Abstract
Background
The precise involvement of the PI3K/mTOR and RAS/MEK pathways in carcinoid tumors is not well defined. Therefore, the purpose of our study was to evaluate the role these pathways play in carcinoid cell proliferation, apoptosis, and secretion and to determine the effects of combined treatment on carcinoid tumor inhibition.
Methods
The human neuroendocrine cell lines BON (pancreatic carcinoid), NCI-H727 (lung carcinoid), and QGP-1 (somatostatinoma) were treated with either the pan-PI3K inhibitor, BKM120, or the dual PI3K-mTOR inhibitor, BEZ235, alone or in combination with the MEK inhibitor, PD0325901; proliferation, apoptosis, and protein expression were assessed. Peptide secretion was evaluated in BON and QGP-1 cells. The anti-proliferative effect of BEZ235, alone or combined with PD0325901, was then tested in vivo.
Results
Both BKM120 and BEZ235 decreased proliferation and increased apoptosis; combination with PD0325901 significantly enhanced the antineoplastic effects of either treatment alone. In contrast, neurotensin (NT) peptide secretion was markedly stimulated with BKM120 treatment, but not BEZ235. The combination of BEZ235 + PD0325901 significantly inhibited the growth of BON xenografts without systemic toxicity.
Conclusions
Both BKM120 and BEZ235 effectively inhibited NET cell proliferation and stimulated apoptosis. However, inhibition of the PI3K pathway alone with BKM120 significantly stimulated NT peptide secretion; this did not occur with the dual inhibition of both PI3K and mTOR using BEZ235 suggesting that this would be a more effective treatment regimen for NETs. Moreover, the combination of BEZ235 and the MEK inhibitor PD0325901 was a safe and more effective therapy in vivo compared with single agents alone.
Keywords: Carcinoid, Neuroendocrine tumor, PI3K, MEK, Molecular targeted therapy
INTRODUCTION
Carcinoid tumors are well-differentiated neuroendocrine tumors (NETs) most commonly occurring in the gastrointestinal tract (1). Although rare, accounting for only 0.49% of all malignancies, their incidence has been increasing (1). Effective treatment of carcinoid tumors remains difficult, as they are often resistant to traditional, cytotoxic chemotherapeutic agents (2). As a result, surgery remains the mainstay of treatment for carcinoid tumors (3). Unfortunately, the indolent nature of these tumors often results in presentation after the development of metastases (3, 4). This usually precludes curative resection and is associated with decreased 5-year survival (1). The poor response to available systemic treatment modalities underscores the need for more effective and targeted therapies in the treatment of carcinoid disease.
The PI3K complex is a ubiquitous lipid kinase composed of an 85 kDa regulatory subunit and a 110 kDa catalytic subunit (5). It exerts its influence through its downstream effectors Akt and mTOR (6). Abnormal signaling in this pathway frequently occurs through constitutive activation of the PI3K subunits or downregulation or mutation of PTEN (7). Upregulation of the PI3K pathway has been identified as a critical component in the growth and progression of numerous cancer types (8, 9) and has been implicated as a key contributor to the development of metastatic disease (6, 10, 11). Recent evidence suggests that abnormal signaling through these pathways is a factor in NETs as well (8, 9). In recent clinical trials, the mTOR inhibitor everolimus was used to treat pancreatic NETs and resulted in significantly prolonged progression-free survival (12).
The RAS/MEK pathway also has a well-defined role in cancer development and progression (13) and is often closely integrated with the PI3K pathway (14). Furthermore, co-targeting of these pathways can enhance the therapeutic response (15–17). While the precise pathway mutations in carcinoid tumors are less clear, the activation of RAS/MEK pathway components, such as the downstream effector ERK, has been identified. For example, in a study evaluating KRAS and BRAF mutations in NETs, only one BRAF mutation and no KRAS mutations were identified; however, the majority of these tumors showed constitutive activation of ERK (18). Despite the recognition of these pathways as potential contributors to carcinoid disease, their specific role in carcinoid tumors is not well defined.
Management of carcinoid tumors is further complicated by their ability to secrete bioactive amines and peptides. These secretory products can cause carcinoid syndrome, which is characterized by severe flushing, diarrhea, bronchospasm, and cardiac fibrosis, and can result in death (3). The PI3K pathway has been linked to the control of microtubule dynamics, indicating its potential involvement in vesicle trafficking and the secretion of these bioactive products (19). Furthermore, PI3K has been identified as a potential negative regulator of peptide secretion. In a recent study, inhibition using the p110α specific inhibitor, PIK-75, resulted in upregulation of neurotensin (NT) peptide secretion from the human carcinoid cell line, BON (20). These findings imply that the use of PI3K inhibitors for the treatment of carcinoid disease may increase secretion, which could result in adverse sequelae. The purpose of our study was to define the roles the PI3K and RAS/MEK pathways play in carcinoid cell proliferation and secretion as well as to determine the effects of combined inhibition on carcinoid tumor growth, apoptosis and peptide secretion.
MATERIALS AND METHODS
Materials
Dulbecco’s modified Eagle medium (DMEM)/F12K was obtained from Invitrogen (Grand Island, NY) and RPMI medium from ATCC (Manassas, VA). Fetal bovine serum was purchased from Atlanta Biologicals (Lawrenceville, GA). The mTOR inhibitor rapamycin was obtained from Calbiochem (San Diego, CA). The Cell Death Detection ELISAplus kit was obtained from Roche (Indianapolis, IN). Annexin V Alexa Fluor 488 was purchased from Invitrogen (Grand Island, NY). The pBabe-puro p110α plasmids were obtained from Addgene (Cambridge, MA). Lipofectamine 2000 was acquired from Invitrogen (Grand Island, NY). NuPAGE 4–12% Bis-Tris gels were also purchased from Invitrogen (Grand Island, NY) and Sequi-blot PVDF membranes from Bio-Rad (Hercules, CA). The following antibodies were obtained from Cell Signaling (Beverly, MA): pAkt, Akt, pERK, ERK, p-p70S6K, p70S6K, pS6, S6, p4E-BP1, 4E-BP1, PARP, and cleaved caspase 3. An enhanced chemiluminescence (ECL) detection system was purchased from GE healthcare (Piscataway, NJ). The NT enzyme immunoassay (EIA) kit was acquired from Phoenix Pharmaceuticals (Belmont, CA). The serotonin ELISA kit was obtained from Immuno-Biological Laboratories Inc. (Minneapolis, MN, USA). 1-Methyl-2-pyrrolidinone (NMP), polyethylene glycol 300, hydroxypropyl methycellulose, and 0.2% Tween 80 were purchased from Sigma Aldrich (Saint Louis, MO).
Small molecule inhibitors
PIK-75, a p110α specific inhibitor of the PI3K complex; rapamycin, an mTOR inhibitor; and PD0325901 and PD98059, MEK inhibitors, were purchased from Cayman Chemical (Ann Arbor, MI). BKM120, a pan-PI3K inhibitor, was purchased from Active Biochem (Maplewood, NJ), and BEZ235, a dual PI3K/mTOR inhibitor, was purchased from LC Laboratories (Woburn, MA).
Cell lines and culture
The human carcinoid cell lines BON (pancreatic carcinoid) (21) and NCI-H727 (lung carcinoid) (22, 23) were used in this study. The neuroendocrine tumor cell line, QGP-1 (human somatostatinoma) (24) was also used since, similar to BON cells, it produces and secretes the NT peptide (24). The cell lines (BON, QGP-1, and NCI-H727) were authenticated in May 2012 at Genetica DNA Laboratories, Inc. and 17 autosomal short tandem repeat (STR) loci and the sex identity locus for the cells were profiled using PowerPlex 18D PCR Amplification Kit (Promega Corporation). BON cells were maintained in a 1:1 mixture of DMEM and nutrient mixture F12K, supplemented with 5% fetal bovine serum (FBS). NCI-H727 and QGP-1 cells were maintained in ATCC-formulated RPMI-1640 medium with 10% FBS. Cells were incubated in 5% CO2 at 37°C.
