Abstract
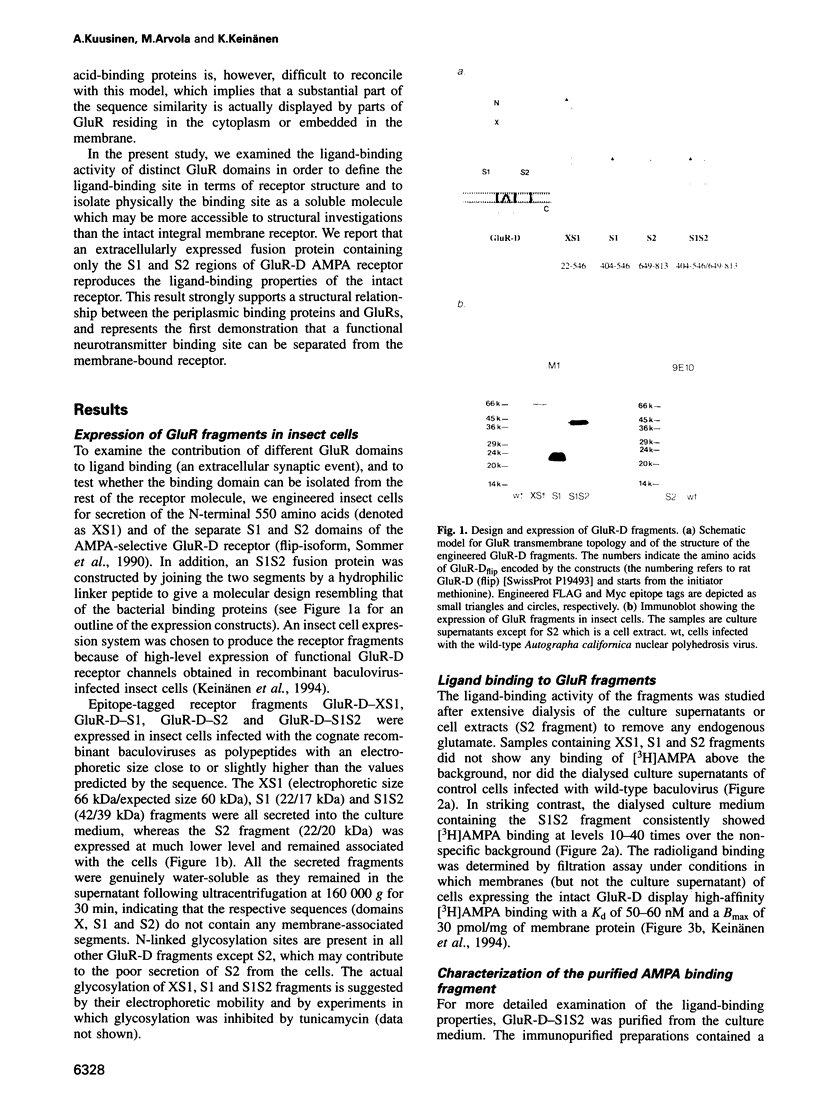
Two discontinuous segments (S1 and S2), separated by membrane-associated domains, in ionotropic glutamate receptor (GluR) subunits show sequence similarity to bacterial periplasmic amino acid-binding proteins, suggesting an evolutionary and structural relationship. Experimental evidence arguing for and against the inferred extracellular location of the S1 and S2 domains in GluRs has been presented. Here, we report that an extracellularly expressed fusion protein consisting of the S1 and S2 domains of alpha-amino-5-methyl-3-hydroxyisoxazolone-4-propionate (AMPA)-selective glutamate receptor GluR-D joined together via a hydrophilic linker peptide specifically reproduces the AMPA-binding properties of GluR-D, whereas the separately expressed segments do not bind ligand. This provides direct evidence that the S1 and S2 segments of GluR-D contain the structural determinants necessary and sufficient for selective agonist binding. Dissection of a functional neurotransmitter binding site as a soluble protein separate from the integral membrane channel will facilitate new approaches to analyse the structure of GluRs.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams M. D., Oxender D. L. Bacterial periplasmic binding protein tertiary structures. J Biol Chem. 1989 Sep 25;264(27):15739–15742. [PubMed] [Google Scholar]
- Bennett J. A., Dingledine R. Topology profile for a glutamate receptor: three transmembrane domains and a channel-lining reentrant membrane loop. Neuron. 1995 Feb;14(2):373–384. doi: 10.1016/0896-6273(95)90293-7. [DOI] [PubMed] [Google Scholar]
- Bruns R. F., Lawson-Wendling K., Pugsley T. A. A rapid filtration assay for soluble receptors using polyethylenimine-treated filters. Anal Biochem. 1983 Jul 1;132(1):74–81. doi: 10.1016/0003-2697(83)90427-x. [DOI] [PubMed] [Google Scholar]
- Collingridge G. L., Lester R. A. Excitatory amino acid receptors in the vertebrate central nervous system. Pharmacol Rev. 1989 Jun;41(2):143–210. [PubMed] [Google Scholar]
- Evan G. I., Lewis G. K., Ramsay G., Bishop J. M. Isolation of monoclonal antibodies specific for human c-myc proto-oncogene product. Mol Cell Biol. 1985 Dec;5(12):3610–3616. doi: 10.1128/mcb.5.12.3610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregor P., Mano I., Maoz I., McKeown M., Teichberg V. I. Molecular structure of the chick cerebellar kainate-binding subunit of a putative glutamate receptor. Nature. 1989 Dec 7;342(6250):689–692. doi: 10.1038/342689a0. [DOI] [PubMed] [Google Scholar]
- Hollmann M., Heinemann S. Cloned glutamate receptors. Annu Rev Neurosci. 1994;17:31–108. doi: 10.1146/annurev.ne.17.030194.000335. [DOI] [PubMed] [Google Scholar]
- Hollmann M., Maron C., Heinemann S. N-glycosylation site tagging suggests a three transmembrane domain topology for the glutamate receptor GluR1. Neuron. 1994 Dec;13(6):1331–1343. doi: 10.1016/0896-6273(94)90419-7. [DOI] [PubMed] [Google Scholar]
- Honoré T., Davies S. N., Drejer J., Fletcher E. J., Jacobsen P., Lodge D., Nielsen F. E. Quinoxalinediones: potent competitive non-NMDA glutamate receptor antagonists. Science. 1988 Aug 5;241(4866):701–703. doi: 10.1126/science.2899909. [DOI] [PubMed] [Google Scholar]
