Abstract
In the developing brain, dendrite branches and dendritic spines form and turn over dynamically. By contrast, most dendrite arbors and dendritic spines in the adult brain are stable for months, years and possibly even decades. Emerging evidence reveals that dendritic spine and dendrite arbor stability have crucial roles in the correct functioning of the adult brain and that loss of stability is associated with psychiatric disorders and neurodegenerative diseases. Recent findings have provided insights into the molecular mechanisms that underlie long-term dendrite stabilization, how these mechanisms differ from those used to mediate structural plasticity and how they are disrupted in disease.
The proper formation and long-term maintenance of neuronal connectivity is crucial for correct functioning of the brain. The size and shape of a neuron’s dendrite arbor determine the number and distribution of receptive synaptic contacts it can make with afferents. During development, dendrites undergo continual dynamic changes in shape to facilitate proper wiring, synapse formation and establishment of neural circuits. Dendrite arbors are highly dynamic during development, extending and retracting branches as they mature, and only a subset of nascent dendrite branches become stabilized1–4 (FIG. 1). During this early wiring period, synapse and dendrite arbor stabilization are intimately connected. For example, synapse formation on a nascent dendrite branch promotes its stabilization, whereas the loss or reduction of synaptic inputs destabilizes target dendrites4–13.
Figure 1. Dendrite branch and dendritic spine dynamics change during development.
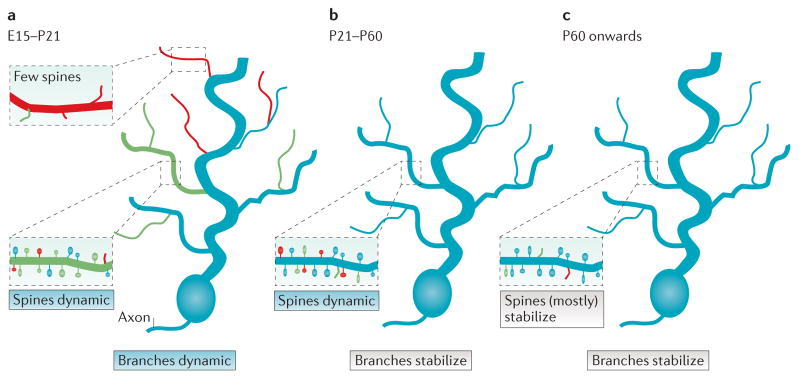
a | During early development in mice (embryonic day 15 (E15) to postnatal day 21 (P21)), dendritic branches are highly dynamic, extending new branches (green) and retracting some existing branches (red). Failure to form productive synaptic contacts (inset, red dendrite segment) results in fewer spines and dendrite branch retraction; more stable branches (inset, green dendrite segment) contain a mix of stable spines, new spines and destabilizing spines. b | As animals enter and transit adolescence (P21–P60), some dendrite branches stabilize, while a fraction of dendritic spines remain dynamic, with a net loss of spines. c | As animals enter adulthood, dendritic spine dynamics slow and most of the spines remain stable.
The structural plasticity of dendrites decreases greatly as circuits mature (FIG. 1). Most dendrite branches become stabilized first while individual dendritic spines continue to form, change shape and turn over as circuits refine14–18. During this period, the formation and pruning of spines is particularly sensitive to experience and activity patterns16,18–20. This is followed by a period of extensive synapse and dendritic spine pruning, which can can last throughout adolescence and early adulthood in some human brain regions17,20–26. In stark contrast to early development, in which stabilization of dendrite branches depends critically on synapse formation, dendritic spine and dendrite branch stability become mechanistically uncoupled during this late refinement period. Such uncoupling is crucial for long-term circuit stability, as it affords mature neurons the ability to fine-tune spine-based synaptic connections, while retaining overall long-term dendrite arbor field integrity and integration within networks. Furthermore, cytoskeletal stability is crucial for maintaining long-lasting synaptic changes such as long-term potentiation (LTP). Examining the distinct mechanisms that mediate spine and dendrite stability is the major focus of this Review.
By adulthood, the dynamic behaviour of spines is greatly reduced. Transcranial two-photon imaging indicates that a large fraction of dendritic spines in the adult rodent cortex are stable for extended time periods of several months and possibly years15–18,27 (FIG. 1). Together, these findings suggest a scenario in which most dendritic spines and dendrite arbors become stabilized for long periods of an organism’s lifetime, perhaps even for decades in humans.
Losses of dendritic spine and dendrite arbor stability in humans are major contributing factors to the pathology of psychiatric illnesses such as schizophrenia and major depressive disorder (MDD), neurodegenerative diseases, such as Alzheimer’s disease, and damage from stroke. Importantly, different patterns of dendritic spine and dendrite branch loss are observed in different psychiatric and neurodegenerative disorders (reviewed in REF. 28), suggesting that spine and branch stabilization mechanisms are differentially disrupted in different disease pathologies. The altered synaptic connectivity resulting from dendrite arbor and dendritic spine destabilization is thought to contribute to the impaired perception, cognition, memory, mood and decision-making that characterize these pathological conditions.
A growing number of recent studies have begun to dissect the mechanisms that mediate long-term dendritic spine and dendrite branch stability. Here, I provide an up-to-date review of the molecules (TABLE 1) and cellular and molecular mechanisms that differentially regulate dendritic spine versus dendrite branch stability and highlight how these mechanisms are targeted by pathology.
Table 1.
Molecules influencing dendritic spine and dendrite arbor stability
| Key molecules | Refs | |
|---|---|---|
| Stabilizes spines | RAC1 | 66 |
| WAVE1 | 69,70 | |
| Cortactin | 76,84,89 | |
| β-adducin | 96,97 | |
| EPHB–FAK | 98 | |
| FYN | 100 | |
| p130CAS | 101 | |
| MARCKS | 78,203 | |
| p140CAP | 85 | |
| Stabilizes dendrites | MAP1A | 60,61 |
| MAP2 | 62,63 | |
| p190RHOGAP | 77,114 | |
| NDR1 and NDR2 | 138 | |
| Cypin | 142,143 | |
| Stabilizes both spines and dendrites | BDNF–TRKB | 108,140,141 |
| Integrin α3β1 | 101,114,115 | |
| ARG | 26,77,114 | |
| EB3 | 85,204 | |
| Destabilizes both spines and dendrites | RHOA–ROCK | 68,71,72,147–151 |
| Amyloid-β-derived diffusible ligands | 175–178,181 | |
| Tau and tau kinases | 179,181 | |
| GATA1 | 185 | |
| Corticosteroids | 205–208 | |
| Increased NMDAR activation | 84,89,198–202 |
BDNF, brain-derived neurotrophic factor; EB3, end-binding protein 3; FAK, focal adhesion kinase; GATA1, GATA-binding factor 1; MAP, microtubule-associated protein; MARCKS, myristoylated alanine-rich C-kinase substrate; NDR, nuclear Dbf2-related kinase; NMDAR, NMDA receptor; p130CAS, p130 Crk-associated substrate; p140CAP, p130 Cas-associated protein; p190RHOGAP, 190 kD RHO GTPase-activating protein A; ROCK, RHO-associated protein kinase.
Determinants of dendrite and spine stability
Dendritic spines are supported by an F-actin framework
At most excitatory synapses, presynaptic boutons synapse onto small dendritic spines that emerge from the dendritic shaft. Spines have diverse shapes, including thin, short and stubby, and mushroom-shaped, with a club-like head and thinner neck15,29–32. Dendritic spines are enriched in filamentous (F) actin, which provides the spine shape, organizes the postsynaptic signalling machinery, drives changes in spine structure and maintains spine stability33–37. The spine cytoskeleton (referred to here as the ‘spinoskeleton’)38 is composed of a mix of linear and branched actin networks, which extend from the base of the spine to the postsynaptic density (PSD)31,39. This actin network fills the spine — it is closely apposed to the spine membrane, and it retains this association during activity-driven remodelling of spine structure31 (FIG. 2). Changes in the amount and structure of F-actin also mediate long-lasting alterations in spine size and synaptic efficacy. For example, repetitive firing of synapses, such as that occurring during high-frequency synaptic stimulation to induce LTP, promotes actin polymerization within the spine, causing the spine to enlarge40,41. Conversely, treatment that weakens synaptic efficacy, such as low-frequency stimulation that results in long-term depression, causes actin loss and dendritic spine shrinkage40,42.
Figure 2. Cytoskeletal structure in dendrites.
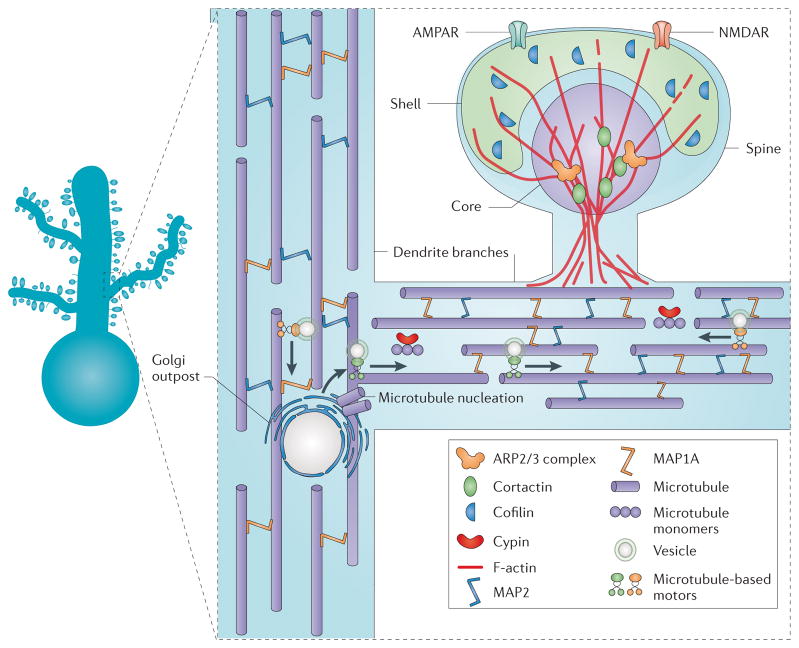
The cytoskeletal ultrastructure in a small region of a dendrite (boxed area on the neuron). Dendritic shafts contain large parallel bundles of microtubules bridged by microtubule-associated protein 1A (MAP1A) and MAP2. The microtubule array near a dendritic branch site serves as a docking site for a Golgi outpost, which services the trafficking of vesicles (cargo) to and from the more terminal regions of dendritic membrane and serves as a site for microtubule nucleation. Cypin helps to promote the assembly and stability of new microtubules. The dendritic spine is supported by network of actin filaments. The more stable central core contains the actin-related protein 2/3 (ARP2/3) complex and the actin nucleation-promoting factor and actin branch stabilizer cortactin. F-actin filaments are less densely packed and more dynamic in the spine shell, which is enriched in the actin-severing protein cofilin. AMPAR, AMPA receptor; NMDAR, NMDA receptor.
Direct measurements using sophisticated live microscopy techniques reveal that actin exists in distinct pools that differ in their movement and stability within the spine36,43,44. Photoactivation studies indicate that actin at the spine tip and periphery, also known as the spinoskeleton ‘shell’, is dynamic, cycling between monomeric globular and polymeric (filamentous) forms that turn over with half-lives of tens of seconds. By contrast, actin in the centre and base of the spine, also known as the spinoskeleton ‘core’, turns over in tens of minutes43. Consistent with this model, when single photoactivated actin molecules were tracked in spines, half remained largely stationary, as would be expected of actin associated with a more stable actin core, whereas one-third underwent slow retrograde movement, which is consistent with a more dynamic pool of actin associated with the shell44. It has recently been noted that distinct actin-binding proteins (ABPs) localize to discrete regions within the spinoskeleton38. For example, cortactin, which both stimulates actin polymerization and stabilizes branched actin networks, localizes to the spinoskeleton core45, whereas cofilin, an F-actin-severing protein that is associated with dynamic F-actin turnover, localizes to the shell46 (FIG. 2). Together, these studies suggest that the periphery of spine structure — near the outer membrane — is likely to be capable of greater morphological change, whereas the central core remains more stable. This is consistent with the observation that spines can be highly motile and could reflect a need, during synaptogenesis, to keep the overall position of new spines constant while the more dynamic outer portion searches for suitable synaptic contacts or adjusts in size in response to new activity patterns.
Microtubule arrays in dendrite branches support morphological structural and membrane trafficking
In the mature nervous system, the cytoskeleton within dendrite branches consists of a packed network of microtubules, which provides structural stability, anchors organelles and serves as a highway for the transport of cargoes, including all manner of dendritic building materials and organelles47. In contrast to microtubules in axons, which are polarized (with the plus ends facing the end of the axon), microtubules within dendrites are of mixed polarity, which may facilitate bidirectional trafficking of cargoes by microtubule-based motors48,49. That said, polarized microtubule arrays have been observed in large-diameter apical dendrites50, possibly reflecting the bias of transport of cargoes away from soma out to their elaborate dendrite arbors. Serial electron micrographic reconstructions reveal that ordered dendritic microtubules lie regularly spaced, parallel to each other and to the dendritic shaft51 (FIG. 2).
A dynamic cytoskeleton, but stable branches and spines
Although the gross structure of dendritic spines and branches is stable for months or years, the individual actin filaments and microtubules that make up their structures turn over in matters of minutes to hours; this is the case in both the developing and mature nervous systems. Over 80% of F-actin in spines turns over every minute36,43,44, whereas 75% of the microtubules in dendrites turn over within tens of minutes52,53. It is important to note that the remaining proportion of F-actin and microtubules undergo depolymerization-mediated turnover in dendrites but at a slower rate — on the order of tens of minutes for spine F-actin and a probably a few hours for dendrite shaft microtubules, as has been measured for axon microtubules52,53.
