Abstract
Purpose
A phase I, dose-finding study of vorinostat in combination with temozolomide (TMZ) was conducted to determine the maximum tolerated dose (MTD), safety, and pharmacokinetics in patients with high-grade glioma (HGG).
Experimental Design
This phase I, dose-finding, investigational study was conducted in two parts. Part 1 was a dose-escalation study of vorinostat in combination with TMZ 150 mg/m2/day × 5 days every 28 days. Part 2 was a dose-escalation study of vorinostat in combination with TMZ 150 mg/m2/day × 5 days of the first cycle and 200 mg/m2/day × 5 days of the subsequent 28-day cycles.
Results
In Part 1, the MTD of vorinostat administered on days 1-7 and 15-21 of every 28 day cycle in combination with TMZ was 500 mg daily. Dose-limiting toxicities (DLTs) included grade 3 anorexia, grade 3 ALT, and grade 5 hemorrhage in the setting of grade 4 thrombocytopenia. In Part 2, the MTD of vorinostat on days 1-7 and 15-21 of every 28 day cycle combined with TMZ was 400 mg daily. No DLTs were encountered, but vorinostat dosing could not be escalated further due to thrombocytopenia. The most common serious adverse events were fatigue, lymphopenia, thrombocytopenia, and thromboembolic events. There were no apparent pharmacokinetic interactions between vorinostat and TMZ. Vorinostat treatment resulted in hyperacetylation of histones H3 and H4 in peripheral mononuclear cells.
Conclusion
Vorinostat in combination with temozolomide is well-tolerated in patients with HGG. A phase I/II trial of vorinostat with radiotherapy and concomitant TMZ in newly diagnosed glioblastoma is underway.
Keywords: High-grade glioma, Temozolomide, Vorinostat, HDAC Inhibitor
Introduction
Histone proteins organize DNA into nucleosomes, which are regular repeating structures of chromatin (1). The acetylation status of histones alters chromatin structure and is regulated by two classes of enzymes, histone deacetylases (HDACs) and histone acetyltransferases (HATs). This acetylation affects the regulation of gene expression by rendering certain genes accessible to transcriptional machinery. There is increasing evidence that HDAC or HAT activity is altered in many cancers, including gliomas. HDAC inhibitors (HDACi) can induce growth arrest, differentiation and/or apoptosis of tumor cells in vitro and in vivo by altering the transcription of a small number of genes and represent a promising novel therapeutic approach to cancer (1, 2).
Vorinostat is a small molecule inhibitor of HDAC that binds directly in the enzyme's active site in the presence of a zinc ion and is approved by the United States Federal Drug Administration (FDA) for use in patients with cutaneous T-cell lymphoma who have progressive, persistent, or recurrent disease on or following two systemic therapies. Vorinostat has activity against high-grade glioma (HGG) lines in vitro and in vivo and appears to pass through the blood brain barrier (3-6). In a phase II study of recurrent glioblastoma (GBM), vorinostat was generally well tolerated and demonstrated modest single agent activity with a six-month progression-free survival rate (PFS6) of 15.2% (7). In addition, tumor samples of GBM patients who received vorinostat prior to surgery indicated that this agent was able to penetrate tumors and inhibited histone acetylation (7).
Acetylation of key lysine residues in core histones leads to a more relaxed chromatin configuration. Therefore, HDAC inhibitors, by allowing acetylation, may provide cytotoxic chemotherapy with enhanced access to DNA resulting in synergistic activity (2). Temozolomide (TMZ) is an alkylating agent with efficacy in HGG; as established by the phase III clinical trial demonstrating that the addition of TMZ to radiation extends overall survival in patients with newly diagnosed GBM compared to radiation alone (8, 9). Treatment of U87 glioma cell lines with vorinostat and temozolomide results in a supra-additive effect on growth inhibition (10). Therefore, the North American Brain Tumor Consortium (NABTC) conducted a phase I study to investigate the combination of vorinostat with temozolomide in patients with HGG.