Generation of stable cell lines
To produce retrovirus, 293FT packaging cells cultured in 60 mm dishes were co-transfected with pBabe-puro plasmid (1 µg) and Ampho packaging plasmid by Lipofectamine 2000 and incubated in growth medium overnight. Subsequently, the cells were cultured in complete medium (growth medium plus 1 mM MEM sodium pyruvate) for 24 h; the supernatant containing the retrovirus was collected, filtered through a 0.45-µm SFCA sterile syringe filter, and used to infect target cells. BON cells in 6-well plates (5 × 105 cells/well) were incubated with the viral supernatant for 24 h; cells were then incubated with growth medium for an additional 24 h. The infected cells were subcultured in 100-mm dishes in fresh medium containing puromycin (2.5 µg/ml). Puromycin-resistant cell pools were collected and the overexpression levels were monitored by western blot.
Cell proliferation
Cells were plated in 24 well plates at a density of 3×104 cells/cm2. Drug treatments were initiated the following morning after allowing the cells to adhere. Media was exchanged with fresh drug media at 36h. Cell proliferation was assessed at 72h directly by cell counting using a Beckman Coulter Cell Viability Analyzer (Fullerton, CA).
DNA fragmentation enzyme linked immunoassay (ELISA)
Cells were plated in 24 well plates at a density of 5×104 cells/cm2. Drug treatments were initiated the following morning and continued for 24h. Apoptosis was measured by DNA fragmentation using the Cell Death Detection ELISAplus as previously described (25).
Annexin V
BON cells were plated in 6 well plates at a density of 9 × 105 cells per well. Drug treatments were initiated the next day and continued for 24 hours. Cells were collected, washed, stained with annexin V Alexa Fluor 488 according to the manufacture's protocol (A13201, Molecular Probes, Invitrogen, Grand Island, NY) and analyzed by flow cytometry (UK Flow Cytometry Core Facility).
Western blot analysis
Cells were plated in 6 well plates at a density of 5×104 cells/cm2. Whole cell lysates were collected following 24h of drug treatment. Total protein was resolved on NuPAGE 4–12% Bis-Tris gels and transferred to Sequi-blot PVDF membranes. Membranes were incubated with specific primary antibodies and subsequently horseradish peroxidase-conjugated secondary antibody. Proteins were visualized using an ECL detection system.
For the in vivo study, three tumors per treatment group were selected at random for analysis. Briefly, pieces of frozen tumors were placed in tubes with ceramic beads. Ice-cold lysis buffer with protease inhibitors was added to the tubes, and tissues were homogenized in a tissue homogenizer (Bullet Blender Advance) at 4°C. Homogenized samples were centrifuged and supernatants collected. Protein concentration of the samples was detected by Bradford assay. Western blots were performed as described above.
NT enzyme immunoassay (EIA)
Cells were plated in 24 well plates at a density of 1×105 cells/cm2 and grown for 48h. Cells were treated with inhibitors in growth medium for 1h. Media were collected and stored at −80°C. Secreted NT peptide was measured by NT EIA as previously described (26, 27).
Serotonin ELISA
Cells were plated in 24 well plates at a density of 1×105 cells/cm2. After 24h, cells were washed and maintained with serum-free medium. The next day, cells were treated with inhibitors in serum-free medium for 1h. Media were collected and stored at −80°C. Secreted serotonin was measured by a serotonin ELISA according to the manufacturer’s instructions. The data for secreted serotonin were normalized by protein concentration from parallel cell lysates.
In vivo studies
2 month-old athymic nude male mice weighing approximately 25 g were used for this study. Mice were acclimated for 1 week at which time xenografts were established by injection of 1×107 BON cells (in 100 µL of sterile PBS) subcutaneously in the flank. The mice were then randomized into four groups (n=15 per group): (a) Vehicle control, (b) BEZ235 (45 mg/kg), (c) PD0325901 (5 mg/kg), or (d) BEZ235 (45 mg/kg) and PD0325901 (5 mg/kg). BEZ235 was dissolved in one part 1-Methyl-2-pyrrolidinone (NMP) to nine parts polyethylene glycol 300. PD0325901 was formulated in 0.5% hydroxypropyl methycellulose plus 0.2% Tween 80. Vehicle control consisted of a combination of NMP, polyethylene glycol 300, and 0.5% hydroxypropyl methycellulose plus 0.2% Tween 80 prepared in the same ratios as used for the drug preparations. All mice were treated by daily oral gavage five days per week for six weeks. Mice were weighed three times/week during the experiment to monitor for toxicity. Tumor size was measured using vernier calipers and volume calculated using the equation: (length × width2) / 2. At the completion of the experiment, mice were sacrificed and tumors excised and weighed. All animal procedures were performed according to protocols approved by the Institutional Animal Care and Use Committee.
Statistical analysis
Study endpoints from the in vitro experiments including cell proliferation, apoptosis, and NT secretion are summarized using bar graphs with means +/− standard error of the mean (SEM). Comparisons across treatment groups utilized the analysis of variance model with contrasts generated from the model to perform specific comparisons including linear trend for increasing dose levels, pairwise comparisons of treatment versus control and combination versus monotherapy. The model also included experiment as an additional factor to account for repeat experiments in these studies. Tumor growth curves and body weight over time were plotted. Two-sample t-tests were used to compare tumor weight, fold change in tumor volume, and fold change in body weight between treatment groups.
RESULTS
PI3K inhibition decreases carcinoid cell proliferation but increases signaling through the RAS/MEK pathway
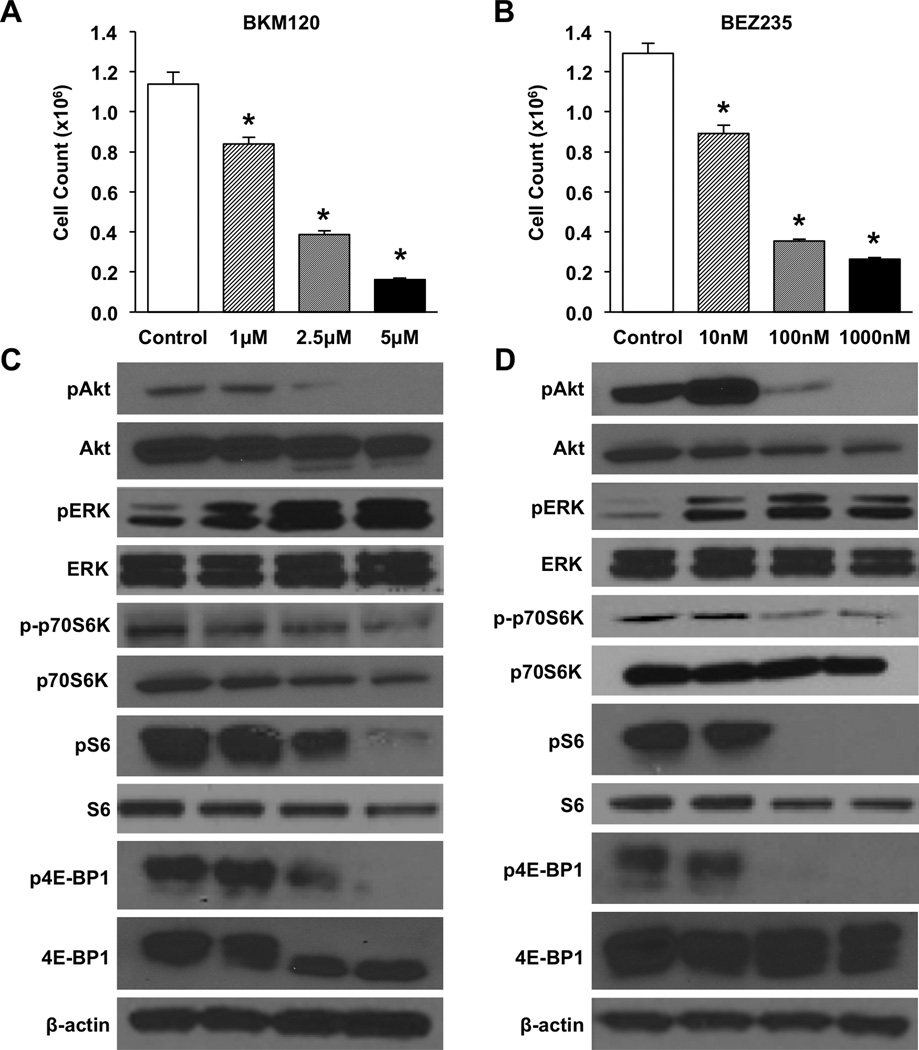
We first determined whether PI3K inhibitors alone were effective in the treatment of NETs. BON carcinoid cells were plated in equal numbers and treated with the pan-PI3K inhibitor BKM120 at various doses (1.0, 2.5, and 5.0 µM) or the dual PI3K/mTOR inhibitor BEZ235 at 10, 100, or 1000 nM. Cells were counted at 72h to evaluate responsiveness to treatment (Fig. 1A, B). Cell number was significantly decreased at all doses for both BKM120 and BEZ235. Furthermore, a dose-dependent trend was observed across the range of doses tested. Two additional NET cell lines, NCI-H727 and QGP-1, were tested as well with a similar decrease in proliferation identified (Supplemental Fig. 2). The role of mTOR was also evaluated using rapamycin treatments (Supplemental Fig. 1). Significant inhibition was identified; however, the effects were less pronounced than with PI3K inhibition. In order to further confirm the benefit of targeted PI3K inhibition, effects on proliferation were evaluated using shRNA directed against the alpha subunit of the PI3K complex. Analysis was performed directly by cell counting at 24h intervals up to 96h (Supplemental Fig. 2C). Significant reduction in proliferation was identified at all time points.
