Abstract
The human kidneys filter 180 liters of blood every day via about 2.5 million glomeruli. The three layers of the glomerular filtration apparatus consists of fenestrated endothelium, specialized extracellular matrix known as the glomerular basement membrane (GBM) and the podocyte foot processes with their modified adherens junctions known as the slit diaphragm (SD). In this study we explored the contribution of podocyte β1 integrin signaling for normal glomerular function. Mice with podocyte specific deletion of integrin β1 (podocin-Cre β1-fl/fl mice) are born normal but cannot complete post-natal renal development. They exhibit detectable proteinuria on day 1 and die within a week. The kidneys of podocin-Cre β1-fl/fl mice exhibit normal glomerular endothelium but show severe GBM defects with multi-laminations and splitting including podocyte foot process effacement. The integrin linked kinase (ILK) is down-stream mediators of integrin β1 activity in epithelial cells. To further explore whether integrin β1-mediated signaling facilitates proper glomerular filtration, we generated mice deficient of ILK in the podocytes (podocin-Cre ILK-fl/fl mice). These mice develop normally but exhibit post-natal proteinuria at birth and die within 15 weeks of age due to renal failure. Collectively, our studies demonstrate that podocyte β1 integrin and ILK signaling is critical for post-natal development and function of the glomerular filtration apparatus.
Keywords: beta1 integrin, ILK, glomerular basement membrane, podocyte, slit diaphragm, filtration barrier, Podocin Cre, permselectivity, proteinuria, FAK
Introduction
The glomerular endothelium, the GBM and podocyte foot processes/slit diaphragm are three distinct components of the filtration apparatus of the kidney. Structural and functional insufficiency of podocytes is implicated as a key determinant in the pathogenesis of several nephritic glomerular diseases including focal segmental glomerulosclerosis (FSGS). Podocytes are highly differentiated and polarized cells characterized by actin-rich foot processes that likely interact with the glomerular basement membrane (GBM) and play an important role in the filtration properties of the glomerulus. Previous studies have suggested that α3β1 integrin mediated interaction of podocytes with the GBM is necessary for proper function of the glomerulus (Kreidberg et al., 1996).
Integrin β1 and the associated integrin linked kinase (ILK) play a crucial role in cell survival, tissue homeostasis and carcinogenesis (Hannigan et al., 2005; Legate et al., 2006). Integrin β1 forms at least 12 different kinds of integrins via their binding to different α chains of the integrin family (Brakebusch et al., 2002; Hynes, 2002). Glomerular podocytes express integrin α3β1 and some data suggests that this integrin facilitates binding to laminin in the GBM (Baraldi et al., 1994). Decreased expression of α3β1 integrin is demonstrated in human diabetic kidney disease, FSGS and several animal models of experimental glomerulonephritis (Chen et al., 2006; Regoli and Bendayan, 1997). Integrin β1 binds to the ILK via its cytoplasmic domain (Hannigan et al., 1996). Integrin-activated ILK induces anti-apoptotic signals (Hannigan et al., 2005; Legate et al., 2006). Such observations suggest that ILK mediated signaling via integrin β1 is likely important for podocyte function.
In this report we demonstrate that specific deletion of integrin β1 in the podocytes of mice (podocin-Cre β1-fl/fl mice) leads to post-natal death with massive proteinuria and podocyte defects. GBM in the new born podocin-Cre β1-fl/fl mice exhibit many structural defects including multi-laminations and splitting. Next, we demonstrate that specific deletion of ILK in podocytes (podocin-Cre ILK-fl/fl mice) leads to proteinuria starting at birth, the development of glomerulosclerosis by 8 weeks of age, and death due to renal failure by 15 weeks of age. Collectively, these results further demonstrate a critical role for podocyte-GBM interactions mediated by β1 integrin and ILK in the normal assembly of the GBM and also proper function of the glomerular filtration apparatus.
Material and methods
Animals
Podocin-Cre mice were kindly provided by Dr. Jordan A. Kreidberg, at the Children's Hospital, Boston. β1 integrin flox mice were purchased from the Jackson Laboratory (Bar Harbor, ME). ILK-flox mice were kindly provided by Dr. Shoukat Dedhar and Dr. Robert Gerszten. For podocyte specific deletion of the target gene, podocin-Cre positive/heterozygous (flox/wt) mice were mated with homozygous (flox/flox) mice. Mice were maintained at the Beth Israel Deaconess Medical Center animal facility under standard conditions. All animal studies were reviewed and approved by the animal care and use committee of Beth Israel Deaconess Medical Center.
Antibodies
Hamster anti-mouse integrin β1,β3 and rat anti-mouse integrin α3 antibody, anti-CD31-FITC conjugated antibody and anti-fibronectin monoclonal antibody were purchased from Becton Dickinson (Franklin Lakes, NJ). The polyclonal anti-podocin antibody was a gift from Dr. Peter Mundel, Mount Sinai School of Medicine, New York. The polyclonal anti-nephrin and anti-COL4A3, -COL4A4 and -COL4A5 antibodies were previously described (Sugimoto et al., 2006). Rat anti-laminin β1, β2 and anti-entactin antibodies were purchased from CHEMICON international (Temecula, CA). Anti-WT1 and anti-YFP polyclonal antibodies are purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Anti-phospho-FAKTyr397 for immunofluorescence and anti-actin antibodies were purchased from Sigma Aldrich (St. Louis, MI). Anti-phospho-FAKTyr397 for both western blot and immunofluorescence was from Invitrogen (Carlsbad, CA). The rabbit polyclonal antibody against total FAK was purchased from Upstate Biotechnology (Lake Placid, NY).
Immunofluorescence
Immunofluorescence was performed as previously described (Hamano et al., 2003). Briefly, frozen sections were fixed in 100% acetone at -20 °C for 10 minutes. After blocking, sections were incubated with primary antibodies for 1 hour at room temperature and subsequently labeled with secondary antibodies (Jackson Immunoresearch, West Grove, PA).