Materials and Methods
This phase I, open-label, dose-finding study was conducted in two parts. Part 1 was a dose-escalation study of vorinostat in combination with TMZ 150 mg/m2/day for 5 days every 28 days. Part 2 was a dose-escalation study of vorinostat in combination with TMZ 150 mg/m2/day for 5 days of the first 28-day cycle and 200 mg/m2/day for 5 days of the subsequent 28-day cycles. The study was approved by the Institutional Review Boards of each participating center and was conducted in accordance with the Declaration of Helsinki. The trial was registered in the National Institutes of Health clinical trials database (NCT00268385). Informed consent was obtained from each patient before participating in the study.
Patient Selection
Patients were eligible for the study if they had histologically proven intracranial HGG (glioblastoma, gliosarcoma, anaplastic astrocytoma, anaplastic oligodendroglioma, anaplastic mixed oligoastrocytoma, and malignant astrocytoma not otherwise specified), were 18 years or older, and had a Karnofsky performance status (KPS) ≥ 60, adequate bone marrow function (WBC ≥ 3,000/μl, ANC ≥ 1,500/mm3, platelet count ≥ 100,000/mm3, and hemoglobin ≥ 10 gm/dl), adequate liver function (SGOT and bilirubin < 2 times the upper limit of normal), adequate renal function (creatinine < 1.5 mg/dL), and were ≥ 3 weeks from completion of radiotherapy. Patients who had previously progressed on TMZ as well as patients who were taking valproic acid (another HDAC inhibitor) within 2 weeks prior to enrollment were excluded. For Part 1, patients with either stable disease after radiotherapy (with or without concurrent TMZ) or following progression on radiotherapy alone (i.e. no concurrent TMZ) were eligible. Any number of prior relapses was allowed. For Part 2, only patients with stable disease after radiotherapy were eligible and the only prior therapies permitted were concomitant TMZ with radiotherapy or radiotherapy alone. Central pathology review was performed by KA.
Study Design
The primary objectives of the study were to define the maximum tolerated dose (MTD) and to characterize the safety profile of vorinostat in combination with TMZ in patients with HGG. Secondary objectives included pharmacokinetic analysis of vorinostat in combination with TMZ. In Part 1 of this study, patients received TMZ 150 mg/m2/day on days 1-5 of every 28 days in addition to variable doses of vorinostat administered either on days 1-14 of every 28 days (Group A) or on days 1-7 and 15-21 of every 28 days (Group B) (Table 1). The TMZ dose was not escalated during Part 1 for safety. In Part 2 of the study, all patients received TMZ 150 mg/m2/day on days 1-5 of the first 28-day cycle, followed by dose escalation to 200 mg/m2/day on days 1-5 of subsequent 28-day cycles. Variable doses of vorinostat were administered in Part 2 on days 1-7 and 15-21 every 28 days (Table 1). Patients in both Parts 1 and 2 of the study were treated for a maximum of 13 cycles of temozolomide in combination with vorinostat but had the option to continue vorinostat monotherapy at the discretion of the treating physician. Vorinostat was supplied by the Cancer Therapy Evaluation Program, Division of Cancer Treatment and Diagnosis, National Cancer Institute under a Cooperative Research and Development Agreement with Merck Sharp and Dohme.
Table 1. Patient characteristics.
| Characteristic | Median or No. (N = 59) | % or Range |
|---|---|---|
| Age | 51 | 25-81 |
| KPS | 90 | 60-100 |
| Race | ||
| Caucasian | 58 | 98 |
| Unknown/Not reported | 1 | 2 |
| Gender | ||
| Male | 34 | 58 |
| Female | 25 | 42 |
| Diagnosis | ||
| Glioblastoma | 39 | 66 |
| Gliosarcoma | 2 | 3 |
| Anaplastic Astrocytoma | 13 | 22 |
| Anaplastic Oligodendroglioma | 1 | 2 |
| Anaplastic Oligoastrocytoma | 4 | 7 |
Safety Assessment
Dose limiting toxicities (DLTs) based on Common Terminology Criteria for Adverse Events (CTCAE), version 3, included grade 3 or higher thrombocytopenia, grade 4 anemia, grade 4 neutropenia, any non-hematologic grade 3 or higher toxicity (except alopecia and grade 3 deep venous thrombosis). In addition, grade 3 or 4 nausea, vomiting, or diarrhea not be controlled by medical therapy were considered DLTs.