Figure 1. PI3K inhibition decreases BON cell proliferation but increases signaling through the RAS/MEK pathway.
Experiments were performed in triplicate and repeated 3 times. BON cells were plated in 24 well plates at a density of 3×104 cells/well. Treatments were initiated the following morning with increasing doses of either (A) BKM120 (0–5 µM) or (B) BEZ235 (0–1000 nM). Media was exchanged for fresh drug media again at 36h. Proliferation was assessed directly by cell counting at 72h (* p < 0.05 vs. control). For Western blot analysis, cells were plated in 6 well plates at a density of 5 ×104 cells/cm2. Whole cell lysates were collected following 24h of drug treatment with either (C) BKM120 (0–5 µM) or (D) BEZ235 (0–1000 nM). β-actin was used as a loading control.
Western blot analysis using whole BON cell lysates was performed to confirm that these effects were the result of targeted inhibition of the PI3K pathway (Fig. 1C and 1D). pAkt levels were progressively decreased with increasing doses of BKM120. Conversely, treatment with BEZ235 at 10 nM resulted in a slight induction of pAkt, possibly as a result of mTOR inhibition with associated loss of negative PI3K inhibition through the S6K feedback loop; this mechanism is supported by the finding of increased pAkt following treatment with mTOR inhibition alone (Supplemental Fig. 1). However, decreased pAkt was observed at the 100 nM and 1000 nM BEZ235 doses. The downstream targets p70S6K, S6, and 4E-BP1 were also analyzed. Phosphorylation of all of these targets was decreased with increasing doses of BKM120. For BEZ235, phosphorylation of these targets was minimally affected at 10 nM; however, phosphorylation of all three targets was decreased at 100 nM and 1000 nM doses.
Inhibition of mTORC1 has been demonstrated to activate RAS/MEK signaling through an S6K-PI3K-RAS feedback loop in several cancer types (28). Given such interactions between the PI3K and RAS/MEK pathways, we also analyzed the levels of pERK to evaluate crosstalk between these two pathways in NETs. Interestingly, levels of pERK were increased at all doses for both the BKM120 and BEZ235 treatment groups indicating compensatory signaling through the RAS/MEK pathway following treatment with a PI3K inhibitor alone.
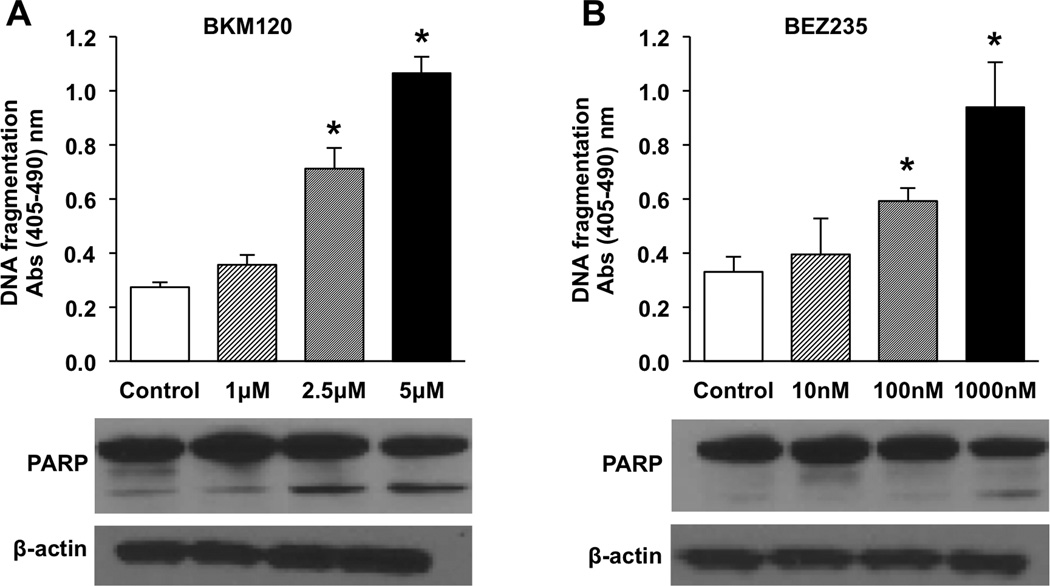
PI3K inhibition results in an increase in apoptosis
We next determined whether the response to treatment was the result of a cytostatic effect or, conversely, if it represented an increase in apoptosis. BON cells were treated with BKM120 at doses of 1.0, 2.5, and 5.0 µM or with BEZ235 at 10, 100, and 1000 nM for 24h and apoptosis measured by DNA fragmentation (Fig. 2). DNA fragmentation was significantly increased at the middle and highest doses tested for both treatment groups. mTOR inhibition alone following treatment with rapaymycin was also assessed (Supplemental Fig. 1). No increase in apoptosis was identified.
Figure 2. PI3K inhibition increases apoptosis in BON cells.
Experiments were performed in triplicate and repeated 3 times. Representative graph is shown. Cells were plated in 24 well plates at a density of 5×104 cells/cm2. Treatments were initiated the following morning with increasing doses of either (A) BKM120 (0–5 µM) or (B) BEZ235 (0–1000 nM) and continued for 24 hours. Apoptosis was measured by DNA fragmentation using the Cell Death Detection ELISAplus (* p < 0.05 vs. control). Whole cell lysates were also collected after 24h of treatment and Western blot performed to analyze for PARP cleavage. β-actin was used as a loading control.
To corroborate this apoptotic effect, Western blot analysis of PARP cleavage was also performed. Increased PARP cleavage corresponded with increases in DNA fragmentation. NCI-H727 and QGP-1 cells were tested to confirm the results in other NET cells. NCI-H727 cells showed a dose dependent trend similar to that identified in BON cells with DNA fragmentation significantly increased at both the middle and highest doses tested; increased DNA fragmentation was also identified in QGP-1 cells (Supplemental Fig. 3).
