% and 8.0%, and MCP-1, 7.8% and 11.1%, respectively. The lower limits of quantitation were TNF-α, 0.4pg/mL; IL-6, 0.154pg/mL; IL-10, 0.78pg/mL; IL1-RA, 25.4pg/mL, and MCP-1, 31.2pg/mL. For the purposes of analysis, samples with values below detection were set to the lower limit of the given assay. After ultracentrifugation, plasma total cholesterol, LDL-cholesterol, HDL-cholesterol, and triglycerides (TG) were measured enzymatically on a Hitachi 912 Analyzer (Roche Diagnostics; Indianapolis, IN).
Statistical Analysis
Unless otherwise specified, data are reported as medians and interquartile range (IQR) for continuous variables and as proportions for categorical variables. Efficacy analyses included, a priori, data from all 60 participants who completed the inpatient endotoxin challenge. On the basis of prior work [17, 20, 25], the trial was designed to have an 80% power to detect a 27% reduction in plasma TNF-α response to endotoxin in a EPA+DHA-treated arm compared to placebo.
The change in baseline parameters following treatment with EPA+DHA or placebo (but before LPS) was compared among groups by Kruskal-Wallis non-parametric tests, and Dunn post-hoc pairwise comparisons. To evaluate endotoxin effects over time, area under the curve (AUC) was calculated for outcome variables using the trapezoidal rule. The area representing the pre-LPS baseline was subtracted out for an incremental, or ΔAUC, which was compared by treatment group using the Kruskal-Wallis test. For temperature, as some subjects’ temperature fell below their baseline following LPS, we subtracted a constant (35.5°C) rather than the individual baseline for the ΔAUC. Correlations between variables were assessed using Spearman’s correlation coefficient (rs). The main focus for analysis of LPS-induced change was the ΔAUC but we present peak responses also to facilitate clinical interpretation. Plasma TNFα was considered the primary endpoint, with additional traits analyzed to provide complementary information about the impact on diverse inflammatory pathways. A P value <0.05 was considered to indicate statistical significance. We did not correct for multiple testing, in part because of the correlations among response variables. Statistical analyses were performed using IBM SPSS Statistics 19 (IBM, Armonk, NY).
3) RESULTS
Pre-LPS characteristics
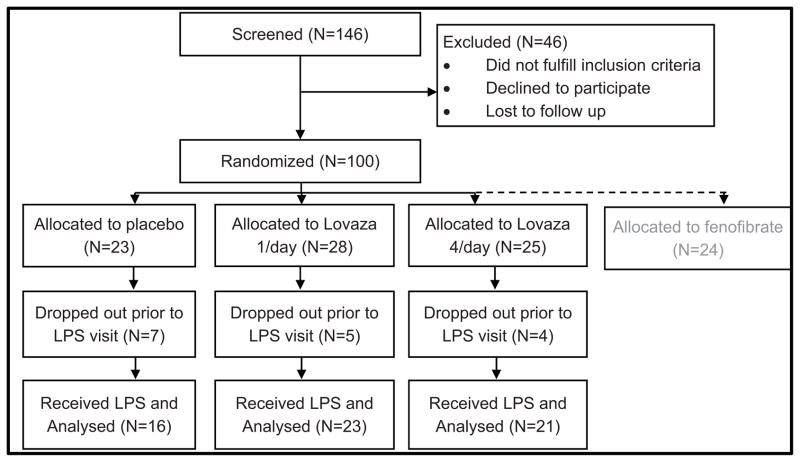
Seventy-six individuals were enrolled in the EPA+DHA portion of the FFAME trial (N=23 Placebo, N=28 Lovaza 1/day, N=25 Lovaza 4/day). A separate arm of the trial, recently reported [21], included individuals randomized to fenofibrate. An overview of the flow of the study is shown in Figure 2. Consistent with a projected completion rate of 80%, 16 individuals dropped out prior to the inpatient endotoxin visit (N= 7 for Placebo, N=5 for Lovaza 1/day, N= 4 for Lovaza 4/day). Details of the reasons for dropping out are described in the Supplementary Methods. There were no serious adverse events; all reported adverse events are listed in Supplement Table S1. Baseline characteristics of FFAME participants who completed the endotoxin visit are shown in Table 1. There were no statistically significant differences in these measures between groups at randomization. Adherence to EPA+DHA supplementation was assessed through pill count, and confirmed in all subjects, with < 20% of study medication remaining on day of LPS visit.
Figure 2. Flow diagram for the FFAME study.
100 subjects were randomized, to allow for an expected 20% rate of dropout, and achieve our goal of 80 completers. A separate fenofibrate arm of the study has been analyzed and reported independently.
Table 1.
Baseline characteristics of participants
| Placebo (N=16) Median (IQR) | Lovaza 1/day (N=23) Median (IQR) | Lovaza 4/day (N=21) Median (IQR) | Group difference P Value | |
|---|---|---|---|---|
| Age (years) | 27 (11) | 27 (8) | 24 (7) | 0.80 |
| Female N (%) | 6 (37.5) | 11 (47.8) | 9 (42.9) | 0.82 |
| Race: | ||||
| White N (%) | 12 (75) | 14 (60) | 13 (62) | |
| African-American N (%) | 2 (12.5) | 6 (26) | 4 (19) | >0.99 |
| Asian N (%) | 2 (12.5) | 3 (13) | 4 (19) | |
| BMI (kg/m2) | 22.8 (5.8) | 23.6 (4.2) | 23.4 (3.8) | 0.85 |
| Systolic blood pressure (mmHg) | 119 (13) | 117 (15) | 113 (11) | 0.19 |
| Heart rate (bpm) | 65 (7) | 64 (19) | 68 (15) | 0.73 |
| Tumor necrosis factor-α (pg/ml) | 0.95 (0.88) | 1.26 (1.02) | 1.14 (1.06) | 0.76 |
| Interleukin-6 (pg/ml) | 1.07 (0.66) | 1.04 (0.73) | 1.00 (0.96) | 0.95 |
| Interleukin-1 receptor agonist (pg/ml) | 141 (57) | 139 (66) | 135 (65) | 0.93 |
| Interleukin-10 (pg/ml) | 0.78 (0.0) | 0.78 (2.8) | 0.78 (0.0) | 0.02 |
| Monocyte chemotactic protein-1 (pg/ml) | 132 (36) | 136 (58) | 146 (57) | 0.97 |
| High-sensitivity C-reactive protein (mg/l) | 0.26 (0.37) | 0.33 (1.05) | 0.48 (0.58) | 0.36 |
| Serum amyloid A (mg/l) | 2.8 (0.15) | 2.8 (0.45) | 2.28 (3.35) | 0.65 |
| Total cholesterol (mg/dl) | 173 (34) | 179 (47) | 170 (57) | 0.98 |
| HDL-cholesterol (mg/dl) | 56 (28) | 57 (23) | 58 (19) | 0.89 |
| LDL-cholesterol (mg/dl) | 97 (23) | 99 (42) | 94 (38) | 0.80 |
| Triglycerides (mg/dl) | 80 (58) | 72 (57) | 80 (63) | 0.59 |
Values given as median (IQR). P Value from Kruskal-Wallis non-parametric test comparing values by treatment group.