A standard 3+3 dose escalation design was used for both parts of the study and MTD was defined as the dose at which fewer than one-third of patients experienced a DLT to vorinostat in combination with TMZ. In Part 1, the MTD was based on assessment of DLT during the first 28 days of treatment. After determining the MTD in Part 1, Part 2 opened. The MTD in Part 2 was based on assessment of DLT in the first 56 days of treatment. In both Parts 1 and 2, additional patients (to a total of 12 patients in each Part) were enrolled at the MTD to further characterize the toxicity profile.
Pharmacokinetic Evaluation
Sample Collection
In Cycle 1, blood (6 mL) was collected from a peripheral vein into anticoagulant-free (vorinostat) or heparinized (TMZ) tubes before treatment and 1, 2, 3, 4, 6 and 8 hours after the first dose on day 1. Blood samples were also drawn before the first dose on day 2 and day 8. In Part 2 of the study, samples were also collected in cycle 2 using a similar schedule. Blood collected for determination of vorinostat was allowed to clot at 4°C for 20-30 minutes Blood collected for determination of TMZ was stabilized with 1.0 N HCl. Blood was separated by centrifugation into serum (vorinostat) or plasma (TMZ), transferred into plastic cryotubes, and stored at –70°C until analysis.
Temozolomide Assay Methodology
TMZ was assayed using high-performance liquid chromatography with UV detection as previously described (11).
Vorinostat Assay Methodology
Vorinostat concentrations were measured by a validated liquid chromatography-electrospray ionization tandem mass spectrometry method [A] as described by Galanis et al (12).
Correlative Studies
Blood samples for acetylation status of histones in peripheral blood mononuclear cells (PBMC) were collected before the initial dose of vorinostat on cycle 1 and subsequently at 1, 2, 6, 8, and 24 hours after administration of the first dose of vorinostat on day 1 of cycle 1; the 24h sample was drawn immediately prior to the first dose of day 2. Additional samples were drawn immediately prior to (baseline) and within 4 hours after administration of vorinostat on day 1 of cycle 2. Samples were collected using BD Vacutainer® CPT™ cell preparation tubes with sodium citrate (Cat # 362761, Beckton Dickinson) at each participating institution. The samples were centrifuged according to manufacturer's specified protocol and the tubes shipped at ambient temperature to the laboratory of VP at MD Anderson Cancer Center.
After partially removing the plasma, the mononuclear cell layer was collected with a Pasteur Pipette and transferred to a 15 mL size conical centrifuge tube per manufacturer specified method. The cells were then washed in PBS by tube inversion, centrifuged at 300 RCF for 15 min and the supernatant aspirated. The cell pellet was resuspended, the cells subsequently lysed and protein extracted. Changes in levels of acetylated histone H3 and H4 in various samples were examined by western blot analysis as previously reported (13).
Statistical Analysis
Baseline characteristics were summarized across all enrolled patients. Safety variables were summarized by descriptive statistics. Adverse events were described in terms of incidence and severity. Vorinostat and temozolomide concentrations and pharmacokinetic parameters are presented in tabular and graphic form. Vorinostat, VA, and VG serum concentration data were analyzed by standard non-compartmental methods using the Program WinNonlin Professional, version 4.1 (Pharsight Corporation, Mountain View, CA). In addition, summary tables depicting individual patient concentrations and individual and mean pharmacokinetic parameters are provided. Normalized OD values of histone H3 and H4 were plotted as a vertical scatter plot and the differences in the means of the values were compared for significance using a two-way ANOVA (for cycle 1 samples) or a paired t-test (for cycle 2 and for cycle 1 baseline versus 24 hour sample comparison).
Results
Patient Characteristics
Fifty-nine eligible patients were enrolled (Table 1). Pathology included 39 GBM, 2 gliosarcoma, 13 AA, 1 AO, and 4 AOA. Median age was 51 (range 25-81) and median KPS was 90 (range 60-100). Thirty-four were male and 25 were female.