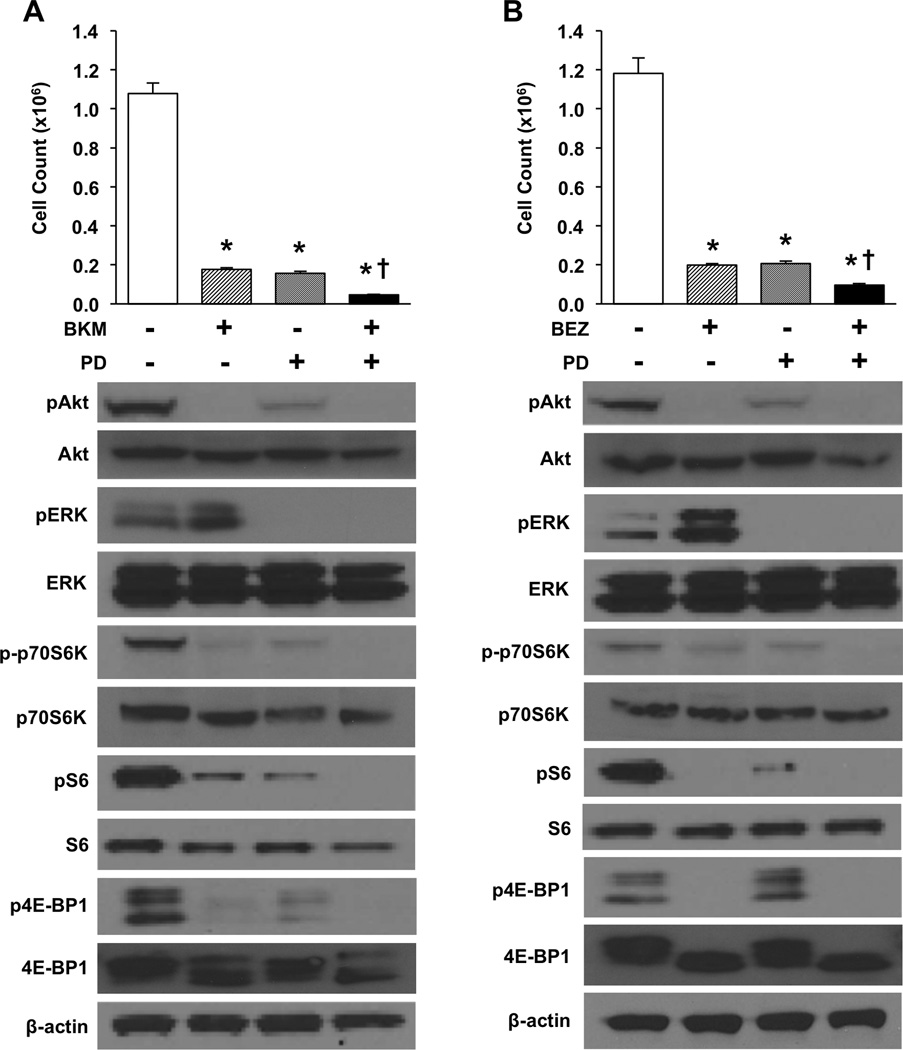
Combination PI3K + MEK inhibitor treatment enhances inhibition of carcinoid cell proliferation
After identifying the upregulation of pERK noted with PI3K treatment alone, we next hypothesized that the addition of an MEK inhibitor (PD0325901) would augment the anti-proliferative response. To test this hypothesis, BON cells were treated with BKM120 (5 µM), PD0325901 (100 nM), or the combination of BKM120 + PD0325901. In another experiment, BON cells were treated with BEZ235 (1000 nM), PD0325901 (100 nM), or the combination of BEZ235 + PD0325901. Cell numbers were assessed using the Coulter counter at 72h (Fig. 3). All individual and combination treatments significantly decreased cell number relative to control (vehicle treatment). Furthermore, the combination significantly reduced BON cell numbers relative to either of the individual treatments. Similar to the BON cell line, all individual and combination treatments significantly decreased NCI-H727 and QGP-1 cell numbers relative to control (Supplemental Fig. 4). mTOR inhibition alone with rapamycin showed an enhanced effect in BON and NCI-H727 cells; however, the response was not as pronounced as with PI3K + MEK inhibition and was absent for QGP-1 cells (Supplemental Fig. 1). Together, these data support enhanced inhibition of cell proliferation with combination PI3K + MEK treatments.
Figure 3. Combination treatment with PI3K and MEK inhibitors results in more pronounced inhibition of BON cell proliferation.
Experiments were performed in triplicate and repeated 3 times. BON cells were plated in 24 well plates at a density of 3×104 cells/well. Treatments were initiated the following morning with either (A) control, BKM120 (5 µM), PD0325901 (100 nM), or combination BKM120 + PD0325901 (5 µM/100 nM), or (B) control, BEZ235 (1000 nM), PD0325901 (100 nM), or combination BEZ235 + PD0325901 (1000 nM/100 nM). Media was exchanged for fresh drug media at 36h. Proliferation was assessed directly by cell counting at 72h (* p < 0.05 vs. control; † p < 0.05 vs. monotherapy). For Western blot analysis, cells were plated in 6 well plates at a density of 5×104 cells/cm2. Whole cell lysates were collected following 24h of drug treatment with either (A) control, BKM120 (5 µM), PD0325901 (100 nM), or combination BKM120 + PD0325901 (5 µM/100 nM), or (B) control, BEZ235 (1000 nM), PD0325901 (100 nM), or combination BEZ235 + PD0325901 (1000 nM/100 nM). β-actin was used as a loading control.
Western blot analysis of BON cell lysate was also performed to evaluate the effects on PI3K and RAS/MEK pathway components. Treatment with a PI3K inhibitor individually or in combination resulted in decreased pAkt and reduced phosphorylation of the downstream effectors p70S6K, S6, and 4E-BP1. Treatment with PD0325901 (100 nM), both alone and in combination, decreased pERK expression. Importantly, the upregulation of pERK observed following treatment with BKM120 or BEZ235 alone was abrogated when combined with the MEK inhibitor.
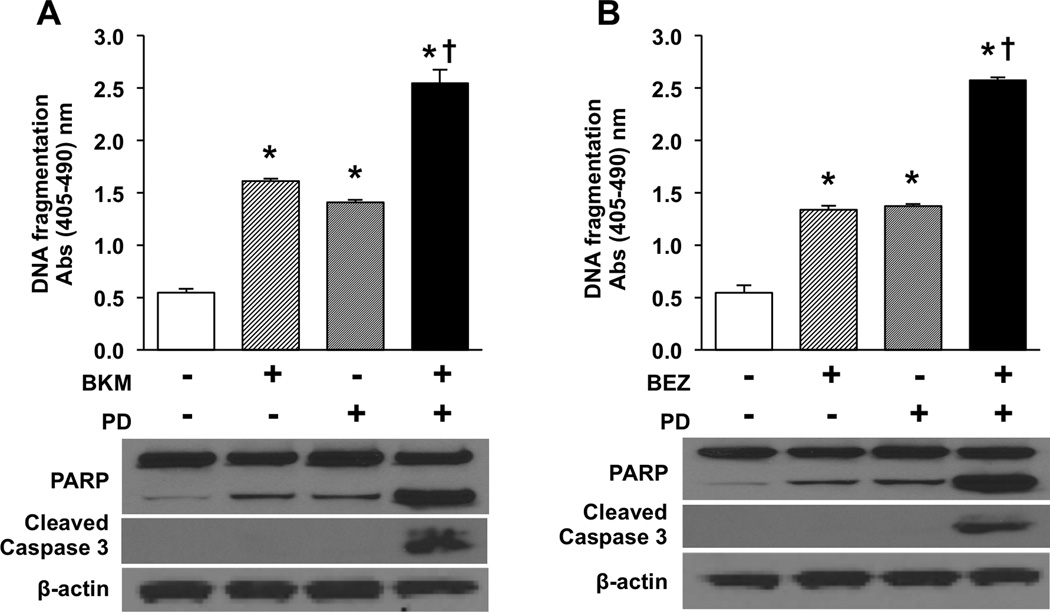
Combination PI3K + MEK inhibition increases apoptosis relative to individual agents
After confirming a decrease in cell number with combination treatment, we next determined whether the enhanced response associated with the MEK inhibitor was due to increased apoptosis. BON cells were treated with either BKM120 (5 µM) or BEZ235 (1000 nM) as single agents or in combination with PD0325901 (100 nM) as described above, and apoptosis measured by DNA fragmentation (Fig. 4). Both the PI3K and MEK inhibitors significantly increased apoptosis when administered as individual treatments. Furthermore, combination PI3K + MEK treatment resulted in a significant increase in apoptosis compared with either individual agents. Similar to the BON cell line, both individual and combination treatments significantly increased DNA fragmentation in NCI-H727 and QGP-1 cells relative to control (Supplemental Fig. 5A and 5B). This enhanced effect was not present following treatment with mTOR + MEK inhibitors (Supplemental Fig. 1).
