Abstract
Embryonic and induced pluripotent stem cells have the ability to differentiate into any somatic cell type, and thus have potential to treat a number of diseases that are currently incurable. Application of these cells for clinical or industrial uses would require an increase in production to yield adequate numbers of viable cells. However, the relatively high costs of cytokines and growth factors required for maintenance of stem cells in the undifferentiated state have the potential to limit translational research. Leukemia inhibitory factor (LIF), a member of the IL-6 cytokine family, is a key regulator in the maintenance of naïve states for both human and mouse stem cells. In this study, we describe a new recombinant human LIF (rhLIF) using a plant-based (rice) expression system. We found that rice-derived rhLIF possessed the same specific activity as commercial E. coli-derived LIF and was capable of supporting mouse embryonic stem cell proliferation in the undifferentiated state as evidenced from pluripotency marker level analysis. Retention of the pluripotent state was found to be indistinguishable between rice-derived rhLIF and other recombinant LIF proteins currently on the market.
Keywords: LIF, embryonic stem cell, recombinant protein expression, glutelin 1, nopaline synthase, growth rate, alkaline phosphatase, Oct4, Nanog, SSEA-1
1. Introduction
Because they grow and differentiate into many cell types, stem cells have the potential to remedy diseases that arise from cell death or dysfunction of specific cell populations. Thus, intense research efforts have focused on the application of stem cells for the treatment of diabetes, heart failure, and neurodegenerative disorders [1, 2]. Embryonic stem cells (ESCs) derived from the inner cell mass of pre-implantation embryos [3] can be cultured and expanded in a pluripotent state in the presence of specific growth factors. Leukemia inhibitory factor (LIF), a member of the IL-6 cytokine family, is a key regulator in the maintenance of stem cell pluripotency [4]. LIF activates gp130/STAT3-dependent signaling, leading to increased transcription of stem cell state regulators while simultaneously inhibiting differentiation [4, 5]. Typically, LIF is supplied to stem cell culture by co-culture with a feeder layer of mitotically-inactivated mouse embryonic fibroblasts (MEFs). However, problems arise with isolating stem cells due to inefficient separation of the two cell types, and variations in stem cell phenotype have been noted due to the amount of secreted LIF based on the preparation and condition of the MEFs, decreasing reproducibility in co-culture systems.
Therefore, mouse and human ESCs, and induced pluripotent stem cells (iPSCs) require addition of exogenous LIF to maintain pluripotency, often from a recombinant source [6, 7]. Currently marketed LIF proteins are estimated to be a large part of the cost for mouse ESC culture [8]. Recently, plant hosts have emerged as powerful systems to express recombinant mammalian proteins in an efficient manner on a large scale [9, 10]. We demonstrate that rice-derive recombinant human LIF (rhLIF) displays similar biochemical characteristics as commercially available rhLIF derived from E. coli. Further, we show that the rice-derived rhLIF is capable of supporting mouse ESC (mESC) proliferation and pluripotency with equal effectiveness as E. coli-derived LIF, demonstrating that rice-derived rhLIF has significantly lower endotoxin levels and is an attractive alternative to LIF proteins currently on the market.
2. Materials and methods
2.1. Rice transformation and rhLIF expression
Microprojectile bombardment-mediated transformation of embryonic calli induced from the mature seeds of rice cultivar Bengal (Oryza sativa, subsp. Japonica) was performed as described [9]. The regenerated transgenic plants were designated as R0 transgenic events, and their progeny in successive generations were designated as R1, R2, etc. Eight R1 seeds from each transgenic event were randomly picked, and placed into eight wells in one column of a 96 deep-well plate. Five hundred microliters of PBS (pH 7.4) were dispensed into wells containing two 10 mm diameter steel beads. Seed proteins were extracted by agitating the plate with a Geno/Grinder 2000 (SPEX CertiPrep, Metuchen, NJ) for 20 min at 1300 strokes/min, followed by centrifugation with a microplate centrifuge at 4,000 rpm for 20 min. The supernatants of protein extracts from eight of the same transgenic events were pooled, and three microliter of pooled protein extracts were spotted on a nitrocellulose membrane. The dot blot was probed with mouse anti-human LIF primary antibody (Cat. No. ab34427, Abcam, Cambridge, MA) as described [9].
2.2. Purification of rhLIF
Procedures were performed at room temperature (22°C) and chemicals were from Sigma (St. Louis, MO) or Amresco (Solon, OH) unless otherwise stated.
Buffers
Buffer A: 58 mM acetic acid (pH 3.0). Buffer B: 25 mM Tris-Cl, 500 mM NaCl, pH 7.4. Buffer C: 25 mM Tris-Cl, 500 mM NaCl, 500 mM α-D-Methyl Mannopyranoside, pH 7.4. Buffer D: 25 mM Sodium Phosphate, 150 mM NaCl, 0.02% Tween 20, pH 7.4, prepared using endotoxin-free water (G-Biosciences, St. Louis, MO).
Extraction
Total rice protein was extracted from de-husked brown rice grain flour expressing rhLIF (600 g) in 3.0 L of buffer A by stirring on a magnetic stirrer for 1 hour. Flour debris were removed by the addition of 40 g of Diatomite filtration aid (CelPure C300, Advanced Minerals Co., Goleta, CA) and filtration through a Whatman #5 filter disc (100 mm) in a Buchner funnel (fraction I).