Fish oil supplementation increased RBC membrane and plasma n-3 PUFA and urinary levels of n-3 PUFA-derived isoprostanes
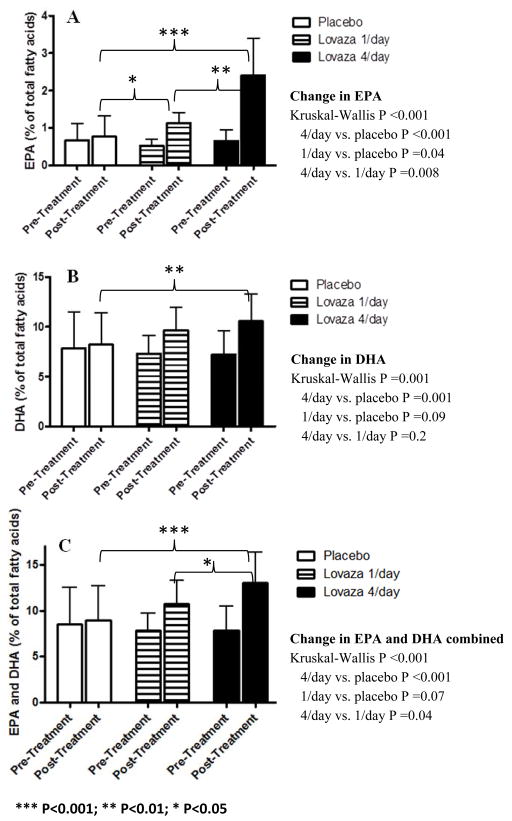
RBC membrane PUFA composition of AA, EPA and DHA was measured at randomization, and after ~8 weeks treatment with Lovaza 1/day, Lovaza 4/day and placebo (Figure 3). At randomization, there were no statistically significant differences between groups in RBC membrane EPA or DHA composition. After intervention, the proportion of RBC EPA and DHA increased in both treatment groups (P <0.005 for all comparisons), while there was no change within the placebo group (P >0.5) resulting in statistically significant differences in the changes between groups (Kruskal-Wallis ANOVA P <0.001 for EPA and P =0.001 for DHA) with post-hoc comparisons showing increases in participants receiving 1/day (P =0.04 and P =0.09 for EPA and DHA respectively) and 4/day (P <0.001 and P =0.001 for EPA and DHA respectively) relative to placebo. The increase in EPA was greater in 4/day vs. 1/day (P =0.008). These data demonstrate a dose-dependent change in n-3 fatty acid composition with EPA+DHA treatment and the achieved RBC levels of n-3 PUFA were consistent with other published trials of similar doses of n-3 PUFA supplementation [26, 27].
Figure 3. Change in red blood cell membrane fatty acids following EPA+DHA treatment; (A) Eicosapentaenoic acid (EPA), (B) Docosahexaenoic acid (DHA) and (C) combined EPA and DHA.
The proportion of EPA and DHA in the red blood cell membrane increased significantly following EPA+DHA supplementation. Arachidonic acid (AA), EPA and DHA expressed as percentage of total fatty acids measured (AA+EPA+DHA).
Concentrations of urinary eicosanoids, normalized to urinary creatinine, were measured at randomization and after EPA+DHA supplementation in the placebo and 4/day groups. We observed a significant difference in the absolute change in urinary eicosanoids over ~8 weeks between placebo and Lovaza 4/day. The prostaglandin-like isoprostane 8,12-iso-iPF3α-VI, a metabolite of EPA, was significantly increased in 4/day compared with placebo (P =0.001), consistent with the increased n-3 PUFA intake (Table 2). In contrast, there was no change in 8,12-iso-iPF2α-VI, an established peroxidation product of arachidonic acid (AA), arguing against a generalized pro-oxidant response. There was a strong correlation between the change in RBC membrane EPA, and the change in 8,12-iso-iPF3α-VI (rs=0.8, P<0.0001).
Table 2.
Changes in blood and urine lipidomics following ~8 weeks of EPA+DHA supplementation
| At Randomization | 8 Weeks Post-Randomization | Absolute Delta | P Value | ||
|---|---|---|---|---|---|
| Blood | |||||
| PC(38:5) (808.584 m/z) | Placebo (N=9) | 2.33 (0.45) | 2.48 (0.48) | −0.06 (0.65) | 0.21 |
| Lovaza 4/day (N=7) | 2.33 (1.09) | 2.86 (0.42) | +0.59 (0.56) | ||
| PC(40:6) (834.600 m/z) | Placebo (N=9) | 0.51 (0.19) | 0.59 (0.19) | +0.06 (0.15) | <0.001 |
| Lovaza 4/day (N=7) | 0.59 (0.23) | 1.14 (0.31) | +0.63 (0.38) | ||
| PC(38:4) (810.600 m/z) | Placebo (N=9) | 2.85 (1.19) | 2.78 (0.84) | +0.06 (0.50) | 0.016 |
| Lovaza 4/day (N=7) | 3.23 (0.92) | 2.37 (0.48) | −0.67 (0.81) | ||
| PC(36:3) (784.584 m/z) | Placebo (N=9) | 3.50 (0.94) | 3.51 (1.09) | +0.04 (0.80) | <0.0001 |
| Lovaza 4/day (N=7) | 3.57 (1.40) | 2.5 (0.81) | −0.99 (0.89) | ||
|
| |||||
| Urine | |||||
| 8,12-iso-iPF2α-VI (ng/mg creatinine) | Placebo (N=16) | 5.43 (6.10) | 6.83 (4.40) | −0.36 (5.57) | 0.20 |
| Lovaza 4/day (N=21) | 6.47 (3.10) | 5.28 (4.10) | −1.13 (3.44) | ||
| 8,12-iso-iPF3α-VI (ng/mg creatinine) | Placebo (N=16) | 0.64 (0.76) | 0.89 (0.57) | 0.22 (0.45) | <0.001 |
| Lovaza 4/day (N=21) | 0.65 (0.44) | 2.52 (2.00) | 1.59 (1.74) | ||
Values given as median (IQR). P Value from Mann Whitney U non-parametric test comparing the absolute delta by treatment group. iPF2α: 8,12-iso-iPF(2 alpha)-VI; iPF3α: 8,12-iso-iPF(3 alpha)-VI
We measured the plasma lipidomic profile at randomization and after supplementation in a subset of the placebo (N=9) and 4/day (N=7) groups (Table 2). There were no baseline differences between groups in EPA- or DHA-related phosphocholine relative levels. There were trends towards an increase in the 4/day n-3 PUFA group in plasma relative levels of the ions of EPA-containing phosphocholine PC(38:5) (808.584 m/z) (Placebo −0.05, n-3 PUFA +0.59, P=0.2) and the DHA-containing phosphocholine PC(40:6) (834.600 m/z) (Placebo +0.06, n-3 PUFA +0.63, P=0.001) compared with placebo. Phosphocholine lipids other than EPA or DHA were significantly decreased in the 4/day group; arachidonic PC(38:4) (810.600 m/z) (Placebo +0.06, n-3 PUFA −0.67, P=0.016) and α-linoleic acid PC(36:3) (784.585) (Placebo +0.04, n-3 PUFA −0.98, P<0.001). Collectively, these lipidomic analyses reveal the anticipated appropriate increases in systemic n-3 fatty acid exposures with EPA+DHA supplementation.
Pre-LPS circulating lipoproteins and inflammatory markers were not significantly altered by EPA+DHA supplementation
During the ~8 week trial period prior to LPS administration, circulating lipoprotein and lipid levels dropped in all groups. However there were no post-supplementation differences in total cholesterol, LDL or HDL between groups. Despite a trend toward greater decrease in TG with Lovaza 4/day, there were no statistically significant differences between treatment groups and placebo in the degree of change although we note TG levels were quite low in this healthy study sample (Table S2).
Prior to LPS, there was an unexpected small increase in IL-6 in all groups [median (IQR) 1.05 (0.7) to 2.9 (2.9), P< 0.001], however there were no differences among groups in the changes of any inflammatory markers over this ~8 week period (Table 3). After ~8 weeks of treatment but prior to LPS, there were no changes in body composition (BMI or body fat %), heart rate or blood pressure (data not shown) with EPA+DHA treatment.