Given the high turnover of molecules that confer structural integrity, how is structural stability and functional integrity achieved? The secret appears to lie in the precise regulation of the molecules that control dendritic cytoskeletal dynamics. Dendritic spines and shafts are replete with proteins that promote the formation of actin filaments and microtubules, respectively, and organize and stabilize these cytoskeletal structures (FIG. 2). In addition to shaping the cytoskeleton, these proteins ensure that only a fraction of the cytoskeleton undergoes remodelling at any given time and that existing actin filaments and microtubule networks can both maintain dendritic structure and serve as scaffolds for their own replenishment. For example, the actin-related protein 2/3 (ARP2/3) complex, which localizes to dendritic spines54, has been shown to nucleate new actin branches from the side of an existing actin filament in vitro55. Selective inactivation of the ARP2/3 complex in excitatory neurons leads to significant spine and synapse loss in cortical and hippocampal neurons, further supporting a crucial role for actin replenishment in the maintenance of spine and synapse structural integrity56.
Dendritic microtubules also contain high concentrations of microtubule-associated proteins (MAPs), including MAP1A and MAP2 (FIG. 2), both of which can promote microtubule polymerization and stabilize existing microtubules57–59. Upregulation of MAP1A and MAP2 expression strongly correlates with dendrite stabilization in cultured neurons, and both proteins are required for activity-dependent enlargement and stabilization of developing dendrite arbors60,61. Importantly, knockout of these proteins in mice disrupts microtubule spacing within dendrites and leads to significant reductions in dendrite arbor size62,63. In addition to their effects on microtubule spacing and stability, MAPs may also protect dendritic microtubules from the activities of endogenous microtubule-severing enzymes, such as katanin, which could otherwise destabilize dendrite arbors by triggering microtubule instability64. Covalent modifications of dendritic microtubules that are associated with microtubule stability include detyrosination of the α-tubulin subunit, which stabilizes the microtubules by making them poor substrates for the kinesin-13 family of microtubule depolymerases65. In summary, structural integrity of spines and dendrites is achieved by a careful balance between stabilization and destabilization mechanisms.
Molecular mechanisms of spine maintenance
The long-term stability of dendrite and spine structure in the mature nervous system thus intimately depends on the actin control machinery, which is regulated by a wide range of extracellular molecules that act on cell surface adhesion receptors, including EPH receptors, immunoglobulin superfamily receptors, cadherins and integrins (FIG. 3). Upon engagement with their extracellular binding partners, these receptors influence a wide range of downstream signalling molecules. These include cytoplasmic non-receptor tyrosine kinases of the focal adhesion kinase (FAK), SRC and ABL families that modulate the activities of RHO-family GTPases and other downstream cytoskeletal stabilizing proteins, and serve to control spinoskeletal structure and ensure long-term spine stability (FIG. 3). Some of these molecules operate throughout neuronal development and into adulthood to control actin dynamics in spines. In many cases, changes in the expression and/or activity of these proteins closely follow changes in synaptic activity. Other spine signalling components become engaged only late in development as network stability emerges, possibly to help stabilize spines for the long-term. Importantly, all of these pathways converge on a single target, the spine actin cytoskeleton, and thus there is significant crosstalk between these mechanisms. The roles of these molecules in regulating actin cytoskeletal dynamics in the spine are discussed in more detail below.
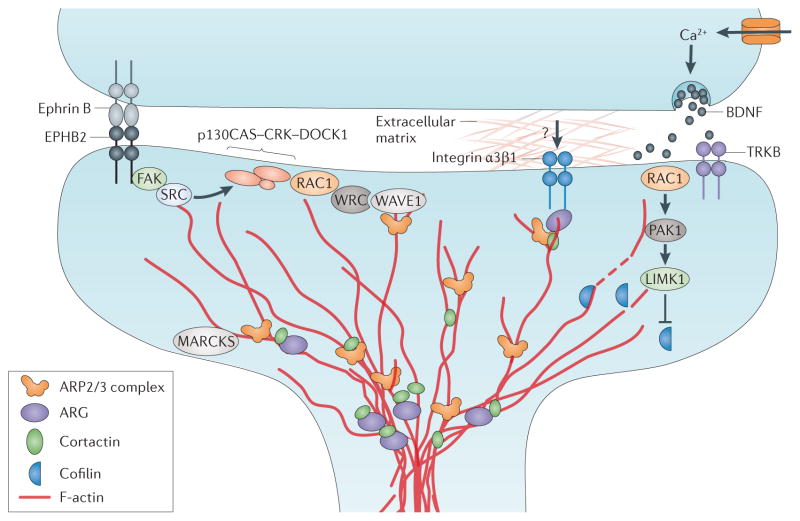
Figure 3. Cytoskeletal signalling pathways that stabilize spines.
Several cytoskeletal signalling pathways mediate dendritic spine stabilization. On the left, presynaptic ephrin B binding to its receptor EPHB2 triggers a focal adhesion kinase (FAK)–SRC kinase relay that activates the p130 Crk-associated substrate (p130CAS)–adaptor molecule CRK–dedicator of cytokinesis protein 1 (DOCK1) complex, which in turn activates the GTPase RAC1. RAC1 releases the WAVE regulatory complex (WRC) from WAVE1, allowing it to activate the actin-related protein 2/3 (ARP2/3) complex to nucleate a new actin branch. In response to an unknown signal, integrin α3β1 activates tyrosine protein kinase ARG and promotes its phosphorylation of and binding to cortactin. Cortactin can activate actin branch nucleation through the ARP2/3 complex and can stabilize the branches that are formed. ARG binding can also stabilize actin filaments and promote cortactin recruitment to actin filaments. On the right, presynaptic depolarization triggers Ca2+-dependent release of brain-derived neurotrophic factor (BDNF), which binds to the receptor tyrosine kinase TRKB to activate RAC1. RAC1 acts through a serine/threonine-protein kinase PAK1–LIM domain kinase 1 (LIMK1) cascade to phosphorylate and inhibit cofilin and block its severing of actin filaments. Myristoylated alanine-rich C kinase substrate (MARCKS) tethers actin filaments to the membrane to stabilize F-actin in spines.
RHO-family GTPases exert differential effects on spine stability
The RHO-family GTPases RHOA, RAC1 and cell division control protein 42 homologue (CDC42) are central regulators of cytoskeletal dynamics in neurons66. All of these GTPases are active in the spine, where they have been shown to mediate spine formation in developing neurons and activity-based changes in spine size67. Beyond this initial period of spinogenesis, these GTPases continue to influence spine shape and stability, even into adulthood. For example, the introduction of dominant-negative RAC1 into hippocampal neurons leads to progressive spine loss, reflecting a requirement for RAC1 in long-term spine stability68. Among its downstream targets, RAC1 activates WAVE (also known as WASF) family proteins to stimulate the ARP2/3 complex, which (as explained above) is associated with F-actin in the spinoskeleton core and acts to nucleate new actin filament branches from existing actin filaments41,66,67 (FIG. 3). WAVE1 also localizes to dendritic spines and knockdown or knockout of Wave1 causes reductions in dendritic spine density69,70. These observations strongly suggest that RAC1 acts through WAVE1 and the ARP2/3 complex to refresh the spinoskeleton core and therefore supports long-term spine stability.
In contrast to RAC1, activated RHO mutants or increased RHOA levels cause reductions in dendritic spine density71,72, whereas RHOA inhibition or knockdown of the RHO activator guanine nucleotide exchange factor 1 (GEF1) increases spine density71,73. Inhibition of the major RHO target RHO-associated protein kinase (ROCK) can block spine loss resulting from increased RHOA protein levels72. These studies suggest that RHO–ROCK signalling antagonizes the stability of previously formed dendritic spines, although the target of RHO signalling in regulating spine stability is not clear. RHO signalling through ROCK stimulates LIM kinase to phosphorylate cofilin and inhibit its actin severing activity74,75, but this might be expected to promote spine stability rather than reduce it (see below).
Studies of the effects of synaptic activity on cytoskeletal stability have revealed a more complex relationship between RHOA signalling and spine stability. Local uncaging of glutamate to stimulate individual spines caused increased RHOA activity in the spine, which mediated activity-based spine enlargement67. By contrast, blocking AMPA receptors in cultured hippocampal neurons with NBQX for several days caused increased RHOA activity, which led to spine loss73. Moreover, several additional studies have shown that although RHOA activation or loss of a RHOA inhibitory cascade in mature neurons leads to a dramatic shrinkage of dendrite arbors, it does not affect dendritic spine stability68,76,77. Overall, it appears that specific activity patterns, developmental timing or other biological factors greatly influence the impact of RHOA–ROCK signalling on dendritic spine stability. Ongoing work in the field should identify how these factors influence RHOA–ROCK activation and its effect on spine stability.
ABPs have key roles in long-term spine stabilization
Dendritic spines are enriched in several ABPs that mediate activity-dependent effects on dendritic spine shape, size and stability, including cortactin, drebrin, ABP1, caldesmon, myristoylated alanine-rich C-kinase substrate (MARCKS) and β-adducin78–82. Cortactin is an F-actin-binding protein that stimulates new actin branch nucleation through the ARP2/3 complex and stabilizes the association of the new branch with the mother filament83. This protein is enriched in the spinoskeleton core, where about 50% is stable (as measured by fluorescence recovery after photobleaching; see FIG. 3)45,84. Cortactin has also been reported to interact with several proteins with known or suspected roles in long-term spine stabilization, including cortactin-binding protein 2, the post-synaptic density (PSD) scaffolding protein SHANK3, p130 Cas-associated protein (p140CAP; also known as SRCIN1) and tyrosine protein kinase ARG (also known as ABL2) (see below)76,85–88.
Because of its interactions with SHANK3 and F-actin within spines, it is perhaps not surprising that cortactin localization and function in spine stability are tightly regulated by synaptic activity. Indeed, chronic stimulation of cultured hippocampal neurons by bath application of NMDA, which is known to destabilize dendritic spines, leads to cortactin redistribution away from postsynaptic sites to dendritic shafts76,84,89,90. Interestingly, activation of the receptor tyrosine kinase TRKB (also known as NTRK2) by its ligand brain-derived neurotrophic factor (BDNF) leads to the opposite effect, increasing cortactin localization to spines. These findings indicate that TRKB and the NMDA receptor (NMDAR) exert opposite effects on spine stability via their different effects on cortactin levels in spines. Together, these findings reveal cortactin as a key effector of spine stability downstream of both stabilizing and destabilizing cues.
Drebrin, MARCKS and ABP1 also have key roles in dendritic spine morphogenesis, particularly in the transition from thin immature dendritic spines to larger, more stable mushroom spines79,91,92. Knockdown of MARCKS in mature hippocampal neuron cultures reduces F-actin content in spines, leading to fewer spines that are smaller in size78. Phosphorylation of MARCKS by protein kinase C (PKC) inhibits its association with the membrane and its ability to crosslink F-actin, and has been implicated in activity-dependent control of spine size and stability93–95. Indeed, direct activation of PKC in mature hippocampal neuron cultures causes spine shrinkage and loss. Importantly, PKC-induced spine destabilization can be blocked using a non-phosphorylatable MARCKS mutant78, indicating that PKC triggers spine destabilization through inhibition of MARCKS.
β-adducin proteins cap actin filaments, and removal of these caps allows the polymerization process to be coordinated by inputs from different signalling pathways into changes in actin structure. Dendritic spines of mice lacking the gene that encodes β-adducin (Add2−/− mice) are relatively normal in appearance but exhibit greatly increased rates of turnover96. Increasing sensory stimulation by housing these mice in enriched environments can induce new spine formation, but a significant fraction of these new spines lack PSD95-positive PSDs, indicating that they are unable to form functional synapses96,97. This increased spine lability and defective synapse formation probably contributes to the observed reductions in spine density in adult Add2−/− mice97. Together, these studies illustrate how the organization of spinoskeletal structure and spine stability, and the influence of synaptic activity on this stability, is coordinated by multiple ABPs.
EPHB signalling through FAK stabilizes mature dendritic spines
Although originally identified as a kinase associated with integrin signalling, FAK can be activated by a large variety of cell surface receptors that regulate spine stability, including EPHB receptors (FIG. 3). Disruption of EPHB2 signalling or knockdown of FAK in established hippocampal neuron cultures leads to a significant loss of mature mushroom-shaped dendritic spines and an increase in immature thin ‘filopodia-like’ spines98. FAK can activate RHOA through 190 kDa GEF (p190RHOGEF; also known as ARHGEF28), and subsequent downstream inactivation of the actin-severing protein cofilin via phosphorylation by LIM kinase may mediate spine stabilization. Consistent with this model, an inactive phosphomimetic cofilin mutant can block the spine destabilization induced by loss of EPHB2–FAK signalling98. In addition, activated FAK also recruits and activates SRC family kinases to phosphorylate p130 Crk-associated substrate (p130CAS; also known as BCAR1), a scaffolding molecule that promotes RAC1 activation99. Interestingly, knockout of the SRC family kinase FYN leads to a progressive age-dependent loss of dendritic spines in mice100, whereas knockdown of p130CAS leads to spine loss in established hippocampal neuron cultures101. These manipulations suggest a mechanism by which spine shrinkage and/or destabilization results from disruption of FAK-mediated RAC1 activation.
BDNF signalling through TRKB influences spine size and stability
BDNF is released from both pre- and postsynaptic compartments during neuronal depolarization or direct stimulation with glutamate, where it has crucial roles in synapse development and stability102–106. During development, BDNF signalling through presynaptic TRKB receptors has been shown to stabilize neurotransmitter release sites and reduce synapse turnover105,106. Interestingly, blockage of NMDARs also disrupts clustering of the presynaptic release sites, but this can be rescued by application of BDNF, suggesting that NMDA-mediated BDNF release is critical for stabilization of the presynaptic compartment105. Although the mechanism by which BDNF–TRKB signalling regulates presynaptic stabilization is not completely clear, it is possible that TRKB signalling to cortactin or other ABPs leads to stabilization of the presynaptic actin network, which in turn stabilizes the synaptic vesicle release machinery.
In the postsynaptic neuron, many actin regulatory pathways central to spine enlargement and stabilization are modulated by BDNF–TRKB signalling. BDNF stimulates activity of serine/threonine-protein kinase PAK (a major target of RAC) and also induces increased inhibitory phosphorylation of cofilin, which is associated with increased spine size and stability (see above). TRKB can also activate the GTPase RAS, which promotes spine enlargement and increased stability107. Interestingly, BDNF signalling through the TRKB receptor can interact with activity-induced molecular pathways to stabilize dendritic spines. For example, exposure of hippocampal slices to modest electrophysiological stimulation and BDNF leads to greater increases in spine F-actin content than either treatment alone, suggesting a synergistic effect. Furthermore, high-frequency stimulation of pre-synaptic afferents in hippocampal slices also increases spine F-actin content, which is attenuated by blocking TRKB signalling108.
