Abstract
Background
Modern research has provided new insights into the biological mechanisms of noise-induced hearing loss, and a number of studies showed the appearance of increased reactive oxygen species (ROS) and reactive nitrogen species (RNS) during and after noise exposure. This study was designed to investigate the noise exposure induced nitrotyrosine change and the mechanism of outer hair cells death in guinea pig cochlea.
Method
Thirty guinea pigs were used in this study. The experimental animals were either exposed for 4 hours per day to broadband noise at 122 dB SPL (A-weighted) for 2 consecutive days or perfused cochleae with 5 mg/ml of the SIN1 solutions, an exogenous NO and superoxide donor, for 30 minutes. Then the cochleae of the animals were dissected. Propidium iodide (PI), a DNA intercalating fluorescent probe, was used to trace morphological changes in OHC nuclei. The distribution of nitrotyrosine (NT) in the organ of Corti and the cochlear lateral wall tissue from the guinea pigs were examined using fluorescence immunohistochemistry method. Whole mounts of organ of Corti were prepared. Morphological and fluorescent changes were examined under a confocal microscope.
Results
Either after noise exposure or after SIN1 perfusion, outer hair cells (OHCs) death with characteristics of both apoptotic and necrotic degradation appeared. Nitrotyrosine immunolabeling could be observed in the OHCs from the control animals. After noise exposure, NT immunostaining became much greater than the control animals in OHCs. The apoptotic OHC has significant increase of nitrotyrosine in and around the nucleus following noise exposure. In the normal later wall of cochleae, relatively weak nitrotyrosine immunolabeling could be observed. After noise exposure, nitrotyrosine immunoactivity became stronger in stria vascularis.
Conclusion
Noise exposure induced increase of nitrotyrosine production is associated with OHCs death suggesting reactive nitrogen species participation in the cochlear pathophysiology of noise-induced hearing loss.
Keywords: noise exposure, outer hair cell, apoptosis, nitrotyrosine, reactive nitrogen species
Noise-induced hearing loss (NIHL) continues to be a significant source of acquired hearing loss in the population of the world. One of the key pathologies underlying NIHL is the loss of outer hair cells (OHCs) in the cochlea.1 OHCs represent one of the key populations of sensory cells in the auditory system, and are responsible for the human ear’s ability to hear low intensity sounds, as well as the ear’s exquisite ability to discriminate sounds of different frequency. Loss of OHCs from noise exposure or other forms of assault leads to a loss of hearing sensitivity, frequency selectivity, and functional hearing when sound is accompanied by background noise. Modern research has provided new insights into the biological mechanisms of NIHL, and with these new insights comes hope for possible prevention and treatment of NIHL.2 A number of studies proved the appearance of increased reactive oxygen species (ROS) and reactive nitrogen species (RNS) during and after noise exposure.
Reactive-nitrogen species (RNS), in particular oxides of nitrogen such as nitrogen monoxide (nitric oxide, NO) and peroxynitrite (ONOO−) can readily react with l-tyrosine (Tyr) and protein-associated tyrosine (TyrProt) to form nitrotyrosine, that is, NO2Tyr and NO2TyrProt, respectively. Despite the uncertainty of what species actually nitrates tyrosine in vivo, detection of NO2Tyr and/or NO2TyrProt may provide evidence for the generation of RNS.3 The increases in tyrosine nitration, whether tyrosine is free or part of a polypeptide chain, reflect the actions of ONOO−.4,5 Although protein tyrosine nitration is a low-yield process in vivo, nitrotyrosine has been revealed as having a higher yield and is a relevant biomarker of NO-dependent oxidative stress. Targets for ROS and RNS damage include proteins, lipids and DNA. They can induce cochlear hair cells damage and even hair cells death. In the current study, we investigated cochlea hair cell death and the associate nitrotyrosine change in guinea pig cochleae before and following broadband noise exposure.
METHODS
Animal preparation and noise exposure
Thirty guinea pigs (albinos of both sexes, 200–250 g) were used in this study. All animals had a positive Preyer’s reflex. The animals were divided into control, SIN1 perfusion and noise exposure groups. Animals in the control group (n=10) and SIN1 perfusion (n=10) were held in a quiet room with food and water.