SDS page and western blot analysis
Samples (1μl of urine samples or purified protein with lysis buffer (Tris 50mM PH7.5, NaCl 0.15M, SDS 0.1%, Triton X-100 1%, Deoxycholate 1% with protease inhibitor) from outer cortex of kidney) were denatured with SDS-sample buffer in boiling water. Denatured samples were separated on 8 or 10% SDS-polyacrylamide gels and blotted onto polyvinylidene fluoride (PVDF) membranes (Immobilon, Bedford, MA) by semi-dry method. The transferred protein was visualized with Coomassie Brilliant Blue (CBB). After blocking with TBS-T (Tris-buffered saline, 0.05% Tween 20) containing 5% non-fat milk, the membranes were incubated with anti-WT1 (1:500 diluted), anti-podocin (1:2000), anti-phospho FAKTyr397 (1:500), anti-total-FAK (1:1000), or anti-actin (1:1000) antibodies room temperature 1 hour (anti-WT1 and anti-podocin) or 4°C over night (others). The membranes were washed three times and incubated with 1:10000 diluted horseradish peroxide (HRP)-conjugated anti-rabbit secondary antibody (Promega, Seattle, WA) at room temperature for 1 hour. The immunoreactive bands were detected with an enhanced chemiluminescence (ECL) detection system (Pierce Biotechnology, Rockford, IL).
Electron microscopy
Kidney tissues were fixed in 0.1M cacodylate acid with 2% glutaraldehyde. Electron microscopy (transmission electron microscopy: TEM and scanning electron microscopy: SEM) was performed as previously described (Sugimoto et al., 2006).
Detection of LacZ expression
Kidney samples (1 mm2) from 6 week old R26Rstop LacZ flox mice (Mao et al., 1999) with or without podocin-Cre were fixed at 4 °C for 4 hours in 4 % paraformaldehyde. Samples were washed 3 times with PBS pH 7.3 and then stained overnight at 37 °C with LacZ staining buffer (1mg/ml X-gal, 35 mM potassium ferrocyanide, 35 mM potassium ferricyanide, 2 mM MgCl2, 0.02% NP-40, 0.01% Na deoxycholate in PBS). After washing with PBS pH 7.3, samples were embedded into paraffin. Sections (10 μm) were then deparaffinized and counter stained with eosin.
Detection of yellow fluorescence protein (YFP) expression
For the YFP staining, kidneys were pre-fixed over night with 4% PFA, and then samples were allowed to equilibrate in 30% sucrose in PBS. The fixed samples were mounted in OCT mounting media. The frozen sections were then blocked directly with 2% BSA PBS and then incubated with 1:200 diluted anti-YFP antibody followed by rhodamine-conjugated secondary antibody. The detection was performed by immunofluorescence microscopy.
Results
Podocin specific gene deletion
To demonstrate podocin specific gene deletion by Cre-recombinase, R26Rstop LacZ flox mice were mated with podocin-Cre mice. In the progeny, Cre-recombinase activates the LacZ reporter gene by the excision of a stop codon between two loxP sites. LacZ labeling reveals that LacZ Rosa-flox/podocin-Cre mice specifically express LacZ in the glomerular podocytes (Fig. 1A) when compared to control mice (Fig. 1B). As a further validation of this expression pattern, we also performed similar experiments with R26Rstop EYFP floxed mice (Srinivas et al., 2001). As in R26Rstop LacZ floxed mice, EYFP reporter gene is induced by the excision of a stop codon in the presence of Cre-recombinase in the R26Rstop EYFP flox mice. Immunofluorescence analysis clearly reveals that podocytes in EYFP Rosa-flox/podocin-Cre mice express YFP protein (Fig. 1 C and D). These results indicate specific activity of podocin promoter driven Cre-recombinase in glomerular podocytes.
Fig. 1. Podocyte specific expression of Cre-recombinase.
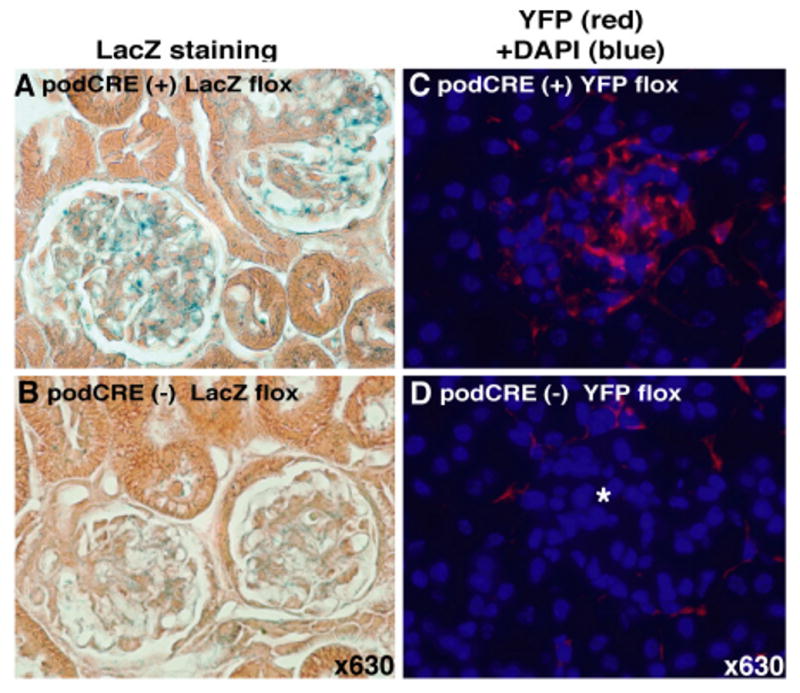
(A-B) LacZ staining for ROSA flox mice with (podCRE(+) LacZ flox) or without (podCRE(-)) the podocin-Cre transgene, clearly shows podocyte specific expression of LacZ. (C-D) YFP expression in EYFP Rosa-flox/podocin-Cre mice (podCre (+) YFP flox). Protein expression of YFP was detected with anti-YFP antibody followed by Rhodamine conjugated secondary antibody. Merged images (Rhodamine plus DAPI) are shown. Star in picture (D) indicates glomerulus.