Figure 4. Combination PI3K + MEK inhibition enhances apoptosis in BON cells compared to either agent individually.
Experiments were performed in triplicate and repeated 3 times. Representative graph is shown. Cells were plated in 24 well plates at a density of 5×104 cells/cm2. Treatments were initiated the following morning with either (A) control, BKM120 (5 µM), PD0325901 (100 nM), or combination BKM120 + PD0325901 (5 µM/100 nM), or (B) control, BEZ235 (1000 nM), PD0325901 (100 nM), or combination BEZ235 + PD0325901 (1000 nM/100 nM) and continued for 24 hours. Apoptosis was measured by DNA fragmentation using the Cell Death Detection ELISAplus (* p < 0.05 vs. control; † p < 0.05 vs. monotherapy). Whole cell lysates were also collected after 24h of treatment and Western blot analysis performed for PARP and caspase 3 cleavage. β-actin was used as a loading control.
To corroborate these findings, BON cells were again treated with single and combination treatments as described. Cells were stained using annexin V Alex Fluor 488 and sorted by flow cytometry (Supplemental Fig. 5C). Consistent with findings from the DNA fragmentation studies, an increase in cell death was identified with all treatment groups. Increased cell death was also significantly increased for BEZ235 + PD0325901 compared to BEZ235 or PD0325901 alone and for BKM120 + PD0325901 compared to PD0325901 alone (Supplemental Fig. 5C). Subsequent Western blot analysis also showed increased PARP cleavage with individual PI3K or MEK inhibitor treatment (Fig. 4). The increase was even more prominent with combination PI3K + MEK inhibition. Additionally, increased cleavage of caspase 3 was identified in the combination treatment groups.
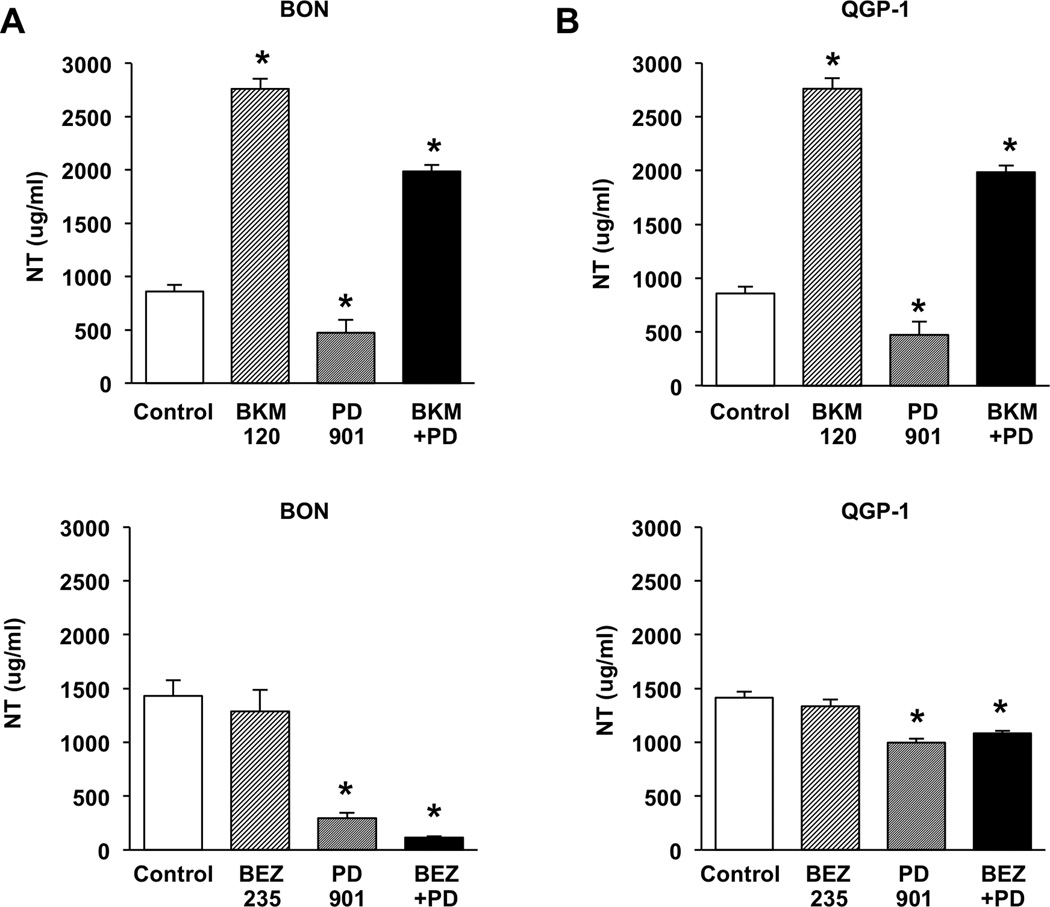
Treatment with BKM120, but not BEZ235 or PD0325901, increases peptide secretion from NET cells
Any treatment regimen considered for NETs must decrease cell proliferation and, importantly, cannot increase peptide secretion which can lead to untoward treatment sequelae. Li, et al (20), in our laboratory has shown that treatment with the p110α PI3K inhibitor PIK75 increases NT secretion, possibly as a result of a loss of inhibitory feedback from the PI3K pathway. We next determined whether the broader spectrum PI3K inhibitors, BKM120 and BEZ235, or the MEK inhibitor, PD0325901, are associated with increased peptide secretion. BON and QGP-1 cells were treated with BKM120 (5 µM) or BEZ235 (1000 nM) as single agents or in combination with PD0325901 (100 nM); media was collected and analyzed for NT secretion (Fig. 5). Individual treatment with BKM120 significantly stimulated NT release in both BON and QGP-1 cells. Conversely, BEZ235 did not increase NT secretion in either cell line, while PD0325901 significantly decreased secretion. When tested in combination, PD0325901 did not completely abrogate the increased secretion associated with BKM120; however, the decreased secretion identified with PD0325901 persisted with combination BEZ235 + PD0325901 treatments.
Figure 5. BKM120 increases NT secretion, while BEZ235 does not cause an increase in NT secretion, and PD0325901 is associated with a decrease in NT secretion.
(A) BON and (B) QGP-1 cells were plated in 24 well plates at a density of 1×105 cells/cm2 and grown for 48h. Cells were treated with either (upper panels) control, BKM120 (5 µM), PD0325901 (100 nM), or combination BKM120 + PD0325901 (5 µM/100 nM), or (lower panels) control, BEZ235 (1000 nM), PD0325901 (100 nM), or combination BEZ235 + PD0325901 (1000 nM/100 nM) in growth medium for 1h. Media were collected and secreted NT peptide measured by NT EIA (* p < 0.05 vs. control).
The ability of carcinoid tumors to secrete serotonin is also well described and has been associated with carcinoid syndrome. Therefore, BON and QGP-1 cells were again treated with single and combination agents as described above and media collected for analysis of serotonin secretion (Supplemental Fig. 6A and 6B). Similar to analysis of NT secretion, serotonin secretion was decreased following treatment with PD0325901. This decrease was maintained following combination BEZ235 + PD0325901 and BKM120 + PD0325901 treatments. Expression of CgA, another secretory product of carcinoid tumors, was also analyzed by Western blot in BON cells (Supplemental Fig. 6C). Decreased expression of CgA was noted in all treatment groups; however, the greatest decrease was identified following treatment with BEZ235 and BEZ235 + PD0325901.
