Abstract
Initially neutral conditioned stimuli (CSs) paired with food often acquire motivating properties. For example, CS presentations may enhance the rate of instrumental responding that normally earns that food reward (Pavlovian-instrumental transfer), or potentiate consumption of that food when the animal is food-sated. Recent evidence suggests that cues associated with the withdrawal of food and food cues (interruption stimuli or ISs) may also potentiate feeding, despite exhibiting some characteristics of conditioned inhibition. Here, we compared the ability of CSs and ISs to modulate both eating food and working for it. If CSs and ISs potentiate eating food by controlling a similar incentive state, both types of cues might also be expected to enhance instrumental responding for food. Although we found substantial potentiation of feeding by both CSs and ISs, and powerful enhancement of instrumental responding by a CS, we found no evidence for such instrumental enhancement by an IS. Furthermore, although an IS produced more FOS expression in the amygdala central nucleus (CeA) than either a previously reinforced CS or a control stimulus after a test for potentiated feeding, an intact CeA was unnecessary for potentiation of feeding by either a CS or an IS. Nevertheless, as in previous studies, CeA was critical to the ability of a CS to enhance instrumental responding. Implications for understanding the nature and basis for incentive learning are discussed.
Keywords: cue-potentiated feeding, Pavlovian-instrumental transfer, amygdala central nucleus, incentive learning
Initially neutral stimuli paired with food often acquire motivating properties. For example, a food-paired cue typically enhances the rate of instrumental lever-pressing that earns that food reward (Pavlovian-instrumental transfer, or PIT; e.g., Holland, 2004, Holmes, Marchand, & Coutureau, 2010). Moreover, a stimulus paired with food while an animal is food-deprived can potentiate consumption of that food later when the animal is food-sated (Holland & Petrovich, 2005; Johnson, 2013; Weingarten, 1983). In addition to the direct evidence of learned incentive motivational function it provides, this observation may provide a basis for understanding what may be a major contributor to the so-called “obesity epidemic”, (e.g., Levitsky & Pacanowski, 2011), overconsumption of unneeded food in an environment rich in enticing food-related cues.
A series of experiments that combined tract tracing, immediate early gene, lesion and other methods identified brain circuitry important for this conditioned potentiation of feeding (reviewed by Holland & Petrovich, 2005; Johnson, 2013). This circuit includes the lateral hypothalamus, basolateral amygdala (BLA), and medial prefrontal cortex, but surprisingly not the amygdala central nucleus (CeA), nucleus accumbens or lateral orbitofrontal cortex, each of which has been implicated in PIT and other aspects of feeding (Holland, Petrovich, & Gallagher, 2002; Petrovich, Holland, & Gallagher, 2005; Petrovich, Ross, Holland, & Gallagher, 2007; Petrovich, Setlow, Holland, & Gallagher, 2002).
Interestingly, cues associated with the withdrawal of food and food cues may also potentiate feeding. Galarce and Holland (2009) first trained rats with a 2-min auditory conditioned stimulus (CS) during which food was presented in a probabilistic fashion. Then, on some trials, that CS was terminated prematurely, whereupon another 10-s auditory cue (termed an “interruption stimulus” or IS) was presented. Later, when the rats were food-sated, that IS was found to potentiate consumption of the food reward, as did the CS, despite evidence from summation and retardation tests that the rats had learned an inhibitory association between the IS and food. Subsequent experimentation (Galarce et al., 2010) replicated the basic observation of IS-potentiated feeding and showed that it shared several properties of CS-potentiated feeding, including specificity to the original food reward and dependence on an intact BLA.
Here, we first compared the ability of CSs and ISs to modulate both eating food and working for it (i.e., cue-potentiated feeding and PIT). If CSs and ISs potentiate eating food by controlling a similar incentive state, both types of cues might also be expected to potentiate instrumental responding for food. Second, we examined the role of CeA in these modulatory functions by examining FOS expression after a test of CS- or IS-potentiated eating (Experiment 1) and by examining the effects of CeA lesions on the modulation of both feeding and instrumental behavior by CSs or ISs (Experiment 2). Although previous experiments showed that CeA lesions do not impair CS-potentiated feeding, those lesions do impair PIT (Corbit & Balleine, 2005; Hall et al., 2001; Holland & Gallagher, 2003). Furthermore, Purgert et al. (2012) found substantial immediate early gene expression in CeA neurons in response to a cue that, like an IS, accompanied the surprising omission of sucrose after presentation of a CS. Thus, it is possible that CeA is importantly involved in the potentiation of feeding and/or food-rewarded instrumental behavior by an IS.
Materials and Methods
Animals
The subjects were male Long-Evans rats (Charles River Laboratories, Raleigh, NC, USA), which weighed 275–325 g on arrival to the laboratory vivarium. After a month of free access to food (2018 Rodent Diet, Harlan Teklad Laboratory, Madison, WI, USA) and water, the rats were given restricted food access until they reached and maintained 85% of their ad libitum weights. Additional experimental manipulations of food access are described later in the behavioral training procedures. During the course of the study, the rats were housed in individual cages in a colony room that was illuminated from 7:00 a.m. to 7:00 p.m. Behavioral training sessions were conducted near the middle of the light-on period. There were 12 rats in Experiment 1 and 28 rats in Experiment 2. The care and experimental treatment of the rats was approved by the Johns Hopkins University Animal Care and Use Committee.
Apparatus
This study used eight training chambers (20.5 cm × 22.0 cm × 22.5 cm), with stainless steel front and back walls and clear acrylic side walls and tops. An illuminated, clear acrylic shallow liquid well, which could hold approximately 1.7ml, was recessed into the center of the front wall. A photocell beam in the liquid recess detected head entries and the time rats spent in the liquid well recess. A retractable response lever could be presented to the left of the liquid well; it was present only in instrumental training and PIT test sessions. A speaker for delivering a 78-db white noise cue, a piezoelectric device for presenting an intermittent (3 Hz) 79-db, 1900-hz tone, and a relay clicker (4 hz) were mounted on the side wall of a sound-resistant shell that enclosed each chamber. A video camera was aimed at the area that included the liquid well recess to record the rat’s behaviors, and a second camera was located under the liquid well to record consummatory responses. To aid in video recording, a panel of infrared lights was placed on top of each experimental chamber. The camera images were digitized, recorded, and shown in real time on four video monitors. Each of these displayed images of four chambers or liquid wells.
Behavioral training procedures
The behavioral training procedures of Experiments 1 and 2 were identical except for the order in which potentiated feeding and PIT tests were conducted, and some differences in the nature of the tests (noted later). All rats first received Pavlovian cue training with a CS, an IS, and an unpaired control stimulus (U). Rats in Experiment 1 then received instrumental lever-press training and PIT testing, followed by food satiation and potentiated feeding testing, whereas rats in Experiment 2 received food satiation and potentiated feeding testing first, followed by redeprivation, lever-press training, and PIT testing.
Pavlovian cue training
The rats were first taught to approach and consume the sucrose reinforcer from the liquid wells. In each of two 64-min sessions there were 16 0.1-ml deliveries of an 8% sucrose solution, which served as the unconditioned stimulus (US). Next, the rats were given 6 60-min training sessions designed to establish a Pavlovian association between a tone and sucrose. In each of these sessions, they received 10 2-min presentations of the intermittent, 1900-hz tone CS. In 9 of these CS trials, 4 USs were presented at random times on a variable time 30 s (VT 30 s) schedule. A single trial was selected as a CS ‘catch’ trial, which permitted assessing liquid well recess entries not confounded by the delivery of sucrose. On that trial, sucrose could not occur in the first 20 s, and the likelihood of sucrose delivery was increased in the remaining 100 s (VT 25 s) to produce the same overall density of reinforcement across all 10 trials.
Next, the rats received twelve 60-min IS training sessions. Each session included one CS catch trial and 9 CS trials (as before), and 6 IS trials. On IS trials, the CS was presented and reinforced with sucrose in the same manner as on CS trials, except that 30 s, 60 s, or 90 s after its onset, the CS was terminated and a 10-s IS was presented. No sucrose presentations were permitted during the IS. A 10-s white noise or a 10-s clicker stimulus (counterbalanced) served as the IS. Regardless of the CS duration, the US delivery density was maintained at VT 30 s during CS. Finally, there were six unpaired cue trials (U), during which 10-s presentations of either the clicker or noise stimulus (whichever did not serve as the IS) was followed by the absence of any cue.