Renal dysfunction in podocin-Cre β1-fl/fl mice at birth
The expression of β1 integrin in glomerular podocytes and capillary walls is absent in podocin-Cre β1-fl/fl mice (Fig. 2, A and B). The α3 integrin associates with β1 integrin in glomerular podocytes to form integrin α3β1 (Baraldi et al., 1994). Immunofluorescence analysis reveals that β1 integrin deletion in the glomerular podocytes results in significant loss of α3 integrin expression in the podocytes, with some expression till observed on non-podocyte cells types such as the endothelial cells (Fig. 2C). In the control mice, α3 integrin expression is noted in the podocytes and also possibly the endothelial area (Fig. 2D). In this regard, integrin α3β1 expression has also been demonstrated in the endothelial cells (Yanez-Mo et al., 1998). The podocin-Cre β1-fl/fl mice are born in an expected Mendelian ratio but do not survive past 1 week of post-natal age (Fig. 2E). The podocin-Cre β1-fl/fl mice exhibit significant proteinuria starting on day 1 after birth (P1) (Fig. 2F). Additionally, Wilm's tumor protein-1 (WT1) and podocin, podocyte specific protein in the kidney, were detected specifically in the urine of podocin-CRE β1-fl/fl mice by western blot analysis (Fig. 2G). Histological analysis by light microscopy of the kidney tissue reveals glomerular disease with defective association of podocyte foot process with the GBM in podocin-Cre β1-fl/fl mice (Fig. 2, H and I). Interestingly, such lesions are not found in embryonic kidneys of podocin-Cre β1-fl/fl mice (data not shown). These results suggest that podocyte defects in the podocin-Cre β1-fl/fl mice first emerge at birth but not during pre-natal development, which eventually leads to death within the first week of post-natal life. All other tissues examined in these mice were normal (data not shown).
Fig. 2. Podocin-Cre/β1-fl/fl mice exhibit proteinuria with poor prognosis.
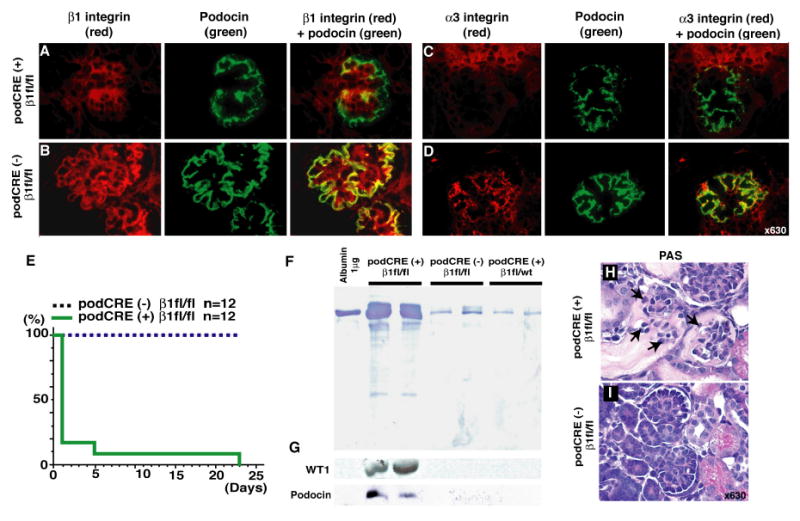
(A-B) β1 integrin expression in podocyte. Merged images (with podocin) clearly shows a lack of β1 integrin expression in podocytes of the glomerulus in podocin-Cre β1-fl/fl mice (podCRE(+) β1fl/fl). (C-D) α3 integrin expression in glomerulus. In podocin-Cre β1-fl/fl mice, markedly decreased expression of α3 integrin was observed. Representative results from 3 mice in each group are shown. The kidney samples from post-natal day1 (P1) mice are used. (E) Survival curve of podocin-Cre β1-fl/fl mice and control mice. (F) Urinary protein excretion at P1 in podocin-Cre β1-fl/fl mice by SDS-page. (G) Detection of WT1 and podocin protein in the urine by western blot analysis using the same membrane as panel (F). (H-I) Periodic Acid Schiff (PAS) staining of kidney from P1 mice. Arrows in panel (H) indicate defective association of podocyte foot process with the GBM. Representative picture from 5 mice in each group are shown.
Loss of podocyte foot processes in podocin-Cre β1-fl/fl mice
To obtain further insight into the renal pathology associated with the podocin-Cre β1-fl/fl mice, we evaluated the expression of several podocyte slit diaphragm associated proteins and glomerular matrix components by indirect immunofluorescence. In the podocin-Cre β1-fl/fl mice, nephrin and podocin expression was weak and patchy when compared to the littermate control mice at P1 (podocin-Cre (-) β1-fl/fl mice) (Fig. 3, A-D). GBM components, such as collagen type IV α3, α4, α5 chains (Fig. 3, E-J) were localized mainly in the GBM of the podocin-Cre β1-fl/fl mice, whereas laminin β1 chain which normally expresses in the mesangium of the normal matured glomeruli, expresses in the GBM of podocin-Cre β1-fl/fl mice (Fig. 3, K and L), suggesting that matrix assembly and maturation of GBM is disrupted in podocin-Cre β1-fl/fl mice. Laminin β2 expresses with normal pattern in the GBM of all groups of mice (Fig. 3, M and N). The expression and distribution of entactin and endothelial marker CD31 reveal insignificant difference in all groups of mice (Fig. 3, O-R). To evaluate compensation by other integrins on the podocytes due to the absence of integrin β1, we evaluated the distribution of integrin β3. Integrin β3 is normally expressed on the glomerular endothelial cells, whereas, in the podocin-Cre β1-fl/fl mice integrin β3 expression is also observed on the podocyte (Fig.3, S and T).
