Abstract
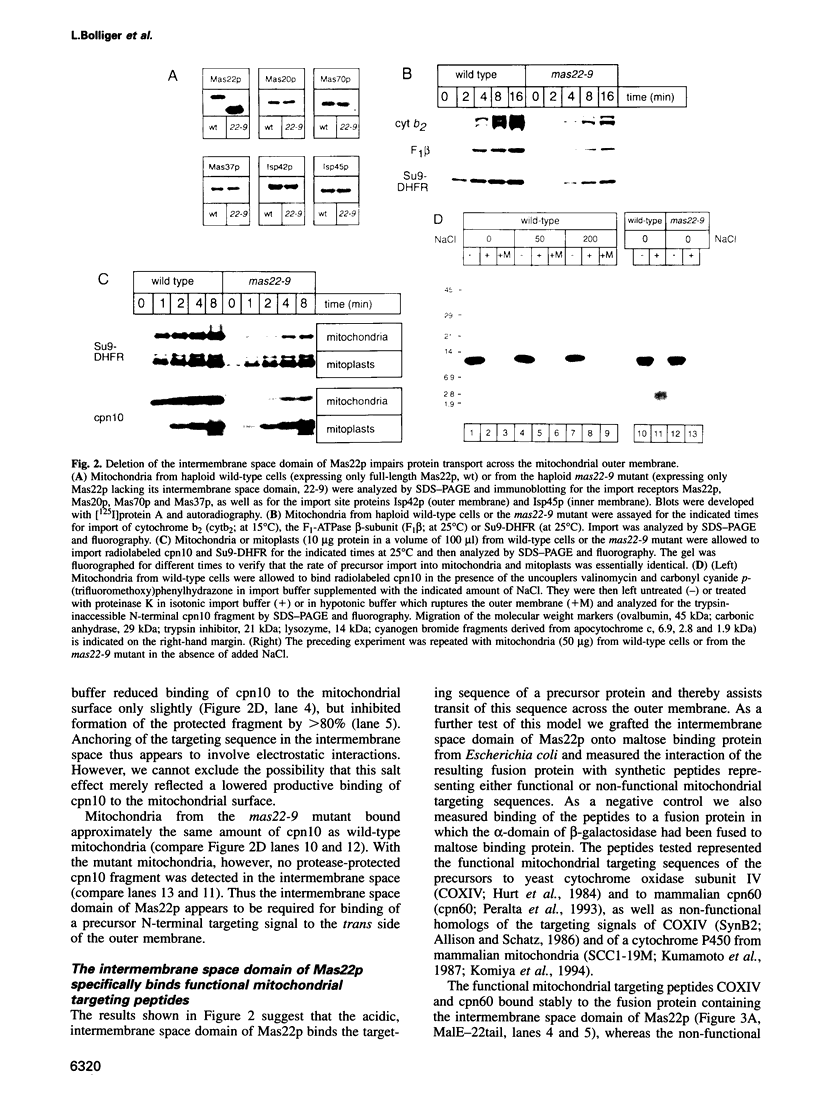
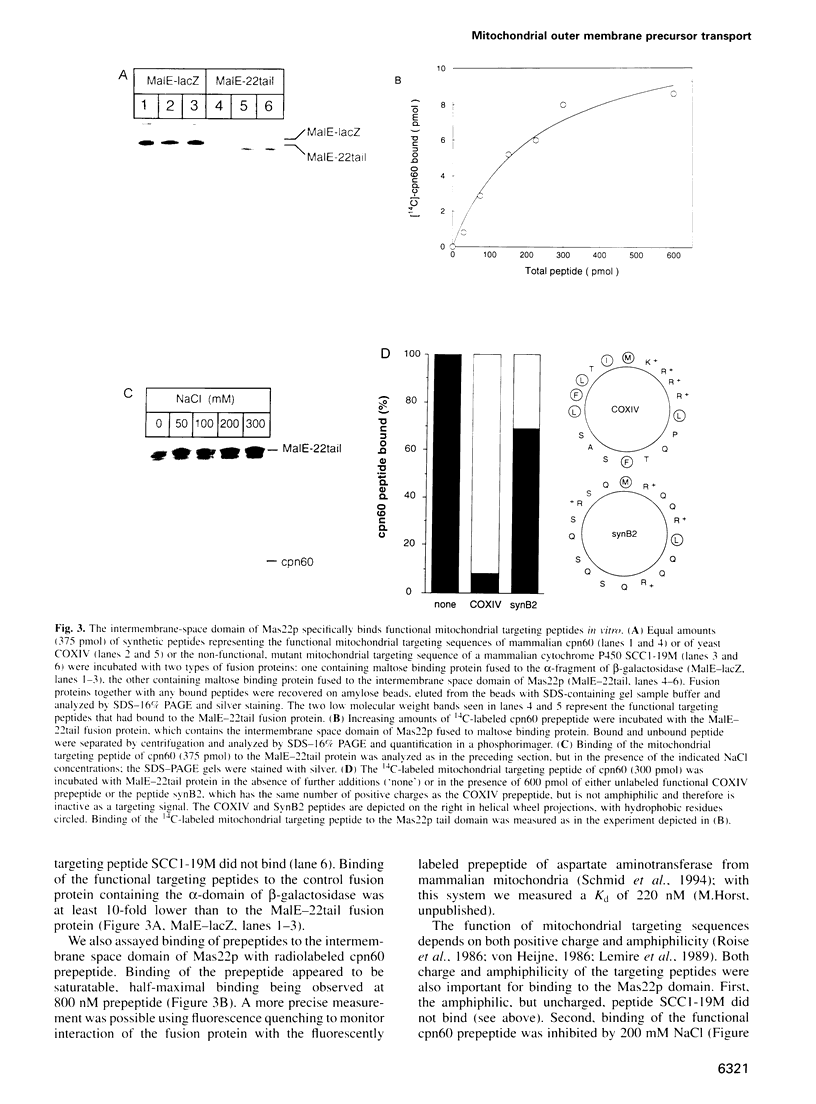
Mitochondrial precursor proteins made in the cytosol bind to a hetero-oligomeric protein import receptor on the mitochondrial surface and then pass through the translocation channel across the outer membrane. This translocation step is accelerated by an acidic domain of the receptor subunit Mas22p, which protrudes into the intermembrane space. This 'trans' domain of Mas22p specifically binds functional mitochondrial targeting peptides with a Kd of < 1 microM and is required to anchor the N-terminal targeting sequence of a translocation-arrested precursor in the intermembrane space. If this Mas22p domain is deleted, respiration-driven growth of the cells is compromised and import of different precursors into isolated mitochondria is inhibited 3- to 8-fold. Binding of precursors to the mitochondrial surface appears to be mediated by cytosolically exposed acidic domains of the receptor subunits Mas20p and Mas22p. Translocation of a precursor across the outer membrane thus appears to involve sequential binding of the precursor's basic and amphiphilic targeting signal to acidic receptor domains on both sides of the membrane.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allison D. S., Schatz G. Artificial mitochondrial presequences. Proc Natl Acad Sci U S A. 1986 Dec;83(23):9011–9015. doi: 10.1073/pnas.83.23.9011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker K. P., Schaniel A., Vestweber D., Schatz G. A yeast mitochondrial outer membrane protein essential for protein import and cell viability. Nature. 1990 Dec 13;348(6302):605–609. doi: 10.1038/348605a0. [DOI] [PubMed] [Google Scholar]
- Beasley E. M., Wachter C., Schatz G. Putting energy into mitochondrial protein import. Curr Opin Cell Biol. 1992 Aug;4(4):646–651. doi: 10.1016/0955-0674(92)90084-p. [DOI] [PubMed] [Google Scholar]
- Becker K., Guiard B., Rassow J., Söllner T., Pfanner N. Targeting of a chemically pure preprotein to mitochondria does not require the addition of a cytosolic signal recognition factor. J Biol Chem. 1992 Mar 15;267(8):5637–5643. [PubMed] [Google Scholar]
- Bernstein H. D., Poritz M. A., Strub K., Hoben P. J., Brenner S., Walter P. Model for signal sequence recognition from amino-acid sequence of 54K subunit of signal recognition particle. Nature. 1989 Aug 10;340(6233):482–486. doi: 10.1038/340482a0. [DOI] [PubMed] [Google Scholar]
- Brunelli J. P., Pall M. L. A series of yeast shuttle vectors for expression of cDNAs and other DNA sequences. Yeast. 1993 Dec;9(12):1299–1308. doi: 10.1002/yea.320091203. [DOI] [PubMed] [Google Scholar]