Integrin β1 signalling through ARG and cortactin mediates dendritic spine stability
Integrins are heterodimeric receptors that are composed of α and β subunits and are widely expressed in the nervous system, with numerous subunits expressed throughout the brain109. They localize to synapses, where they link pre- and postsynaptic membranes to the extracellular matrix, and are involved in signalling complexes that regulate the cytoskeletal structure. The integrin β1 subunit is localized particularly to the centre of the PSD110. Importantly, integrins appear to be involved in the stabilization of LTP, which is associated with a rapid, long-lasting increase in polymerized F-actin and an increase in spine stability111–113. Indeed, inhibition or loss of integrin α3β1 destabilizes spines and reduces synapse density during adolescence and early adulthood (postnatal day 21 (P21)–P42) in mice101,114,115. The extracellular molecules that activate integrins at synapses are not known but may include both traditional extracellular matrix molecules, such as laminins and thrombospondins, and guidance cues, such as netrins and reelin, which can interact with integrins116–119.
Integrins signal through the ABL family kinases, ABL and ARG in vertebrates, to coordinate changes in cytoskeletal structure120. Importantly, biochemical and genetic experiments indicate that the integrin β1 cytoplasmic tail binds directly to ARG and that this interaction is crucial for dendritic spine and dendrite stability (FIG. 3). ARG is particularly abundant in the nervous system121, where it localizes to dendritic spines76,114,122, and Arg−/− mice exhibit widespread losses of spine and synapse density throughout the cortex and hippocampus (20–35% depending on the brain region) as they mature to adulthood77,123. Interestingly, these phenotypes closely resemble those observed in conditional knockout mice that have neurons lacking the integrin subunit α3 or β1 (REFS 101,114,115), which is consistent with a role for ARG as a major downstream mediator of spine stabilization by integrin α3β1.
ARG acts by binding cooperatively to actin filaments and preventing their depolymerization124,125. ARG binding to actin filaments also changes their helical pitch126, promoting increased cortactin binding, which may further stabilize filaments125. Knockdown of ARG in cultured hippocampal neurons leads to cortactin loss from spines, a parallel decrease in spine F-actin content and a 50% loss of spines76. These data suggest that binding of an ARG–cortactin complex to F-actin stabilizes actin, which promotes spine stability. Indeed, fusion of just the ARG F-actin-binding domains to cortactin results in its accumulation in spines and rescues spine instability in ARG knockdown neurons76. Interestingly, the synapses that remain on ARG knockdown neurons are enlarged and exhibit increased mini-excitatory postsynaptic current amplitudes, consistent with increased signalling by postsynaptic AMPA receptors and NMDARs. NMDAR antagonists prevent cortactin depletion from spines and rescue spine loss resulting from ARG knockdown in cultured neurons. These observations suggest that ARG may also attenuate NMDAR activity to counter its antagonism of cortactin localization to spines. One possibility is that ARG may regulate NMDAR subunit phosphorylation127,128, which is known to regulate localization and trafficking of NMDARs129. Thus, ARG appears to serve as a general brake on the spine destabilizing influence of excessive NMDAR signalling.
Control of dendrite arbor stabilization
The size and shape of a dendrite arbor determine where it can receive inputs and how it can integrate into neural networks. Dendrite branches are especially stable in mature neurons, and this stability is essential for the durability of neural networks. Dendrite branches are supported and maintained through the combined actions of an extensive network of microtubules and associated proteins.
Role of microtubule-based trafficking in stabilizing dendrite arbors
Drosophila melanogaster dendrite-arborizing sensory neurons undergo complete dendrite arbor loss during metamorphosis, and this model system has been particularly useful for studying the mechanisms that control microtubule networks in dendrite branch stability. Severing of individual dendritic branches is initiated by a localized depletion of microtubules and thinning of the dendrite branch, followed by severing and fragmentation of the branch130. Genetic studies indicate that the microtubule-severing protein Katanin 60 is crucial for this process131. These observations underscore the central role of microtubule integrity for maintaining overall dendrite arbor stability.
Dendrites are composed of large amounts of proteins and lipids, which must be continually replenished. Although this is partially accomplished by local protein synthesis, other components must be synthesized and processed elsewhere and trafficked to dendrites. Microtubules play crucial parts in the protein and lipid trafficking events that are required to sustain dendrite structure, and perturbations that disrupt trafficking of microtubule-based cargoes have devastating effects on dendrite formation during development and stability. For example, disruptions in microtubule orientation and spacing correlate with disruptions of local dendrite architecture and have been noted in neurons from individuals with neurodevelopmental disorders132,133. Moreover, the Golgi apparatus in the soma is highly polarized towards dendrites, and Golgi outposts, which serve as local sites for post-Golgi trafficking, and recycling of membrane-based cargoes as well as new microtubule nucleation, are localized at many dendrite arbor branch points134,135 (FIG. 2). Mutations in the microtubule-based motors dynein and kinesin also significantly disrupt trafficking within the dendrites as well as proper localization of Golgi outposts, and lead to deficits in dendrite arbor formation and stabilization136,137. Nuclear Dbf2-related kinase 1 (NDR1; also known as STK38) and NDR2 (also known as STK38L), which are key regulators of dendrite arbor development in vertebrates, have recently been shown to target several proteins with known or suspected roles in protein trafficking138.
Activity and BDNF signalling through the TRKB receptor regulate microtubule-binding proteins to stabilize dendrites
Neuronal activity is a potent stimulator of dendritic remodelling and stabilization in developing neurons, and the neurotrophic factor BDNF, signalling through TRKB, appears to be an important mediator of this effect60,61,139 (FIG. 4). Experimental approaches such as membrane depolarization, repetitive electrophysiological stimulation and direct application of NMDA have all been shown to promote BDNF release at synapses in cultured hippocampal neurons102–104. Moreover, brain-specific ablation of BDNF or its receptor TRKB leads to a striking shrinkage of cortical excitatory neuron dendrites from early adolescence to adulthood140,141. The molecular mechanisms by which synaptic activity stimulates neurotrophic factor signalling that leads to dendrite microtubule stability are coming into focus. Direct application of BDNF or nerve growth factor to cultured neurons is sufficient to induce expression of the microtubule-stabilizing proteins MAP1A and MAP2 (REFS 58–61). In addition, BDNF also induces expression of cypin, a guanine deaminase that promotes microtubule assembly, and dendrite formation and stability. These data suggest that BDNF signalling through TRKB converges on microtubule-binding proteins to mediate dendrite stability142,143.
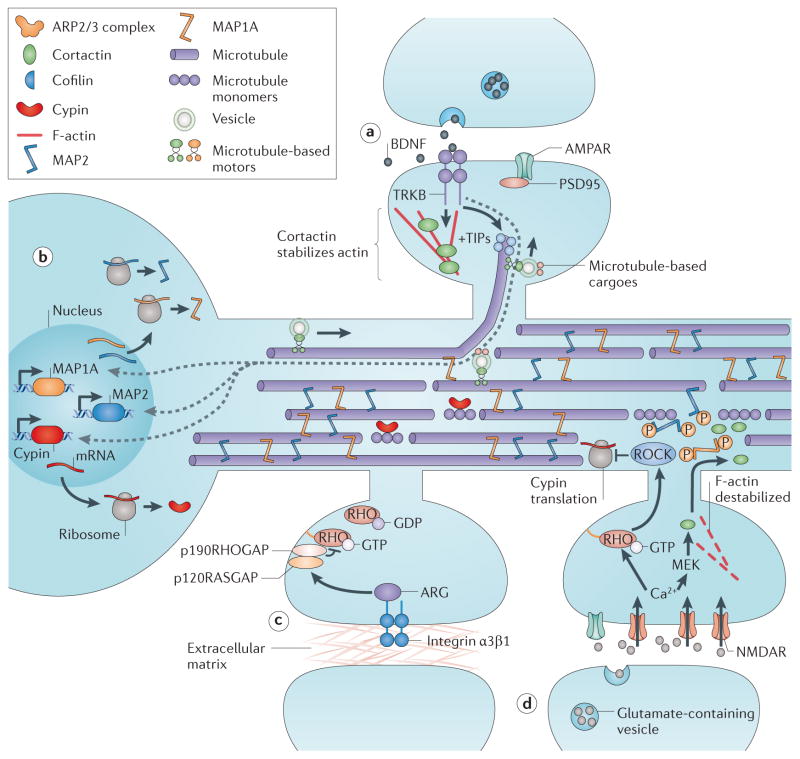
Figure 4. Pathways that mediate crosstalk between dendritic spines and dendrite arbors.
Several pathways mediate crosstalk between the spine and shaft cytoskeletons. a | Brain-derived neurotrophic factor (BDNF) signalling through the receptor tyrosine kinase TRKB triggers assembly of microtubules containing plus end tip-binding proteins (+TIPs), such as end-binding protein 3 (EB3), to target to spines and allow delivery of cargoes that stabilize spine structure. BDNF also promotes accumulation of cortactin in spines to stabilize F-actin structure. b | BDNF triggers increased expression of microtubule-associated protein 1A (MAP1A), MAP2 and cypin, all of which promote microtubule stabilization in the dendrite shaft. c | Integrin α3β1 signalling through the receptor tyrosine kinase ARG and 190 kD RHO GTPase-activating protein A (p190RHOGAP) attenuates RHO activity to preserve dendrite shaft structure. d | Increased AMPA recptor (AMPAR) and NMDA receptor (NMDAR) activity, such as that associated with stroke, results in Ca2+ influx that activates RHO–RHO-associated protein kinase (ROCK) signalling which phosphorylates MAPs and dissociates them from microtubules. Increased levels of ROCK also inhibit localized cypin translation, which further destabilizes spines. NMDAR hyperactivation also triggers cortactin relocalization to the dendrite shaft, which destabilizes dendritic spine F-actin. MEK, MAPK/ERK kinase.
Integrin signalling to p190RHOGAP attenuates RHO activity to stabilize dendrite branches
In addition to regulating dendritic spine stability, as discussed above, integrin α3β1 signalling through the ABL and ARG kinases is crucial for dendrite branch stabilization (FIG. 4). Dendrite arbors of cortical or hippocampal excitatory neurons lacking either ARG or both kinases develop normally until P21, approaching their fully mature size. As the mice reach adulthood, however, dendrite arbor size reduces significantly, leading to an overall thinning or shrinkage of the cortex and hippocampus77,144. Similar phenotypes are observed in hippocampal CA1 neurons deficient in the integrin subunit α3 or β1, both of which are key upstream regulators of ABL and ARG. However, no defects in dendrite branch stability are observed in animals with disruption of integrin subunit α5 or β3, suggesting that integrin α3β1 interacts selectively with ARG to control dendrite stability.
The 190 kD RHO GTPase-activating protein (GAP) A (p190RHOGAP) is a major ARG substrate in the postnatal brain145. ARG-mediated phosphorylation of p190RHOGAP promotes its binding to membrane-associated p120RASGAP, which allows p190RHOGAP to inhibit membrane-bound active RHO145,146. p190RHOGAP phosphorylation and p190RHOGAP–p120RASGAP complex formation are decreased in the brains of Arg−/− mice, leading to net increases in RHO activity. Importantly, dominant-negative RHOA or reducing the gene-dosage of Rock2 suppresses the dendrite branch loss observed in Arg−/− mice76,77. Together, these studies indicate that the integrin α3β1–ARG–p190RHOGAP axis preserves dendrite structure in early adulthood by attenuating RHO activity. Identification and characterization of the integrin ligand that activates this pathway should reveal how and why this pathway is activated to stabilize branches and spines.
Pathological dendritic destabilization by RHO–ROCK signalling
Although most dendrite arbors exhibit long-term stability, there are circumstances under which dendrite stability is compromised, such as in neurodegenerative diseases and after an excitotoxic insult during stroke (see below). The GTPase RHO appears to be a central mediator of this dendrite destabilization. For example, expression of constitutively active RHO, or reduced activation of RHO inhibitors, such as p190RHOGAP (see above), destabilizes growing147–150 and mature68,151 dendrite arbors (FIG. 4). RHO-mediated activation of ROCK probably acts through several mechanisms to disrupt dendrite architecture. Inhibition of ROCK can promote microtubule assembly and the stabilization of dendrite-like neuronal processes in cells, suggesting that ROCK antagonizes dendrite stability by disrupting microtubules152. Indeed, ROCK phosphorylates MAP2 on a site in its microtubule-binding region. ROCK-mediated phosphorylation of a similar conserved site in tau disrupts its interactions with microtubules153. MAP2 phosphorylation in other contexts has been shown to reduce its ability to bind microtubules and promote their assembly154,155. Activated RHO also suppresses translation of the microtubule stabilizer cypin (see above), but cypin overexpression in cultured hippocampal neurons can overcome the reduction in dendrite arbors induced by increased RHO activity151. These studies indicate that the loss of MAP- and cypin-mediated microtubule stabilization contributes to RHO-mediated dendrite arbor destabilization.
Coordination of spine and dendrite stability
Although the mechanisms that regulate dendritic spine and dendrite branch stability have been discussed separately so far, there are several instances in which signalling between the compartments influences stability. This crosstalk is particularly evident under conditions in which activity-driven circuit remodelling requires a coordinated shaping and stabilizing of spine and dendrite structure.
Activity-driven spine enlargement and stabilization
Activity and activity-mediated neurotrophic factor signalling affects dendritic spine size and stability throughout a neuron’s lifetime, including mature cortical and hippocampal neurons. Growing microtubule ends are enriched in ‘plus end tip-binding proteins’, notably end-binding protein 3 (EB3; also known as MAPRE3). Live-imaging studies involving tracking of the growing microtubule tips using EB3-fluorescent protein fusions have found that neuronal activity results in EB3-labelled microtubule tips making transient visits to dendritic spines85,156–158 (FIG. 4). Microtubule invasion of individual spines is accompanied by increased accumulation of PSD95 and the EB3-binding protein p140CAP, both of which are required for spine maintenance85.157. These events are accompanied by increased spine F-actin content and spine enlargement, both of which are features that are generally associated with greater stability. By contrast, disruption of microtubule dynamics using drugs or by knocking down EB3 leads to reductions of PSD95 and p140CAP in spines, destabilizing some of them and decreasing the size of those remaining85,156,158.