Animals in the noise exposure group (n=10) were placed in the noise exposure chamber with food and water and exposed for 4 hours per day to broadband noise at 122 dB SPL (A-weighted) for 2 consecutive days. Two animals at a time were noise-exposed in separate cages. Noise-induced hearing loss was estimated using auditory brain-stem response thresholds before and after noise exposure. This exposure level does result in permanent threshold shift.6 Guinea pigs were prepared for the detection of the hair cell death and change of nitrotyrosine in the organ of Corti and cochlear lateral wall immediately following noise exposure.
SIN1 perfusion
As the active metabolite of molsidomine, SIN-1 chloride produces both NO and superoxide and can therefore be used to generate peroxynitrite under physiological conditions.7,8 Ten guinea pigs were used to detect hair cells death following SIN1 perfusion. The animals were anesthetized with a mixture of ketamine (15 mg/kg) and xylazine (5 mg/kg). Both the left and right cochleae were surgically exposed using a conventional ventral approach. Two small openings were drilled into the bony shell over the scala vestibuli and the scala tympani in the basal turn of the cochlea. The right cochleae were perfused with 5 mg/ml of the freshly prepared SIN1 solutions for 30 minutes. The left cochleae were perfused with artifical perilymph for 30 minutes. The animals were sacrificed and the cochleae harvested.
Specimen preparation
Immediately following the second day’s noise exposure, the animals were anesthetized and cardiac perfusion was performed follwed by further vascular perfusion with 4% paraformaldehyde. Both experimental animals and normal controls were similarly sacrificed and studied. Following decapitation, the temporal bones were immediately removed and transferred into 4% paraformaldehyde in 0.01 mol/L phosphate-buffered saline (PBS, pH 7.4). Under a dissection microscope, the round and oval windows and the bone near the apex were opened, followed by gentle local perfusion from the apex. The tissue was kept in the fixative for 12 hours. The cochlea was gently isolated after fixing. For whole mount preparations, the lateral wall tissues and the organ of Corti were harvested after removal of the bony capsule. The tissue was then prepared for immunohistochemistry.
Immunohistochemistry
The fixed cochleae were washed in 0.02 PBS (pH 7.4), permeabilized in 0.5% Triton x-100 (Sigma, USA) for 1 hour and immuno blocked in 19% goat serum in 1% bovine albumin in 0.02 mol/L PBS for 1 hour. The specimens were incubated overnight in anti-3-nitrotyrosine (mouse monoclonal antibody, 39B6, Alexis biochemicals, diluted 1:500 with 1% BSA-PBS). The specimens were washed in 1% BSA-PBS for 30 minutes and incubated in Alexa fluor 488 anti-mouse IgG for NT (diluted 1: 100 with 1% BSA-PBS). After washing in 0.02 PBS for 30 minutes, the surface prepared tissues were mounted. Negative controls were incubated tissues with 1% BSA-PBS instead of the primary antibody.
Propidium iodide staining of the nuclea
In addition to immunoactive labeling of nitrotyrosine, specimens were double labeled with propidium iodide (PI, diluted 1: 100 with 0.02 mol/L PBS, Molecular Probes), a DNA intercalator, to observe nuclear morphology and to determine if nitrotyrosine distributes in the nuclear area.
Fluorescence and confocal microscopy
The fluorescent signals from tissues were observed with a laser scanning confocal microscope (Bio-Rad, MRC 1024ES) and images were taken using the same settings for gain and illumination power. Cochleae were double labeled with red fluorescence (PI-labeled nuclei) and green fluorescence (Alexa Fluor 488 anti-rabbit antibody labeled nitrotyrosin). A series of approximately 40–60 laser confocal images were taken for each section of the organ of Corti beginning at the top of the OHC stereocilia and stepping progressively through the OHCs body to the bottom of the OHCs at depth intervals of 1.0 μm. The images in the figures are presented as projection sets of 40–60 images.
Statistical analysis
Data were averaged within the ten experimental and ten control animals, and were shown as mean ± standard deviation (SD). Quantitative analysis for fluorescence intensity of nitrotyrosine was performed using Adobe Photoshop 7.0® software (Adobe, USA) on single confocal optical sections. Differences among the different groups were evaluated using a two-tailed Student’s t-test by SPSS 15.0 (SPSS Inc., USA). P ≤0.05 was considered statistically significant.
RESULTS
Noise exposure induced hair cells death
Cochleae exposed to broadband noise demonstrated variable OHC damage. The broadband sound damage was mainly confined to the first and second turns. Examination of PI labeling nuclei in damaged OHCs revealed two distinct morphological alterations, nuclear condensation or nuclear swelling. Nuclear condensation is associated with apoptosis, whereas nuclear swelling is a characteristic of necrosis. There is no apoptosis and necrosis in normal OHCs in guinea pig cochlea without noise exposure (Figure 1A). Noise-exposure caused apoptosis and necrosis of some OHCs. The apoptotic OHCs had condensed nuclei with labeling of PI and the necrotic OHCs were characterized by a swollen nucleus with labeling of PI (Figure 1B). SIN-1, an exogenous NO and superoxide donor, perfusion induced apoptosis and necrosis of some OHCs (Figure 1C).
Figure 1.
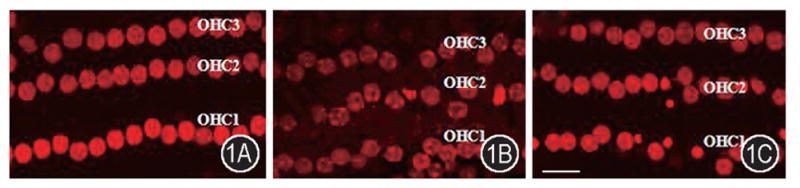
Noise exposure induced out hair cell death. A: Normal organ of Corti. OHCs were arranged in rows with nuclei stained (red) by PI labeling. The apoptotic and necrotic cells were not observed in the normal OHCs. B: Noise-exposed cochlea. Noise-exposure caused apoptosis and necrosis of some OHCs. The apoptotic OHCs had condensed nuclei and the necrotic OHCs were characterized by a swollen nucleus. C: SIN1-perfused cochlea. SIN1 perfusion induced apoptosis and necrosis of some OHCs. OHC1, 2 and 3: first, second and third rows of outer hair cells. Scale bar = 10 μm.
Nitrotyrosine immunolabeling in the unstimulated and loud sound-stimulated organ of Corti
Nitrotyrosine (NT) immunolabeling could be observed in both the cochleae from the control group and the noise exposure group. For the unstimulated cochlea, a slight background staining was observed in a normal-hearing, nonexposed, cochlea (Figure 2A). After exposing the animals for 4 hours per day to broadband noise at 122 dB SPL (A-weighted) for two consecutive days, NT immunostaining was much greater in outer hair cells (Figure 2B). Notice the labeling along the three rows of the OHC. And there is no NT immunostaining in the outer hair cells of control animal (Figure 2C).
Figure 2.
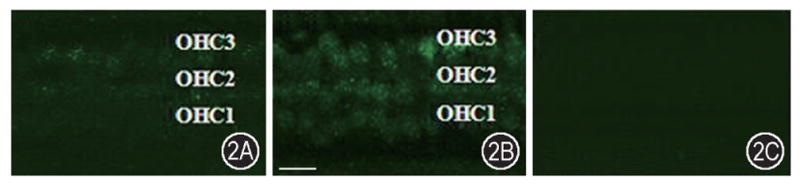
Nitrotyrosine increase in OHCs following noise exposure. A: Immunostaining of anti-nitrotyrosine in the organ of Corti of the control animals showed a relatively weak staining of NT in the outer hair cells. B: Significant NT immunostaining increase was observed in the outer hair cells of guinea pig cochleae following noise exposure. C: No immunoreactive staining was observed in control animals without the primary antibody. Scale bar = 30 μm.
To quantitatively determine the NT increase in OHCs following noise exposure, the mean fluorescent intensity was measured using photoshop software in a user-selected window approximately covering the OHCs (approximately 12 OHCs) in images of organ of Corti in the second turn of the cochleae. One ear from each animal was used in the 10 control animals and the 10 noise exposure animals showing the change of fluorescent signals in the OHCs of control animals. The quantitative analysis showed nitrotyrosine signals of OHCs significant increases (P <0.01) in the noise exposure group compared with that of the control group.
Nitrotyrosine change in apoptotic hair cells
Anti-nitrotyrosine and propidium iodide double labeling shows the nitrotyrosine change in the apoptotic outer hair cells after noise exposure. An apoptotic OHC was observed following noise exposure (Figure 3A). A significant NT increase was observed in an OHC (Figure 3B). Anti-NT antibody and propidium iodide double labeling showed an apoptotic OHC with a condensed nuclei having a significant increase of nitrotyrosine in and around the nucleus (Figure 3C). The strong fluorescence shows the labeled NT at the apoptotic OHC.
Figure 3.
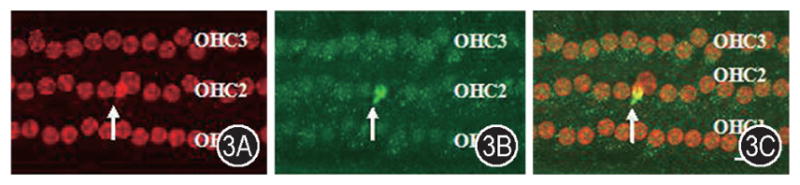
Anti-nitrotyrosine and propidium iodide double labeling showed the nitrotyrosine change in the outer hair cells after noise exposure. A: The organ of corti was stained with propidium iodide showing nucleoli after noise exposure. Apoptotic (arrow) OHCs were observed following noise exposure. B: Anti-NT antibody labeling shows the change of nitrotyrosine following noise exposure. A significant NT increase was observed in an OHC. C: Anti-NT antibody and propidium iodide double labeling shows that the apoptotic OHC with a condensed nuclei has a significant increase of nitrotyrosine in and around nucleus. OHC1, 2 and 3: first, second and third rows of outer hair cells. Scale bar = 20 μm.
Nitrotyrosine immunolabeling in the cochlear lateral wall from unstimulated and loud sound-stimulated guinea pig cochleae
To determine nitrotyrosine distributions in the cochlear lateral wall, the surface prepared lateral walls were double labeled with anti-nitrotyrosine and with propidium iodide. In the unstimulated guinea pig lateral wall, relatively weak NT immunolabeling was observed in stria vascularis (Figure 4A–4C). The NT immunoactivity became stronger in stria vascularis in the tissue specimens from noise-exposed animals (Figure 4D–4F). There was no apparent immunoreactivity in the capillary lumen of the cochlear stria vascularis.
Figure 4.
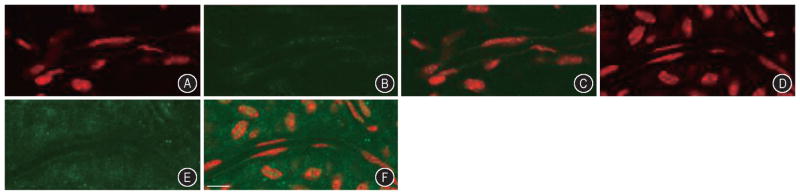
Anti-nitrotyrosine and propidium iodide double staining in the stria sections in the cochlear lateral wall of the control and noise exposure guinea pig. A–C: Relatively weak staining of NT (green) in the stria vascularis in the control animals. D–F: Enhanced NT immunostaining in the stria vascularis in the noise exposure animal. A and D: propidum iodide (PI) Labeling; B and E: Anti-NT antibody staining; C and F: Anti-NT antibody and PI double labeling. Scale bar = 30 μm.
Quantitative analysis comparing the intensity of NT fluorescence in the control and noise-exposed groups showed significant (P <0.01) NT increase in the stria vascularis following noise exposure.
DISCUSSION
Cell death occurs through one of the two processes, either as necrosis or apoptosis. Necrotic cell death is a passive form of cell death and is associated with cell swelling, which eventually results in the rupture of the cell and the spillage of the cell’s contents, causing damage to surrounding tissue and initiation of an inflammatory response. Apoptotic cell death is an active, highly regulated form of cell death. Apoptosis may occur as a means for the body to neatly eliminate unwanted or damaged/dying cells that could potentially damage neighboring healthy cells. These unwanted cells include excessive cells formed during development and old and damaged cells in adult tissues.9 Throughout the process of apoptosis, the cell membrane remains intact, and the cell condenses and pulls away from neighboring cells resulting in minimal damage to surrounding tissues.
Necrosis can occur in the cochlea after traumatic noise exposure. The swollen cells that are found in the cochlea after traumatic noise are examples of necrotic cell death. The presence of swollen OHC immediately after a traumatic exposure led to the belief that necrosis was the primary mode of cell death in the noise-exposed cochlea.8 It was not until recently that the existence of apoptosis in the noise-damaged cochlea was described.10–12 Our results confirmed the existence of apoptosis and necrosis in the noise-exposed cochlea and indicated that both apoptosis and necrosis are involved in the creation of the OHC lesion (Figure 1).
The cochlea are known to be highly demanding of energy, and high level noise exposure to cochlea can create large amounts of superoxide generated as an unwanted byproduct. The increased superoxide can then react with other molecules to generate higher levels of ROS and RNS during and after noise exposure.1 RNS, in particular oxides of nitrogen such as nitrogen monoxide (NO) and peroxynitrite (ONOO−) can damage DNA and the cell membrane and act as a putative trigger for apoptosis. The end result is cell death from a combination of necrosis and apoptosis.1,12
Peroxynitrite is a transient species with a biological half-life (10–20 ms) even shorter than that of NO (1–30 seconds),13 and cannot be directly measured. Peroxynitrite has the ability to nitrite free tyrosine and tyrosine residues in proteins to form nitrotyrosine (NT).14 Nitric oxide synthase (NOS) produces NO. NO readily diffuses through the cytosol and cell membrane, allowing it to react with superoxide (O2−), produced by NADPH oxidase in the mitochondria, to form ONOO−. Therefore, the increase in tyrosine nitration reflects the actions of ONOO. NT can be a marker for peroxynitrite-induced neurotoxicity.15
In this study, we have found a weak distribution of nitrotyrosine (NT), in the cochlear lateral wall tissue and the organ of Corti in the normal (no noise exposure) animals. NT was observed in the outer hair cells (OHCs). Although the complete actions of peroxynitrite remain to be determined, we clearly found NT to be elevated in OHCs following noise exposure. This is also consistent with our earlier demonstration of elevated NO levels in OHCs. The earlier demonstration of reactive species16 can now clearly be attributed in part to peroxynitrite and elevated NO.
Using anti-nitrotyrosine and propidium iodide double staining, we found that the nitrotyrosine change in the apoptotic outer hair cells after noise exposure. The strong fluorescence indicating labeled NT at the apoptotic OHC (Figure 3). At the level of the HC, noise stresses can lead to overdriving of the mitochondria, excitotoxicity at the junctions between the IHC and afferent auditory nerve fibers, and ischemia/reperfusion effects on the cochlea’s blood supply. Each of these actions can lead to increases in ROS and RNS, which can cause protein, lipid breakdown and damage DNA and the cell membrane and act as a putative trigger for apoptosis. In the cochlea it has been found that hydroxyl radicals significantly increase in the cochlea with noise.17 Nitrotyrosine and 4-hydroxy-2-nonel increased within 10 days following noise exposure.18
In the current study, we observed an increased NT signal in OHCs following exposure to the broadband noise. The increase of nitrotyrosine means large quantities of peroxynitrite are produced under loud sound stimulation. This excess peroxynitrite is the principal pathogenic pathway result from the reaction of NO with oxygen and oxygen radicals.18 Some studies have suggested that mitochondria contain NO synthase and are capable of producing biologically significant quantities of NO to regulate energy metabolism and perform other physiologic functions or to become involved in pathologic processes.19 NO-related free radicals can damage the organelles leading to cell death through irreversible inhibition of mitochondrial respiration.20,21 The high concentration of NT in the apoptotic OHCs indicate that peroxynitrite increase leading to apoptotic hair cell death.
