Abstract
Purpose
Poorer survival from head and neck squamous cell carcinoma (HNSCC) in African Americans (AA) may be due to disparity in the prevalence of Human Papillomavirus (HPV) but earlier studies often failed to control other etiological factors. We aimed to elucidate whether racial disparities in HPV prevalence and overall survival were due to confounding from smoking or alcohol use.
Materials and Methods
385 patients with SCC of the mouth, pharynx, nose, or larynx who had surgical resection at Wayne State University affiliated hospitals were identified through a population-based cancer registry. Formalin fixed paraffin embedded tissue blocks were used to determine the presence of HPV DNA and its genotype using a sensitive broad-spectrum PCR technique. Patients’ demographics, tumor characteristics and vital status were obtained through record linkage with the registry data and smoking and alcohol information was abstracted from medical record. Cox’s proportional hazard model and unconditional logistic models were employed to analyze the overall survival and tumor HPV-positivity, respectively.
Results
HPV positivity in oropharyngeal cancer was substantially lower in AA than in other racial groups (odds ratio 0.14, 95% confidence interval (CI) 0.05–0.37) and adjustment for smoking or alcohol did not change this association. However, a significantly increased hazard ratio of death in AA oropharyngeal cancer patients (univariable hazard ratio (HR) 2.55, 95% CI 1.42–4.59) decreased to almost unity (HR 1.49, 95% CI 0.75–2.93) after adjustment for HPV and smoking.
Conclusions
Lower HPV prevalence in AA largely accounts for their poorer survival from oropharyngeal cancer, but not other HNSSC.
Introduction
Over 644,000 incident cases and 350,000 deaths of head and neck squamous cell carcinoma (HNSSSC) are estimated to occur every year worldwide [1]. In the USA, HNSSC account for about 3% to 5% of all cancers [2]. In 2013 alone, an estimated 53,640 people will develop HNSSC, with 11,520 of these patients dying due to HNSCC [2]. Despite the overall declining trend in the incidence of head and neck cancer, reflecting the decreasing trend in tobacco consumption [3], incidence of cancer from the oropharyngeal sites, especially the tonsil and the base of the tongue, are rising, most notably in ages 40–55 [4,5,6]. Recently Human Papilloma Virus (HPV) has emerged as a contributing risk factor for HNSSC, specifically in the oropharynx [5,7–10]. HPV-associated tumors tend to respond more favorably to chemoradiation, and have better outcomes than HPV-negative HNSSC [5,11–14]. Moreover, racial disparities among African Americans and Non-African Americans exist in HPV-associated HNSSC [15,16]. The National Cancer Institute defines racial disparities as adverse differences in incidence, prevalence, mortality, survivorship and tumor burden. According to the 2010 US Census Bureau, the black population grew 15.4% from 2000 to 2010 and make up 13.6% of the US population [17]. African Americans show a 50% higher age-adjusted HNSSC mortality rate compared to whites, with younger age at onset, more advanced stage at diagnosis and poorer survival in blacks than in whites [18].
Socioeconomic factors [19–21], less access to health care, high-risk sexual practices [22,23], host immunity/genetics [24], and tobacco and alcohol consumption[25, 26] have all been linked to racial differences [18]. However the reason for racial disparities between incidence and outcome is still not fully elucidated. Racial differences in HPV prevalence in HNSCC have been reported and these differences have been attributed to poorer survival in African American patients [5,6]. The objective to our study is to extend our previous cancer-registry based study [6] in order to reevaluate the effects of race on HPV prevalence and overall survival in HNSCC patients, by taking advantage of more detailed information about smoking and alcohol drinking that could be retrieved from individual medical records.
Materials and Methods
Details concerning the 385 study subjects included in the parent study and laboratory methods were described elsewhere [6]. Briefly, patients with squamous cell carcinoma (SCC) of the mouth, pharynx, nose, or larynx who had surgical resection at Wayne State University affiliated hospitals were identified through a population-based cancer registry, the Metropolitan Detroit Cancer Surveillance System (MDCSS). Formalin fixed paraffin embedded tissue blocks were used to determine the presence of HPV DNA and its genotype using a sensitive broad-spectrum PCR technique (SPF10-LiPA25) [27,28]. Patients’ demographics, tumor characteristics and vital status (as of September 6, 2012) were obtained through record linkage with MDCSS data and smoking and alcohol information was abstracted from medical record. The study was approved with a waiver for informed consent by WSU Human Investigation Committee.
Primary sites of cancer were grouped into oropharyngeal or other sites based on International Classification of Diseases for Oncology (ICDO) 4 digit topology codes. The former included C019–C020 (base and dorsal surface of tongue), C051 (soft palate), C052 (uvula), C090–C103 and C108–C109 (all tonsil sites and all oropharynx sites except branchial cleft). Races were divided into two groups, African Americans (AA) vs. others who were primarily Caucasians except 4 Asians. We combined Asians to Caucasians as they have not been found with poorer survival from these cancers, like AA [29].
Differences in frequency distribution of patients’ and tumor characteristics between the racial groups were tested by chi-square test. Odds ratios (OR) and 95% CI for HPV-positivity were calculated by unconditional logistic model according to patients’ racial groups, smoking status and alcohol use. Smoking or alcohol-adjusted-ORs for HPV-positivity in AA compared with the other races were also computed including marital status as a covariate due to its differential distribution by HPV status reported previously [6] Time at risk in survival analysis was defined as the time from diagnosis to the date of death or the date of last vital status confirmation ascertained by MDCSS. The Kaplan-Meier method and log rank test were used to analyze overall survival by racial group and Cox’s proportional hazard models were employed to estimate multivariable hazard ratios (HR) for deaths and the corresponding 95% confidence intervals (CI) associated with African American race, adjusted for smoking, HPV-status or both in addition to basic covariates. Potential basic covariate (cancer stage, other primaries, age, sex, marital status, etc.) were tested by adding to the univariable model with racial group one at a time and covariates that altered the regression coefficient for race for at least 10% were selected, leaving only marital status in the multivariable model. Because smoking and alcohol use was closely correlated each other and because smoking had stronger effect on survival than alcohol use, only smoking was entered in multivariable models. The proportional hazard assumption was checked by inclusion of a time-dependent covariate, log (follow-up time) multiplied by HPV status. Statistical analyses were performed by SAS 9.2. All statistical tests were 2-sided.
Results
Table 1 shows selected characteristics of 385 patients (161 AA and 224 other races). Gender and age distributions were not significantly different between these two groups (p =0.20 and p = 0.4406, respectively). Sixty nine percent of AA were not married as oppose to 50% of Non-African Americans were not married (p = 0.0003). No difference was seen in SEER summary stage (p = 0.6119) and anatomic sites (p = 0.5897) between AA and Non-AA. 88.2% of AA had current or past use of cigarette smoking and 85.3% of Non-AA had current or past use of cigarette smoking (p= 0.387). 78.2% of African Americans had current or past use of alcohol and 67.4% of Non-African Americans had current of past use of alcohol (p = 0.094). Similar differences in smoking and alcohol use were observed between AA and other races even limited to oropharyngeal site only (data not shown).
Table 1.
Patients’ and tumor characteristics according by race (African Americans vs. other)
| Characteristics | Characteristics | Other races | African American | P-values | ||
|---|---|---|---|---|---|---|
| No. of subjects | % | No. of subjects | % | |||
| Age | 20–44 yrs | 26 | 11.6 | 17 | 10.6 | |
| 45–54 yrs | 66 | 29.5 | 56 | 34.8 | ||
| 55–64 yrs | 70 | 31.3 | 54 | 33.5 | ||
| 65+ yrs | 62 | 27.7 | 34 | 21.1 | 0.4406 | |
| Gender | Female | 72 | 32.1 | 42 | 26.1 | |
| Male | 152 | 67.9 | 119 | 73.9 | 0.1992 | |
| Marital Status | Not-married | 113 | 50.5 | 111 | 68.9 | |
| Married | 111 | 49.6 | 50 | 31.1 | 0.0003 | |
| Primary sites | Other sites | 179 | 79.9 | 125 | 77.6 | |
| Oro-pharynx* | 45 | 20.1 | 36 | 22.4 | 0.5897 | |
| Tumor stage | Local | 54 | 24.1 | 45 | 28.0 | |
| Regional | 122 | 54.5 | 80 | 49.7 | ||
| Distant | 48 | 21.4 | 36 | 22.4 | 0.6119 | |
| Tumor differentiation | Well-Moderate | 111 | 49.6 | 78 | 48.5 | |
| Poor- Non | 113 | 50.5 | 83 | 51.6 | 0.8304 | |
| Multiple primaries | Absence | 137 | 61.2 | 104 | 64.6 | |
| Presence | 87 | 38.8 | 57 | 35.4 | 0.492 | |
| Cigarette smoking | Never | 23 | 10.3 | 13 | 8.1 | |
| Past | 67 | 29.9 | 39 | 24.2 | ||
| Current | 124 | 55.4 | 103 | 64.0 | ||
| Ever unspecified | 1 | 0.5 | 2 | 1.2 | ||
| Unknown | 9 | 4.0 | 4 | 2.5 | 0.3827 | |
| Not smoking | 90 | 40.2 | 52 | 32.3 | ||
| Currently smoking | 124 | 55.4 | 103 | 64.0 | ||
| Unknown | 10 | 4.5 | 6 | 3.7 | 0.237 | |
| Alcohol use | Never | 60 | 26.8 | 28 | 17.4 | |
| Past | 50 | 22.3 | 35 | 21.7 | ||
| Current | 101 | 45.1 | 91 | 56.5 | ||
| Ever unspecified | 1 | 0.5 | 2 | 1.2 | ||
| Unknown | 12 | 5.4 | 5 | 3.1 | 0.0944 | |
| Never | 60 | 26.8 | 28 | 17.4 | ||
| Ever | 152 | 67.9 | 128 | 79.5 | ||
| Unknown | 12 | 5.4 | 5 | 3.1 | 0.04 | |
Oro-pharynx was defined by ICD-O 4 digit topology codes; C019–C020 (base and dorsal surface of tongue), C051 (soft palate)-C052
Table 2 shows that of those who had oropharyngeal cancer, 53.1% were currently smoking, and 37.2% of those smokers were HPV positive, whereas among non-smokers 65.8% were HPV positive (OR 3.25, 1.3–8.08). 71.4% of oropharyngeal cancer patient who never drank alcohol were HPV-positive, whereas 44.6% of drinkers were HPV positive (OR 3.10 0.88–10.92). For oropharyngeal site, 25% of AA were HPV positive whereas 71.1% of non-AA were HPV positive (OR=0.14, 95% CI 0.05–0.37). Adjustment for current smoking or ever alcohol use had a negligible effect in the OR for HPV positivity. For non-oropharyngeal HNSSC sites, HPV-positivity was fairly stable across strata of smoking, alcohol use, and race resulting in ORs close to unity.
Table 2.
Odds ratios (OR) and 95 % confidence intervals (CI) for HPV positivity associated with smoking, alcohol use and African American race
| Characteristics | HPV prevalence | |||||||
|---|---|---|---|---|---|---|---|---|
| No. of subjects | Oropharyngeal* | No. of subjects | Other | |||||
| % HPV+ | OR** | (95% CI) | % HPV+ | OR** | (95% CI) | |||
| Current smoking | ||||||||
| Yes | 43 | 37.2 | 1.00 | - | 184 | 22.8 | 1.00 | - |
| No | 38 | 65.8 | 3.25 | (1.30–8.08) | 104 | 23.1 | 1.01 | (0.57–1.80) |
| Alcohol use | ||||||||
| Ever | 65 | 44.6 | 1.00 | - | 215 | 21.4 | 1.00 | - |
| Never | 14 | 71.4 | 3.10 | (0.88–10.92) | 74 | 27.0 | 1.36 | (0.74–2.50) |
| Race | ||||||||
| Other | 45 | 71.1 | 1.00 | - | 179 | 22.9 | 1.00 | - |
| African American | 36 | 25.0 | 0.14 | (0.05–0.37) | 125 | 24.8 | 1.11 | (0.65–1.90) |
| Smoking -adjusted | 0.13 | (0.05–0.38) | Smoking -adjusted | 1.12 | (0.63–1.99) | |||
| Alcohol-adjusted | 0.16 | (0.06–0.46) | Alcohol-adjusted | 1.13 | (0.64–2.01) | |||
Oro-pharynx was defined by ICD-O 4 digit topology codes; C019–C020 (base and dorsal surface of tongue), C051 (soft palate)-C052
Smoking or alcohol adjusted ORs exclude subjects with current smoking or alcohol use unknown and include marital status at diagnosis as a covariate
When HPV types were compared between AA and other racial groups, there were no differences in distributions with the predominant genotype of HPV16 or its combinations (82.5 % in AA and 86.3% in others) (Table 3).
Table 3.
Distribution of HPV types in African Americans and other racial groups
| HPV types | Other races N (%) | African Americans N (%) |
|---|---|---|
| 6 | 2 (2.74) | 0 |
| 6, 33 | 0 | 1 (2.50) |
| 11 | 0 | 2 (5.00) |
| 16 | 60 (82.19) | 30 (75.00) |
| 16,18 | 1 (1.37) | 2 (5.00) |
| 16,31 | 1 (1.37) | 0 |
| 16,52 | 1 (1.37) | 1 (2.50) |
| 18 | 2 (2.74) | 0 |
| 31 | 1 (1.37) | 0 |
| 33 | 2 (2.74) | 0 |
| 35 | 0 | 1 (2.50) |
| 45 | 0 | 1 (2.50) |
| 51 | 0 | 1 (2.50) |
| 56 | 1 (1.37) | 1 (2.50) |
| X | 2 (2.74) | 0 |
| Total | 73 | 40 |
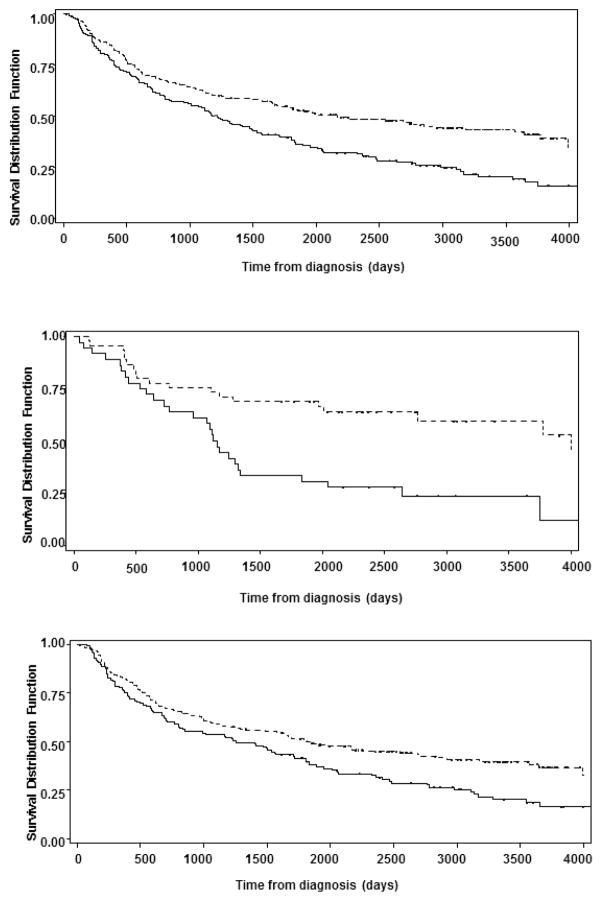
The results of Kaplan-Meier analysis indicate that overall survival from all HNSCC was statistically significantly worse in African American than the others (P=0.0002) (Fig 1a). For oropharyngeal cancer, there was a pronounced racial difference by race, with the median survival of 3.11 years in [AA] and 10.94 years in the other racial group (P=0.0012) (Fig 1b) whereas the corresponding difference was relatively smaller for non-oropharyngeal HNSSSC with the median survival of 3.44 years in African Americans and 5.06 years in the others (P=0.0068) (Fig 1c).
Figure 1.
Figure 1a (top): Kaplan-Meyer Curves for overall survival between African Americans and Non-African Americans. The horizontal axis represents the time of diagnosis in number days and the vertical axis represents the survival distribution in percentage. Broken Line: Non-African Americans, Solid Line: African American
Figure 1b (middle): Kaplan-Meyer Curves for overall survival in patients with oropharyngeal cancers in African Americans and Non-African Americans. The horizontal axis represents the time of diagnosis in number days and the vertical axis represents the survival distribution in percentage. Broken Line: Non-African Americans, Solid Line: African American
Figure 1c (bottom): Kaplan-Meyer Curves for overall survival for non-oropharyngeal cancers. The horizontal axis represents the time of diagnosis in number days and the vertical axis represents the survival distribution in percentage. Broken Line: Non-African Americans, Solid Line: African American
Univariable HR for death based on Cox’s proportional model was 1.61 (95% CI 1.25–2.06) for AA compared with other races all sites combined. The corresponding HR for AA was highly statistically significant in oropharyngeal site (HR=2.55, 95% CI 1.42–4.59), whereas it was 1.46 (95% CI 1.11–1.93) for non-oropharyngeal site. Adjustment for a basic covariate weakened these HRs, with stronger effect on non-oropharyngeal site. Additional adjustment for smoking had little effect on these estimates, while additional adjustment for HPV status drastically reduced the HR for AA in oropharyngeal site (HR=1.55, 95% CI: 0.79–3.06). After adjusting for both factors, HRs associated with AA race in both anatomic sites became close to each other, but it was only statistically significant for non-oropharyngeal site (HR=1.56, 95% CI: 1.17–2.06) (Table 4).
Table 4.
Mutivariable hazard ratios (HR) and 95% confidence intervals (CI) for deaths associated with African American race by Cox’s proportional Hazard models
| Hazard ratios ** (95% CI) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cancer type | Racial groups | Alive/dead | Univariable | Basic covariate adjusted | & Smoking adjusted | & HPV adjusted | & Smoking-HPV-adjusted | |||||
| All | Other | 97/127 | 1.00 | - | 1.00 | - | 1.00 | - | 1.00 | - | 1.00 | - |
| African | 37/124 | 1.61 | (1.25–2.06) | 1.48 | (1.15–1.91) | 1.47 | (1.14–1.89) | 1.45 | (1.13–1.87) | 1.61 | (1.25–2.07) | |
| Oropharyngeal | Other | 25/20 | 1.00 | - | 1.00 | - | 1.00 | - | 1.00 | - | 1.00 | - |
| African | 8/28 | 2.55 | (1.42–4.59) | 2.51 | (1.38–4.56) | 2.22 | (1.21–4.08) | 1.55 | (0.79–3.06) | 1.49 | (0.75–2.93) | |
| Other | Other | 72/107 | 1.00 | - | 1.00 | - | 1.00 | - | 1.00 | - | 1.00 | - |
| African | 29/96 | 1.46 | (1.11–1.93) | 1.32 | (0.99–1.75) | 1.33 | (1.00–1.77) | 1.33 | (1.00–1.76) | 1.56 | (1.17–2.06) | |
Oro-pharynx was defined by ICD-O 4 digit topology codes; C019–C020 (base and dorsal surface of tongue), C051 (soft palate)-C052
Basic-covariate adjusted HRs include marital status at diagnosis and additional smoking adjusted HR include an indicator variable for those with missing information for current smoking.
Discussion
Our study corroborates what other studies have shown; that African Americans have a decreased overall survival when compared to non-AA [13,15,29,30]. In fact, just as shown in our study, others have found that racial disparity in overall survival is larger for oropharyngeal site than the other HNSSSC sites [30]. Recent data from Surveillance Epidemiology, End Results (SEER) program also show that white-black differences in 5 year-relative survival were more pronounced for oropharyngeal site than the other head and neck site.29 Interestingly, a report from a major US cancer center indicates that this racial difference in oropharyngeal cancer survival is apparent for recently diagnosed cases only (1995 or later) [1], which coincides with the recent increase in HPV-related HNSCC [5].
Racial differences in survival may arise from differential distributions of traditional prognostic factors. SEER data as well as data from individual academic medical centers have described that AA are more likely diagnosed with advanced disease than Caucasians [14,15,29]. On the contrary, stage distribution was very similar between AA and Caucasians in our study population. This was probably due to one of our eligibility criteria-limiting patients to those who underwent surgery without any neo-adjuvant therapies, resulting in a rather homogenous patient population. We also found no difference in the distribution of HNSSSC by age group and gender by racial group. Instead, we found a significant difference in marital status between AA and Caucasians, whereas we reported previously that marital status was also associated with HPV status [6]. Although it is under-appreciated, not being married or living alone has been associated with poorer prognosis of cancer across various cancer sites, including HNSCC [19,31 ]. Underuse of optimal treatment by those socially deprived patients may account for a part of the observed differences [19,20,21].
The data concerning racial differences in smoking and alcohol use in HNSCC patients have been very mixed in earlier studies. Two studies, where information was obtained by in person interview [26] or presumably from medical records [14] reported that AA patients have higher prevalence of both smoking and alcohol use. Another medical record based study reported that alcohol use was more frequent in AA than Caucasians while both had similar history of tobacco use [18]. On the other hand, studies where medical record [15] or a self-administered questionnaire [32] was source of information, did not find a difference in either. The results of our study with a modest difference in alcohol only were close to the last three studies. It is also noteworthy that the data from US national surveys indicate equivalent or lower prevalence of cigarette and alcohol use in AA compared with Caucasians [3, 25].
The present investigation also confirmed that almost all (>90%) of our HNSCC patients were in fact smokers. This implies that our previous report using smoking-related comorbidities as a surrogate marker [6] substantially underestimated the effects of smoking.
HNSCC now represents the most common diagnosed HPV-associated cancer in the US surpassing cervical cancer [9] and thus there is public health interest in expanding the indications of HPV prophylactic vaccines to these cancers. However, the data concerning HPV-positivity in HNSCC by racial group are still limited. Most earlier studies include only a small number of AA patients, ranging from 5 to 67 [8,11,12,14] often not yielding a statistically significant difference. Our study convincingly demonstrated that tumors from oropharyngeal site of AA were indeed less often positive to HPV and that that was not accompanied by substantial differences of smoking or alcohol use. This indicates that other behavioral factors in acquiring oral HPV infection or biological differences in the risk of HPV progression to cancer may account for the racial difference [8,12].
Several surveys conducted in the US have shown that whites more often practice oral sex than blacks [22,23], but a recent National Health and Nutrition and Examination Survey revealed that HPV DNA was more often found in oral exfoliated cells from AA than in those from Caucasians [7]. There are other unanswered questions, e.g., whether other behavioral factors, such as certain hygiene practices or eating habits, influence the likelihood of acquisition or persistence of HPV infection in the oropharynx and whether there are racial differences in such behaviors if any. Second, HPV-associated HNSCC preferentially develops from oropharyngeal site, which houses substantial lymphoid tissues, e.g., tonsils and adenoid. Although AA and Caucasians have anthropometrically different facial and cranial structures [33,34], there is sparse information as to racial difference in inner mouth structures. Moreover, AA and low socio-economic status (SES) children have been reported to be more frequently diagnosed with sleep–disordered breathing caused by enlarged tonsils and adenoids, which often lead to surgical removal of those organs [35]. Such procedures may affect the risk of HPV-associated oropharyngeal cancer. Even though they are not surgically removed, chronic inflamed or hypertrophic tonsils and adenoids may lead to sustained secretion of inflammatory mediators from their lymphoid follicles, which process antiviral properties, such as interferon [36]. Finally, there is a possibility that expression of key host molecules necessary for HPV entry to epithelial cells [37,38] is genetically controlled and possibly, racially different.
Strengths of the present study include the inclusion of a relatively large number of AA patients compared with other studies, use of a highly sensitive broad spectrum HPV detection method, rather uniform patient care by a single medical center and long follow-up time by cancer registry. However, the limitations of our study lie in the retrospective study design based on HNSSSC patients who had surgical resection of the tumor as first therapy at one of three Wayne State University affiliated hospitals [6]. A recent study from a different hospital group in the Metropolitan Detroit supported our observations concerning racial difference in HPV prevalence and overall survival, but suggested that disparity may remain in HPV-negative oropharyngeal cancer [39]. Information in our medical records was not sufficient for in-depth analysis of smoking alcohol, e.g., pack-years of smoking, amount/frequency of alcohol drinking and duration of abstinence. Also other important information to shed light on potential causes of racial differences, such as sexual behaviors and history of tonsillectomy, was not available from medical charts. In addition, cancer registry information was not complete for systemic therapies, which may have given in independent doctors’ offices or other institutions. Lastly, when stratified by anatomic site and race, our sample size was not necessarily sufficient to detect a modest size of survival differences.
Conclusions
The present study clearly demonstrated that poorer survival from oropharyngeal cancer in AA was largely attributable to a lower fraction of HPV-positive cancer. However, after adjustment for HPV and smoking, some residual disadvantage in HNSSSC survival was still present in AA, compared with other racial groups. Altogether, including non-oropharyngeal sites, AA experienced poorer survival than others. These residual racial differences may be attributable to higher prevalence of uncontrolled comorbidities [40] and lower use of/and adherence to adjuvant treatments [20,21] due to their socially deprived status or lower health literacy. These factors need to be addressed in order to improve outcomes of HNSCC in this minority population.
Acknowledgments
This work was supported in part by the National Cancer Institute (Contract number N01-PC-35145 to IK) and a grant from the Institut National du Cancer (INCa) (SPLIT project grant N°2011/196 to SF).
We thank Jean-Paul Brunsveld and Jacqueline van Harmelen from DDL for technical assistance in this study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Dahlstrom KR, Calzada G, Hanby JD, et al. An evolution in demographics, treatment, and outcomes of oropharyngeal cancer at a major cancer center: 2 staging system in need of repair. Cancer. 2013;119:81–89. doi: 10.1002/cncr.27727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cancer.Net. http://www.cancer.net/cancer-types/head-and-neck-cancer/statistics.
- 3.Centers for Disease Control and Prevention (CDC) Current cigarette smoking among adults - United States, 2011. MMWR Morb Mortal Wkly Rep. 2012;61:889–894. [PubMed] [Google Scholar]
- 4.Brown LM, Check DP, Devesa SS. Oropharyngeal cancer incidence trends: diminishing racial disparities. Cancer Causes Control. 2011;22:753–763. doi: 10.1007/s10552-011-9748-1. [DOI] [PubMed] [Google Scholar]
- 5.Chaturvedi AK, Engels EA, Anderson WF, et al. Incidence trends for human papillomavirus-related and -unrelated oral squamous cell carcinomas in the United States. J Clin Oncol. 2008;26:612–619. doi: 10.1200/JCO.2007.14.1713. [DOI] [PubMed] [Google Scholar]
- 6.Sethi S, Ali-Fehmi R, Franceschi S, et al. Characteristics and survival of head and neck cancer by HPV status: a cancer registry-based study. Int J Cancer. 2012;131:1179–1186. doi: 10.1002/ijc.26500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gillison ML, Broutian T, Pickard RK, et al. Prevalence of oral HPV infection in the United States, 2009–2010. JAMA. 2012;307:693–703. doi: 10.1001/jama.2012.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gillison ML, D’Souza G, Westra W, et al. Distinct risk factor profiles for human papillomavirus type 16-positive and human papillomavirus type 16-negative head and neck cancers. J Natl Cancer Inst. 2008;100:407–420. doi: 10.1093/jnci/djn025. [DOI] [PubMed] [Google Scholar]
- 9.Jemal A, Simard EP, Dorell C, et al. Annual Report to the Nation on the Status of Cancer, 1975–2009, Featuring the Burden and Trends in Human Papillomavirus (HPV)-Associated Cancers and HPV Vaccination Coverage Levels. J Natl Cancer Inst. 2013;105:175–201. doi: 10.1093/jnci/djs491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ryerson AB, Peters ES, Coughlin SS, et al. Burden of potentially human papillomavirus-associated cancers of the oropharynx and oral cavity in the US, 1998–2003. Cancer. 2008;113:2901–2909. doi: 10.1002/cncr.23745. [DOI] [PubMed] [Google Scholar]
- 11.Fakhry C, Westra WH, Li S, et al. Improved survival of patients with human papillomavirus-positive head and neck squamous cell carcinoma in a prospective clinical trial. J Natl Cancer Inst. 2008;100:261–269. doi: 10.1093/jnci/djn011. [DOI] [PubMed] [Google Scholar]
- 12.Gillison ML, Koch WM, Capone RB, et al. Evidence for a causal association between human papillomavirus and a subset of head and neck cancers. J Natl Cancer Inst. 2000;92:709–720. doi: 10.1093/jnci/92.9.709. [DOI] [PubMed] [Google Scholar]
- 13.Sedaghat AR, Zhang Z, Begum S, et al. Prognostic significance of human papillomavirus in oropharyngeal squamous cell carcinomas. Laryngoscope. 2009;119:1542–1549. doi: 10.1002/lary.20533. [DOI] [PubMed] [Google Scholar]
- 14.Settle K, Taylor R, Wolf J, et al. Race impacts outcome in stage III/IV squamous cell carcinomas of the head and neck after concurrent chemoradiation therapy. Cancer. 2009;115:1744–1752. doi: 10.1002/cncr.24168. [DOI] [PubMed] [Google Scholar]
- 15.Ragin CC, Langevin SM, Marzouk M, et al. Determinants of head and neck cancer survival by race. Head Neck. 2011;33:1092–1098. doi: 10.1002/hed.21584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang XI, Thomas J, Zhang S. Changing trends in human papillomavirus-associated head and neck squamous cell carcinoma. Ann Diagn Pathol. 2012;16:7–12. doi: 10.1016/j.anndiagpath.2011.07.003. [DOI] [PubMed] [Google Scholar]
- 17.United States Census. 2010 http://www.census.gov/2010census/
- 18.Gourin CG, Podolsky RH. Racial disparities in patients with head and neck squamous cell carcinoma. Laryngoscope. 2006;116:1093–1106. doi: 10.1097/01.mlg.0000224939.61503.83. [DOI] [PubMed] [Google Scholar]
- 19.Cavalli-Björkman N, Qvortrup C, Sebjørnsen S, et al. Lower treatment intensity and poorer survival in metastatic colorectal cancer patients who live alone. Br J Cancer. 2012;107:189–194. doi: 10.1038/bjc.2012.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hu CY, Delclos GL, Chan W, et al. Assessing the initiation and completion of adjuvant chemotherapy in a large nationwide and population-based cohort of elderly patients with stage-III colon cancer. Med Oncol. 2011;28:1062–1074. doi: 10.1007/s12032-010-9644-7. [DOI] [PubMed] [Google Scholar]
- 21.Ayanian JZ, Zaslavsky AM, Fuchs CS, et al. Use of adjuvant chemotherapy and radiation therapy for colorectal cancer in a population-based cohort. J Clin Oncol. 2003;21:1293–1300. doi: 10.1200/JCO.2003.06.178. [DOI] [PubMed] [Google Scholar]