MTD and Safety
The MTD of vorinostat on days 1-14 of every 28 days in combination with TMZ 150 mg/m2/day for 5 days every 28 days was 300 mg daily (Group A). However, the exposure of vorinostat achieved with this dose was low based on PK analysis. To increase the exposure, a schedule of vorinostat administered on days 1-7 and 15-21 of every 28 day cycle in combination with TMZ was examined (Group B). On this schedule, the MTD of vorinostat was 500 mg daily in combination with TMZ. In Part 2, the MTD of vorinostat on days 1-7 and 15-21 of every 28 day cycle combined with TMZ 150 mg/m2/day on days 1-5 of the first cycle and 200 mg/m2/day on days 1-5 of subsequent cycles was 400 mg daily.
Table 2 summarizes the DLTs encountered at each dose level. In Part 1B, DLTs included grade 3 anorexia, grade 3 ALT, and grade 5 hemorrhage in the setting of grade 4 thrombocytopenia (resulting in the only death on this study). In Part 2, no DLTs were encountered at the MTD. Four patients were treated at one dose level above MTD, but none of the patients were able to dose escalate TMZ to 200 mg/m2 due to thrombocytopenia.
Table 2. Dose escalation and dose limiting toxicities.
| Dose Level | Vorinostat dose | TMZ dose | DLT |
|---|---|---|---|
| Part 1A | |||
| Level 1 (starting dose) | 200 mg BID on Days 1-14 of every 28 days | 150 mg/m2 on Days 1-5 of every 28 days | 2/6 DLTs: grade 3 fatigue in 2 patients |
| Level 2 | 300 mg BID on Days 1-14 of every 28 days | 150 mg/m2 on Days 1-5 of every 28 days | 2/3 DLT: 1 grade 3 thrombocytopenia, 1 grade 3 fatigue |
| Level 2a | 200 mg TID on Days 1-14 of every 28 days | 150 mg/m2 on Days 1-5 of every 28 days | 2/3 DLTs: 1 grade 3 nausea, 1 grade 4 thrombocytopenia |
| Level -1 (MTD) | 300 mg QD on Days 1-14 of every 28 days | 150 mg/m2 on Days 1-5 of every 28 days | No DLTs were encountered (16 patients) |
| Part 1B | |||
| Level 1 (starting dose) | 400 mg QD on Days 1-7 and 14-21 of every 28 days | 150 mg/m2 on Days 1-5 of every 28 days | No DLTs were encountered (3 patients) |
| Level 2 (MTD) | 500 mg QD on Days 1-7 and 14-21 of every 28 days | 150 mg/m2 on Days 1-5 of every 28 days | 3/12 DLTs: grade 3 anorexia, grade 3 ALT, grade 4 thrombocytopenia + grade 5 hemorrhage |
| Part 2 | |||
| Level 1 (MTD) | 400 mg QD on Days 1-7 and 14-21 of every 28 days | 150 mg/m2 on Days 1-5 of first 28 day cycle and 200 mg/m2 on Days 1-5 of subsequent 28 day cycle | No DLTs (6 patients) |
| Level 2 | 500 mg QD on Days 1-7 and 14-21 of every 28 days | 150 mg/m2 on Days 1-5 of first 28 day cycle and 200 mg/m2 on Days 1-5 of subsequent 28 day cycle | 4 patients treated but unable to escalate to 200 mg/m2 for subsequent cycles mainly due to thrombocytopenia |
Table 3 lists the grade 3 or higher adverse events related to combination therapy according to dose levels. The most common serious adverse events considered possibly, probably, or definitely related to vorinostat in combination with TMZ were fatigue (15%), lymphopenia (8%), thrombocytopenia (8%), and thromboembolic event (5%).
Table 3. Grade 3 or 4 events with relationship possible or higher to combination therapy (Vorinostat + TMZ).
