Abstract
Emotional hyper-reactivity can inhibit maternal responsiveness in female rats and other animals. Maternal behavior in postpartum rats is disrupted by increasing norepinephrine release in the ventral bed nucleus of the stria terminalis (BSTv) with the α2-autoreceptor antagonist, yohimbine, or the more selective α2-autoreceptor antagonist, idazoxan (Smith et al., 2012). Because high noradrenergic activity in the BSTv can also increase anxiety-related behaviors, increased anxiety may underlie the disrupted mothering of dams given yohimbine or idazoxan. To assess this possibility, anxiety-related behaviors in an elevated plus maze were assessed in postpartum rats after administration of yohimbine or idazoxan. It was further assessed if the α2-autoreceptor agonist clonidine (which decreases norepinephrine release) would, conversely, reduce dams’ anxiety. Groups of diestrous virgins were also examined. It was found that peripheral or intra-BSTv yohimbine did increase anxiety-related behavior in postpartum females. However, BSTv infusion of idazoxan did not reproduce yohimbine’s anxiogenic effects and anxiety was not reduced by peripheral or intra-BSTv clonidine. Because yohimbine is a weak 5HT1A receptor agonist, other groups of females received BSTv infusion of the 5HT1A receptor agonist 8OH-DPAT, but it did not alter their anxiety-related behavior. Lastly, levels of norepinephrine and serotonin in tissue punches from the BSTv did not differ between postpartum and diestrous rats, but serotonin turnover was lower in mothers. These results suggest that the impaired maternal behavior after BSTv infusion of yohimbine or idazoxan cannot both be readily explained by an increase in dams’ anxiety, and that BSTv α2-autoreceptor modulation alone has little influence anxiety-related behaviors in postpartum or diestrous rats.
Keywords: anxiety, bed nucleus of the stria terminalis, lactation, maternal behavior, norepinephrine, serotonin
The bed nucleus of the stria terminalis (BST) is an integral component of the neural network mediating anxiety-related behaviors. Research on this topic in male laboratory rats has revealed that the BST is responsible for generating anxiety-related responses to relatively diffuse, unconditioned threats (Davis, Walker, Miles & Grillon, 2010). In support, destroying the BST in rats is anxiolytic in some behavioral paradigms (Hammack, Richey, Watkins & Maier, 2004; Lee & Davis, 1997; Waddell, Morris & Bouton, 2006), as is suppressing its activity with an AMPA antagonist (Walker & Davis, 1997), 5-HT1A agonist (Levita, Hammack, Mania, Li, Davis, & Rainnie, 2004), or calcium channel blocker (Resstel, Alves, Reis, Crestani, Corrêa, & Guimarães, 2008). Conversely, electrically stimulating the BST or inhibiting its GABA synthesis has been observed to be anxiogenic (Casada & Dafny, 1991; Sajdyk, Johnson, Fitz & Shekhar, 2008). This association between BST activity and anxiety is conserved across species and extends to non-human and human primates (Alvarez, Chen, Bodurka, Kaplan & Grillon, 2011; Kalin, Shelton, Rox, Oakes & Davidson, 2005; Somerville, Whalen & Kelley, 2010; Yassa, Hazlett, Stark & Hoehn-Saric, 2012).
A handful of other studies, however, do not demonstrate such results and even include reports that BST lesions do not affect anxiety-related behaviors at all (Pezuk, Aydin, Aksoy, & Canbeyli, 2008; Schulz & Canbeyli, 2000; Treit, Aujla, & Menard, 1998). Such incongruous results probably exist because of variation in the placement or size of the BST lesions or drug infusions, which is relevant because the BST is a heterogeneous structure of interconnected subnuclei possessing distinct neurochemical, neuroanatomical, and neurophysiological characteristics that may differentially influence anxiety (Dong & Swanson, 2006a–c; Egli & Winder, 2003; Phelix, Liposits, and Paull, 1992; Woodhams, Roberts, Polak & Crow, 1983). In fact, instead of promoting anxiety, activity of the BST may sometimes help animals cope with anxiogenic stimuli. An early indication of this was that BST lesions could exacerbate restraint-induced stomach ulcers (Henke, 1984). More recently, Campeau et al. (1997) reported that presenting a stimulus indicating safety from electric shock increased c-fos expression selectively in the subcomissural or ventral BST (BSTv) of male rats, which could reflect disinhibition of BSTv GABAergic cells that suppress brain sites otherwise potentiating anxiety (Day, Nebel, Sasse, & Campeau, 2005).
We recently conducted a study of anxiety-related behaviors in postpartum female rats that produced results consistent with this view. Maternal experience produces very long-term effects on females’ emotional reactivity (Byrnes & Bridges, 2006; Pawluski, Lieblich & Galea, 2009; Wartella et al., 2003), but we found that anxiety-related behaviors are also acutely suppressed in mother rats if they have physical contact with their offspring before testing, while mothers who are separated from their litters for as little as 4 hours before testing are significantly more anxious (Lonstein, 2005; Miller, Piasecki & Lonstein, 2011; Smith & Lonstein, 2008). When brain c-fos activity was examined after litter-separated and unseparated dams were exposed to the anxiogenic stimuli of an elevated-plus maze, we found that the lower-anxiety (unseparated) dams had significantly greater c-fos activity in the BSTv compared to higher-anxiety dams (Smith & Lonstein, 2008). That is, BSTv activation was associated with lower anxiety-related behaviors. This increased Fos in the BSTv of unseparated dams occurred only if dams were placed in the elevated-plus maze and not if they were left in the home cage with pups. Therefore, this increase in BSTv Fos expression depended on dams undergoing a potentially anxiogenic experience and was not due to their ongoing maternal behavior in the home cage before testing (Smith & Lonstein, 2008).
The BSTv is neurochemically unique because it contains the densest plexus of noradrenergic terminals in the mammalian forebrain (Fendt, Siegl & Steiniger-Brach, 2005; Kilts & Anderson, 1986; Woulfe, Flumerfelt & Hrycyshyn, 1990), primarily arising from A1 and A2 neurons of the medulla (Phelix et al., 1992; Riche, De Pommery & Menetrey, 1990; Roder & Ciriello 1994; Woulfe et al. 1990). Norepinephrine is tonically released in the BSTv and increases in response to anxiogenic or other aversive stimuli (Cecci, Khoshbousi & Morilak, 2002; Deyama et al., 2011; Fendt et al., 2005; Onaka & Yagi, 1998; Pacak, McCarty, Palkovitz, Kopin & Goldstein, 1995; Park et al., 2012). This elevated noradrenergic activity in the BSTv is associated with increased anxiety-related behaviors (Cecchi et al., 2002; Fendt et al., 2005; Morilak, Cecci & Khoshbouei, 2003; Naka, Ide, Nakako, Hirata, Majima, Deyama, Takeda, Yoshioka & Minami, 2013), but anxiety and other emotional behaviors can be suppressed by lesioning the noradrenergic fibers in the BSTv (Onaka & Yagi, 1998) or by reducing its norepinephrine release with the α2-autoreceptor agonist, clonidine (Deyama et al., 2011; Fendt et al., 2005; Schweimer, Fendt & Schnitzler, 2005).
Norepinephrine in the BSTv suppresses local glutamate release, increases local GABA release, and thereby silences most BSTv neurons (Casada & Dafny, 1993; Egli et al., 2005; Forray, Bustos & Gysling, 1999). This can occur through activation of postsynaptic α1, α2a–c and β receptors (Dumont & Williams, 2004; Egli et al., 2005; Forray et al. 1999). Because up to 90% of neurons in the BSTv are GABAergic (Mugnaini & Oertel, 1985; Sun & Cassell, 1993), noradrenergic inhibition could render these cells unable to suppress downstream sites whose activity would otherwise increase anxiety (Campeau et al., 1997). We have hypothesized that basal release of norepinephrine in the BSTv, or its release in response to potentially anxiogenic stimuli, is blunted in mother rats that have recent interaction with pups and that this blunting disinhibits BSTv GABAergic output cells to help attenuate maternal anxiety (Lonstein & Miller, 2008). This is analogous to the downregulation of the noradrenergic system of the maternal PVN that contributes to the postpartum suppression of the hypothalamic-pituitary-adrenal axis response to stress (Douglas, 2005; Toufexis & Walker, 1996; Toufexis et al., 1998; Windle et al. 1997). Most A1 and A2 noradrenergic projections to the PVN collateralize to the BSTv (Bienkowski & Rinaman, 2008; Woulfe, Hrycyshyn & Flumerfelt, 1988), so norepinephrine release may be similarly blunted in both sites of the maternal brain.
The BSTv is not only involved in anxiety-related behaviors, but is also a component of the neural network for maternal behaviors in laboratory rats. Interacting with pups increases Fos expression in the BSTv (Numan & Numan, 1995; Fleming, Suh, Korsmit & Rusak, 1994; Kalinichev, Rosenblatt, Nakabeppu & Morell, 2000) and lesions of the BSTv, but not the supracommissural or dorsal BST (BSTd), severely impair maternal retrieving (Numan & Numan, 1996). We recently reported that BSTv infusion of the α2-autoreceptor antagonist yohimbine, which increases norepinephrine release (Forray, Andres, Bustos & Gysling, 1995; Forray, Bustos & Gysling, 1997; Palij & Stamford 1993; Park, Kile & Wightman, 2009), eliminated dams’ retrieval of pups but did not significantly affect other maternal behaviors including dams’ latency to seek contact with pups or the duration of time spent licking or nursing them (Smith, Holschbach, Olsewicz & Lonstein, 2012). BSTv infusion of the more selective α2-autoreceptor antagonist, idazoxan, produced similar effects in addition to a disruption in nursing, all strongly implicating BSTv norepinephrine in maternal behavior (Smith et al., 2012). Because norepinephrine in the BSTv has roles in both anxiety and mothering, it is intriguing to posit that this neurochemical system underlies the incompatibility of these behaviors. Indeed, while some anxiety probably promotes maternal attention to the needs of the pups (Bosch, 2011; Pryce, 1992), emotional over-reactivity is thought to interfere with mothering (Fleming & Leubke, 1981; Lonstein, 2007). If so, the BSTv noradrenergic manipulations we previously observed to impair mothering might be predicted to significantly increase dams’ anxiety-related behaviors.
In the present studies, we first determined the effects of systemic or BSTv administration of yohimbine on elevated plus-maze behaviors in postpartum laboratory rats. To assess if some of yohimbine’s effects on anxiety-related behavior could be mediated by its activity on receptors other than the α2 autoreceptor, we studied the effects of BSTv infusion of the more selective idazoxan, as well as the 5-HT1A receptor agonist, 8-OH-DPAT (Millan et al., 1998; Newman-Tancredi et al., 1998; Winter & Rabin, 1992). We also examined if systemic or BSTv infusion of the α2 autoreceptor agonist, clonidine, would produce the opposite effect of yohimbine or idazoxan by reducing anxiety-related behavior. To determine if any drug effects on anxiety-related behavior depended on the females’ reproductive state, each study also included groups of diestrous rats. Lastly, tissue concentrations of norepinephrine and serotonin in the BSTv of unmanipulated postpartum and diestrous rats were measured to determine if basal production of these neurochemicals could be associated with any reproductive state differences in anxiety behavior of the control or drug-treated females in the previous experiments.
Methods
Subjects
Subjects were Long-Evans female rats born and raised in our colony, descended from rats purchased from Harlan Laboratories (Indianapolis, IN). Food and water were continuously available. Animals were housed in clear polypropylene cages (48 × 28 × 16 cm) with wood shavings for bedding. The colony room was maintained at 22 ± 1° C and a 12:12 light/dark cycle beginning at 0800. Starting at approximately 70 days of age, females for the postpartum groups were monitored daily with a vaginal impedance meter that measures changes in electrical resistance of the vaginal walls across the estrous cycle (Fine Science Tools, Foster City, CA). On a day of proestrus they were placed overnight with a sexually experienced Long-Evans male from our colony. Pregnant females were group housed (2–3 females per cage) until one week before giving birth (Experiments 1, 3, 7) or until day of stereotaxic surgery between days 15–18 of pregnancy (Experiments 2, 4–6), after which they were housed alone. The day of parturition was assigned as Day 0 postpartum. Within 24 hr after parturition, litters were culled to contain 4 males and 4 females.
Other groups of rats remained as virgins and were singly housed at least two weeks before testing (Experiments 1 and 3) or sacrifice (Experiment 7), or one week before stereotaxic surgery plus at least an additional week after surgery (Experiments 2, 4–6). Virgin females received daily vaginal smears starting approximately one week before behavioral testing. To avoid handling on the day of testing, virgins were tested (or sacrificed in Experiment 7) the day after we detected a day of estrus; a subsequent vaginal smear immediately after testing (or sacrifice in Experiment 7) was used to confirm females’ diestrous state and the small number found to not be in diestrus were removed from the studies. Diestrus was chosen as the day of testing because plasma ovarian hormone levels at this stage are similar to early postpartum rats, which are in lactational diestrus (Tsukamura & Maeda, 2001). All procedures were performed in accordance with the principles of laboratory animal care and approved by the Institutional Animal Care and Use Committee at Michigan State University.
