Abstract
Purpose of the Review
In this review, examples of recent progress in HIV-1 vaccine research are discussed.
Recent Findings
New insights from the immune correlates analyses of the RV144 efficacy trial have accelerated vaccine development with leads to follow in non-human primate studies and improved vaccine designs. Several new vaccine vector approaches offer promise in exquisite control of acute infection and in improving the breadth of T cell responses. New targets of broadly neutralizing antibodies (BnAbs) have been elucidated, and improved understanding of how the human host controls BnAb development have emerged from BnAb knockin mice and from analyses of BnAb maturation and virus evolution in subjects followed from the time of HIV-1 transmission to BnAb induction.
Summary
Based on these observations, it is clear that development of a successful HIV-1 vaccine will require new vaccine approaches and iterative testing of immunogens in well-designed animal and human trials.
Keywords: immune correlates, broad neutralizing antibodies, T cells, viral diversity
Introduction
After more than 30 years since the discovery of HIV-1 as the cause of AIDS, no vaccine exists for clinical use to prevent HIV-1 infection. The reasons are numerous and include the ability of HIV-1 to integrate into host DNA, the error-prone nature of HIV-1 reverse transcriptase leading to remarkably high HIV-1 diversity over time, and the host’s inability to mount antibodies to targets within conserved envelope regions that confer broad neutralization. Recent insights of ways vaccines can potentially stimulate protective T and B cell immunity, the identification of new targets for broad neutralizing antibodies (BnAbs), and the discovery of new mechanisms of host control of HIV-1 BnAb induction offer renewed hope for the development of a safe and effective preventive HIV-1 vaccine. In this review, we discuss recent progress in understanding the immune correlates of risk for HIV-1 infection and protection in both human and non-human primate vaccine trials, and discuss progress in understanding the nature of roadblocks that prevent the induction of B cell responses to conserved Env regions that are BnAb targets. Finally, the paths forward for HIV-1 vaccine development are discussed.
Immune Correlates Analysis of the RV144 Vaccine Efficacy Trial
In 2009, the RV144 phase 3 efficacy trial conducted in Thailand revealed an estimated efficacy of 31.2% with the ALVAC vCP1521-AIDSVAX B/E vaccine regimen [1]. The impetus to explore the immune correlates of risk for HIV-1 infection in this first clinical trial demonstrating efficacy led to the performance of a two-year study by an international team [2]. In 2012, two immune correlates of risk were reported [2]. First, IgG antibodies to HIV-1 Env V1-V2 correlated inversely with infection risk, raising the hypothesis that antibodies binding to the V1V2 Env region were involved in preventing infection. To investigate this further, four V2 Env monoclonal antibodies (mAbs) from RV144 vaccinees were isolated that do not neutralize HIV-1 CRF01_AE tier 2 viruses from trial breakthrough infections, but do bind to the surface of CRF01_AE tier 2 virus-infected CD4+ T cells and mediate antibody-dependent cellular cytotoxicity (ADCC) [3]. Epitope mapping and structural studies of these RV144 V2 mAbs demonstrated that they bind to the same V2 Env region (including K169) as the previously described anti-V1V2 BnAbs PG9 and CH01[4, 5]. However, unlike the V1V2 Bnabs, the RV144 V1 mAbs do not bind glycans (3). Moreover, the V2 region where RV144 mAbs bind can vary in conformation, existing as an alpha-helix in the context of RV144 V2 mabs [3] and a beta strand in the context of V1V2 BnAbs [6] (Figure 1). A molecular genetic analysis of viruses infecting RV144 vaccinees and placebos demonstrated vaccine-induced immune responses were associated with a signature in V2 (aa 169), with vaccine efficacy of 48% against viruses matching the vaccine at position 169 [7]. These studies have raised the hypothesis that one potential mechanism of protection in the RV144 trial is V2-antibody-mediated ADCC of infected CD4+ T cells [2, 3].
Figure 1.
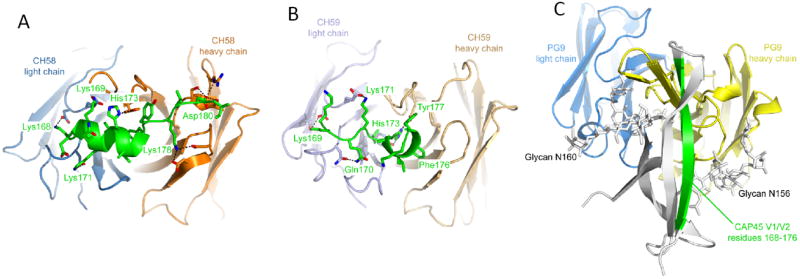
Structures of Antibodies CH58 and CH59 Bound to an HIV-1 gp120 V2 Peptide Compared to V1V2 BnAb PG9 bound to a V1V2 scaffold. RV144 vaccine-elicited antibodies CH58 and CH59 recognize alternative conformations of V2 compared to BnAb PG9.(A). Ribbon representation of the CH58 antigen-binding fragment in complex with an A244 V2 peptide as viewed end on looking at the Fab antibody conforming site. Heavy chain is colored orange, light chain is blue, and V2 peptide is green. The sequence of the peptide is shown, with modeled residues in green. The side chains of residues involved in hydrogen bonds or salt bridges are shown as sticks, with the interactions depicted as dashed lines.(B) Structure of CH59 in complex with peptide, depicted as in (A). The heavy chain is tan, and the light chain is light blue.(C) Structure of BnAb PG9 in complex with the V1V2 domain from HIV-1 strain CAP45. The PG9 structure is shown as ribbons with heavy and light chains (colored yellow and blue, respectively) in the same orientation as in (A) and (B). The V1V2 domain is shown as a gray ribbon with residues 168–176 colored green and N-linked glycans attached to residues Asn156 and Asn160 shown as sticks. Reprinted with permission from ref. [3].