- Dekker P. J., Keil P., Rassow J., Maarse A. C., Pfanner N., Meijer M. Identification of MIM23, a putative component of the protein import machinery of the mitochondrial inner membrane. FEBS Lett. 1993 Sep 6;330(1):66–70. doi: 10.1016/0014-5793(93)80921-g. [DOI] [PubMed] [Google Scholar]
- Dumont M. E., Ernst J. F., Sherman F. Coupling of heme attachment to import of cytochrome c into yeast mitochondria. Studies with heme lyase-deficient mitochondria and altered apocytochromes c. J Biol Chem. 1988 Nov 5;263(31):15928–15937. [PubMed] [Google Scholar]
- Emtage J. L., Jensen R. E. MAS6 encodes an essential inner membrane component of the yeast mitochondrial protein import pathway. J Cell Biol. 1993 Sep;122(5):1003–1012. doi: 10.1083/jcb.122.5.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glick B. S., Brandt A., Cunningham K., Müller S., Hallberg R. L., Schatz G. Cytochromes c1 and b2 are sorted to the intermembrane space of yeast mitochondria by a stop-transfer mechanism. Cell. 1992 May 29;69(5):809–822. doi: 10.1016/0092-8674(92)90292-k. [DOI] [PubMed] [Google Scholar]
- Glick B., Wachter C., Schatz G. Protein import into mitochondria: two systems acting in tandem? Trends Cell Biol. 1991 Oct;1(4):99–103. doi: 10.1016/0962-8924(91)90037-a. [DOI] [PubMed] [Google Scholar]
- Hannavy K., Rospert S., Schatz G. Protein import into mitochondria: a paradigm for the translocation of polypeptides across membranes. Curr Opin Cell Biol. 1993 Aug;5(4):694–700. doi: 10.1016/0955-0674(93)90142-d. [DOI] [PubMed] [Google Scholar]
- Harkness T. A., Nargang F. E., van der Klei I., Neupert W., Lill R. A crucial role of the mitochondrial protein import receptor MOM19 for the biogenesis of mitochondria. J Cell Biol. 1994 Mar;124(5):637–648. doi: 10.1083/jcb.124.5.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haucke V., Lithgow T., Rospert S., Hahne K., Schatz G. The yeast mitochondrial protein import receptor Mas20p binds precursor proteins through electrostatic interaction with the positively charged presequence. J Biol Chem. 1995 Mar 10;270(10):5565–5570. doi: 10.1074/jbc.270.10.5565. [DOI] [PubMed] [Google Scholar]
- Horst M., Hilfiker-Rothenfluh S., Oppliger W., Schatz G. Dynamic interaction of the protein translocation systems in the inner and outer membranes of yeast mitochondria. EMBO J. 1995 May 15;14(10):2293–2297. doi: 10.1002/j.1460-2075.1995.tb07223.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurt E. C., Pesold-Hurt B., Schatz G. The cleavable prepiece of an imported mitochondrial protein is sufficient to direct cytosolic dihydrofolate reductase into the mitochondrial matrix. FEBS Lett. 1984 Dec 10;178(2):306–310. doi: 10.1016/0014-5793(84)80622-5. [DOI] [PubMed] [Google Scholar]
- Hwang S. T., Schatz G. Translocation of proteins across the mitochondrial inner membrane, but not into the outer membrane, requires nucleoside triphosphates in the matrix. Proc Natl Acad Sci U S A. 1989 Nov;86(21):8432–8436. doi: 10.1073/pnas.86.21.8432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hönlinger A., Kübrich M., Moczko M., Gärtner F., Mallet L., Bussereau F., Eckerskorn C., Lottspeich F., Dietmeier K., Jacquet M. The mitochondrial receptor complex: Mom22 is essential for cell viability and directly interacts with preproteins. Mol Cell Biol. 1995 Jun;15(6):3382–3389. doi: 10.1128/mcb.15.6.3382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvis J. A., Ryan M. T., Hoogenraad N. J., Craik D. J., Høj P. B. Solution structure of the acetylated and noncleavable mitochondrial targeting signal of rat chaperonin 10. J Biol Chem. 1995 Jan 20;270(3):1323–1331. doi: 10.1074/jbc.270.3.1323. [DOI] [PubMed] [Google Scholar]
- Kiebler M., Becker K., Pfanner N., Neupert W. Mitochondrial protein import: specific recognition and membrane translocation of preproteins. J Membr Biol. 1993 Sep;135(3):191–207. doi: 10.1007/BF00211091. [DOI] [PubMed] [Google Scholar]
- Kiebler M., Keil P., Schneider H., van der Klei I. J., Pfanner N., Neupert W. The mitochondrial receptor complex: a central role of MOM22 in mediating preprotein transfer from receptors to the general insertion pore. Cell. 1993 Aug 13;74(3):483–492. doi: 10.1016/0092-8674(93)80050-o. [DOI] [PubMed] [Google Scholar]
- Komiya T., Hachiya N., Sakaguchi M., Omura T., Mihara K. Recognition of mitochondria-targeting signals by a cytosolic import stimulation factor, MSF. J Biol Chem. 1994 Dec 9;269(49):30893–30897. [PubMed] [Google Scholar]
- Kumamoto T., Morohashi K., Ito A., Omura T. Site-directed mutagenesis of basic amino acid residues in the extension peptide of P-450(SCC) precursor: effects on the import of the precursor into mitochondria. J Biochem. 1987 Oct;102(4):833–838. doi: 10.1093/oxfordjournals.jbchem.a122122. [DOI] [PubMed] [Google Scholar]
- Kunz J., Henriquez R., Schneider U., Deuter-Reinhard M., Movva N. R., Hall M. N. Target of rapamycin in yeast, TOR2, is an essential phosphatidylinositol kinase homolog required for G1 progression. Cell. 1993 May 7;73(3):585–596. doi: 10.1016/0092-8674(93)90144-f. [DOI] [PubMed] [Google Scholar]
- Lemire B. D., Fankhauser C., Baker A., Schatz G. The mitochondrial targeting function of randomly generated peptide sequences correlates with predicted helical amphiphilicity. J Biol Chem. 1989 Dec 5;264(34):20206–20215. [PubMed] [Google Scholar]
- Lithgow T., Glick B. S., Schatz G. The protein import receptor of mitochondria. Trends Biochem Sci. 1995 Mar;20(3):98–101. doi: 10.1016/s0968-0004(00)88972-0. [DOI] [PubMed] [Google Scholar]
- Lithgow T., Junne T., Suda K., Gratzer S., Schatz G. The mitochondrial outer membrane protein Mas22p is essential for protein import and viability of yeast. Proc Natl Acad Sci U S A. 1994 Dec 6;91(25):11973–11977. doi: 10.1073/pnas.91.25.11973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lithgow T., Schatz G. Import of the cytochrome oxidase subunit Va precursor into yeast mitochondria is mediated by the outer membrane receptor Mas20p. J Biol Chem. 1995 Jun 16;270(24):14267–14269. doi: 10.1074/jbc.270.24.14267. [DOI] [PubMed] [Google Scholar]
- Martin J., Mahlke K., Pfanner N. Role of an energized inner membrane in mitochondrial protein import. Delta psi drives the movement of presequences. J Biol Chem. 1991 Sep 25;266(27):18051–18057. [PubMed] [Google Scholar]
- Mayer A., Lill R., Neupert W. Translocation and insertion of precursor proteins into isolated outer membranes of mitochondria. J Cell Biol. 1993 Jun;121(6):1233–1243. doi: 10.1083/jcb.121.6.1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer A., Neupert W., Lill R. Mitochondrial protein import: reversible binding of the presequence at the trans side of the outer membrane drives partial translocation and unfolding. Cell. 1995 Jan 13;80(1):127–137. doi: 10.1016/0092-8674(95)90457-3. [DOI] [PubMed] [Google Scholar]
- Miller B. R., Cumsky M. G. An unusual mitochondrial import pathway for the precursor to yeast cytochrome c oxidase subunit Va. J Cell Biol. 1991 Mar;112(5):833–841. doi: 10.1083/jcb.112.5.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moczko M., Ehmann B., Gärtner F., Hönlinger A., Schäfer E., Pfanner N. Deletion of the receptor MOM19 strongly impairs import of cleavable preproteins into Saccharomyces cerevisiae mitochondria. J Biol Chem. 1994 Mar 25;269(12):9045–9051. [PubMed] [Google Scholar]
- Nakai M., Endo T. Identification of yeast MAS17 encoding the functional counterpart of the mitochondrial receptor complex protein MOM22 of Neurospora crassa. FEBS Lett. 1995 Jan 3;357(2):202–206. doi: 10.1016/0014-5793(94)01362-5. [DOI] [PubMed] [Google Scholar]
- Nargang F. E., Künkele K. P., Mayer A., Ritzel R. G., Neupert W., Lill R. 'Sheltered disruption' of Neurospora crassa MOM22, an essential component of the mitochondrial protein import complex. EMBO J. 1995 Mar 15;14(6):1099–1108. doi: 10.1002/j.1460-2075.1995.tb07093.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neupert W., Hartl F. U., Craig E. A., Pfanner N. How do polypeptides cross the mitochondrial membranes? Cell. 1990 Nov 2;63(3):447–450. doi: 10.1016/0092-8674(90)90437-j. [DOI] [PubMed] [Google Scholar]