Combination BEZ235 + PD0325901 treatment most effectively suppresses in vivo subcutaneous BON xenograft growth
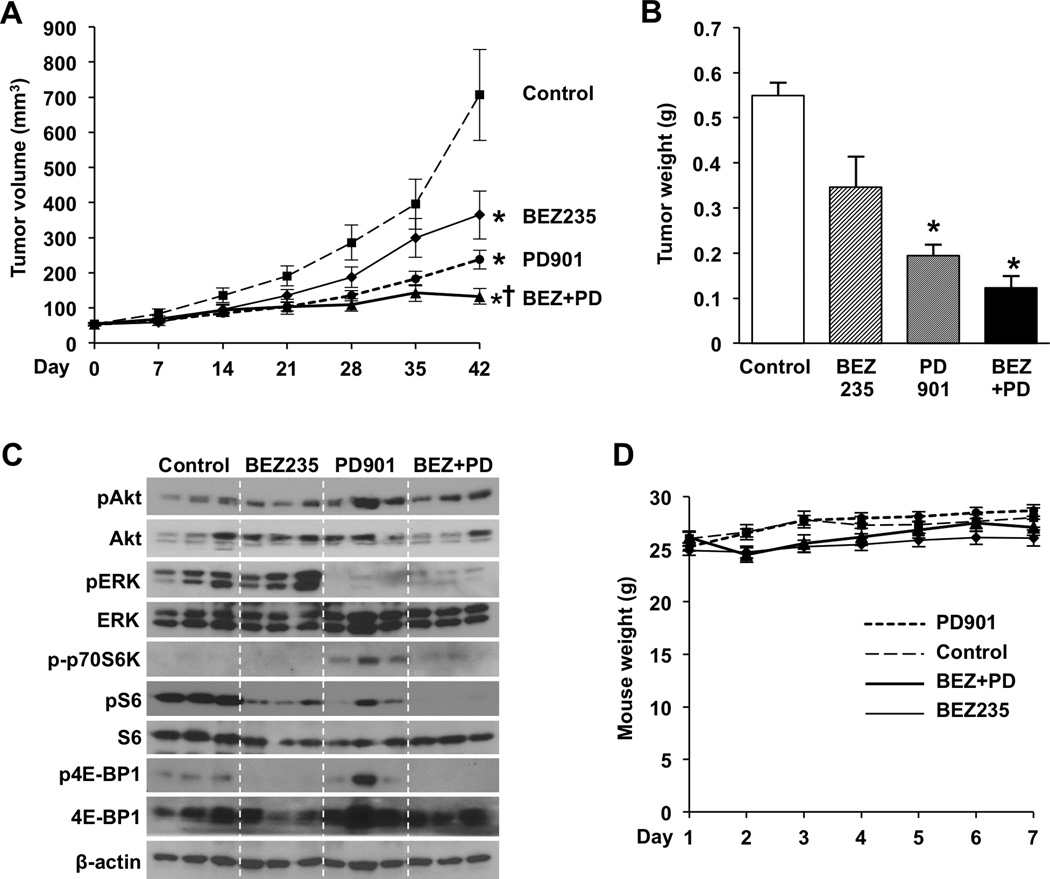
Both BEZ235 and BKM120 significantly increased NET cell death in vitro; however, BKM120 resulted in increased NET peptide secretion. Therefore, we next focused our studies on BEZ235 and the efficacy and safety of BEZ235 treatment alone or combined with PD0325901 in vivo. Subcutaneous BON xenografts were established in athymic nude mice and allowed to grow over a 14-day period. Groups of 15 mice were then randomized to treatment with vehicle (control), BEZ235 (45 mg/kg), PD0325901 (5 mg/kg), or the combination of BEZ235 (45 mg/kg) + PD0325901 (5 mg/kg). Tumor volumes at the initiation of therapy were comparable between all treatment groups (average volume 52.3 mm3 – 54.2 mm3). Mice were sacrificed after 6 weeks of treatment when control tumors had reached the maximal size limitations (average volume = 653 mm3) as indicated by our institutional guidelines.
Measurement of tumor volumes showed significant inhibition of tumor growth for individual BEZ235 and PD0325901 treatments compared to control (Fig. 6A). Additionally, combination treatment with BEZ235 + PD0325901 significantly inhibited tumor growth compared to both control and individual treatments. At study completion, tumor volumes in the combination treatment group increased by only 2.4-fold compared to the 12.5-fold increase in the control group. Comparisons of tumor weight were not significant for BEZ235 relative to control (p=0.133) or for combination treatment compared to PD0325901 alone (p=0.051), although they did follow a similar pattern as tumor volume (Fig. 6B).
Figure 6. Combination treatment with BEZ235 and PD0325901 most effectively suppresses in vivo growth of subcutaneous carcinoid xenografts.
Subcutaneous injections of 1 × 106 BON cells in 100 uL of sterile PBS were performed in athymic nude mice. After allowing time for xenograft establishment, mice were randomized into either vehicle control, BEZ235 (45 mg/kg), PD0325901 (5 mg/kg), or combination BEZ235 + PD0325901 (45 mg/kg, 5 mg/kg) treatment groups (n=15 per group). Treatments were administered by oral gavage once daily, 5 days per week, for 6 weeks. (A) Tumor size was measured with Vernier calipers and the equation (length × width2) / 2 was used to calculate volume (* p < 0.05 vs. control; † p < 0.05 vs. monotherapy). (B) Tumors were excised at the end of the treatment period and weighed (* p < 0.05 vs. control; † p < 0.05 vs. BEZ235 monotherapy). (C) Protein was collected from harvested xenografts and expression analyzed by Western blot. β-actin was used as a loading control. (D) To monitor for toxicity, mice were weighed weekly for 6 weeks.
In order to confirm on-target effects following treatments in vivo, the BON xenografts were harvested and analyzed by Western blot (Fig. 6C). Phosphorylation of Akt, p70S6K, S6, 4E-BP1, and ERK were evaluated. Although pAkt had variable expression, decreased phosphorylation of the downstream targets p70S6K, S6, and 4E-BP1 were identified following treatment with BEZ235 and BEZ235 + PD0325901. Decreased phosphorylation of ERK was also noted for groups treated with PD0325901 and BEZ235 + PD0325901. These findings support the targeted effects of these inhibitors in vivo.
Four mice died early in the treatment period, two in the BEZ235 treatment group and two in the combination treatment group. Mortality occurred shortly after oral gavage in these mice, and necropsy showed mottling of the lungs, which is consistent with administration of the treatment into the trachea. As a more accurate assessment of toxicity, there was no significant reduction in weight between the mice in the various treatment groups compared to control nor were other signs of acute or delayed toxicity present (Fig. 6D). Collectively, these data indicate that the dose and treatment schedule tested in this experiment are well tolerated by the mice and demonstrate effective BON tumor inhibition.
DISCUSSION
In this study, we evaluated the role of the PI3K and RAS/MEK pathways in NET proliferation, apoptosis, and secretion. First, we showed that PI3K pathway inhibition results in both decreased proliferation and increased apoptosis in multiple NET cell lines, while concomitantly increasing signaling through the RAS/MEK pathway. Second, we demonstrated that using the MEK inhibitor, PD0325901, to block this compensatory signaling enhanced the antineoplastic effects over either agent alone. Interestingly, we found that, similar to the p110α specific PI3K inhibitor PIK75, the pan-PI3K inhibitor BKM120, stimulated peptide release from the functioning BON carcinoid cell line; however, the dual PI3K/mTOR inhibitor, BEZ235, did not increase secretion. Treatment with the MEK inhibitor, PD0325901, decreased NT secretion; this decrease was sustained when used in combination with BEZ235. Finally, we demonstrated that combination treatment with BEZ235 and PD0325901 is both safe and effective for the in vivo treatment of mice bearing BON xenografts.