Stereotaxic Surgery
Subjects in Experiments 2 and 4–6 received permanent intracranial cannulae using methods previously described (Miller & Lonstein, 2005; Miller, Piasecki, Peabody & Lonstein, 2010). Briefly, females were weighed and anesthetized with ketamine (90 mg/kg IP; Butler, Dublin, OH) and xylazine (4 mg/kg IM; Butler, Dublin, OH). Holes were drilled bilaterally above the BSTv (A/P 0.0, M/L ± 1.2) and a 5-mm long, 22-gauge bilateral stainless steel guide cannula (Plastics One, Roanoke, VA) was implanted above the BST and kept patent with a dummy stylet extending 1 mm beyond the end of the cannulae. A dust cap covered the entire apparatus. After surgery, subjects received buprenorphine (0.015 mg/kg IP; Reckitt Benckiser, Parsippany NJ) and were placed on a heating pad until recovery from anesthesia.
Intraperitoneal Injection or Intra-BSTv Infusion of Drugs
Subjects in Experiments 2 and 4–6 were habituated to the handling and intracranial infusion procedures similar to that previously described (Miller & Lonstein, 2005; Smith et al., 2012). Females were removed from their home cages in the colony room, placed in a clean carrying cage, and transported to an adjacent room where they were gently handled for 5 min. Their dust cap and stylet were removed, cleaned with ethanol, and replaced. Nothing was infused and subjects were returned to their home cages and colony room. Infusion and behavioral testing occurred the next day. Because we expected clonidine to reduce anxiety, dams in Experiments 3 and 4 had their litters removed 4 hr before testing to potentially increase dams’ anxiety and avoid a ceiling effect (Lonstein, 2005; Smith & Lonstein, 2008). To better allow comparison across the clonidine and yohimbine experiments, dams receiving yohimbine in Experiments 1 and 2 had their litter mildly perturbed 4 hr before testing, which involved lifting the pups out of the cage and immediately replacing them. Virgins in Experiments 1–4 had the experimenter’s hand placed in their cage to simulate the brief perturbation that dams experienced. To avoid any additional handling or stress on the day of testing in Experiments 1 and 3, subjects’ body weights were obtained 1–2 days before IP injection and elevated plus-maze testing.
Females received the following drugs (all purchased from Sigma, USA): Experiment 1 – intraperitoneal (IP) injection of vehicle (2:1 propylene glycol:saline) or 1 or 5 mg/kg yohimbine hydrochloride dissolved in vehicle; Experiment 2 – BSTv infusion of 125 nl vehicle per hemisphere or 2 or 6 μg yohimbine hydrochloride divided in half and delivered in 125 nl vehicle per hemisphere; Experiment 3 – IP saline or 5 or 10 μg/kg clonidine hydrochloride dissolved in saline; Experiment 4 – BSTv infusion of 125 nl saline per hemisphere or 200 or 2000 ng clonidine hydrochloride divided in half and delivered in 125 nl saline per hemisphere; Experiment 5 – BSTv infusion of 125 nl saline per hemisphere or 10 or 20 μg idazoxan hydrochloride, divided in half and delivered in 125 nl saline per hemisphere; Experiment 6 – BSTv infusion of 125 nl 2% TWEEN-80 in saline per hemisphere or 0.66 μg (2 nmol) or 6.6 μg (20 nmol) (±)-8-OH-DPAT hydrobromide, divided in half and delivered in 125 nl of the vehicle. Doses were chosen based on their ability to influence anxiety or other behaviors when injected intraperitoneal or site-specifically into the rodent brain (Bertrand, Lehmann, Lazarus, Jeltsch & Cassel, 2000; Carli, Tatarczynska, Cervo & Samanin, 1993; Clark, 1991; De Almeida, Giovenardi, Charchat & Lucion, 1998; Da Silva, Poltronieri, Nascimento, Zangrossi & Viana, 2011; Dodge & Badura, 2002; Fendt et al., 2005; Gulia, Kumar & Mallick, 2002; Guo et al., 1996; Schweimer et al., 2005; Solderpalm & Engel, 1988; Toufexis et al., 1999). The intra-BSTv infusions were done slowly over the course of 1 min through a 16-mm-long bilateral 28-gauge injector connected by polyethylene tubing to a 0.5 μl Hamilton syringe (Hamilton, Reno, NV). After infusion, the injector remained for an additional 60–90 sec and was then slowly removed. The stylet and dust cap were then replaced.
Elevated plus-maze testing
Based on the post-administration times of testing in previous studies using these drugs (see citations in paragraph above), after systemic injections of yohimbine or clonidine the animals were placed back in their home cages and 30 min later brought to a nearby 10 × 10 ft room for elevated plus-maze testing. After intra-BSTv infusions of yohimbine, clonidine, or idazoxan animals were immediately brought to the testing room and placed on the elevated plus maze. Animals with BSTv infusion of 8-OH-DPAT were tested 10 min after infusion. The elevated plus maze was constructed of black plastic (for details see Lonstein, 2005; Smith & Lonstein, 2008) and the testing room was illuminated with a 100-watt light bulb. Females were removed from their home cage, placed in the center of the maze facing an open arm, and released for the 10-min test. We previously demonstrated that differences between diestrous virgins and postpartum rats in elevated plus-maze behavior are more pronounced during a 10-min test, rather than a 5-min test (Lonstein, 2005). After subjects were released into the maze, their home cages were removed from the testing room. A mirror placed above the maze reflected the images into a Panasonic low-light-sensitive video camera interfaced with a VCR. Behavior was recorded with a computerized data acquisition system while subjects were being videotaped, or the videotapes were later transcribed. Durations of time and frequency of entries into the open and closed arms were recorded. The frequencies of closed arm entries and total arm entries were used as indicators of general locomotor activity (Pellow, Chopin, File & Briley, 1985). A subject was considered to have entered an arm or the central square if at least its head and two forepaws were within that space (Beiderbeck, Neumann & Veenema, 2007; Neumann, Torner & Wigger, 2000; Smith & Lonstein, 2008; Turgeon, Anderson, & O’Loughlin, 2010). The elevated plus maze was cleaned with a 70% ethanol and allowed to dry between subjects.
Verification of Infusion Sites
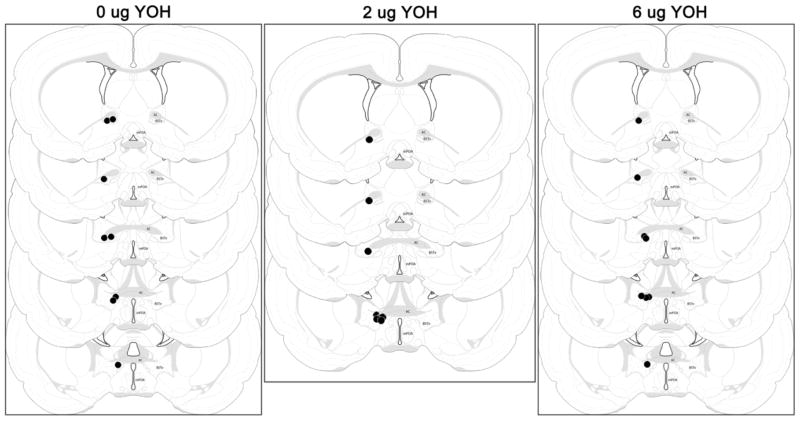
Within a week after testing, cannulated subjects were overdosed with sodium pentobarbital and perfused with 100 ml of 0.9% saline. Brains were removed and postfixed overnight in 10% formalin and then submerged in 20% sucrose for at least 2 days. Brains were cut into 40 μm sections and stained with Neutral Red. Infusion sites were analyzed at 100x magnification and mapped onto plates from Swanson’s (1998) atlas of the rat brain. An infusion was considered a BSTv hit only if it terminated in the subcommissural BST between ~0.0 and −0.51 mm from bregma, where the densest noradrenergic fibers and concentration of norepinephrine are found (Fendt et al. 2005). Some females from every group in the four BSTv infusion studies (i.e., 24 groups of cannulated rats) were found to have either unilateral infusions, bilateral infusions outside the BSTv most often in the anterior commissure or dorsal BST, asymmetrical infusions with only one cannula prong in the BSTv, or undetectable infusions so were removed from data analyses (n = 155 misses across all four studies; see figures for final sample sizes).
Neurochemical Analysis
In Experiment 7, unmanipulated diestrous virgin females and postpartum day 7 or 8 females that were continuously housed with their pups (ns = 9/group) were removed from their home cages and placed in a plastic bag pre-filled with CO2 for up to 30 sec. Immediately after cessation of movement, rats were decapitated and their brains quickly removed and frozen in cold 2-methylbutane. Such brief exposure to CO2 immediately before sacrifice does not affect brain amine content when measured in tissue punches (Jones, Arters & Berger-Sweeney, 1999) and the entire procedure starting with removing the subjects from their home cage to freezing their extracted brains required less than 2.5 min for each rat. Brains were stored at −80° C until each was cut on a cryostat to obtain a 1 mm-thick section beginning approximately −0.15 mm from bregma to contain the rostrocaudal extent of the BST (Swanson, 1998). The BSTv was bilaterally punched from the brain slices using an 18-gauge stainless steel tube. The BSTd was also punched for use as a comparison brain site that contains little norepinephrine (Herr, Park, McElligott, Belle, Carelli & Wightman, 2012; Park et al., 2012). Punches were weighed and placed in centrifuge tubes filled with 200 nl of cold 0.1M perchloric acid and homogenized. Samples were centrifuged at 10,000 rpm for 10 min, the supernatant collected, and stored at −80°C until analysis using HPLC.
Monoamine analysis was conducted at a core facility at Michigan State University using a commercially available system (ESA Biosciences, Inc, Chelmsford, MA) consisting of a solvent delivery module (model 584), an autosampler (model 542) cooled to 4°C and a Coulochem III detector that was equipped with a 5021A conditioning cell (electrode I) and a 5011A high sensitivity analytical cell (electrodes II and III). Both cells used flow-through porous graphite electrodes. Hydrodynamic voltammograms were obtained to determine the optimum potential for detection. The highest signal-to-noise results were obtained when electrode I was set at +200 mV, electrode II at +100 mV and electrode III at −280 mV. Chromatograms were obtained by monitoring the reduction current for working electrode III. The monoamines and metabolites were separated on an HR-80 (C18, 3 μm particle size, 80 mm length × 4.6 mm I.D.) reversed-phase column (ESA Biosciences, Inc). The mobile phase was a commercial Cat-A-Phase II (ESA Biosciences, Inc) that consisted of a proprietary mixture of acetonitrile, methanol, phosphate buffer and an ion pairing agent (pH 3.2). The optimum flow rate for the separation was 1.1 mL/min. The separation column was maintained at 35°C. Concentrations of analytes were determined by an external calibration using standards to generate a standard curve. The limit of detection was 0.1 ng/ml for norepinephrine and 0.5 ng/ml for serotonin and its metabolite 5-HIAA. The ratio of serotonin/5-HIAA was calculated as an indicator of serotonin turnover. The norepinephrine metabolite, MHPG, was not detectable.
Data Analyses
Behavioral durations and frequencies were analyzed with two-way ANOVAs using reproductive state and drug dose as factors. In cases of significant main effects (p < 0.05), pair-wise post-hoc comparisons were performed using p-corrected Fisher’s LSD tests with significant differences indicated by p < 0.017. In cases of significant interactions between reproductive state and drug on the percentage of open-arm time or frequency, one-way ANOVAs followed by Fisher’s LSD tests were conducted within reproductive state. Neurochemical data were analyzed with t-tests, with significance indicated by p < 0.05. One postpartum subject was mistakenly omitted from analysis of serotonin and 5-HIAA, resulting in n = 8 for these neurochemicals. Additionally, the BSTd of one virgin subject was improperly weighed so excluded from analysis, resulting in n = 8 in this group for this brain site.
Results
Experiment 1 - Elevated plus-maze behavior after IP injection of yohimbine
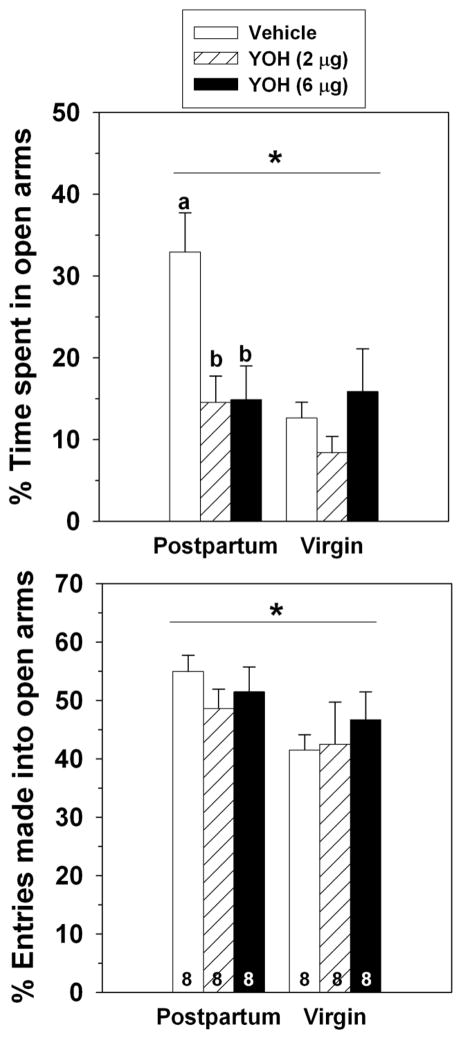
Postpartum rats spent a greater percentage of time in the open arms of the elevated plus maze compared to diestrous virgins (F(1,41) = 6.22, p = 0.02), but the percentage of total entries that were made into the open arms did not differ between groups (F(1,41) = 0.40, p = 0.53; Figure 1). Dams also made more entries into closed arms compared to virgins (F(1,41) = 12.07, p = 0.001; Table 1).
Figure 1.
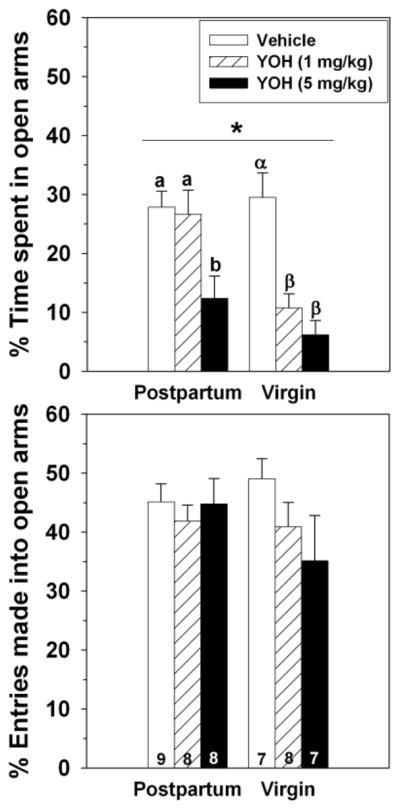
Effects of 0, 1 or 5 mg/kg of yohimbine administered IP on the percentage of time postpartum and virgin females spent in the open arms (top) and percentage of entries made into open arms (bottom) of an elevated plus maze. * = significant main effect of reproductive state, p < 0.05. Groups with different Latin or Greek letters above bars indicate significant differences within reproductive state, p < 0.05. Sample sizes are indicated at bottom of bars on bottom panel.
Table 1.
Elevated plus-maze behaviors of postpartum and diestrous virgin female rats after IP or BSTv administration of yohimbine, clonidine, idazoxan or 8-OH-DPAT.
| Drug/Site | Drug Dose | Significant Effects | ||
|---|---|---|---|---|
| Yohimbine – I.P. | 0 | 1 mg/kg | 5 mg/kg | |
| Closed arm entries | State, Dose | |||
| Virgin | 14 ± 1a | 9 ± 2a | 4 ± 1b | |
| Postpartum | 17 ± 1a | 18 ± 3a | 8 ± 3b | |
| All arm entries | State, Dose, State x Dose | |||
| Virgin | 27 ± 2a | 15 ± 3b | 7 ± 2c | |
| Postpartum | 31 ± 2a | 31 ± 4b | 13 ± 3c | |
| Yohimbine - BSTv | 0 | 2 μg | 6 μg | |
| Closed arm entries | State, Dose | |||
| Virgin | 12 ± 1a | 6 ± 1b | 7 ± 1b | |
| Postpartum | 14 ± 1a | 10 ± 2b | 8 ± 1b | |
| All arm entries | State, Dose | |||
| Virgin | 20 ± 2a | 12 ± 2b | 13 ± 2b | |
| Postpartum | 31 ± 2a | 20 ± 3b | 17 ± 2b | |
| Clonidine – I.P. | 0 | 5 μg/kg | 10 μg/kg | |
| Closed arm entries | State, Dose, State x Dose | |||
| Virgin | 9 ± 2ab | 13 ± 1a | 7 ± 1b | |
| Postpartum | 16 ± 1ab | 15 ± 1a | 14 ± 2b | |
| All arm entries | State, State x Dose | |||
| Virgin | 12 ± 2 | 20 ± 2 | 10 ± 2 | |
| Postpartum | 29 ± 3 | 25 ± 1 | 27 ± 3 | |
| Clonidine - BSTv | 0 | 200 ng | 2000 ng | |
| Closed arm entries | Dose | |||
| Virgin | 13 ± 3a | 12 ± 2ab | 8 ± 2b | |
| Postpartum | 12 ± 2a | 10 ±2ab | 7 ± 1b | |
| All arm entries | Dose | |||
| Virgin | 23 ± 5a | 20 ± 3ab | 13 ± 2b | |
| Postpartum | 23 ± 3a | 18 ± 3ab | 15 ± 2b | |
| Idazoxan - BSTv | 0 | 10 μg | 20 μg | |
| Closed arm entries | Dose | |||
| Virgin | 13 ± 1a | 10 ± 1b | 9 ± 1b | |
| Postpartum | 15 ± 2a | 11 ± 1b | 9 ± 2b | |
| All arm entries | Dose | |||
| Virgin | 25 ± 3a | 17 ± 2b | 16 ± 3b | |
| Postpartum | 28 ± 3a | 21 ± 2b | 20 ± 4b | |
| 8-OH-DPAT - BSTv | 0 | 0.66 μg | 6.6 μg | |
| Closed arm entries | - | |||
| Virgin | 13 ± 2 | 15 ± 2 | 15 ± 2 | |
| Postpartum | 12 ± 2 | 14 ± 2 | 15 ± 2 | |
| All arm entries | - | |||
| Virgin | 21 ± 4 | 26 ± 4 | 26 ± 3 | |
| Postpartum | 25 ± 4 | 24 ± 4 | 26 ± 4 |
Numbers with different superscript letters indicate significant differences related to the main effect of the drug dose, collapsed across reproductive state. See text for additional details of the statistical analyses.
Yohimbine significantly decreased the percentage of time females spent in the open arms of the elevated plus maze (F(2,41) = 16.65, p < 0.0001), with females receiving 5 mg/kg yohimbine spending significantly less time in the open arms compared to vehicle-injected females (Figure 1). There was also a significant interaction between reproductive state and yohimbine on the percentage of time spent in the open arms (F(2,41) = 3.52, p = 0.04), likely due to the greater sensitivity of virgins (one-way ANOVA: F(2,19) = 16.14, p < 0.0001) than dams (one-way ANOVA: F(2,22) = 5.84, p = 0.009) to the lower dose of yohimbine. Yohimbine did not affect the percentage of entries that were made into open arms (F(2,41) = 1.51, p = 0.23), nor was there an interaction between reproductive state or yohimbine on this measure (F(2,41) = 1.24, p = 0.32).
Collapsed across reproductive state, 5 mg/kg yohimbine significantly reduced the frequencies of closed arm entries (F(2,41) = 14.89, p < 0.0001) and total arm entries (F(2,41) = 27.01, p < 0.0001) compared to the other doses of yohimbine (Table 1). There was a significant interaction between yohimbine and reproductive state on total arm entries (F(2,41) = 8.03, p = 0.003), with both doses of yohimbine affecting virgins (one-way ANOVA: F(2,19) = 22.07, p < 0.0001), but only the high dose reducing total entries in dams (one-way ANOVA: F(2,22) = 12.25, p = 0.0003).
Experiment 2 - Elevated plus-maze behavior after BSTv infusion of yohimbine
The locations of the correctly placed BSTv infusions are indicated in Figure 2. Postpartum females spent a significantly greater percentage of time in the open arms (F(1,42) = 7.64, p = 0.008) and had a higher percentage of entries into the open arms (F(1,42) = 5.03, p = 0.03) compared to diestrous virgins (Figure 3). The number of closed (F(1,42) = 6.17, p = 0.017) and total (F(1,42) = 23.14, p < 0.0001) arm entries was also higher in postpartum rats compared to virgins (Table 1).
Figure 2.
Schematic representation of infusion sites for postpartum rats receiving 0, 2, or 6 μg yohimbine into the BSTv. The bilateral infusion sites (black circles) are depicted unilaterally. AC – anterior commissure, BSTv – ventral bed nucleus of the stria terminalis, mPOA – medial preoptic area. ac = anterior commissure, 3V = third ventricle. From Brain Maps: Structure of the Rat Brain, levels 17–21, by L.W. Swanson, 1998, Amsterdam: Elsevier Science., Copyright 1998 by Elsevier Science. Adapted with permission.
Figure 3.
Effects of 0, 2 or 6 μg of yohimbine administered into the BSTv on the percentage of time postpartum and virgin females spent in the open arms (top) and percentage of entries made into the open arms (bottom) of an elevated plus maze. * = significant main effect of reproductive state, p < 0.05. Groups with different letters above bars indicate significant differences within reproductive state, p < 0.05. Sample sizes are indicated at bottom of bars on bottom panel.
Intra-BSTv yohimbine decreased the percentage of time females spent in the open arms (F(2,42) = 4.68, p = 0.015; Figure 3), with those receiving 1 μg yohimbine spending significantly less time in the open arms compared to females infused with vehicle. There was a significant interaction between reproductive state and yohimbine on the percentage of time spent in the open arms (F(2,42) = 4.14, p = 0.023), such that open-arm time was reduced in dams by both doses of yohimbine (one-way ANOVA: F(2,21) = 6.64, p = 0.006) while virgins were already relatively low on this measure and not further affected (one-way ANOVA: F(2,21) = 1.22, p = 0.32). The percentage of entries made into open arms was not affected by yohimbine (F(2,42) = 0.34, p = 0.71), nor was there an interaction between reproductive state or yohimbine on this measure (F(2,42) = 0.55, p = 0.58). Intra-BSTv yohimbine at both doses significantly reduced the number of closed (F(2,42) = 9.76, p = 0.0003) and total (F(2,42) = 16.77, p < 0.001) arm entries compared to vehicle (Table 1). There was no significant interaction between reproductive state and yohimbine on the number of closed (F(2,42) = 0.65, p = 0.52) or total (F(2,42) = 1.82, p = 0.17) arm entries.
Experiment 3 – Elevated plus-maze behavior after IP injection of clonidine
There was a significant main effect of reproductive state with postpartum rats spending a greater percentage of time in the open arms (F(1,36) = 30.04, p < 0.0001) and making a higher percentage of open-arm entries compared to virgins (F(1,36) = 12.91, p = 0.001; Figure 4). The number of entries made into the closed arms (F(1,36) = 30.17, p < 0.0001) and the total number of arms entered (F(1,36) = 58.02, p < 0.0001) were also higher in dams than virgins (Table 1).
Figure 4.
Effects of 0, 5 or 10 μg/kg of clonidine administered IP on the percentage of time postpartum and virgin females spent in the open arms (top) and percentage of entries made into the open arms (bottom) of an elevated plus maze. * = significant main effect of reproductive state, p < 0.05. Sample sizes are indicated at bottom of bars on bottom panel.
Contrary to what was expected, there was no main effect of clonidine on the percentage of time spent in the open arms (F(1,36) = 0.91, p = 0.41; Figure 4). There was no significant interaction between reproductive state and clonidine on this measure (F(1,36 = 0.71, p = 0.50). Clonidine also did not affect the percentage of entries that were made into the open arms (F(2,36) = 0.11, p = 0.90) or total number of entries into all arms (F(2,36) = 1.70, p = 0.20). The number of entries made into closed arms was significantly lower in females receiving 10 μg/kg compared to 5 μg/kg clonidine (F(2,36) = 4.60, p = 0.02). A significant interaction between reproductive state and clonidine was also found for the number of entries into the closed arms (F(2,36) = 4.68, p = 0.02; Table 1) such that virgins given 5 μg/kg of clonidine entered the closed arms of the maze more frequently than the other groups (one-way ANOVA: F(2,18) = 8.03, p = 0.003) whereas clonidine did not affect the frequency of closed arm entries made by dams (one-way ANOVA: F(2,18) = 0.37, p = 0.70).