| Dose Levels | 200 mg BID on Days 1-14 | 200 mg TID on Days 1-14 | 300 mg BID on Days 1-14 | 300 mg QD on Days 1-14 | 400 mg QD on Days 1-7 and 15-21 | 500 mg QD on Days 1-7 and 15-21 | Total (%) n = 59 |
|---|---|---|---|---|---|---|---|
| Abdominal pain | 1 | 1 | 3% | ||||
| ALT elevation | 1 | 2% | |||||
| Anemia | 1 | 2% | |||||
| Anorexia | 1 | 1 | 3% | ||||
| Constipation | 1 | 2% | |||||
| Creatinine elevation | 1 | 2% | |||||
| Fatigue | 3 | 1 | 3 | 1 | 1 | 15% | |
| Headache | 1 | 2% | |||||
| Hematoma | 1 | 2% | |||||
| Hyperglycemia | 1 | 2% | |||||
| Hyponatremia | 1 | 2% | |||||
| Intracranial hemorrhage | 1 | 2% | |||||
| Lymphopenia | 1 | 2 | 1 | 1 | 8% | ||
| Nausea | 1 | 1 | 3% | ||||
| Neutropenia | 1 | 1 | 3% | ||||
| Peripheral sensory neuropathy | 1 | 2% | |||||
| Thrombocytopenia | 1 | 1 | 1 | 1 | 1 | 8% | |
| Thromboembolic event | 1 | 2 | 5% | ||||
| Vomiting | 1 | 1 | 3% | ||||
| Leukopenia | 1 | 1 | 3% | ||||
| Wound infection | 1 | 2% |
Pharmacokinetics
Pharmacokinetic analysis of TMZ was performed on 16 patients enrolled in the study (Supplementary Table A). Peak plasma concentrations were achieved 1.95 ± 0.91 hrs after the oral dose, and the mean TMZ half-life was 1.95 ± 1.95 hrs. These pharmacokinetic findings are comparable to a comparison trial of single agent TMZ in advanced cancers (14) suggesting no significant interaction with vorinostat.
Pharmacokinetic analysis of vorinostat and the vorinostat metabolites (VG and VA) was performed on 56 patients enrolled in the study. Results for each vorinostat dose are summarized in Table 4. Peak plasma concentrations of vorinostat were achieved 2.2 ± 1.5 hrs after the oral dose. The mean vorinostat half-life and oral clearance values for all patients were 2.1 ± 1.3 hrs and 430 ± 150 L/hr, respectively. Peak plasma concentrations of VA and VG were achieved 3.3 hrs and 2.5 hrs, respectively, after the oral vorinostat dose. The mean VA and VG half-life values for all patients were 8.9 ± 6.4 hrs and 2.1 ± 0.7 hrs, respectively. While there was substantial variability in drug disposition at each dose level, Cmax and AUC values appeared to increase in proportion to dose level. Accumulation of vorinostat and its metabolites in serum was assessed following seven days of treatment with vorinostat by comparing the Day 8 pretreatment concentration values with the Day 1-C8hr value for BID and TID dosing or the Day 1-C24hr value for QD dosing. No accumulation was observed for vorinostat and VG (R < 1). Modest accumulation (BID or TID- R = 2.1; QD- R = 1.6) of 4-anilino-4-oxobutanoic acid was consistent with the 8.9 hour half-life value.
Table 4. Pharmacokinetics of vorinostat and its metabolites.
| Dose (mg) | 200 | 300 | 400 | 500 | |
|---|---|---|---|---|---|
| N | 9 | 19 | 12 | 16 | |
| Vorinostat | Tmax (h) | 2.2 ± 1.2 | 2.4 ± 1.6 | 2.4 ± 1.8 | 1.9 ± 1.3 |