Fig. 3. Podocyte slit diaphragm proteins and basement membrane components expression in the podocin-Cre β1-fl/fl mice.

Immunofluorescence analysis for (A-B) Nephrin, (C-D) Podocin, (E-J) Collagen typeIV α3-5, (K-N) Laminin β1-2, (O-P) Entactin, (Q-R) CD31. Laminin β1 are localized distinctly along GBM in podocin-Cre β1-fl/fl mice. The representative results of podocin-Cre β1-fl/fl mice (podCRE(+) β1fl/fl) and control (podCRE(-) β1fl/fl) from 3 mice in each group are shown. The kidney samples from P1 mice are used. (S-T) Compensatory upregulation of integrin β3 in podocytes of podocin-Cre β1-fl/fl mice. The representative results from 2 embryos (E 18.5) in each group are shown.
Ultrastructural analysis of the glomerulus by TEM reveals a multi-laminated GBM with extensive splitting and abnormal assembly (Fig. 4, A and B). Lower number of mature foot processes was detected in the podocin-Cre β1-fl/fl mice by SEM analysis (Fig. 4, C and D). These results indicate that deletion of integrin β1 in the glomerular podocyte results in podocyte insufficiency and also in the emergence of abnormal GBM. This observation prompted us to further explore the role of integrin β1 mediated signaling in the normal function of the kidney glomerulus. Hence, we next focused on the role of ILK in the glomerular podocyte.
Fig. 4. Ultrastructural analysis of the glomerulus in the podocin-Cre β1-fl/fl mice.
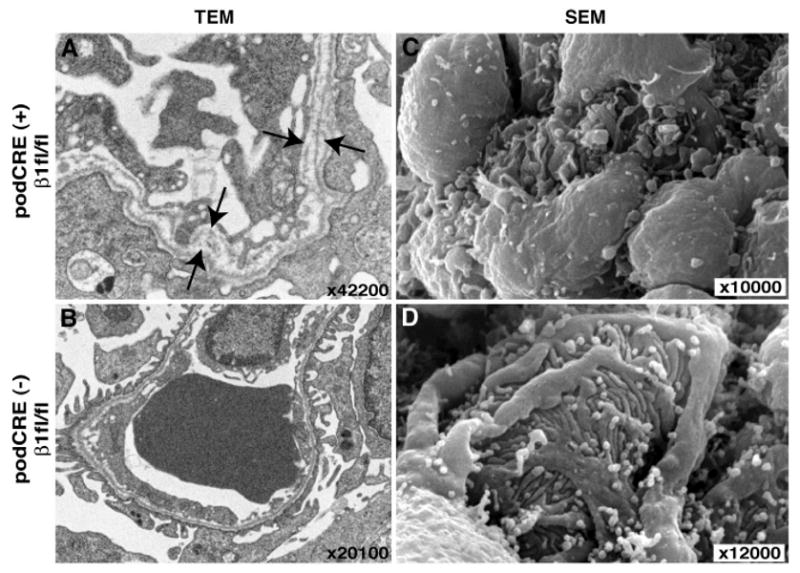
(A-B) Transmission electron microscopy (TEM) analysis. Arrows in panel (A) show severe multilamination in podocin-Cre β1-fl/fl mice. All of GBM in the glomerulus of podocin-Cre β1-fl/fl showed multi-lamination in GBM. (C-D) Scanning electron microscope (SEM) analysis. In panel (C), formation of podocyte foot process was severely disrupted in podocin-Cre β1-fl/fl mice. The representative results of podocin-Cre β1-fl/fl mice (podCRE(+) β1fl/fl) and control (podCRE(-) β1fl/fl) from 2 mice in each group are shown. The kidney samples from P1 mice were used here.
Podocin-Cre ILK-fl/fl mice develop focal segmental glomerulosclerosis
ILK binds directly to the intracellular domain of integrin β1 and mediates outside-in signaling to facilitate cell survival (Hannigan et al., 1996). To evaluate the role of integrin β1 mediated intracellular signaling in the podocytes, we deleted ILK in glomerular podocytes. The podocin-Cre ILK-fl/fl mice exhibit proteinuria at birth and die between the age of 4 and 15 weeks (Fig. 5A). By gross examination, starting at 4 weeks of age, the kidneys look small and pale (Fig. 5B). Proteinuria is detected from P1 (Fig. 5C) and increases with time until their death at 15 weeks of age (Fig. 5D). From 1 week of age, WT1 protein was detected in the urine of podocin-Cre ILK-fl/fl mice (Fig. 5E). Podocin, a podocyte specific protein in the kidney, was also detected in the urine of podocin-Cre ILK-fl/fl mice starting from 2 weeks of age (Fig. 5E).
Fig. 5. Focal segmental glomerulosclerosis in the kidneys from podocin-CRE ILK-fl/fl mice.
