Abstract
Background
The mechanisms underlying the co-occurrence of the functional somatic syndromes are largely unknown. No empirical study has explicitly examined how genetic and environmental factors influence the comorbidity of these syndromes. We aimed to examine how the comorbidity of functional somatic syndromes is influenced by genetic and environmental factors that are in common to the syndromes.
Methods
A total of 31,318 twins in the Swedish Twin Registry aged 41–64 underwent screening interviews via a computer-assisted telephone system from 1998 to 2002. Four functional somatic syndromes (chronic widespread pain, chronic fatigue, irritable bowel syndrome, and recurrent headache) and 2 psychiatric disorders (major depression and generalized anxiety disorder) were assessed using structured questions based on standard criteria for each illness in a blinded manner.
Results
Multivariate twin analyses revealed that a common pathway model with 2 latent traits that were shared by the 6 illnesses fit best to the women's data. One of the 2 latent traits loaded heavily on the psychiatric disorders, whereas the other trait loaded on all 4 of the functional somatic syndromes, particularly chronic widespread pain, but not on the psychiatric disorders. All illnesses except the psychiatric disorders were also affected by genetic influences that were specific to each.
Conclusions
The co-occurrence of functional somatic syndromes in women can be best explained by affective and sensory components in common to all these syndromes, as well as by unique influences specific to each of them. The findings clearly suggest a complex view of the multifactorial pathogenesis of these illnesses.
INTRODUCTION
It is a general observation in clinical practice that patients with persistent pain or fatigue frequently have comorbid symptoms that show similar clinical features. These symptoms or syndromes include fibromyalgia, chronic fatigue syndrome, irritable bowel syndrome, and recurrent headache. An increasing literature has revealed that considerable overlap (Aaron & Buchwald, 2001) exists among these illnesses, which are often referred to as functional somatic syndromes (Barsky & Borus, 1999; Henningsen, Zipfel, & Herzog, 2007; Wessely, Nimnuan, & Sharpe, 1999), although there is no consensus definition or criteria. These syndromes also share characteristics such as a female predominance and frequent co-occurrence with psychiatric disorders, most notably major depression and generalized anxiety disorder.
In our previous report (Kato, Sullivan, Evengard, & Pedersen, 2006a), we demonstrated elevated risks of comorbidity with chronic widespread pain in the general population. Furthermore, the extent to which the observed associations are influenced by familial (i.e., genetic and environmental) factors may be illness-specific. Nevertheless, the etiological structure in the mechanisms underlying the co-occurrence of these syndromes remains unknown. Typically, the discussion over functional somatic syndromes can be characterized as reflecting conceptualizations from “lumpers” and “splitters” (Deary, 1999; Wessely & White, 2004): The former tend to emphasize the commonalities among the syndromes, whereas the latter tend to emphasize the distinctness of each syndrome. Some studies support the notion that these syndromes can be clustered, by using statistical modeling (Ciccone & Natelson, 2003; Nimnuan, Rabe-Hesketh, Wessely, & Hotopf, 2001; Nisenbaum, Reyes, Unger, & Reeves, 2004). On the other hand, various studies have found differences among the syndromes (Evengard et al., 1998; Moss-Morris & Spence, 2006; Parker, Wessely, & Cleare, 2001; Romans, Belaise, Martin, Morris, & Raffi, 2002). Thus, at issue is to investigate the balance between splitting and lumping views of these syndromes (Henningsen et al., 2007). To date, no empirical study has explicitly examined whether and to what extent functional somatic syndromes can be attributable to one or a few factors in common to the syndromes, and how important genetic and environmental influences are for the common factor(s), if any.
The present study employed a quantitative genetic approach using twins, a valuable source of information about genetic and environmental bases of complex disorders (Boomsma, Busjahn, & Peltonen, 2002). The twins were ascertained from the population-based Swedish Twin Registry, which enabled us to minimize selection biases to which clinical samples are prone (e.g., those seeking medical care tend to have more concurrent disorders). By using the same population-based sample as in our previous report (Kato et al., 2006a), we aimed in this study to evaluate the multivariate structure of genetic and environmental factors for the comorbidities of 4 most common functional somatic syndromes (chronic widespread pain, chronic fatigue, irritable bowel syndrome, and recurrent headache) and 2 psychiatric disorders (major depression and generalized anxiety disorder), all of which were assessed via standard criteria. Based on our previous findings (Kato et al., 2006a), we already know that the co-occurrence of these illnesses are influenced, in part, by familial factors that are shared among them. However, we now will be able to go further and reveal the structure and relative importance of genetic and environmental influences for the co-occurrence.
METHODS
Subjects
Subjects were participants in the Swedish Twin Registry, which comprises all twin births in Sweden from 1886 to 2000, with data on place of birth, current vital status, and address of more than 160,000 individuals (Lichtenstein et al., 2002; Lichtenstein et al., 2006). All living, contactable, and consenting twins born in Sweden between January 1935, and December 1958 were interviewed via the telephone from March 1998 to December 2002. Thus, the interviewees were between 41 and 64 years of age at the time of the interview. The interviews were conducted by trained personnel with adequate medical background using a computer based data collection system. All participants provided verbal informed consent during this telephone interview which was later confirmed by postcard. Zygosity was based on responses to questions regarding physical similarity in childhood. This method has been validated repeatedly as having 98% or higher accuracy by using DNA markers (Lichtenstein et al., 2002). This study was approved by the ethical committee of Karolinska Institutet.