Ammonium sulfate precipitation
Fraction I was chilled to 4°C and precipitated with 1.09 kg of solid ammonium sulfate (65% saturation, 0.398 g/mL) and centrifuged at 22,000 × g (Beckman JA-18) at 4°C for 20 minutes. Pellets were discarded and ammonium sulfate concentration in the supernatant was increased to 80% saturation by addition of 0.097 g ammonium sulfate per mL of supernatant (315 g total). This solution was centrifuged as above and the pellets were dissolved in buffer B (90 mL), and then subjected to diafiltration through a 5 kDa molecular weight cutoff tangential flow filtration unit (Pellicon XL, Millipore) to produce fraction II.
Concanavalin A Chromatography
Fraction II was loaded onto a column containing 100 mL of Concanavalin A Sepharose (GE Healthcare, 3.5×15 cm) equilibrated with Buffer B. The column was washed with 3-column volumes of buffer B and eluted with 3-column volumes of buffer C at a flow rate of 120 ml/h; 7-ml fractions were collected. rhLIF eluted as the only peak and was pooled at ¾ peak height. The pool was dialyzed into buffer D using a 10 kDa molecular weight cutoff dialysis cassette (Thermo Fisher Scientific, Rockford, IL), distributed in aliquots, and frozen in liquid nitrogen for storage at −80°C (fraction III). Bacterial endotoxin contamination of this material was determined using a commercial kit (Pyrogene Endotoxin Detection Assay, Lonza, Walkersville, MD).
2.3. M1 growth inhibition assay
LIF potency was assessed by M1 leukemia cell differentiation [11]. Briefly, 2-fold dilutions of rice-derived rhLIF or E. coli-derived rhLIF (LIF1010, Millipore, Billerica, MA) were tested for M1 differentiation as evidenced by inhibition of growth. Log-phase M1 cells (ATCC #TIB-192) were seeded at a cell density of 7.0×104 in 1 ml of growth media and incubated at 37°C/6% CO2 for 120 hrs. An EC50, (the concentration of LIF to inhibit growth of 50% of M1 cells) was derived using a 4-parameter model to determine units per milligram active LIF (50 units was defined as 50% maximal response) [11].
2.4. Mouse ESC culture
Mouse C57BL/6N Lex3.13 ESCs were maintained on 0.1% gelatin-coated 6-well plates without feeder cells in Embryo Max DMEM (Millipore) supplemented with 15% ESC-qualified fetal bovine serum (Hyclone/Thermo), 2 mM L-glutamine (Gibco), 0.1 mM β-mercaptoethanol (Sigma), 0.1 mM MEM non-essential amino acid stock (Gibco), and 1000U mouse leukemia inhibitory factor (LIF, Millipore, ESG1106). ESCs were grown at 37°C in a humidified incubator in 5% CO2 and passaged every two days (~70–80% confluency).
2.5. LIF Comparison
Mouse ESCs were passaged as normal, then resuspended in media supplemented with either 1000U/mL E. coli-derived recombinant mouse LIF (Millipore, catalog no. ESG1106), 10 ng/mL E. coli-derived recombinant human LIF (Millipore, LIF1010), or 10 ng/mL rice-derived rhLIF (InVitria, 777LIF048). Cells were grown for 2, 4, 10, or 12 passages as indicated.
2.6. Quantitative RT-PCR
RNA was extracted and genomic DNA eliminated from mESCs using the Qiagen RNAeasy Kit. Complementary DNA was prepared from mouse ESC RNA by reverse transcription with Super Script II RT (Invitrogen) as recommended by the manufacturer. 20 ng of cDNA was amplified in triplicates using 2× SYBR Green Master Mix (Invitrogen). PCR was 95°C for 10 minutes, 40 cycles of 95°C for 15 seconds followed by 55°C for 1 minute.
3. Results and discussion
3.1. Genetic transformation, expression analysis, and purification of rhLIF
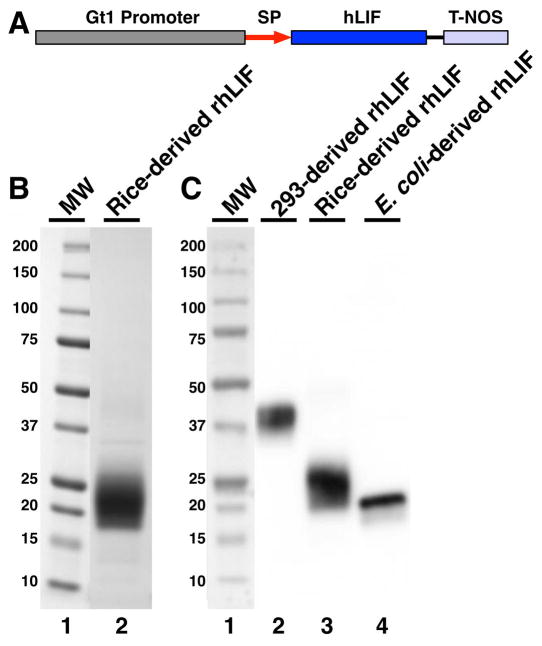
The expression cassette containing sequences encoding human LIF protein altered with codons biased towards favorable codon usage in the rice proteome is presented in Figure 1A. The rice cultivar Bengal (Oryza sativa L. subsp. Japonica) was transformed with the LIF expression cassette (Figure 1A) using microprojectile bombardment-mediated transformation [12]. To identify rhLIF expression, pooled seed protein from eight seeds of each fertile event were analyzed using immuno-dot blot with an anti-human LIF antibody (Supplementary Figure 1). Of 249 transgenic events with R1 seeds, 22 were identified as positive, and two (VB50-40 and VB50-199) with high-level expression of rhLIF were selected to proceed to the next generation.