We previously examined the production and distribution of NO by employing a sensitive NO fluorescent indicator (DAF-2DA) and showed a baseline production of NO was found in various cells of the lateral wall, including in marginal and endothelial cells.22 In the current study, we have examined the distribution of nitric oxide derivative, nitrotyrosine, in the cochlear lateral wall tissue from the guinea pig. We observed relatively weak nitrotyrosine distributions in the stria vascularis of the unstimulated animals (Figures 4B and 4C). In contrast, upregulated nitrotyrosine immunoactivity was detected in the stria vascularis following exposure to the broadband noise. In whole mounts of the lateral wall, the NT increase with noise was significant compared to the control (Figures 4E and 4F). This is consistent with our previous observation21 that increased NO signal was observed following exposure to noise. We have observed increased nitrotyrosine activity in stria vacularis, which might result in oxidative injury due to peroxynitrite related free radical damage.
Taken together, our data indicate that noise exposure leads to hair cell death and significant production of nitrotyrosine in the OHCs and stria vascularis. This is consistent with the known increase of NO production by loud sound stress and suggests that NO derived free radicals, peroxynitrite, participate in the cochlear pathophysiology of noise-induced hearing loss.
Acknowledgments
This research was supported by the grants from National Natural Science Foundation of China (No. 30973305 and No. 81170908), Nuttal Alfred’s Grant (NIH NIDCD DC 000105 and Shi Xiaorui’s Grant (NIH NIDCD R01-DC010844).
Contributor Information
HAN Wei-ju, Department of Otolaryngology Head and Neck Surgery, Chinese People’s Liberation Army General Hospital, Beijing 100853, China.
SHI Xiao-rui, Oregon Hearing Research Center, OHSU, SW Sam Jackson Park Road, Portland, OR, USA.
Alfred Nuttall, Oregon Hearing Research Center, OHSU, SW Sam Jackson Park Road, Portland, OR, USA. KHRI, University of Michigan, Ann Arbor, MI, USA.
References
- 1.Henderson D, Bielefeld EC, Harris KC, Hu BH. The role of oxidative stress in noise-induced hearing loss. Ear Hear. 2006;27:1–19. doi: 10.1097/01.aud.0000191942.36672.f3. [DOI] [PubMed] [Google Scholar]
- 2.Op de Beeck K, Schacht J, Van Camp G. Apoptosis in acquired and genetic hearing impairment: the programmed death of the hair cell. Hear Res. 2011;281:18–27. doi: 10.1016/j.heares.2011.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tsikas D, Caidahl K. Recent methodological advances in the mass spectrometric analysis of free and protein-associated 3-nitrotyrosine in human plasma. J Chromatogr B Analyt Technol Biomed Life Sci. 2005;814:1–9. doi: 10.1016/j.jchromb.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 4.Crow JP, Beckman JS. The role of peroxynitrite in nitric oxide-mediated toxicity. Curr Top Microbiol Immunol. 1995;196:57–73. doi: 10.1007/978-3-642-79130-7_7. [DOI] [PubMed] [Google Scholar]
- 5.Crow JP, Ischiropoulos H. Detection and quantitation of nitrotyrosine residues in proteins: in vivo marker of peroxynitrite. Meth Enzymol. 1996;269:185–194. doi: 10.1016/s0076-6879(96)69020-x. [DOI] [PubMed] [Google Scholar]
- 6.Shi X, Han W, Yamamoto H, Omelchenko I, Nuttall A. Nitric oxide and mitochondrial status in noise-induced hearing loss. Free Radic Res. 2007;41:1313–1325. doi: 10.1080/10715760701687117. [DOI] [PubMed] [Google Scholar]
- 7.Rosenkranz B, Winkelmann BR, Parnham MJ. Clinical pharmacokinetics of molsidomine. Clin Pharmokinet. 1996;30:372–384. doi: 10.2165/00003088-199630050-00004. [DOI] [PubMed] [Google Scholar]
- 8.Lomonosova EE, Kirsch M, Rauen U, de Groot H. The critical role of hepes in SIN-1 cytotoxicity, peroxynitrite versus hydrogen peroxide. Free Radic Biol Med. 1998;24:522–528. doi: 10.1016/s0891-5849(97)00295-5. [DOI] [PubMed] [Google Scholar]
- 9.Majno G, Joris I. Apoptosis, oncosis, and necrosis: an overview of cell death. Am J Pathol. 1995;146:3–15. [PMC free article] [PubMed] [Google Scholar]
- 10.Saunders JC, Dear SP, Schneider ME. The anatomical consequences of acoustic injury: A review and tutorial. J Acoust Soc Am. 1985;78:833–860. doi: 10.1121/1.392915. [DOI] [PubMed] [Google Scholar]
- 11.Hu BH, Henderson D, Nicotera TM. Involvement of apoptosis in progression of cochlear lesion following exposure to intense noise. Hear Res. 2002;166:62–71. doi: 10.1016/s0378-5955(02)00286-1. [DOI] [PubMed] [Google Scholar]
- 12.Han W, Shi X, Nuttall AL. AIF and endoG translocation in noise exposure induced hair cell death. Hear Res. 2006;211:85–95. doi: 10.1016/j.heares.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 13.Denicola A, Souza JM, Radi R. Diffusion of peroxynitrite across erythrocyte membranes. Proc Natl Acad Sci USA. 1998;95:3566–3571. doi: 10.1073/pnas.95.7.3566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ischiropoulos H. Biological selectivity and functional aspects of protein tyrosine nitration. Biochem Biophys Res Commun. 2003;305:776–783. doi: 10.1016/s0006-291x(03)00814-3. [DOI] [PubMed] [Google Scholar]
- 15.Kuhn DM, Sakowski SA, Sadidi M, Geddes TJ. Nitrotyrosine as a marker for peroxynitrite-induced neurotoxicity: the beginning or the end of the end of dopamine neurons? J Neurochem. 2004;89:529–536. doi: 10.1111/j.1471-4159.2004.02346.x. [DOI] [PubMed] [Google Scholar]
- 16.Shi X, Dai C, Nuttall AL. Altered expression of inducible nitric oxide synthase (iNOS) in the cochlea. Hear Res. 2003;177:43–52. doi: 10.1016/s0378-5955(02)00796-7. [DOI] [PubMed] [Google Scholar]
- 17.Ohlemiller KK, Wright JS, Dugan LL. Early elevation of cochlear reactive oxygen species following noise exposure. Audiol Neuro-Otol. 1999;4:229–236. doi: 10.1159/000013846. [DOI] [PubMed] [Google Scholar]
- 18.Yamashita D, Jiang HY, Schacht J, Miller JM. Delayed production of free radicals following noise exposure. Brain Res. 2004;1019:201–209. doi: 10.1016/j.brainres.2004.05.104. [DOI] [PubMed] [Google Scholar]
- 19.Murphy MP. Nitric oxide and cell death. Biochim Biophys Acta. 1999;1411:401–414. doi: 10.1016/s0005-2728(99)00029-8. [DOI] [PubMed] [Google Scholar]
- 20.Brown GC. Nitric oxide as a competitive inhibitor of oxygen consumption in the mitochondrial respiratory chain. Acta Physiol Scand. 2000;168:667–674. doi: 10.1046/j.1365-201x.2000.00718.x. [DOI] [PubMed] [Google Scholar]
- 21.Bierlefeld EC, Hangauer D, Henderson D. Protection from impulse noise-induced hearing loss with novel Src-protein tyrosine kinase inhibitors. Neurosci Res. 2011;71:348–354. doi: 10.1016/j.neures.2011.07.1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shi X, Ren T, Nuttall AL. Nitric oxide distribution and production in the guinea pig cochlea. Hear Res. 2001;153:23–31. doi: 10.1016/s0378-5955(00)00254-9. [DOI] [PubMed] [Google Scholar]