| Cmax (ng/ml) | 114 ± 56 | 239 ± 95 | 292 ± 186 | 327 ± 107 | |
| t1/2 (h) | 1.96 ± 0.75 | 1.86 ± 1.40 | 1.98 ± 0.94 | 2.49 ± 1.75 | |
| AUC0-8h (ng/ml*h) | 388 ± 160 | 672 ± 209 | 933 ± 357 | 1240 ± 300 | |
| Cl/F (L/h) | 518 ± 174 | 452 ± 155 | 417 ± 145 | 351 ± 94 | |
|
| |||||
| SAHA-acid | Tmax (h) | 2.9 ± 1.2 | 2.9 ± 1.9 | 3.6 ± 1.6 | 3.8 ±1.7 |
| Cmax (ng/ml) | 401 ± 160 | 788 ± 260 | 803 ± 454 | 1060 ± 420 | |
| t1/2 (h) | 7.4 ± 9.2 | 9.4 ± 8.2 | 9.9 ± 4.4 | 8.4 ± 2.1 | |
| AUC0-8h (ng/ml*h) | 1850 ± 760 | 3360 ± 1120 | 3360 ± 1280 | 5500 ± 2110 | |
|
| |||||
| SAHA-glucuronide | Tmax (h) | 2.5 ± 1.2 | 2.5 ± 1.7 | 2.7 ± 2.0 | 2.4 ± 1.3 |
| Cmax (ng/ml) | 933 ± 340 | 1690 ± 690 | 1760 ± 520 | 2380 ± 1410 | |
| t1/2 (h) | 1.9 ± 0.6 | 1.7 ± 0.7 | 2.3 ± 0.7 | 2.5 ± 0.5 | |
| AUC0-8h (ng/ml*h) | 3360 ± 1440 | 5160 ± 1640 | 6340 ± 1400 | 10110 ± 6040 | |
Correlative Studies
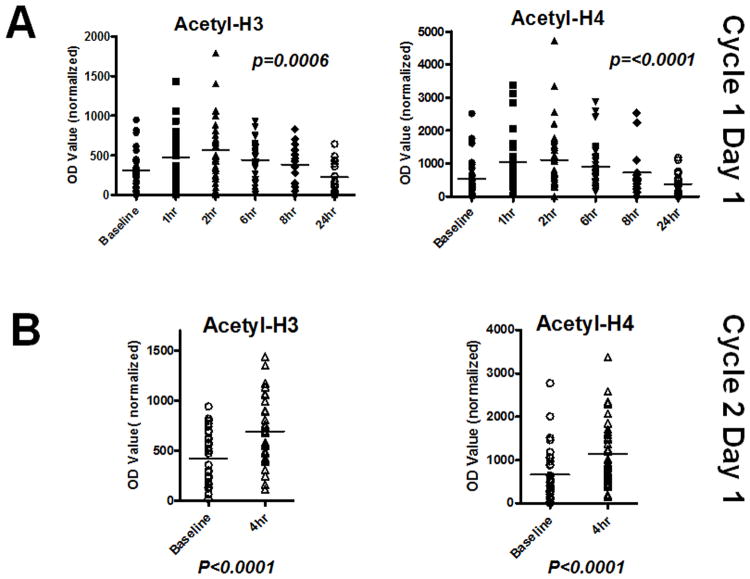
Samples were available from 33 patients for assessment of histone H3 and H4 acetylation for the baseline and at least 3 consecutive post-treatment samples for each patient for cycle 1. Vorinostat treatment resulted in hyperacetylation of both proteins in these samples with the highest average levels being seen at the 2 hour time point as shown in Figure 1A although the highest values in the individual patients occurred at either the 1 hour or 2 hour time point. Of note, there was an apparent decrease in mean of acetylation levels at 24 hours compared to the baseline levels but this was not statistically significant (p=0.35). A similar appearance of histone hyperacetylation was seen during cycle 2 with the post-treatment levels being significantly higher than pretreatment baseline. These findings are consistent with the expected effects of vorinostat in PBMC.
Figure 1.
A. Levels of acetylation of histone H3 (N=33) and H4 (N=37) in PBMC isolated from patients at baseline and at various time points indicated after Cycle 1 dose 1 of vorinostat as determined by western blot analysis. Differences in means of acetylation levels at various time points were assessed for statistical significance. B. Levels of histone H3 (N=30) and H4 (N=34) acetylation in Cycle 2 pre and post dose samples were assessed for treatment related changes.
Clinical Outcomes
The median number of cycles of combination therapy was 6 (Table 5), with two patients (5%) remaining on study on vorinostat monotherapy as of February 1, 2012, more than one and a half years since initiating treatment on protocol. Of the 57 patients who have stopped treatment on protocol, 29 patients (51%) experienced disease progression and 18 patients (32%) completed treatment per protocol criteria and decided not to continue on vorinostat monotherapy. The remainder stopped early due to adverse events.