Figure 1.
Rice-derived rhLIF is extensively glycosylated. (A) Diagram of the linear gene expression cassette for hLIF protein expression. Indicated are the rice seed storage protein glutelin 1 gene promoter (Gt1 promoter), the Gt1 signal peptide (SP), the codon-optimized human leukemia inhibitory factor (hLIF), and the nopaline synthase gene terminator of Agrobacterium tumefaciens (T-NOS). Mature LIF protein sequence containing 180 amino acids (SwissProt accession number P15018) was back-translated with the codons optimized towards the codon-usage preference of rice genes (http://www.kazusa.or.jp/codon) and synthesized by DNA 2.0 (Menlo Park, CA) and ligated into the expression vector pAPI405 containing rice seed storage protein glutelin 1 gene promoter (Gt1) including its signal peptide encoding sequence (GenBank accession no. Y00687) and the nopaline synthase (nos) gene terminator of Agrobacterium tumefaciens. Plasmid pAPI146 expressing the hygromycin-resistance gene under the control of a rice callus-specific beta-glucanase (Gns9) gene promoter was used as a selection marker [9]. (B) Five micrograms of purified rice-derived rhLIF were electrophoresed on 4–20% SDS-PAGE (Life Technologies, Carlsbad, CA), stained using colloidal Coomassie blue dye (SimplyBlue™ SafeStain, Life Technologies, Carlsbad, CA), and destained in water. Lane 1, molecular weight markers (BioRad laboratories, Hercules, CA); lane 2, purified rhLIF pool. Rice-derived rhLIF runs as a smear between 19 and 30 kDa. (C) Comparison of rhLIF expressed in human cells, rice, and E. coli. Proteins were electrophoresed as above, and blotted to nitrocellulose for Western blot detection with a mouse anti-LIF monoclonal antibody. Image detection was using a Protein Simple Fluorochem Q imager (Sunnyvale, CA). Lane 1, molecular weight markers (BioRad Laboratories, Hercules, CA, MW); lane 2, recombinant human LIF expressed in human embryonic kidney 293 cells (Symansis, Timaru, New Zealand); lane 3, rice-derived recombinant human LIF (InVitria); lane 4, E. coli-derived recombinant human LIF (Abcam, Cambridge, MA). 50 ng of each purified protein was loaded in lanes 2–4.
Purification of rhLIF by lectin affinity chromatography took advantage of its extensive glycosylation (Figure 1B, C and Supplementary Figure 2). Ammonium sulfate precipitation between 65–80% saturation afforded a 4.6-fold purification (assuming 100% recovery) and aided subsequent Concanavalin A-affinity chromatography by eliminating trace rice glycoproteins. The purified fraction III rhLIF (Figure 1B, lane 2) was determined to be >97% pure by densitometry. Bacterial endotoxin contamination was <0.002 endotoxin units/μg of purified rhLIF, which is less than those commonly found on the market (Table 1).
Table 1.
Endotoxin levels in LIF from different commercial sources*
| Vendor | Source | Endotoxin Specification (EU/μg) |
|---|---|---|
| Santa Cruz Biotechnology (catalog no. sc-4988) | E. coli | Not specified |
| Millipore (catalog no. LIF1010) | E. coli | < 1 |
| Life Technologies (catalog no. PHC9481) | E. coli | < 1 |
| ORF Genetics (catalog no. 01-A0880) | barley grain | < 0.05 |
| Symansis (catalog no. 3014C) | 293 cells | Not specified |
| Sigma-Aldrich (catalog no. L5283) | E. coli | < 1 |
| InVitria**(catalog no. 777LIF048) | rice grain | < 0.002 |
Endotoxin levels were cited from product certificates of analysis from each vendor indicated.
Endotoxin level was measured in this study using the Pyrogene Endotoxin Detection Assay from Lonza (Walkersville, MD).
3.2. Rice-derived rhLIF activates M1 cell differentiation and induces mESC proliferation
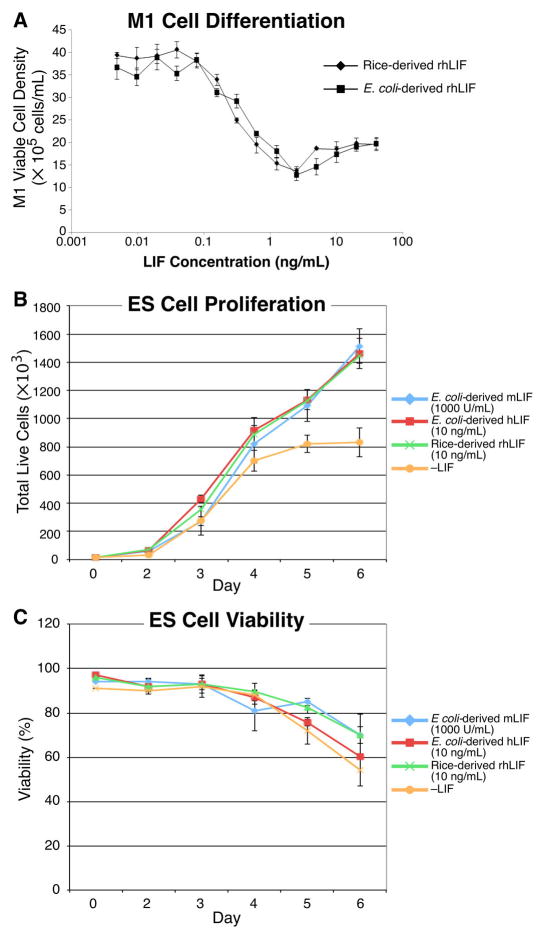
We compared the specific activity of rice-derived rhLIF to that produced in E. coli by a modified M1 cell differentiation assay that quantitates inhibition of cell growth during LIF-dependent differentiation of mouse myeloid leukemia M1 cells into a macrophage lineage [13]. The EC50 of rice-derived rhLIF exhibited a slightly higher potency than E. coli-derived rhLIF (Figure 2A). Rice-derived rhLIF was found to have a mean activity of 2.4 ± 0.26 × 108 units/mg LIF while the E. coli-derived rhLIF showed a specific activity of 1.54 ± 0.17 × 108 units/mg.
Figure 2.
Rice-derived rhLIF exhibits potent M1 differentiation activity and maintains high mESC proliferation rate. (A) Mouse myeloid leukemia M1 cells were obtained from ATCC (Catalog no. TIB-192, Manassas, VA) and were cultured in a fortified RPMI 1640 medium containing 10 mM HEPES (Life Technologies, Carlsbad, CA), 4.5 g/L glucose, 1 mM sodium pyruvate, 1.5× RPMI vitamins and amino acids (Sigma Aldrich, St. Louis, MO) and 10% FBS (Thermo Fisher, Waltham, MA). Log-phase mouse M1 cells were plated in the presence of 0.004 to 40 ng/mL of rice-derived or E. coli-derived recombinant human LIF as described in Methods. Both of the sourced recombinant human LIF proteins exhibited potent inhibition of M1 proliferation as indicated by the EC50 (EC50 0.21 ± 0.02 ng/mL versus 0.34 ± 0.12 ng/mL for the rice-derived rhLIF and E. coli-derived rhLIF, respectively, p = 0.063). The rice-derived rhLIF exhibited a statistically insignificant enhanced potency of M1 growth inhibition (P=0.06). Data shown is a representative experiment of three independent experiments. Error bars represent 1 standard deviation. (B and C) Embryonic stem cells were plated in triplicate at 10,000 cells/well in the presence of E. coli-derived recombinant mouse LIF, E. coli-derived recombinant human LIF, rice-derived recombinant human LIF, or without LIF (–LIF). Growth was monitored by daily cell count and viability measurements using a TC-10 automated cell counter (BioRad). (B) Cell proliferation of mESCs cultured in rice-derived rhLIF was indistinguishable from proliferation rates in cells cultured in E. coli-derived recombinant mouse and human LIF, with doubling times of approximately 11 hours. Cells cultured without LIF ceased proliferating at 5 days. (C) Viability of mESCs cultured in rice-derived rhLIF was indistinguishable from viability of cells cultured in E. coli-derived recombinant mouse and human LIF.
Growth rates and viability of mESCs were compared between cultures treated with rice-derived rhLIF or E. coli-derived mouse and human LIF for up to 6 days. Cultures grown in the presence of E. coli-derived mouse and human LIF and the rice-derived rhLIF grew at nearly identical rates for at least 6 days with doubling times of approximately 11 hours (Figure 2B). In addition, we observed no significant differences in viability using trypan blue exclusion of mESCs cultured in the different LIF proteins (Figure 2C). In the absence of LIF, cessation of growth was observed at day 5 (Figure 2B) and was accompanied by a reduced viability (Figure 2C) and change in cellular morphology (Figure 3D). These data indicate that the rice-derived rhLIF maintains growth of mESCs to the same extent as currently marketed mouse or human LIF proteins.
Figure 3.
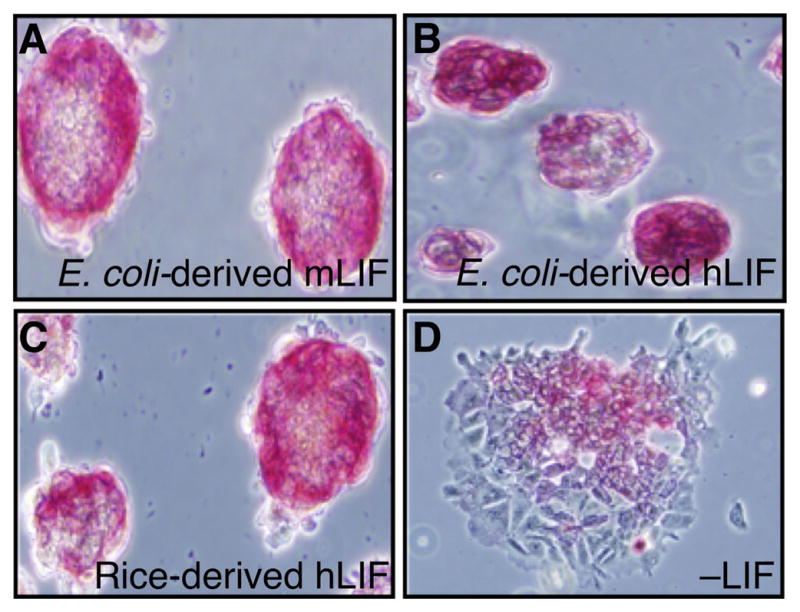
mESCs cultured in rice-derived rhLIF express alkaline phosphatase. mESCs cultured for 12 passages in rice-derived rhLIF or E. coli-derived recombinant mouse and human LIF were stained with alkaline phosphatase (Stemgent) following manufactures instructions and immediately photographed. Cells were grown in E. coli-derived recombinant mouse LIF (A), E. coli-derived recombinant human LIF (B), rice-derived recombinant human LIF (C), or without LIF (D).
3.3. ESCs grown in the presence of rice-derived rhLIF retain markers of pluripotency
ESCs grow in characteristic tight clusters while exhibiting alkaline phosphatase staining [14, 15]. We exploited this marker for comparing mESCs grown in E. coli-derived human or mouse recombinant LIF or rice-derived rhLIF. Alkaline phosphatase was easily detected in mESCs that were cultured in E coli-derived recombinant mouse LIF (Figure 3A), human LIF (Figure 3B), or rice-derived rhLIF (Figure 3C), and there were no apparent differences in staining patterns. However, alkaline phosphatase was far less detectable in mESCs grown without LIF (Figure 3D), indicating that these cells expressed less of this pluripotency marker.
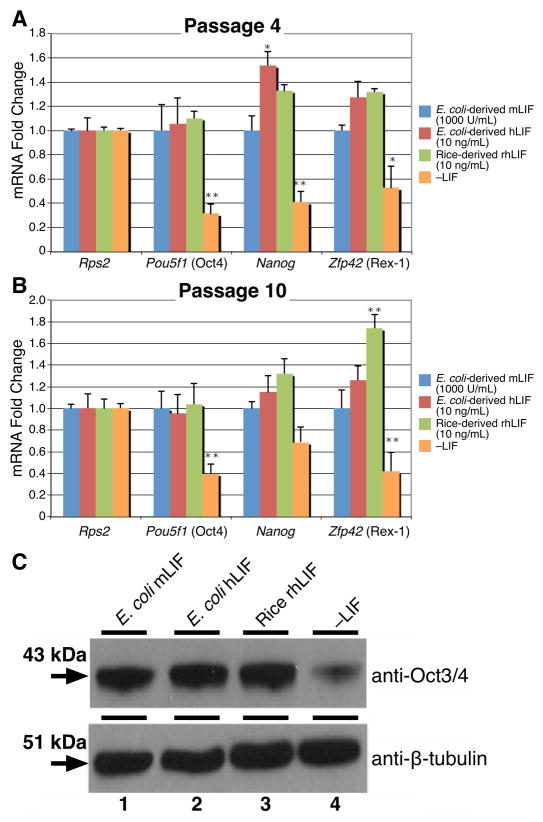
We further examined the retention of pluripotency by determining mRNA expression levels of the transcription factors Pou5f1 (Oct4), Nanog, and Zfp42 (Rex1). As expected, mESCs cultured in the presence of any of the LIF proteins showed significantly higher expression of the stem cell state regulators than the –LIF control at passages 4 and 10 (Figure 4A, B, Pou5f1, Nanog, Zfp42). The rice-derived rhLIF was statistically indistinguishable from the E. coli-derived available recombinant human LIF in the expression of mRNAs encoding the pluripotent state regulators, Pou5f1 and Nanog for both passages 4 and 10. Oct4 protein levels were comparable between the cells cultured in the LIF proteins while the –LIF control had significantly decreased expression (Figure 4C).
Figure 4.
mESCs cultured in rice-derived rhLIF retain high expression of pluripotency transcription factors. (A) and (B) mESCs cultured in rice-derived rhLIF or E. coli-derived recombinant mouse and human LIF were examined for the expression of stem cell state regulators (Rps2, Pou5f1, Nanog, and Zfp42) using qRT-PCR at passages 4 (A) and 10 (B). mESCs cultured in LIF showed significantly higher expression of Pou5f1, Nanog, and Zfp42 than cells cultured without LIF. A single asterisk (*) indicates P<0.05; double asterisk (**) P<0.01. Statistics were calculated using One-way ANOVA followed by Dunnett’s test. Primers were Pou5f1: 5′-GCCGTCTTTCCACCAGGCCC-3′, 5′-TCGAGGATCCACCCAGCCCG-3′; Nanog: 5′-CCTGGTCCCCACAGTTTGCCT-3′, 5′-GCAGGTCTTCAGAGGAAGGGCG-3′; and Zfp42: 5′-GCCTCACTGTGCTGCCTCCAAG-3′, 5′-TTCCGCCCGGCCCTTTCTGG-3′. The ribosomal protein S2 (Rps2) served as a loading control and reference gene. Relative expression was calculated using the ΔΔCt method [16]. ΔΔCt was derived by normalizing the sample mean cycle threshold (Ct) with its respective control Rps2 Ct. C. Western blots with protein extracts (5 μg) lysed with RIPA buffer (50mM Tris-HCl, pH 8.8, 150mM NaCl, 0.1% SDS, 0.5% deoxycholate, 0.5% NP-40) were loaded onto 10% SDS-polyacrylamide gels from cells grown in E. coli-derived recombinant mouse LIF (lane 1), E. coli-derived recombinant human LIF (lane 2), rice-derived recombinant human LIF (lane 3), or without LIF (lane 4). SDS-PAGE gel was transferred to PVDF (Millipore) and probed using an anti-Oct3/4 monoclonal antibody (Santa Cruz sc-5279) (top). Western blot using an anti-β-tubulin antibody for loading comparison (bottom).
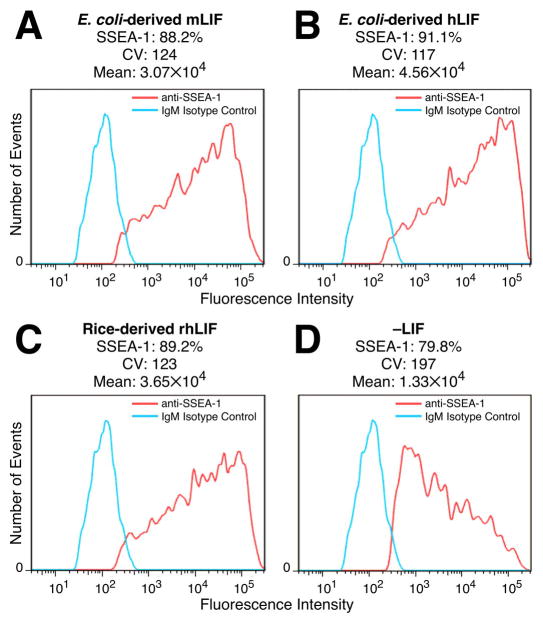
The maintenance of the pluripotent state of mESCs cultured in the presence of rice-derived LIF was further confirmed by expression of the SSEA-1 surface antigen [14] (Figure 5C). Withdrawal of LIF from the mESC cultures induced a significant decrease in detectable SSEA-1 (Figure 5D). Cells cultured in the presence of either the E. coli-derived LIF (Figure 5A, B) or the rice-derived rhLIF (Figure 5C) exhibited indistinguishable levels of SSEA-1. These results indicate that mESCs cultured with rice-derived rhLIF exhibit robust growth, acceptable viability, and maintain pluripotency markers.
Figure 5.
Rice-derived rhLIF maintains expression of the cell surface pluripotency marker SSEA-1 in mESCs. mESCs were cultured in E. coli-derived recombinant mouse LIF (A), E. coli-derived recombinant human LIF (B), rice-derived recombinant human LIF (C), or without LIF (D). Approximately 2×106 cells were resuspended in FACS buffer (1×PBS, 10% FBS, 0.1% NaN3) and incubated on ice with either anti-SSEA1 coupled to Alexa Fluor 647 (BD Biosciences #562277) or purified mouse IgM κ isotype control (Biolegend #4011618) for 45 minutes. Expression analysis was performed on a BD LSR II flow cytometer. The Y-axes indicate number of cell events (no scale) and the X-axes indicate fluorescence intensity on a logarithmic scale. Indicated above each panel is the percent of cells that were SSEA-1-positive, the coefficient of variation (CV), and the mean fluorescence intensity (Mean).
4. Conclusions
We have expressed rhLIF produced in rice grain and demonstrate an efficient purification scheme. Ride-derived rhLIF displays bioequivalency to E. coli-derived rhLIF products currently on the market for the culture of mouse stem cells. We believe that through the high product yields, exceptional protein purity, and extremely low endotoxin, this new rice-derived rhLIF protein could promote stem cell propagation for clinical applications. Rice-derived rhLIF is currently commercially available at http://www.InVitria.com.
Supplementary Material
Several rice lines express rhLIF.391 transgenic plants were generated through three batches of transformation, and 249 transgenic plants were fertile to set the seeds. To identify the transgenic events that resulted in rhLIF expression, pooled seed protein extracts of each fertile event were analyzed using immuno-dot blot with an anti-human LIF antibody (Figure 1B). Of 249 transgenic events with R1 seeds, 22 were identified as positive, and two (VB50-40 and VB50-199) with high-level expression of rhLIF were selected to proceed to the next generation. (A) Immuno-dot blot expression screening of transgenic rice R1 seeds expressing rhLIF. Rice seed total soluble proteins were extracted with 250 μl of PBS buffer, pH 7.4 per seed at room temperature for 1 h followed by centrifugation. Equal amounts of protein extracts from eight R1 seeds of the same transgenic event were pooled, and spotted onto a nitrocellulose membrane. Spots A1–E12 and F1 each contained 3 μl of pooled protein extracts from 61 transgenic rice R1 plants. The spot F2 contained 3 μl of non-transgenic Bengal seed protein extract. The spots F3–F9 represent protein extracts from individual R1 seeds of transgenic event VB50-40. Spots F10, F11, and F12 contained 30, 50, and 100 ng of E. coli-derived recombinant LIF, respectively. (B) Immuno-dot blot expression screening of individual transgenic rice R2 seeds to select homozygous lines expressing rhLIF. Rice seed total soluble proteins were extracted as above. Protein extracts (3 μl) from 12 individual R2 seeds of different transgenic R1 plants were spotted onto a nitrocellulose membrane in the same row. Rows A–H represent eight transgenic rice R1 lines. Lines in which all the progeny seeds show rhLIF expression (e. g., rows A, C, F, and G) are considered homozygous, while the lines in which some progeny seeds show no expression of rhLIF (e. g., B, D, E, and H) are considered heterozygous.
Rice-derived rhLIF is abundantly expressed in rice grains. The rhLIF expressed in rice grains was characterized by SDS-PAGE and Western blot analysis. Stained SDS–PAGE did not reveal distinct protein bands in transgenic seed extracts attributable to rhLIF expression (Supplementary Figure 2A.). However, Western blotting revealed strong immunoreactivity as a smear of bands from 20 to 50 kDa (Supplementary Figure 2B, bottom). The E. coli-derived non-glycosylated rhLIF showed a single immunoreactive band of about 19 kDa. The heterogeneity of rice seed-derived rhLIF was likely due to variable glycosylation of rhLIF. (A) SDS-PAGE stained with Coomassie blue. Total soluble proteins were extracted from rice seeds of transgenic plants and non-transgenic rice cultivar Bengal with Na-acetate, pH 5.0. Lane 1 contains 0.1 μg of E. coli-derived recombinant human LIF (Abcam, Cambridge, MA). Lanes 2–9 contained protein from the indicated rice strains extracted with either Na-acetate (50 mMNaAc, pH 5, lanes 2–5). Proteins were resolved on 4–20% Tris–glycine SDS–PAGE gradient gels (Invitrogen) without protein concentration, enrichment or purification and stained with Coomassie blue. (B) Western blot analysis of recombinant human LIF expressed in rice grains. An identical gel as (A) was transferred to nitrocellulose for Western blot detection by mouse monoclonal anti-hLIF antibody (50 ng/mL, Abcam, Cambridge, MA).
Highlights.
LIF comprises up to 90% of the cost of stem cell propagation
The high cost of LIF decreases the ability to experiment with stem cells
Rice-derived proteins are a less expensive, high purity source of useful proteins
Mouse embryonic stem cells grown in rice-derived recombinant human LIF are indistinguishable from those grown in other media
Acknowledgments
The authors would like to thank the team members at Ventria Bioscience Inc. and InVitria (including Diane Nguyen, Javier Herrera, Weiqiang Zhang, Nathan Fortner, Jordan Kraft, Michael Barnett and Cameron Austin) and at the Texas Tech University Health Sciences Center (Rafael Rosales and Atia Amatullah) for their contributions and excellent technical assistance to the project. We thank Daniel Webster for the gift of the anti-β-tubulin antibody. Work at TTUHSC was supported by a grant from the National Institutes of Health (HD037109) to CCM.
Footnotes
Author Contributions
CCM, RA, and NH directed the project and drafted the manuscript. BAY and RA designed and performed experiments and analyzed data. HGD developed the purification protocol and purified the protein. DZ expressed rhLIF in rice grain. SP, DZ, and HGD participated in the analysis of the rice-derived recombinant rhLIF. All authors commented on or contributed to the manuscript.
Competing Interests
BAY and CCM are not affiliated with InVitria or Ventria Bioscience Inc. and have no financial interest in this research. Other authors are employees of InVitria or Ventria Bioscience Inc. as indicated in the author addresses. The authors declare that they have no competing interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Santos FAP, Abescasis M, Gimble J, Chase L, Campbell A, Boucher S, Vemuri M, Silva C, Cabral J. Toward a clinical-grade expansion of mesenchymal stem cells from human sources: a microcarrier-based culture system under xeno-free conditions. Tissue Eng Part C Methods. 2011;17(12):1201–10. doi: 10.1089/ten.tec.2011.0255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Murry CKG. Differentiation of embryonic stem cells to clinically relevant populations: lessons from embryonic development. Cell. 2008;132(4):661–80. doi: 10.1016/j.cell.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 3.Friel RvdSS, Mee P. Embryonic stem cells: understanding their history, cell biology and signaling. Advanced Drug Delivery Reviews. 2005;57(13):1894–1903. doi: 10.1016/j.addr.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 4.Burdon TSA, Savatier P. Signaling, cell cycle and pluripotency in embyronic stem cells. Trends in Cell Biology. 2002;12(9):432–438. doi: 10.1016/s0962-8924(02)02352-8. [DOI] [PubMed] [Google Scholar]
- 5.Chambers ITS. The transcriptional foundation of pluripotency. Development. 2009;136(14):2311–22. doi: 10.1242/dev.024398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Williams RHD, Pease S, Wilson T, Stewart C, Gearing D, Gough N. Myeloid leukemia inhibitory factor maintains the developmental potential of embryonic stem cells. Nature. 1988;336(6200):684–7. doi: 10.1038/336684a0. [DOI] [PubMed] [Google Scholar]
- 7.Hirai H, FM, Kikyo N. Establishment of LIF-dependent human iPS cells closely related to basic FGF-dependent authentic iPS cells. PLoS ONE. 2012;7:6. doi: 10.1371/journal.pone.0039022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Feng S, et al. Establishment of an exogenous LIF-free culture system for mouse embryonic stem cells. Cloning Stem Cells. 2009;11(3):437–443. doi: 10.1089/clo.2009.0008. [DOI] [PubMed] [Google Scholar]