Second, the RV144 immune correlates analysis demonstrated that high titer anti-Env IgA antibodies directly correlated with infection risk [2]. An association study demonstrated that in the presence of low titer anti-Env IgA antibodies, ADCC levels inversely correlated with infection risk in RV144, suggesting that anti-Env IgA antibodies may mitigate the protective effects of IgG antibodies, as well as potentially other effector activities such as CD4+ T cell helper responses [2]. Serum IgA antibodies isolated from RV144 vaccinees indeed can block ADCC activity of IgG anti-Env antibodies [8].
To better understand how the two RV144 immune correlates of infection risk may confer protection, immunoprophylaxis studies in the non-human primate vaccine challenge model are underway. In addition, future clinical efficacy trials are planned in Southern Africa with vaccine constructs similar to the RV144 regimen but with HIV-1 subtype C sequences to more closely match the circulating strains, and these trials will be important to confirm the two RV144 antibody correlates in other at-risk heterosexual populations. Since the estimated vaccine efficacy (31.2%) observed in the RV144 trials was quite low and short in duration [1], important areas of investigation are formulating Env immunogens with alternative adjuvants than alum that trigger TLR4, TLR7/8 and other innate signaling pathways to improve vaccine potency [9-11], optimizing heterologous prime-boost regimens to target the desired immune T cell and Ab responses, broadening the epitope specificities and HIV-1 subtypes recognized with mosaic antigen approaches, and discovering ways to construct Env immunogens that can generate BnAbs.
Vector-Based Vaccines
The failure of the Step trial evaluating a single-strain MRKAd5 HIV-1 gag/pol/nef vaccine dampened enthusiasm that a vaccine inducing high CD8+ T cell response rates but no Env-specific antibodies could induce immune protection against HIV-1 acquisition [11, 12]. However, recent insights have emerged from this phase 2b efficacy trial that guide decision-making moving forward. Despite the lack of efficacy, the sieve analysis of viral sequences from breakthrough strains provided evidence for selective pressure by the vaccine [13], and vaccine recipients with T cells recognizing more than two Gag epitopes pre-infection showed significantly lower plasma viremia following infection (Janes H et al, submitted). These findings suggest that induction of CD8+ T cells of certain specificities may have a beneficial effect in early control of infection, as has been recently shown in the rhesus macaque model [14]. Moreover, further analysis of the Step study indicated a time-dependent increased infection rate in uncircumcised vaccine recipients with Ad5-seropositivity prior to immunization [11, 15]. Although to date the major detectable effect of previous Ad5 immunity in vaccinees has been its blunting effect on both the innate immune response to vaccination [16] and the T cell response to HIV-1 [17], safety concerns now limit further consideration of the Ad5 vector as an HIV-1 vaccine. Other vector approaches have been pursued and show promise in the NHP vaccine model and early phase clinical trials.
Hansen et al have constructed and evaluated recombinant rhesus cytomegalovirus (RhCMV) vectors expressing the full SIV genome and shown 50% of vaccinated rhesus macaques exert complete early control against mucosal challenge with the highly pathogenic SIVmac251 [18]. Immune protection was correlated with peak levels of effector memory SIV-specific CD8+ T cells pre-challenge in the absence of SIV Env-specific antibodies. Interestingly, depletion of CD8+ or CD4+ T cells with mAbs did not lead to recrudescence of viremia, suggesting that the vaccine-induced response eliminated the viral challenge [18, 19]. While concerns exist that immunization of healthy humans with recombinant human CMV/HIV-1 vectors may cause CMV infectious complications, engineering more attenuated CMV vectors as well as other types of vectors that are safe and can induce similar cellular immune responses are key to develop.
In this regard, Barouch et al reported that rhesus macaques immunized with a heterologous vector regimen using recombinant adenovirus serotype 26 (Ad26) and modified vaccinia Ankara (MVA) expressing SIV Gag-Pol and Env, as well as an Ad35/Ad26 regimen, resulted in ≥80% reduction in per-exposure probability of infection against repetitive heterologous mucosal low-dose SHIV challenge [20]. Correlates of infection risk were total and V2-specific plasma Env antibody titers and tier 1 neutralizing antibodies. ADCC and Gag-specific interferon-secreting T cell breadth and frequencies correlated with viral control in animals who became infected [20]. Whether the protective immune responses underlying the correlates analyses in this and the RV144 trial have a common mechanism is not clear, but these studies, like the RhCMV studies above, are of interest because of the levels of protection suggested by use of these vectors.
Vaccine Insert Design to Overcome HIV-1 Diversity
One of the earliest vaccine approaches to overcome HIV-1 diversity that has undergone extensive clinical evaluation is the NIH Vaccine Research Center (VRC) DNA and rAd5 vectors. This regimen consists of a DNA prime containing clade B gag-pol-nef genes and clades A, B and C env genes, followed by a rAd5 vector expressing the same genes except nef [21, 22]. In a recent phase 2 trial of this vaccine regimen (HVTN 204), HIV-specific T cells secreting IFN-γ were seen in ~70% of vaccinees, and ~90% of subjects mounted high titer Env binding antibodies [22] and BnAbs to tier 1 clade B HIV-1 strains but not to tier 2 HIV-1 strains. However, binding antibodies directed to the homologous Env clade A V1V2 region were seen in 38% of vaccinees (Tomaras, G et al. personal communication). This vaccine regimen is currently under evaluation in a phase 2b efficacy trial (HVTN 505) in Ad5 seronegative, circumcised men in the U.S. Accrual will be completed in April 2013, and efficacy outcome revealed within the next two years. This efficacy trial is the only one testing the immune correlates hypotheses raised in the RV144 efficacy trial, and new candidate vaccine regimens designed to extend this analysis will enter clinical studies in 2015.
A number of newer vaccine constructs designed to overcome HIV-1 diversity in CD4+ and CD8+ T cell recognition include ancestral center-of-the-tree [23], consensus [24], conserved [25, 26] and mosaic approaches [27, 28]. Conserved vaccines seek to include the most conserved CD8+ cytotoxic T cell epitopes in vaccines to increase viral quasispecies coverage [29] and recent data suggest conserved T cell epitopes are more immunogenic when presented within full-length HIV-1 immunogens [30].
Mosiac vaccines are optimized for both CD4 and CD8 T cell recognition by a process of in silico homologous recombination, selecting 2-4 full gene sequences with the most conserved epitope variants of sequences annotated in the HIV-1 Los Alamos Database (www.lanl.gov), and ensuring that the joining sequences of each epitope are natural sequences [27, 28]. Comparison of mosaic and consensus immunogens for breadth and depth of T cell epitope diversity recognition has demonstrated the superiority of 2- and 3-valent mosaics over consensus immunogens [31, 32]. A conserved vaccine has already entered phase 1 testing [29], and clinical trials with mosaic HIV-1 vaccines in pox or Ad26 vectors will begin this year (B. Haynes, B. Korber, L. Baden, personal communication; D.Barouch, N. Michael and B. Korber, personal communication).
Broad Neutralizing Antibodies: Understanding Targets, Host Control, and Maturation Pathways
Recently, the HIV-1 vaccine field has extensively embraced recombinant human antibody cloning for production of human BnAbs from chronically HIV-1-infected subjects [33-35]. Improved recombinant antibody technology has combined with new methods for isolating HIV-1 Env-reactive memory B cells from antigen-specific B cell sorts [36-38], from plasma cell sorts [35, 39, 40] and from clonal memory B cell cultures [3-5]. As a result, a large number of human BnAbs have been identified that target 1 of 4 major conserved areas in the HIV-1 envelope, including 1) the gp120 CD4 binding site (CD4bs) region [41-45], 2) the membrane proximal external region (MPER) of gp41 [38, 46], and 3) two new gp120 BnAb peptide-glycan epitopes, one in the Env gp120 V1V2 loop [4-6]; and the other in the V3 region [47-49] (Figure 2). The latter BnAb group is especially potent, eliciting NHP protection from SHIV infection in passive immunoprophylaxis studies at plasma levels as low as 2 ug/ml [50].
Figure 2.
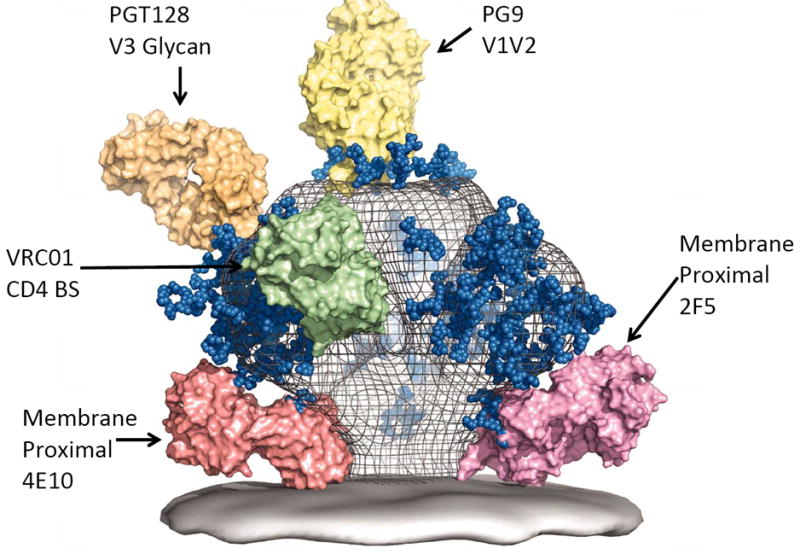
A model of the HIV-1 Env spike with select BnAbs Fab molecules bound to Env BnAb binding sites. Adapted with permission from ref. [49].
Nevertheless, a critical issue in HIV-1 vaccine development is that current vaccines do not induce BnAbs. They arise after many years of HIV-1 infection in only ~20% of subjects [51-54] and typically have more than one BnAb lineage in a given subject [55]. BnAbs may be difficult to induce by vaccination in part because carbohydrates can mask neutralizing epitopes, and immunodominant non-neutralizing Env epitopes can divert B cell responses from neutralizing epitopes (reviewed in [56]).
Early observations of two first generation BnAbs (MPER antibodies 2F5 and 4E10) revealed long heavy chain (H) third complementarity determining regions (CDR3s) and autoreactivity with non-HIV-1 antigens [57]. These findings led to the hypothesis that host tolerance mechanisms may prevent BnAb induction [57, 58]. Using 2F5 MPER BnAb homologous recombinant mice, Verkoczy et al. [59, 60] demonstrated that indeed most mAb 2F5-bearing B cells are deleted in the bone marrow and a minor cell population (~5%) survive in the periphery as anergic B cells [60]. Similar observations have been made with MPER BnAb 4E10 knock-in mice by Nemazee et al [61] and L. Verkoczy and B. Haynes (personal communication). Kelsoe et al have recently identified kynureninase (KYNU) and splicing factor 3b subunit 3 (SF3B3) as the primary high affinity autoantigens recognized by 2F5 and 4E10 BnAbs, respectively [62]. Thus, one major factor limiting some BnAb induction is tolerance deletion due to mAb autoreactivity.
Although HIV-1 antibodies from chronically infected subjects may [34] or may not [41, 46] be polyreactive, all reported BnAbs share one or more of the following unusual B cell receptor traits--polyreactivity/autoreactivity, high levels of somatic mutations, and long HCDR3s— traits that expose antibodies to tolerance control mechanisms [63] (reviewed in [56]). Thus, unusual antibodies undergoing complex maturation pathways are required to achieve binding to the four conserved areas of HIV-1 Env to which BnAbs bind. Moreover, in some cases, only one or a few VH/VL antibody pairs can be used. For example, for the VRC01-like antibodies that bind to the CD4bs in a manner similar to CD4, only VH1-02 or VH1-46 have been found in this BnAb type [41, 43]. Similarly, mAb 4E10 and Cap206-CH12 both bind to the same MPER site and use VH1-69 and Vκ3-20 even though they were derived from two separate individuals [38, 64]. Thus, despite the vast redundancy in the human B cell repertoire, for some of the BnAb sites, only a few VH and VL pairs will suffice. Moreover, these findings strongly suggest that one reason BnAbs are not readily made is that their unusual traits predispose their precursors to be limited in development, and even when allowed to develop, are in limited numbers such that the BnAb response is always subdominant to other non-neutralizing Env responses.
The full BnAb maturation pathways are being unraveled by isolation of mature antibodies from more distal BnAb clonal lineages and inferring their intermediate ancestor antibodies and the unmutated common ancestor (UCA, the putative naïve B cell receptor) using computational analysis and pyrosequencing [5, 39, 65]. One proposed reason that BnAbs are difficult to induce with immunization is that there are “holes” in the B cell germline repertoire and the BnAb UCA antibodies (naïve B cell receptors) are not available to bind Envs to drive the BnAb lineages [66-69]. However, Env constructs have been recently found to bind to VIV2 UCAs [3, 5] and gp41 MPER BnAbs [70].
Sequential virus envelopes isolated by BnAbs from SHIV-infected rhesus macaques and used as immunogens have been proposed to recreate viral evolution pathways [71]. A recent study has taken a new approach to Env immunogen design by mapping both BnAb and virus evolution from the time of transmission to development of plasma BnAb activity in the same HIV-1-infected subject [44]. The transmitted/founder virus bound well to the BnAb lineage UCA, as well as to all descendants of the BnAb clonal lineage. Moreover, the trajectory of virus evolution was mapped and a series of Env mutations were identified that developed concomitant with antibody evolution, revealing the pattern of simultaneous Env-BnAb evolution [44]. Such an analysis has permitted the precise identification of Env variants associated with induction of the BnAb lineage. A strategy has been proposed to use such data to drive otherwise unfavorable subdominant BnAb lineages to be dominant responses, termed B cell lineage immunogen design [56], by administering sequential or swarms of immunogens designed to bind optimally to each stage of the BnAb maturation pathway and to selectively induce affinity maturation in BnAb and not other non-BnAb lineages. Another proposed strategy is based on optimization of immunogens in the form of the native trimer [72]. Evidence that some Envs may be more able to induce BnAbs than others has emerged from studies demonstrating a high frequency of BnAbs in rhesus macaques infected with SHIV AD8 [73] and from immunogenicity studies indicating that transmitter/founder Envs induce greater neutralizing breadth than chronic Envs [74].
Conclusion
A highly effective HIV-1 vaccine will likely need to harness T cell and B cell immunity to protect against both virions and virus-infected cells. Progress has been made in using replicating vectors for induction of T cells that can exert early control of virus replication in non-human primates and in defining the immune correlates of infection risk in the ALVAC/AIDSVAX B/E® vaccine efficacy trial. The field eagerly awaits the results and insights from the DNA prime, rAd5 boost HVTN 505 efficacy trial, which like the RV144 trial, is unlikely to induce BnAbs. The ability to induce BnAbs remains the holy grail of HIV-1 vaccine development, invigorated by discoveries of BnAb specificities and their ability to protect in vivo at remarkably low plasma levels. However, host tolerance controls of BnAbs requires new methods of immunogen design that can selectively target members of the BnAb lineage and are tailored to induce subdominant BnAb rather than dominant non-BnAb responses. In summary, major strategies being pursued are several approaches for the induction of BnAbs, augmentation of the quality and quantity of non-neutralizing V1V2 antibodies as seen in the RV144 immune correlates study, and development of vaccine vectors that better represent critical T cell epitopes and the viral diversity of circulating strains. Hopefully, one or more of these paths will lead to a sufficiently efficacious vaccine that can be deployed as a preventive vaccine either alone or in combination with other prevention modalities [75] to ultimately end the HIV-1 epidemic.
Key Points.
Immune correlates analyses in the RV144 trial and in rhesus macaque protection model have provided new directions for vaccine development.
New targets for broad neutralizing antibodies have been described.
New vectors are inducing new degrees of protection in non-human primates.
Host controls of neutralizing antibody induction have been described and have pointed the way to new strategies for broad neutralizing antibody induction.
Acknowledgments
The authors thank Kim R. McClammy for expert secretarial assistance. This work supported by the Center for HIV/AIDS Vaccine Immunogen-Discovery (CHAVI-ID) grant UM1 AI100645, the Duke Center for AIDS Research AI 067854-02, by NIH Grants UM1 AI068618 and U0169481, and Vaccine Development Center grants from the Bill and Melinda Gates Foundation Collaboration for AIDS Vaccine Development.
References
- 1.Rerks-Ngarm S, Pitisuttithum P, Nitayaphan S, Kaewkungwal J, Chiu J, Paris R, et al. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N Engl J Med. 2009;361(23):2209–2220. doi: 10.1056/NEJMoa0908492. [DOI] [PubMed] [Google Scholar]
- 2**.Haynes BF, Gilbert PB, McElrath MJ, Zolla-Pazner S, Tomaras GD, Alam SM, et al. Immune-correlates analysis of an HIV-1 vaccine efficacy trial. N Engl J Med. 2012;366(14):1275–1286. doi: 10.1056/NEJMoa1113425. Report of results of the RV144 immune correlates trial first pointing to V1V2 as a immune correlate of transmission risk. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3*.Liao HX, Bonsignori M, Alam SM, McLellan JS, Tomaras GD, Moody MA, et al. Vaccine Induction of Antibodies against a Structurally Heterogeneous Site of Immune Pressure within HIV-1 Envelope Protein Variable Regions 1 and 2. Immunity. 2013;38(1):176–186. doi: 10.1016/j.immuni.2012.11.011. Key paper defining functional characteristics of RV144 V1V2 antibodies. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Walker LM, Phogat SK, Chan-Hui PY, Wagner D, Phung P, Goss JL, et al. Broad and potent neutralizing antibodies from an African donor reveal a new HIV-1 vaccine target. Science. 2009;326(5950):285–289. doi: 10.1126/science.1178746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bonsignori M, Hwang KK, Chen X, Tsao CY, Morris L, Gray E, et al. Analysis of a clonal lineage of HIV-1 envelope V2/V3 conformational epitope-specific broadly neutralizing antibodies and their inferred unmutated common ancestors. J Virol. 2011;85(19):9998–10009. doi: 10.1128/JVI.05045-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6*.McLellan JS, Pancera M, Carrico C, Gorman J, Julien JP, Khayat R, et al. Structure of HIV-1 gp120 V1/V2 domain with broadly neutralizing antibody PG9. Nature. 2011;480(7377):336–343. doi: 10.1038/nature10696. First report of a V1V2 broad neutralizing antibody co-crystal structure. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rolland M, Edlefsen PT, Larsen BB, Tovanabutra S, Sanders-Buell E, Hertz T, et al. Increased HIV-1 vaccine efficacy against viruses with genetic signatures in Env V2. Nature. 2012;490(7420):417–420. doi: 10.1038/nature11519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tomaras GD, Ferrari G, Shen X, Alam M, Liao H-X, Pollara J, et al. HIV-1 vaccine-induced envelope gp120 C1-region IgA blocks binding and effector function of gp120 IgG. Proc Natl Acad Sci U S A. 2013 doi: 10.1073/pnas.1301456110. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Evans TG, McElrath MJ, Matthews T, Montefiori D, Weinhold K, Wolff M, et al. QS-21 promotes an adjuvant effect allowing for reduced antigen dose during HIV-1 envelope subunit immunization in humans. Vaccine. 2001;19(15-16):2080–2091. doi: 10.1016/s0264-410x(00)00415-1. [DOI] [PubMed] [Google Scholar]
- 10.Goepfert PA, Tomaras GD, Horton H, Montefiori D, Ferrari G, Deers M, et al. Durable HIV-1 antibody and T-cell responses elicited by an adjuvanted multi-protein recombinant vaccine in uninfected human volunteers. Vaccine. 2007;25(3):510–518. doi: 10.1016/j.vaccine.2006.07.050. [DOI] [PubMed] [Google Scholar]
- 11.Buchbinder SP, Mehrotra DV, Duerr A, Fitzgerald DW, Mogg R, Li D, et al. Efficacy assessment of a cell-mediated immunity HIV-1 vaccine (the Step Study): a double-blind, randomised, placebo-controlled, test-of-concept trial. Lancet. 2008;372(9653):1881–1893. doi: 10.1016/S0140-6736(08)61591-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McElrath MJ, Haynes BF. Induction of immunity to human immunodeficiency virus type-1 by vaccination. Immunity. 2010;33(4):542–554. doi: 10.1016/j.immuni.2010.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13*.Rolland M, Tovanabutra S, deCamp AC, Frahm N, Gilbert PB, Sanders-Buell E, et al. Genetic impact of vaccination on breakthrough HIV-1 sequences from the STEP trial. Nat Med. 2011;17(3):366–371. doi: 10.1038/nm.2316. Key paper reporting genetic analysis of breakthrough viruses in RV144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mudd PA, Martins MA, Ericsen AJ, Tully DC, Power KA, Bean AT, et al. Vaccine-induced CD8+ T cells control AIDS virus replication. Nature. 2012;491(7422):129–133. doi: 10.1038/nature11443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Duerr A, Huang Y, Buchbinder S, Coombs RW, Sanchez J, del Rio C, et al. Extended follow-up confirms early vaccine-enhanced risk of HIV acquisition and demonstrates waning effect over time among participants in a randomized trial of recombinant adenovirus HIV vaccine (Step Study) J Infect Dis. 2012;206(2):258–266. doi: 10.1093/infdis/jis342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zak DE, Andersen-Nissen E, Peterson ER, Sato A, Hamilton MK, Borgerding J, et al. Merck Ad5/HIV induces broad innate immune activation that predicts CD8(+) T-cell responses but is attenuated by preexisting Ad5 immunity. Proc Natl Acad Sci U S A. 2012;109(50):E3503–3512. doi: 10.1073/pnas.1208972109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17*.Frahm N, DeCamp AC, Friedrich DP, Carter DK, Defawe OD, Kublin JG, et al. Human adenovirus-specific T cells modulate HIV-specific T cell responses to an Ad5-vectored HIV-1 vaccine. J Clin Invest. 2012;122(1):359–367. doi: 10.1172/JCI60202. Key papers (16, 17) defining Ad5 effects in persons with previous immunity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18**.Hansen SG, Ford JC, Lewis MS, Ventura AB, Hughes CM, Coyne-Johnson L, et al. Profound early control of highly pathogenic SIV by an effector memory T-cell vaccine. Nature. 2011;473(7348):523–527. doi: 10.1038/nature10003. Key paper demonstrating 50% protection from SIV challenge by rCMV vector-SIV insert vaccination. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Picker LJ, Hansen SG, Lifson JD. New paradigms for HIV/AIDS vaccine development. Annu Rev Med. 2012;63:95–111. doi: 10.1146/annurev-med-042010-085643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20*.Barouch DH, Liu J, Li H, Maxfield LF, Abbink P, Lynch DM, et al. Vaccine protection against acquisition of neutralization-resistant SIV challenges in rhesus monkeys. Nature. 2012;482(7383):89–93. doi: 10.1038/nature10766. Interesting study reporting pox prime, rAd26 boost can delay transmission in rhesus macaques. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.De Rosa SC, Thomas EP, Bui J, Huang Y, deCamp A, Morgan C, et al. HIV-DNA priming alters T cell responses to HIV-adenovirus vaccine even when responses to DNA are undetectable. J Immunol. 2011;187(6):3391–3401. doi: 10.4049/jimmunol.1101421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Churchyard GJ, Morgan C, Adams E, Hural J, Graham BS, Moodie Z, et al. A phase IIA randomized clinical trial of a multiclade HIV-1 DNA prime followed by a multiclade rAd5 HIV-1 vaccine boost in healthy adults (HVTN204) PLoS One. 2011;6(8):e21225. doi: 10.1371/journal.pone.0021225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rolland M, Jensen MA, Nickle DC, Yan J, Learn GH, Heath L, et al. Reconstruction and function of ancestral center-of-tree human immunodeficiency virus type 1 proteins. J Virol. 2007;81(16):8507–8514. doi: 10.1128/JVI.02683-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gaschen B, Taylor J, Yusim K, Foley B, Gao F, Lang D, et al. Diversity considerations in HIV-1 vaccine selection. Science. 2002;296(5577):2354–2360. doi: 10.1126/science.1070441. [DOI] [PubMed] [Google Scholar]
- 25.Letourneau S, Im EJ, Mashishi T, Brereton C, Bridgeman A, Yang H, et al. Design and pre-clinical evaluation of a universal HIV-1 vaccine. PLoS One. 2007;2(10):e984. doi: 10.1371/journal.pone.0000984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rolland M, Nickle DC, Mullins JI. HIV-1 group M conserved elements vaccine. PLoS Pathog. 2007;3(11):e157. doi: 10.1371/journal.ppat.0030157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fischer W, Perkins S, Theiler J, Bhattacharya T, Yusim K, Funkhouser R, et al. Polyvalent vaccines for optimal coverage of potential T-cell epitopes in global HIV-1 variants. Nat Med. 2007;13(1):100–106. doi: 10.1038/nm1461. [DOI] [PubMed] [Google Scholar]
- 28.Korber BT, Letvin NL, Haynes BF. T-cell vaccine strategies for human immunodeficiency virus, the virus with a thousand faces. J Virol. 2009;83(17):8300–8314. doi: 10.1128/JVI.00114-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hanke T, McMichael AJ. HIV-1: from escapism to conservatism. Eur J Immunol. 2011;41(12):3390–3393. doi: 10.1002/eji.201190072. [DOI] [PubMed] [Google Scholar]
- 30.Stephenson KE, SanMiguel A, Simmons NL, Smith K, Lewis MG, Szinger JJ, et al. Full-length HIV-1 immunogens induce greater magnitude and comparable breadth of T lymphocyte responses to conserved HIV-1 regions compared with conserved-region-only HIV-1 immunogens in rhesus monkeys. J Virol. 2012;86(21):11434–11440. doi: 10.1128/JVI.01779-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Santra S, Liao HX, Zhang R, Muldoon M, Watson S, Fischer W, et al. Mosaic vaccines elicit CD8+ T lymphocyte responses that confer enhanced immune coverage of diverse HIV strains in monkeys. Nat Med. 2010;16(3):324–328. doi: 10.1038/nm.2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Barouch DH, O’Brien KL, Simmons NL, King SL, Abbink P, Maxfield LF, et al. Mosaic HIV-1 vaccines expand the breadth and depth of cellular immune responses in rhesus monkeys. Nat Med. 2010;16(3):319–323. doi: 10.1038/nm.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wardemann H, Yurasov S, Schaefer A, Young JW, Meffre E, Nussenzweig MC. Predominant autoantibody production by early human B cell precursors. Science. 2003;301(5638):1374–1377. doi: 10.1126/science.1086907. [DOI] [PubMed] [Google Scholar]
- 34.Mouquet H, Scheid JF, Zoller MJ, Krogsgaard M, Ott RG, Shukair S, et al. Polyreactivity increases the apparent affinity of anti-HIV antibodies by heteroligation. Nature. 2010;467(7315):591–595. doi: 10.1038/nature09385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wrammert J, Smith K, Miller J, Langley WA, Kokko K, Larsen C, et al. Rapid cloning of high-affinity human monoclonal antibodies against influenza virus. Nature. 2008;453(7195):667–671. doi: 10.1038/nature06890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Scheid JF, Mouquet H, Feldhahn N, Walker BD, Pereyra F, Cutrell E, et al. A method for identification of HIV gp140 binding memory B cells in human blood. J Immunol Methods. 2009;343(2):65–67. doi: 10.1016/j.jim.2008.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gray ES, Moody MA, Wibmer CK, Chen X, Marshall D, Amos J, et al. Isolation of a monoclonal antibody that targets the alpha-2 helix of gp120 and represents the initial autologous neutralizing-antibody response in an HIV-1 subtype C-infected individual. J Virol. 2011;85(15):7719–7729. doi: 10.1128/JVI.00563-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Morris L, Chen X, Alam M, Tomaras G, Zhang R, Marshall DJ, et al. Isolation of a human anti-HIV gp41 membrane proximal region neutralizing antibody by antigen-specific single B cell sorting. PLoS One. 2011;6(9):e23532. doi: 10.1371/journal.pone.0023532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liao HX, Chen X, Munshaw S, Zhang R, Marshall DJ, Vandergrift N, et al. Initial antibodies binding to HIV-1 gp41 in acutely infected subjects are polyreactive and highly mutated. J Exp Med. 2011;208(11):2237–2249. doi: 10.1084/jem.20110363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moody MA, Zhang R, Walter EB, Woods CW, Ginsburg GS, McClain MT, et al. H3N2 influenza infection elicits more cross-reactive and less clonally expanded anti-hemagglutinin antibodies than influenza vaccination. PLoS One. 2011;6(10):e25797. doi: 10.1371/journal.pone.0025797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wu X, Yang ZY, Li Y, Hogerkorp CM, Schief WR, Seaman MS, et al. Rational design of envelope identifies broadly neutralizing human monoclonal antibodies to HIV-1. Science. 2010;329(5993):856–861. doi: 10.1126/science.1187659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhou T, Georgiev I, Wu X, Yang ZY, Dai K, Finzi A, et al. Structural basis for broad and potent neutralization of HIV-1 by antibody VRC01. Science. 2010;329(5993):811–817. doi: 10.1126/science.1192819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43*.Scheid JF, Mouquet H, Ueberheide B, Diskin R, Klein F, Oliveira TY, et al. Sequence and structural convergence of broad and potent HIV antibodies that mimic CD4 binding. Science. 2011;333(6049):1633–1637. doi: 10.1126/science.1207227. Important study defining VH-restriction for CD4 binding site antibodies. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44**.Liao HX, Lynch R, Zhou T, Gao F, Alam M, Boyd SD, et al. Co-evolution of a broadly neutralizing HIV-1 antibody and founder virus. Nature. 2013 doi: 10.1038/nature12053. in press. Study defining the evolutionally pathways of both transmitted/founder virus and CD4 binding site broad neutralizing antibodies in the same patient – thus pointing the way to sequential Envs for vaccine design. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Corti D, Langedijk JP, Hinz A, Seaman MS, Vanzetta F, Fernandez-Rodriguez BM, et al. Analysis of memory B cell responses and isolation of novel monoclonal antibodies with neutralizing breadth from HIV-1-infected individuals. PLoS One. 2010;5(1):e8805. doi: 10.1371/journal.pone.0008805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Huang J, Ofek G, Laub L, Louder MK, Doria-Rose NA, Longo NS, et al. Broad and potent neutralization of HIV-1 by a gp41-specific human antibody. Nature. 2012;491(7424):406–412. doi: 10.1038/nature11544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47**.Walker LM, Huber M, Doores KJ, Falkowska E, Pejchal R, Julien JP, et al. Broad neutralization coverage of HIV by multiple highly potent antibodies. Nature. 2011;477(7365):466–470. doi: 10.1038/nature10373. Key paper reporting the new target for BnAbs, V3 glycans, and the most potent BnAbs to date. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pejchal R, Doores KJ, Walker LM, Khayat R, Huang PS, Wang SK, et al. A potent and broad neutralizing antibody recognizes and penetrates the HIV glycan shield. Science. 2011;334(6059):1097–1103. doi: 10.1126/science.1213256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49**.Burton DR, Poignard P, Stanfield RL, Wilson IA. Broadly neutralizing antibodies present new prospects to counter highly antigenically diverse viruses. Science. 2012;337(6091):183–186. doi: 10.1126/science.1225416. Key recent review of the progress made in structures of new BnAbs co-crystalized with HIV-1 Env. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50*.Moldt B, Rakasz EG, Schultz N, Chan-Hui PY, Swiderek K, Weisgrau KL, et al. Highly potent HIV-specific antibody neutralization in vitro translates into effective protection against mucosal SHIV challenge in vivo. Proc Natl Acad Sci U S A. 2012;109(46):18921–18925. doi: 10.1073/pnas.1214785109. Key paper demonstrating very low levels of plasma BnAbs protect against SHIV challenge. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dhillon AK, Donners H, Pantophlet R, Johnson WE, Decker JM, Shaw GM, et al. Dissecting the neutralizing antibody specificities of broadly neutralizing sera from human immunodeficiency virus type 1-infected donors. J Virol. 2007;81(12):6548–6562. doi: 10.1128/JVI.02749-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Simek MD, Rida W, Priddy FH, Pung P, Carrow E, Laufer DS, et al. Human immunodeficiency virus type 1 elite neutralizers: individuals with broad and potent neutralizing activity identified by using a high-throughput neutralization assay together with an analytical selection algorithm. J Virol. 2009;83(14):7337–7348. doi: 10.1128/JVI.00110-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gray ES, Madiga MC, Hermanus T, Moore PL, Wibmer CK, Tumba NL, et al. The neutralization breadth of HIV-1 develops incrementally over four years and is associated with CD4+ T cell decline and high viral load during acute infection. J Virol. 2011;85(10):4828–4840. doi: 10.1128/JVI.00198-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tomaras GD, Binley JM, Gray ES, Crooks ET, Osawa K, Moore PL, et al. Polyclonal B cell responses to conserved neutralization epitopes in a subset of HIV-1-infected individuals. J Virol. 2011;85(21):11502–11519. doi: 10.1128/JVI.05363-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bonsignori M, Montefiori DC, Wu X, Chen X, Hwang KK, Tsao CY, et al. Two distinct broadly neutralizing antibody specificities of different clonal lineages in a single HIV-1-infected donor: implications for vaccine design. J Virol. 2012;86(8):4688–4692. doi: 10.1128/JVI.07163-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56*.Haynes BF, Kelsoe G, Harrison SC, Kepler TB. B-cell-lineage immunogen design in vaccine development with HIV-1 as a case study. Nat Biotechnol. 2012;30(5):423–433. doi: 10.1038/nbt.2197. Important overview of host controls of BnAb induction and the B cell lineage design vaccine design strategy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Haynes BF, Fleming J, St Clair EW, Katinger H, Stiegler G, Kunert R, et al. Cardiolipin polyspecific autoreactivity in two broadly neutralizing HIV-1 antibodies. Science. 2005;308(5730):1906–1908. doi: 10.1126/science.1111781. [DOI] [PubMed] [Google Scholar]
- 58.Haynes BF, Moody MA, Verkoczy L, Kelsoe G, Alam SM. Antibody polyspecificity and neutralization of HIV-1: a hypothesis. Hum Antibodies. 2005;14(3-4):59–67. [PMC free article] [PubMed] [Google Scholar]
- 59.Verkoczy L, Diaz M, Holl TM, Ouyang YB, Bouton-Verville H, Alam SM, et al. Autoreactivity in an HIV-1 broadly reactive neutralizing antibody variable region heavy chain induces immunologic tolerance. Proc Natl Acad Sci U S A. 2010;107(1):181–186. doi: 10.1073/pnas.0912914107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Verkoczy L, Chen Y, Bouton-Verville H, Zhang J, Diaz M, Hutchinson J, et al. Rescue of HIV-1 broad neutralizing antibody-expressing B cells in 2F5 VH × VL knockin mice reveals multiple tolerance controls. J Immunol. 2011;187(7):3785–3797. doi: 10.4049/jimmunol.1101633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ota T, Doyle-Cooper C, Hudson K, Aoki-Ota M, Cooper AB, Doores KJ, et al. Knock-in mice expressing broadly neutralizing HIV antibodies B12 and 4E10. B Cell Development and Function Keystone Conference; February 10-15; 2013. Abstract X1 2064(112) [Google Scholar]
- 62.Yang G, Holl TM, Liu Y, Li Y, Lu X, Nicely NI, et al. Identification of autoantigens recognized by the 2F5 and 4E10 broadly neutralizing HIV-1 antibodies. J Exp Med. 2013;210(2):241–256. doi: 10.1084/jem.20121977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tobin GJ, Trujillo JD, Bushnell RV, Lin G, Chaudhuri AR, Long J, et al. Deceptive imprinting and immune refocusing in vaccine design. Vaccine. 2008;26(49):6189–6199. doi: 10.1016/j.vaccine.2008.09.080. [DOI] [PubMed] [Google Scholar]
- 64.Muster T, Steindl F, Purtscher M, Trkola A, Klima A, Himmler G, et al. A conserved neutralizing epitope on gp41 of human immunodeficiency virus type 1. J Virol. 1993;67(11):6642–6647. doi: 10.1128/jvi.67.11.6642-6647.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65**.Wu X, Zhou T, Zhu J, Zhang B, Georgiev I, Wang C, et al. Focused evolution of HIV-1 neutralizing antibodies revealed by structures and deep sequencing. Science. 2011;333(6049):1593–1602. doi: 10.1126/science.1207532. Landmark paper demonstrating the remarkable depth of affinity maturation of the VRC1501 CD1594 binding site BnAbs. Landmark paper demonstrating the remarkable depth of affinity maturation of the VRC01 CD4 binding site BnAb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Xiao X, Chen W, Feng Y, Zhu Z, Prabakaran P, Wang Y, et al. Germline-like predecessors of broadly neutralizing antibodies lack measurable binding to HIV-1 envelope glycoproteins: implications for evasion of immune responses and design of vaccine immunogens. Biochem Biophys Res Commun. 2009;390(3):404–409. doi: 10.1016/j.bbrc.2009.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Xiao X, Chen W, Feng Y, Dimitrov DS. Maturation Pathways of Cross-Reactive HIV-1 Neutralizing Antibodies. Viruses. 2009;1(3):802–817. doi: 10.3390/v1030802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dimitrov DS. Therapeutic antibodies, vaccines and antibodyomes. MAbs. 2010;2(3):347–356. doi: 10.4161/mabs.2.3.11779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hoot S, McGuire AT, Cohen KW, Strong RK, Hangartner L, Klein F, et al. Recombinant HIV envelope proteins fail to engage germline versions of anti-CD4bs bNAbs. PLoS Pathog. 2013;9(1):e1003106. doi: 10.1371/journal.ppat.1003106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Alam SM, Liao HX, Dennison SM, Jaeger F, Parks R, Anasti K, et al. Differential reactivity of germ line allelic variants of a broadly neutralizing HIV-1 antibody to a gp41 fusion intermediate conformation. J Virol. 2011;85(22):11725–11731. doi: 10.1128/JVI.05680-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Malherbe DC, Doria-Rose NA, Misher L, Beckett T, Puryear WB, Schuman JT, et al. Sequential immunization with a subtype B HIV-1 envelope quasispecies partially mimics the in vivo development of neutralizing antibodies. J Virol. 2011;85(11):5262–5274. doi: 10.1128/JVI.02419-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72*.Burton DR, Ahmed R, Barouch DH, Butera ST, Crotty S, Godzik A, et al. A Blueprint for HIV Vaccine Discovery. Cell Host Microbe. 2012;12(4):396–407. doi: 10.1016/j.chom.2012.09.008. Important overview of pathways and strategies for HIV-1 vaccine development. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73**.Shingai M, Donau OK, Schmidt SD, Gautam R, Plishka RJ, Buckler-White A, et al. Most rhesus macaques infected with the CCR5-tropic SHIV(AD8) generate cross-reactive antibodies that neutralize multiple HIV-1 strains. Proc Natl Acad Sci U S A. 2012;109(48):19769–19774. doi: 10.1073/pnas.1217443109. Remarkable study demonstrating the SHIV AD8 induces glycan antibodies in most SHIV AD8-infected rhesus macaques. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Liao HX, Tsao CY, Alam SM, Muldoon M, Vandergrift N, Ma BJ, et al. Antigenicity and Immunogenicity of Transmitted/Founder, Consensus and Chronic Envelope Glycoproteins of Human Immunodeficiency Virus Type 1. J Virol. 2013 doi: 10.1128/JVI.02297-12. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75**.Cohen MS, Chen YQ, McCauley M, Gamble T, Hosseinipour MC, Kumarasamy N, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. 2011;365(6):493–505. doi: 10.1056/NEJMoa1105243. Landmark paper demonstrating treatment of HIV-1 negative partners of HIV-1-infected subjects with retroviral therapy prevents transmission by 96%. [DOI] [PMC free article] [PubMed] [Google Scholar]