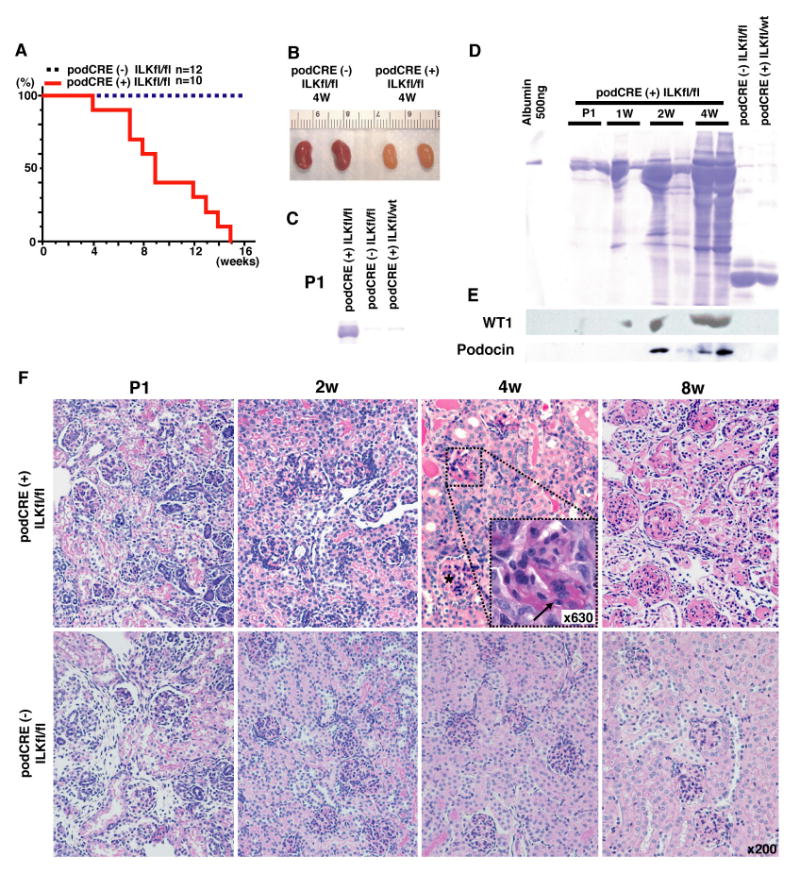
(A) Survival curve of podocin-Cre ILK-fl/fl mice (podCRE(+) ILKfl/fl) and control mice (podCRE(-) ILKfl/fl). (B) Gross appearance at 4 weeks of age podocin-Cre ILK-fl/fl mice or control mice. podocin-Cre ILK-fl/fl mice have pale and atrophic kidneys compared to control mice. (C) Urinary albumin excretion at post natal (P1) in podocin-Cre ILK-fl/fl mice. (D) Progressive urinary albumin excretion in podocin-Cre ILK-fl/fl mice. (E) Detection of WT1 and podocin protein in urine by western blot analysis on same membrane as panel (D). (F) Progressive focal segmental glomerulosclerosis in podocin-Cre ILK-fl/fl mice. At 4 weeks of age, progressive FSGS with interstitial alteration was developed in podocin-Cre ILK-fl/fl mice. The arrow in the panel of 4 weeks aged podocin-Cre ILK-fl/fl mice showed segmental lesions (×630). Star in same panel shows normal glomerulus. The representative results of podocin-Cre ILK-fl/fl mice and control from 3 mice in each group are shown.
Histological analysis of podocin-Cre ILK-fl/fl mice by light microscopy shows normal kidneys at birth, but starting at 4 weeks of age, progressive focal segmental glomerulosclerosis with associated tubulo-interstitial damage is observed (Fig. 5F). At 2 weeks of age, the expression of slit diaphragm proteins nephrin and podocin is normal in the podocin-Cre ILK-fl/fl mice when compared to the littermate control mice (podocin-Cre (-) ILK-fl/fl mice) (Fig. 6, A-D). The expression of type IV collagen is the same in all groups of mice (Fig. 6, E-J). Laminin β1, which is expressed in the mesangial area but absent in the GBM of the control mice, is significantly induced in the GBM of the podocin-Cre ILK-fl/fl mice (Fig. 6, K and L), while the expression of laminin β2 is the same in all groups of mice (Fig. 6, M and N). Entactin expression is also the same in all groups of mice (Fig. 6, O and P). Endothelial marker CD31 was decreased in podocin-Cre ILK-fl/fl mice (Fig. 6Q), compared with control mice (Fig. 6R).
Fig. 6. Podocyte slit diaphragm proteins and basement membrane components expression in the podocin-Cre ILK-fl/fl mice.

Immunofluorescence analysis for (A-B) Nephrin, (C-D) Podocin, (E-J) Collagen type IV α3-5, (K-N) Laminin β1-2, (O-P) Entactin, (Q-R) CD31. (S-T) Fibronectin (red) co-stained with WT1 (green). In panel (K-L), it is clear that laminin β1 is localized in GBM in podocin-Cre ILK-fl/fl mice but not in the control mice. The representative results of podocin-Cre ILK-fl/fl mice (podCRE(+) ILKfl/fl) and control (podCRE(-) ILKfl/fl) from 3 mice in each group are shown. The kidney samples from 2 weeks old mice were used here (A-T). (U-V) TEM analysis in P1 mice. podocin-Cre ILK-fl/fl mice shows occasional foot process effacement. (W-X) TEM analysis for mice 2 weeks of age. Nodular thickening (arrow heads in panel (W)) in the GBM of podocin-Cre ILK-fl/fl mice are apparent. (Y-Z) SEM analysis for mice 2 weeks of age. Microvillous formations in podocin-CRE ILK-fl/fl mice are present. The representative results of podocin-Cre ILK-fl/fl mice (podCRE(+) ILKfl/fl) and control (podCRE(-) ILKfl/fl) from 2 mice in each group are shown.
Fibronectin accumulates during the progression of glomerulosclerosis (Sharma and Ziyadeh, 1994). Fibronectin protein expression was induced along the glomerular capillary walls in podocin-Cre ILK-fl/fl mice (Fig. 6S) but not in the control mice (Fig. 6T). Ultrastructural analysis reveals that podocin-Cre ILK-fl/fl mice exhibit occasional effacement of glomerular foot processes at P1 (Fig. 6, U and V). There was insignificant difference between the GBM structure of podocin-Cre ILK-fl/fl mice and control mice at P1 (Fig. 6, U and V). However, at 2 weeks of age, podocin-Cre ILK-fl/fl mice exhibit significant nodular thickening of the GBM, when compared to control mice (Fig. 6, W and X). Scanning electron microscopy analysis of the glomeruli shows microvillous formation in podocin-Cre ILK-fl/fl mice (Fig. 6, Y and Z).