- 9.Zhang DNS, Bryan P, Pettit S, Nguyen D, Santos M, Huang N. Expression, purification, and characertization of recombinant human transferrin from rice (Oryza sativa L.) Protein Expr Purif. 2010;74(1):69–79. doi: 10.1016/j.pep.2010.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang D, et al. Characterization of transferrin receptor-mediated endocytosis and cellular iron delivery of recombinant human serum transferrin from rice (Oryza sativa L.) BMC Biotechnology. 2012;12:92. doi: 10.1186/1472-6750-12-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Umemiya-Okada TNT, Matsui T, Ito M, Taniguchi T, Nakao Y. Expression and regulation of the leukemia inhibitory factor/D factor gene in human T-cell leukemia virus type 1 infected T-cell lines. Cancer Res. 1992;52(24):6961–5. [PubMed] [Google Scholar]
- 12.Huang N, WL, Nandi S, Bowman E, Huang J, Sutliff T, Rodriguez R. The tissue-specific activity of a rice beta-glucanase promoter (Gns9) is used to select rice transformants. Plant Sci. 2001;161:589–595. [Google Scholar]
- 13.Ralph P, HM, Litcofsky P, Springer T. Expression and induction in vitro of macrophage differentiation antigens on murine cell lines. J Immunol. 1983;130(1):108–14. [PubMed] [Google Scholar]
- 14.Wobus A, HH, Jakel P, Schoneich J. Characterization of a pluripotent stem cell line derived from a mouse embryo. Experimental Cell Research. 1984;152(1):212–9. doi: 10.1016/0014-4827(84)90246-5. [DOI] [PubMed] [Google Scholar]
- 15.Solter DKB. Monoclonal antibody defining a stage-specific mouse embryonic antigen (SSEA-1) Proceedings of the National Academy of Sciences of the United States of America. 1978;75(11):5565–9. doi: 10.1073/pnas.75.11.5565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCt Method. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Several rice lines express rhLIF.391 transgenic plants were generated through three batches of transformation, and 249 transgenic plants were fertile to set the seeds. To identify the transgenic events that resulted in rhLIF expression, pooled seed protein extracts of each fertile event were analyzed using immuno-dot blot with an anti-human LIF antibody (Figure 1B). Of 249 transgenic events with R1 seeds, 22 were identified as positive, and two (VB50-40 and VB50-199) with high-level expression of rhLIF were selected to proceed to the next generation. (A) Immuno-dot blot expression screening of transgenic rice R1 seeds expressing rhLIF. Rice seed total soluble proteins were extracted with 250 μl of PBS buffer, pH 7.4 per seed at room temperature for 1 h followed by centrifugation. Equal amounts of protein extracts from eight R1 seeds of the same transgenic event were pooled, and spotted onto a nitrocellulose membrane. Spots A1–E12 and F1 each contained 3 μl of pooled protein extracts from 61 transgenic rice R1 plants. The spot F2 contained 3 μl of non-transgenic Bengal seed protein extract. The spots F3–F9 represent protein extracts from individual R1 seeds of transgenic event VB50-40. Spots F10, F11, and F12 contained 30, 50, and 100 ng of E. coli-derived recombinant LIF, respectively. (B) Immuno-dot blot expression screening of individual transgenic rice R2 seeds to select homozygous lines expressing rhLIF. Rice seed total soluble proteins were extracted as above. Protein extracts (3 μl) from 12 individual R2 seeds of different transgenic R1 plants were spotted onto a nitrocellulose membrane in the same row. Rows A–H represent eight transgenic rice R1 lines. Lines in which all the progeny seeds show rhLIF expression (e. g., rows A, C, F, and G) are considered homozygous, while the lines in which some progeny seeds show no expression of rhLIF (e. g., B, D, E, and H) are considered heterozygous.
Rice-derived rhLIF is abundantly expressed in rice grains. The rhLIF expressed in rice grains was characterized by SDS-PAGE and Western blot analysis. Stained SDS–PAGE did not reveal distinct protein bands in transgenic seed extracts attributable to rhLIF expression (Supplementary Figure 2A.). However, Western blotting revealed strong immunoreactivity as a smear of bands from 20 to 50 kDa (Supplementary Figure 2B, bottom). The E. coli-derived non-glycosylated rhLIF showed a single immunoreactive band of about 19 kDa. The heterogeneity of rice seed-derived rhLIF was likely due to variable glycosylation of rhLIF. (A) SDS-PAGE stained with Coomassie blue. Total soluble proteins were extracted from rice seeds of transgenic plants and non-transgenic rice cultivar Bengal with Na-acetate, pH 5.0. Lane 1 contains 0.1 μg of E. coli-derived recombinant human LIF (Abcam, Cambridge, MA). Lanes 2–9 contained protein from the indicated rice strains extracted with either Na-acetate (50 mMNaAc, pH 5, lanes 2–5). Proteins were resolved on 4–20% Tris–glycine SDS–PAGE gradient gels (Invitrogen) without protein concentration, enrichment or purification and stained with Coomassie blue. (B) Western blot analysis of recombinant human LIF expressed in rice grains. An identical gel as (A) was transferred to nitrocellulose for Western blot detection by mouse monoclonal anti-hLIF antibody (50 ng/mL, Abcam, Cambridge, MA).