Assessment Procedures
Chronic widespread pain was defined using our previously reported algorithm (Kato et al., 2006a; Kato, Sullivan, Evengard, & Pedersen, 2006b) based on the classification criteria for fibromyalgia proposed by the American College of Rheumatology (Wolfe et al., 1990) without clinical examinations. Interviewees were asked about continuous pain during 3 consecutive months in both the upper and lower body and in both the right and left sides of the body. Those who further endorsed pain on body axis (assessed as back pain in the last 12 months) in addition to the previous questions were defined as chronic widespread pain cases. Detailed information about the criteria for chronic widespread pain in this study was reported elsewhere (Kato et al., 2006b).
Screening of chronic fatigue was based on our criteria that emulated closely the 1994 criteria for chronic fatigue syndrome (Fukuda et al., 1994) without clinical examinations. We defined fatigue as the presence of self-reported abnormal tiredness in the absence of an exclusionary condition. Exclusionary conditions were determined from multiple sources (e.g., Swedish national registers and medical records), as described elsewhere (Evengard, Jacks, Pedersen, & Sullivan, 2005). Subjects were considered having chronic impairing fatigue when they endorsed that they had felt abnormally tired in the last 6 months and that they experienced impairment. The definition of chronic impairing fatigue corresponds to “CF-B” in our previous report (Evengard et al., 2005).
Irritable bowel syndrome was assessed by criteria used in previous studies in the Swedish Twin Registry (Svedberg, Johansson, Wallander, Hamelin, & Pedersen, 2002), which is in line with the Rome criteria (Thompson, Dotevall, Drossman, Heaton, & Kruis, 1989). In brief, subjects who endorsed experiencing recurrent discomfort in the stomach or intestine at least 7 days per month with one of the 6 symptoms were defined as having irritable bowel syndrome.
A lifetime history of recurrent headache not associated with infection, fever, or hangover was assessed through the stem question, “Do you or have you ever suffered from recurrent headaches, that were not caused by infection, fever, or hangovers?”, which has been used in previous studies in the Swedish Twin Registry (Svensson, Waldenlind, Ekbom, & Pedersen, 2004).
Major depression and generalized anxiety disorder were assessed by using the Computerized Composite International Diagnostic Interview-Short Form (CIDI-SF) adapted from its original design for 12-month prevalence to assess lifetime prevalence (Kessler, Andrews, Mroczek, Ustun, & Wittchen, 1998). Details on these criteria were described elsewhere (Kendler, Gatz, Gardner, & Pedersen, 2006b; Mackintosh, Gatz, Wetherell, & Pedersen, 2006).
Statistical Analyses
Patterns of similarity in monozygotic and dizygotic pairs are the basis of inferences regarding the likely importance of genetic and family environmental influences. Because monozygotic twins share all their genes and dizygotic twins share only half (of their segregating genes) on average, comparison of similarities in monozygotic and dizygotic twins permits estimating the relative importance of genes and environment. Using structural equation modeling, individual differences in liability to disease may be decomposed into 3 latent sources of variation: additive genetic, shared environmental, and nonshared environmental variance. Additive genetic influences are indicated when monozygotic twins are more similar than dizygotic twins. Shared (family) environmental influences (environmental influences that make family members similar) contribute equally to the similarity of monozygotic and dizygotic twins. Nonshared (unique) environmental influences, which contribute to the dissimilarity of twins, include measurement errors as well. As a preliminary analysis, we first calculated phenotypic tetrachoric correlations stratified by sex, using the PROC FREQ procedure in SAS statistical software version 9 (SAS Institute, Inc, Cary, NC).
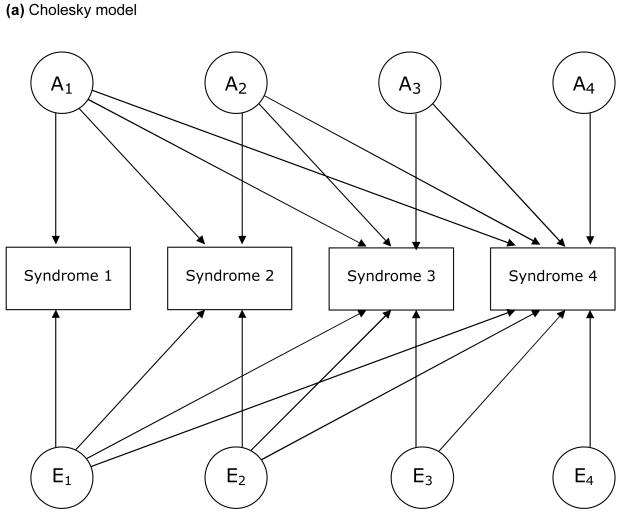
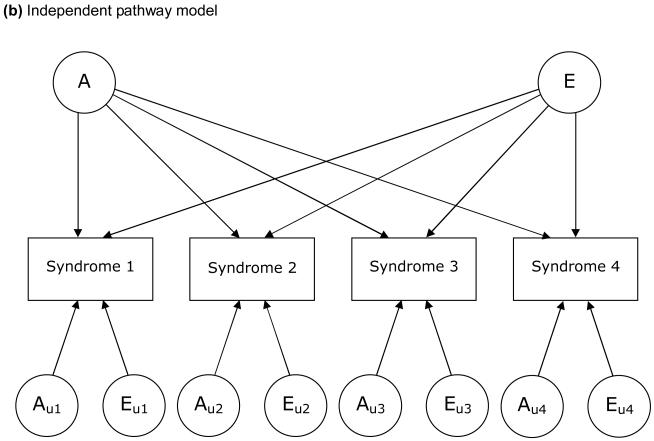
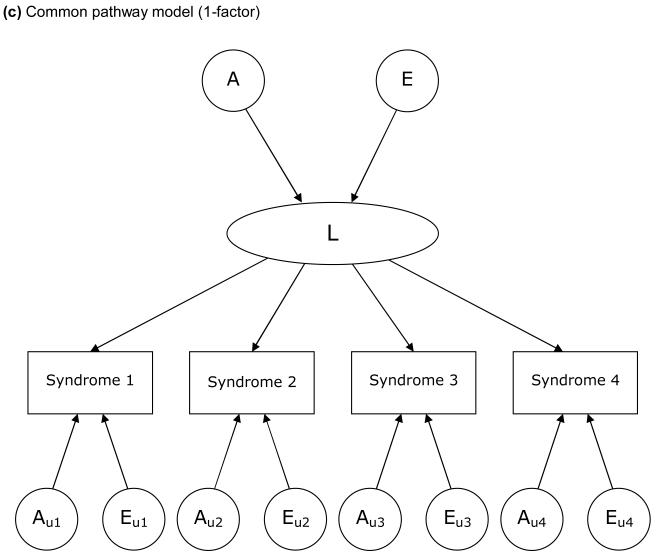
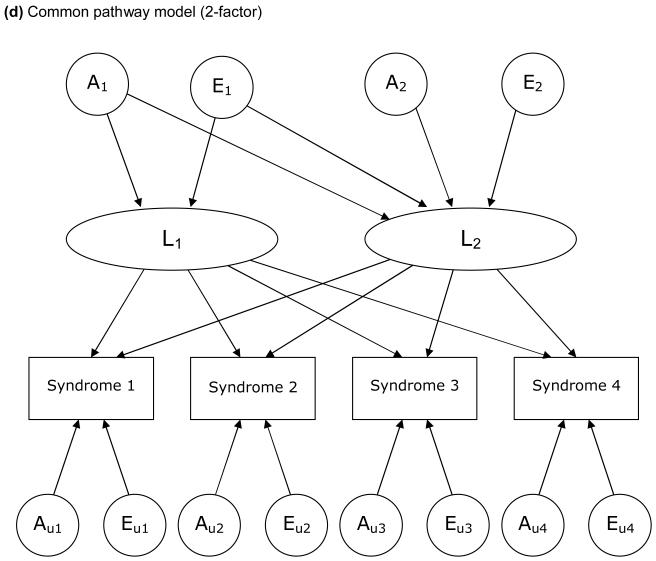
The models examined in this study are based on a liability threshold model (Falconer, 1965). We examined 3 types of model, each of which depicts a particular hypothesis about genetic and environmental structure for comorbidities. FIGURE 1 illustrates these 3 models, in which 4 variables for each are included for simplicity. First, we examined a Cholesky decomposition (also known as a triangular decomposition) model, which included additive genetic (A), shared environmental, and nonshared environmental (E) components (Figure 1a). In a Cholesky model, the first factor loads on all the variables, the second factor loads on all but the first one, and so on. Second, we examined an independent pathway model which included 1 common factor of genetic and environmental components, as well as unique factors for each variable (Figure 1b). Third, we examined a common pathway model, where all variables were influenced by a single latent factor that was itself influenced by genetic and environmental components (Figure 1c). We further extended our analysis of common pathway models with 2 (Figure 1d) and 3 latent factors. We compared the Cholesky model, independent pathway model, and common pathway models with 1 to 3 latent factors in terms of Akaike Information Criterion (AIC) (Akaike, 1987). A smaller, more negative value of AIC indicates that the model fits the data better than other models with a greater AIC. Maximum likelihood model fitting was applied to raw data, by using the Mx program (Neale, Boker, Xie, & Maes, 2004). In all analyses, we adjusted trait prevalence (threshold of liability to each illness) for age at the interview and sex using logistic regression coefficients computed by the PROC LOGISTIC procedure in SAS. Standardized path coefficients were obtained by fixing the total variance of each variable as well as the variance of latent factors (in common pathway models) to be unity. Through an iterative procedure, we ran each model 6 times, using parameter estimates for one model as the starting values for the next model, and picked the model with the best fit (typically the last).
Figure 1.
Examples of models for underlying structures of comorbidities (multivariate AE models).
A: additive genetic factors; E: nonshared environmental factors. L: latent factor. Factors with a subscript “u” are unique to each syndrome. For simplicity, only one twin of a pair is shown and shared environmental factors are excluded.
RESULTS
Of 41,355 eligible individuals under 65 years of age, 31,318 responded to the interview. Both members of 12,248 twin pairs (24,496 pair-wise respondents) and 6,822 single respondents were included. Of the pair-wise respondents, 3,260 pairs were monozygotic, 4,470 pairs same-sexed dizygotic, 4,518 pairs opposite-sexed dizygotic, and 127 pairs of unknown zygosity. The mean age ± standard deviation of the sample at the time of the interview was 53.7 ± 5.7 years, and 53.1% were women.
TABLE 1 summarizes the prevalence estimates for the 4 functional somatic syndromes and the 2 psychiatric disorders. Recurrent headache showed the highest prevalence both in women and men, followed by major depression. TABLE 2 shows phenotypic associations (tetrachoric correlations) stratified by sex. The pattern of correlations was generally similar in men and women. The strongest associations were between chronic widespread pain and chronic impairing fatigue, and between generalized anxiety disorder and major depression. Most other associations were moderate, and all were significantly greater than zero (P < .001). Due to the scarcity of male pairs concordant for 2 of the syndromes, only women were used in the subsequent analyses.
Table 1.
Sex-specific estimates of prevalence for the 6 illnesses in subjects aged 41–64.
| Illness | CWP | CF | IBS | Headache | GAD | MD | |
|---|---|---|---|---|---|---|---|
| N of respondents | Women | 16,320 | 16,278 | 15,405 | 16,362 | 16,440 | 16,348 |
| Men | 14,749 | 14,710 | 13,797 | 14,780 | 14,878 | 14,765 | |
| N of cases | Women | 1,115 | 1,312 | 1,368 | 5,330 | 862 | 4,689 |
| Men | 302 | 511 | 709 | 2,625 | 387 | 2,369 | |
| Prevalence (%) | Women | 6.8 | 8.1 | 8.9 | 32.6 | 5.2 | 28.7 |
| Men | 2.0 | 3.5 | 5.1 | 17.8 | 2.6 | 16.0 | |
CWP: chronic widespread pain, CF: chronic impairing fatigue, IBS: irritable bowel syndrome, GAD: generalized anxiety disorder, MD: major depression. Subjects who did not provide usable answers for screening were excluded.
Table 2.
Phenotypic (tetrachoric) correlations among the 6 illnesses in subjects aged 41–64.
| Men | CWP | CF | IBS | Headache | GAD | MD |
|---|---|---|---|---|---|---|
| Women | ||||||
| CWP | 0.51 | 0.34 | 0.31 | 0.32 | 0.23 | |
| CF | 0.59 | 0.33 | 0.29 | 0.39 | 0.38 | |
| IBS | 0.42 | 0.37 | 0.24 | 0.31 | 0.24 | |
| Headache | 0.36 | 0.29 | 0.23 | 0.23 | 0.22 | |
| GAD | 0.25 | 0.30 | 0.24 | 0.16 | 0.53 | |
| MD | 0.20 | 0.27 | 0.22 | 0.22 | 0.49 |
CWP: chronic widespread pain, CF: chronic impairing fatigue, IBS: irritable bowel syndrome, GAD: generalized anxiety disorder, MD: major depression. Men are shown above the diagonal (N = 14,878) and women below (N = 16,440). All correlations are statistically significant at P <.001.
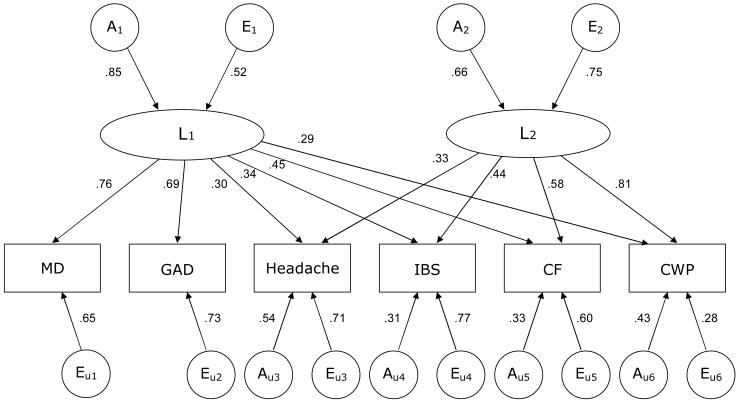
In the model fitting (TABLE 3), we first tested each model by dropping shared environmental factors, resulting in no significant changes in fit. Thus, only additive genetic factors (A) and nonshared environmental factors (E) were included in the models thereafter (“AE models”). When we compared the 5 models (i.e., models a, b, c(i), c(ii), and c(iii) in Table 3) in terms of AIC, the 2-factor common pathway model c(ii) fit best, i.e., had the smallest AIC. We then dropped non-significant paths from the 2-factor common pathway model in order to obtain the most parsimonious model (FIGURE 2). Dropping the paths from A1 and E1 to the second latent factor L2 did not significantly worsen the goodness-of-fit (c(ii)' in Table 3), indicating that these 2 latent factors are independent of each other both genetically and environmentally. The first latent factor L1 was more greatly influenced by genetic factor A1 than by environmental factor E1, whereas L2 was slightly more influenced by environmental factor E2 than by genetic factor A2. Only L1 loaded on major depression and generalized anxiety disorder. In addition, paths from unique genetic factors to these disorders were not significant, suggesting that genetic influences on major depression and generalized anxiety disorder can be accounted for by the common genetic factor A1 through L1. On the other hand, L2 loaded primarily on chronic widespread pain, followed by chronic impairing fatigue. For all 4 functional somatic syndromes, the loading from L2 was greater than that from L1. The proportion of variance explained by the 2 latent factors was 74% (=0.292 + 0.812) for chronic widespread pain, 54% for chronic impairing fatigue, 31% for irritable bowel syndrome, and 20% for recurrent headache. Note that the loadings from L1 and L2 were standardized for each variable, which means that the values of path coefficients indicate the relative importance for liability to each syndrome or disorder and should not be interpreted as reflecting the actual magnitude of the impacts of L1 and L2.
Table 3.
Comparison of goodness-of-fit among 5 full models examined.
| Type of model | −2 log-likelihood | Degrees of freedom | Chi-square differences1) | P | AIC | |
|---|---|---|---|---|---|---|
| a. Cholesky model | 36186.052 | 49726 | Ref | −63265.948 | ||
| b. Independent pathway model | 36212.874 | 49738 | 26.822 | 0.0082 | −63263.126 | |
| c. Common pathway model | (i) Full 1-factor | 36392.842 | 49743 | 251.92 | < 0.0001 | −63093.158 |
| (ii) Full 2-factor | 36149.033 | 49734 | 8.12 | 0.703 | −63318.967 | |
| (ii)' Best-fit 2-factor | 36150.019 | 49740 | 9.16 | 0.937 | −63329.981 | |
| (iii) Full 3-factor | 36140.913 | 49723 | Ref | −63305.087 | ||
The best-fit model is shown in Figure 2. AIC: Akaike Information Criterion
1) A difference in −2 log-likelihood is known to asymptotically distribute as a chi-square distribution with degrees of freedom calculated as the difference in the degrees of freedom for the models compared. Comparisons are model b versus model a, and model c (iii) versus model c (i) – c(iia).
Figure 2.
Best-fit (2-factor common pathway) model with age-adjusted, standardized parameter estimates for the 6 illnesses in women aged 41–64.
A: additive genetic factors; E: nonshared environmental factors. Factors with a subscript “u” are unique to each illness. L1, L2: latent factors. MD: major depression. GAD: generalized anxiety disorder. IBS: irritable bowel syndrome. CF: chronic impairing fatigue. CWP: chronic widespread pain. The total variances of L1 and L2 as well as each variable were fixed at 1. Non-significant paths were dropped from the model. For simplicity, only one twin of a pair is shown.
DISCUSSION
In this large, population-based study using a genetically informative sample of women, we found that the co-occurrence of functional somatic syndromes (chronic widespread pain, chronic impairing fatigue, irritable bowel syndrome, and recurrent headache) is due to combined effects of 2 latent traits: One of the traits is predominantly defined as psychiatric and more influenced by genetic factors, whereas the other is not related to psychiatric disorders but particularly to chronic widespread pain and is more influenced by environmental factors. In addition, we found that each syndrome is also influenced by genetic and environmental factors that are specific to each. To our knowledge, the present study is the first to reveal the structure and relative importance of genetic and environmental influences on complex etiological mechanisms underlying the comorbidities of as yet unexplained functional somatic syndromes.
Researchers have widely debated whether the illnesses known as functional somatic syndromes can be clustered as a single, either physical or mental disorder, or each of these illnesses should be considered as a distinct entity (Wessely & White, 2004). The empirical evidence for women provided by the present study demonstrates the entire etiological structure wherein both psychiatric and non-psychiatric (or physical) traits influence the co-occurrence. Both the latent traits in common to all the syndromes and those factors unique to each syndrome play some role. Furthermore, the relative importance of the 2 latent traits for the syndromes varies from illness to illness, ranging between 20% (recurrent headache) and 74% (chronic widespread pain). Our findings suggest a possible solution to controversies over the pathogenesis of functional somatic syndromes. That is, these syndromes co-occur because they share 2 etiological components, whereas the syndromes are distinguishable because factors unique to each are also important. Given that these unique factors are predominantly environmental for all the syndromes except chronic widespread pain (Figure 2), it is likely that differences in symptoms and characteristics observed in each syndrome are primarily due to non-familial, environmental influences experienced by each individual. Collectively, the results in this study lend support for multifactorial pathogenesis in both the etiology of the syndromes and their co-occurrence.
Latent trait L1, which is common to all 6 illnesses, indicates that functional somatic syndromes share underlying mechanisms in part with major depression and generalized anxiety disorder. The present finding that these psychiatric disorders contribute to the associations among the functional somatic syndromes through a latent trait with a substantial genetic loading is consistent with our previous findings on the association between major depression, generalized anxiety disorder, and chronic widespread pain (Kato et al., 2006a). Given that emotional instability is a predictor of depression (Kendler, Gatz, Gardner, & Pedersen, 2006a), the present results are also in line with findings that the association between premorbid emotional instability and chronic fatigue was mediated by genetic factors (Kato, Sullivan, Evengard, & Pedersen, 2006c). Furthermore, our recent study has demonstrated that premorbid emotional instability is a predictor of not only chronic fatigue but also chronic widespread pain and other related symptoms, and that these prospective associations are partly mediated by genetic influences (Charles, Gatz, Kato, & Pedersen, in press). Taking into consideration these longitudinal studies as well as the present study,, the latent trait L1 is likely related to the predisposition to these psychiatric disorders, and the genetic factor A1 is likely a set of genes responsible for its biological mechanisms, e.g., serotonin transporter genes (Camilleri et al., 2002; Juhasz et al., 2003; Narita et al., 2003; Offenbaecher et al., 1999). Similarly, environmental factor E1 may be individual experiences such as stressful life events and social learning. It should be of note, however, that the present results do not mean that psychiatric disorders cause functional somatic syndromes, but rather that these illnesses are likely to share psychobiological pathways in the pathogenesis, e.g., responses to stress or regulatory mechanisms in the brain.
We found a distinct, second latent trait L2, which itself is more influenced by environmental factors than by genetic ones. Given the substantial loading of L2 on chronic widespread pain, we speculate that this trait is related primarily to pain sensation without its affective dimension. Exaggerated pain is common in patients with fibromyalgia (Price et al., 2002; Staud, Vierck, Cannon, Mauderli, & Price, 2001), and studies have consistently supported the contribution of central mechanisms to pain hypersensitivity (Staud & Smitherman, 2002). Abnormalities of central pain processing similar to fibromyalgia have also been observed in patients with irritable bowel syndrome (Verne & Price, 2002) and recurrent headache (Okifuji, Turk, & Marcus, 1999), although the evidence for central sensitization in chronic fatigue is limited. These findings suggest abnormal pain processing in the central nervous system as a plausible pathophysiologic characteristic in common to functional somatic syndromes. In addition, a study using brain imaging for fibromyalgia patients showed that neither the presence nor the level of depression modulated the sensory dimension of pain; however, depression was associated with neuronal activation in brain regions processing the affective dimension of pain, suggesting that pain in fibromyalgia is regulated by the two distinct, affective and sensory dimensions (Giesecke et al., 2005). Thus, the 2 latent traits can be considered analogous to the 2 dimensions of pain and we propose our model be called the “affective-sensory model” of functional somatic syndromes.
The present study has notable strengths such as using a genetically informative sample of twins who can be considered a representative of Swedish population. In addition, 4 major functional somatic syndromes and 2 psychiatric disorders were assessed by using a structured interview in a blinded manner, which allows us to minimize recall and interviewer biases. Furthermore, the final best-fit model was selected through a series of statistical tests in a conventional manner, which is free from a priori knowledge or biases due to investigators' medical specialty (Wessely et al., 1999). Nevertheless, some limitations should also be noted. First, our assessment was based on self-reports via telephone interviews, without physical examinations by a physician. Thus, the results may not be directly comparable to those using clinical samples. Second, the data were obtained on only one occasion, although some of the illnesses were diagnosed as lifetime occurrence. Thus, temporal order is unclear. Third, our study design does not preclude the possibility that subjects with these syndromes were heterogeneous and could be categorized into subgroups. For example, the influences of L2 on chronic fatigue may reflect subgroups of subjects who suffered from joint and muscle pain as well as fatigue. To answer this question, further investigations using statistical techniques such as latent class analysis will be needed (Sullivan, Pedersen, Jacks, & Evengard, 2005). Fourth, it is possible that the proportion of measurement error in the estimates of specific environmental influences (Eu) differs from illness to illness. Thus, care should be taken when comparing estimates for Eu between or across the illnesses examined. Finally, gene-environment interaction was not addressed in the present analyses. Thus, the estimates reported here may be regarded as a first approximation of the relative contributions of genetic and environmental influences.
In conclusion, the co-occurrence of functional somatic syndromes can be best explained by distinct affective and sensory components that impact all these syndromes, as well as by influences specific to each. The findings suggest that a complex view of the multifactorial pathogenesis of these illnesses is clearly required.
ACKNOWLEDGMENTS
This study was supported by NS-041483. The Swedish Twin Registry is supported by grants from the Swedish Department of Higher Education, the Swedish Research Council, and AstraZeneca. We declare that we have no conflict of interest. Dr Kato had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. The funding source had no role in the design or conduct of the study; collection, management, analysis, or interpretation of the data; or preparation, review, or approval of the manuscript.
This study was supported by NS-041483. The Swedish Twin Registry is supported by grants from the Swedish Department of Higher Education, the Swedish Research Council, and AstraZeneca.
REFERENCES
- Aaron LA, Buchwald D. A review of the evidence for overlap among unexplained clinical conditions. Annals of Internal Medicine. 2001;134(9 Pt 2):868–881. doi: 10.7326/0003-4819-134-9_part_2-200105011-00011. [DOI] [PubMed] [Google Scholar]
- Akaike H. Factor analysis and AIC. Psychometrika. 1987;52:317–332. [Google Scholar]
- Barsky AJ, Borus JF. Functional somatic syndromes. Annals of Internal Medicine. 1999;130(11):910–921. doi: 10.7326/0003-4819-130-11-199906010-00016. [DOI] [PubMed] [Google Scholar]
- Boomsma D, Busjahn A, Peltonen L. Classical twin studies and beyond. Nature Reviews Genetics. 2002;3(11):872–882. doi: 10.1038/nrg932. [DOI] [PubMed] [Google Scholar]
- Camilleri M, Atanasova E, Carlson PJ, Ahmad U, Kim HJ, Viramontes BE, et al. Serotonin-transporter polymorphism pharmacogenetics in diarrhea-predominant irritable bowel syndrome. Gastroenterology. 2002;123(2):425–432. doi: 10.1053/gast.2002.34780. [DOI] [PubMed] [Google Scholar]
- Charles ST, Gatz M, Kato K, Pedersen NL. Physical health twenty-five years later: the predictive ability of neuroticism. Health Psychology. doi: 10.1037/0278-6133.27.3.369. in press. [DOI] [PubMed] [Google Scholar]
- Ciccone DS, Natelson BH. Comorbid illness in women with chronic fatigue syndrome: a test of the single syndrome hypothesis. Psychosomatic Medicine. 2003;65(2):268–275. doi: 10.1097/01.psy.0000033125.08272.a9. [DOI] [PubMed] [Google Scholar]
- Deary IJ. A taxonomy of medically unexplained symptoms. Journal of Psychosomatic Research. 1999;47(1):51–59. doi: 10.1016/s0022-3999(98)00129-9. [DOI] [PubMed] [Google Scholar]
- Evengard B, Jacks A, Pedersen NL, Sullivan PF. The epidemiology of chronic fatigue in the Swedish Twin Registry. Psychological Medicine. 2005;35(9):1317–1326. doi: 10.1017/S0033291705005052. [DOI] [PubMed] [Google Scholar]
- Evengard B, Nilsson CG, Lindh G, Lindquist L, Eneroth P, Fredrikson S, et al. Chronic fatigue syndrome differs from fibromyalgia. No evidence for elevated substance P levels in cerebrospinal fluid of patients with chronic fatigue syndrome. Pain. 1998;78(2):153–155. doi: 10.1016/S0304-3959(98)00134-1. [DOI] [PubMed] [Google Scholar]
- Falconer DS. Inheritance of liability to certain diseases estimated from incidence among relatives. Annals of Human Genetics. 1965;29:51–71. [Google Scholar]
- Fukuda K, Straus SE, Hickie I, Sharpe MC, Dobbins JG, Komaroff AL. The chronic fatigue syndrome: a comprehensive approach to its definition and study. International Chronic Fatigue Syndrome Study Group. Annals of Internal Medicine. 1994;121(12):953–959. doi: 10.7326/0003-4819-121-12-199412150-00009. [DOI] [PubMed] [Google Scholar]
- Giesecke T, Gracely RH, Williams DA, Geisser ME, Petzke FW, Clauw DJ. The relationship between depression, clinical pain, and experimental pain in a chronic pain cohort. Arthritis and Rheumatism. 2005;52(5):1577–1584. doi: 10.1002/art.21008. [DOI] [PubMed] [Google Scholar]
- Henningsen P, Zipfel S, Herzog W. Management of functional somatic syndromes. Lancet. 2007;369(9565):946–955. doi: 10.1016/S0140-6736(07)60159-7. [DOI] [PubMed] [Google Scholar]
- Juhasz G, Zsombok T, Laszik A, Gonda X, Sotonyi P, Faludi G, et al. Association analysis of 5-HTTLPR variants, 5-HT2a receptor gene 102T/C polymorphism and migraine. Journal of Neurogenetics. 2003;17(2–3):231–240. [PubMed] [Google Scholar]
- Kato K, Sullivan PF, Evengard B, Pedersen NL. Chronic widespread pain and its comorbidities: a population-based study. Archives of Internal Medicine. 2006a;166(15):1649–1654. doi: 10.1001/archinte.166.15.1649. [DOI] [PubMed] [Google Scholar]
- Kato K, Sullivan PF, Evengard B, Pedersen NL. Importance of genetic influences on chronic widespread pain. Arthritis and Rheumatism. 2006b;54(5):1682–1686. doi: 10.1002/art.21798. [DOI] [PubMed] [Google Scholar]
- Kato K, Sullivan PF, Evengard B, Pedersen NL. Premorbid predictors of chronic fatigue. Archives of General Psychiatry. 2006c;63(11):1267–1272. doi: 10.1001/archpsyc.63.11.1267. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Gatz M, Gardner CO, Pedersen NL. Personality and major depression: a Swedish longitudinal, population-based twin study. Archives of General Psychiatry. 2006a;63(10):1113–1120. doi: 10.1001/archpsyc.63.10.1113. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Gatz M, Gardner CO, Pedersen NL. A Swedish national twin study of lifetime major depression. American Journal of Psychiatry. 2006b;163(1):109–114. doi: 10.1176/appi.ajp.163.1.109. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Andrews G, Mroczek DK, Ustun B, Wittchen H-U. The World Health Organization Composite International Diagnostic Interview Short Form (CIDI-SF) International Journal of Methods in Psychiatric Research. 1998;7:171–185. [Google Scholar]
- Lichtenstein P, De Faire U, Floderus B, Svartengren M, Svedberg P, Pedersen NL. The Swedish Twin Registry: a unique resource for clinical, epidemiological and genetic studies. Journal of Internal Medicine. 2002;252(3):184–205. doi: 10.1046/j.1365-2796.2002.01032.x. [DOI] [PubMed] [Google Scholar]
- Lichtenstein P, Sullivan PF, Cnattingius S, Gatz M, Johansson S, Carlstrom E, et al. The Swedith Twin Registry in the third millenium: an update. Twin Research and Human Genetics. 2006;9:875–882. doi: 10.1375/183242706779462444. [DOI] [PubMed] [Google Scholar]
- Mackintosh MA, Gatz M, Wetherell JL, Pedersen NL. A twin study of lifetime generalized anxiety disorder (GAD) in older adults: Genetic and environmental influences shared by neuroticism and GAD. Twin Research and Human Genetics. 2006;9(1):30–37. doi: 10.1375/183242706776402902. [DOI] [PubMed] [Google Scholar]
- Moss-Morris R, Spence M. To “lump” or to “split” the functional somatic syndromes: can infectious and emotional risk factors differentiate between the onset of chronic fatigue syndrome and irritable bowel syndrome? Psychosomatic Medicine. 2006;68(3):463–469. doi: 10.1097/01.psy.0000221384.07521.05. [DOI] [PubMed] [Google Scholar]
- Narita M, Nishigami N, Narita N, Yamaguti K, Okado N, Watanabe Y, et al. Association between serotonin transporter gene polymorphism and chronic fatigue syndrome. Biochemical and Biophysical Research Communications. 2003;311(2):264–266. doi: 10.1016/j.bbrc.2003.09.207. [DOI] [PubMed] [Google Scholar]
- Neale MC, Boker SM, Xie G, Maes HHM. Mx: Statistical Modeling. Virginia Commonwealth University Department of Psychiatry; Richmond, VA: 2004. [Google Scholar]
- Nimnuan C, Rabe-Hesketh S, Wessely S, Hotopf M. How many functional somatic syndromes? Journal of Psychosomatic Research. 2001;51(4):549–557. doi: 10.1016/s0022-3999(01)00224-0. [DOI] [PubMed] [Google Scholar]
- Nisenbaum R, Reyes M, Unger ER, Reeves WC. Factor analysis of symptoms among subjects with unexplained chronic fatigue: what can we learn about chronic fatigue syndrome? Journal of Psychosomatic Research. 2004;56(2):171–178. doi: 10.1016/S0022-3999(03)00039-4. [DOI] [PubMed] [Google Scholar]
- Offenbaecher M, Bondy B, de Jonge S, Glatzeder K, Kruger M, Schoeps P, et al. Possible association of fibromyalgia with a polymorphism in the serotonin transporter gene regulatory region. Arthritis and Rheumatism. 1999;42(11):2482–2488. doi: 10.1002/1529-0131(199911)42:11<2482::AID-ANR27>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Okifuji A, Turk DC, Marcus DA. Comparison of generalized and localized hyperalgesia in patients with recurrent headache and fibromyalgia. Psychosomatic Medicine. 1999;61(6):771–780. doi: 10.1097/00006842-199911000-00009. [DOI] [PubMed] [Google Scholar]
- Parker AJR, Wessely S, Cleare AJ. The neuroendocrinology of chronic fatigue syndrome and fibromyalgia. Psychological Medicine. 2001;31(8):1331–1345. doi: 10.1017/s0033291701004664. [DOI] [PubMed] [Google Scholar]
- Price DD, Staud R, Robinson ME, Mauderli AP, Cannon R, Vierck CJ. Enhanced temporal summation of second pain and its central modulation in fibromyalgia patients. Pain. 2002;99(1–2):49–59. doi: 10.1016/s0304-3959(02)00053-2. [DOI] [PubMed] [Google Scholar]
- Romans S, Belaise C, Martin J, Morris E, Raffi A. Childhood abuse and later medical disorders in women. An epidemiological study. Psychotherapy & Psychosomatics. 2002;71(3):141–150. doi: 10.1159/000056281. [DOI] [PubMed] [Google Scholar]
- Staud R, Smitherman ML. Peripheral and central sensitization in fibromyalgia: pathogenetic role. Current Pain and Headache Reports. 2002;6(4):259–266. doi: 10.1007/s11916-002-0046-1. [DOI] [PubMed] [Google Scholar]
- Staud R, Vierck CJ, Cannon RL, Mauderli AP, Price DD. Abnormal sensitization and temporal summation of second pain (wind-up) in patients with fibromyalgia syndrome. Pain. 2001;91(1–2):165–175. doi: 10.1016/s0304-3959(00)00432-2. [DOI] [PubMed] [Google Scholar]
- Sullivan PF, Pedersen NL, Jacks A, Evengard B. Chronic fatigue in a population sample: definitions and heterogeneity. Psychological Medicine. 2005;35(9):1337–1348. doi: 10.1017/S0033291705005210. [DOI] [PubMed] [Google Scholar]
- Svedberg P, Johansson S, Wallander MA, Hamelin B, Pedersen NL. Extra-intestinal manifestations associated with irritable bowel syndrome: a twin study. Alimentary Pharmacology & Therapeutics. 2002;16(5):975–983. doi: 10.1046/j.1365-2036.2002.01254.x. [DOI] [PubMed] [Google Scholar]
- Svensson DA, Waldenlind E, Ekbom K, Pedersen NL. Heritability of migraine as a function of definition. Journal of Headache and Pain. 2004;5(3):171–176. [Google Scholar]
- Thompson WG, Dotevall G, Drossman DA, Heaton KW, Kruis W. Irritable-Bowel-Syndrome: guidelines for the diagnosis. Gastroenterology International. 1989;2:92–95. [Google Scholar]
- Verne GN, Price DD. Irritable bowel syndrome as a common precipitant of central sensitization. Current Rheumatology Reports. 2002;4(4):322–328. doi: 10.1007/s11926-002-0041-x. [DOI] [PubMed] [Google Scholar]
- Wessely S, Nimnuan C, Sharpe M. Functional somatic syndromes: one or many? Lancet. 1999;354(9182):936–939. doi: 10.1016/S0140-6736(98)08320-2. [DOI] [PubMed] [Google Scholar]
- Wessely S, White PD. There is only one functional somatic syndrome. British Journal of Psychiatry. 2004;185:95–96. doi: 10.1192/bjp.185.2.95. [DOI] [PubMed] [Google Scholar]
- Wolfe F, Smythe HA, Yunus MB, Bennett RM, Bombardier C, Goldenberg DL, et al. The American College of Rheumatology 1990 criteria for the classification of fibromyalgia: report of the Multicenter Criteria Committee. Arthritis and Rheumatism. 1990;33(2):160–172. doi: 10.1002/art.1780330203. [DOI] [PubMed] [Google Scholar]