Focal adhesion kinase (FAK) activation in podocin-CRE ILK-fl/fl mice
FAK controls actin reorganization and cell-cell or cell-matrix adhesion downstream of integrin β1 (Dedhar and Hannigan, 1996). Therefore, we evaluated FAK activation in podocytes with disrupted ILK signaling. Immunofluorescence study reveals that phosphorylated FAKTyr397 is induced and elevated in the glomerulus, including the podocytes, of the podocin-Cre ILK-fl/fl mice when compared to control mice (Fig. 7, A-E). Such increased phoshorylation of FAK is not observed in podocin-CRE β1-fl/fl mice (data not shown). Also, total FAK expression is also increased in the podocytes of podocin-Cre ILK-fl/fl mice (Fig. 7, F and G). Western blot analysis using the samples from outer cortex of kidney reveals increased protein expression of FAK and phosphorylated FAKTyr397 in podocin-Cre ILK-fl/fl mice (Fig. 7H), suggesting that the deletion of ILK in podocyte results in the induction of compensation adhesion pathway by integrin-FAK system in the kidney glomerulus. These observations suggest that loss of ILK results in compensatory upregulation of FAK activation but this action does not protect the kidney from glomerular dysfunction and failure.
Fig. 7. Activation of integrin β1-FAK signaling in podocin-Cre ILK-fl/fl mice.

Phospho- FAKTyr397 is induced in podocin-Cre ILK-fl/fl mice (A and D) compared to control mice (B and E). In panel (C), negative control was shown (secondary antibody only without primary antibody in podocin-Cre ILK-fl/fl mice). The primary antibodies from Sigma (#1) or from Invitrogen (#2) are used in panel (A-B) or (D-E), respectively. (F-G) Total FAK expression is also increased in the podocin-Cre ILK-fl/fl mice. Enlarged image in panel (F) clearly indicates increased expression of FAK in podocytes. Merged image with entactin are also shown for indicating the glomerlar structure (D-G). (H) Western blot anaysis for phosphorylated FAKTyr397 and total-FAK. Podocin is shown as the control for glomerular proteins. Actin and Coomassie Brilliant Blue staining are shown as the loading control. (I-L) Integrin β1 and α3 expressions in the glomerulus in the podocin-Cre ILK-fl/fl mice. (I-J) Integrin β1 is localized in mesangial area, endothelial cell and capillary walls. There is no apparent difference in the expression of integrin β1 between podocin-Cre ILK-fl/fl and control mice. (K-L) Compared to control mice, integrin α3 is occasionally upregulated in capillary walls of glomerulus in podocin-Cre ILK-fl/fl mice. The representative results of podocin-Cre ILK-fl/fl mice (podCRE(+) ILKfl/fl) and control (podCRE(-) ILKfl/fl) from 3 mice in each group are shown. The samples from 2 weeks old mice are used (A-L).
Previous reports suggests that conditional deletion of ILK in the skin and heart results in the decreased expression of integrin β1 (Lorenz et al., 2007; White et al., 2006), indicating the presence of inside-out regulatory pathway connecting the ILK- integrin β1 system. Therefore, we evaluated the expression of integrin β1 and α3 in the podocin-Cre ILK-fl/fl mice. Immunofluorescence study reveals that expression of integrin β1 is not altered in podocin-Cre ILK-fl/fl mice when compared to control mice (Fig. 7, I and J), while integrin α3 are upregulated and deposits granularly in the capillary wall of glomeruli in podocin-Cre ILK-fl/fl mice (Fig. 7, K and L), similar to the previous report (El-Aouni et al., 2006).
Discussion
Fetal renal function is characterized by low glomerular filtration rate (GFR) and is regulated by low blood pressure (low mean arterial pressure), low renal blood flow and high renal vascular resistance (Toth-Heyn et al., 2000). Upon birth, the neonate kidney is immediately subjected to a relatively higher blood pressure resulting in a higher GFR. Our study suggests that the deletion of integrin β1 and ILK in podocytes does not result in any defects in pre-natal kidney development, but immediately after birth the kidneys of these mice cannot keep up with the increased filtration needs. It is likely that such increased intra-glomerular microcirculation and resultant pressure after birth leads to podocyte detachment from the capillary wall causing proteinuria. Interestingly, our results also demonstrate that such podocyte/slit diaphragm defects are accompanied by significant structural defects in the GBM and matrix assembly. GBM is contributed by the glomerular endothelial cells and also the podocytes during kidney development as two separate layers (Abrahamson, 1987; McCarthy, 1997). Ultimately, these two layers fuse to form a mature GBM (Abrahamson, 1987; McCarthy, 1997). In the podocin-CRE β1-fl/fl mice, the GBM remains as two layers and in many areas it is further disrupted.
Laminins and type IV collagens are important basement membrane constituents that bind to integrins containing the β1 chain (Miner and Yurchenco, 2004). Embryoid bodies that lack integrin β1 cannot form heterotrimeric laminins due to an absence of the α1 and α5 laminin chain recruitment into the assembled basement membrane (Aumailley et al., 2000; Miner and Yurchenco, 2004). Several different experiments from the Yurchenco group have demonstrated that integrin β1 is required for the proper assembly of basement membranes (Li and Yurchenco, 2006). Integrin β1 facilitates the recruitment of laminin initially and then possibly type IV collagen to organize the basement membrane structure at the basolateral aspect of the cell. Podocytes express integrin α3β1 and bind to laminin-10 and laminin-11 in the GBM (Miner and Yurchenco, 2004). Laminin β1 is localized in GBM of developing glomeruli but not in mature glomeruli (Abrahamson et al., 2003). In our studies, we demonstrate that laminin β1 expresses in the GBM of mature glomeruli of both mutant mice, favoring the notion that integrin β1 signaling impact GBM composition and assembly.
Deletion of integrin α3 leads to many systemic effects including kidney defects, and the mice develop proteinuria and die during the neo-natal period (Kreidberg et al., 1996), a phenotype also observed in the podocin-Cre β1-fl/fl mice. The GBM defects and podocyte abnormalities in the α3 integrin null mice are similar to the podocin-Cre β1-fl/fl mice reported here and also similar to the podocin-Cre α3 integrin fl/fl mice reported elsewhere (Sachs et al., 2006). Interestingly, the podocin-Cre α3 integrin fl/fl mice die much later than the podocin-Cre β1-fl/fl mice. Collectively, our results, in association with published reports, strongly suggest that the predominant β1 containing integrin on the podocytes is the integrin α3β1 and this integrin is crucial for post-natal development and function of the podocyte and proper assembly of the GBM.
ILK and FAK are important down stream regulators of integrin β1 action (Dedhar and Hannigan, 1996; Hannigan et al., 2005). Previous studies have demonstrated that deletion of ILK in podocytes results in GBM defects and also progressive renal disease (Dai et al., 2006; El-Aouni et al., 2006). ILK binds with α-actinin-4 and regulates slit diaphragm function (Dai et al., 2006). In these reports, deletion of ILK in the podocytes results in death starting at 10 weeks of age with most mice live up to 4-8 months of age. In our independent experiments, deletion of ILK in podocytes leads to a much more severe kidney phenotype; death is observed starting at 4 weeks of age, and all mice die by 15 weeks of age. Our results confirm that the absence of ILK also leads to GBM defects, suggesting that the role of integrin β1 in the assembly of the GBM might involve active signaling networks mediated by ILK.
In this regard, it is interesting that the podocytes of the podocin-Cre ILK-fl/fl mice reveal an increase in FAK phosphorylation but not in those of the podocin-Cre β1-fl/fl mice. Such activation of FAK in podocin-Cre ILK-fl/fl mice are opposite to observations reported for conditional deletion of ILK in heart and skin (Lorenz et al., 2007; White et al., 2006). In the skin and heart models, ILK-deletion results in decreased expression of integrin β1 (Lorenz et al., 2007; White et al., 2006), while in our study ILK-ablation in podocyte dose not alter the expression of integrin β1. Therefore, kidney might react differently from skin and heart when ILK is deleted. Such FAK compensation in the podocytes of the podocin-Cre ILK-fl/fl mice might explain why these mice live longer than the podocin-Cre β1-fl/fl mice. In this regard, systemic deletion of FAK in mice leads to a defect in mesoderm development and embryonic lethality (Ilic et al., 1995), hence, kidney function cannot be evaluated. Collectively, our results provide unequivocal proof for the crucial role of podocyte associated integrin β1 and ILK in the assembly of GBM and also podocyte function.
Acknowledgments
This work was partially supported by NIH Grants DK 55001, DK 62987, DK 13193 and DK 61688. This work was also supported by the BIDMC Department of Medicine research funds to the Division of Matrix Biology.
Footnotes
The role of integrin signaling in kidney podocytes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abrahamson DR. Structure and development of the glomerular capillary wall and basement membrane. Am J Physiol. 1987;253:F783–94. doi: 10.1152/ajprenal.1987.253.5.F783. [DOI] [PubMed] [Google Scholar]
- Abrahamson DR, Prettyman AC, Robert B, John PL. Laminin-1 reexpression in Alport mouse glomerular basement membranes. Kidney Int. 2003;63:826–34. doi: 10.1046/j.1523-1755.2003.00800.x. [DOI] [PubMed] [Google Scholar]
- Aumailley M, Pesch M, Tunggal L, Gaill F, Fassler R. Altered synthesis of laminin 1 and absence of basement membrane component deposition in (beta)1 integrin-deficient embryoid bodies. J Cell Sci. 2000;113(Pt 2):259–68. doi: 10.1242/jcs.113.2.259. [DOI] [PubMed] [Google Scholar]
- Baraldi A, Zambruno G, Furci L, Manca V, Vaschieri C, Lusvarghi E. Beta-1 integrins in the normal human glomerular capillary wall: an immunoelectron microscopy study. Nephron. 1994;66:295–301. doi: 10.1159/000187826. [DOI] [PubMed] [Google Scholar]
- Brakebusch C, Bouvard D, Stanchi F, Sakai T, Fassler R. Integrins in invasive growth. J Clin Invest. 2002;109:999–1006. doi: 10.1172/JCI15468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CA, Hwang JC, Guh JY, Chang JM, Lai YH, Chen HC. Reduced podocyte expression of alpha3beta1 integrins and podocyte depletion in patients with primary focal segmental glomerulosclerosis and chronic PAN-treated rats. J Lab Clin Med. 2006;147:74–82. doi: 10.1016/j.lab.2005.08.011. [DOI] [PubMed] [Google Scholar]
- Dai C, Stolz DB, Bastacky SI, St-Arnaud R, Wu C, Dedhar S, Liu Y. Essential role of integrin-linked kinase in podocyte biology: Bridging the integrin and slit diaphragm signaling. J Am Soc Nephrol. 2006;17:2164–75. doi: 10.1681/ASN.2006010033. [DOI] [PubMed] [Google Scholar]
- Dedhar S, Hannigan GE. Integrin cytoplasmic interactions and bidirectional transmembrane signalling. Curr Opin Cell Biol. 1996;8:657–69. doi: 10.1016/s0955-0674(96)80107-4. [DOI] [PubMed] [Google Scholar]