Table 3.
There were no significant differences between the EPA+DHA treated groups and placebo in baseline levels of inflammatory markers or in the change in variables over the ~8 week pre-LPS treatment period.
| At Randomization | 8 Weeks Post-Randomization | Absolute Delta | P Value | ||
|---|---|---|---|---|---|
| Tumor necrosis factor-α (pg/ml) | Placebo | 0.95 (0.88) | 1.12 (1.20) | −0.08 (0.56) | 0.58 |
| Lovaza 1/day | 1.26 (1.02) | 1.25 (0.97) | −0.06 (0.25) | ||
| Lovaza 4/day | 1.14 (1.06) | 0.99 (0.70) | −0.07 (0.55) | ||
|
| |||||
| Interleukin-6 (pg/ml) | Placebo | 1.07 (0.66) | 3.30 (2.82) | 2.3 (2.13) | 0.08 |
| Lovaza 1/day | 1.04 (0.73) | 3.55 (3.82) | 2.31 (3.76) | ||
| Lovaza 4/day | 1.00 (0.96) | 2.39 (2.69) | 1.13 (1.71) | ||
|
| |||||
| Interleukin-1 receptor agonist (pg/ml) | Placebo | 141 (57) | 130 (67) | 0.65 (32.82) | 0.89 |
| Lovaza 1/day | 139 (66) | 148 (89) | 6.34 (60.16) | ||
| Lovaza 4/day | 135 (65) | 143 (93) | −13.78 (51.98) | ||
|
| |||||
| Monocyte chemotactic protein-1 (pg/ml) | Placebo | 132 (36) | 138 (43) | 3.4 (25.68) | 0.71 |
| Lovaza 1/day | 136 (58) | 146 (57) | 9.67 (49.07) | ||
| Lovaza 4/day | 146 (57) | 143 (44) | 9.18 (36.87) | ||
|
| |||||
| High-sensitivity C-reactive protein (mg/l) | Placebo | 0.26 (0.37) | 0.42 (0.81) | 0.04 (0.23) | 0.87 |
| Lovaza 1/day | 0.33 (1.05) | 0.54 (0.90) | 0.01 (0.47) | ||
| Lovaza 4/day | 0.48 (0.58) | 0.48 (1.0) | 0 (0.67) | ||
|
| |||||
| Serum amyloid A (mg/l) | Placebo | 2.8 (0.15) | 2.8 (1.03) | 0 (0) | 0.13 |
| Lovaza 1/day | 2.8 (0.45) | 2.8 (1.8) | 0 (1.2) | ||
| Lovaza 4/day | 2.28 (3.35) | 2.8 (0.95) | 0 (0.75) | ||
Values given as median (IQR). Placebo N=16; Lovaza 1/day N=23; Lovaza 4/day N=21. P Value from Kruskal-Wallis non-parametric test comparing the absolute delta by treatment group
High-dose but not low-dose EPA+DHA attenuated the temperature response to LPS
Following LPS, subjects exhibited an expected modest acute inflammatory response [21, 28], with a small but significant increase in body temperature, peaking ~4 hours post-LPS (Figure 4). The temperature response differed significantly across groups (Kruskal-Wallis P=0.035), with reduced ΔAUC in 4/day compared with placebo (post-hoc adjusted P=0.046) but no significant difference in 1/day vs. placebo. There was no effect of EPA+DHA treatment on blood pressure or heart rate in response to LPS (Supplement Figure 1, A–C).
Figure 4. Temperature response to LPS in EPA+DHA treated groups compared to placebo.
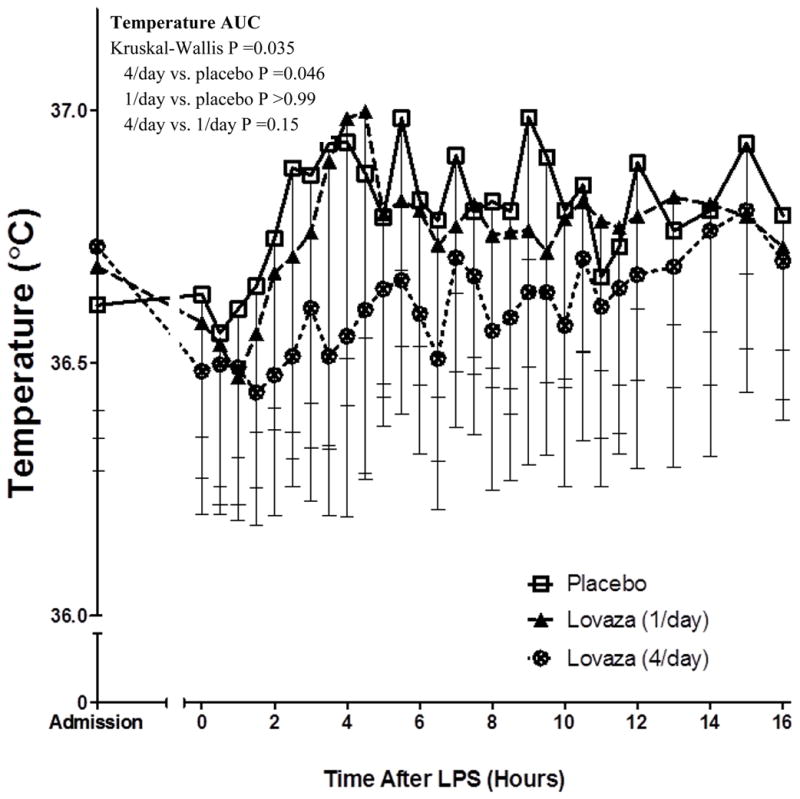
Subjects treated with high-dose EPA+DHA (Lovaza 4/day) had a significantly reduced temperature response compared with placebo (AUC P=0.046). This reduction was not observed in the low-dose EPA+DHA group
Neither EPA+DHA treatments had statistically significant impact on the inflammatory biomarker response to endotoxemia
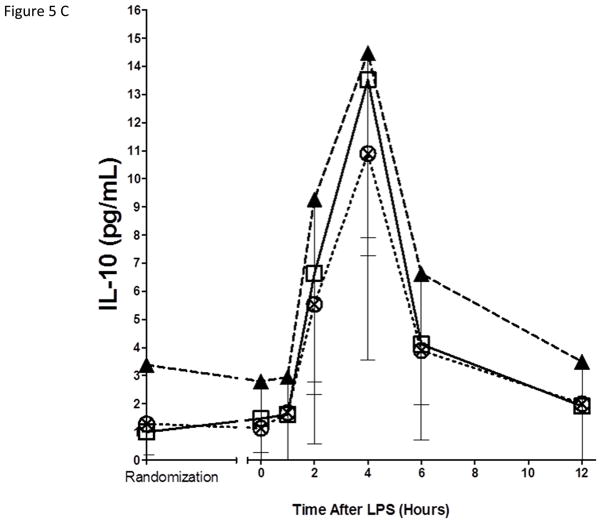
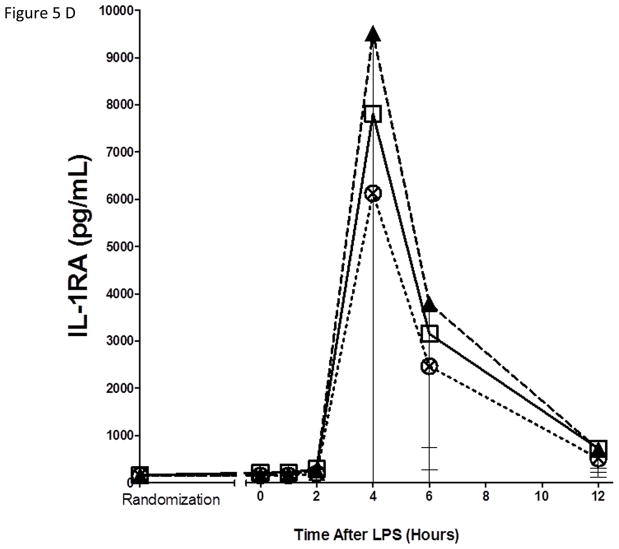
As expected, in the placebo group, endotoxemia induced an acute increase in plasma cytokines (TNFα, 13-fold; IL-6, 12-fold; IL-10, 14-fold; IL1RA, 54-fold), chemokines (MCP-1 10-fold) and acute phase proteins (CRP, 20-fold; SAA, 17-fold) (Figure 5). There were no statistically significant differences among treatment groups in the ΔAUC of our primary inflammatory variable, TNFα (Figure 5 A), or in other inflammatory biomarkers (Figure 5, B–G). There was a consistent trend towards a reduction in several inflammatory biomarkers (TNFα, IL-6, IL-10, IL-1ra, MCP-1, CRP and SAA) with Lovaza 4/day (Figure 5 A–G); however, this did not reach statistical significance vs. placebo for any individual biomarker. The 1/day group did not display any trend toward a reduced inflammatory biomarker response.
Figure 5. Inflammatory responses to LPS in high-dose and low-dose EPA+DHA treated subjects compared to placebo.
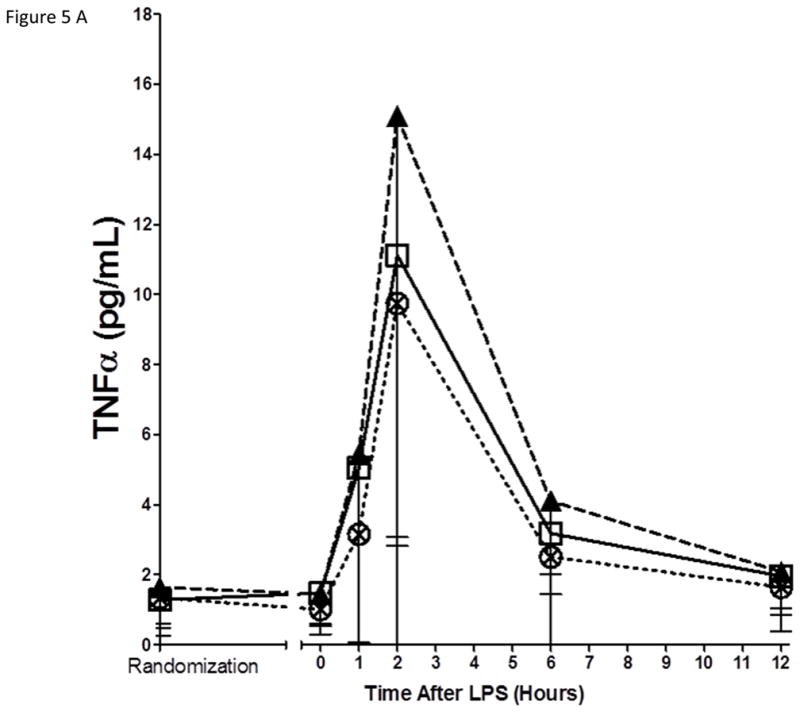
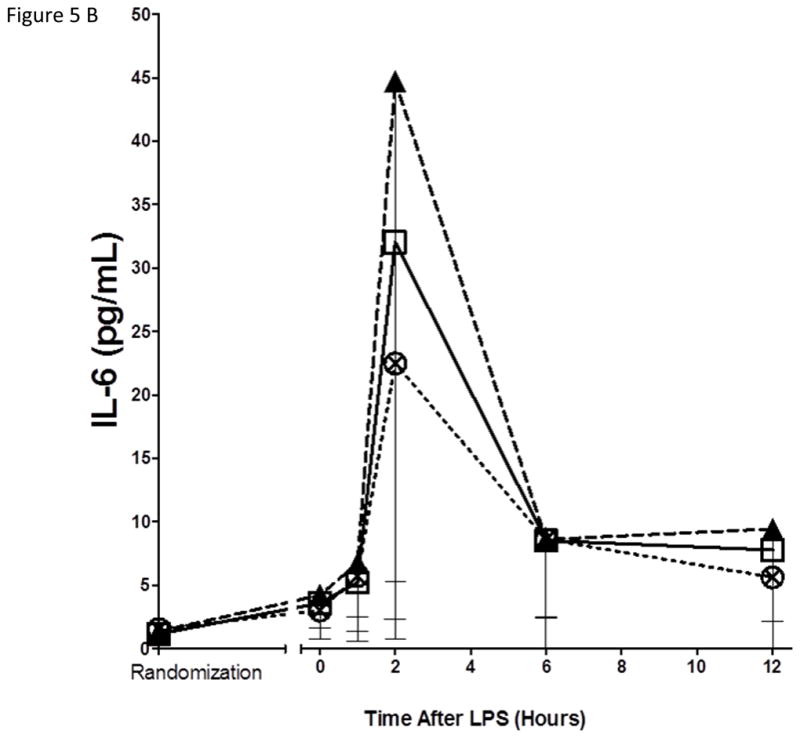
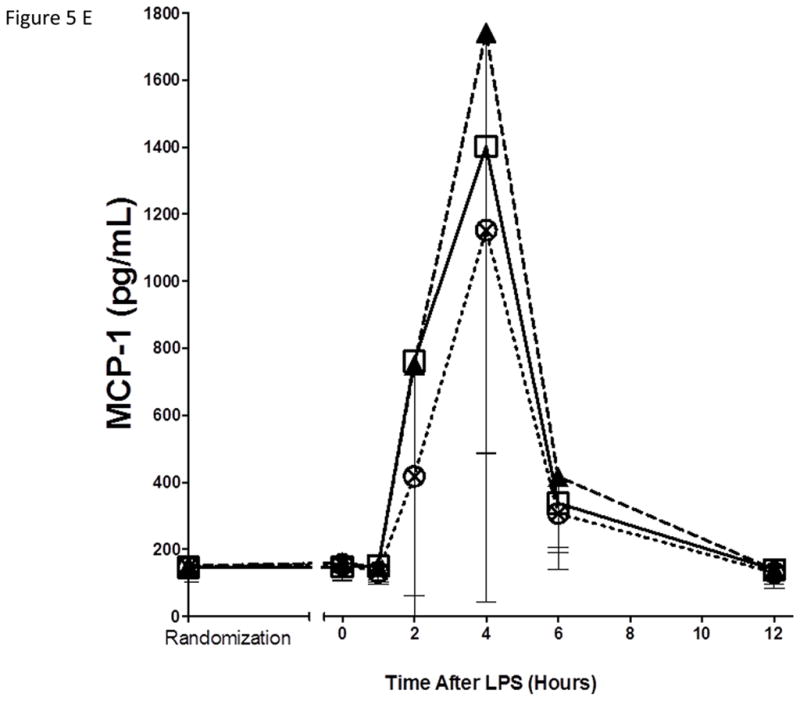
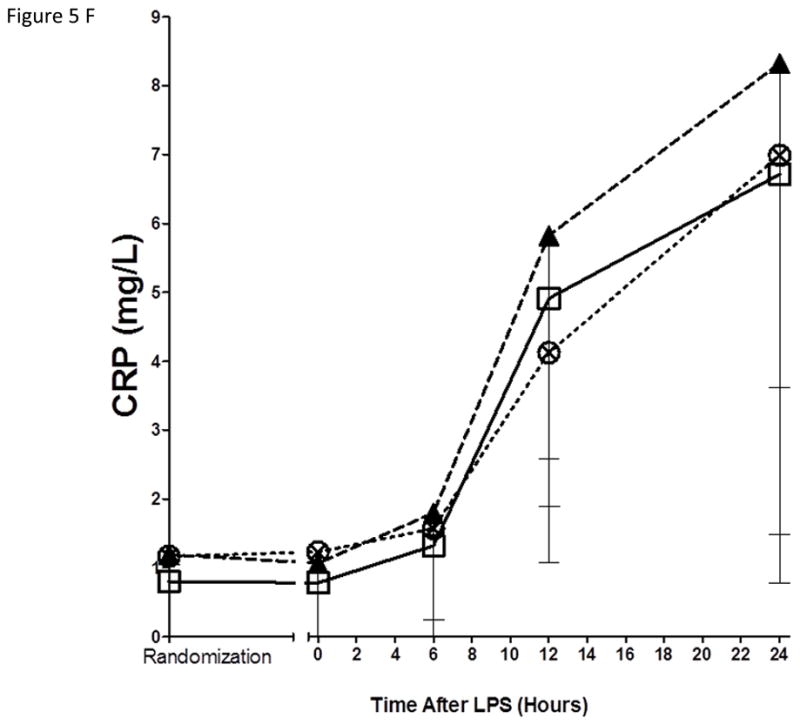
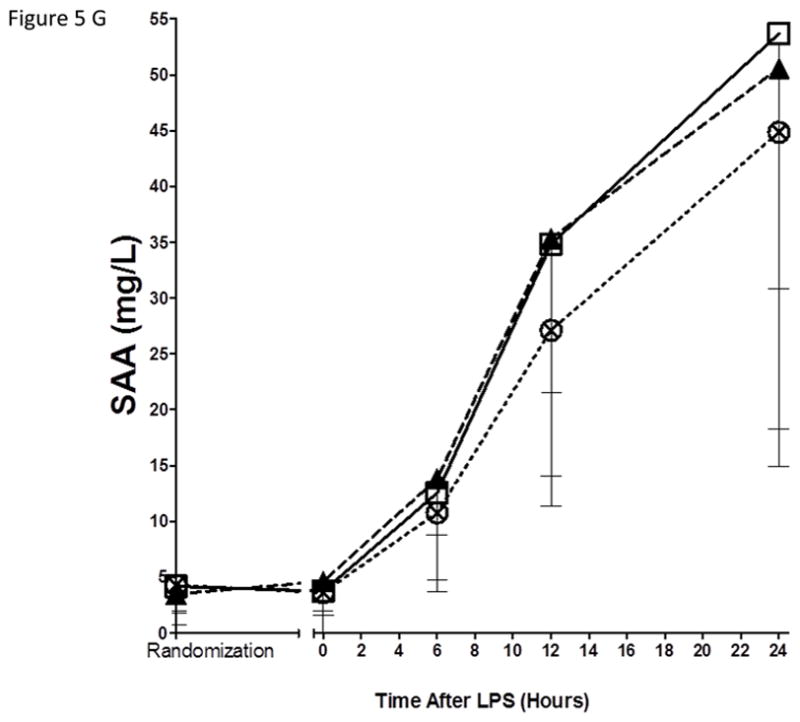
(A) Tumor necrosis factor-α (TNFα); (B) Interleukin-6 (IL-6); (C) Interleukin-10 (IL-10); (D) Interleukin-1 receptor agonist (IL-1RA); (E) Monocyte chemotactic protein-1 (MCP-1); (F) C-reactive Protein (CRP); (G) Serum amyloid A (SAA).
⊟ Placebo
 Lovaza (1/day)
Lovaza (1/day)
⊗ Lovaza (4/day)
Trend across inflammatory biomarkers with high-dose EPA+DHA treatment
Because of an apparent pattern of lower responses for many inflammatory measures with high-dose EPA+DHA, we explored the patterns of these trends across 9 endotoxemia-modulated inflammatory responses (temperature, pain, TNFα, IL-6, IL-10, IL-1RA, MCP-1, CRP, SAA). For Lovaza 4/day, 9 of 9 ΔAUC responses were lower than the placebo group (Table 4). In contrast, 3 of 9 ΔAUC responses were lower for 1/day vs. placebo.
Table 4.
Pattern of inflammatory responses in EPA+DHA treated groups.
| Variable | Δ AUC Placebo | Δ AUC Lovaza 4/day | Response in Lovaza 4/day compared to placebo | Δ AUC Lovaza 1/day | Response in Lovaza 1/day compared to placebo |
|---|---|---|---|---|---|
| Tumor necrosis factor-α | 37.63 | 33.36 | ↓ | 53.40 | ↑ |
| Interleukin-6 | 113.5 | 99.0 | ↓ | 125.1 | ↑ |
| Interleukin-10 | 43.82 | 40.36 | ↓ | 50.55 | ↑ |
| Interleukin-1 receptor agonist | 28617.4 | 22351.3 | ↓ | 35077.7 | ↑ |
| Monocyte chemotactic protein-1 | 2977.1 | 1804.1 | ↓ | 3617.1 | ↑ |
| C-reactive protein | 76.05 | 62.69 | ↓ | 91.25 | ↑ |
| Serum amyloid A | 632.3 | 501.7 | ↓ | 597.7 | ↓ |
| Temperature | 1266.6 | 1159.8 | ↓ | 1215.7 | ↓ |
| Pain | 28.8 | 14.6 | ↓ | 21.6 | ↓ |
Δ AUC calculated as area under the curve, adjusted for baseline levels
4) DISCUSSION
We used a low-dose evoked endotoxemia model in healthy volunteers to examine putative anti-inflammatory effects of fish oil-derived n-3 PUFA supplementation. After 6–8 weeks of treatment, there were measureable differences in red blood cell membrane and plasma fatty acids, as well as urinary prostaglandins in the n-3 PUFA treated groups, indicating appropriate systemic changes in fatty acid composition related to EPA+DHA supplementation. These did not translate into any measureable differences in pre-LPS clinical or inflammatory variables. Although there was no statistically significant impact of either n-3 PUFA dose on LPS-induced increases in plasma TNFα, the primary response variable, treatment with 4/day, but not 1/day attenuated endotoxemia-induced fever and produced a pattern of lower responses across all measured inflammatory biomarkers compared to placebo.
There are several lines of evidence linking cardiometabolic disease with inflammation. Obesity is associated with adipose dysfunction, and increased local production of TNFα and other inflammatory mediators, which contribute to the development of insulin resistance and metabolic dysfunction [29]. Atherosclerosis is characterized by localized inflammation within atherosclerotic plaque as well as increased systemic inflammation [30]. While a robust inflammatory response to pathogens is crucial for survival, chronic activation as observed in metabolic disease may itself be pathogenic. With considerable overlap between the inflammatory immune response and the nutrient-sensitive metabolic response, it is likely that an imbalance in input from either direction results in dysregulated inflammatory signaling [29]. Thus, chronic over-nutrition or sub-optimal nutrition in the context of reduced physical activity may disturb inflammatory equilibrium, with pathogenic consequences.
Given the close inter-relationship between innate immune and nutrient-responsive inflammatory signaling, and the strong link between diet-induced obesity and cardiometabolic disease, manipulation of nutrient intake may be a key modulator of disease. Long-chain n-3 PUFA have been associated with protection against disease in both epidemiological [6] and interventional studies [7, 8, 31]. However, recent studies and meta-analyses have been inconclusive as to the efficacy of fish-oil supplementation and fish consumption in disease reduction [32, 33], with interpretation confounded by considerable heterogeneity in dose, type of n-3 PUFA used (e.g. EPA vs. DHA), population studied, habitual fish consumption, n-3 status, and potential interaction with other drugs (e.g. statins) [34].