Mutations resulting in aberrant activation of signaling pathways provide a selective advantage for cancer cells compared to their normal counterparts. Upregulation of the PI3K pathway, in particular, has been demonstrated as a key contributor to tumor progression in a number of cancer types (9). In carcinoid tumors, compromised regulation of the PI3K complex has been attributed to activation of Akt or loss of PTEN expression (29–31). In addition, global inhibition of PI3K with LY294002 or selective siRNA knockdown of Akt1 has previously been shown to inhibit carcinoid cell proliferation (32, 33). In our current study, we extend these findings by confirming that the pan-PI3K inhibitor, BKM120, and the dual PI3K/mTOR inhibitor, BEZ235, decrease proliferation in multiple NET cell lines. We further show that, at least in vitro, the mechanism may include increased cell death rather than strictly a cytostatic effect; however, these cytotoxic effects are less evident in vivo.
The abnormal activation of the RAS/MEK pathway also occurs in many cancers, including carcinoid tumors (18, 34). This was demonstrated in a study of 40 primary gastroenteropancreatic NETs, in which activated ERK was identified in all specimens (18). In addition to the individual contribution of the RAS/MEK pathway, significant crosstalk with the PI3K pathway can also occur. For example, treatment with the mTORC inhibitor, RAD001, can increase RAS/MEK pathway signaling through an S6K-PI3K-RAS feedback loop (28). Zitzmann et al (15) also showed that combination treatment with an mTOR inhibitor and a high dose RAF inhibitor is capable of enhancing anti-tumor effects in neuroendocrine cells. In our study, we demonstrate that, in addition to mTOR, inhibition of the PI3K complex alone also results in compensatory signaling through the RAS/MEK pathway, as indicated by an increase in pERK expression following treatment with both BKM120 and BEZ235 at all doses tested. We further show that combining PI3K and MEK inhibition significantly increases DNA fragmentation and is associated with increased PARP and caspase 3 cleavage. Furthermore, combination treatment using BEZ235 and PD0325901 in vivo is both a safe and effective treatment and offers a greater therapeutic benefit than either agent alone. Together these data provide a strong argument for the use of dual PI3K and MEK inhibition as treatment for NETs.
A unique feature of carcinoid tumors is their ability to secrete bioactive substances. When the tumor is localized to the bowel, these substances are metabolized by the liver, thereby preventing sequelae; however, when liver metastases are present, these substances are released systemically and can result in carcinoid syndrome with potentially life-threatening effects (35). As shown by Li et al (20), the loss of negative regulation from the PI3K pathway results in an increase in NET peptide secretion, a process that appears to be dependent upon the p110α subunit. In our study, we show that while the pan-PI3K inhibitor, BKM120, significantly increases carcinoid cell apoptosis, peptide secretion is also stimulated. Furthermore, the decreased secretion observed with PD0325901 treatment only partially counteracts this increase when used in combination with BKM120. This augmentation of carcinoid cell secretion has the potential to produce adverse effects in the clinical setting. In contrast, we demonstrate that the dual PI3K/mTOR inhibitor, BEZ235, significantly inhibits NET growth but, in contrast to BKM120, does not stimulate peptide release. These results argue that dual inhibition with BEZ235 and PD0325901 is unlikely to be associated with adverse effects related to enhanced secretion and may actually provide a therapeutic benefit in those patients with carcinoid syndrome.
In summary, we show that PI3K inhibition effectively decreases carcinoid cell growth and induces apoptosis; however, compensatory signaling occurs through the RAS/MEK pathway. Dual inhibition of the PI3K and RAS/MEK pathways blocks this feedback activation and results in an enhanced therapeutic benefit compared to either agent alone. Furthermore, we show that the increased secretion that can occur following treatment with PI3K complex inhibitors does not occur with the dual PI3K/mTOR inhibitor BEZ235 and, in addition, that MEK inhibition can actually decrease secretion. Finally, we confirm the safety and efficacy of dual PI3K and RAS/MEK inhibition in vivo. Collectively, our data strongly support a combination strategy of dual PI3K/mTOR and MEK inhibition as an effective treatment for NETs.
Supplementary Material
TRANSLATIONAL RELEVANCE.
The increasing incidence of NETs, the frequently advanced stage at the time of presentation, and the poor response to traditional chemotherapeutic agents indicate an important need for more effective systemic therapies. Molecular targeted therapies, such as mTOR inhibitors, have shown promise as a therapeutic alternative. In this study, we evaluated the efficacy of a pan-PI3K inhibitor (BKM120) and a dual PI3K/mTOR inhibitor (BEZ235), either alone or in combination with an MEK inhibitor (PD0325901) on proliferation and peptide secretion in human NET cells. Our findings indicate that the dual inhibition of PI3K and mTOR, using BEZ235, effectively increased NET cell apoptosis and, in contrast to PI3K inhibition alone with BKM120, did not stimulate NET peptide secretion. Furthermore, the combination of BEZ235 with the MEK inhibitor represented a safe and more effective treatment regimen in vivo compared with single agents alone. Therefore, our findings suggest that the dual inhibition of PI3K and mTOR pathways may represent a novel treatment strategy for advanced NETs and that combination with an MEK inhibitor may further enhance the anti-proliferative effect.
Acknowledgments
Financial Support: This work is supported by the P20CA153043 (GI SPORE), R37AG010885-21, and R01 DK048498-18 grants from the National Institutes of Health.
Footnotes
Disclosures: The authors have no conflicts of interest regarding publication of this manuscript.
REFERENCES
- 1.Modlin IM, Lye KD, Kidd M. A 5-decade analysis of 13,715 carcinoid tumors. Cancer. 2003;97:934–959. doi: 10.1002/cncr.11105. [DOI] [PubMed] [Google Scholar]
- 2.Kulke MH. Clinical presentation and management of carcinoid tumors. Hematol Oncol Clin North Am. 2007;21:433–455. vii–viii. doi: 10.1016/j.hoc.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 3.Valentino J, Evers B Mark. Recent Advances in the Diagnosis and Treatment of Gastrointestinal Carcinoids. In: Cameron JL, editor. Advances in Surgery. Elsevier Inc.; 2011. pp. 285–300. [DOI] [PubMed] [Google Scholar]
- 4.Rorstad O. Prognostic indicators for carcinoid neuroendocrine tumors of the gastrointestinal tract. J Surg Oncol. 2005;89:151–160. doi: 10.1002/jso.20179. [DOI] [PubMed] [Google Scholar]
- 5.Cantley LC. The phosphoinositide 3-kinase pathway. Science. 2002;296:1655–1657. doi: 10.1126/science.296.5573.1655. [DOI] [PubMed] [Google Scholar]
- 6.Gulhati P, Cai Q, Li J, Liu J, Rychahou PG, Qiu S, et al. Targeted inhibition of mammalian target of rapamycin signaling inhibits tumorigenesis of colorectal cancer. Clin Cancer Res. 2009;15:7207–7216. doi: 10.1158/1078-0432.CCR-09-1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Silva SR, Zaytseva YY, Jackson LN, Lee EY, Weiss HL, Bowen KA, et al. The effect of PTEN on serotonin synthesis and secretion from the carcinoid cell line BON. Anticancer Res. 2011;31:1153–1160. [PMC free article] [PubMed] [Google Scholar]