Experiment 4 - Elevated plus-maze behavior after BSTv infusion of clonidine
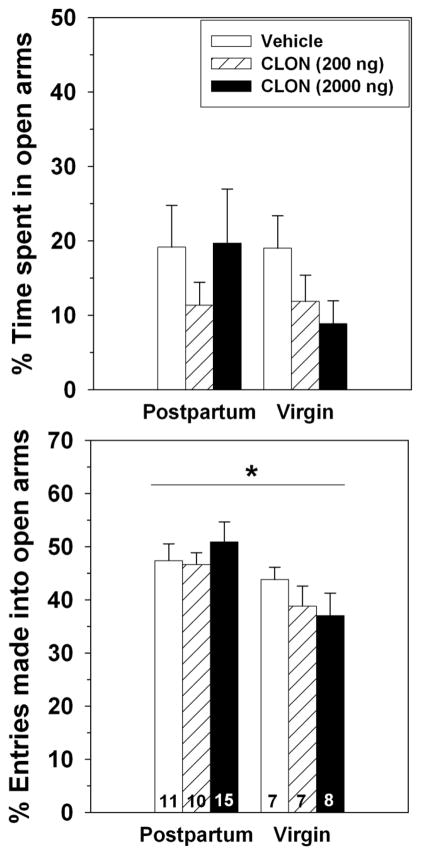
Correctly-placed BSTv infusions of clonidine were similar in location to those in Experiment 1. There was no significant main effect of reproductive state on the percentage of time spent in the open arms (F(1,52) = 0.50, p = 0.49), but dams did make a greater percentage of their entries into the open arms compared to that found for virgins (F(1,52) = 7.78, p = 0.007; Figure 5). There was no difference between dams and virgins in the number closed (F(1,52) = 0.75, p = 0.39) or total (F(1,52) = 0.023, p = 0.88) arm entries (Table 1).
Figure 5.
Effects of 0, 200 or 2000 ng of clonidine administered into the BSTv on the percentage of time postpartum and virgin females spent in the open arms (top) and percentage of entries made into the open arms (bottom) of an elevated plus maze. * = significant main effect of reproductive state, p < 0.05. Sample sizes are indicated at bottom of bars on bottom panel.
Intra-BSTv clonidine did not significantly affect the percentage of time females spent in the open arms (F(2,52) = 0.73, p = 0.49) or their percentage of open-arm entries (F(2,52) = 0.29, p = 0.75). Females receiving the 2000 ng dose of clonidine made significantly fewer closed (F(2,52) = 3.98, p = 0.03) and total (F(2,52) = 4.51, p = 0.02) arm entries compared to females receiving vehicle (Table 1).
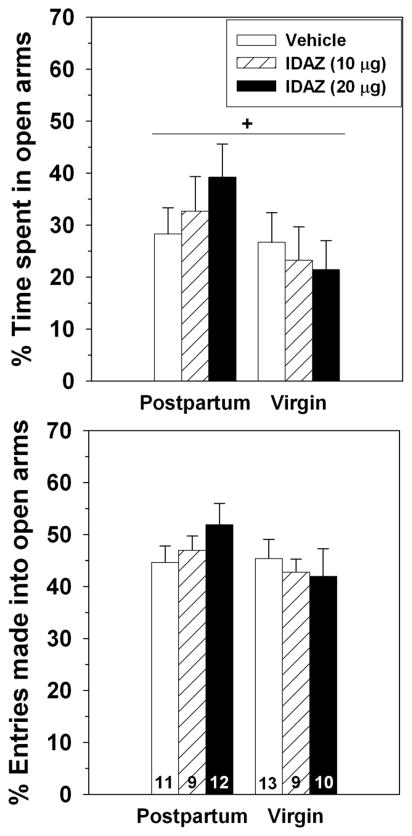
Experiment 5 – Elevated plus-maze behavior after BSTv infusion of idazoxan
BSTv infusions of idazoxan were similar in location to those in Experiment 1. Dams tended to spend a greater percentage of time in the open arms than did virgins (F(1,58) = 3.77, p = 0.06), but there was no significant main effect of reproductive state on the percentage of entries made into the open arms (F(1,58) = 1.93, p = 0.17; Figure 6). There was no difference between dams and virgin on the number of closed (F(1,58) = 0.84, p = 0.36) or total (F(1,58) = 2.01, p = 0.16) entries (Table 1).
Figure 6.
Effects of 0, 10 or 20 μg of idazoxan administered into the BSTv on the percentage of time postpartum and virgin females spent in the open arms (top) and percentage of entries made into the open arms (bottom) of an elevated plus maze. + = marginally significant main effect of reproductive state, p = 0.057. Sample sizes are indicated at bottom of bars on bottom panel.
In contrast to yohimbine, idazoxan affected neither the percentage of time females spent in the open arms (F(2,58) = 0.13, p = .88) nor the percentage of open-arm entries (F(2,58) = 0.18, p = 0.84). There were also no significant interactions between reproductive state and idazoxan on these measures (Table 1). Idazoxan significantly reduced the number of closed (F(2,58) = 7.99, p = .0009) and total (F(2,58) = 4.88, p = 0.01) arm entries. The interactions between dose and reproductive state on either closed (F(2,58) = 0.29, p = 0.75) or total (F(2,58) = 0.012, p = 0.99) arm entries were not significant.
Experiment 6 - Elevated plus-maze behavior after BSTv infusion of 8-OH-DPAT
BSTv infusions of 8-OH-DPAT were very similar in location to those found in the experiments above. Dams and virgins did not differ in their percentage of time spent in open arms (F(1,44) = 0.13, p = 0.72) but dams tended to make a greater percentage of open-arm entries than did virgins (F(1,44) = 3.68, p = 0.06; Figure 7). Reproductive state did not influence the number of closed (F(1,44) = 0.24, p = 0.62) or total (F(1,44) = 0.49, p = 0.83) arm entries (Table 1).
Figure 7.
Effects of 0, 0.66 or 6.60 μg of 8-OH-DPAT administered into the BSTv on the percentage of time postpartum and virgin females spent in the open arms (top) and percentage of entries made into the open arms (bottom) of an elevated plus maze. + = marginally significant main effect of reproductive state, p = 0.061. Sample sizes are indicated at bottom of bars on bottom panel.
There was no significant main effect of 8-OH-DPAT on the percentage of entries made into open arms (F(2,44) = 0.03, p = 0.97), females’ open arm frequency (F(2,44) = 0.27, p = 0.77), or the number of closed (F(2,44) = 0.86, p = 0.43) or total (F(2,44) = 0.31, p = 0.73) entries. There were no significant interactions between reproductive state and 8-OH-DPAT on any measure (Table 1).
Experiment 7 – Norepinephrine and serotonin content in the BST
Postpartum and virgin females did not differ in the norepinephrine content of their BSTv (t16 = 0.92, p > 0.37) or BSTd (t15 = 0.09, p = 0.72) (Table 2). Dams tended to have lower serotonin in the BSTv (t16 = 1.78, p = 0.09), but not in the BSTd (t15 = 0.18, p = 0.85), compared to virgins. However, serotonin turnover in the BSTv (t14 = 3.71, p < 0.003), but not BSTd (t13 = 1.61, p = 0.13), was significantly lower in dams than in virgins.
Table 2.
Neurochemical measures of norepinephrine and serotonin in tissue punches of the BSTv and BSTd from postpartum and diestrous virgin female rats.
| Postpartum | Virgin | t | |
|---|---|---|---|
| Norepinephrine (ng/mg) | |||
| BSTv | 10.88 ± 0.75 | 11.86 ± 0.75 | 0.92 |
| BSTd | 1.23 ± 0.20 | 1.33 ± 0.17 | 0.09 |
| Serotonin (ng/mg) | |||
| BSTv | 0.71 ± 0.06 | 0.88 ± 0.07 | 1.78 |
| BSTd | 0.72 ± 0.08 | 0.69 ± 0.01 | 0.18 |
| 5-HIAA (ng/mg) | |||
| BSTv | 0.75 ± 0.08 | 0.73 ± 0.12 | 0.11 |
| BSTd | 0.76 ± 0.09 | 0.49 ± 0.11 | 1.84 |
| Serotonin/5HIAA | |||
| BSTv | 0.93 ± 0.06 | 1.37 ± 0.11 | 3.71* |
| BSTd | 1.02 ± 0.06 | 2.09 ± 0.71 | 1.61 |
p < 0.05.
Discussion
Notable findings from the present experiments include: 1) the α2-autoreceptor antagonist yohimbine was significantly anxiogenic after intraperitoneal or BSTv administration in postpartum female rats, and anxiogenic after intraperitoneal injection in diestrous virgin rats, 2) intraperitoneal or BSTv administration of the α2-autoreceptor agonist clonidine was not anxiolytic in either group of females, 3) unlike yohimbine, BSTv infusion of the more selective α2-autoreceptor antagonist idazoxan did not increase females’ anxiety-related behavior, 4) BSTv infusion of the 5HT1A receptor agonist 8OH-DPAT also did not alter anxiety, and 5) postpartum and diestrous virgin rats did not differ in the norepinephrine or serotonin content in their BSTv or BSTd, but dams had lower serotonin turnover in the BSTv than did virgins.
Previous research from our laboratory somewhat unexpectedly revealed that high Fos expression in the BSTv was associated with lower anxiety-related behavior in postpartum female rats (Smith & Lonstein, 2008). This result is consistent with the emerging literature demonstrating heterogeneity in how the BST subregions regulate anxiety (cf. Ventura-Silva, Pêgo, Sousa, Marques, Rodrigues, Marques, Cerqueira, Almeida, & Sousa, 2012), as well as with data showing that the BSTv is one of few brains sites activated when animals sense safety rather than threat (Campeau et al., 1997; however, see Christianson, Jennings, Ragole, Flyer, Benison, Barth, Watkins, & Maier, 2011) and is involved in their ability to “cope” with familiar threats (Heinke, 1984). The BSTv has both short and long GABAergic projections (Fisher, Buchwald, Hull & Levine, 1988; Kudo, Uchigashima, Miyazaki, Konno, Yamasaki, Yanagawa, Minami, & Watanabe, 2012; Roland & Sawchencko, 1993) and BSTv inhibition of downstream sites that normally contribute to anxiety may be responsible for this effect. More than 60% of the BSTv cells expressing Fos in low-anxiety dams exposed to anxiogenic stimuli contain glutamate decarboxlyase (GAD67; Smith & Lonstein, unpublished data), the rate-limiting enzyme for GABA synthesis. If these GABAergic cells are projection neurons, sites receiving this inhibitory input that suppresses anxiety could include the central amygdala, mammillary region and periaqueductal gray (Dong & Swanson, 2006c; Gonzalo-Ruiz, Alonso, Sanz & Llinas, 1992; Sun and Cassell, 1993). In fact, intraperitoneal yohimbine is not anxiogenic in male rats if the BSTv and BSTd are disconnected from the central amgydala (Cai, Bakalli & Rinaman, 2012). Because most BSTv neurons are both GABAergic and disinhibited in the absence of norepinephrine (Casada & Dafny, 1993; Egli et al., 2005; Forray et al., 1999), we hypothesized that interactions with pups reduces norepinephrine release in the postpartum BSTv and this keeps anxiety low. The postpartum downregulation of the noradrenergic system in the PVN provided a precedent for this suggestion (Toufexis & Walker, 1996; Toufexis et al., 1998; Windle et al. 1997).
The results of Experiment 1 provided support for this hypothesis such that intraperitoneal administration of yohimbine significantly increased dams’ anxiety-related behaviors. Yohimbine increases norepinephrine release in the BSTv and elsewhere (Forray, Andres, Bustos & Gysling, 1995; Forray, Bustos & Gysling, 1997; Palij & Stamford 1993; Park, Kile & Wightman, 2009) and is anxiogenic in male rats and humans (Cole, Hillman, Seidelmann, Klewer, & Jones, 1995; Morgan et al., 1993; Pellow et al., 1985; Vasa et al., 2009). The only other study of emotion-related behavior in postpartum rats after yohimbine administration by Toufexis et al. (1999) is consistent with our results, with them reporting that yohimbine (1.5 and 2.5 mg/kg IP) greatly increased acoustic startle in postpartum rats compared to vehicle-injected dams. Our results from Experiment 2 pinpointed the BSTv as a site of yohimbine’s anxiogenic action in dams and thus adds females to the reports of significantly altered elevated plus-maze behavior after a neurochemical manipulation of the BST (Gomes, Resstel & Guimaraes, 2011; Naka et al., 2013; Rodi et al., 2008; Sahuque et al., 2006; Sajdyk et al., 2008; Sink, Walker, Yang & Davis, 2011).
In contrast to Experiments 1 and 2, the subsequent experiments did not support the hypothesis that BSTv noradrenergic activity affects postpartum anxiety or that the yohimbine effects we observed were even mediated by the α2 autoreceptor. We first found that decreasing norepinephrine release by intraperitoneal or intra-BSTv clonidine was not anxiolytic in our females (Experiments 3 and 4). This was partly unexpected because clonidine reduces anxiety in male rats after IP (Soderpalm & Engel, 1988) or inta-BSTv (Fendt et al., 2005; Schweimer et al., 2005) administration. A sex difference in response to clonidine could underlie the discrepancy (Del Rio, Verlardo, Zizzo, Marrama, & Della Casa, 1993; Limberg, Eldridge, Proctor, Sebranek, & Schrage, 2010), perhaps driven by the greater number of brainstem noradrenergic cells and higher anxiety- or stress-induced activity these cells in females compared to males (Curtis, Bethea & Valentino, 2006; Luque, De Blas, Segovia & Guillamon, 1992). However, we previously reported that selectively lesioning the noradrenergic innervation of the BSTv did not reduce anxiety in virgin female rats (Smith et al., 2012; also Carvalho, 2010) and there are some notable inconsistencies in how noradrenergic manipulations affect anxiety behaviors even in male laboratory rodents (Itoi & Sugimoto, 2010). Generally consistent with our results, Toufexis et al. (1999) found that clonidine (2 or 4 μg/kg) was only mildly anxiolytic in their postpartum rats, in contrast to their strong anxiogenic effects of yohimbine discussed above. These weak effects of clonidine are interesting because some serotonergic anxiolytics are relatively ineffective in dams compared to virgins (Fernandez-Guasti, Ferreira, & Picazo, 1998; Fernandez-Guasti, Picazo, & Ferreira, 2001). This might extend to anxiolytics targeting the noradrenergic system but we could provide no evidence for this in the current study because the anxiety-related behavior of neither dams nor virgins was affected by clonidine. Perhaps exposing females to a severe acute or chronic stressor before elevated plus-maze testing would have revealed differences in their response to clonidine (Morilak, Barrera, Echevarria, Garcia, Hernandez, Ma, Petre, 2005).
We found in Experiment 5 that yohimbine’s anxiogenic effects were not reproduced by the more selective α2-autoreceptor antagonist, idazoxan (Newman-Trancridi et al. 1998; Winter & Rabin 1992). Similar to yohimbine, idazoxan increases norepinephrine release in the BSTv (Herr et al., 2012) and is anxiogenic in an elevated plus maze after peripheral injection in male rats and mice (Cole et al. 1995; Soderpalm & Engel, 1989; Wright, Heaton, Upton, & Marsden, 1992). Even though idazoxan did not affect open-arm behaviors in our females, it did reduce the frequency of closed-arm entries, which is usually thought to reflect general locomotion (Pellow et al., 1985), and helps confirm our doses of idazoxan were behaviorally relevant. Nonetheless, the lack of effects of idazoxan on open-arm behavior indicated that yohimbine’s mechanism of action did not involve the α2 noradrenergic receptor. We then considered that yohimbine’s moderate agonism of the 5HT1A receptor was responsible, but intra-BSTv infusion of 8-OH-DPAT – which is often anxiolytic but sometimes anxiogenic in male and female rats (Cheeta, Kenny & File, 2000; De Almedia, Giovenardi, Charchat & Lucion, 1998; Dos Santos, de Andrade & Zangrossi, 2008; Rogel-Salazar & López-Rubalcava, 2011) - had no detectable effect in our females in Experiment 6. It appears that the anxiogenic effect of yohimbine in postpartum or diestrous rats cannot be explained by its effects only on the noradrenergic α2 receptor, a point made in other reports of the physiological or behavioral effects of yohimbine (e.g., Cheng, Costall, Ge & Naylor, 1993; Conrad et al., 2012; Davis et al., 2008; Powell, Palomo, Carasso, Bakshi & Geyer, 2005; Sanger, 1988). Furthermore, these results collectively suggest that a sex difference could exist in how the BST or its noradrenergic or serotonergic innervations influence anxiety-related behavior, which would be very valuable to assess in future studies directly comparing the sexes.
Because noradrenergic activity in some brain areas is downregulated during the postpartum period (Douglas, 2005), anxiety-related behavior in mothers was expected to be less sensitive to a given dose of yohimbine, clonidine or idazoxan than that found in virgins (see Mann & Bridges, 1992; Stern, Goldman & Levine, 1973; Toufexis et al., 1998; Toufexis, Tesolin, Huang & Walker, 1999). We did find in Experiment 1 that the percentage of time dams spent in open arms was decreased only by the high dose of IP yohimbine while virgins were significantly affected by both doses. The locomotor activity data from the IP yohimbine and IP clonidine experiments also indicated that dams were less sensitive than virgins. Toufexis et al. (1999) found that dams’ acoustic startle was less sensitive to 2.5 mg/kg yohimbine (2.5. mg/kg) than that of cycling virgins, but that dams were more sensitive to a lower dose of yohimbine (0.75 mg/kg). A similar pattern might have been revealed in our study if doses of yohimbine lower than 1.0 mg/kg were included. Our analysis of norepinephrine and serotonin content in the BSTv (Experiment 7) aimed to provide a neurochemical basis for such reproductive state differences in sensitivity to yohimbine and the other drugs examined. There were no significant differences between groups in either neurotransmitter, but serotonin turnover in the BSTv was lower in dams than in virgins. This difference might have contributed to dams’ lower responsiveness to yohimbine and further implicates serotonin in yohimbine’s anxiogenic effects. If this difference in serotonin turnover did contribute, differences between dams and virgins also would have been expected in response to BSTv 5HT1A receptor agonism with 8-OH-DPAT. As mentioned above, some serotonergic anxiolytics including 8-OH-DPAT are less effective in dams than virgins after peripheral injection (Fernandez-Guasti, Ferreira, & Picazo, 1998; Fernandez-Guasti, Picazo, & Ferreira, 2001). The influence of BST serotonin on anxiety is complex (Hammack, Guo, Hazra, Dabrowska, Myers & Rainnie, 2009) and perhaps other doses of 8-OH-DPAT, or some other 5HT1A agonist infused into the BSTv, would have revealed a reproductive state difference (Levita et al., 2004). Another possibility is that the BSTv is not a site where serotonin receptor modulators could act to produce such effects on anxiety in female rats.
When collapsed across treatment, dams in each experiment displayed less anxiety-related behavior than did virgins, as indicated by differences in open-arm time or frequency. This is consistent with the large literature demonstrating that postpartum rats are often less anxious than cycling virgin females (Lonstein, 2007; Bosch, 2011). It can be seen in our data, though, that in some experiments the control dams and virgins were not notably different on either open-arm measure. It is unknown why this was the case, but it is not related to any potential stress of the IP or BSTv injections because previous experiments from our laboratory using these same methods found differences between control dams and virgins in open-arm behavior (Figueira et al., 2008; Miller et al., 2010). Other laboratories also sometimes find no significant differences between postpartum and virgin females in their open-arm behaviors, with no obvious explanation (e.g., Boccia & Pedersen, 2001; Neumann et al., 2000; Young & Cook, 2004).
The BSTv is critical for the display of maternal behaviors (Numan & Numan, 1995; Numan & Numan, 1996). We have proposed that the BSTv is an interface between the neural networks underlying maternal care and anxiety, mediating the tradeoffs between the competing motivational states sometimes involved in these behaviors (Lonstein, 2007; Lonstein & Miller, 2008). BSTv infusion of the same doses of yohimbine or idazoxan as those used here severely impaired maternal behaviors in postpartum rats (Smith et al., 2012). Because we found herein that idazoxan was not anxiogenic, dams’ disrupted mothering after idazoxan (and even perhaps yohimbine) cannot be due to their elevated anxiety. Thus, the BSTv of postpartum rats can clearly influence both anxiety and mothering - and yohimbine infused into the BSTv affects both – but our data collectively suggest this occurs independently through partly non-overlapping neurochemical systems and probably different neural populations within the BSTv. Our focus here was on the BSTv, but numerous other brain sites are candidates for where norepinephrine acting alone or with other neurotransmitters influences anxiety and maternal behaviors in female rats. The paraventricular and dorsomedial hypothalamic nuclei, medial preoptic area, and periaqueductal gray all have relatively high norepinephrine concentrations (Kilts & Anderson, 1986; Crowley, O’Donohue & Jacobowitz, 1978) and are known to regulate females’ anxiety-related behaviors (e.g., Jurek, Slattery, Maloumby, Hillerer, Koszinowski, Neumann & van den Burg, 2012; Rivera-Arce, Morales-Crespo, Vargas-Pinto, Velázquez, & Jorge, 2006; Spiteri, Ogawa, Musatov, Pfaff & Agmo, 2012; Lovick, 2008) and maternal care toward pups (e.g., Insel & Harbaugh, 1989; Bridges, Mann & Coppeta, 1999; Numan, 1974; Lonstein & Stern, 1997).
Acknowledgments
This research was supported by NICHD grant R01HD057962 to JSL and a NICHD predoctoral National Research Service Award F32MH83344 to CDS. The authors appreciate the advice of Dr. Sheba Mohan-Kumar and the technical assistance of Mr. Robert Burnett with the HPLC analysis.
References
- Alvarez RP, Chen G, Bodurka J, Kaplan R, Grillon C. Phasic and sustained fear in humans elicits distinct patterns of brain activity. Neuroimage. 2011;55:389–400. doi: 10.1016/j.neuroimage.2010.11.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beiderbeck DI, Neumann ID, Veenema AH. Differences in intermale aggression are accompanied by opposite vasopressin release patterns within the septum in rats bred for low and high anxiety. European Journal of Neuroscience. 2007;26:3597–605. doi: 10.1111/j.1460-9568.2007.05974.x. [DOI] [PubMed] [Google Scholar]
- Bertrand F, Lehmann O, Lazarus C, Jeltsch H, Cassel JC. Intraseptal infusions of 8-OH-DPAT in the rat impairs water-maze performances: effects on memory or anxiety? Neuroscience Letters. 2000;279:45–48. doi: 10.1016/s0304-3940(99)00948-9. [DOI] [PubMed] [Google Scholar]
- Bienkowski MS, Rinaman L. Noradrenergic inputs to the paraventricular hypothalamus contribute to hypothalamic-pituitary-adrenal axis and central Fos activation in rats after acute systemic endotoxin exposure. Neuroscience. 2008;156:1093–102. doi: 10.1016/j.neuroscience.2008.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boccia ML, Pedersen CA. Brief vs. long maternal separations in infancy: contrasting relationships with adult maternal behavior and lactation levels of aggression and anxiety. Psychoneuroendocrinology. 2001;26:657–72. doi: 10.1016/s0306-4530(01)00019-1. [DOI] [PubMed] [Google Scholar]
- Bosch OJ. Maternal nurturing is dependent on her innate anxiety: the behavioral roles of brain oxytocin and vasopressin. Hormones and Behavior. 2011;59:202–212. doi: 10.1016/j.yhbeh.2010.11.012. [DOI] [PubMed] [Google Scholar]
- Bridges RS, Mann PE, Coppeta JS. Hypothalamic involvement in the regulation of maternal behaviour in the rat: inhibitory roles for the ventromedial hypothalamus and the dorsal/anterior hypothalamic areas. Journal of Neuroendocrinology. 1999;11:259–266. doi: 10.1046/j.1365-2826.1999.00322.x. [DOI] [PubMed] [Google Scholar]
- Byrnes EM, Bridges RS. Reproductive experience alters anxiety-like behavior in the female rat. Hormones and Behavior. 2006;50:70–76. doi: 10.1016/j.yhbeh.2006.01.006. [DOI] [PubMed] [Google Scholar]
- Cai L, Bakalli H, Rinaman L. Yohimbine anxiogenesis in the elevated plus maze is disrupted by bilaterally disconnecting the bed nucleus of the stria terminalis from the central nucleus of the amygdala. Neuroscience. 2012;223:200–208. doi: 10.1016/j.neuroscience.2012.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campeau S, Falls WA, Cullinan WE, Helmreich DL, Davis M, Watson SJ. Elicitation and reduction of fear: behavioural and neuroendocrine indices and brain induction of the immediate-early gene c-fos. Neuroscience. 1997;78:1087–104. doi: 10.1016/s0306-4522(96)00632-x. [DOI] [PubMed] [Google Scholar]
- Carli M, Tatarczynska E, Cervo L, Samanin R. Stimulation of hippocampal 5-HT1A receptors causes amnesia and anxiolytic-like but not antidepressant-like effects in the rat. European Journal of Pharmacology. 1993;234:215–21. doi: 10.1016/0014-2999(93)90956-i. [DOI] [PubMed] [Google Scholar]
- Carvalho AF, Reyes AR, Sterling RC, Unterwald E, Van Bockstaele EJ. Contribution of limbic norepinephrine to cannabinoid-induced aversion. Psychopharmacology. 2010;211:479–491. doi: 10.1007/s00213-010-1923-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casada JH, Dafny N. Responses of neurons in bed nucleus of the stria terminalis to microiontophoretically applied morphine, norepinephrine and acetylcholine. Neuropharmacology. 1993;32:279–284. doi: 10.1016/0028-3908(93)90112-g. [DOI] [PubMed] [Google Scholar]
- Cecchi M, Khoshbouei H, Morilak DA. Modulatory effects of norepinephrine, acting on alpha 1 receptors in the central nucleus of the amygdala, on behavioral and neuroendocrine responses to acute immobilization stress. Neuropharmacology. 2002;43:1139–1147. doi: 10.1016/s0028-3908(02)00292-7. [DOI] [PubMed] [Google Scholar]
- Cheeta S, Kenny PJ, File SE. The role of 5-HT1A receptors in mediating the anxiogenic effects of nicotine following lateral septal administration. European Journal of Neuroscience. 2000;12:3797–802. doi: 10.1046/j.1460-9568.2000.00246.x. [DOI] [PubMed] [Google Scholar]
- Cheng CH, Costall B, Ge J, Naylor RJ. The profiles of interaction of yohimbine with anxiolytic and putative anxiolytic agents to modify 5-HT release in the frontal cortex of freely-moving rats. British Journal of Pharmacology. 1993;110:1079–84. doi: 10.1111/j.1476-5381.1993.tb13924.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christianson JP, Jennings JH, Ragole T, Flyer JG, Benison AM, Barth DS, Watkins LR, Maier SF. Safety signals mitigate the consequences of uncontrollable stress via a circuit involving the sensory insular cortex and bed nucleus of the stria terminalis. Biological Psychiatry. 2011;70:458–64. doi: 10.1016/j.biopsych.2011.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark AJ, Butcher SP, Winn P. Evidence for functional separation of alpha-1 and alpha-2 noradrenaline receptors by pre-synaptic terminal re-uptake mechanisms. Psychopharmacology. 1991;103:366–74. doi: 10.1007/BF02244291. [DOI] [PubMed] [Google Scholar]
- Cole BJ, Hillmann M, Seidelmann D, Klewer M, Jones GH. Effects of benzodiazepine receptor partial inverse agonists in the elevated plus maze test of anxiety in the rat. Psychopharmacology. 1995;121:118–26. doi: 10.1007/BF02245598. [DOI] [PubMed] [Google Scholar]
- Conrad KL, Davis AR, Silberman Y, Sheffler DJ, Shields AD, Saleh SA, Sen N, Matthies HJ, Javitch JA, Lindsley CW, Winder DG. Yohimbine Depresses Excitatory Transmission in BNST and Impairs Extinction of Cocaine Place Preference Through Orexin-Dependent, Norepinephrine-Independent Processes. Neuropsychopharmacology. 2012;37:2253–2266. doi: 10.1038/npp.2012.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowley WR, O’Donohue TL, Jacobowitz DM. Changes in catecholamine content in discrete brain nuclei during the estrous cycle of the rat. Brain Research. 1978;147:315–326. doi: 10.1016/0006-8993(78)90842-9. [DOI] [PubMed] [Google Scholar]
- Curtis AL, Bethea T, Valentino RJ. Sexually dimorphic responses of the brain norepinephrine system to stress and corticotropin-releasing factor. Neuropsychopharmacology. 2006;31:544–54. doi: 10.1038/sj.npp.1300875. [DOI] [PubMed] [Google Scholar]
- Da Silva ES, Poltronieri SC, Nascimento JO, Zangrossi H, Jr, Viana MB. Facilitation of 5-HT(2A/2C)-mediated neurotransmission in the ventromedial hypothalamic nucleus decreases anxiety in the elevated T-maze. Behavioral Brain Research. 2011;216:692–8. doi: 10.1016/j.bbr.2010.09.016. [DOI] [PubMed] [Google Scholar]
- Davis AR, Shields AD, Brigman JL, Norcross M, McElligott ZA, Holmes A, Winder DG. Yohimbine impairs extinction of cocaine-conditioned place preference in an alpha2-adrenergic receptor independent process. Learning and Memory. 2008;15:667–76. doi: 10.1101/lm.1079308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M, Walker DL, Miles L, Grillon C. Phasic vs sustained fear in rats and humans: role of the extended amygdala in fear vs anxiety. Neuropsychopharmacology. 2010;35:105–35. doi: 10.1038/npp.2009.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day HE, Nebel S, Sasse S, Campeau S. Inhibition of the central extended amygdala by loud noise and restraint stress. European Journal of Neuroscience. 2005;21:441–54. doi: 10.1111/j.1460-9568.2005.03865.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Almeida RM, Giovenardi M, Charchat H, Lucion AB. 8-OH-DPAT in the median raphe nucleus decreases while in the medial septal area it may increase anxiety in female rats. Neuroscience and Biobehavioral Reviews. 1998;23:259–64. doi: 10.1016/s0149-7634(98)00026-8. [DOI] [PubMed] [Google Scholar]
- Del Rio G, Verlardo A, Zizzo G, Marrama P, Della Casa L. Sex differences in catecholamine response to clonidine. International Journal of Obsity and Related Metabolic Disorders. 1993;17:465–9. [PubMed] [Google Scholar]
- Deyama S, Ide S, Kondoh N, Yamaguchi T, Yoshioka M, Minami M. Inhibition of noradrenaline release by clonidine in the ventral bed nucleus of the stria terminalis attenuates pain-induced aversion in rats. Neuropharmacology. 2011;61:156–60. doi: 10.1016/j.neuropharm.2011.03.023. [DOI] [PubMed] [Google Scholar]
- Dodge JC, Badura LL. Infusion of alpha-2-adrenergic agents into the paraventricular and arcuate nuclei of the hypothalamus in the Siberian hamster: opposing effects on basal prolactin. Neuroendocrinology. 2002;75:175–184. doi: 10.1159/000048235. [DOI] [PubMed] [Google Scholar]
- Dong HW, Swanson LW. Projections from bed nuclei of the stria terminalis, dorsomedial nucleus: implications for cerebral hemisphere integration of neuroendocrine, autonomic, and drinking responses. Journal of Comparative Neurology. 2006a;494:75–107. doi: 10.1002/cne.20790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong HW, Swanson LW. Projections from bed nuclei of the stria terminalis, magnocellular nucleus: implications for cerebral hemisphere regulation of micturition, defecation, and penile erection. Journal of Comparative Neurology. 2006b;494:108–41. doi: 10.1002/cne.20789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong HW, Swanson LW. Projections from bed nuclei of the stria terminalis, anteromedial area: cerebral hemisphere integration of neuroendocrine, autonomic, and behavioral aspects of energy balance. Journal of Comparative Neurology. 2006c;494:142–78. doi: 10.1002/cne.20788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dos Santos L, de Andrade TG, Zangrossi H. 5-HT1A receptors in the dorsal hippocampus mediate the anxiogenic effect induced by the stimulation of 5-HT neurons in the median raphe nucleus. European Journal of Neuropsychopharmacology. 2008;18:286–94. doi: 10.1016/j.euroneuro.2007.07.007. [DOI] [PubMed] [Google Scholar]
- Douglas AJ. Central noradrenergic mechanisms underlying acute stress responses of the Hypothalamo-pituitary-adrenal axis: adaptations through pregnancy and lactation. Stress. 2005;8:5–18. doi: 10.1080/10253890500044380. [DOI] [PubMed] [Google Scholar]
- Dumont EC, Williams JT. Noradrenaline triggers GABAA inhibition of bed nucleus of the stria terminalis neurons projecting to the ventral tegmental area. Journal of Neuroscience. 2004;24:8198–8204. doi: 10.1523/JNEUROSCI.0425-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn JD. Plasma corticosterone responses to electrical stimulation of the bed nucleus of the stria terminalis. Brain Research. 1987;407:327–31. doi: 10.1016/0006-8993(87)91111-5. [DOI] [PubMed] [Google Scholar]
- Duvarci S, Bauer EP, Paré D. The bed nucleus of the stria terminalis mediates inter-individual variations in anxiety and fear. Journal of Neuroscience. 2009;29:10357–61. doi: 10.1523/JNEUROSCI.2119-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egli RE, Kash TL, Choo K, Savchenko V, Matthews RT, Blakely RD, Winder DG. Norepinephrine modulates glutamatergic transmission in the bed nucleus of the stria terminalis. Neuropsychopharmacology. 2005;30:657–68. doi: 10.1038/sj.npp.1300639. [DOI] [PubMed] [Google Scholar]
- Fendt M, Siegl S, Steiniger-Brach B. Noradrenaline transmission within the ventral bed nucleus of the stria terminalis is critical for fear behavior induced by trimethylthiazoline, a component of fox odor. Journal of Neuroscience. 2005;25:5998–6004. doi: 10.1523/JNEUROSCI.1028-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández-Guasti A, Picazo O, Ferreira A. Blockade of the anxiolytic action of 8-OH-DPAT in lactating rats. Pharmacology, Biochemistry and Behavior. 1998;59:45–50. doi: 10.1016/s0091-3057(97)00392-4. [DOI] [PubMed] [Google Scholar]
- Fernández-Guasti A, Ferreira A, Picazo O. Diazepam, but not buspirone, induces similar anxiolytic-like actions in lactating and ovariectomized Wistar rats. Pharmacology, Biochemistry and Behavior. 2001;70:85–93. doi: 10.1016/s0091-3057(01)00586-x. [DOI] [PubMed] [Google Scholar]
- Figueira RJ, Peabody MF, Lonstein JS. Oxytocin receptor activity in the ventrocaudal periaqueductal gray modulates anxiety-related behavior in postpartum rats. Behavioral Neuroscience. 2008;122:618–628. doi: 10.1037/0735-7044.122.3.618. [DOI] [PubMed] [Google Scholar]
- Fisher RS, Buchwald NA, Hull CD, Levine MS. GABAergic basal forebrain neurons project to the neocortex: the localization of glutamic acid decarboxylase and choline acetyltransferase in feline corticopetal neurons. Journal of Comparative Neurology. 1988;272:489–502. doi: 10.1002/cne.902720404. [DOI] [PubMed] [Google Scholar]
- Fleming AS, Suh EJ, Korsmit M, Rusak B. Activation of Fos-like immunoreactivity in the medial preoptic area and limbic structures by maternal and social interactions in rats. Behavioral Neuroscience. 1994;108:724–34. doi: 10.1037//0735-7044.108.4.724. [DOI] [PubMed] [Google Scholar]
- Forray MI, Andrés ME, Bustos G, Gysling K. Regulation of endogenous noradrenaline release from the bed nucleus of stria terminalis. Biochemistry and Pharmacology. 1995;49:687–92. doi: 10.1016/0006-2952(94)00498-b. [DOI] [PubMed] [Google Scholar]
- Forray MI, Bustos G, Gysling K. Regulation of norepinephrine release from the rat bed nucleus of the stria terminalis: in vivo microdialysis studies. Journal of Neuroscience Research. 1997;50:1040–1046. doi: 10.1002/(SICI)1097-4547(19971215)50:6<1040::AID-JNR15>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Forray MI, Bustos G, Gysling K. Noradrenaline inhibits glutamate release in the rat bed nucleus of the stria terminalis: in vivo microdialysis studies. Journal of Neuroscience Research. 1999;55:311–320. doi: 10.1002/(SICI)1097-4547(19990201)55:3<311::AID-JNR6>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Gomes FV, Resstel LB, Guimarães FS. The anxiolytic-like effects of cannabidiol injected into the bed nucleus of the stria terminalis are mediated by 5-HT1A receptors. Psychopharmacology. 2011;213:465–73. doi: 10.1007/s00213-010-2036-z. [DOI] [PubMed] [Google Scholar]
- Gonzalo-Ruiz A, Alonso A, Sanz JM, Llinás RR. Afferent projections to the mammillary complex of the rat, with special reference to those from surrounding hypothalamic regions. Journal of Comparative Neurology. 1992;321:277–99. doi: 10.1002/cne.903210208. [DOI] [PubMed] [Google Scholar]
- Gulia KK, Kumar VM, Mallick HN. Role of the lateral septal noradrenergic system in the elaboration of male sexual behavior in rats. Pharmacology, Biochemistry and Behavior. 2002;72:817–823. doi: 10.1016/s0091-3057(02)00771-2. [DOI] [PubMed] [Google Scholar]
- Guo TZ, Poree L, Golden W, Stein J, Fujinaga M, Maze M. Antinociceptive response to nitrous oxide is mediated by supraspinal opiate and spinal alpha 2 adrenergic receptors in the rat. Anesthesiology. 1996;85:846–852. doi: 10.1097/00000542-199610000-00020. [DOI] [PubMed] [Google Scholar]
- Hammack SE, Richey KJ, Watkins LR, Maier SF. Chemical lesion of the bed nucleus of the stria terminalis blocks the behavioral consequences of uncontrollable stress. Behavioral Neuroscience. 2004;118:443–8. doi: 10.1037/0735-7044.118.2.443. [DOI] [PubMed] [Google Scholar]
- Henke PG. The bed nucleus of the stria terminalis and immobilization-stress: unit activity, escape behaviour, and gastric pathology in rats. Behavioral Brain Research. 1984;11:35–45. doi: 10.1016/0166-4328(84)90006-8. [DOI] [PubMed] [Google Scholar]
- Herr NR, Park J, McElligott ZA, Belle AM, Carelli RM, Wightman RM. In vivo voltammetry monitoring of electrically evoked extracellular norepinephrine in subregions of the bed nucleus of the stria terminalis. Journal of Neurophysiology. 2012;107:1731–7. doi: 10.1152/jn.00620.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel TR, Harbaugh CR. Lesions of the hypothalamic paraventricular nucleus disrupt the initiation of maternal behavior. Physiology & Behavior. 1989;45:1033–1041. doi: 10.1016/0031-9384(89)90234-5. [DOI] [PubMed] [Google Scholar]
- Itoi K, Sugimoto N. The brainstem noradrenergic systems in stress, anxiety and depression. Journal of Neuroendocrinology. 2010;22:355–61. doi: 10.1111/j.1365-2826.2010.01988.x. [DOI] [PubMed] [Google Scholar]
- Jones DM, Arters J, Berger-Sweeney J. Carbon dioxide-induced anesthesia has no effect on brain biogenic amine concentrations in mice. Laboratory Animal Science. 1999;49:316–318. [PubMed] [Google Scholar]
- Jurek B, Slattery DA, Maloumby R, Hillerer K, Koszinowski S, Neumann ID, van den Burg EH. Differential contribution of hypothalamic MAPK activity to anxiety-like behaviour in virgin and lactating rats. PLoS One. 2012;7:e37060. doi: 10.1371/journal.pone.0037060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalin NH, Shelton SE, Fox AS, Oakes TR, Davidson RJ. Brain regions associated with the expression and contextual regulation of anxiety in primates. Biological Psychiatry. 2005;58:796–804. doi: 10.1016/j.biopsych.2005.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalinichev M, Rosenblatt JS, Nakabeppu Y, Morrell JI. Induction of c-fos-like and fosB-like immunoreactivity reveals forebrain neuronal populations involved differentially in pup-mediated maternal behavior in juvenile and adult rats. Journal of Comparative Neurology. 2000;416:45–78. doi: 10.1002/(sici)1096-9861(20000103)416:1<45::aid-cne5>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- Kilts CD, Anderson CM. The simultaneous quantification of dopamine, norepinephrine, and epinephrine in micropunched rat brain nuclei by online trace enrichment HPLC with electrochemical detection: Distribution of catecholamines in the limbic system. Neuroschemistry International. 1986;9:437–445. doi: 10.1016/0197-0186(86)90086-0. [DOI] [PubMed] [Google Scholar]
- Kudo T, Uchigashima M, Miyazaki T, Konno K, Yamasaki M, Yanagawa Y, Minami M, Watanabe M. Three types of neurochemical projection from the bed nucleus of the stria terminalis to the ventral tegmental area in adult mice. Journal of Neuroscience. 2012;32:18035–46. doi: 10.1523/JNEUROSCI.4057-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y, Davis M. Role of the hippocampus, the bed nucleus of the stria terminalis, and the amygdala in the excitatory effect of corticotropin-releasing hormone on the acoustic startle reflex. Journal of Neuroscience. 1997;17:6434–46. doi: 10.1523/JNEUROSCI.17-16-06434.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levita L, Hammack SE, Mania I, Li XY, Davis M, Rainnie DG. 5-hydroxytryptamine1A-like receptor activation in the bed nucleus of the stria terminalis: electrophysiological and behavioral studies. Neuroscience. 2004;128:583–96. doi: 10.1016/j.neuroscience.2004.06.037. [DOI] [PubMed] [Google Scholar]
- Limberg JK, Eldridge MW, Proctor LT, Sebranek JJ, Schrage WG. Alpha-adrenergic control of blood flow during exercise: effect of sex and menstrual phase. Journal of Applied Physiology. 2010;109:1360–8. doi: 10.1152/japplphysiol.00518.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonstein JS. Reduced anxiety in postpartum rats requires recent physical interactions with pups, but is independent of suckling and peripheral sources of hormones. Hormones and Behavior. 2005;47:241–255. doi: 10.1016/j.yhbeh.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Lonstein JS. Regulation of anxiety during the postpartum period. Frontiers in Neuroendocrinology. 2007;28:115–141. doi: 10.1016/j.yfrne.2007.05.002. [DOI] [PubMed] [Google Scholar]
- Lonstein JS, Miller SM. Infant touch, neurochemistry, and postpartum anxiety. In: Bridges RS, editor. Neurobiology of the Parental Brain. Elsevier Press; 2008. pp. 145–162. [Google Scholar]
- Lonstein JS, Stern JM. Role of the midbrain periaqueductal gray in maternal nurturance and aggression: c-fos and electrolytic lesion studies in lactating rats. Journal of Neuroscience. 1997;17:3364–3378. doi: 10.1523/JNEUROSCI.17-09-03364.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovick TA. GABA in the female brain -- oestrous cycle-related changes in GABAergic function in the periaqueductal grey matter. Pharmacology, Biochemistry & Behavior. 2008;90:43–50. doi: 10.1016/j.pbb.2007.12.014. [DOI] [PubMed] [Google Scholar]
- Luque JM, De Blas MR, Segovia S, Guillamon A. Sexual dimorphism of the dopamine-beta-hydroxylase-immunoreactive neurons in the rat locus ceruleus. Developmental Brain Research. 1992;67:211–215. doi: 10.1016/0165-3806(92)90221-h. [DOI] [PubMed] [Google Scholar]
- Mann PE, Bridges RS. Neural and endocrine sensitivities to opioids decline as a function of multiparity in the rat. Brain Research. 1992;580:241–8. doi: 10.1016/0006-8993(92)90950-e. [DOI] [PubMed] [Google Scholar]
- Millan MJ, Newman-Tancredi A, Audinot V, Cussac D, Lejeune F, et al. Agonist and antagonist actions of yohimbine as compared to fluparoxan at alpha(2)-adrenergic receptors (AR)s, serotonin (5-HT)(1A), 5-HT(1B), 5-HT(1D) and dopamine D(2) and D(3) receptors. Significance for the modulation of frontocortical monoaminergic transmission and depressive states. Synapse. 2000;35:79–95. doi: 10.1002/(SICI)1098-2396(200002)35:2<79::AID-SYN1>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Miller SM, Lonstein JS. Dopamine d1 and d2 receptor antagonism in the preoptic area produces different effects on maternal behavior in lactating rats. Behavioral Neuroscience. 2005;119:1072–1083. doi: 10.1037/0735-7044.119.4.1072. [DOI] [PubMed] [Google Scholar]
- Miller SM, Piasecki CC, Lonstein JS. Use of the light-dark box to compare the anxiety-related behavior of virgin and postpartum female rats. Pharmacology Biochemistry and Behavior. 2011;100:130–137. doi: 10.1016/j.pbb.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller SM, Piasecki CC, Peabody MF, Lonstein JS. GABAA receptor antagonism in the ventrocaudal periaqueductal gray increases anxiety in the anxiety-resistant postpartum rat. Pharmacology Biochemistry and Behavior. 2010;95:457–465. doi: 10.1016/j.pbb.2010.03.007. [DOI] [PubMed] [Google Scholar]
- Morgan CA, Southwick SM, Grillon C, Davis M, Krystal JH, Charney DS. Yohimbine-facilitated acoustic startle reflex in humans. Psychopharmacology. 1993;110:342–346. doi: 10.1007/BF02251291. [DOI] [PubMed] [Google Scholar]
- Morilak DA, Cecchi M, Khoshbouei H. Interactions of norepinephrine and galanin in the central amygdala and lateral bed nucleus of the stria terminalis modulate the behavioral response to acute stress. Life Sciences. 2003;73:715–726. doi: 10.1016/s0024-3205(03)00392-8. [DOI] [PubMed] [Google Scholar]
- Morilak DA, Barrera G, Echevarria DJ, Garcia AS, Hernandez A, Ma S, Petre CO. Role of brain norepinephrine in the behavioral response to stress. Progress in Neuropsychopharmacology and Biological Psychiatry. 2005;29:1214–1224. doi: 10.1016/j.pnpbp.2005.08.007. [DOI] [PubMed] [Google Scholar]
- Mugnaini E, Oertel WH. An atlas of the distribution of GABAergic neurons and terminals in the rat CNS as revealed by GAD immunohistochemistry. In: Bjorklund A, Hokfelt T, editors. Handbook of Chemical Neuroanatomy, Vol. 4, GABA and Neuropeptides in the CNS, Part I. Amsterdam: Elsevier; 1985. pp. 436–608. [Google Scholar]
- Naka T, Ide S, Nakako T, Hirata M, Majima Y, Deyama S, Takeda H, Yoshioka M, Minami M. Activation of β-adrenoceptors in the bed nucleus of the stria terminalis induces food intake reduction and anxiety-like behaviors. Neuropharmacology. 2012;67C:326–330. doi: 10.1016/j.neuropharm.2012.11.021. [DOI] [PubMed] [Google Scholar]
- Neumann ID, Torner L, Wigger A. Brain oxytocin: differential inhibition of neuroendocrine stress responses and anxiety-related behaviour in virgin, pregnant and lactating rats. Neuroscience. 2000;95:567–75. doi: 10.1016/s0306-4522(99)00433-9. [DOI] [PubMed] [Google Scholar]
- Newman-Tancredi A, Nicolas JP, Audinot V, Gavaudan S, Verriele L, et al. Actions of alpha2 adrenoceptor ligands at alpha2A and 5-HT1A receptors: the antagonist, atipamezole, and the agonist, dexmedetomidine, are highly selective for alpha2A adrenoceptors. Naunyn Schmiedebergs Archives Pharmacology. 1998;358:197–206. doi: 10.1007/pl00005243. [DOI] [PubMed] [Google Scholar]
- Numan M. Medial preoptic area and maternal behavior in the female rat. Journal of Comparative and Physiological Psychology. 1974;87:746–759. doi: 10.1037/h0036974. [DOI] [PubMed] [Google Scholar]
- Numan M, Numan MJ. A lesion and neuroanatomical tract-tracing analysis of the role of the bed nucleus of the stria terminalis in retrieval behavior and other aspects of maternal responsiveness in rats. Developmental Psychobiology. 1996;29:23–51. doi: 10.1002/(SICI)1098-2302(199601)29:1<23::AID-DEV2>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Numan M, Numan MJ. Importance of pup-related sensory inputs and maternal performance for the expression of Fos-like immunoreactivity in the preoptic area and ventral bed nucleus of the stria terminalis of postpartum rats. Behavioral Neuroscience. 1995;109:135–149. doi: 10.1037//0735-7044.109.1.135. [DOI] [PubMed] [Google Scholar]
- Onaka T, Yagi K. Role of noradrenergic projections to the bed nucleus of the stria terminalis in neuroendocrine and behavioral responses to fear-related stimuli in rats. Brain Research. 1998;788:287–93. doi: 10.1016/s0006-8993(98)00012-2. [DOI] [PubMed] [Google Scholar]
- Pacak K, McCarty R, Palkovits M, Kopin IJ, Goldstein DS. Effects of immobilization on in vivo release of norepinephrine in the bed nucleus of the stria terminalis in conscious rats. Brain Research. 1995;688:242–6. doi: 10.1016/0006-8993(95)00566-9. [DOI] [PubMed] [Google Scholar]
- Palij P, Stamford JA. Real-time monitoring of endogenous noradrenaline release in rat brain slices using fast cyclic voltammetry. 2. Operational characteristics of the alpha 2 autoreceptor in the bed nucleus of stria terminalis, pars ventralis. Brain Research. 1993;607:134–40. doi: 10.1016/0006-8993(93)91498-h. [DOI] [PubMed] [Google Scholar]
- Park J, Kile BM, Wightman RM. In vivo voltammetric monitoring of norepinephrine release in the rat ventral bed nucleus of the stria terminalis and anteroventral thalamic nucleus. European Journal of Neuroscience. 2009;30:2121–33. doi: 10.1111/j.1460-9568.2009.07005.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J, Wheeler RA, Fontillas K, Keithley RB, Carelli RM, Wightman RM. Catecholamines in the bed nucleus of the stria terminalis reciprocally respond to reward and aversion. Biological Psychiatry. 2012;71:327–34. doi: 10.1016/j.biopsych.2011.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawluski JL, Lieblich SE, Galea LA. Offspring-exposure reduces depressive-like behaviour in the parturient female rat. Behavioral Brain Research. 2009;197:55–61. doi: 10.1016/j.bbr.2008.08.001. [DOI] [PubMed] [Google Scholar]
- Pezuk P, Aydin E, Aksoy A, Canbeyli R. Effects of BNST lesions in female rats on forced swimming and navigational learning. Brain Research. 2008;1228:199–207. doi: 10.1016/j.brainres.2008.06.071. [DOI] [PubMed] [Google Scholar]
- Pellow S, Chopin P, File SE, Briley M. Validation of open:closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. Journal of Neuroscience Methods. 1985;14:149–167. doi: 10.1016/0165-0270(85)90031-7. [DOI] [PubMed] [Google Scholar]
- Phelix CF, Liposits Z, Paull WK. Monoamine innervation of bed nucleus of stria terminalis: an electron microscopic investigation. Brain Research Bulletin. 1992;28:949–965. doi: 10.1016/0361-9230(92)90218-m. [DOI] [PubMed] [Google Scholar]
- Powell SB, Palomo J, Carasso BS, Bakshi VP, Geyer MA. Yohimbine disrupts prepulse inhibition in rats via action at 5-HT1A receptors, not alpha2-adrenoceptors. Psychopharmacology. 2005;180:491–500. doi: 10.1007/s00213-005-2193-7. [DOI] [PubMed] [Google Scholar]
- Pryce CR. A comparative systems model of the regulation of maternal motivation in mammals. Animal Behaviour. 1992;43:417–441. [Google Scholar]
- Resstel LB, Alves FH, Reis DG, Crestani CC, Corrêa FM, Guimarães FS. Anxiolytic-like effects induced by acute reversible inactivation of the bed nucleus of stria terminalis. Neuroscience. 2008;154:869–76. doi: 10.1016/j.neuroscience.2008.04.007. [DOI] [PubMed] [Google Scholar]
- Riche D, De Pommery J, Menetrey D. Neuropeptides and catecholamines in efferent projections of the nuclei of the solitary tract in the rat. Journal of Comparative Neurology. 1990;293:399–424. doi: 10.1002/cne.902930306. [DOI] [PubMed] [Google Scholar]
- Rivera-Arce JC, Morales-Crespo L, Vargas-Pinto N, Velázquez KT, Jorge JC. Central effects of the anabolic steroid 17alpha methyltestosterone in female anxiety. Pharmacology, Biochemistry & Behavior. 2006;84:275–281. doi: 10.1016/j.pbb.2006.05.009. [DOI] [PubMed] [Google Scholar]
- Roder S, Ciriello J. Collateral axonal projections to limbic structures from ventrolateral medullary A1 noradrenergic neurons. Brain Research. 1994;638:182–188. doi: 10.1016/0006-8993(94)90648-3. [DOI] [PubMed] [Google Scholar]
- Rodi D, Zucchini S, Simonato M, Cifani C, Massi M, Polidori C. Functional antagonism between nociceptin/orphanin FQ (N/OFQ) and corticotropin-releasing factor (CRF) in the rat brain: evidence for involvement of the bed nucleus of the stria terminalis. Psychopharmacology. 2008;196:523–31. doi: 10.1007/s00213-007-0985-7. [DOI] [PubMed] [Google Scholar]
- Rogel-Salazar G, López-Rubalcava C. Evaluation of the anxiolytic-like effects of clomipramine in two rat strains with different anxiety vulnerability (Wistar and Wistar-Kyoto rats): participation of 5-HT1A receptors. Behavioral Pharmacology. 2011;22:136–46. doi: 10.1097/FBP.0b013e328343d7c5. [DOI] [PubMed] [Google Scholar]
- Roland BL, Sawchenko PE. Local origins of some GABAergic projections to the paraventricular and supraoptic nuclei of the hypothalamus in the rat. Journal of Comparative Neurology. 1993;332:123–43. doi: 10.1002/cne.903320109. [DOI] [PubMed] [Google Scholar]
- Sahuque LL, Kullberg EF, Mcgeehan AJ, Kinder JR, Hicks MP, et al. Anxiogenic and aversive effects of corticotropin-releasing factor (CRF) in the bed nucleus of the stria terminalis in the rat: role of CRF receptor subtypes. Psychopharmacology. 2006;186:122–32. doi: 10.1007/s00213-006-0362-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sajdyk T, Johnson P, Fitz S, Shekhar A. Chronic inhibition of GABA synthesis in the bed nucleus of the stria terminalis elicits anxiety-like behavior. Journal of Psychopharmacology. 2008;22:633–41. doi: 10.1177/0269881107082902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger DJ. Behavioural effects of the alpha 2-adrenoceptor antagonists idazoxan and yohimbine in rats: comparisons with amphetamine. Psychopharmacology. 1988;96:243–9. doi: 10.1007/BF00177568. [DOI] [PubMed] [Google Scholar]
- Schulz D, Canbeyli RS. Lesion of the bed nucleus of the stria terminalis enhances learned despair. Brain Research Bulletin. 2000;52:83–87. doi: 10.1016/s0361-9230(00)00235-5. [DOI] [PubMed] [Google Scholar]
- Schweimer J, Fendt M, Schnitzler HU. Effects of clonidine injections into the bed nucleus of the stria terminalis on fear and anxiety behavior in rats. European Journal of Pharmacology. 2005;507:117–124. doi: 10.1016/j.ejphar.2004.11.044. [DOI] [PubMed] [Google Scholar]
- Sink KS, Walker DL, Yang Y, Davis M. Calcitonin gene-related peptide in the bed nucleus of the stria terminalis produces an anxiety-like pattern of behavior and increases neural activation in anxiety-related structures. Journal of Neuroscience. 2011;31:1802–10. doi: 10.1523/JNEUROSCI.5274-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CD, Lonstein JS. Contact with infants modulates anxiety-generated c-fos activity in the brains of postpartum rats. Behavioral Brain Research. 2008;190:193–200. doi: 10.1016/j.bbr.2008.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CD, Holschbach MA, Olsewicz J, Lonstein JS. Effects of noradrenergic alpha-2 receptor antagonism or noradrenergic lesions in the ventral bed nucleus of the stria terminalis and medial preoptic area on maternal care in female rats. Psychopharmacology. 2012;224:263–76. doi: 10.1007/s00213-012-2749-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soderpalm B, Engel JA. Biphasic effects of clonidine on conflict behavior: involvement of different alpha-adrenoceptors. Pharmacology Biochemistry and Behavior. 1988;30:471–477. doi: 10.1016/0091-3057(88)90482-0. [DOI] [PubMed] [Google Scholar]
- Soderpalm B, Engel JA. Alpha 2-adrenoceptor antagonists potentiate the anticonflict and the rotarod impairing effects of benzodiazepines. Journal of Neural Transmission. 1988;76:191–204. doi: 10.1007/BF01260504. [DOI] [PubMed] [Google Scholar]
- Somerville LH, Whalen PJ, Kelley WM. Human bed nucleus of the stria terminalis indexes hypervigilant threat monitoring. Biological Psychiatry. 2010;68:416–24. doi: 10.1016/j.biopsych.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiteri T, Ogawa S, Musatov S, Pfaff DW, Agmo A. The role of the estrogen receptor α in the medial preoptic area in sexual incentive motivation, proceptivity and receptivity, anxiety, and wheel running in female rats. Behavioral Brain Research. 2012;230:11–20. doi: 10.1016/j.bbr.2012.01.048. [DOI] [PubMed] [Google Scholar]
- Stern JM, Goldman L, Levine S. Pituitary-adrenal responsiveness during lactation in rats. Neuroendocrinology. 1973;12:179–91. doi: 10.1159/000122167. [DOI] [PubMed] [Google Scholar]
- Sun N, Cassell MD. Intrinsic GABAergic neurons in the rat central extended amygdala. Journal of Comparative Neurology. 1993;330:381–404. doi: 10.1002/cne.903300308. [DOI] [PubMed] [Google Scholar]
- Swanson LW. Brain Maps: Structure of the Rat Brain. Amsterdam: Elsevier Science; 1998. [Google Scholar]
- Toufexis DJ, Walker CD. Noradrenergic facilitation of the adrenocorticotropin response to stress is absent during lactation in the rat. Brain Research. 1996;737:71–7. doi: 10.1016/0006-8993(96)00627-0. [DOI] [PubMed] [Google Scholar]
- Toufexis DJ, Rochford J, Walker CD. Lactation-induced reduction in rats’ acoustic startle is associated with changes in noradrenergic neurotransmission. Behavioral Neuroscience. 1999;113:176–184. doi: 10.1037//0735-7044.113.1.176. [DOI] [PubMed] [Google Scholar]
- Toufexis DJ, Tesolin S, Huang N, Walker C. Altered pituitary sensitivity to corticotropin-releasing factor and arginine vasopressin participates in the stress hyporesponsiveness of lactation in the rat. Journal of Neuroendocrinology. 1999;11:757–756. doi: 10.1046/j.1365-2826.1999.00381.x. [DOI] [PubMed] [Google Scholar]
- Toufexis DJ, Thrivikraman KV, Plotsky PM, Morilak DA, Huang N, Walker CD. Reduced noradrenergic tone to the hypothalamic paraventricular nucleus contributes to the stress hyporesponsiveness of lactation. Journal of Neuroendocrinology. 1998;10:417–427. doi: 10.1046/j.1365-2826.1998.00223.x. [DOI] [PubMed] [Google Scholar]
- Treit D, Aujla H, Menard J. Does the bed nucleus of the stria terminalis mediate fear behaviors? Behavioral Neuroscience. 1998;112:379–86. doi: 10.1037//0735-7044.112.2.379. [DOI] [PubMed] [Google Scholar]
- Tsukamura H, Maeda K. Non-metabolic and metabolic factors causing lactational anestrus: rat models uncovering the neuroendocrine mechanism underlying the suckling-induced changes in the mother. Progress in Brain Research. 2001;133:187–205. doi: 10.1016/s0079-6123(01)33014-5. [DOI] [PubMed] [Google Scholar]
- Turgeon SM, Anderson N, O’Loughlin K. Phencyclidine (PCP) produces sexually dimorphic effects on voluntary sucrose consumption and elevated plus maze behavior. Pharmacology Biochemistry and Behavior. 2010;95:173–178. doi: 10.1016/j.pbb.2010.01.001. [DOI] [PubMed] [Google Scholar]
- Vasa RA, Pine DS, Masten CL, Vythilingam M, Collin C, Charney DS, et al. Effects of yohimbine and hydrocortisone on panic symptoms, autonomic responses, and attention to threat in healthy adults. Psychopharmacology. 2009;204:445–455. doi: 10.1007/s00213-009-1475-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventura-Silva AP, Pêgo JM, Sousa JC, Marques AR, Rodrigues AJ, Marques F, Cerqueira JJ, Almeida OF, Sousa N. Stress shifts the response of the bed nucleus of the stria terminalis to an anxiogenic mode. European Journal of Neuroscience. 2012;36:3396–406. doi: 10.1111/j.1460-9568.2012.08262.x. [DOI] [PubMed] [Google Scholar]
- Waddell J, Morris RW, Bouton ME. Effects of bed nucleus of the stria terminalis lesions on conditioned anxiety: aversive conditioning with long-duration conditional stimuli and reinstatement of extinguished fear. Behavioral Neuroscience. 2006;120:324–336. doi: 10.1037/0735-7044.120.2.324. [DOI] [PubMed] [Google Scholar]
- Walker DL, Davis M. Double dissociation between the involvement of the bed nucleus of the stria terminalis and the central nucleus of the amygdala in startle increases produced by conditioned versus unconditioned fear. Journal of Neuroscience. 1997;17:9375–9383. doi: 10.1523/JNEUROSCI.17-23-09375.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wartella J, Amory E, Lomas LM, Macbeth A, McNamara I, Stevens L, Lambert KG, Kinsley CH. Single or multiple reproductive experiences attenuate neurobehavioral stress and fear responses in the female rat. Physiology & Behavior. 2003;79:373–81. doi: 10.1016/s0031-9384(03)00150-1. [DOI] [PubMed] [Google Scholar]
- Windle RJ, Brady MM, Kunanandam T, Da Costa AP, Wilson BC, et al. Reduced response of the hypothalamo-pituitary-adrenal axis to alpha1-agonist stimulation during lactation. Endocrinology. 1997;138:3741–8. doi: 10.1210/endo.138.9.5405. [DOI] [PubMed] [Google Scholar]
- Winter JC, Rabin RA. Yohimbine as a serotonergic agent: evidence from receptor binding and drug discrimination. Journal of Pharmacology and Experimental Therapeutics. 1992;263:682–689. [PubMed] [Google Scholar]
- Woodhams PL, Roberts GW, Polak JM, Crow TJ. Distribution of neuropeptides in the limbic system of the rat: the bed nucleus of the stria terminalis, septum and preoptic area. Neuroscience. 1983;8:677–703. doi: 10.1016/0306-4522(83)90003-9. [DOI] [PubMed] [Google Scholar]
- Woulfe JM, Hrycyshyn AW, Flumerfelt BA. Collateral axonal projections from the A1 noradrenergic cell group to the paraventricular nucleus and bed nucleus of the stria terminalis in the rat. Experimental Neurology. 1988;102:121–124. doi: 10.1016/0014-4886(88)90084-2. [DOI] [PubMed] [Google Scholar]
- Woulfe JM, Flumerfelt BA, Hrycyshyn AW. Efferent connections of the A1 noradrenergic cell group: a DBH immunohistochemical and PHA-L anterograde tracing study. Experimental Neurology. 1990;109:308–322. doi: 10.1016/s0014-4886(05)80022-6. [DOI] [PubMed] [Google Scholar]
- Wright IK, Heaton M, Upton N, Marsden CA. Comparison of acute and chronic treatment of various serotonergic agents with those of diazepam and idazoxan in the rat elevated X-maze. Psychopharmacology. 1992;107:405–414. doi: 10.1007/BF02245168. [DOI] [PubMed] [Google Scholar]
- Yassa MA, Hazlett RL, Stark CE, Hoehn-Saric R. Functional MRI of the amygdala and bed nucleus of the stria terminalis during conditions of uncertainty in generalized anxiety disorder. Journal of Psychiatric Research. 2012;46:1045–1052. doi: 10.1016/j.jpsychires.2012.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young BJ, Cook CJ. Stress-induced modification of anxiety in rats is dependent on reproductive status. Physiology and Behavior. 2004;80:569–575. doi: 10.1016/j.physbeh.2003.10.014. [DOI] [PubMed] [Google Scholar]