While the precise pathways linking n-3 PUFA and disease prevention are not known, there are several plausible mechanisms, including competitive inhibition by n-3 PUFA of pro-inflammatory eicosanoid production [35], suppression of pro-inflammatory oxidant species [36], transcriptional modulation of lipid-responsive and inflammatory genes [37, 38], alteration of fatty acid membrane properties [39], alteration of receptor signaling e.g. through the specific G-protein coupled signaling receptor GPR120 [40], and through modulation of the gut microbiota [41]. The relative roles, if any, of these proposed mechanisms remain unclear. In addition, the optimal dose required for preventing chronic inflammation in cardiometabolic disease while maintaining optimal innate immune responsiveness is not known.
Much of the uncertainty surrounding the efficacy of n-3 PUFA in disease modulation is likely related to the considerable heterogeneity in intake, population, drug interactions, and existing disease status. We hypothesized that an evoked endotoxemia model in healthy individuals would increase the ability to detect systemic anti-inflammatory effects of n-3 PUFA supplementation. We chose to use a pharmaceutical-grade preparation of EPA and DHA (Lovaza) to minimize confounding by natural variation in the composition of non-pharmaceutical fish oil preparations. Intravenous infusion of very high doses of fish oil has been shown to blunt clinical and inflammatory response to endotoxin [42, 43], with reduced endotoxin-induced fever following fish oil infusion, but the clinical relevance of this extreme dosing is unclear. In FFAME, we observed a significantly reduced temperature response in subjects treated with high-dose EPA+DHA (3600mg/day), but not with low-dose (900mg/day). The high-dose corresponds to the recommended dose for individuals with hypertriglyceridemia, while the low-dose is recommended for individuals with documented CHD [44]. While both doses are relatively high in a nutritional context, intake of 900mg/day EPA+DHA is achievable with regular consumption of fatty fish consistent with dietary recommendations [45]. While higher doses are recommended in hypertriglyceridemia, it remains unclear whether these doses should be recommended in an anti-inflammatory context. While in vivo oxidation of n-3 PUFA may be protective by reducing generation of more deleterious oxidized n-6 PUFA [23, 36], consumption of oxidized lipids may have pro-inflammatory effects [46]. Optimal dosage may be dependent both on the health status of the individual, and background diet, in particular intake of n-6 PUFA [35]. Notably, the low-dose group did not show any trend toward lower inflammation during endotoxemia; in fact for many biomarkers the trend was toward increased responses. In contrast, the impact of high-dose fish oil on fever responses and its pattern of lower inflammatory responses during endotoxemia suggest that n-3 PUFA supplementation might have clinically relevant anti-inflammatory effects when used at higher doses, but not at the lower doses used for cardioprotection. However, we emphasize that neither EPA+DHA dose had significant impact on the primary endotoxemia response variable, plasma TNFα.
Our study had several strengths. Understanding causality is problematic in the context of inflammatory cardiometabolic disease, as the chronically elevated inflammation observed in individuals presenting with overt cardiovascular disease or diabetes may in part be a result of the disease process itself. Thus, we used a model of evoked inflammation in healthy individuals. Administration of bacterial-derived LPS activates TLR4, which acts as a general activation signal for multiple downstream inflammatory responses [47] related to both innate immune and metabolic signaling. By controlling the initiation of the inflammatory insult, we are able to define downstream inflammatory events which aids in inferring causality. The LPS model has been used extensively by our group and others [16, 17, 19, 20, 28, 48–50] to interrogate inflammatory, lipid, and insulin signaling responses to endotoxemia. We used a pharmaceutical preparation of EPA+DHA, which removed potential confounding by other compounds present in fish oil preparations, and allows easier interpretation for clinical applications, particularly as we used low- and high-dose regimens that are used commonly in clinical practice.
Our trial also has limitations. Although our pre-trial power calculations suggested that 20 participants per group would be sufficient to detect a 27% reduction in TNFα in the EPA+DHA-treated group compared with placebo, our findings reveal that our study was in fact under-powered to detect significant differences in our primary outcome; instead of the anticipated 80% power, we actually only had ~30% power given the observed heterogeneous responses. We have recently reported race-differences in inflammatory responses to endotoxemia in a large sample [49]; because the FFAME study recruited individuals of European, African, and Asian ancestry, this heterogeneity may have impacted power to detect differences related to EPA+DHA treatment. Although small, a slightly higher drop-out rate in the placebo compared with the EPA+DHA groups may have introduced bias. Based on these data, we suggest that replication of this study in a larger, ethnically homogenous population should achieve power to definitively address hypotheses of differences in inflammatory responses with EPA+DHA supplementation, and dose-related effects. It will also be important to explore potentially different effects of EPA and DHA in future work given evidence that EPA and DHA have distinct effects on lipids and inflammation [51–53], and thus may exert specific anti-inflammatory effects through unique mechanisms.
Conclusions
During experimental endotoxemia, neither high-dose nor low-dose EPA+DHA had significant impact on the primary response variable, plasma TNFα. However, high-dose EPA+DHA at doses used for treatment of lipid disorders, but not at low-dose used for cardioprotection, did reduce the febrile response and may suppress diverse inflammatory cytokines, chemokines, and acute phase protein responses. Thus, n-3 PUFA supplementation might modulate inflammatory responses in a dose-related manner that is specifically relevant to current dosing strategies in cardiometabolic disease but this hypothesis requires further trials.
Supplementary Material
Acknowledgments
We would like to thank the participants of the FFAME study, the staff at the UPenn Clinical Translational Research Center and the support of the UPenn Investigational Drug Services. Study materials (LOVAZA and matching placebo) were provided to MPR by GlaxoSmithKline. GlaxoSmithKline had no role in the design and conduct of the study; in the collection, analysis, and interpretation of the data; or in the preparation, review, or approval of the manuscript. No other authors declare a conflict of interest.
Funding: The project described was supported by the National Institutes of Health (NIH) through Grant UL1RR024134 and P20-DK 019525 (both to the University of Pennsylvania) as well as a NIH-NHLBI SCCOR Project grant (P50-HL-083799) to MPR. JFF is supported by a postdoctoral fellowship grant from the American Heart Association (12POST11840017). MPR is also supported by RO1-HL-111694, RO1-HL-113147, RO1-DK-090505, UO1-HL-108636 and K24-HL-107643. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. JLG, AK and ME are supported by the Medical Research Council, UK (UD99999906). GlaxoSmithKline provided Lovaza and matching placebo.
Abbreviations
- FFAME
Fenofibrate and omega-3 Fatty Acid Modulation of Endotoxemia Study
- n-3 PUFA
omega-3 polyunsaturated fatty acid
- AA
arachidonic acid
- EPA
eicosapentaenoic acid
- DHA
docosahexaenoic acid
- LPS
lipopolysaccharide
- TNFα
Tumor necrosis factor-α
- IL-6
Interleukin-6
- IL-10
Interleukin-10
- IL-1RA
Interleukin-1 receptor agonist
- MCP-1
Monocyte chemotactic protein-1
- CRP
C-reactive Protein
- SAA
Serum amyloid A
Footnotes
Clinical Trial Registry: FDA clinicaltrials.gov registration number: NCT01048502
5) REFERENCES
- 1.de Luca C, Olefsky JM. Stressed out about obesity and insulin resistance. Nature medicine. 2006;12:41–42. doi: 10.1038/nm0106-41. discussion 42. [DOI] [PubMed] [Google Scholar]
- 2.de Luca C, Olefsky JM. Inflammation and insulin resistance. FEBS letters. 2008;582:97–105. doi: 10.1016/j.febslet.2007.11.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Calder PC, Ahluwalia N, Brouns F, Buetler T, et al. Dietary factors and low-grade inflammation in relation to overweight and obesity. The British journal of nutrition. 2011;106(Suppl 3):S5–78. doi: 10.1017/S0007114511005460. [DOI] [PubMed] [Google Scholar]
- 4.Moreira AP, Texeira TF, Ferreira AB, do Peluzio MC, de Alfenas RC. Influence of a high-fat diet on gut microbiota, intestinal permeability and metabolic endotoxaemia. The British journal of nutrition. 2012;108:801–809. doi: 10.1017/S0007114512001213. [DOI] [PubMed] [Google Scholar]
- 5.Wang C, Harris WS, Chung M, Lichtenstein AH, et al. n-3 Fatty acids from fish or fish-oil supplements, but not alpha-linolenic acid, benefit cardiovascular disease outcomes in primary- and secondary-prevention studies: a systematic review. The American journal of clinical nutrition. 2006;84:5–17. doi: 10.1093/ajcn/84.1.5. [DOI] [PubMed] [Google Scholar]
- 6.Kromhout D, Bosschieter EB, de Lezenne Coulander C. The inverse relation between fish consumption and 20-year mortality from coronary heart disease. N Engl J Med. 1985;312:1205–1209. doi: 10.1056/NEJM198505093121901. [DOI] [PubMed] [Google Scholar]
- 7.Yokoyama M, Origasa H, Matsuzaki M, Matsuzawa Y, et al. Effects of eicosapentaenoic acid on major coronary events in hypercholesterolaemic patients (JELIS): a randomised open-label, blinded endpoint analysis. Lancet. 2007;369:1090–1098. doi: 10.1016/S0140-6736(07)60527-3. [DOI] [PubMed] [Google Scholar]
- 8.Investigators GP. Dietary supplementation with n-3 polyunsaturated fatty acids and vitamin E after myocardial infarction: results of the GISSI-Prevenzione trial. Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto miocardico. Lancet. 1999;354:447–455. [PubMed] [Google Scholar]
- 9.Mozaffarian D, Marchioli R, Macchia A, Silletta MG, et al. Fish oil and postoperative atrial fibrillation: the Omega-3 Fatty Acids for Prevention of Post-operative Atrial Fibrillation (OPERA) randomized trial. JAMA. 2012;308:2001–2011. doi: 10.1001/jama.2012.28733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kromhout D, Giltay EJ, Geleijnse JM. Alpha Omega Trial, G. n-3 fatty acids and cardiovascular events after myocardial infarction. N Engl J Med. 2010;363:2015–2026. doi: 10.1056/NEJMoa1003603. [DOI] [PubMed] [Google Scholar]
- 11.Patel PS, Forouhi NG, Kuijsten A, Schulze MB, et al. The prospective association between total and type of fish intake and type 2 diabetes in 8 European countries: EPIC-InterAct Study. The American journal of clinical nutrition. 2012;95:1445–1453. doi: 10.3945/ajcn.111.029314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tierney AC, McMonagle J, Shaw DI, Gulseth HL, et al. Effects of dietary fat modification on insulin sensitivity and on other risk factors of the metabolic syndrome--LIPGENE: a European randomized dietary intervention study. International journal of obesity (2005) 2011;35:800–809. doi: 10.1038/ijo.2010.209. [DOI] [PubMed] [Google Scholar]
- 13.Xin W, Wei W, Li X. Effects of fish oil supplementation on inflammatory markers in chronic heart failure: a meta-analysis of randomized controlled trials. BMC cardiovascular disorders. 2012;12:77. doi: 10.1186/1471-2261-12-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rangel-Huerta OD, Aguilera CM, Mesa MD, Gil A. Omega-3 long-chain polyunsaturated fatty acids supplementation on inflammatory biomakers: a systematic review of randomised clinical trials. The British journal of nutrition. 2012;107(Suppl 2):S159–170. doi: 10.1017/S0007114512001559. [DOI] [PubMed] [Google Scholar]
- 15.Agwunobi AO, Reid C, Maycock P, Little RA, Carlson GL. Insulin resistance and substrate utilization in human endotoxemia. J Clin Endocrinol Metab. 2000;85:3770–3778. doi: 10.1210/jcem.85.10.6914. [DOI] [PubMed] [Google Scholar]
- 16.Hudgins LC, Parker TS, Levine DM, Gordon BR, et al. A single intravenous dose of endotoxin rapidly alters serum lipoproteins and lipid transfer proteins in normal volunteers. Journal of lipid research. 2003;44:1489–1498. doi: 10.1194/jlr.M200440-JLR200. [DOI] [PubMed] [Google Scholar]
- 17.Anderson PD, Mehta NN, Wolfe ML, Hinkle CC, et al. Innate immunity modulates adipokines in humans. J Clin Endocrinol Metab. 2007;92:2272–2279. doi: 10.1210/jc.2006-2545. [DOI] [PubMed] [Google Scholar]
- 18.McGillicuddy FC, de la Llera Moya M, Hinkle CC, Joshi MR, et al. Inflammation impairs reverse cholesterol transport in vivo. Circulation. 2009;119:1135–1145. doi: 10.1161/CIRCULATIONAHA.108.810721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shah R, Lu Y, Hinkle CC, McGillicuddy FC, et al. Gene profiling of human adipose tissue during evoked inflammation in vivo. Diabetes. 2009;58:2211–2219. doi: 10.2337/db09-0256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mehta NN, McGillicuddy FC, Anderson PD, Hinkle CC, et al. Experimental endotoxemia induces adipose inflammation and insulin resistance in humans. Diabetes. 2010;59:172–181. doi: 10.2337/db09-0367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mulvey CK, Ferguson JF, Tabita-Martinez J, Kong S, et al. Peroxisome Proliferator-Activated Receptor-alpha Agonism With Fenofibrate Does Not Suppress Inflammatory Responses to Evoked Endotoxemia. J Am Heart Assoc. 2012;1:e002923. doi: 10.1161/JAHA.112.002923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Song WL, Lawson JA, Reilly D, Rokach J, et al. Neurofurans, novel indices of oxidant stress derived from docosahexaenoic acid. J Biol Chem. 2008;283:6–16. doi: 10.1074/jbc.M706124200. [DOI] [PubMed] [Google Scholar]
- 23.Song WL, Paschos G, Fries S, Reilly MP, et al. Novel eicosapentaenoic acid-derived F3-isoprostanes as biomarkers of lipid peroxidation. J Biol Chem. 2009;284:23636–23643. doi: 10.1074/jbc.M109.024075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Song WL, Lawson JA, Wang M, Zou H, FitzGerald GA. Noninvasive assessment of the role of cyclooxygenases in cardiovascular health: a detailed HPLC/MS/MS method. Methods Enzymol. 2007;433:51–72. doi: 10.1016/S0076-6879(07)33003-6. [DOI] [PubMed] [Google Scholar]
- 25.Pleiner J, Schaller G, Mittermayer F, Zorn S, et al. Simvastatin prevents vascular hyporeactivity during inflammation. Circulation. 2004;110:3349–3354. doi: 10.1161/01.CIR.0000147774.90396.ED. [DOI] [PubMed] [Google Scholar]
- 26.Harris WS, Sands SA, Windsor SL, Ali HA, et al. Omega-3 fatty acids in cardiac biopsies from heart transplantation patients: correlation with erythrocytes and response to supplementation. Circulation. 2004;110:1645–1649. doi: 10.1161/01.CIR.0000142292.10048.B2. [DOI] [PubMed] [Google Scholar]
- 27.Harris WS, Pottala JV, Sands SA, Jones PG. Comparison of the effects of fish and fish-oil capsules on the n 3 fatty acid content of blood cells and plasma phospholipids. The American journal of clinical nutrition. 2007;86:1621–1625. doi: 10.1093/ajcn/86.5.1621. [DOI] [PubMed] [Google Scholar]
- 28.Mehta NN, Heffron SP, Patel PN, Ferguson J, et al. A human model of inflammatory cardio-metabolic dysfunction; a double blind placebo-controlled crossover trial. J Transl Med. 2012;10:124. doi: 10.1186/1479-5876-10-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444:860–867. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- 30.Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. 2005;352:1685–1695. doi: 10.1056/NEJMra043430. [DOI] [PubMed] [Google Scholar]
- 31.Browning LM, Walker CG, Mander AP, West AL, et al. Incorporation of eicosapentaenoic and docosahexaenoic acids into lipid pools when given as supplements providing doses equivalent to typical intakes of oily fish. The American journal of clinical nutrition. 2012;96:748–758. doi: 10.3945/ajcn.112.041343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rizos EC, Ntzani EE, Bika E, Kostapanos MS, Elisaf MS. Association between omega-3 fatty acid supplementation and risk of major cardiovascular disease events: a systematic review and meta-analysis. JAMA. 2012;308:1024–1033. doi: 10.1001/2012.jama.11374. [DOI] [PubMed] [Google Scholar]
- 33.Kwak SM, Myung SK, Lee YJ, Seo HG Korean Meta-analysis Study, G. Efficacy of omega-3 fatty acid supplements (eicosapentaenoic acid and docosahexaenoic acid) in the secondary prevention of cardiovascular disease: a meta-analysis of randomized, double-blind, placebo-controlled trials. Archives of internal medicine. 2012;172:686–694. doi: 10.1001/archinternmed.2012.262. [DOI] [PubMed] [Google Scholar]
- 34.de Lorgeril M, Salen P, Defaye P, Rabaeus M. Recent findings on the health effects of omega-3 fatty acids and statins, and their interactions: do statins inhibit omega-3? BMC Med. 2013;11:5. doi: 10.1186/1741-7015-11-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bagga D, Wang L, Farias-Eisner R, Glaspy JA, Reddy ST. Differential effects of prostaglandin derived from omega-6 and omega-3 polyunsaturated fatty acids on COX-2 expression and IL-6 secretion. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:1751–1756. doi: 10.1073/pnas.0334211100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Calzada C, Colas R, Guillot N, Guichardant M, et al. Subgram daily supplementation with docosahexaenoic acid protects low-density lipoproteins from oxidation in healthy men. Atherosclerosis. 2010;208:467–472. doi: 10.1016/j.atherosclerosis.2009.07.049. [DOI] [PubMed] [Google Scholar]
- 37.Jump DB, Clarke SD, Thelen A, Liimatta M. Coordinate regulation of glycolytic and lipogenic gene expression by polyunsaturated fatty acids. Journal of lipid research. 1994;35:1076–1084. [PubMed] [Google Scholar]
- 38.Sampath H, Ntambi JM. Polyunsaturated fatty acid regulation of genes of lipid metabolism. Annual review of nutrition. 2005;25:317–340. doi: 10.1146/annurev.nutr.25.051804.101917. [DOI] [PubMed] [Google Scholar]
- 39.Chapkin RS, Kim W, Lupton JR, McMurray DN. Dietary docosahexaenoic and eicosapentaenoic acid: emerging mediators of inflammation. Prostaglandins, leukotrienes, and essential fatty acids. 2009;81:187–191. doi: 10.1016/j.plefa.2009.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Oh DY, Talukdar S, Bae EJ, Imamura T, et al. GPR120 is an omega-3 fatty acid receptor mediating potent anti-inflammatory and insulin-sensitizing effects. Cell. 2010;142:687–698. doi: 10.1016/j.cell.2010.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ghosh S, Decoffe D, Brown K, Rajendiran E, et al. Fish oil attenuates omega-6 polyunsaturated Fatty Acid-induced dysbiosis and infectious colitis but impairs LPS dephosphorylation activity causing sepsis. PLoS ONE. 2013;8:e55468. doi: 10.1371/journal.pone.0055468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pittet YK, Berger MM, Pluess TT, Voirol P, et al. Blunting the response to endotoxin in healthy subjects: effects of various doses of intravenous fish oil. Intensive Care Med. 2010;36:289–295. doi: 10.1007/s00134-009-1689-8. [DOI] [PubMed] [Google Scholar]
- 43.Pluess TT, Hayoz D, Berger MM, Tappy L, et al. Intravenous fish oil blunts the physiological response to endotoxin in healthy subjects. Intensive Care Med. 2007;33:789–797. doi: 10.1007/s00134-007-0591-5. [DOI] [PubMed] [Google Scholar]
- 44.Lichtenstein AH, Appel LJ, Brands M, Carnethon M, et al. Summary of American Heart Association Diet and Lifestyle Recommendations revision 2006. Arteriosclerosis, thrombosis, and vascular biology. 2006;26:2186–2191. doi: 10.1161/01.ATV.0000238352.25222.5e. [DOI] [PubMed] [Google Scholar]
- 45.Gebauer SK, Psota TL, Harris WS, Kris-Etherton PM. n-3 fatty acid dietary recommendations and food sources to achieve essentiality and cardiovascular benefits. The American journal of clinical nutrition. 2006;83:1526S–1535S. doi: 10.1093/ajcn/83.6.1526S. [DOI] [PubMed] [Google Scholar]
- 46.Awada M, Soulage CO, Meynier A, Debard C, et al. Dietary oxidized n-3 PUFA induce oxidative stress and inflammation: role of intestinal absorption of 4-HHE and reactivity in intestinal cells. Journal of lipid research. 2012;53:2069–2080. doi: 10.1194/jlr.M026179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shi H, Kokoeva MV, Inouye K, Tzameli I, et al. TLR4 links innate immunity and fatty acid-induced insulin resistance. J Clin Invest. 2006;116:3015–3025. doi: 10.1172/JCI28898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Suffredini AF, Fromm RE, Parker MM, Brenner M, et al. The cardiovascular response of normal humans to the administration of endotoxin. N Engl J Med. 1989;321:280–287. doi: 10.1056/NEJM198908033210503. [DOI] [PubMed] [Google Scholar]
- 49.Ferguson JF, Patel PN, Shah RY, Mulvey CK, et al. Race and gender variation in response to evoked inflammation. J Transl Med. 2013;11:63. doi: 10.1186/1479-5876-11-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.van Eijk LT, Dorresteijn MJ, Smits P, van der Hoeven JG, et al. Gender differences in the innate immune response and vascular reactivity following the administration of endotoxin to human volunteers. Crit Care Med. 2007;35:1464–1469. doi: 10.1097/01.CCM.0000266534.14262.E8. [DOI] [PubMed] [Google Scholar]
- 51.Jacobson TA, Glickstein SB, Rowe JD, Soni PN. Effects of eicosapentaenoic acid and docosahexaenoic acid on low-density lipoprotein cholesterol and other lipids: a review. Journal of clinical lipidology. 2012;6:5–18. doi: 10.1016/j.jacl.2011.10.018. [DOI] [PubMed] [Google Scholar]
- 52.Oliver E, McGillicuddy FC, Harford KA, Reynolds CM, et al. Docosahexaenoic acid attenuates macrophage-induced inflammation and improves insulin sensitivity in adipocytes-specific differential effects between LC n-3 PUFA. The Journal of nutritional biochemistry. 2012;23:1192–1200. doi: 10.1016/j.jnutbio.2011.06.014. [DOI] [PubMed] [Google Scholar]
- 53.Jaudszus A, Gruen M, Watzl B, Ness C, et al. Evaluation of suppressive and pro-resolving effects of EPA and DHA in human primary monocytes and T-helper cells. Journal of lipid research. 2013;54:923–935. doi: 10.1194/jlr.P031260. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.