Intriguingly, these microtubule ‘visits’ to dendritic spines are greatly increased by membrane depolarization or stimulation with BDNF85,156–158 (FIG. 4). A decrease in BDNF-induced structural reinforcement of the spine may explain the reductions in hippocampal spine density reported in mice with reduced gene-dosage of the BDNF receptor Trkb159. Conversely, treatment of cultured neurons with a chemical long-term depression induction protocol arrests the mobility and targeting of EB3-positive microtubule tips, preventing them from entering the spine160. Under these conditions, EB3 relocates to the dendritic shaft, where it associates with microtubule-bound MAP2, and the loss of microtubule targeting to the spines leads to spine shrinkage and loss.
Stabilization of dendrites by synaptic activity
As mentioned above, new synapse formation is essential for dendrite stabilization in developing neurons, but maintenance of this synaptic input appears to be crucial for ongoing dendritic stability18,19, Accordingly, a reduction in synaptic inputs, by experimental deafferentation or removal of sensory inputs, leads to dendrite loss in a wide variety of model systems7–9,77,161. Both Ca2+/calmodulin-dependent kinase II (CaMKII) and MEK (MAPK/ERK kinase; also known as MAP2K) signalling modules are crucial for activity-dependent dendrite stabilization in cultured sympathetic neurons60. Interestingly, arrest of dynamic dendrite behaviour and premature stabilization of dendrite arbors can also be achieved via expression of a constitutively activated form of the postsynaptic signalling protein CaMKII139. CaMKIIα has many substrates that can affect the actin cytoskeleton within the spine, including the RAC activators T lymphoma invasion and metastasis-inducing protein 1 (REF. 162) and kalirin 7 (REF. 163), and the RAS GTPase activating protein SYNGAP164. Although CaMKIIα and its substrates are predominantly localized to dendritic spines165, it can relocalize to dendritic shafts upon stimulation in cultured neurons166. The exact mechanism by which CaMKIIα functions to stabilize dendrites awaits further study.
Events that destabilize spines and branches
Several human brain diseases that arise later in life are associated with losses of dendritic spine density and dendrite arbor complexity, including psychiatric diseases, such as schizophrenia and depression, and neurodegenerative diseases, such as Alzheimer’s disease167. The consequent loss of connectivity at both the local and inter-regional level is thought to contribute to disease symptoms. Emerging evidence suggests that the pathology associated with these diseases specifically targets dendritic spine and dendrite branch stabilization mechanisms.
Roles of ADDLs and tau in spine and dendrite instability in Alzheimer’s disease
Extensive synapse loss and dendrite arbor atrophy is observed in Alzheimer’s disease168–171, and the reductions in synapse number strongly correlate with the magnitude of cognitive and memory impairment172,173.
Work over the past several years has focused on soluble amyloid-β (Aβ)-derived diffusible ligands (ADDLs; also known as Aβ oligomers) as key triggers for spine loss and dendritic atrophy in Alzheimer’s disease pathology174 (FIG. 5). Application of ADDLs to cultured neurons or slices has been found to lead to a significant loss of dendritic spines175–177. Insight into the mechanism underlying this effect has been gained from the finding that binding of ADDLs to the cellular form of the prion protein (PrPC) triggers FYN-mediated phosphorylation of the GluN2B (also known as NR2B) subunit of the NMDAR178. This initially leads to an increase in the number of NMDARs at the postsynaptic surface followed by a persistent loss of surface NMDAR by endocytosis175,176,178 (FIG. 5). The initial rise in NMDAR surface activity may contribute to spine destabilization, possibly by triggering the loss of drebrin and cortactin from the spine175. However, NMDAR activation probably also mediates spine destabilization by signalling through calcineurin179 to activate cofilin180, which as mentioned above, regulates actin disassembly. Both calcineurin and cofilin have been found to be required for ADDL-induced spine destabilization176. ADDL treatment also disrupts the surface localization of other receptors, such as EPHB2 (discussed above), that are also important regulators of spine stability175.
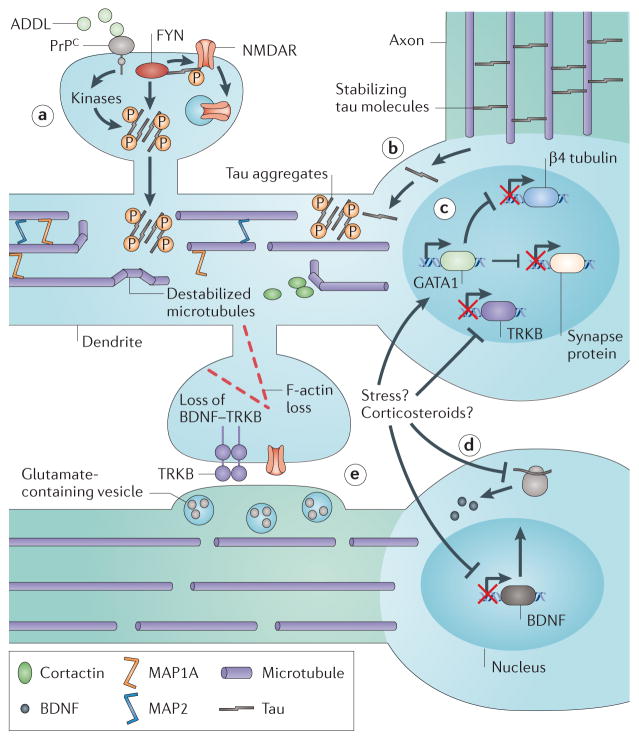
Figure 5. Disruption of spine and dendrite stabilization mechanisms in disease.
Disease pathology specifically disrupts dendritic spine and dendrite arbor stabilization. a | In Alzheimer’s disease, amyloid-β-derived diffusible ligands (ADDLs) bind to the cellular prion protein (PrPC) receptor and activate receptor tyrosine kinase FYN as well as other kinases. This triggers transient NMDA receptor (NMDAR) phosphorylation, followed by NMDAR internalization and tau hyperphosphorylation and accumulation in spines and shafts. b | There is also redistribution of tau from axons to dendrites. c | In major depressive disorder (MDD), upregulation of GATA-binding factor 1 (GATA1) inhibits expression of the dendrite shaft component β4 tubulin and also several genes required for synaptic function. d | Stress and increased corticosteroid levels, which are known risk factors for MDD, inhibit expression of brain-derived neurotrophic factor (BDNF) in the presynaptic neuron and its receptor the receptor tyrosine kinase TRKB in the postsynaptic neuron. e | This disrupts spine and dendrite stabilization by this BDNF–TRKB axis. AMPAR, AMPA receptor.
ADDLs also trigger significant dendrite arbor shrinkage179. ADDLs stimulate the activities of several kinases (including MARK1, p70S6K, BRSK and FYN) that can phosphorylate the microtubule-binding protein tau181,182. Hyperphosphorylated tau relocalizes from a primarily axonal distribution to the soma and some dendrites (FIG. 5), an effect that is associated with a significant reduction in microtubules and localized dystrophic beading of the dendrites179,181. Interestingly, these tau missorting events are also accompanied by reductions in spine density181. Although a small portion of tau is normally found in spines, where it tethers FYN to the PSD183, hyperphosphorylated tau accumulates in spines, where it interferes with NMDA localization to the spine and synaptic anchoring, which contributes to cytoskeletal destabilization183,184. Thus, ADDL-induced tau phosphorylation contributes to destabilization of both dendrite arbors and dendritic spines, albeit through distinct mechanisms.
Dendritic spine destabilization and dendrite atrophy in depression
MDD is associated with a reduction in synapse density185 and reduced dendrite arbor size in the prefrontal cortex and hippocampus186–189. A recent study found that levels of GATA-binding factor 1 (GATA1), a transcriptional repressor and master regulator of several genes that has crucial roles in dendritic spine and dendrite arbor maintenance, are reduced in the brains of individuals with MDD185 (FIG. 5). Several GATA1 target genes are significantly downregulated in the MDD brain, including β4 tubulin (the major β-tubulin subunit in neurons) and the membrane trafficking regulators RAB3A and RAB4B185. These reductions may cause a decrease in the size and stability of the microtubule network and perturb the trafficking of key dendritic building blocks that support dendrite structure. This model is supported by the observation that GATA1 overexpression alone is sufficient to significantly reduce dendrite arbor size in cortical neurons185.
Chronic stress and the associated increase in circulating corticosteroid levels are major risk factors for the development of MDD. The application of restraint stress and the administration of exogenous corticosterone have both been used as models for MDD states in rodents. Both treatments reduce BDNF and TRKB levels in the hippocampus and prefrontal cortex190–194 (FIG. 5), which probably explains the reduction in BDNF in the brains of patients with MDD195. Reduced BDNF signalling would be expected to compromise the BDNF-mediated support of dendritic spine and dendrite arbor stability via mechanisms detailed above, thereby contributing to the neuronal destabilization found in this disease. Recent work shows that chronic corticosterone treatment also decreases expression of caldesmon, an F-actin-stabilizing molecule, increasing F-actin turnover in dendritic spines and resulting in smaller, more unstable spines82.
Disruption of spine and dendrite stability in stroke
Ischaemic events such as stroke cause destabilization of spines and dendrites on neurons within and adjacent to the infarct area. This destabilization may clear dendrites away from areas in which damage or death of afferents has disrupted connectivity, thus freeing up molecular components and metabolic resources to remodel the dendrite arbor during the recovery phase. In a murine ischaemia model, the reductions in dendrite arbor size in the peri-infarct region that follow stroke are closely matched by growth of distal dendrites during the recovery period196, suggesting that the neuron may attempt to reach some homeostatic ‘set point’ in dendrite size and synaptic inputs as it reintegrates into networks.
Increased NMDAR activity and the resulting excito-toxicity has long been known to mediate neuronal damage and death after stroke (for a review, see REF. 197). Application of NMDA to cultured neurons or focal application of NMDA to specific brain areas in vivo has been widely used to model this glutamate-induced excitotoxicity.
Whereas prolonged, excessive stimulation leads to neuronal death, excitotoxic but sublethal NMDAR stimulation of neurons, either in culture or focally in vivo, causes spine loss, dendrite branch swelling and dendrite shrinkage84,89,198–202. These changes are closely associated with disruptions in actin and microtubule structure in spines and dendrites, respectively89,127,203. In spines, the cytoskeletal destabilization appears to be linked to disrupted functioning of actin regulatory proteins. Accordingly, bath application of NMDA to cultured hippocampal neurons causes cortactin to relocalize from dendritic spines to the dendritic shaft, and this correlates with the loss of spine stability84,89. In addition, inhibitors of the cathepsin B family of proteases attenuate NMDA-induced spine loss, Indeed, NMDAR stimulation leads to reduced levels of the spine stabilizing protein MARCKS (see above), but this decrease can be prevented by cathepsin B inhibition, and this suggests that the spine collapse induced by NMDAR stimulation results from proteolysis by cathepsin B of MARCKS and possibly other key actin regulatory proteins203. Increased NMDAR stimulation also disrupts the targeting of dynamic microtubules to spines, which prevents delivery of key proteins, including PSD95 and p140CAP160, which are required for spine stability.
Exactly how increased NMDAR stimulation triggers dendrite branch destabilization is less clear. One potential candidate for mediating these effects is the GTPase RHOA. As noted above, focal synaptic stimulation can activate RHOA in the spine, which can then diffuse into the dendritic shaft67. The summed signalling from a group of spines in aggregate, such as that following excessive NMDAR stimulation, could trigger RHOA–ROCK signalling at levels sufficient to disrupt MAP function154,155 and cypin expression151, thereby destabilizing the dendritic cytoskeleton. In support of this, the ROCK inhibitor fasudil can reduce the severity of NMDA-induced damage to the dendrite-rich inner plexiform layer of the retina202. Moreover, RHO activation and NMDA stimulation have both been shown to decrease cypin levels in cultured neurons200. Finally, it is possible that NMDA-mediated disruption of microtubule structure affects dendrites by disrupting cargo delivery to the dendrite.
Conclusions
Neurons are continually in a balance between stabilization and destabilization. In the normal healthy brain, this balance is predominantly tipped in favour of stabilization, but the loss of this balance towards excessive and unregulated destabilization is a major factor in various brain disorders. The identification of pathways that mediate stabilization of spines and dendrites and the evidence that they are disrupted in brain diseases raise their profiles as possible targets for therapeutic intervention. However, our understanding of these mechanisms, which are vital for our brain function over several decades, is far from complete.
In the future, one challenge will be to understand how different activity patterns influence these stabilization mechanisms at the biochemical and physiological level and whether these can be manipulated to increase stability. Genome-wide searches for genes associated with psychiatric and neurodegenerative disorders will continue to identify mutations and polymorphisms in genes associated with dendrite instability. Understanding how these genes contribute to stabilization and how their functions are altered in susceptible individuals should reveal new methods to identify those at risk of disease and also suggest preventive or therapeutic approaches for these diseases.
Fortunately, our understanding of the biochemical and cellular mechanisms that shape and maintain dendrite structure is accelerating, as methods to control neuronal activity and fluorescent probes for measuring biochemical events in neurons become even more widely used. The goal of these studies should be to both reveal the timing and distribution of key signalling events and causally relate them to changes in dendritic spine and dendrite structure and function. Along the way, we should learn why different diseases are associated with different patterns of dendritic spine and dendrite destabilization. It is possible that in diseases in which multiple pathways are disrupted (for example, Alzheimer’s disease), ‘combination therapies’, such as those increasingly used to treat cancer, may prove especially efficacious to treat some diseases.
Acknowledgments
I apologize to those whose excellent work I could not cite owing to word and reference limits. I am grateful to members of my laboratory, especially M. Kerrisk, A. Levy, M. Omar and Y-C. Lin for critical feedback, and to three anonymous reviewers for helpful suggestions on content. Work in my laboratory is supported by US National Institutes of Health grants NS39475, CA133346, and multi-PI grant GM100411 (joint with T. Boggon).