Instrumental response training
All rats were trained to press the left lever. Each rat’s lever presses were reinforced on a fixed ratio 2 (FR 2) schedule until 100 lever presses were made, at which time the rat was removed from the chamber. All rats met this criterion within 60 min. They then received 6 daily 30-min sessions of instrumental training, in which they were reinforced with sucrose on a variable interval 30 s (VI 30 s) schedule. Finally, in preparation for the PIT tests, the rats were given a single IS training reminder session, identical to those received in Pavlovian training, followed by another VI 30-s session.
Pavlovian-instrumental transfer (PIT) testing
Each rat received two 20-min PIT tests to evaluate the abilities of the CS, IS, and U to enhance instrumental lever pressing. One PIT test included the CS and U, and the other including the IS and U. A 30-min VI 30 s lever press training session was given between the two PIT tests. In Experiment 1, all rats received the IS PIT test first, whereas in Experiment 2 the test order was counterbalanced. During each test there were two presentations of the CS (or IS) and two presentations of U, in order CS/IS, U, U, CS/IS. Although lever pressing was reinforced on a VI 30 s schedule throughout both PIT test sessions, no other USs were delivered on any trial. To facilitate comparisons across stimuli, in the test sessions, the durations of CS, IS, and U (which differed in training) were equated. In Experiment 1, all stimuli were 120 s in duration, whereas in Experiment 2 they were 20 s long.
At the conclusion of PIT testing, the rats in Experiment 1 received a 60-min IS training reminder session, in preparation for subsequent food satiation and potentiated feeding testing.
Potentiated feeding testing
Rats were first given 7 days free access to their normal chow in their home cages. In Experiment 1, the sated rats then received a single 20-min potentiated feeding test, designed to assess consumption induced by CS, IS, or U. To satiate rats further on the sucrose US itself, rats were given 10 min unlimited access to 15 ml sucrose placed in cups attached to the floor of the experimental chamber, in front of the liquid well. After 10 min had elapsed, the rats were removed from the experimental chambers. The cups were removed and set aside for subsequent measurement of liquid consumption, and the liquid wells filled with 1.6 ml sucrose before the rats were replaced in the chambers for 20 min. In the first 2 min period of this test, no stimuli (other than sucrose) were presented. Over the next 18 min, the rats received 14 20-s stimulus presentations, 7 of U, and 7 of either IS (n=4), CS (n=4), or additional U presentations (n=4). We chose 20-s test presentations to match the procedures used by Galarce & Holland (2009) and Galarce et al. (2010). Those studies showed that consumption was potentiated during 20-s CS or IS presentations compared to consumption during stimulus-free periods. The inclusion of U trials permitted attribution of CS- or IS-potentiated consumption to the training histories of those stimuli rather than the simple presentation of a stimulus. Because all rats received identical presentations of the U control stimulus, differences in FOS expression among the 3 groups could be attributed to effects of the different target stimuli (CS, IS, or U) presented to those groups. In Experiment 2, each rat received two potentiated feeding tests, one with CS and U, and one with IS and U, identical to the CS and IS tests of Experiment 1. These two tests were given 1 day apart and their order was counterbalanced.
The rats were tested in squads of 4. During the test, the consumption behaviors of the rats were monitored (via the liquid well video cameras) by an experimenter who sampled each camera image once each s throughout the test session. When the liquid in a well was close to depletion, an additional 0.1 ml was delivered manually by signaling a computer program that activated the corresponding infusion pump and noted the timing and number of these deliveries, which served as a record of the pattern and quantity of sucrose consumed by each rat. To reduce the chance of experimenter bias, the experimenter was blind to whether a cue was being delivered or not, and to what types of cues each rat received.
Response measures and data analysis
Pavlovian response measures
The measure of conditioned responding was the percentage of time the sucrose well photobeam was broken during presentations of the CS on catch trials, and during IS and U presentations. The percentages of time spent in the sucrose well during the 10-s period before each stimulus presentation were also recorded. To reduce the effects of individual differences in tendencies to enter the sucrose well, elevation scores were constructed by subtracting pre-stimulus responding from responding during each cue.
PIT measures
The measures of PIT produced by the CS, IS and U were elevation scores computed by subtracting the rate of lever-press responding in the stimulus-free period prior to each test trial from the response rate during the first 20 s of cue presentation during that trial. We chose a 20-s test interval to match the sampling procedure used in the potentiated feeding test. In addition, we contrasted the elevation scores obtained on CS or IS trials with those found on U trials to determine if the CS or IS potentiated lever-pressing beyond the rates produced in the presence of another auditory stimulus that had not accompanied sucrose presentation or unexpected sucrose omission.
Potentiated feeding measures
The measure of potentiated feeding was the difference between the rate of sucrose deliveries needed to maintain a constant sucrose well volume during stimulus presentations and the rate needed during stimulus-free periods. As for PIT test data, we also contrasted the elevation scores obtained on CS or IS trials with those found on U trials to determine if the CS or IS potentiated consumption beyond the levels observed in the presence of another auditory stimulus (U) that had not accompanied sucrose presentation or unexpected sucrose omission. Finally, following Galarce and Holland (2009) and Galarce et al. (2010), we presented data only from the last 4 of each 20-s stimulus presentations and the 40-s stimulus-free periods prior to each of those stimulus presentations. First, despite home cage chow satiation and the availability of sucrose 10 min prior to the test, consumption continued at a high rate during the first few minutes of the test session. Second, initial presentations of the IS alone caused many of the rats to withdraw from the food cup. This tendency might reflect conditioned inhibitory tendencies (Galarce & Holland, 2009) or the novelty of IS-alone presentations.
Behavioral data analysis
All response measures were subjected to repeated measures analyses of variance (ANOVAs) using the Greenhouse-Geisser correction to guard against any violations of sphericity. Individual contrasts used the Tukey HSD procedure. In Experiment 1, ANOVA of the potentiated feeding test data used a mixed design, with test group as a between-subjects variable, and test interval (baseline, U, or “target” stimulus, the CS or IS) as a within-subject variable. In Experiment 2, all ANOVAs were of mixed design, with lesion (excitotoxic or sham lesion of CeA) as a between-subjects variable.
Analysis of FOS expression
FOS immunochemistry
Amygdala tissue of the rats in Experiment 1 was processed for FOS expression. Ninety min after the start of the potentiated feeding test, the rats were killed by exsanguination under deep isoflurane anesthesia, and perfused with 0.9 % saline followed by 4% paraformaldehyde in 0.1 M phosphate buffer (PB). Brains were removed, post-fixed and cryoprotected overnight in 4% paraformaldehyde in 0.1 M PB containing 12% sucrose, and stored at −80°C. Brains were then sliced on a freezing microtome and 30-μm coronal sections through the amygdala were collected for FOS immunohistochemical staining, which followed a protocol similar to that used by Lee et al. (2005). The primary antibody was rabbit FOS antibody (1:5000 dilution; Santa Cruz Biotechnology, no. sc-52). After the primary antibody incubation (48–72 hrs at 4 °C), sections were rinsed in PBS, incubated in goat antirabbit IgG-biotinylated secondary antibody (1:250 dilution; Vector Laboratories) for 1–1.5 hrs, rinsed in PBS and then incubated in avidin–biotin peroxidase conjugate (Vector laboratories) for 1–2 hrs. After several rinses in PBS, sections were reacted using a Vector DAB substrate kit for peroxidase (Vector Laboratories) to visualize FOS. Sections were mounted on slides, dehydrated in ascending concentrations of alcohol, and coverslipped with Permount (Fisher Scientific).
FOS+ cell counting
Cell counting was conducted blind with respect to the test condition. Six sections from different rostral–caudal levels (−1.80, −2.12, −2.30, −2.80, −3.14, and −3.60 relative to bregma) of each rat brain contributed to the analysis. Images of the FOS-stained sections were acquired using a MicroPublisher RTV camera (QImaging, Burnaby, BC, Canada). Borders of the CeA and BLA were drawn on the images of FOS-stained sections, and FOS+ cells were counted with the aid of an image analysis system (NIH Image 1.63), which contrasted potential FOS+ cells with the background density in each section. Borders of the amygdalar subregions were defined according to the Paxinos and Watson (1998) Rat Brain Atlas.
Lesion Surgery and evaluation procedures
Surgery
In Experiment 2, surgery was performed after a month’s acclimatization to the colony and prior to food deprivation. The surgery was performed under aseptic conditions with isoflurane anesthesia, and all infusions were made with a Hamilton 2.0-μl syringe and 25-gauge needle. Bilateral CeA lesions (18 rats) were made with ibotenic acid (10 mg/ml in PBS, pH=7.4; Sigma St. Louis, MO, USA) using the coordinates 2.2 mm posterior of bregma, 4.3 mm from the midline, and 8.1 mm (0.15 μl/site) ventral from the skull surface at the injection site. Ten rats that received bilateral sham lesions underwent the same surgical procedures, but no infusions were made once the needle was in position. After surgery, each rat received a single subcutaneous injection of buprenorphine (0.02 mg/kg) to ameliorate pain, and was allowed to recover with free access to food for 10 days prior to food deprivation.