- Ohba M., Schatz G. Disruption of the outer membrane restores protein import to trypsin-treated yeast mitochondria. EMBO J. 1987 Jul;6(7):2117–2122. doi: 10.1002/j.1460-2075.1987.tb02478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peralta D., Lithgow T., Hoogenraad N. J., Høj P. B. Prechaperonin 60 and preornithine transcarbamylase share components of the import apparatus but have distinct maturation pathways in rat liver mitochondria. Eur J Biochem. 1993 Feb 1;211(3):881–889. doi: 10.1111/j.1432-1033.1993.tb17621.x. [DOI] [PubMed] [Google Scholar]
- Pfanner N., Rassow J., van der Klei I. J., Neupert W. A dynamic model of the mitochondrial protein import machinery. Cell. 1992 Mar 20;68(6):999–1002. doi: 10.1016/0092-8674(92)90069-o. [DOI] [PubMed] [Google Scholar]
- Ramage L., Junne T., Hahne K., Lithgow T., Schatz G. Functional cooperation of mitochondrial protein import receptors in yeast. EMBO J. 1993 Nov;12(11):4115–4123. doi: 10.1002/j.1460-2075.1993.tb06095.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roise D., Horvath S. J., Tomich J. M., Richards J. H., Schatz G. A chemically synthesized pre-sequence of an imported mitochondrial protein can form an amphiphilic helix and perturb natural and artificial phospholipid bilayers. EMBO J. 1986 Jun;5(6):1327–1334. doi: 10.1002/j.1460-2075.1986.tb04363.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rospert S., Junne T., Glick B. S., Schatz G. Cloning and disruption of the gene encoding yeast mitochondrial chaperonin 10, the homolog of E. coli groES. FEBS Lett. 1993 Dec 13;335(3):358–360. doi: 10.1016/0014-5793(93)80419-u. [DOI] [PubMed] [Google Scholar]
- Schmid D., Baici A., Gehring H., Christen P. Kinetics of molecular chaperone action. Science. 1994 Feb 18;263(5149):971–973. doi: 10.1126/science.8310296. [DOI] [PubMed] [Google Scholar]
- Schägger H., von Jagow G. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal Biochem. 1987 Nov 1;166(2):368–379. doi: 10.1016/0003-2697(87)90587-2. [DOI] [PubMed] [Google Scholar]
- Stuart R. A., Neupert W. Apocytochrome c: an exceptional mitochondrial precursor protein using an exceptional import pathway. Biochimie. 1990 Feb-Mar;72(2-3):115–121. doi: 10.1016/0300-9084(90)90136-5. [DOI] [PubMed] [Google Scholar]
- Söllner T., Rassow J., Wiedmann M., Schlossmann J., Keil P., Neupert W., Pfanner N. Mapping of the protein import machinery in the mitochondrial outer membrane by crosslinking of translocation intermediates. Nature. 1992 Jan 2;355(6355):84–87. doi: 10.1038/355084a0. [DOI] [PubMed] [Google Scholar]
- von Heijne G. Mitochondrial targeting sequences may form amphiphilic helices. EMBO J. 1986 Jun;5(6):1335–1342. doi: 10.1002/j.1460-2075.1986.tb04364.x. [DOI] [PMC free article] [PubMed] [Google Scholar]