Glossary
- Golgi outposts
Clusters of Golgi-like cisternae that reside in neuronal processes and act as local trafficking centres for membrane-bound vesicles
Footnotes
Competing interests statement
The author declares no competing financial interests.
References
- 1.Dailey ME, Smith SJ. The dynamics of dendritic structure in developing hippocampal slices. J Neurosci. 1996;16:2983–2994. doi: 10.1523/JNEUROSCI.16-09-02983.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wong WT, Faulkner-Jones BE, Sanes JR, Wong RO. Rapid dendritic remodeling in the developing retina: dependence on neurotransmission and reciprocal regulation by Rac and Rho. J Neurosci. 2000;20:5024–5036. doi: 10.1523/JNEUROSCI.20-13-05024.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wong WT, Wong RO. Rapid dendritic movements during synapse formation and rearrangement. Curr Opin Neurobiol. 2000;10:118–124. doi: 10.1016/s0959-4388(99)00059-8. [DOI] [PubMed] [Google Scholar]
- 4.Wu GY, Zou DJ, Rajan I, Cline H. Dendritic dynamics in vivo change during neuronal maturation. J Neurosci. 1999;19:4472–4483. doi: 10.1523/JNEUROSCI.19-11-04472.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clark WL. Inquiries into the anatomical basis of olfactory discrimination. Proc R Soc Lond B. 1957;146:299–319. doi: 10.1098/rspb.1957.0013. [DOI] [PubMed] [Google Scholar]
- 6.Cline H, Haas K. The regulation of dendritic arbor development and plasticity by glutamatergic synaptic input: a review of the synaptotrophic hypothesis. J Physiol. 2008;586:1509–1517. doi: 10.1113/jphysiol.2007.150029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coleman PD, Riesen AH. Evironmental effects on cortical dendritic fields. I. Rearing in the dark. J Anat. 1968;102:363–374. [PMC free article] [PubMed] [Google Scholar]
- 8.Jones WH, Thomas DB. Changes in the dendritic organization of neurons in the cerebral cortex following deafferentation. J Anat. 1962;96:375–381. [PMC free article] [PubMed] [Google Scholar]
- 9.Matthews MR, Powell TP. Some observations on transneuronal cell degeneration in the olfactory bulb of the rabbit. J Anat. 1962;96:89–102. [PMC free article] [PubMed] [Google Scholar]
- 10.Wiesel TN, Hubel DH. Effects of visual deprivation on morphology and physiology of cells in the cats lateral geniculate body. J Neurophysiol. 1963;26:978–993. doi: 10.1152/jn.1963.26.6.978. [DOI] [PubMed] [Google Scholar]
- 11.Rajan I, Witte S, Cline HT. NMDA receptor activity stabilizes presynaptic retinotectal axons and postsynaptic optic tectal cell dendrites in vivo. J Neurobiol. 1999;38:357–368. doi: 10.1002/(sici)1097-4695(19990215)38:3<357::aid-neu5>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 12.Niell CM, Meyer MP, Smith SJ. In vivo imaging of synapse formation on a growing dendritic arbor. Nature Neurosci. 2004;7:254–260. doi: 10.1038/nn1191. [DOI] [PubMed] [Google Scholar]
- 13.Vaughn JE. Fine structure of synaptogenesis in the vertebrate central nervous system. Synapse. 1989;3:255–285. doi: 10.1002/syn.890030312. [DOI] [PubMed] [Google Scholar]
- 14.Oray S, Majewska A, Sur M. Dendritic spine dynamics are regulated by monocular deprivation and extracellular matrix degradation. Neuron. 2004;44:1021–1030. doi: 10.1016/j.neuron.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 15.Majewska AK, Newton JR, Sur M. Remodeling of synaptic structure in sensory cortical areas in vivo. J Neurosci. 2006;26:3021–3029. doi: 10.1523/JNEUROSCI.4454-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Holtmaat A, Wilbrecht L, Knott GW, Welker E, Svoboda K. Experience-dependent and cell-type-specific spine growth in the neocortex. Nature. 2006;441:979–983. doi: 10.1038/nature04783. [DOI] [PubMed] [Google Scholar]
- 17.Holtmaat AJ, et al. Transient and persistent dendritic spines in the neocortex in vivo. Neuron. 2005;45:279–291. doi: 10.1016/j.neuron.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 18.Trachtenberg JT, et al. Long-term in vivo imaging of experience-dependent synaptic plasticity in adult cortex. Nature. 2002;420:788–794. doi: 10.1038/nature01273. [DOI] [PubMed] [Google Scholar]
- 19.Zuo Y, Yang G, Kwon E, Gan WB. Long-term sensory deprivation prevents dendritic spine loss in primary somatosensory cortex. Nature. 2005;436:261–265. doi: 10.1038/nature03715. [DOI] [PubMed] [Google Scholar]
- 20.Yang G, Pan F, Gan WB. Stably maintained dendritic spines are associated with lifelong memories. Nature. 2009;462:920–924. doi: 10.1038/nature08577. The landmark studies described in references 14–20 use live imaging to describe and characterize dendrite arbor and dendritic spine dynamics in living brain tissue over periods ranging from hours to many months and assess how dynamics are affected by developmental periods and sensory inputs. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huttenlocher PR. Synaptic density in human frontal cortex - developmental changes and effects of aging. Brain Res. 1979;163:195–205. doi: 10.1016/0006-8993(79)90349-4. [DOI] [PubMed] [Google Scholar]
- 22.Huttenlocher PR. Morphometric study of human cerebral cortex development. Neuropsychologia. 1990;28:517–527. doi: 10.1016/0028-3932(90)90031-i. [DOI] [PubMed] [Google Scholar]
- 23.Rakic P, Bourgeois JP, Eckenhoff MF, Zecevic N, Goldman-Rakic PS. Concurrent overproduction of synapses in diverse regions of the primate cerebral cortex. Science. 1986;232:232–235. doi: 10.1126/science.3952506. [DOI] [PubMed] [Google Scholar]
- 24.Rakic P, Bourgeois JP, Goldman-Rakic PS. Synaptic development of the cerebral cortex: implications for learning, memory, and mental illness. Prog Brain Res. 1994;102:227–243. doi: 10.1016/S0079-6123(08)60543-9. [DOI] [PubMed] [Google Scholar]
- 25.Markus EJ, Petit TL. Neocortical synaptogenesis, aging, and behavior: lifespan development in the motor-sensory system of the rat. Exp Neurol. 1987;96:262–278. doi: 10.1016/0014-4886(87)90045-8. [DOI] [PubMed] [Google Scholar]
- 26.Gourley SL, Olevska A, Warren MS, Taylor JR, Koleske AJ. Arg kinase regulates prefrontal dendritic spine refinement and cocaine-induced plasticity. J Neurosci. 2012;32:2314–2323. doi: 10.1523/JNEUROSCI.2730-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zuo Y, Lin A, Chang P, Gan WB. Development of long-term dendritic spine stability in diverse regions of cerebral cortex. Neuron. 2005;46:181–189. doi: 10.1016/j.neuron.2005.04.001. This study associates the formation and stabilization of new spines with learning of new tasks, supporting the idea that spines may help to encode memories. [DOI] [PubMed] [Google Scholar]
- 28.Kulkarni VA, Firestein BL. The dendritic tree and brain disorders. Mol Cell Neurosci. 2012;50:10–20. doi: 10.1016/j.mcn.2012.03.005. [DOI] [PubMed] [Google Scholar]
- 29.Harris KM. Structure, development, and plasticity of dendritic spines. Curr Opin Neurobiol. 1999;9:343–348. doi: 10.1016/s0959-4388(99)80050-6. [DOI] [PubMed] [Google Scholar]
- 30.Bourne J, Harris KM. Do thin spines learn to be mushroom spines that remember? Curr Opin Neurobiol. 2007;17:381–386. doi: 10.1016/j.conb.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 31.Izeddin I, et al. Super-resolution dynamic imaging of dendritic spines using a low-affinity photoconvertible actin probe. PLoS ONE. 2011;6:e15611. doi: 10.1371/journal.pone.0015611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tashiro A, Yuste R. Structure and molecular organization of dendritic spines. Histol Histopathol. 2003;18:617–634. doi: 10.14670/HH-18.617. [DOI] [PubMed] [Google Scholar]
- 33.Fifkova E, Delay RJ. Cytoplasmic actin in neuronal processes as a possible mediator of synaptic plasticity. J Cell Biol. 1982;95:345–350. doi: 10.1083/jcb.95.1.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Matus A, Ackermann M, Pehling G, Byers HR, Fujiwara K. High actin concentrations in brain dendritic spines and postsynaptic densities. Proc Natl Acad Sci USA. 1982;79:7590–7594. doi: 10.1073/pnas.79.23.7590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fischer M, Kaech S, Knutti D, Matus A. Rapid actin-based plasticity in dendritic spines. Neuron. 1998;20:847–854. doi: 10.1016/s0896-6273(00)80467-5. [DOI] [PubMed] [Google Scholar]
- 36.Star EN, Kwiatkowski DJ, Murthy VN. Rapid turnover of actin in dendritic spines and its regulation by activity. Nature Neurosci. 2002;5:239–246. doi: 10.1038/nn811. [DOI] [PubMed] [Google Scholar]
- 37.Hotulainen P, Hoogenraad CC. Actin in dendritic spines: connecting dynamics to function. J Cell Biol. 2010;189:619–629. doi: 10.1083/jcb.201003008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Racz B, Weinberg RJ. Microdomains in forebrain spines: an ultrastructural perspective. Mol Neurobiol. 2012;47:77–89. doi: 10.1007/s12035-012-8345-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Korobova F, Svitkina T. Molecular architecture of synaptic actin cytoskeleton in hippocampal neurons reveals a mechanism of dendritic spine morphogenesis. Mol Biol Cell. 2010;21:165–176. doi: 10.1091/mbc.E09-07-0596. This paper uses platinum replica electron microscopy to reveal the cytoskeletal ultrastructure in dendritic spines. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Okamoto K, Nagai T, Miyawaki A, Hayashi Y. Rapid and persistent modulation of actin dynamics regulates postsynaptic reorganization underlying bidirectional plasticity. Nature Neurosci. 2004;7:1104–1112. doi: 10.1038/nn1311. [DOI] [PubMed] [Google Scholar]
- 41.Matsuzaki M, Honkura N, Ellis-Davies GC, Kasai H. Structural basis of long-term potentiation in single dendritic spines. Nature. 2004;429:761–766. doi: 10.1038/nature02617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhou Q, Homma KJ, Poo MM. Shrinkage of dendritic spines associated with long-term depression of hippocampal synapses. Neuron. 2004;44:749–757. doi: 10.1016/j.neuron.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 43.Honkura N, Matsuzaki M, Noguchi J, Ellis-Davies GC, Kasai H. The subspine organization of actin fibers regulates the structure and plasticity of dendritic spines. Neuron. 2008;57:719–729. doi: 10.1016/j.neuron.2008.01.013. This study uses photoactivation of green fluorescent protein-tagged actin to demonstrate that dendritic spines contain a more stable F-actin core and a dynamic pool of F-actin at the periphery. [DOI] [PubMed] [Google Scholar]
- 44.Tatavarty V, Kim EJ, Rodionov V, Yu J. Investigating sub-spine actin dynamics in rat hippocampal neurons with super-resolution optical imaging. PLoS ONE. 2009;4:e7724. doi: 10.1371/journal.pone.0007724. This study uses single-molecule photoactivation combined with total internal reflection fluorescence microscopy to monitor the dynamics of single actin molecules in dendritic spines. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Racz B, Weinberg RJ. The subcellular organization of cortactin in hippocampus. J Neurosci. 2004;24:10310–10317. doi: 10.1523/JNEUROSCI.2080-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Racz B, Weinberg RJ. Spatial organization of cofilin in dendritic spines. Neuroscience. 2006;138:447–456. doi: 10.1016/j.neuroscience.2005.11.025. [DOI] [PubMed] [Google Scholar]
- 47.Conde C, Caceres A. Microtubule assembly, organization and dynamics in axons and dendrites. Nature Rev Neurosci. 2009;10:319–332. doi: 10.1038/nrn2631. [DOI] [PubMed] [Google Scholar]
- 48.Baas PW, Deitch JS, Black MM, Banker GA. Polarity orientation of microtubules in hippocampal neurons: uniformity in the axon and nonuniformity in the dendrite. Proc Natl Acad Sci USA. 1988;85:8335–8339. doi: 10.1073/pnas.85.21.8335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Burton PR. Dendrites of mitral cell neurons contain microtubules of opposite polarity. Brain Res. 1988;473:107–115. doi: 10.1016/0006-8993(88)90321-6. [DOI] [PubMed] [Google Scholar]
- 50.Kwan AC, Dombeck DA, Webb WW. Polarized microtubule arrays in apical dendrites and axons. Proc Natl Acad Sci USA. 2008;105:11370–11375. doi: 10.1073/pnas.0805199105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sasaki S, Stevens JK, Bodick N. Serial reconstruction of microtubular arrays within dendrites of the cat retinal ganglion cell: the cytoskeleton of a vertebrate dendrite. Brain Res. 1983;259:193–206. doi: 10.1016/0006-8993(83)91250-7. [DOI] [PubMed] [Google Scholar]
- 52.Okabe S, Hirokawa N. Turnover of fluorescently labelled tubulin and actin in the axon. Nature. 1990;343:479–482. doi: 10.1038/343479a0. [DOI] [PubMed] [Google Scholar]
- 53.Edson KJ, Lim SS, Borisy GG, Letourneau PC. FRAP analysis of the stability of the microtubule population along the neurites of chick sensory neurons. Cell Motil Cytoskeleton. 1993;25:59–72. doi: 10.1002/cm.970250108. [DOI] [PubMed] [Google Scholar]
- 54.Racz B, Weinberg RJ. Organization of the Arp2/3 complex in hippocampal spines. J Neurosci. 2008;28:5654–5659. doi: 10.1523/JNEUROSCI.0756-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pollard TD, Borisy GG. Cellular motility driven by assembly and disassembly of actin filaments. Cell. 2003;112:453–465. doi: 10.1016/s0092-8674(03)00120-x. [DOI] [PubMed] [Google Scholar]