Lesion evaluation
At the conclusion of Experiment 2, the rats were anesthetized heavily with isoflurane and perfused intracardially with 0.9% saline, followed by 10% (v/v) Formalin in 0.1M PBS. Brains were removed and stored in 0.1M PBS and 20% (w/v) sucrose. 40-μm slices were collected and Nissl-stained to verify lesion placements. CeA lesions were evaluated from photographs of Nissl-stained sections at 4 coronal planes of CeA. Outlines of the lesion extents were drawn on digital images from Paxinos and Watson (1998) using Adobe Photoshop 11.0.2. Calculation of percent damage was performed within Photoshop by comparing the area of the intersection of lesion and region extent with the area within the region’s borders.
Results
Experiment 1
Behavior
Acquisition
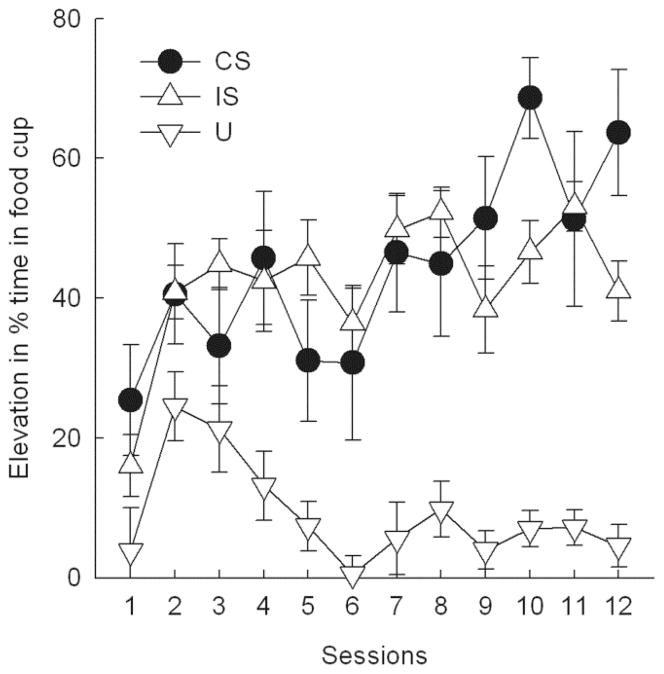
Performance on catch trials did not change appreciably during the initial nondiscriminative conditioning of the CS, F(5, 50) = 2.03, p = .090, reaching elevation scores of about 10%. However, over the course of subsequent IS training, acquisition to the CS was rapid, and responding on the CS trials was readily discriminated from that on U trials. A cue X session ANOVA of elevation scores showed significant main effects of cue, F(2, 22) = 64.69, p < .001, session, F(11, 121) = 4.02, p < .001, and their interaction, F(22, 242) = 2.55, p < .001. Responding to the IS was maintained at levels comparable to CS responding until the last 4-session block of training, when IS responding was significantly lower than CS (catch trial) responding, F(1, 22) = 11.86, p = .005. The high levels of responding to IS during acquisition are not surprising because IS was presented immediately after CS and US termination, and hence responding during IS likely reflects substantial ‘carryover’ of sucrose well responding controlled by the CS and the US. Furthermore, it is notable that during CS periods immediately prior to IS presentations (which often included US presentations), the rats had their heads in the liquid wells nearly 100% of the time (not shown), so rats’ responding was more reduced by IS presentations than comparisons with the catch trial data might suggest. Pre-stimulus responding (overall mean ± sem percentage time in the sucrose well was 15.1 ± 2.5%) did not change significantly over the course of training nor did that responding differ across the trial types, ps > .24.
All rats acquired instrumental lever-pressing rapidly. By the last session of VI 30-s training, the rats responded at a rate of 12.9 ± 1 lever presses/min. Similarly, the rats made 15.1 ± 1.6 lever presses/min in the VI 30-s reminder session that occurred between the two PIT tests.
Pavlovian-Instrumental (PIT) transfer tests
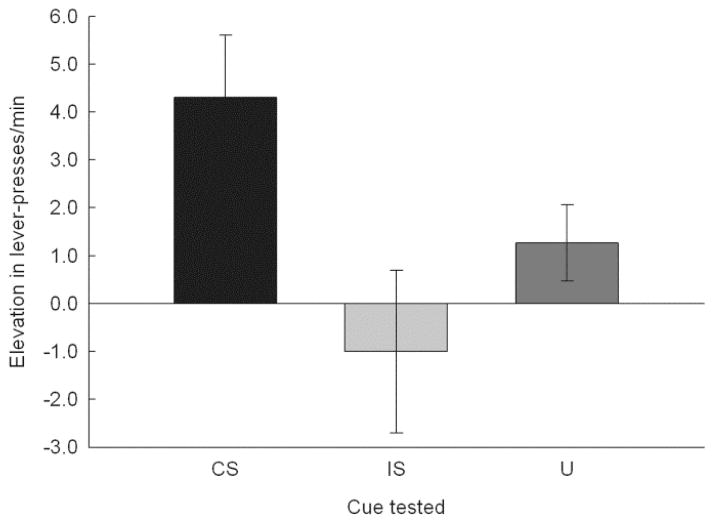
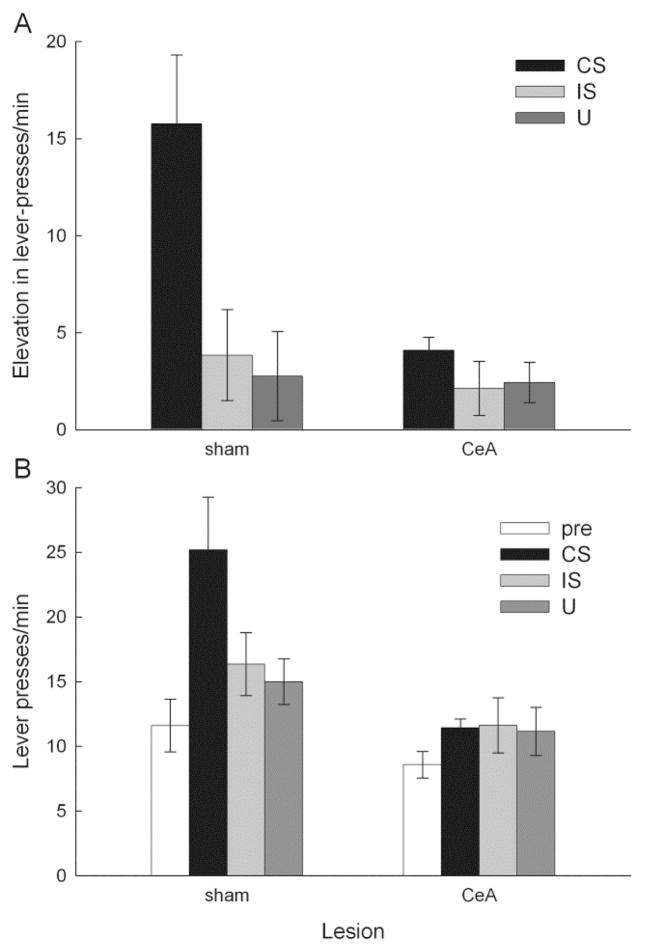
Figure 2 shows the results of the two PIT tests. Presentation of the CS, but not of the IS, enhanced rates of instrumental lever pressing above baseline rates and above responding to the control cue (U). A one-way repeated measures ANOVA of the elevation scores showed a significant effect of cue, F(2, 22) = 4.09, p = .031, and individual contrasts showed that elevation during the CS was significantly greater than that during either IS or U, ps < .050, but elevation during IS and U did not differ, p = .277. Pre-stimulus (baseline) responding was 12.0 ± 1.1 lever presses/min and did not differ across trial types, F < 1, p = .857. Comparison of responding during the various stimuli to responding prior to those stimuli showed significant elevation over baseline only during the CS, p = .007, other ps > .167.
Figure 2.
Mean ± s.e.m performance in the Pavlovian-instrumental transfer tests of Experiment 1. Entries are expressed as the elevation in lever-press rate during presentations of the conditioned stimulus (CS), interruption stimulus (IS), and unpaired (U) stimulus over the lever-press rate during pre-stimulus periods. Elevation scores were significantly (p < .050) greater for CS trials than for either IS or U trials.
Potentiation of feeding test
Rats gained considerable weight over the 7 days free access to chow that preceded the potentiated feeding test, from 336 ± 8 g (deprived) to 428 ± 7 g (sated).
Because the primary purpose of the potentiated feeding tests was the evaluation of amygdala FOS, consumption was tested in a session that included either the CS or IS “target” stimulus, but not both. However, all rats also received presentations of the U control stimulus in that session. These presentations allowed for within–subject comparisons of the effects of the CS or IS on consumption, relative to that control stimulus. Finally, one group of rats received only presentations of U during the test session; both the “target” and control stimuli were U for those rats.
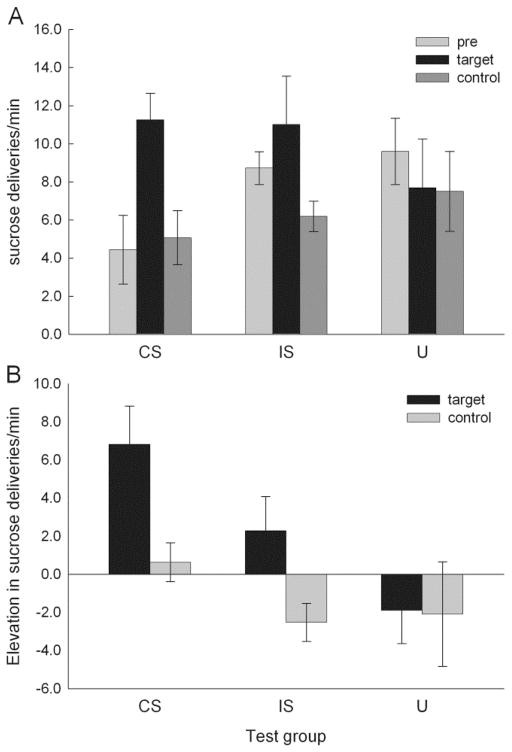
Consistent with Galarce and Holland’s (2009)’s observations, total sucrose consumption did not differ (F < 1, p = .570) across the 3 test conditions (18.4 ± 1.4, 18.2 ± 4.4, and 22.6 ± 2.4 ml, that is, 9.2 ± 0.7, 9.1 ± 2.2, and 11.3 ± 1.2 deliveries/min over the entire 20 min test, for groups CS, IS, and U, respectively). However, consumption was differentially distributed across stimulus and non-stimulus periods in those three conditions. All 12 rats showed greater consumption during CS or IS periods than during U or pre-stimulus periods, but only 4 rats (2 each in groups CS and U) showed more responding during U periods than during pre-stimulus periods. Figure 3 shows consumption over the final 4 trials of each type in the potentiated feeding test. A group (CS, IS or U) X period (pre-stimulus, target, or U) ANOVA of the data shown in Figure 3A showed a significant effect of period, F(2,18) = 5.07, p = .018. A group X stimulus (target or control) ANOVA of elevation scores (cue consumption minus baseline consumption; Figure 3B) cue showed significant effects of both group, F(2,9) = 4.74, p = .039, and stimulus, F(1,9) = 6.89, p = .028, with no significant group X stimulus interaction, F(2, 9) = 1.63, p = .248. Individual contrasts of elevation scores for target versus control stimuli were significant for both CS and IS targets, ps < .050, but not for U (for which both target and control were the same event), p = .941. Elevation scores for CS and IS targets did not differ, p = .702.
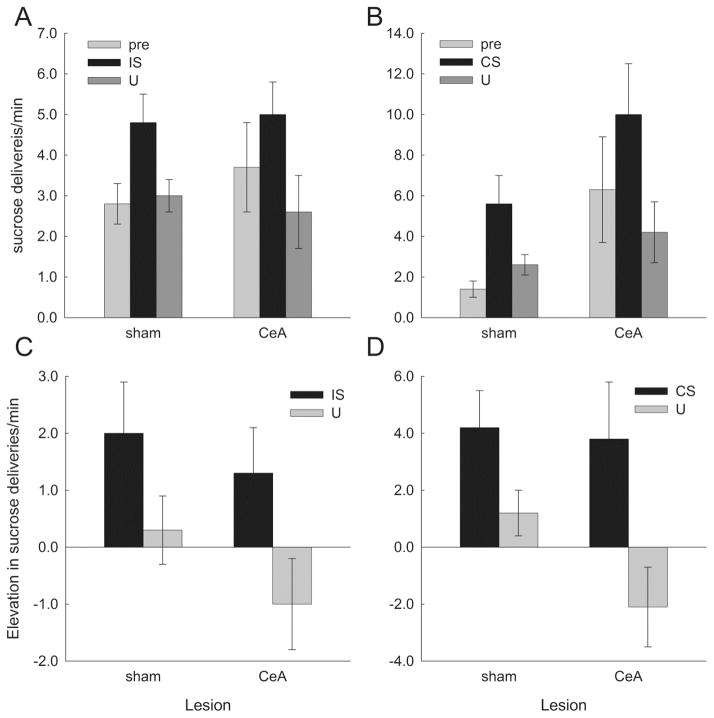
Figure 3.
Consumption in the potentiated feeding tests of Experiment 1. In Figure 3A, entries are the mean ± s.e.m. rate of 0.1-ml deliveries needed to maintain constant sucrose levels in the food wells during the pre-stimulus, target stimulus, and control stimulus periods in each of the three test conditions. Rats in the CS test condition were tested with the conditioned stimulus and the control stimulus, rats in the IS condition were tested with the interruption stimulus and the control stimulus, and rats in the U condition were tested with the unpaired control stimulus as both target and control stimulus. Consumption was significantly (p < .050) greater during the target stimuli than during the control stimuli for both CS and IS targets, but not for the U target (which was the same as the control stimulus). Figure 3B shows the same data, expressed as the difference between sucrose delivery rates during the CS, IS, or U and pre-stimulus delivery rates (elevation scores). As with absolute consumption scores, these elevation scores were significantly (p < .050) greater for the target stimuli than for the control stimuli for both CS and IS targets, but not for the U target.
Although both CS and IS significantly elevated consumption relative to the U control stimulus, some caution may be in order in interpreting these results because baseline (pre-stimulus) consumption appeared to be lower in the CS-tested rats than in the other rats; ANOVA of pre-stimulus consumption scores alone showed a marginal effect of test group, F(2, 9) = 3.26, p = .086. Indeed, post-hoc contrasts of consumption during the target cues with pre-stimulus baseline consumption were significant (p < .05) only for the CS-tested rats, other ps > .244. Nevertheless, we believe that the most appropriate evaluation of the effects of the CS and IS is provided by comparison with consumption in the presence of the U control stimulus, rather than with pre-stimulus consumption. Importantly, consumption during U did not differ across the groups, F(1,9) = .63, p = .553, pre-stimulus consumption did not differ between target (CS or IS) and U trials in testing, F(2,9) = 1.38, p = .300, and ANOVAs and contrasts of absolute consumption scores during CS, IS, and U yielded the same pattern of statistical significance as the analyses of elevation scores reported in the preceding paragraph.
FOS expression
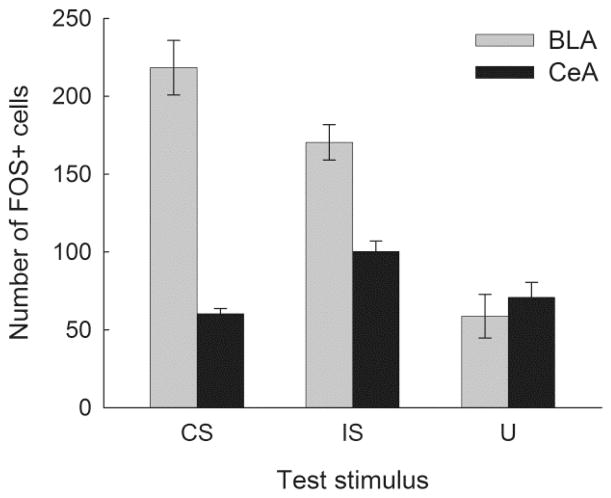
Figure 4 shows FOS counts in CeA and BLA in the rats tested for sucrose consumption in the presence of the CS, IS, and U. Recall that the test included U presentations for all rats. Separate one-way ANOVAs for each region showed significant effects of test region, Fs(2, 9) > 12.63, ps < .003. Tukey HSD tests showed that in BLA, FOS+ counts were lower in the U test condition than in either the CS or IS test conditions, ps < .002, which did not differ, p = .102. By contrast, in CeA, IS FOS+ counts were significantly higher than in either the U or CS conditions, ps < .041, which did not differ, p = .149. Note that these between-group differences in FOS+ counts were not confounded with the amount of sucrose consumption itself: total sucrose consumption during the entire FOS test did not differ across those groups. CSs and ISs instead altered the distribution of consumption such that rats consumed more during cue periods than during no-cue periods. These results are consistent with previous demonstrations of greater IEG activity in BLA after CS presentations than after U presentations in potentiated feeding tests BLA, but no such difference in CeA (Petrovich et al., 2005), and with demonstrations that lesions of BLA, but not CeA, impair conditioned potentiation of feeding by a CS (Holland, et al., 2002). Similarly, the observation of greater BLA FOS+ counts after IS than after U is consistent with Galarce et al.’s (2010) observation of impairment of IS-potentiated feeding in rats with BLA lesions.
Figure 4.
FOS expression after the potentiated feeding test in Experiment 1. Rats in the CS test condition were tested with the conditioned stimulus and the control stimulus, rats in the IS condition were tested with the interruption stimulus and the control stimulus, and rats in the U condition were tested with the unpaired control stimulus as both target and control stimulus. Entries are the mean ± s.e.m. numbers of cells that expressed FOS protein. FOS counts in the basolateral amygdala (BLA) were significantly (p < .002) lower in rats in the U condition than in rats tested in either the CS or IS condition. By contrast, FOS counts in the central amygdala (CeA) were significantly (p < .050) greater in rates tested in the IS condition than in rats tested in either the CS or U condition.
The greater CeA FOS+ counts observed in the IS condition than in either the CS or U test conditions suggests that, unlike with CS-potentiated feeding (Holland et al., 2002; Petrovich et al., 2005), CeA may be importantly involved in IS-potentiated feeding. Thus, in Experiment 2 we examined the effects of CeA lesions on IS-induced eating. In that experiment, we also examined the effects of CeA lesions on CS-potentiated feeding, and on the modulation of instrumental responding by the CS and IS.
Experiment 2
Lesions
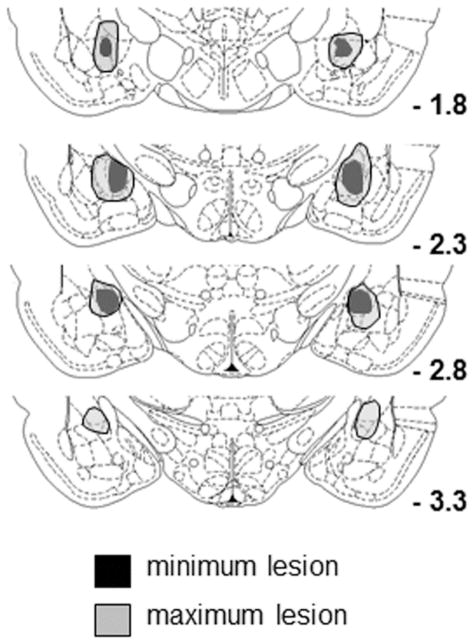
Rats with lesions that included substantial bilateral damage to BLA (n=3) and rats with damage encompassing less than 40% of the area of CeA in either hemisphere (n = 7) were dropped from the study. There were eight rats with acceptable lesions, averaging 63.8 ± 3.2% damage (Figure 5). None of the ten sham-lesioned rats showed any damage, and all were included in the study.
Figure 5.
Drawings of largest and smallest lesions of the amygdala central nucleus in Experiment 2. The numbers indicate the mm posterior to bregma for each section. Brain drawings adapted from The Rat Brain in Stereotaxic Coordinates (4th edition), pp. pp. 189, 192, 194, and 196 by G. Paxinos & C. Watson, 1998, San Diego, CA: Academic Press. Copyright 1998 by Elsevier Academic Press. Adapted with permission.
Behavior
Acquisition
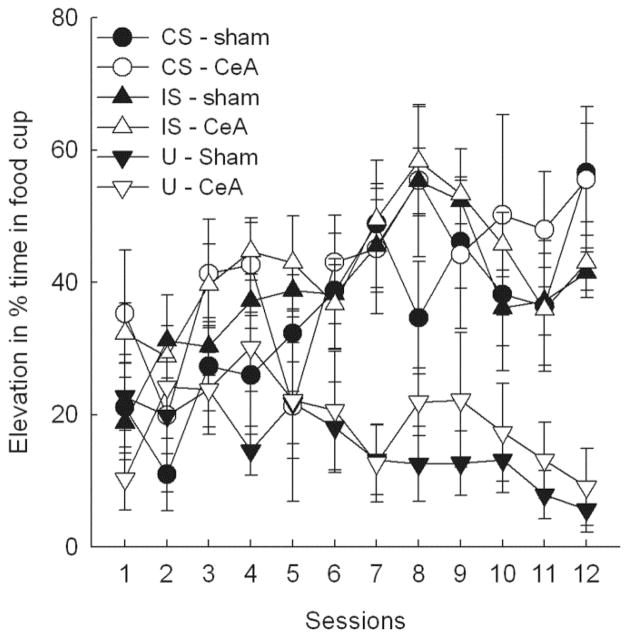
Performance on CS catch trials did not change significantly over the course of the initial nondiscriminative conditioning of the CS, F(5, 80) = 2.07, p = .078, reaching elevation scores of 19.5 ± 7.9% and 27.6 ± 8.8% in sham-lesioned and CeA-lesioned rats, respectively. However, over the course of subsequent IS training, acquisition to the CS was rapid, and CS was readily discriminated from U trials. A lesion X cue X session ANOVA of elevation scores showed significant main effects of cue, F(2, 32) = 40.32, p < .001, session, F(11, 176) = 3.43, p < .001, and their interaction, F(22, 352) = 4.08, p < .001. No effect or interaction involving lesion was significant, ps > .320. Pre-stimulus responding did not change significantly over the course of IS training, F(11, 176) = 1.43, p = .165, nor did that responding differ across lesion or trial types, ps > .424. The overall percentage time in the sucrose well in pre-CS periods was 14.0 ± 3.0% in the sham-lesioned rats and 11.1 ± 1.5% in the CeA-lesioned rats.
Potentiation of feeding test
The primary purpose of Experiment 2 was to evaluate the effects of CeA lesions on potentiated feeding produced by an IS or a CS. All rats received tests of consumption with both an IS and a CS, in counterbalanced order. As in Experiment 1, all rats also received presentations of the U control stimulus in each session, permitting within-subject, within-session comparisons of the effects of CS or IS on consumption, relative to that control.
The week of free access to chow prior to testing increased the rats’ weights substantially. The sham-lesioned rats’ weights increased from 380 ± 16 g (deprived) to 506 ± 15 g (sated) and the CeA-lesioned rats increased from 388 ± 19 g to 516 ± 17 g.
Prior to each test session, the rats were given 10 min access to 15 ml of sucrose in cups placed in the experimental chambers. Sham-lesioned rats consumed 9.5 ± 1.0 ml prior to the IS test and 7.9 ± 0.4 ml prior to the CS test, while CeA-lesioned rats consumed 12.2 ± 0.6 ml and 10.8 ±1.5 ml, respectively. Lesion X test order X test type ANOVA of these data showed no significant effects or interactions (lowest p = .127 for the main effect of lesion, all other ps > .301.
As in Experiment, the total sucrose consumption did not differ across the two test conditions (13.2 ± 2.4 ml and 12.6 ± 3.2 ml in the CS and IS tests, respectively, in the sham-lesioned rats, and 18.4 ± 4.6 ml and 13.6 ± 2.6 ml in the CeA-lesioned rats). A lesion X test ANOVA showed no effect of either lesion, F(1, 16) = 2.58, p = .128, or test, F(1, 16) = 3.20, p = .092. However, consumption was differentially distributed across CS, IS, U and pre-stimulus periods. The CeA lesions had no effect on the ability of either CS or IS to potentiate sucrose consumption, and the magnitudes of potentiation of feeding by CS and by IS were comparable. Consumption during CS and IS periods was elevated over consumption during U periods, as in Experiment 1, as well as over consumption during pre-stimulus periods. Figure 7 shows consumption over the final 4 trials of each type in the potentiated feeding tests, as in Experiment 1. An initial lesion X test order (a counterbalancing variable) X test type (CS or IS) X period (pre-stimulus, target, or control) ANOVA showed only a significant main effect of period, F(1, 14) = 15.53, p < .001. Neither the main effect of lesion, F(1, 14) = 2.31, p = .151, nor that of test type, F(1, 14) = 2.68, p = .124, was significant, but the interaction of lesion and test type was marginally significant, F(1, 14) = 4.39, p = .055. No other interactions with lesion, ps > .134, or test type, ps > .160, approached significance. There were no significant main effects of, nor interactions with, test order, ps > .209, so that variable was dropped from subsequent analyses.
Figure 7.
Consumption in the potentiated feeding tests of Experiment 2, in rats with lesions of the amygdala central nucleus (CeA) or sham lesions of that region. Figures 7A and 7C show consumption during the interruption stimulus (IS) test, and Figures 7B and 7D show consumption during the conditioned stimulus (CS) test. In Figure 7A, entries are the mean ± s.e.m. rate of 0.1-ml deliveries needed to maintain constant sucrose levels in the food wells during the pre-stimulus, IS, and control stimulus (U) periods. Consumption was significantly greater during the IS than during U in both CeA-lesioned (p = .007) and sham-lesioned (p = .024) rats, and there was no effect of lesion (p > .500). Figure 7B shows mean ± s.e.m. sucrose delivery rates during the pre-stimulus, CS, and U periods. Consumption was significantly (p < .050) greater during the CS than during U in both CeA-lesioned and sham-lesioned rats, which did not differ (p = .303). Figures 7C and 7D show the same data as Figures 7A and 7B, expressed as the elevation in sucrose delivery rates during stimulus periods over delivery rates in the pre-stimulus periods. The main effects of cue (IS vs. U or CS vs. U) were significant (p < .001) but not the effect of lesion nor any of its interactions (ps > .157.)
Because the design of the test sessions stressed within-subject comparisons of consumption during the target (CS or IS) and control (U) stimuli, we continued our analysis by conducting separate lesion X period (pre-stimulus, target stimulus, or control stimulus) ANOVAs for the CS and IS consumption tests. Consider first consumption in the IS test (Figure 7A). A lesion X period ANOVA showed a significant effect of period, F(2, 32) = 7.77, p = .002, but no effect of lesion or lesion X period interaction, Fs < 1, ps > .503. Individual comparisons showed that consumption during the IS was significantly greater than during the U stimulus in both sham-lesioned, p = .024, and CeA-lesioned, p = .007, rats, and greater during IS than during the pre-stimulus periods in sham-lesioned rats, p = .010. Consumption during U was no greater than pre-stimulus consumption in either sham-lesioned, p = .715, or CeA-lesioned, p = .217, rats. Consumption during IS was also not significantly elevated over pre-stimulus levels in CeA-lesioned rats, p = .119. Neither consumption during U nor pre-stimulus consumption differed as a function of lesion, Fs < 1 ps > .672. A lesion X stimulus (IS or U) ANOVA of elevation scores (cue consumption minus baseline consumption; Figure 7C) showed a significant effect of stimulus, F(1, 16) = 14.13, p = .002, but not of lesion or stimulus X lesion interaction, ps > .314. Contrasts of IS and control (U) elevation scores were also significant for sham-lesioned and CeA-lesioned rats, taken separately, ps < .029.
Next consider consumption in the CS test (Figure 7B). A lesion X period ANOVA showed only a significant effect of period, F(2, 32) = 9.47, p < .001. Although overall consumption was marginally greater in the CeA-lesioned rats, F(1, 16) = 3.89, p = .066, the lesion X period interaction was not significant, F(2, 32) = 1.24, p = .303, suggesting that the lesion did not amplify (or depress) the effect of IS specifically. Subsequent individual comparisons showed that, in both sham- and CeA-lesioned rats, consumption during the CS was significantly greater than consumption during either the U stimulus or the pre-stimulus periods, ps < .050, which did not differ from each other, ps > .813. A lesion X stimulus (CS or U) ANOVA of elevation scores (cue consumption minus baseline consumption; Figure 7D) showed a significant effect of stimulus, F(1, 16) = 10.79, p = .005, but no effect of lesion or lesion X stimulus interaction, ps > .222. Contrasts of CS and U elevation scores were also significant for sham-lesioned and CeA-lesioned rats, taken separately, ps < .05.
Finally, to compare the magnitudes of potentiation of feeding by CS and IS, we performed a lesion X test type (CS or IS) X cue (target or control) ANOVA of elevation scores across the two consumption tests. This ANOVA showed a significant main effect of cue, F(1, 16) = 21.54, p < .001, but no effects of lesion, F(1, 16) = 2.21, p = .157, test type, F(1, 16) = 2.10, p = .167, or any interactions, ps > .236. Separate test type X cue ANOVAs for sham-lesioned and CeA-lesioned rats also showed significant effects of cue, ps < .022, but not of test type, ps > .152 or test type X cue interactions, ps > .179. Thus, the magnitudes of CS- and IS-potentiation of consumption were similar, both within and between sham-lesioned and CeA-leioned rats.
Instrumental lever pressing and PIT testing
Prior to lever-press training, the rats were deprived to 85% of their new, post-satiation weights (sham, 430 ± 15 g; CeA lesion, 439 ± 17 g), which were substantially higher than their original 85% weights (noted earlier). All rats acquired instrumental lever-pressing rapidly. By the last session of VI 30-s training, the sham-lesioned and CeA-lesioned rats responded at rates of 12.8 ± 2.2% and 9.2 ± 1.4 responses/min, respectively. A lesion X session ANOVA showed a significant effect of sessions, F(5, 80) = 4.39, p < .001, but neither the effect of lesion, F < 1, p = .426, nor the lesion X session interaction, F(5, 80) = 1.93, p = .098, was significant.
Figure 8 shows the combined results of the two PIT tests, which occurred in counterbalanced order. The data shown are from the first 20 s of stimulus presentations, comparable to the period sampled for the potentiated feeding tests. Presentation of the CS, but not of the IS, enhanced rates of instrumental lever pressing above baseline rates and above responding to the control cue (U). It is notable that these rats had been tested with the IS alone previously in the consumption tests, so it is unlikely that the failure of IS to enhance lever pressing reflects a response to novelty of IS-alone presentations, as might have been the case in Experiment 1. As in previous studies, CeA lesions interfered with the ability of the CS to produce PIT, but those lesions had no effect on responding during any other cue or baseline periods. A lesion X cue ANOVA of the elevation scores (Figure 8A) showed significant effects of lesion, F(1, 16) = 5.55, p = .032, cue, F(2, 32) = 6.49, p = .004, and their interaction, F(2, 32) = 3.65, p = .037. Individual contrasts showed that CS elevation scores of sham-lesioned rats were greater than those scores for each other lesion/stimulus combination, ps < .010, which did not differ from each other. Similarly, a lesion X period (pre-stimulus, CS, IS, or U) ANOVA of absolute response rates (Figure 8B) showed significant effects of lesion, F(1, 16) = 6.84, p = .019, period, F(3, 48) = 6.41, p < .001, and their interaction, F(3, 48) = 3.45, p = .024. Individual contrasts again showed that mean responding of sham rats during CS presentations was significantly higher than responding during any other lesion/period combination, p < .025, none of which differed from each other, ps > .100.
Figure 8.
Mean ± s.e.m performance in the Pavlovian-instrumental transfer tests of Experiment 2. In Figure 8A, entries are expressed as the elevation in lever-press rate during presentations of the conditioned stimulus (CS), interruption stimulus (IS), and unpaired (U) stimulus over the lever-press rate during pre-stimulus periods. The elevation produced by the CS in sham-lesioned rats was significantly (p < .010) greater than any other elevation score. Figure 8B shows the absolute lever-press rates during the pre-stimulus and stimulus periods in those tests. The response rate during CS presentations in sham rats was significantly (p < .025) higher than responding during any other lesion/period combination.
Discussion
The results of these experiments replicated our previous observations (Galarce & Holland, 2009; Galarce et al., 2010) that a stimulus that previously accompanied the termination of a sucrose-paired CS and cancellation of subsequent sucrose deliveries enhances consumption of sucrose when the rats are food-sated. The effect was robust, nearly as large as that controlled by the CS, and was observed in both between-subject and within-subject designs, either before or after testing the same stimulus for PIT. Nevertheless, this “interruption stimulus” (IS) failed to modulate the rate of instrumental lever pressing for sucrose in tests of Pavlovian-instrumental transfer (PIT) in the same rats, whereas the CS produced substantial PIT. Thus, whereas the CS enhanced both consummatory and appetitive instrumental behavior, the IS potentiated only consummatory behavior.
In Experiment 1, presentation of an IS in a potentiated feeding test produced more FOS expression in the CeA than presentation of either a previously reinforced CS or a control stimulus. This outcome, which occurred despite similar levels of overall sucrose consumption during the consumption test period, led us to examine the effects of CeA lesions on IS-potentiated feeding. However, in Experiment 2, lesions of the CeA sufficient to eliminate PIT produced by a CS had no effect on the ability of either a CS or an IS to potentiate feeding. Although there were some differences in the procedures of Experiments 1 and 2 (a single potentiated feeding test was administered after PIT testing in Experiment 1, but two potentiated feeding tests were administered prior to PIT testing in Experiment 2), it seems unlikely that those differences were the source of the apparent inconsistency between these two results. First, in previous experiments with CSs, the order of PIT and potentiated feeding testing affected neither the magnitude nor the sensitivity to CeA lesions of either phenomenon (Holland & Gallagher, 2003). Second, in Experiment 2, performance in the first test was similar to that in the second test. Thus, although CeA activity is apparently engaged selectively by an IS, that activity is not critical for an IS’s ability to potentiate consumption.
The IS’s ability to enhance food consumption suggests that stimulus possessed positive incentive value, whereas its inability to either enhance or suppress instrumental responding suggests that it lacked such value. By contrast, evidence from our laboratory indicates that IS training and other related training procedures endow cues with negative incentive value. First, Galarce and Holland (2009) found that in the absence of food (and even early in a food consumption test), an IS presented alone suppresses food cup entry below baseline levels, and when combined with a CS, inhibits the food cup responses usually elicited by the CS. Second, Holland (2013) found that whereas a CS acted as a conditioned reinforcer for instrumental responding, an IS acted as a conditioned punisher, even when both were shown separately to enhance feeding.
Why might a stimulus with negative value enhance consumption? One possibility is that IS enhances eating because it generates a negative state, that is, it produces frustration- or stress-induced eating (e.g., Levine & Morley, 1981; Polivy & Herman, 1999; Tamashiro et al., 2007). Indeed, in Experiment 1, the induction after IS presentation of high levels of FOS in CeA, a brain region often assigned a major role in stress, aversive learning and action (e.g., LeDoux, 2000), is consistent with that view. However, the lack of an effect of CeA lesions on IS-potentiation of consumption in Experiment 2 argues against the notion that the IS’s aversiveness is responsible for that potentiation. Amygdala lesions reduce many frustration-induced enhancements of behavior (e.g., Henke, 1973; Henke & Maxwell, 1973; Kemble & Beckman, 1970), suppress stress responses and generally impair the acquisition or performance of negative affective responses in learning (e.g., Fanselow & Poulos, 2005; Roozendaal, McEwen, & Chattarji, 2009). Of course, not all stress effects are mediated by CeA (Roozendaal, et al., 2009), and IS-potentiated feeding might be such a case. Nevertheless, the reinforcer-specificity of IS-potentiated consumption (Galarce et al., 2010) also seems inconsistent with most descriptions of more general frustration- or stress-induced eating.
Galarce et al. (2010) suggested that IS-potentiated consumption is an adaptive response to the anticipation of scarcity of a particular resource, whereas if the food is already absent, working to earn further signals of its absence, as in a conditioned reinforcement test, hardly seems adaptive. Although the IS might well be aversive, given the impending depletion or removal of a currently-available food resource, the most appropriate behavior in the presence of that food is to maximize consumption while it is still available. Indeed, one might invoke concepts of reward prediction error and describe the presence of the reinforcer, despite signals to the contrary, as an unexpected delight. Indeed the mere threat of unavailability of a food item can induce increased consumption of that item, at least in some individuals. Urbszat, Herman, and Polivy (2002) warned participants in a putative dieting study that beginning the next day they would not be permitted to consume certain forbidden foods, such as pizza, chips, and cookies. However, before the diet could start the participants were told that they needed to rate the tastiness of one of those food items (cookies). Participants were told they could consume as many of those cookies as they needed to make an accurate rating. So-called restrained eaters (Herman & Polivy, 1980) consumed more of those cookies than either unrestrained eaters facing the upcoming diet, or restrained eaters who were not faced with the threat of subsequent deprivation of such forbidden foods. Thus, food scarcity and abundance, and cues for those conditions, may interact in many ways to alter food consumption, and may contribute to the increased prevalence in obesity seen in recent decades.
The potentiation of food consumption by an IS in food-sated rats despite substantial evidence for that cue’s aversive or food-inhibitory properties remains puzzling and worthy of investigation. One step is to better characterize the conditions necessary for establishing an IS as a potentiator of consumption. For example, neither an unpaired cue nor a stimulus trained in a standard conditioned inhibition procedure (e.g., light→food, tone + light → no food) potentiates feeding in food-sated rats (Holland, 2013), and cues with aversive properties established by pairing with shock suppress, rather than enhance, food consumption, even when obvious response competition (e.g., from freezing) is eliminated (Petrovich, Ross, Mody, Holland, & Gallagher, 2009). Elsewhere, we have found that the IS’s correlation with the reduction in food delivery alone is not sufficient for it to induce eating under our test conditions; instead it must also accompany the termination of the CS (Galarce & Holland, 2009). It is possible that the ability of an IS to potentiate consumption is mediated to some extent by the establishment of associations between IS and CS, at the same time that IS is linked to scarcity of food- and/or food-cues. Such mediation would be consistent with observations that IS- and CS-potentiation of feeding share US-specificity and BLA-dependence.
Further investigation of the brain correlates of IS-potentiated feeding would also be valuable. For example, to the extent that CS- and IS-potentiating of feeding share mechanisms, we would expect that manipulations that affect the former, such as lesions of medial prefrontal cortex (Petrovich, Ross, Holland, & Gallagher, 2009), functional disconnection of the lateral hypothalamus and BLA (Petrovich, et al., 2002), genetic or pharmacological alteration of MCH receptor activity (Johnson, 2011, but see Petrovich, Hobin, & Reppucci, 2012) and manipulations of the feeding-related gut peptide ghrelin (Konoski, Fortin, Ricks, & Grill, 2013; Walker, Ibia, & Zigman, 2012), might also affect IS-potentiated feeding. Is potentiation of feeding by an IS produced solely by such shared mechanisms, or are there brain systems uniquely engaged by such a stimulus that are critical to its orexigenic function?
Finally, the dissociation among CS and IS effects on food consumption, PIT, and conditioned reinforcement observed in our recent experiments suggests that describing all of these phenomena as examples of “incentive learning” adds little to our understanding. Although it is always difficult to compare the magnitudes of two phenomena that are not measured on the same scale, it seems reasonable to conclude from our present observations that whereas the CS for food potentiated both consuming and working for food reward, the IS potentiated only feeding. Thus, from a traditional perspective, “incentive” states controlled by CSs and ISs must differ, as do those responsible for enhancement of instrumental behavior in PIT and for potentiation of consumption itself. What do we mean by “incentive learning” if the abilities of a cue to make you eat more of a food item and to work more for that item are not correlated? Furthermore, the pattern of brain lesion effects on PIT, cue-potentiated feeding, conditioned reinforcement, and other phenomena often ascribed to “incentive learning” reveals several double- and triple dissociations (e.g., reviewed in Holland & Petrovich, 2005). The case of ISs may be especially problematic for a simple view because some behavioral measures imply that those cues are negative incentive stimuli and others imply they are positive incentives. More extensive investigation and comparison of behavioral, brain, and other physiological determinants of a range of examples of modulation of ongoing behavior by cues previously associated with motivationally significant events may lead to more satisfying understanding of brain-behavior relations.
Figure 1.
Acquisition of food cup responding during training of the conditioned stimulus (CS), interruption stimulus (IS), and unpaired (U) stimulus in Experiment 1. Entries are expressed as mean ± s.e.m. elevation in the percentage of time spent in the food well during stimulus presentations compared to pre-stimulus periods. Over the final 4 sessions, responding to the CS was significantly (p < .005) greater than responding to the IS, which in turn was significantly greater than responding to U.
Figure 6.
Acquisition of food cup responding during training of the conditioned stimulus (CS), interruption stimulus (IS), and unpaired (U) stimulus in Experiment 2. Some rats had previously received lesions of the amygdala central nucleus (CeA) and others had received sham lesions of that region. Entries are expressed as mean ± s.e.m. elevation in the percentage of time spent in the food well during stimulus presentations compared to pre-stimulus periods. Although the stimulus X session interaction was significant (p < .001), neither the effect of lesion nor any of its interactions was significant.
Acknowledgments
Notes: This research was supported in part by NIH grant MH53667. We thank Caitlin Hepps Keeney, Weidong Hu, and Jun Hyun Park for their technical assistance.
References
- Corbit LH, Balleine BW. Double dissociation of basolateral and central amygdala lesions on the general and outcome-specific forms of Pavlovian-instrumental transfer. Journal of Neuroscience. 2005;25:962–970. doi: 10.1523/JNEUROSCI.4507-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanselow MS, Poulos AM. The neuroscience of mammalian associative learning. Annual Review of Psychology. 2005;56:207–234. doi: 10.1146/annurev.psych.56.091103.070213. [DOI] [PubMed] [Google Scholar]
- Galarce EM, Holland PC. Effects of cues associated with meal interruption on feeding behavior. Appetite. 2009;52:693–702. doi: 10.1016/j.appet.2009.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galarce EM, McDannald MA, Holland PC. The basolateral amygdala mediates the effects of cues associated with meal interruption on feeding behavior. Brain Research. 2010;1350:112–122. doi: 10.1016/j.brainres.2010.02.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall J, Parkinson JA, Connor TM, Dickinson A, Everitt BJ. Involvement of the central nucleus of the amygdala and nucleus accumbens core in mediating Pavlovian influences on instrumental behavior. European Journal of Neuroscience. 2001;13:1984–1992. doi: 10.1046/j.0953-816x.2001.01577.x. [DOI] [PubMed] [Google Scholar]
- Henke PG. Effects of reinforcement omission on rats with lesions in the amygdala. Journal of Comparative and Physiological Psychology. 1973;84:187–193. doi: 10.1037/h0035015. [DOI] [PubMed] [Google Scholar]
- Henke PG, Maxwell D. Lesions in the amygdala and the frustration effect. Physiology and Behavior. 1973;10:647–650. doi: 10.1016/0031-9384(73)90137-6. [DOI] [PubMed] [Google Scholar]
- Herman CP, Polivy J. Restrained eating. In: Stunkard A, editor. Obesity. Philadelphia: W. B. Saunders; 1980. pp. 208–225. [Google Scholar]
- Holland PC. Relations between Pavlovian-instrumental transfer and reinforcer devaluation. Journal of Experimental Psychology: Animal Behavior Processes. 2004;30:104–117. doi: 10.1037/0097-7403.30.2.104. [DOI] [PubMed] [Google Scholar]
- Holland PC. Double dissociation of choice for earning and consuming foods in the presence of a cue associated with the withdrawal of food and food cues. 2013. Unpublished manuscript. [Google Scholar]
- Holland PC, Gallagher M. Double dissociation of the effects of lesions of basolateral and central amygdala on CS-potentiated feeding and Pavlovian-instrumental transfer. European Journal of Neuroscience. 2003;17:1680–1694. doi: 10.1046/j.1460-9568.2003.02585.x. [DOI] [PubMed] [Google Scholar]
- Holland PC, Petrovich GD. A neural systems analysis of the potentiation of feeding by conditioned stimuli. Physiology and Behavior. 2005;86:747–761. doi: 10.1016/j.physbeh.2005.08.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland PC, Petrovich GD, Gallagher M. The effects of amygdala lesions on conditioned stimulus-potentiated eating in rats. Physiology & Behavior. 2002;76:117–129. doi: 10.1016/s0031-9384(02)00688-1. [DOI] [PubMed] [Google Scholar]
- Holmes NM, Marchand AR, Coutureau E. Pavlovian to instrumental transfer: a neurobehavioural perspective. Neuroscience and Biobehavioral Reviews. 2010;34:1277–1295. doi: 10.1016/j.neubiorev.2010.03.007. [DOI] [PubMed] [Google Scholar]
- Johnson AW. Melanin concentrating hormone (MCH) influences cue-driven food intake under conditions of satiety. Appetite. 2011;57:S21. [Google Scholar]
- Johnson AW. Eating beyond metabolic need: how environmental cues influence feeding behavior. Trends in Neurosciences. 2013;36:101–109. doi: 10.1016/j.tins.2013.01.002. [DOI] [PubMed] [Google Scholar]
- Kanoski SE, Fortin SM, Ricks KM, Grill HJ. Ghrelin signaling in the ventral hippocampus stimulates learned and motivational aspects of feeding via PI3K-Akt signaling. Biological Psychiatry. 2013;73:915–923. doi: 10.1016/j.biopsych.2012.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemble ED, Beckman GJ. Runway performance of rats following amygdaloid lesions. Physiology & Behavior. 1970;5:45–47. doi: 10.1016/0031-9384(70)90011-9. [DOI] [PubMed] [Google Scholar]
- LeDoux JE. Emotion circuits in the brain. Annual Review of Neuroscience. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- Lee HJ, Groshek F, Petrovich GD, Cantalini JP, Gallagher M, Holland PC. Role of amygdalo-nigral circuitry in conditioning of a visual stimulus paired with food. Journal of Neuroscience. 2005;25:3881–3888. doi: 10.1523/JNEUROSCI.0416-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine AS, Morley JE. Stress-induced eating in rats. American Journal of Physiology. 1981;241:R72–R76. doi: 10.1152/ajpregu.1981.241.1.R72. [DOI] [PubMed] [Google Scholar]
- Levitsky DA, Pacanowski CR. Free will and the obesity epidemic. Public Health and Nutrition. 2011;15:126–141. doi: 10.1017/S1368980011002187. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 4. San Diego, CA: Academic Press; 1998. [Google Scholar]
- Petrovich GD, Hobin MP, Reppucci CJ. Selective FOS induction in hypothalamic orexin/hypocretin, but not melanin-concentrating hormone neurons, by a learned food-cue that stimulates feeding in sated rats. Neuroscience. 2012;224:70–80. doi: 10.1016/j.neuroscience.2012.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrovich GD, Holland PC, Gallagher M. Amygdalar and prefrontal pathways to the lateral hypothalamus are activated by a learned cue that stimulates eating. Journal of Neuroscience. 2005;25:8295–8302. doi: 10.1523/JNEUROSCI.2480-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrovich GD, Ross CA, Holland PC, Gallagher M. Medial prefrontal cortex is necessary for an appetitive contextual conditioned stimulus to promote eating in sated rats. Journal of Neuroscience. 2007;27:6436–6441. doi: 10.1523/JNEUROSCI.5001-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrovich GD, Ross CA, Mody P, Holland PC, Gallagher M. Central, but not basolateral amygdala, is critical for control of feeding by aversive learned cues. Journal of Neuroscience. 2009;29:15205–15212. doi: 10.1523/JNEUROSCI.3656-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrovich GD, Setlow B, Holland PC, Gallagher M. Amygdalo-hypothalamic circuit allows learned cues to override satiety and promote eating. Journal of Neuroscience. 2002;22:8748–8753. doi: 10.1523/JNEUROSCI.22-19-08748.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polivy J, Herman CP. Distress and eating: why do dieters overeat? International Journal of Eating Disorders. 1999;26:153–164. doi: 10.1002/(sici)1098-108x(199909)26:2<153::aid-eat4>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Purgert RJ, Wheeler DS, McDannald MA, Holland PC. Role of amygdala central nucleus in aversive learning produced by shock or by unexpected omission of food. Journal of Neuroscience. 2012;32:2461–2472. doi: 10.1523/JNEUROSCI.5090-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roozendaal B, McEwen BS, Chattarji S. Stress, memory, and the amygdala. Nature Reviews Neuroscience. 2009;10:434–445. doi: 10.1038/nrn2651. [DOI] [PubMed] [Google Scholar]
- Tamashiro KL, Hegeman MA, Nguyen MM, Melhorn SJ, Ma LY, Woods SC, Sakai RR. Dynamic body weight and body composition changes in response to subordination stress. Physiology & Behavior. 2007;91:440–448. doi: 10.1016/j.physbeh.2007.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbszat D, Herman CP, Polivy J. Eat, drink, and be merry, for tomorrow we diet: effects of anticipated deprivation on food intake in restrained and unrestrained eaters. Journal of Abnormal Psychology. 2002;111:396–401. doi: 10.1037//0021-843x.111.2.396. [DOI] [PubMed] [Google Scholar]
- Walker AK, Ibia IE, Zigman JM. Disruption of cue-potentiated feeding in mice with blocked ghrelin signaling. Physiology & Behavior. 2012;108:34–43. doi: 10.1016/j.physbeh.2012.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weingarten HP. Conditioned cues elicit feeding in sated rats: A role for learning in meal initiation. Science. 1983;220:431–433. doi: 10.1126/science.6836286. [DOI] [PubMed] [Google Scholar]