- 22.Auslander BA, Biro FM, Succop PA, et al. Racial/ethnic differences in patterns of sexual behavior and STI risk among sexually experienced adolescent girls. J Pediatr Adolesc Gynecol. 2009;22:33–39. doi: 10.1016/j.jpag.2008.01.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Johnson EH, Jackson LA, Hinkle Y, et al. What is the significance of black-white differences in risky sexual behavior? J Natl Med Assoc. 1994;86:745–759. [PMC free article] [PubMed] [Google Scholar]
- 24.Smith SC, Jr, Clark LT, Cooper RS, et al. American Heart Association Obesity, Metabolic Syndrome, and Hypertension Writing Group. Discovering the full spectrum of cardiovascular disease: Minority Health Summit 2003: report of the Obesity, Metabolic Syndrome, and Hypertension Writing Group. Circulation. 2005;111:e134–e139. doi: 10.1161/01.CIR.0000157743.54710.04. [DOI] [PubMed] [Google Scholar]
- 25.Stahre M, Naimi T, Brewer R, et al. Measuring average alcohol consumption: the impact of including binge drinks in quantity-frequency calculations. Addiction. 2006;101:1711–1718. doi: 10.1111/j.1360-0443.2006.01615.x. [DOI] [PubMed] [Google Scholar]
- 26.Stingone JA, Funkhouser WK, Weissler MC, et al. Racial differences in the relationship between tobacco, alcohol, and squamous cell carcinoma of the head and neck. Cancer Causes Control. 2013;24:649–664. doi: 10.1007/s10552-012-9999-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kleter B, van Doorn LJ, ter Schegget J, et al. Novel short-fragment PCR assay for highly sensitive broad-spectrum detection of anogenital human papillomaviruses. Am J Pathol. 1998;153:1731–1739. doi: 10.1016/S0002-9440(10)65688-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kleter B, van Doorn LJ, Schrauwen L, et al. Development and clinical evaluation of a highly sensitive PCR-reverse hybridization line probe assay for detection and identification of anogenital human papillomavirus. J Clin Microbiol. 1999;37:2508–2517. doi: 10.1128/jcm.37.8.2508-2517.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Howlader N, Noone AM, Krapcho M, et al. SEER Cancer Statistics Review, 1975–2009 (Vintage 2009 Populations) National Cancer Institute; Bethesda, MD: Apr, 2012. http://seer.cancer.gov/csr/1975_2009_pops09/, based on November 2011 SEER data submission, posted to the SEER web site. [Google Scholar]
- 30.Settle K, Posner MR, Schumaker LM, et al. Racial survival disparity in head and neck cancer results from low prevalence of human papillomavirus infection in black oropharyngeal cancer patients. Cancer Prev Res. 2009;2:776–781. doi: 10.1158/1940-6207.CAPR-09-0149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pinquart M, Duberstein PR. Associations of social networks with cancer mortality: a meta-analysis. Crit Rev Oncol Hematol. 2010;75:122–137. doi: 10.1016/j.critrevonc.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen LM, Li G, Reitzel LR, et al. Matched-pair analysis of race or ethnicity in outcomes of head and neck cancer patients receiving similar multidisciplinary care. Cancer Prev Res. 2009;2:782–791. doi: 10.1158/1940-6207.CAPR-09-0154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Porter JP. The average African American male face: an anthropometric analysis. Arch Facial Plast Surg. 2004;6:78–81. doi: 10.1001/archfaci.6.2.78. [DOI] [PubMed] [Google Scholar]
- 34.Faustini MM, Hale C, Cisneros GJ. Mesh diagram analysis: developing a norm for African Americans. Angle Orthod. 1997;67:121–128. doi: 10.1043/0003-3219(1997)067<0121:MDADAN>2.3.CO;2. [DOI] [PubMed] [Google Scholar]
- 35.Boss EF, Smith DF, Ishman SL. Racial/ethnic and socioeconomic disparities in the diagnosis and treatment of sleep-disordered breathing in children. Int J Pediatr Otorhinolaryngol. 2011;75:299–307. doi: 10.1016/j.ijporl.2010.11.006. [DOI] [PubMed] [Google Scholar]
- 36.Richtsmeier WJ. Human interferon production in tonsil and adenoid tissue cultures. Am J Otolaryngol. 1983;4:325–333. doi: 10.1016/s0196-0709(83)80019-2. [DOI] [PubMed] [Google Scholar]
- 37.Horvath CA, Boulet GA, Renoux VM, et al. Mechanisms of cell entry by human papillomaviruses: an overview. Virol J. 2010;7:11. doi: 10.1186/1743-422X-7-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Letian T, Tianyu Z. Cellular receptor binding and entry of human papillomavirus. Virol J. 2010;7:2. doi: 10.1186/1743-422X-7-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Worsham MJ, Stephen JK, Chen KM, et al. Improved survival with HPV among African Americans with oropharyngeal cancer. Clin Cancer Res. 2013;19:2486–2492. doi: 10.1158/1078-0432.CCR-12-3003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rose BS, Jeong JH, Nath SK, et al. Population-based study of competing mortality in head and neck cancer. J Clin Oncol. 2011;29:3503–3509. doi: 10.1200/JCO.2011.35.7301. [DOI] [PubMed] [Google Scholar]