Table 5. Clinical outcomes.
| Median or No. (% or range) N = 59 total patients on study | |
|---|---|
| Median No. of Cycles | 6 (1-NR*) |
| No. Patients Off Treatment | 57 (97) |
| Reasons for Stopping Treatment | N = 57 who stopped treatment |
| Adverse event/side effects/complications | 6 (11) |
| Disease progression | 29 (51) |
| Patient withdrawal or refusal after beginning protocol therapy | 3 (5) |
| Treatment completed per protocol criteria | 18 (32) |
| Death on study | 1 (2) |
NR = not reached; two patients remain on vorinostat monotherapy after completing treatment per protocol criteria
Conclusion
This is the first clinical study to evaluate the safety and tolerability of combining an HDAC inhibitor with TMZ in HGG. We initially tested vorinostat dosing on days 1-14 of every 28 day cycles in combination with TMZ. No pharmacokinetic interactions between temozolomide and vorinostat were seen. However, the exposure of vorinostat at the MTD achieved with this schedule was low based on PK analysis. To increase the exposure of vorinostat in combination with temozolomide, a schedule of vorinostat administered on days 1-7 and 15-21 of every 28 day cycle in combination with TMZ was examined. We determined MTDs based on this new dosing schedule.
Hyperacetylation of histones H3 and H4 is the primary effect of vorinostat's HDAC inhibitory activity. However, assessment of these changes in human glioma tissue is challenging. We demonstrated the feasibility of assessing hyperacetylation in PBMC as a potential surrogate of tumor tissue. Vorinostat treatment caused histone hyperacetylation which had its highest average at approximately 2 hours from the initial dose of the agent subsiding to baseline levels over 8 hours later. Given that vorinostat is known to cross the blood-brain barrier, it is possible that a similar duration of effect may be seen in glioma tissue as well. Such assessment may be potentially useful in future studies to determine if the changes correlate with clinical outcome thus providing surrogate markers of clinical benefit.
The combination of vorinostat and TMZ was well tolerated by many patients. The median number of cycles of combination therapy was 6 suggesting that the regimen can be administered for an extended period with appropriate management of side effects. Based on the information obtained from this phase I clinical trial, the Alliance in Clinical Trials in Oncology Cooperative Group (ACTION) and the Adult Brain Tumor Consortium (ABTC) are currently conducting an intergroup phase II trial of vorinostat with radiotherapy and concomitant TMZ in newly diagnosed GBM.
Supplementary Material
Statement Of Translational Relevance.
Vorinostat, a histone deacetylase inhibitor, has activity against high-grade glioma in preclinical studies and may work synergistically with cytotoxic chemotherapy. In this phase I trial, we evaluated escalating doses of vorinostat in combination with temozolomide. We show that vorinostat can be administered safely on days 1-7 and 15-21 of every 28-day cycle in doses up to 500 mg daily when combined with temozolomide 150 mg/m2/day × 5 days in 28-day cycles and in doses up to 400 mg daily when combined with temozolomide 150 mg/m2/day × 5 days for the first 28-day cycle and 200 mg/m2/day × 5 days for the subsequent 28-day cycles. Pharmacokinetic analysis revealed no significant interactions between vorinostat and temozolomide. Based on these results a phase I/II trial of vorinostat in combination with temozolomide and radiotherapy for newly-diagnosed glioblastoma is in progress.
Acknowledgments
The authors would like to acknowledge MDACC and the Johns Hopkins Data Management Centers
Funding: This study was sponsored by NIH Grant number U01 CA062399. Merck provided funding for pharmacokinetic analysis.
Footnotes
Conflicts of Interest: Eudocia Q. Lee: Advisory board for Novartis. Royalties from UpToDate, DEMOS Publishers.
Vinay K. Puduvalli: research grant support from Merck, Genentech and Celgene.
Advisory board for Novartis
Joel M. Reid: funding from Merck for PK analysis
John G. Kuhn: none reported
Kathleen R. Lamborn: none reported
Timothy F. Cloughesy: Merck, consultant and honoraria from speakers bureau, Genentech honoraria from ad board, Roche honoraria from ad board and consultant, Merck Sorano consultant, Celgene consultant, Novartis consultant
Susan M. Chang: Research support from Schering
Jan Drappatz: none reported
W. K. Alfred Yung; none reported
Mark R. Gilbert: Advisory boards and honoraria for Merck, Genentech and Abbott
H. Ian Robins: consultant to Genentech and Abbott
Frank S. Lieberman: none reported
Andrew B. Lassman: Consultant, Speaker, and/or Research Funding from Abbott, Campus Bio, