Abstract
Objectives
This study considered the association between sexual maturation and adiposity in children and adolescents, and examined the contribution of sexual maturation to ethnic differences in total and depot-specific body fat.
Methods
The sample included 382 White and African American 5–18-year-olds. Body mass index (BMI), waist circumference (WC) and sexual maturity status (breast/genital and pubic hair stage) were assessed in a clinical setting. Total body fat (TBF) was measured by dual-energy X-ray absorptiometry and abdominal subcutaneous (SAT) and visceral adipose tissue (VAT) were measured by magnetic resonance imaging. Analysis of covariance adjusted for age was used to examine the association between sexual maturity status and adiposity, and linear regression adjusted for age was used to examine the influence of sexual maturation on ethnic differences in adiposity. Analysis of VAT also controlled for TBF. Significance was accepted at P<0.05.
Results
Breast/genital stage was significantly associated with BMI, WC, TBF, and SAT in girls of both ethnic groups and in White boys. Breast stage was associated with VAT. Stage of pubic hair was significantly associated with TBF and VAT in White girls only. In girls, sexual maturation attenuated the ethnic effects on BMI and WC, but the ethnic effect in VAT persisted. In boys, sexual maturation did not attenuate ethnic differences on VAT and did not predict WC or SAT. Sexual maturity status independently explained variance in adiposity in girls only.
Conclusions
Sexual maturity status is an important determinant of pediatric adiposity and attenuates ethnic differences in girls’ adiposity.
Pediatric obesity is a major clinical and public health challenge. Increased adiposity during puberty is a significant predictor of adult obesity (Guo et al., 2002) and associated comorbidities including coronary heart disease (Baker et al., 2007). Pubertal changes in estrogen, androgen, and growth hormone levels also influence adiposity (Loomba-Albrecht and Styne 2009). Stage of sexual maturation at the time of examination may thus influence total body fat (TBF) (Himes et al., 2004; Roemmich et al., 2002) and depot-specific adiposity, including subcutaneous (SAT) and visceral adipose tissue (VAT) (Loomba-Albrecht and Styne 2009). In a study of 7–16-year-old White and Hispanic youth, pubertal status explained 18.6 and 12.4% of the variance in SAT and VAT, respectively, and had a greater influence on SAT and VAT than age, ethnicity or gender (Brambilla et al., 2006). In contrast, pubertal status was moderately correlated with SAT and VAT in boys but not in girls among youth aged 13.5 ± 0.4 years, and explained only 6.8 and 3.7% of the variance in SAT and VAT, respectively (Benfield et al., 2008). Pubertal status, when adjusted for age, did not explain the variance in body mass index (BMI), waist circumference (WC), TBF, or VAT among a sample of 138 8–12 year-old African American girls (Hoffman et al., 2005).
Pubertal status is influenced by ethnicity: African American boys and girls begin puberty before White boys and girls whether assessed by stage of breasts/genitals or pubic hair (Sun et al., 2002). Overweight and obesity are more prevalent among African American girls (41.3%) and boys (36.9%) compared to White youth (27.9%) (Ogden et al., 2012). African Americans also have more abdominal SAT (Lee et al., 2008), a major component of body fat. In contrast, White youth tend to have more VAT than African American youth (Liska et al., 2007; Taksali et al., 2007). Of relevance to discussions of ethnic variation in adiposity, comparisons of White and African American youth rarely control for variation in sexual maturity status. The purpose of this study was to examine the association between sexual maturation and adiposity among African American and White youth and to estimate the contribution of variation in sexual maturity status to observed ethnic differences in total and depot-specific body fat.
METHODS
Participants
Participants were drawn from a sample of 423 White and African American youth 5–18 years of age. All were involved in a cross-sectional study of the correlates of abdominal adiposity. Exclusion criteria included being unwilling or unable to communicate with study staff to provide an informed consent, or having a chronic medical condition or pregnancy which could interfere with the measurements in this study. Additional exclusion criteria for the present analysis included ethnicity other than White or African American (n=12), outlier value (>±3 SD from the mean for age and sex) for a primary analysis variable (n=1), or incomplete magnetic resonance imaging (MRI) or dual-energy X-ray absorptiometry (DXA) scans (n=28) due to participant refusal, motion artifacts, or participant weight exceeding equipment limits. The present analysis included 382 youth (84 African American and 96 White boys, and 118 African American and 84 White girls). Informed consent was obtained from parents or guardians along with written assent from the participants. The Pennington Biomedical Research Center (PBRC) institutional review board approved all study procedures.
Anthropometry
Height and weight were measured in the PBRC outpatient clinic using established and standardized procedures. The BMI (kg/m2) was calculated. BMI percentiles were determined using the SAS macro program developed from the 2000 CDC Growth Charts for the United States (Centers for Disease Control and Prevention, 2011). WC was measured to the nearest 0.1 cm at the midpoint between the iliac crest and the lowest rib; the average of two measurements was used for analysis (closest two of three if the difference exceeded 0.5 cm).
Sexual maturity status
Sexual maturation was assessed by a physician (for participants < 9 years) or self-assessed (for participants ≥9 years) using the criteria developed by Tanner (Tanner, 1986). A series of drawings depicted progressive stages of pubertal development from 1 (no development) to 5 (complete development) for female breasts or male genitalia and from 1 (no development) to 6 (complete development) for pubic hair development (Tanner, 1986). Stages 5 and 6 of pubic hair development were collapsed into a single category to represent the mature state. Moderate to high concurrence has been previously demonstrated between physician assessment and self-assessment in this pediatric age range (Matsudo and Matsudo, 1994).
Body composition
TBF (kilograms) was assessed by DXA on a Hologic QDR 4500A whole-body scanner (Bedford, MA); analyses were conducted with QDR software for Windows V11.2. Abdominal SAT and VAT were assessed by MRI on a General Electric (GE) Signa Excite (3.0 Tesla) (GE Medical Systems, Waukesha, WI) scanner. Scans were obtained from the highest point of the liver to the bottom pole of the right kidney. Depending on the stature, five to eight slices with a slice gap of 4.78 cm were analyzed for each participant. Using the AnalyzeV® software package (CNSoftware, Rochester, MN), one trained technician manually drew SAT and VAT areas. Area (cm2) for each slice was calculated by the number of pixels multiplied by voxel width by voxel height. Volume (ml) at each slice was then calculated by the SAT or VAT area multiplied by the voxel depth by the slice gap by 0.000001. The five to eight slice volumes were summed for total SAT or VAT volume for each participant. A sub-set of 20 images at the L4–L5 slice was reanalyzed (blinded) for intrarater reliability. The average coefficient of variation, calculated as the ratio of the standard deviation to the mean of each set of two measurements, was 0.99±1.00 for SAT area and 6.63±6.35 for VAT area. Pearson correlation coefficients between first and second analysis were high (r=0.99 for SAT area; r=0.97 for VAT area).
Statistical analysis
Analyses were performed using SAS VR statistical package V9.3 (SAS Institute, Cary, NC). Analysis of covariance with age as the covariate was used to examine the relationship between sexual maturity status (stage of breast/genital and pubic hair) with each measure of adiposity (BMI, WC, TBF, SAT, and VAT). The analysis of VAT also controlled for TBF. P values for linear trends across maturity categories are reported.
Sex-specific linear regression analysis was used to examine the relationship between sexual maturity status (breast/genital stage) and ethnicity (White vs. African American) with each measure of adiposity (BMI, WC, TBF, SAT, and VAT). Adiposity variables were transformed using the natural logarithm to correct for skewness. Model 1 included the independent variable of ethnicity with age as a covariate. Age squared, age cubed, and the age by ethnicity interaction were included if significant (P<0.05). Model 2 included the independent variables of sexual maturity status and ethnicity with age as a covariate. Age squared, age cubed, and interaction terms (age by ethnicity, age by maturation) were included if significant (P<0.05). For analysis of VAT, TBF was also included as a covariate and the TBF by age interaction term was included if significant (P<0.05). Sex-specific univariate analysis was used to estimate the variance explained by sexual maturity status for each measure of adiposity.
RESULTS
Descriptive characteristics are reported by ethnicity and sex in Table 1. Means and standard deviations in the total sample were as follows: age, 12.3±3.5 years; BMI, 23.1±6.6 kg/m2; BMI percentile, 72.6±28.0%; WC, 74.4±16.9 cm; TBF, 16.8±11.8 kg; SAT, 4255.2±3878.7 ml; and VAT, 146.5±148.2 ml.
TABLE 1.
Descriptive characteristics of African American and Caucasian 5–18-year-old girls and boys
| White girls | African American girls | White boys | African American boys | |
|---|---|---|---|---|
| n | 84 | 118 | 96 | 84 |
| Age, years | 12.4 (3.4) | 12.3 (3.6) | 12.3 (3.5) | 12.0 (3.4) |
| Tanner stage, % (by pubic hair development) | ||||
| I | 25.0 | 19.5 | 33.3 | 32.1 |
| II | 16.7 | 9.3 | 16.7 | 13.1 |
| III | 4.8 | 11.9 | 6.3 | 13.1 |
| IV | 14.3 | 15.3 | 10.4 | 9.5 |
| V | 39.3 | 44.1 | 33.3 | 32.1 |
| Tanner stage, % (by breast/genital development) | ||||
| I | 23.8b | 17.8 | 26.0 | 34.5 |
| II | 17.9 | 13.6 | 26.0 | 17.9 |
| III | 22.6 | 15.3 | 14.6 | 14.3 |
| IV | 23.8 | 22.9 | 20.8 | 20.2 |
| V | 11.9 | 30.5 | 12.5 | 13.1 |
| BMI, kg/m2 | 21.9 (5.2) | 25.1 (7.8)a | 21.6 (5.6) | 22.9 (6.6) |
| BMI percentile, % | 70.0 (26.0) | 78.1 (27.7)a | 67.8 (29.5) | 73.0 (27.9) |
| Waist circumference, cm | 72.0 (13.9) | 77.1 (18.0)a | 73.5 (16.1) | 73.9 (18.8) |
| Body fat, kg | 16.9 (9.4) | 20.8 (14.1)a | 13.7 (10.2) | 14.5 (11.0) |
| Body fat percent, % | 31.1 (8.4)b | 31.8 (9.6)a | 24.0 (9.1) | 24.0 (10.0) |
| SAT volume, ml | 4246.6 (3153.8)a | 5626.5 (4767.2)a | 3171.3 (3075.2) | 3576.3 (3428.2) |
| VAT volume, ml | 162.3 (137.0) | 139.4 (128.0) | 167.7 (190.7)b | 116.6 (124.6) |
Parentheses indicate standard deviation. BMI—body mass index; SAT—subcutaneous adipose tissue; VAT—visceral adipose tissue.
Significant difference by ethnicity, within sex.
Significant difference by sex, within ethnicity.
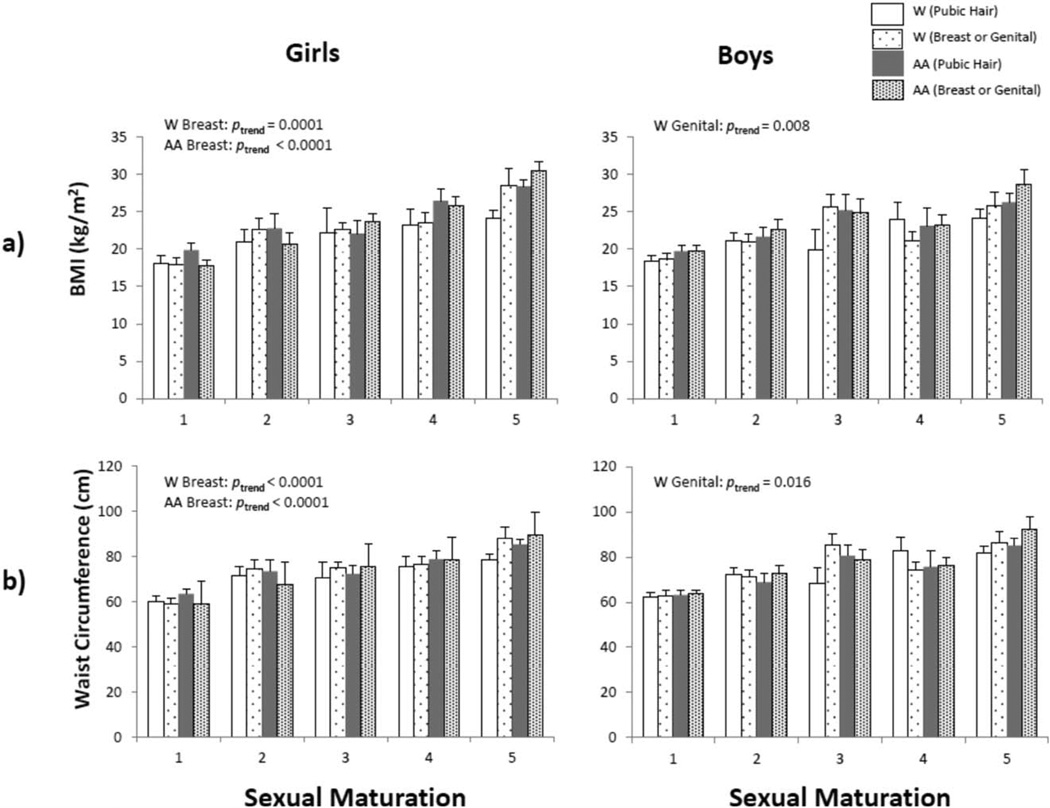
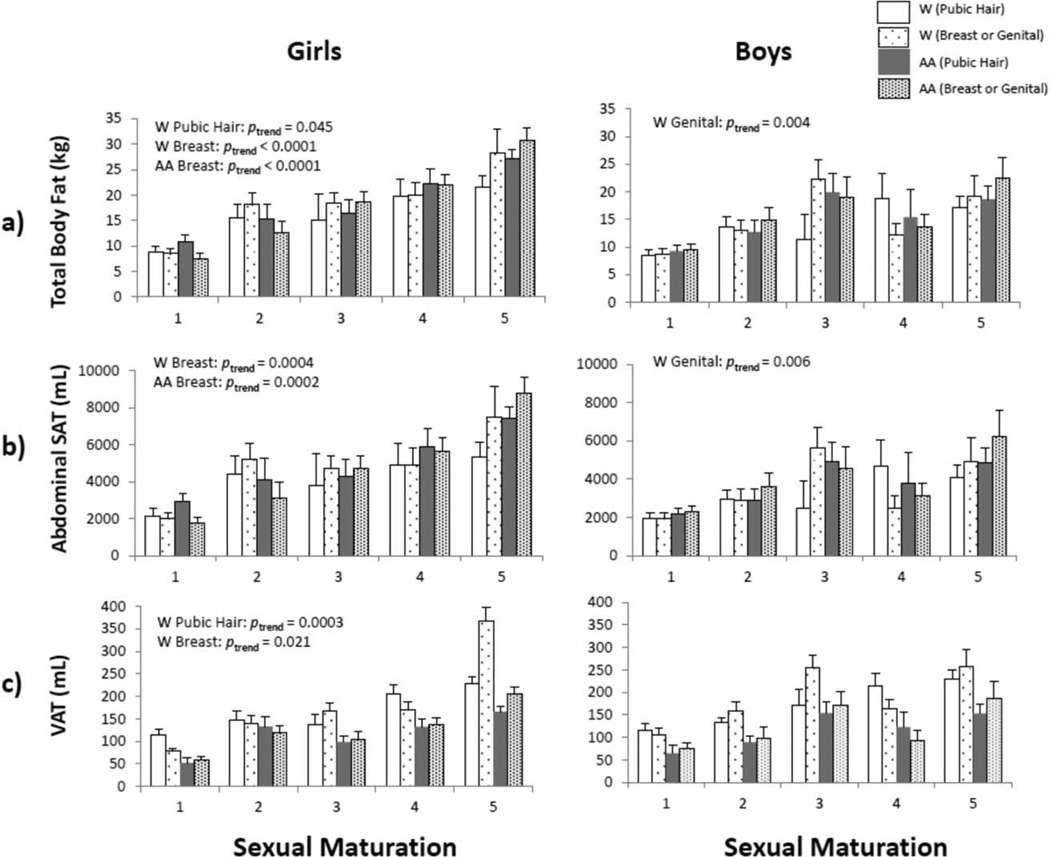
Breast stage in girls was significantly and positively associated with BMI, WC, TBF, and SAT in White and African American girls (all ptrend <0.001), and with VAT in White girls (ptrend=0.021) (Figs. 1 and 2). Genital stage was significantly related to increased BMI (ptrend=0.008), WC (ptrend=0.016), TBF (ptrend=0.004), and SAT (ptrend=0.006) in White boys, but not in African American boys. Pubic hair stage was not associated with adiposity in White or African American boys.
Fig. 1.
Mean values of age-adjusted a) BMI and b) WC, stratified by sex, ethnicity, and sexual maturation assessed by the criteria of Tanner (development of breasts/genitals). AA indicates African American. Windicates White.
Fig. 2.
Mean values of age-adjusted a) TBF, b) SAT, and c) VAT (also adjusted for TBF), stratified by sex, ethnicity, and sexual maturation assessed by the criteria of Tanner (development of breasts/genitals). AA indicates African American. Windicates White.
Linear regression models for girls adjusted for age indicated ethnicity as a significant predictor of BMI, WC, and VAT (Table 2). African American girls had higher BMI and WC, but lower VAT, compared to White girls. When sexual maturity status was added to the model (breast stage), ethnic differences between White and African American girls disappeared. Sexual maturity status (breast stage) significantly and positively predicted BMI, WC, TBF, and SAT in girls. Ethnic effects persisted for VAT among girls and sexual maturity status (breast stage) did not significantly predict VAT. Results of the univariate analyses indicated that sexual maturity status (breast stage) explained 33.7% of the variance in BMI, 34.5% of the variance in WC, 35.1% of the variance in body fat, 25.1% of the variance in SAT, and 16.8% of the variance in VAT in girls. In contrast, ethnicity explained less than 5% of the variance in each measure of adiposity. In the full model which included age and ethnicity, sexual maturity status (breast stage) explained more of the variance in several measures of adiposity than ethnicity in girls (partial R2=37% for BMI, 37% for WC, 43% for TBF, and 32% for SAT), with the exception of body fat-adjusted VAT (partial R2=0%).
TABLE 2.
Linear regression analyses of sexual maturation effects on adipositya
| BMI (kg/m2) |
Waist circumference (cm) |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Model 1 |
Model 2 |
Model 1 |
Model 2 |
|||||||||
| β | SE | Partial R2 | β | SE | Partial R2 | β | SE | Partial R2 | β | SE | Partial R2 | |
| Girls | ||||||||||||
| Ethnicityb | 0.22g | 0.04 | 0.05 | 0.05 | 0.03 | 0.002 | 0.15e | 0.03 | 0.023 | −0.002 | 0.03 | <0.0001 |
| Maturationc | – | – | – | 0.85g | 0.02 | 0.37 | – | – | – | 0.80g | 0.02 | 0.37 |
| Boys | ||||||||||||
| Ethnicity | 0.12 | 0.03 | 0.01 | 0.12 | 0.03 | 0.01 | 0.02 | 0.03 | 0.001 | 0.02 | 0.03 | 0.001 |
| Maturation | – | – | – | 20.20 | 0.02 | 0.01 | – | – | – | −0.12 | 0.02 | 0.003 |
| Total body fat (kg) |
SAT (ml) |
|||||||||||
| Model 1 |
Model 2 |
Model 1 |
Model 2 |
|||||||||
| β | SE | Partial R2 | β | SE | Partial R2 | β | SE | Partial R2 | β | SE | Partial R2 | |
| Girls | ||||||||||||
| Ethnicity | 0.10 | 0.08 | 0.01 | −0.05 | 0.08 | 0.002 | 0.12 | 0.11 | 0.01 | −0.04 | 0.11 | 0.001 |
| Maturation | – | – | – | 0.80g | 0.05 | 0.43 | – | – | – | 0.79g | 0.07 | 0.32 |
| Boys | ||||||||||||
| Ethnicity | 0.02 | 0.10 | 0.001 | 0.03 | 0.10 | 0.001 | 0.05 | 0.12 | 0.003 | 0.06 | 0.12 | 0.003 |
| Maturation | – | – | – | −0.28e | 0.07 | 0.02 | – | – | – | −0.24 | 0.09 | 0.01 |
| VAT (mL) |
||||||||||||
| Model 1d |
Model 2d |
|||||||||||
| β | SE | Partial R2 | β | SE | Partial R2 | |||||||
| Girls | ||||||||||||
| Ethnicity | −0.12 | 0.11 | 0.06 | −0.25g | 0.07 | 0.06 | ||||||
| Maturation | – | – | – | 0.0003 | 0.05 | 0.0001 | ||||||
| Boys | ||||||||||||
| Ethnicity | −0.23g | 0.07 | 0.06 | −0.23g | 0.06 | 0.06 | ||||||
| Maturation | – | – | – | −0.21f | 0.05 | 0.01 | ||||||
Note: Model 1 included ethnicity and age, with age squared, age cubed, and ethnicity × age interaction allowed in the model if P<0.05. Model 2 included sexual maturation, ethnicity, and age, with age squared, age cubed, ethnicity × age interaction, and maturation × age interaction allowed in the model if P<0.05. BMI—body mass index; SAT—subcutaneous adipose tissue; VAT—visceral adipose tissue.
Adiposity variables were transformed using the natural logarithm.
Ethnicity was coded as 0 = white and 1 = African American.
Sexual maturation was assessed as 5 ordinal stages based on the development of breasts (girls) or genitals (boys).
VAT controlled for total body fat, and body fat × age was allowed in the model if P<0.05.
indicates P<0.05
indicates <0.01
indicates P<0.001.
The linear regression models adjusted for age among boys indicated that ethnicity was a significant predictor of VAT. African American boys had lower VAT compared to White boys. When sexual maturity status (genital stage) was added to the model, ethnicity remained significantly related to VAT, and sexual maturity status (genital stage) significantly predicted VAT and TBF. Neither ethnicity nor sexual maturity status (genital stage) significantly predicted BMI, WC, or SAT in boys. Results of the univariate analyses indicated that sexual maturity status (genital stage) explained 13.7% of the variance in BMI, 19.8% in WC, 9.1% in body fat, 7.1% in SAT, and 5.2% in VAT in boys. In contrast, ethnicity explained none of the variance in each measure of adiposity among boys in univariate analyses. When age and ethnicity were controlled, sexual maturity (genital stage) explained a negligible amount of the variance in all measures of adiposity in boys.
DISCUSSION
This study examined the association between sexual maturity status and adiposity in youth. Later stage of sexual maturation was significantly associated with greater amounts of adiposity even when controlling for age (Figs. 1 and 2), although sex and ethnic differences emerged. Advanced sexual maturation (breast stage) was associated with greater BMI, WC, TBF, and SAT in White and African American girls. Later stage of sexual maturation (pubic hair stage) was associated with greater TBF and VAT in only White girls. Advanced sexual maturation (genital stage) was associated with greater BMI, WC, TBF, and SAT in only White boys. Corresponding relationships were not significant in African American boys.
This study also determined the influence of sexual maturity status on ethnic differences in total and depot-specific adiposity. African American girls had higher BMI and WC compared to White girls when age was statistically controlled. However, when sexual maturity status (breast stage) was added to the model, the ethnic differences in these adiposity variables disappeared. The sole exception was VAT. White girls had more VAT than African American girls whether sexual maturity status was or was not included. The results were partially consistent with the lack of differences in TBF, SAT, or VAT between White and African American obese youth (11.8±0.5 years) after adjustment for stage of puberty (indicator not specified) (Tershakovec et al., 2003).
Among boys, ethnic differences in VAT were evident when age was statistically controlled. Adding sexual maturity status (genital stage) in the analysis did not attenuate the ethnic differences. White boys had higher VAT even when controlling for sexual maturity status. The persistence of ethnicity effects on VAT was consistent with an earlier study of boys 11–18 years: African American boys had more SAT but less VAT than White boys, despite similar age, stage of puberty (pubic hair and genital) and BMI (Lee et al., 2011).
In contrast to ethnicity, sexual maturity status explained more of the variance in BMI, WC, TBF, SAT, and VAT in both girls and boys in univariate analyses, but there were sex differences in the explained variances. Sexual maturity status explained 25.1 and 16.8% of the variance in SAT and VAT in girls, respectively, compared to 7.1 and 5.2%, respectively, in boys. These observations were similar to estimates in a study of 7–16-year-old boys and girls: sexual maturity status (indicator not specified) explained 18.6 and 12.4% of the variance in SAT and VAT, respectively (Brambilla et al., 2006). Sexual maturity status (breast and pubic hair) explained less variance, 6.8% for SAT and 3.7% for VAT, in a large cohort of British children; however, maturity status (pubic hair) was correlated with SAT and VAT in boys but not girls (Benfield et al., 2008). The variable findings may reflect the indicator of sexual maturity status used in the respective analysis. In the study of British youth (Benfield et al., 2008), stages of breast/genital and pubic hair were collapsed into prepubertal (stage 1), early pubertal (stages 2–3) and late pubertal (stages 4–5). This study used the five specific stages of breast and genital development in girls and boys, respectively. This is an important analytical issue as stages of breast and pubic hair in girls and genital and pubic hair in boys, though related, are not equivalent within each sex (Malina et al., 2004). Stages of breast and genital development are also not equivalent (Malina et al., 2004).
This study also examined the independent variance in adiposity attributable to sexual maturity status (breast/ genital stage), controlling for ethnicity. The independent variance in adiposity explained by sexual maturity status ranged from 6 to 43% in girls and from 2 to 9% in boys. The results emphasize a need to consider age, ethnicity and TBF when examining the independent association between sexual maturity status and adiposity.
Differences in the associations between sexual maturation and adiposity were noted, but varied depending on the indicator used. Breast stage was associated with every measure of adiposity (in girls), and genital stage (in White boys) was associated with each adiposity measure except VAT. Stage of pubic hair was associated only with TBF and VAT in White girls and with none of the adiposity measures in boys of either ethnic group. Breast stage is generally considered more reliable than pubic hair stage because breast tissue requires action of the estrogen in the hypothalamic–pituity–gonadal axis (Euling et al., 2008). However, given the increased prevalence of obesity in children and adolescents, the potential for error in assessing breast stage is increased, especially self-assessments or assessments by clinicians with limited experience. Some individuals may have difficulty distinguishing the development of the breast per se from increased subcutaneous adiposity. Nevertheless, differences in relationships between adiposity with pubertal stages warrant further study.
A cross-sectional trend among boys in the present study may warrant further study. The present data suggest a decline in TBF, SAT, and VAT among White and African American boys in stage 4 of genital and pubic hair (Fig. 2). Most boys reach peak height velocity at Tanner Stage three or four, which may explain a relative decrease in body fat. However, there is considerable variation in the sexual maturation stage represented at peak height velocity (Malina et al., 2004), and this study was cross-sectional so that inferences about changes in adiposity across stages of pubertal maturation need to be made with care. The findings may be an artifact of the cross-sectional data, but observations from the Zurich Longitudinal Study (n=100) indicate the majority of boys were in pubic hair stage 3 (61) and genital stage 4 (55) at the time of peak height velocity (Prader et al., 1984). Estimated rates of growth in subcutaneous adiposity (by skinfold thicknesses or radiography) tend to decelerate at the time of peak height velocity, and percentage body fat also declines at this time (Malina et al., 2004). It is not certain if corresponding changes occur in VAT and SAT, but a need for longitudinal study across the interval of sexual maturation and the growth spurt is implicated.
The process of sexual maturation may influence relationships between depot-specific adipose tissue and metabolic parameters. For instance, in overweight and obese prepubertal children, insulin sensitivity was associated with SAT but not with VAT (Maffeis et al., 2008). Similarly, VAT was related to glucose metabolism after puberty but not before (Brambilla et al., 1999). In post-pubertal adolescents, VAT was related to insulin sensitivity and high density lipoprotein cholesterol levels as seen among adults (Brambilla et al., 1994). Many studies are cross-sectional so that causal implications are not warranted. The studies, however, implicate a need for comprehensive longitudinal study of associations between depot-specific adiposity and metabolic health during pubertal maturation in normal weight and obese youth.
A major strength of this study was the broad age range, pubertal status spanning pre- to post-puberty, and the variety of adiposity indicators in White and African American youth. MRI and DXA were used to precisely assess depot-specific and TBF, respectively. Nevertheless, the study has several limitations. First, the sample was cross-sectional and thus limited to stage of sexual maturity at the time of assessment, without indication of the timing or tempo of sexual maturation. The direction of the relationship between sexual maturity status and adiposity is also unclear. Physiological and hormonal changes during puberty influence adiposity (Euling et al., 2008). It has been suggested that obesity per se may accelerate the timing to onset of puberty, as demonstrated in United States girls (Euling et al., 2008), although the evidence indicates that body fat has limited influence (de Ridder et al., 1992). Second, both physician- and self-assessments of pubertal status were used. Concordance of self- versus physician-assessed maturation varied with indicator and among stages (Matsudo and Matsudo, 1994). As noted earlier, assessment of breast stage may be problematic in obese girls, as it may be difficult to distinguish adipose tissue per se from the elevation of the breast. A related issue may be the fact that criteria for stages of puberty described by Tanner (1962, 1975) were based primarily on studies of White youth (Malina et al., 2004). Variation in the development of the secondary sex characteristics per se associated with ethnicity warrants attention. Finally, there were limitations to the sample. Two ethnic groups were included in this study, so it is not known how sexual maturation contributes to adiposity differences across other ethnic groups. The four heaviest participants were excluded due to exceeding weight limits of the DXA, which created a ceiling effect in the adiposity measures that may have attenuated the associations between ethnicity, maturation and adiposity. Additionally, the mean BMI of African American girls was equivalent to overweight in adults, so the results may differ in populations with lower BMI.
CONCLUSION
Sexual maturity status is an important consideration for adiposity in White and African American girls and in White boys. Sexual maturity status explained ethnic variation in adiposity among girls, but not in boys. There is a need for longitudinal research to examine the influence of individual differences in the timing and tempo of sexual maturation and its relationship with changes in adiposity, as well as the influence of early pubertal onset on cardiometabolic health in youth.
Acknowledgments
Contract grant sponsor: NIH; Contract grant number: NIH-NIDDK-1RC1DK086881-01; Contract grant sponsor: NIH Nutrition Obesity Research Center; Contract grant number: NIH-2P30DK072476; Contract grant sponsor: NIH NIDDK National Research Service Award; Contract grant number: T32DK064584-06; Contract grant sponsor: Louisiana Public Facilities Authority Endowed Chair in Nutrition.
Abbreviations
- BMI
body mass index
- DXA
dual energy x-ray absorptiometry
- MRI
magnetic resonance imaging
- SAT
subcutaneous adipose tissue
- TBF
total body fat
- VAT
visceral adipose tissue
- WC
waist circumference
Footnotes
Amanda Staiano wrote the first draft of this manuscript and all authors provided critical review. No one received an honorarium, grant, or other form of payment to produce the manuscript. Each author listed on the manuscript has seen and approved the submission of the manuscript and takes full responsibility for the manuscript.
LITERATURE CITED
- Baker JL, Olsen LW, Sørensen TIA. Childhood body-mass index and the risk of coronary heart disease in adulthood. New Eng J Med. 2007;357:2329–2337. doi: 10.1056/NEJMoa072515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benfield LL, Fox KR, Peters DM, Blake H, Rogers I, Grant C, Ness A. Magnetic resonance imaging of abdominal adiposity in a large cohort of British children. Int J Obes (Lond) 2008;32:91–99. doi: 10.1038/sj.ijo.0803780. [DOI] [PubMed] [Google Scholar]
- Brambilla P, Bedogni G, Moreno LA, Goran MI, Gutin B, Fox KR, Peters DM, Barbeau P, De Simone M, Pietrobelli A. Crossvalidation of anthropometry against magnetic resonance imaging for the assessment of visceral and subcutaneous adipose tissue in children. Int J Obes (Lond) 2006;30:23–30. doi: 10.1038/sj.ijo.0803163. [DOI] [PubMed] [Google Scholar]
- Brambilla P, Manzoni P, Agostini G, Beccaria L, Ruotolo G, Sironi S, Del Maschio A, Chiumello G. Persisting obesity starting before puberty is associated with stable intraabdominal fat during adolescence. Int J Obes (Lond) 1999;23:299–303. doi: 10.1038/sj.ijo.0800815. [DOI] [PubMed] [Google Scholar]
- Brambilla P, Manzoni P, Sironi S, Simone P, Del Maschio A, di Natale B, Chiumello G. Peripheral and abdominal adiposity in childhood obesity. Int J Obes Relat Metab Disord. 1994;18:795–800. [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. A SAS program for the CDC growth charts. Atlanta, GA: Centers for Disease Control and Prevention; 2011. [Google Scholar]
- de Ridder CM, de Boer RW, Seidell JC, Nieuwenhoff CM, Jeneson JA, Bakker CJ, Zonderland ML, Erich WB. Body fat distribution in pubertal girls quantified by magnetic resonance imaging. Int J Obes Relat Metab Disord. 1992;16:443–449. [PubMed] [Google Scholar]
- Euling SY, Herman-Giddens ME, Lee PA, Selevan SG, Juul A, Sørensen TIA, Dunkel L, Himes JH, Teilmann G, Swan SH. Examination of US puberty-timing data from 1940 to 1994 for secular trends: panel findings. Pediatrics. 2008;121(S3):S172–S191. doi: 10.1542/peds.2007-1813D. [DOI] [PubMed] [Google Scholar]
- Guo SS, Wu W, Chumlea WC, Roche AF. Predicting overweight and obesity in adulthood from body mass index values in childhood and adolescence. Am J Clin Nutr. 2002;76:653–658. doi: 10.1093/ajcn/76.3.653. [DOI] [PubMed] [Google Scholar]
- Himes JH, Obarzanek E, Baranowski T, Wilson DM, Rochon J, McClanahan BS. Early sexual maturation, body composition, and obesity in African-American girls. Obesity. 2004;12(S9):64S–72S. doi: 10.1038/oby.2004.270. [DOI] [PubMed] [Google Scholar]
- Hoffman WH, Barbeau P, Litaker MS, Johnson MH, Howe CA, Gutin B. Tanner staging of secondary sexual characteristics and body composition, blood pressure, and insulin in black girls. Obesity. 2005;13:2195–2201. doi: 10.1038/oby.2005.272. [DOI] [PubMed] [Google Scholar]
- Lee S, Kuk JL, Hannon TS, Arslanian SA. Race and gender differences in the relationships between anthropometrics and abdominal fat in youth. Obesity. 2008;16:1066–1071. doi: 10.1038/oby.2008.13. [DOI] [PubMed] [Google Scholar]
- Lee S, Kim Y, Kuk JL, Boada FE, Arslanian S. Whole-body MRI and ethnic differences in adipose tissue and skeletal muscle distribution in overweight black and white adolescent boys. J Obes. 2011:1–5. doi: 10.1155/2011/159373. 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liska D, Dufour S, Zern TL, Taksali S, Calí AMG, Dziura J, Shulman GI, Pierpont BM, Caprio S. Interethnic differences in muscle, liver and abdominal fat partitioning in obese adolescents. PLoS One. 2007;2:e569. doi: 10.1371/journal.pone.0000569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loomba-Albrecht LA, Styne DM. Effect of puberty on body composition. Curr Opin Endocrinol Diabetes Obes. 2009;16:10–15. doi: 10.1097/med.0b013e328320d54c. [DOI] [PubMed] [Google Scholar]
- Maffeis C, Manfredi R, Trombetta M, Sordelli S, Storti M, Benuzzi T, Bonadonna RC. Insulin sensitivity is correlated with subcutaneous but not visceral body fat in overweight and obese prepubertal children. J Clin Endocrinol Metab. 2008;93:2122–2128. doi: 10.1210/jc.2007-2089. [DOI] [PubMed] [Google Scholar]
- Malina RM, Bouchard C, Bar-Or O. Growth, maturation, and physical activity. 2nd ed. Champaign, IL: Human Kinetics; 2004. [Google Scholar]
- Matsudo SMM, Matsudo VKR. Self-assessment and physician assessment of sexual maturation in Brazilian boys and girls: Concordance and reproducibility. Am J Hum Biol. 1994;6:451–455. doi: 10.1002/ajhb.1310060406. [DOI] [PubMed] [Google Scholar]
- Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of obesity and trends in body mass index among US children and adolescents, 1999–2010. JAMA. 2012;307:483–490. doi: 10.1001/jama.2012.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prader A, Largo RH, Wolf C. Timing of pubertal growth and maturation in the first Zurich longitudinal growth study. Acta Paediatr Hung. 1984;25:155–159. [PubMed] [Google Scholar]
- Roemmich JN, Clark PA, Lusk M, Friel A, Weltman A, Epstein LH, Rogol AD. Pubertal alterations in growth and body composition. VI. Pubertal insulin resistance: relation to adiposity, body fat distribution and hormone release. Int J Obes Relat Metab Disord. 2002;26:701–709. doi: 10.1038/sj.ijo.0801975. [DOI] [PubMed] [Google Scholar]
- Sun SS, Schubert CM, Chumlea WC, Roche AF, Kulin HE, Lee PA, Himes JH, Ryan AS. National estimates of the timing of sexual maturation and racial differences among US children. Pediatrics. 2002;110:911–919. doi: 10.1542/peds.110.5.911. [DOI] [PubMed] [Google Scholar]
- Taksali SE, Caprio S, Dziura J, Dufour S, Cali AMG, Goodman TR, Papademetris X, Burgert TS, Pierpont BM, Savoye M, Shaw M, Seyal AA, Weiss R. High visceral and low abdominal subcutaneous fat stores in the obese adolescent: a determinant of an adverse metabolic phenotype. Diabetes. 2007;57:367–371. doi: 10.2337/db07-0932. [DOI] [PubMed] [Google Scholar]
- Tanner JM. Growth at adolescence. 2nd edn. Oxford: Blackwell Scientific Publications; 1962. [Google Scholar]
- Tanner JM. Growth and endocrinology of the adolescent. In: Gardner L, editor. Endocrine and Genetic Diseases of Childhood and Adolescence. 2nd edn. Philadelphia: WB Saunders; 1975. pp. 14–64. [Google Scholar]
- Tanner JM. Normal growth and techniques of growth assessment. Clin Endocrinol Metab. 1986;15:411–451. doi: 10.1016/s0300-595x(86)80005-6. [DOI] [PubMed] [Google Scholar]
- Tershakovec AM, Kuppler KM, Zemel BS, Katz L, Weinzimer S, Harty MP, Stallings VA. Body composition and metabolic factors in obese children and adolescents. Int J Obes. 2003;27:19–24. doi: 10.1038/sj.ijo.0802185. [DOI] [PubMed] [Google Scholar]