- 56.Kim IH, et al. Disruption of Arp2/3 results in asymmetric structural plasticity of dendritic spines and progressive synaptic and behavioral abnormalities. J Neurosci. 2013;33:6081–6092. doi: 10.1523/JNEUROSCI.0035-13.2013. This interesting paper shows that inactivation of the ARP2/3 complex in mature neurons leads to loss of plasticity-associated spine enlargement and gradual spine shrinkage and loss. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.De Camilli P, Miller PE, Navone F, Theurkauf WE, Vallee RB. Distribution of microtubule-associated protein 2 in the nervous system of the rat studied by immunofluorescence. Neuroscience. 1984;11:817–846. [PubMed] [Google Scholar]
- 58.Huber G, Matus A. Differences in the cellular distributions of two microtubule-associated proteins, MAP1 and MAP2, in rat brain. J Neurosci. 1984;4:151–160. doi: 10.1523/JNEUROSCI.04-01-00151.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bloom GS, Schoenfeld TA, Vallee RB. Widespread distribution of the major polypeptide component of MAP 1 (microtubule-associated protein 1) in the nervous system. J Cell Biol. 1984;98:320–330. doi: 10.1083/jcb.98.1.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vaillant AR, et al. Signaling mechanisms underlying reversible, activity-dependent dendrite formation. Neuron. 2002;34:985–998. doi: 10.1016/s0896-6273(02)00717-1. [DOI] [PubMed] [Google Scholar]
- 61.Szebenyi G, et al. Activity-driven dendritic remodeling requires microtubule-associated protein 1A. Curr Biol. 2005;15:1820–1826. doi: 10.1016/j.cub.2005.08.069. This very elegant study demonstrates that activity-dependent dendrite elaboration depends critically on MAP1A. [DOI] [PubMed] [Google Scholar]
- 62.Teng J, et al. Synergistic effects of MAP2 and MAP1B knockout in neuronal migration, dendritic outgrowth, and microtubule organization. J Cell Biol. 2001;155:65–76. doi: 10.1083/jcb.200106025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Harada A, Teng J, Takei Y, Oguchi K, Hirokawa N. MAP2 is required for dendrite elongation, PKA anchoring in dendrites, and proper PKA signal transduction. J Cell Biol. 2002;158:541–549. doi: 10.1083/jcb.200110134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sudo H, Baas PW. Acetylation of microtubules influences their sensitivity to severing by katanin in neurons and fibroblasts. J Neurosci. 2010;30:7215–7226. doi: 10.1523/JNEUROSCI.0048-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Peris L, et al. Motor-dependent microtubule disassembly driven by tubulin tyrosination. J Cell Biol. 2009;185:1159–1166. doi: 10.1083/jcb.200902142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Govek EE, Newey SE, Van Aelst L. The role of the Rho GTPases in neuronal development. Genes Dev. 2005;19:1–49. doi: 10.1101/gad.1256405. [DOI] [PubMed] [Google Scholar]
- 67.Murakoshi H, Wang H, Yasuda R. Local, persistent activation of Rho GTPases during plasticity of single dendritic spines. Nature. 2011;472:100–104. doi: 10.1038/nature09823. This landmark paper uses fluorescence resonance energy transfer-based probes and genetic knockdown to implicate RHO and CDC42 in activity-based spine head enlargement. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nakayama AY, Harms MB, Luo L. Small GTPases Rac and Rho in the maintenance of dendritic spines and branches in hippocampal pyramidal neurons. J Neurosci. 2000;20:5329–5338. doi: 10.1523/JNEUROSCI.20-14-05329.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kim Y, et al. Phosphorylation of WAVE1 regulates actin polymerization and dendritic spine morphology. Nature. 2006;442:814–817. doi: 10.1038/nature04976. [DOI] [PubMed] [Google Scholar]
- 70.Soderling SH, et al. A WAVE-1 and WRP signaling complex regulates spine density, synaptic plasticity, and memory. J Neurosci. 2007;27:355–365. doi: 10.1523/JNEUROSCI.3209-06.2006. References 69 and 70 implicate WAVE1 as a critical downstream target of RAC1 in the control of dendritic spine formation and stability. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tashiro A, Minden A, Yuste R. Regulation of dendritic spine morphology by the rho family of small GTPases: antagonistic roles of Rac and Rho. Cereb Cortex. 2000;10:927–938. doi: 10.1093/cercor/10.10.927. [DOI] [PubMed] [Google Scholar]
- 72.Xing L, Yao X, Williams KR, Bassell GJ. Negative regulation of RhoA translation and signaling by hnRNP-Q1 affects cellular morphogenesis. Mol Biol Cell. 2012;23:1500–1509. doi: 10.1091/mbc.E11-10-0867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kang MG, Guo Y, Huganir RL. AMPA receptor and GEF-H1/Lfc complex regulates dendritic spine development through RhoA signaling cascade. Proc Natl Acad Sci USA. 2009;106:3549–3554. doi: 10.1073/pnas.0812861106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yang N, et al. Cofilin phosphorylation by LIM-kinase 1 and its role in Rac-mediated actin reorganization. Nature. 1998;393:809–812. doi: 10.1038/31735. [DOI] [PubMed] [Google Scholar]
- 75.Arber S, et al. Regulation of actin dynamics through phosphorylation of cofilin by LIM-kinase. Nature. 1998;393:805–809. doi: 10.1038/31729. [DOI] [PubMed] [Google Scholar]
- 76.Lin YC, Yeckel MF, Koleske AJ. Abl2/Arg controls dendritic spine and dendrite arbor stability via distinct cytoskeletal control pathways. J Neurosci. 2013;33:1846–1857. doi: 10.1523/JNEUROSCI.4284-12.2013. This paper demonstrates that ARG dampens activity-dependent disruption of cortactin localization to stabilize dendritic spines and independently attenuates RHO activity to stabilize dendrite arbors. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sfakianos MK, et al. Inhibition of Rho via Arg and p190RhoGAP in the postnatal mouse hippocampus regulates dendritic spine maturation, synapse and dendrite stability, and behavior. J Neurosci. 2007;27:10982–10992. doi: 10.1523/JNEUROSCI.0793-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Calabrese B, Halpain S. Essential role for the PKC target MARCKS in maintaining dendritic spine morphology. Neuron. 2005;48:77–90. doi: 10.1016/j.neuron.2005.08.027. [DOI] [PubMed] [Google Scholar]
- 79.Shirao T, Inoue HK, Kano Y, Obata K. Localization of a developmentally regulated neuron-specific protein S54 in dendrites as revealed by immunoelectron microscopy. Brain Res. 1987;413:374–378. doi: 10.1016/0006-8993(87)91032-8. [DOI] [PubMed] [Google Scholar]
- 80.Yamazaki H, Takahashi H, Aoki T, Shirao T. Molecular cloning and dendritic localization of rat SH3P7. Eur J Neurosci. 2001;14:998–1008. doi: 10.1046/j.0953-816x.2001.01727.x. [DOI] [PubMed] [Google Scholar]
- 81.Matsuoka Y, Li X, Bennett V. Adducin is an in vivo substrate for protein kinase C: phosphorylation in the MARCKS-related domain inhibits activity in promoting spectrin-actin complexes and occurs in many cells, including dendritic spines of neurons. J Cell Biol. 1998;142:485–497. doi: 10.1083/jcb.142.2.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tanokashira D, et al. Glucocorticoid suppresses dendritic spine development mediated by down-regulation of caldesmon expression. J Neurosci. 2012;32:14583–14591. doi: 10.1523/JNEUROSCI.2380-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Macgrath SM, Koleske AJ. Cortactin in cell migration and cancer at a glance. J Cell Sci. 2012;125:1621–1626. doi: 10.1242/jcs.093781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Iki J, Inoue A, Bito H, Okabe S. Bi-directional regulation of postsynaptic cortactin distribution by BDNF and NMDA receptor activity. Eur J Neurosci. 2005;22:2985–2994. doi: 10.1111/j.1460-9568.2005.04510.x. [DOI] [PubMed] [Google Scholar]
- 85.Jaworski J, et al. Dynamic microtubules regulate dendritic spine morphology and synaptic plasticity. Neuron. 2009;61:85–100. doi: 10.1016/j.neuron.2008.11.013. This study, along with references 156–158, indicates that microtubule targeting to dendritic spines can be regulated by activity and by BDNF and that this targeting stabilizes spines by promoting the accumulation of key spine stabilizing proteins. [DOI] [PubMed] [Google Scholar]
- 86.Naisbitt S, et al. Shank, a novel family of postsynaptic density proteins that binds to the NMDA receptor/PSD-95/GKAP complex and cortactin. Neuron. 1999;23:569–582. doi: 10.1016/s0896-6273(00)80809-0. [DOI] [PubMed] [Google Scholar]
- 87.Du Y, Weed SA, Xiong WC, Marshall TD, Parsons JT. Identification of a novel cortactin SH3 domain-binding protein and its localization to growth cones of cultured neurons. Mol Cell Biol. 1998;18:5838–5851. doi: 10.1128/mcb.18.10.5838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chen YK, Hsueh YP. Cortactin-binding protein 2 modulates the mobility of cortactin and regulates dendritic spine formation and maintenance. J Neurosci. 2012;32:1043–1055. doi: 10.1523/JNEUROSCI.4405-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hering H, Sheng M. Activity-dependent redistribution and essential role of cortactin in dendritic spine morphogenesis. J Neurosci. 2003;23:11759–11769. doi: 10.1523/JNEUROSCI.23-37-11759.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Seese RR, et al. LTP induction translocates cortactin at distant synapses in wild-type but not Fmr1 knockout mice. J Neurosci. 2012;32:7403–7413. doi: 10.1523/JNEUROSCI.0968-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Takahashi H, et al. Drebrin-dependent actin clustering in dendritic filopodia governs synaptic targeting of postsynaptic density-95 and dendritic spine morphogenesis. J Neurosci. 2003;23:6586–6595. doi: 10.1523/JNEUROSCI.23-16-06586.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Haeckel A, Ahuja R, Gundelfinger ED, Qualmann B, Kessels MM. The actin-binding protein Abp1 controls dendritic spine morphology and is important for spine head and synapse formation. J Neurosci. 2008;28:10031–10044. doi: 10.1523/JNEUROSCI.0336-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Malenka RC, Bear MF. LTP and LTD: an embarrassment of riches. Neuron. 2004;44:5–21. doi: 10.1016/j.neuron.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 94.Larsson C. Protein kinase C and the regulation of the actin cytoskeleton. Cell Signal. 2006;18:276–284. doi: 10.1016/j.cellsig.2005.07.010. [DOI] [PubMed] [Google Scholar]
- 95.Hartwig JH, et al. MARCKS is an actin filament crosslinking protein regulated by protein kinase C and calcium-calmodulin. Nature. 1992;356:618–622. doi: 10.1038/356618a0. [DOI] [PubMed] [Google Scholar]
- 96.Bednarek E, Caroni P. β-Adducin is required for stable assembly of new synapses and improved memory upon environmental enrichment. Neuron. 2011;69:1132–1146. doi: 10.1016/j.neuron.2011.02.034. This paper shows the critical importance of β-adducin in controlling new synapse formation upon environmental enrichment. [DOI] [PubMed] [Google Scholar]
- 97.Jung Y, Mulholland PJ, Wiseman SL, Judson Chandler L, Picciotto MR. Constitutive knockout of the membrane cytoskeleton protein β adducin decreases mushroom spine density in the nucleus accumbens but does not prevent spine remodeling in response to cocaine. Eur J Neurosci. 2012;37:1–9. doi: 10.1111/ejn.12037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Shi Y, Pontrello CG, DeFea KA, Reichardt LF, Ethell IM. Focal adhesion kinase acts downstream of EphB receptors to maintain mature dendritic spines by regulating cofilin activity. J Neurosci. 2009;29:8129–8142. doi: 10.1523/JNEUROSCI.4681-08.2009. This study describes an important role for FAK as an intermediary between EPHB signalling and cofilin phosphorylation in the control of dendritic spine stability. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Tomar A, Schlaepfer DD. Focal adhesion kinase: switching between GAPs and GEFs in the regulation of cell motility. Curr Opin Cell Biol. 2009;21:676–683. doi: 10.1016/j.ceb.2009.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Babus LW, et al. Decreased dendritic spine density and abnormal spine morphology in Fyn knockout mice. Brain Res. 2011;1415:96–102. doi: 10.1016/j.brainres.2011.07.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bourgin C, Murai KK, Richter M, Pasquale EB. The EphA4 receptor regulates dendritic spine remodeling by affecting β1-integrin signaling pathways. J Cell Biol. 2007;178:1295–1307. doi: 10.1083/jcb.200610139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hartmann M, Heumann R, Lessmann V. Synaptic secretion of BDNF after high-frequency stimulation of glutamatergic synapses. EMBO J. 2001;20:5887–5897. doi: 10.1093/emboj/20.21.5887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kohara K, Kitamura A, Morishima M, Tsumoto T. Activity-dependent transfer of brain-derived neurotrophic factor to postsynaptic neurons. Science. 2001;291:2419–2423. doi: 10.1126/science.1057415. [DOI] [PubMed] [Google Scholar]
- 104.Kojima M, et al. Biological characterization and optical imaging of brain-derived neurotrophic factor-green fluorescent protein suggest an activity-dependent local release of brain-derived neurotrophic factor in neurites of cultured hippocampal neurons. J Neurosci Res. 2001;64:1–10. doi: 10.1002/jnr.1080. [DOI] [PubMed] [Google Scholar]