- Kang C. H., Shin W. C., Yamagata Y., Gokcen S., Ames G. F., Kim S. H. Crystal structure of the lysine-, arginine-, ornithine-binding protein (LAO) from Salmonella typhimurium at 2.7-A resolution. J Biol Chem. 1991 Dec 15;266(35):23893–23899. [PubMed] [Google Scholar]
- Kaplan R. S., Pedersen P. L. Sensitive protein assay in presence of high levels of lipid. Methods Enzymol. 1989;172:393–399. doi: 10.1016/s0076-6879(89)72025-5. [DOI] [PubMed] [Google Scholar]
- Keinänen K., Köhr G., Seeburg P. H., Laukkanen M. L., Oker-Blom C. High-level expression of functional glutamate receptor channels in insect cells. Biotechnology (N Y) 1994 Aug;12(8):802–806. doi: 10.1038/nbt0894-802. [DOI] [PubMed] [Google Scholar]
- Keinänen K., Wisden W., Sommer B., Werner P., Herb A., Verdoorn T. A., Sakmann B., Seeburg P. H. A family of AMPA-selective glutamate receptors. Science. 1990 Aug 3;249(4968):556–560. doi: 10.1126/science.2166337. [DOI] [PubMed] [Google Scholar]
- Kuryatov A., Laube B., Betz H., Kuhse J. Mutational analysis of the glycine-binding site of the NMDA receptor: structural similarity with bacterial amino acid-binding proteins. Neuron. 1994 Jun;12(6):1291–1300. doi: 10.1016/0896-6273(94)90445-6. [DOI] [PubMed] [Google Scholar]
- Luckow V. A., Lee S. C., Barry G. F., Olins P. O. Efficient generation of infectious recombinant baculoviruses by site-specific transposon-mediated insertion of foreign genes into a baculovirus genome propagated in Escherichia coli. J Virol. 1993 Aug;67(8):4566–4579. doi: 10.1128/jvi.67.8.4566-4579.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monaghan D. T., Bridges R. J., Cotman C. W. The excitatory amino acid receptors: their classes, pharmacology, and distinct properties in the function of the central nervous system. Annu Rev Pharmacol Toxicol. 1989;29:365–402. doi: 10.1146/annurev.pa.29.040189.002053. [DOI] [PubMed] [Google Scholar]
- Nakanishi N., Shneider N. A., Axel R. A family of glutamate receptor genes: evidence for the formation of heteromultimeric receptors with distinct channel properties. Neuron. 1990 Nov;5(5):569–581. doi: 10.1016/0896-6273(90)90212-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nohno T., Saito T., Hong J. S. Cloning and complete nucleotide sequence of the Escherichia coli glutamine permease operon (glnHPQ). Mol Gen Genet. 1986 Nov;205(2):260–269. doi: 10.1007/BF00430437. [DOI] [PubMed] [Google Scholar]
- O'Hara P. J., Sheppard P. O., Thøgersen H., Venezia D., Haldeman B. A., McGrane V., Houamed K. M., Thomsen C., Gilbert T. L., Mulvihill E. R. The ligand-binding domain in metabotropic glutamate receptors is related to bacterial periplasmic binding proteins. Neuron. 1993 Jul;11(1):41–52. doi: 10.1016/0896-6273(93)90269-w. [DOI] [PubMed] [Google Scholar]
- Oh B. H., Kang C. H., De Bondt H., Kim S. H., Nikaido K., Joshi A. K., Ames G. F. The bacterial periplasmic histidine-binding protein. structure/function analysis of the ligand-binding site and comparison with related proteins. J Biol Chem. 1994 Feb 11;269(6):4135–4143. [PubMed] [Google Scholar]
- Oh B. H., Pandit J., Kang C. H., Nikaido K., Gokcen S., Ames G. F., Kim S. H. Three-dimensional structures of the periplasmic lysine/arginine/ornithine-binding protein with and without a ligand. J Biol Chem. 1993 May 25;268(15):11348–11355. [PubMed] [Google Scholar]
- Raymond L. A., Blackstone C. D., Huganir R. L. Phosphorylation and modulation of recombinant GluR6 glutamate receptors by cAMP-dependent protein kinase. Nature. 1993 Feb 18;361(6413):637–641. doi: 10.1038/361637a0. [DOI] [PubMed] [Google Scholar]
- Roche K. W., Raymond L. A., Blackstone C., Huganir R. L. Transmembrane topology of the glutamate receptor subunit GluR6. J Biol Chem. 1994 Apr 22;269(16):11679–11682. [PubMed] [Google Scholar]
- Rogers J., Early P., Carter C., Calame K., Bond M., Hood L., Wall R. Two mRNAs with different 3' ends encode membrane-bound and secreted forms of immunoglobulin mu chain. Cell. 1980 Jun;20(2):303–312. doi: 10.1016/0092-8674(80)90616-9. [DOI] [PubMed] [Google Scholar]
- Seeburg P. H. The TINS/TiPS Lecture. The molecular biology of mammalian glutamate receptor channels. Trends Neurosci. 1993 Sep;16(9):359–365. doi: 10.1016/0166-2236(93)90093-2. [DOI] [PubMed] [Google Scholar]
- Sommer B., Keinänen K., Verdoorn T. A., Wisden W., Burnashev N., Herb A., Köhler M., Takagi T., Sakmann B., Seeburg P. H. Flip and flop: a cell-specific functional switch in glutamate-operated channels of the CNS. Science. 1990 Sep 28;249(4976):1580–1585. doi: 10.1126/science.1699275. [DOI] [PubMed] [Google Scholar]
- Stern-Bach Y., Bettler B., Hartley M., Sheppard P. O., O'Hara P. J., Heinemann S. F. Agonist selectivity of glutamate receptors is specified by two domains structurally related to bacterial amino acid-binding proteins. Neuron. 1994 Dec;13(6):1345–1357. doi: 10.1016/0896-6273(94)90420-0. [DOI] [PubMed] [Google Scholar]
- Taverna F. A., Wang L. Y., MacDonald J. F., Hampson D. R. A transmembrane model for an ionotropic glutamate receptor predicted on the basis of the location of asparagine-linked oligosaccharides. J Biol Chem. 1994 May 13;269(19):14159–14164. [PubMed] [Google Scholar]
- Wada K., Dechesne C. J., Shimasaki S., King R. G., Kusano K., Buonanno A., Hampson D. R., Banner C., Wenthold R. J., Nakatani Y. Sequence and expression of a frog brain complementary DNA encoding a kainate-binding protein. Nature. 1989 Dec 7;342(6250):684–689. doi: 10.1038/342684a0. [DOI] [PubMed] [Google Scholar]
- Wang L. Y., Taverna F. A., Huang X. P., MacDonald J. F., Hampson D. R. Phosphorylation and modulation of a kainate receptor (GluR6) by cAMP-dependent protein kinase. Science. 1993 Feb 19;259(5098):1173–1175. doi: 10.1126/science.8382377. [DOI] [PubMed] [Google Scholar]
- Wo Z. G., Oswald R. E. Transmembrane topology of two kainate receptor subunits revealed by N-glycosylation. Proc Natl Acad Sci U S A. 1994 Jul 19;91(15):7154–7158. doi: 10.1073/pnas.91.15.7154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood M. W., VanDongen H. M., VanDongen A. M. Structural conservation of ion conduction pathways in K channels and glutamate receptors. Proc Natl Acad Sci U S A. 1995 May 23;92(11):4882–4886. doi: 10.1073/pnas.92.11.4882. [DOI] [PMC free article] [PubMed] [Google Scholar]