- El-Aouni C, Herbach N, Blattner SM, Henger A, Rastaldi MP, Jarad G, Miner JH, Moeller MJ, St-Arnaud R, Dedhar S, Holzman LB, Wanke R, Kretzler M. Podocyte-specific deletion of integrin-linked kinase results in severe glomerular basement membrane alterations and progressive glomerulosclerosis. J Am Soc Nephrol. 2006;17:1334–44. doi: 10.1681/ASN.2005090921. [DOI] [PubMed] [Google Scholar]
- Hamano Y, Zeisberg M, Sugimoto H, Lively JC, Maeshima Y, Yang C, Hynes RO, Werb Z, Sudhakar A, Kalluri R. Physiological levels of tumstatin, a fragment of collagen IV alpha3 chain, are generated by MMP-9 proteolysis and suppress angiogenesis via alphaV beta3 integrin. Cancer Cell. 2003;3:589–601. doi: 10.1016/s1535-6108(03)00133-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannigan G, Troussard AA, Dedhar S. Integrin-linked kinase: a cancer therapeutic target unique among its ILK. Nat Rev Cancer. 2005;5:51–63. doi: 10.1038/nrc1524. [DOI] [PubMed] [Google Scholar]
- Hannigan GE, Leung-Hagesteijn C, Fitz-Gibbon L, Coppolino MG, Radeva G, Filmus J, Bell JC, Dedhar S. Regulation of cell adhesion and anchorage-dependent growth by a new beta 1-integrin-linked protein kinase. Nature. 1996;379:91–6. doi: 10.1038/379091a0. [DOI] [PubMed] [Google Scholar]
- Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110:673–87. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- Ilic D, Furuta Y, Kanazawa S, Takeda N, Sobue K, Nakatsuji N, Nomura S, Fujimoto J, Okada M, Yamamoto T. Reduced cell motility and enhanced focal adhesion contact formation in cells from FAK-deficient mice. Nature. 1995;377:539–44. doi: 10.1038/377539a0. [DOI] [PubMed] [Google Scholar]
- Kreidberg JA, Donovan MJ, Goldstein SL, Rennke H, Shepherd K, Jones RC, Jaenisch R. Alpha 3 beta 1 integrin has a crucial role in kidney and lung organogenesis. Development. 1996;122:3537–47. doi: 10.1242/dev.122.11.3537. [DOI] [PubMed] [Google Scholar]
- Legate KR, Montanez E, Kudlacek O, Fassler R. ILK, PINCH and parvin: the tIPP of integrin signalling. Nat Rev Mol Cell Biol. 2006;7:20–31. doi: 10.1038/nrm1789. [DOI] [PubMed] [Google Scholar]
- Li S, Yurchenco PD. Matrix assembly, cell polarization, and cell survival: analysis of peri-implantation development with cultured embryonic stem cells. Methods Mol Biol. 2006;329:113–25. doi: 10.1385/1-59745-037-5:113. [DOI] [PubMed] [Google Scholar]
- Lorenz K, Grashoff C, Torka R, Sakai T, Langbein L, Bloch W, Aumailley M, Fassler R. Integrin-linked kinase is required for epidermal and hair follicle morphogenesis. J Cell Biol. 2007;177:501–13. doi: 10.1083/jcb.200608125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao X, Fujiwara Y, Orkin SH. Improved reporter strain for monitoring Cre recombinase-mediated DNA excisions in mice. Proc Natl Acad Sci U S A. 1999;96:5037–42. doi: 10.1073/pnas.96.9.5037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy KJ. Morphogenesis of the glomerular filter: the synchronous assembly and maturation of two distinct extracellular matrices. Microsc Res Tech. 1997;39:233–53. doi: 10.1002/(SICI)1097-0029(19971101)39:3<233::AID-JEMT4>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Miner JH, Yurchenco PD. Laminin functions in tissue morphogenesis. Annu Rev Cell Dev Biol. 2004;20:255–84. doi: 10.1146/annurev.cellbio.20.010403.094555. [DOI] [PubMed] [Google Scholar]
- Regoli M, Bendayan M. Alterations in the expression of the alpha 3 beta 1 integrin in certain membrane domains of the glomerular epithelial cells (podocytes) in diabetes mellitus. Diabetologia. 1997;40:15–22. doi: 10.1007/s001250050637. [DOI] [PubMed] [Google Scholar]
- Sachs N, Kreft M, van den Bergh Weerman MA, Beynon AJ, Peters TA, Weening JJ, Sonnenberg A. Kidney failure in mice lacking the tetraspanin CD151. J Cell Biol. 2006;175:33–9. doi: 10.1083/jcb.200603073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma K, Ziyadeh FN. The emerging role of transforming growth factor-beta in kidney diseases. Am J Physiol. 1994;266:F829–42. doi: 10.1152/ajprenal.1994.266.6.F829. [DOI] [PubMed] [Google Scholar]
- Srinivas S, Watanabe T, Lin CS, William CM, Tanabe Y, Jessell TM, Costantini F. Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC Dev Biol. 2001;1:4. doi: 10.1186/1471-213X-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugimoto H, Mundel TM, Sund M, Xie L, Cosgrove D, Kalluri R. Bone-marrow-derived stem cells repair basement membrane collagen defects and reverse genetic kidney disease. Proc Natl Acad Sci U S A. 2006;103:7321–6. doi: 10.1073/pnas.0601436103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth-Heyn P, Drukker A, Guignard JP. The stressed neonatal kidney: from pathophysiology to clinical management of neonatal vasomotor nephropathy. Pediatr Nephrol. 2000;14:227–39. doi: 10.1007/s004670050048. [DOI] [PubMed] [Google Scholar]
- White DE, Coutu P, Shi YF, Tardif JC, Nattel S, St Arnaud R, Dedhar S, Muller WJ. Targeted ablation of ILK from the murine heart results in dilated cardiomyopathy and spontaneous heart failure. Genes Dev. 2006;20:2355–60. doi: 10.1101/gad.1458906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanez-Mo M, Alfranca A, Cabanas C, Marazuela M, Tejedor R, Ursa MA, Ashman LK, de Landazuri MO, Sanchez-Madrid F. Regulation of endothelial cell motility by complexes of tetraspan molecules CD81/TAPA-1 and CD151/PETA-3 with alpha3 beta1 integrin localized at endothelial lateral junctions. J Cell Biol. 1998;141:791–804. doi: 10.1083/jcb.141.3.791. [DOI] [PMC free article] [PubMed] [Google Scholar]