- 105.Hu B, Nikolakopoulou AM, Cohen-Cory S. BDNF stabilizes synapses and maintains the structural complexity of optic axons in vivo. Development. 2005;132:4285–4298. doi: 10.1242/dev.02017. [DOI] [PubMed] [Google Scholar]
- 106.Marshak S, Nikolakopoulou AM, Dirks R, Martens GJ, Cohen-Cory S. Cell-autonomous TrkB signaling in presynaptic retinal ganglion cells mediates axon arbor growth and synapse maturation during the establishment of retinotectal synaptic connectivity. J Neurosci. 2007;27:2444–2456. doi: 10.1523/JNEUROSCI.4434-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Yasuda R, et al. Supersensitive Ras activation in dendrites and spines revealed by two-photon fluorescence lifetime imaging. Nature Neurosci. 2006;9:283–291. doi: 10.1038/nn1635. [DOI] [PubMed] [Google Scholar]
- 108.Rex CS, et al. Brain-derived neurotrophic factor promotes long-term potentiation-related cytoskeletal changes in adult hippocampus. J Neurosci. 2007;27:3017–3029. doi: 10.1523/JNEUROSCI.4037-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Pinkstaff JK, Detterich J, Lynch G, Gall C. Integrin subunit gene expression is regionally differentiated in adult brain. J Neurosci. 1999;19:1541–1556. doi: 10.1523/JNEUROSCI.19-05-01541.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Mortillo S, et al. Compensatory redistribution of neuroligins and N-cadherin following deletion of synaptic β1-integrin. J Comp Neurol. 2012;520:2041–2052. doi: 10.1002/cne.23027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kramar EA, Bernard JA, Gall CM, Lynch G. α3 integrin receptors contribute to the consolidation of long-term potentiation. Neuroscience. 2002;110:29–39. doi: 10.1016/s0306-4522(01)00540-1. [DOI] [PubMed] [Google Scholar]
- 112.Chan CS, et al. α3-integrins are required for hippocampal long-term potentiation and working memory. Learn Mem. 2007;14:606–615. doi: 10.1101/lm.648607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Chan CS, Weeber EJ, Kurup S, Sweatt JD, Davis RL. Integrin requirement for hippocampal synaptic plasticity and spatial memory. J Neurosci. 2003;23:7107–7116. doi: 10.1523/JNEUROSCI.23-18-07107.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Warren MS, et al. Integrin β1 signals through Arg to regulate postnatal dendritic arborization, synapse density, and behavior. J Neurosci. 2012;32:2824–2834. doi: 10.1523/JNEUROSCI.3942-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kerrisk ME, Greer CA, Koleske AJ. Integrin a3 is required for late postnatal stability of dendrite arbors, dendritic spines and synapses, and mouse behavior. J Neurosci. 2013;133:6742–6752. doi: 10.1523/JNEUROSCI.0528-13.2013. References 114 and 115 describe key roles for integrin α3β1 in the control of dendrite and dendritic spine stability. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Stanco A, et al. Netrin-1–α3β1 integrin interactions regulate the migration of interneurons through the cortical marginal zone. Proc Natl Acad Sci USA. 2009;106:7595–7600. doi: 10.1073/pnas.0811343106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Yebra M, et al. Recognition of the neural chemoattractant Netrin-1 by integrins α6β4 and α3β1 regulates epithelial cell adhesion and migration. Dev Cell. 2003;5:695–707. doi: 10.1016/s1534-5807(03)00330-7. [DOI] [PubMed] [Google Scholar]
- 118.Dulabon L, et al. Reelin binds α3β1 integrin and inhibits neuronal migration. Neuron. 2000;27:33–44. doi: 10.1016/s0896-6273(00)00007-6. [DOI] [PubMed] [Google Scholar]
- 119.DeFreitas MF, et al. Identification of integrin α3β1 as a neuronal thrombospondin receptor mediating neurite outgrowth. Neuron. 1995;15:333–343. doi: 10.1016/0896-6273(95)90038-1. [DOI] [PubMed] [Google Scholar]
- 120.Bradley WD, Koleske AJ. Regulation of cell migration and morphogenesis by Abl-family kinases: emerging mechanisms and physiological contexts. J Cell Sci. 2009;122:3441–3454. doi: 10.1242/jcs.039859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Koleske AJ, et al. Essential roles for the Abl and Arg tyrosine kinases in neurulation. Neuron. 1998;21:1259–1272. doi: 10.1016/s0896-6273(00)80646-7. [DOI] [PubMed] [Google Scholar]
- 122.Moresco EM, Scheetz AJ, Bornmann WG, Koleske AJ, Fitzsimonds RM. Abl family nonreceptor tyrosine kinases modulate short-term synaptic plasticity. J Neurophysiol. 2003;89:1678–1687. doi: 10.1152/jn.00892.2002. [DOI] [PubMed] [Google Scholar]
- 123.Gourley SL, Koleske AJ, Taylor JR. Loss of dendrite stabilization by the Abl-related gene (Arg) kinase regulates behavioral flexibility and sensitivity to cocaine. Proc Natl Acad Sci USA. 2009;106:16859–16864. doi: 10.1073/pnas.0902286106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Wang Y, Miller AL, Mooseker MS, Koleske AJ. The Abl-related gene (Arg) nonreceptor tyrosine kinase uses two F-actin-binding domains to bundle F-actin. Proc Natl Acad Sci USA. 2001;98:14865–14870. doi: 10.1073/pnas.251249298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Macgrath SM, Koleske AJ. Arg/Abl2 modulates the affinity and stoichiometry of binding of cortactin to f-actin. Biochemistry. 2012;51:6644–6653. doi: 10.1021/bi300722t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Galkin VE, Orlova A, Koleske AJ, Egelman EH. The Arg non-receptor tyrosine kinase modifies F-actin structure. J Mol Biol. 2005;346:565–575. doi: 10.1016/j.jmb.2004.11.078. [DOI] [PubMed] [Google Scholar]
- 127.Lau LF, Huganir RL. Differential tyrosine phosphorylation of N-methyl-D-aspartate receptor subunits. J Biol Chem. 1995;270:20036–20041. doi: 10.1074/jbc.270.34.20036. [DOI] [PubMed] [Google Scholar]
- 128.Moon IS. Relative extent of tyrosine phosphorylation of the NR2A and NR2B subunits in the rat forebrain postsynaptic density fraction. Mol Cells. 2003;16:28–33. [PubMed] [Google Scholar]
- 129.Prybylowski K, et al. The synaptic localization of NR2B-containing NMDA receptors is controlled by interactions with PDZ proteins and AP-2. Neuron. 2005;47:845–857. doi: 10.1016/j.neuron.2005.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Williams DW, Truman JW. Cellular mechanisms of dendrite pruning in Drosophila: insights from in vivo time-lapse of remodeling dendritic arborizing sensory neurons. Development. 2005;132:3631–3642. doi: 10.1242/dev.01928. [DOI] [PubMed] [Google Scholar]
- 131.Lee HH, Jan LY, Jan YN. Drosophila IKK-related kinase Ik2 and Katanin p60-like 1 regulate dendrite pruning of sensory neuron during metamorphosis. Proc Natl Acad Sci USA. 2009;106:6363–6368. doi: 10.1073/pnas.0902051106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Bodick N, Stevens JK, Sasaki S, Purpura DP. Microtubular disarray in cortical dendrites and neurobehavioral failure. II. Computer reconstruction of perturbed microtubular arrays. Brain Res. 1982;281:299–309. doi: 10.1016/0165-3806(82)90129-8. [DOI] [PubMed] [Google Scholar]
- 133.Purpura DP, Bodick N, Suzuki K, Rapin I, Wurzelmann S. Microtubule disarray in cortical dendrites and neurobehavioral failure. I. Golgi and electron microscopic studies. Brain Res. 1982;281:287–297. doi: 10.1016/0165-3806(82)90128-6. [DOI] [PubMed] [Google Scholar]
- 134.Horton AC, et al. Polarized secretory trafficking directs cargo for asymmetric dendrite growth and morphogenesis. Neuron. 2005;48:757–771. doi: 10.1016/j.neuron.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 135.Ori-McKenney KM, Jan LY, Jan YN. Golgi outposts shape dendrite morphology by functioning as sites of acentrosomal microtubule nucleation in neurons. Neuron. 2012;76:921–930. doi: 10.1016/j.neuron.2012.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Satoh D, et al. Spatial control of branching within dendritic arbors by dynein-dependent transport of Rab5-endosomes. Nature Cell Biol. 2008;10:1164–1171. doi: 10.1038/ncb1776. [DOI] [PubMed] [Google Scholar]
- 137.Zheng Y, et al. Dynein is required for polarized dendritic transport and uniform microtubule orientation in axons. Nature Cell Biol. 2008;10:1172–1180. doi: 10.1038/ncb1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Ultanir SK, et al. Chemical genetic identification of NDR1/2 kinase substrates AAK1 and Rabin8 Uncovers their roles in dendrite arborization and spine development. Neuron. 2012;73:1127–1142. doi: 10.1016/j.neuron.2012.01.019. This study uses the most cutting edge chemical genetic approach to identify several targets of the NDR1 and NDR2 in the control of dendrite formation and stability. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Wu GY, Cline HT. Stabilization of dendritic arbor structure in vivo by CaMKII. Science. 1998;279:222–226. doi: 10.1126/science.279.5348.222. [DOI] [PubMed] [Google Scholar]
- 140.Gorski JA, Zeiler SR, Tamowski S, Jones KR. Brain-derived neurotrophic factor is required for the maintenance of cortical dendrites. J Neurosci. 2003;23:6856–6865. doi: 10.1523/JNEUROSCI.23-17-06856.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Xu B, et al. Cortical degeneration in the absence of neurotrophin signaling: dendritic retraction and neuronal loss after removal of the receptor TrkB. Neuron. 2000;26:233–245. doi: 10.1016/s0896-6273(00)81153-8. [DOI] [PubMed] [Google Scholar]
- 142.Akum BF, et al. Cypin regulates dendrite patterning in hippocampal neurons by promoting microtubule assembly. Nature Neurosci. 2004;7:145–152. doi: 10.1038/nn1179. [DOI] [PubMed] [Google Scholar]
- 143.Kwon M, Fernandez JR, Zegarek GF, Lo SB, Firestein BL. BDNF-promoted increases in proximal dendrites occur via CREB-dependent transcriptional regulation of cypin. J Neurosci. 2011;31:9735–9745. doi: 10.1523/JNEUROSCI.6785-10.2011. This study shows that BDNF can induce transcription of the microtubule- and dendrite-stabilizing protein cypin. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Moresco EM, Donaldson S, Williamson A, Koleske AJ. Integrin-mediated dendrite branch maintenance requires Abelson (Abl) family kinases. J Neurosci. 2005;25:6105–6118. doi: 10.1523/JNEUROSCI.1432-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Hernandez SE, Settleman J, Koleske AJ. Adhesion-dependent regulation of p190RhoGAP in the developing brain by the Abl-related gene tyrosine kinase. Curr Biol. 2004;14:691–696. doi: 10.1016/j.cub.2004.03.062. [DOI] [PubMed] [Google Scholar]
- 146.Bradley WD, Hernandez SE, Settleman J, Koleske AJ. Integrin signaling through Arg activates p190RhoGAP by promoting its binding to p120RasGAP and recruitment to the membrane. Mol Biol Cell. 2006;17:4827–4836. doi: 10.1091/mbc.E06-02-0132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Li Z, Aizenman CD, Cline HT. Regulation of rho GTPases by crosstalk and neuronal activity in vivo. Neuron. 2002;33:741–750. doi: 10.1016/s0896-6273(02)00621-9. [DOI] [PubMed] [Google Scholar]
- 148.Li Z, Van Aelst L, Cline HT. Rho GTPases regulate distinct aspects of dendritic arbor growth in Xenopus central neurons in vivo. Nature Neurosci. 2000;3:217–225. doi: 10.1038/72920. [DOI] [PubMed] [Google Scholar]
- 149.Ruchhoeft ML, Ohnuma S, McNeill L, Holt CE, Harris WA. The neuronal architecture of Xenopus retinal ganglion cells is sculpted by rho-family GTPases in vivo. J Neurosci. 1999;19:8454–8463. doi: 10.1523/JNEUROSCI.19-19-08454.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Lee T, Winter C, Marticke SS, Lee A, Luo L. Essential roles of Drosophila RhoA in the regulation of neuroblast proliferation and dendritic but not axonal morphogenesis. Neuron. 2000;25:307–316. doi: 10.1016/s0896-6273(00)80896-x. [DOI] [PubMed] [Google Scholar]
- 151.Chen H, Firestein BL. RhoA regulates dendrite branching in hippocampal neurons by decreasing cypin protein levels. J Neurosci. 2007;27:8378–8386. doi: 10.1523/JNEUROSCI.0872-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Hirose M, et al. Molecular dissection of the Rho-associated protein kinase (p160ROCK)-regulated neurite remodeling in neuroblastoma N1E-115 cells. J Cell Biol. 1998;141:1625–1636. doi: 10.1083/jcb.141.7.1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Amano M, et al. Identification of Tau and MAP2 as novel substrates of Rho-kinase and myosin phosphatase. J Neurochem. 2003;87:780–790. doi: 10.1046/j.1471-4159.2003.02054.x. [DOI] [PubMed] [Google Scholar]
- 154.Murthy AS, Flavin M. Microtubule assembly using the microtubule-associated protein MAP-2 prepared in defined states of phosphorylation with protein kinase and phosphatase. Eur J Biochem. 1983;137:37–46. doi: 10.1111/j.1432-1033.1983.tb07792.x. [DOI] [PubMed] [Google Scholar]
- 155.Yamamoto H, Fukunaga K, Tanaka E, Miyamoto E. Ca2+- and calmodulin-dependent phosphorylation of microtubule-associated protein 2 and tau factor, and inhibition of microtubule assembly. J Neurochem. 1983;41:1119–1125. doi: 10.1111/j.1471-4159.1983.tb09060.x. [DOI] [PubMed] [Google Scholar]