- 8.Luo J, Manning BD, Cantley LC. Targeting the PI3K-Akt pathway in human cancer: rationale and promise. Cancer Cell. 2003;4:257–262. doi: 10.1016/s1535-6108(03)00248-4. [DOI] [PubMed] [Google Scholar]
- 9.Vivanco I, Sawyers CL. The phosphatidylinositol 3-Kinase AKT pathway in human cancer. Nat Rev Cancer. 2002;2:489–501. doi: 10.1038/nrc839. [DOI] [PubMed] [Google Scholar]
- 10.Rychahou PG, Jackson LN, Silva SR, Rajaraman S, Evers BM. Targeted molecular therapy of the PI3K pathway: therapeutic significance of PI3K subunit targeting in colorectal carcinoma. Ann Surg. 2006;243:833–842. doi: 10.1097/01.sla.0000220040.66012.a9. discussion 43-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rychahou PG, Kang J, Gulhati P, Doan HQ, Chen LA, Xiao SY, et al. Akt2 overexpression plays a critical role in the establishment of colorectal cancer metastasis. Proc Natl Acad Sci U S A. 2008;105:20315–20320. doi: 10.1073/pnas.0810715105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yao JC, Shah MH, Ito T, Bohas CL, Wolin EM, Van Cutsem E, et al. Everolimus for advanced pancreatic neuroendocrine tumors. N Engl J Med. 2011;364:514–523. doi: 10.1056/NEJMoa1009290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McCubrey JA, Steelman LS, Chappell WH, Abrams SL, Wong EW, Chang F, et al. Roles of the Raf/MEK/ERK pathway in cell growth, malignant transformation and drug resistance. Biochim Biophys Acta. 2007;1773:1263–1284. doi: 10.1016/j.bbamcr.2006.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shaw RJ, Cantley LC. Ras, PI(3)K and mTOR signalling controls tumour cell growth. Nature. 2006;441:424–430. doi: 10.1038/nature04869. [DOI] [PubMed] [Google Scholar]
- 15.Zitzmann K, Ruden J, Brand S, Goke B, Lichtl J, Spottl G, et al. Compensatory activation of Akt in response to mTOR and Raf inhibitors - a rationale for dual-targeted therapy approaches in neuroendocrine tumor disease. Cancer Lett. 2010;295:100–109. doi: 10.1016/j.canlet.2010.02.018. [DOI] [PubMed] [Google Scholar]
- 16.She QB, Halilovic E, Ye Q, Zhen W, Shirasawa S, Sasazuki T, et al. 4E-BP1 is a key effector of the oncogenic activation of the AKT and ERK signaling pathways that integrates their function in tumors. Cancer Cell. 2010;18:39–51. doi: 10.1016/j.ccr.2010.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Valentino JD, Elliott VA, Zaytseva YY, Rychahou PG, Mustain WC, Wang C, et al. Novel small interfering RNA cotargeting strategy as treatment for colorectal cancer. Surgery. 2012;152:277–285. doi: 10.1016/j.surg.2012.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tannapfel A, Vomschloss S, Karhoff D, Markwarth A, Hengge UR, Wittekind C, et al. BRAF gene mutations are rare events in gastroenteropancreatic neuroendocrine tumors. Am J Clin Pathol. 2005;123:256–260. [PubMed] [Google Scholar]
- 19.Akiyama H, Kamiguchi H. Phosphatidylinositol 3-kinase facilitates microtubule-dependent membrane transport for neuronal growth cone guidance. J Biol Chem. 2010;285:41740–41748. doi: 10.1074/jbc.M110.156489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li J, Song J, Cassidy MG, Rychahou P, Starr ME, Liu J, et al. PI3K p110alpha/Akt signaling negatively regulates secretion of the intestinal peptide neurotensin through interference of granule transport. Mol Endocrinol. 2012;26:1380–1393. doi: 10.1210/me.2012-1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Evers BM, Townsend CM, Jr, Upp JR, Allen E, Hurlbut SC, Kim SW, et al. Establishment and characterization of a human carcinoid in nude mice and effect of various agents on tumor growth. Gastroenterology. 1991;101:303–311. doi: 10.1016/0016-5085(91)90004-5. [DOI] [PubMed] [Google Scholar]
- 22.Takahashi T, Nau MM, Chiba I, Birrer MJ, Rosenberg RK, Vinocour M, et al. p53: a frequent target for genetic abnormalities in lung cancer. Science. 1989;246:491–494. doi: 10.1126/science.2554494. [DOI] [PubMed] [Google Scholar]
- 23.Brandt DW, Pandol SJ, Deftos LJ. Calcium-stimulated parathyroid hormone-like protein secretion: potentiation through a protein kinase-C pathway. Endocrinology. 1991;128:2999–3004. doi: 10.1210/endo-128-6-2999. [DOI] [PubMed] [Google Scholar]
- 24.Doihara H, Nozawa K, Kojima R, Kawabata-Shoda E, Yokoyama T, Ito H. QGP-1 cells release 5-HT via TRPA1 activation; a model of human enterochromaffin cells. Mol Cell Biochem. 2009;331:239–245. doi: 10.1007/s11010-009-0165-7. [DOI] [PubMed] [Google Scholar]
- 25.Rychahou PG, Murillo CA, Evers BM. Targeted RNA interference of PI3K pathway components sensitizes colon cancer cells to TNF-related apoptosis-inducing ligand (TRAIL) Surgery. 2005;138:391–397. doi: 10.1016/j.surg.2005.05.012. [DOI] [PubMed] [Google Scholar]
- 26.Li J, O'Connor KL, Cheng X, Mei FC, Uchida T, Townsend CM, Jr, et al. Cyclic adenosine 5'-monophosphate-stimulated neurotensin secretion is mediated through Rap1 downstream of both Epac and protein kinase A signaling pathways. Mol Endocrinol. 2007;21:159–171. doi: 10.1210/me.2006-0340. [DOI] [PubMed] [Google Scholar]
- 27.Li J, Chen LA, Townsend CM, Jr, Evers BM. PKD1, PKD2, and their substrate Kidins220 regulate neurotensin secretion in the BON human endocrine cell line. J Biol Chem. 2008;283:2614–2621. doi: 10.1074/jbc.M707513200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Carracedo A, Ma L, Teruya-Feldstein J, Rojo F, Salmena L, Alimonti A, et al. Inhibition of mTORC1 leads to MAPK pathway activation through a PI3K-dependent feedback loop in human cancer. J Clin Invest. 2008;118:3065–3074. doi: 10.1172/JCI34739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pitt SC, Davis R, Kunnimalaiyaan M, Chen H. AKT and PTEN expression in human gastrointestinal carcinoid tumors. Am J Transl Res. 2009;1:291–299. [PMC free article] [PubMed] [Google Scholar]
- 30.Voortman J, Lee JH, Killian JK, Suuriniemi M, Wang Y, Lucchi M, et al. Array comparative genomic hybridization-based characterization of genetic alterations in pulmonary neuroendocrine tumors. Proc Natl Acad Sci U S A. 2010;107:13040–13045. doi: 10.1073/pnas.1008132107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang L, Ignat A, Axiotis CA. Differential expression of the PTEN tumor suppressor protein in fetal and adult neuroendocrine tissues and tumors: progressive loss of PTEN expression in poorly differentiated neuroendocrine neoplasms. Appl Immunohistochem Mol Morphol. 2002;10:139–146. doi: 10.1097/00129039-200206000-00008. [DOI] [PubMed] [Google Scholar]
- 32.Pitt SC, Chen H, Kunnimalaiyaan M. Inhibition of phosphatidylinositol 3-kinase/Akt signaling suppresses tumor cell proliferation and neuroendocrine marker expression in GI carcinoid tumors. Ann Surg Oncol. 2009;16:2936–2942. doi: 10.1245/s10434-009-0591-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pitt SC, Chen H, Kunnimalaiyaan M. Phosphatidylinositol 3-kinase-Akt signaling in pulmonary carcinoid cells. J Am Coll Surg. 2009;209:82–88. doi: 10.1016/j.jamcollsurg.2009.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shah T, Hochhauser D, Frow R, Quaglia A, Dhillon AP, Caplin ME. Epidermal growth factor receptor expression and activation in neuroendocrine tumours. J Neuroendocrinol. 2006;18:355–360. doi: 10.1111/j.1365-2826.2006.01425.x. [DOI] [PubMed] [Google Scholar]
- 35.Modlin IM, Shapiro MD, Kidd M. Carcinoid tumors and fibrosis: an association with no explanation. Am J Gastroenterol. 2004;99:2466–2478. doi: 10.1111/j.1572-0241.2004.40507.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.