- 156.Gu J, Firestein BL, Zheng JQ. Microtubules in dendritic spine development. J Neurosci. 2008;28:12120–12124. doi: 10.1523/JNEUROSCI.2509-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Hu X, et al. BDNF-induced increase of PSD-95 in dendritic spines requires dynamic microtubule invasions. J Neurosci. 2011;31:15597–15603. doi: 10.1523/JNEUROSCI.2445-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Hu X, Viesselmann C, Nam S, Merriam E, Dent EW. Activity-dependent dynamic microtubule invasion of dendritic spines. J Neurosci. 2008;28:13094–13105. doi: 10.1523/JNEUROSCI.3074-08.2008. References 156–158, along with reference 85, indicate that microtubule targeting to dendritic spines can be regulated by activity and by BDNF and that this targeting stabilizes spines by promoting the accumulation of key spine stabilizing proteins. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.von Bohlen und Halbach O, Minichiello L, Unsicker K. Haploinsufficiency in trkB and/or trkC neurotrophin receptors causes structural alterations in the aged hippocampus and amygdala. Eur J Neurosci. 2003;18:2319–2325. doi: 10.1046/j.1460-9568.2003.02953.x. [DOI] [PubMed] [Google Scholar]
- 160.Kapitein LC, et al. NMDA receptor activation suppresses microtubule growth and spine entry. J Neurosci. 2011;31:8194–8209. doi: 10.1523/JNEUROSCI.6215-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Le Gros Clark W. Inquiries into the anatomical basis of olfactory discrimination. Proc R Soc Lond B. 1957;146:299–319. doi: 10.1098/rspb.1957.0013. [DOI] [PubMed] [Google Scholar]
- 162.Fleming IN, Elliott CM, Buchanan FG, Downes CP, Exton JH. Ca2+/calmodulin-dependent protein kinase II regulates Tiam1 by reversible protein phosphorylation. J Biol Chem. 1999;274:12753–12758. doi: 10.1074/jbc.274.18.12753. [DOI] [PubMed] [Google Scholar]
- 163.Xie Z, et al. Kalirin-7 controls activity-dependent structural and functional plasticity of dendritic spines. Neuron. 2007;56:640–656. doi: 10.1016/j.neuron.2007.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Chen HJ, Rojas-Soto M, Oguni A, Kennedy MB. A synaptic Ras-GTPase activating protein (p135 SynGAP) inhibited by CaM kinase II. Neuron. 1998;20:895–904. doi: 10.1016/s0896-6273(00)80471-7. [DOI] [PubMed] [Google Scholar]
- 165.Takemoto-Kimura S, et al. Differential roles for CaM kinases in mediating excitation-morphogenesis coupling during formation and maturation of neuronal circuits. Eur J Neurosci. 2010;32:224–230. doi: 10.1111/j.1460-9568.2010.07353.x. [DOI] [PubMed] [Google Scholar]
- 166.Lemieux M, et al. Translocation of CaMKII to dendritic microtubules supports the plasticity of local synapses. J Cell Biol. 2012;198:1055–1073. doi: 10.1083/jcb.201202058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Lin YC, Koleske AJ. Mechanisms of synapse and dendrite maintenance and their disruption in psychiatric and neurodegenerative disorders. Annu Rev Neurosci. 2010;33:349–378. doi: 10.1146/annurev-neuro-060909-153204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Flood DG. Region-specific stability of dendritic extent in normal human aging and regression in Alzheimer’s disease. II. Subiculum. Brain Res. 1991;540:83–95. doi: 10.1016/0006-8993(91)90494-g. [DOI] [PubMed] [Google Scholar]
- 169.Flood DG, Buell SJ, Horwitz GJ, Coleman PD. Dendritic extent in human dentate gyrus granule cells in normal aging and senile dementia. Brain Res. 1987;402:205–216. doi: 10.1016/0006-8993(87)90027-8. [DOI] [PubMed] [Google Scholar]
- 170.Flood DG, Guarnaccia M, Coleman PD. Dendritic extent in human CA2-3 hippocampal pyramidal neurons in normal aging and senile dementia. Brain Res. 1987;409:88–96. doi: 10.1016/0006-8993(87)90744-x. [DOI] [PubMed] [Google Scholar]
- 171.Anderton BH, et al. Dendritic changes in Alzheimer’s disease and factors that may underlie these changes. Prog Neurobiol. 1998;55:595–609. doi: 10.1016/s0301-0082(98)00022-7. [DOI] [PubMed] [Google Scholar]
- 172.Falke E, et al. Subicular dendritic arborization in Alzheimer’s disease correlates with neurofibrillary tangle density. Am J Pathol. 2003;163:1615–1621. doi: 10.1016/S0002-9440(10)63518-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Terry RD, et al. Physical basis of cognitive alterations in Alzheimer’s disease: synapse loss is the major correlate of cognitive impairment. Ann Neurol. 1991;30:572–580. doi: 10.1002/ana.410300410. [DOI] [PubMed] [Google Scholar]
- 174.Haass C, Selkoe DJ. Soluble protein oligomers in neurodegeneration: lessons from the Alzheimer’s amyloid β-peptide. Nature Rev Mol Cell Biol. 2007;8:101–112. doi: 10.1038/nrm2101. [DOI] [PubMed] [Google Scholar]
- 175.Lacor PN, et al. Aβ oligomer-induced aberrations in synapse composition, shape, and density provide a molecular basis for loss of connectivity in Alzheimer’s disease. J Neurosci. 2007;27:796–807. doi: 10.1523/JNEUROSCI.3501-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Shankar GM, et al. Natural oligomers of the Alzheimer amyloid-β protein induce reversible synapse loss by modulating an NMDA-type glutamate receptor-dependent signaling pathway. J Neurosci. 2007;27:2866–2875. doi: 10.1523/JNEUROSCI.4970-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Calabrese B, et al. Rapid, concurrent alterations in pre- and postsynaptic structure induced by naturally-secreted amyloid-β protein. Mol Cell Neurosci. 2007;35:183–193. doi: 10.1016/j.mcn.2007.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Um JW, et al. Alzheimer amyloid-β oligomer bound to postsynaptic prion protein activates Fyn to impair neurons. Nature Neurosci. 2012;15:1227–1235. doi: 10.1038/nn.3178. References 175–178 describe the destabilizing effects of ADDLs on dendritic spine stability and the involvement of FYN and the NMDAR in this process. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Wu HY, et al. Amyloid β induces the morphological neurodegenerative triad of spine loss, dendritic simplification, and neuritic dystrophies through calcineurin activation. J Neurosci. 2010;30:2636–2649. doi: 10.1523/JNEUROSCI.4456-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Wang Y, Shibasaki F, Mizuno K. Calcium signal-induced cofilin dephosphorylation is mediated by Slingshot via calcineurin. J Biol Chem. 2005;280:12683–12689. doi: 10.1074/jbc.M411494200. [DOI] [PubMed] [Google Scholar]
- 181.Zempel H, Thies E, Mandelkow E, Mandelkow EM. Aβ oligomers cause localized Ca2+ elevation, missorting of endogenous Tau into dendrites, Tau phosphorylation, and destruction of microtubules and spines. J Neurosci. 2010;30:11938–11950. doi: 10.1523/JNEUROSCI.2357-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Larson M, et al. The complex. PrPc-Fyn couples human oligomeric Aβ with pathological tau changes in Alzheimer’s disease. J Neurosci. 2012;32:16857–16871. doi: 10.1523/JNEUROSCI.1858-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Ittner LM, et al. Dendritic function of tau mediates amyloid-β toxicity in Alzheimer’s disease mouse models. Cell. 2010;142:387–397. doi: 10.1016/j.cell.2010.06.036. [DOI] [PubMed] [Google Scholar]
- 184.Hoover BR, et al. Tau mislocalization to dendritic spines mediates synaptic dysfunction independently of neurodegeneration. Neuron. 2010;68:1067–1081. doi: 10.1016/j.neuron.2010.11.030. References 181–184 elaborate on the effects of ADDLs on tau phosphorylation and redistribution and the ensuing destabilization of dendrite arbors. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Kang HJ, et al. Decreased expression of synapse-related genes and loss of synapses in major depressive disorder. Nature Med. 2012;18:1413–1417. doi: 10.1038/nm.2886. This landmark study documents synapse loss in MDD and identifies a genetic regulatory network that underlies synapse and dendrite arbor destablization. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Drevets WC, et al. Subgenual prefrontal cortex abnormalities in mood disorders. Nature. 1997;386:824–827. doi: 10.1038/386824a0. [DOI] [PubMed] [Google Scholar]
- 187.Drevets WC. Functional anatomical abnormalities in limbic and prefrontal cortical structures in major depression. Prog Brain Res. 2000;126:413–431. doi: 10.1016/S0079-6123(00)26027-5. [DOI] [PubMed] [Google Scholar]
- 188.Cotter D, et al. Reduced neuronal size and glial cell density in area 9 of the dorsolateral prefrontal cortex in subjects with major depressive disorder. Cereb Cortex. 2002;12:386–394. doi: 10.1093/cercor/12.4.386. [DOI] [PubMed] [Google Scholar]
- 189.Cotter D, Mackay D, Landau S, Kerwin R, Everall I. Reduced glial cell density and neuronal size in the anterior cingulate cortex in major depressive disorder. Arch Gen Psychiatry. 2001;58:545–553. doi: 10.1001/archpsyc.58.6.545. [DOI] [PubMed] [Google Scholar]
- 190.Smith MA, Makino S, Kvetnansky R, Post RM. Stress and glucocorticoids affect the expression of brain-derived neurotrophic factor and neurotrophin-3 mRNAs in the hippocampus. J Neurosci. 1995;15:1768–1777. doi: 10.1523/JNEUROSCI.15-03-01768.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Nibuya M, Takahashi M, Russell DS, Duman RS. Repeated stress increases catalytic TrkB mRNA in rat hippocampus. Neurosci Lett. 1999;267:81–84. doi: 10.1016/s0304-3940(99)00335-3. [DOI] [PubMed] [Google Scholar]
- 192.Gourley SL, Kedves AT, Olausson P, Taylor JR. A history of corticosterone exposure regulates fear extinction and cortical NR2B, GluR2/3, and BDNF. Neuropsychopharmacology. 2009;34:707–716. doi: 10.1038/npp.2008.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Gourley SL, et al. Action control is mediated by prefrontal BDNF and glucocorticoid receptor binding. Proc Natl Acad Sci USA. 2012;109:20714–20719. doi: 10.1073/pnas.1208342109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Ray B, et al. Restraint stress and repeated corticotrophin-releasing factor receptor activation in the amygdala both increase amyloid-β precursor protein and amyloid-β peptide but have divergent effects on brain-derived neurotrophic factor and pre-synaptic proteins in the prefrontal cortex of rats. Neuroscience. 2011;184:139–150. doi: 10.1016/j.neuroscience.2011.03.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Schmidt HD, Duman RS. The role of neurotrophic factors in adult hippocampal neurogenesis, antidepressant treatments and animal models of depressive-like behavior. Behav Pharmacol. 2007;18:391–418. doi: 10.1097/FBP.0b013e3282ee2aa8. [DOI] [PubMed] [Google Scholar]
- 196.Brown CE, Boyd JD, Murphy TH. Longitudinal in vivo imaging reveals balanced and branch-specific remodeling of mature cortical pyramidal dendritic arbors after stroke. J Cereb Blood Flow Metab. 2010;30:783–791. doi: 10.1038/jcbfm.2009.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Kalia LV, Kalia SK, Salter MW. NMDA receptors in clinical neurology: excitatory times ahead. Lancet Neurol. 2008;7:742–755. doi: 10.1016/S1474-4422(08)70165-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Halpain S, Hipolito A, Saffer L. Regulation of F-actin stability in dendritic spines by glutamate receptors and calcineurin. J Neurosci. 1998;18:9835–9844. doi: 10.1523/JNEUROSCI.18-23-09835.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Hasbani MJ, Schlief ML, Fisher DA, Goldberg MP. Dendritic spines lost during glutamate receptor activation reemerge at original sites of synaptic contact. J Neurosci. 2001;21:2393–2403. doi: 10.1523/JNEUROSCI.21-07-02393.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Tseng CY, Firestein BL. The role of PSD-95 and cypin in morphological changes in dendrites following sublethal NMDA exposure. J Neurosci. 2011;31:15468–15480. doi: 10.1523/JNEUROSCI.2442-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Hasbani MJ, Hyrc KL, Faddis BT, Romano C, Goldberg MP. Distinct roles for sodium, chloride, and calcium in excitotoxic dendritic injury and recovery. Exp Neurol. 1998;154:241–258. doi: 10.1006/exnr.1998.6929. [DOI] [PubMed] [Google Scholar]
- 202.Kitaoka Y, et al. Involvement of RhoA and possible neuroprotective effect of fasudil, a Rho kinase inhibitor, in NMDA-induced neurotoxicity in the rat retina. Brain Res. 2004;1018:111–118. doi: 10.1016/j.brainres.2004.05.070. [DOI] [PubMed] [Google Scholar]
- 203.Graber S, Maiti S, Halpain S. Cathepsin B-like proteolysis and MARCKS degradation in sub-lethal NMDA-induced collapse of dendritic spines. Neuropharmacology. 2004;47:706–713. doi: 10.1016/j.neuropharm.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 204.Sweet ES, et al. PSD-95 alters microtubule dynamics via an association with EB3. J Neurosci. 2011;31:1038–1047. doi: 10.1523/JNEUROSCI.1205-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Morales-Medina JC, Sanchez F, Flores G, Dumont Y, Quirion R. Morphological reorganization after repeated corticosterone administration in the hippocampus, nucleus accumbens and amygdala in the rat. J Chem Neuroanat. 2009;38:266–272. doi: 10.1016/j.jchemneu.2009.05.009. [DOI] [PubMed] [Google Scholar]
- 206.Woolley CS, Gould E, McEwen BS. Exposure to excess glucocorticoids alters dendritic morphology of adult hippocampal pyramidal neurons. Brain Res. 1990;531:225–231. doi: 10.1016/0006-8993(90)90778-a. [DOI] [PubMed] [Google Scholar]
- 207.Wellman CL. Dendritic reorganization in pyramidal neurons in medial prefrontal cortex after chronic corticosterone administration. J Neurobiol. 2001;49:245–253. doi: 10.1002/neu.1079. [DOI] [PubMed] [Google Scholar]
- 208.Gourley SL, Swanson AM, Koleske AJ. Corticosteroid-induced neural remodeling predicts behavioral vulnerability and resilience. J Neurosci. 2013;33:3107–3112. doi: 10.1523/JNEUROSCI.2138-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]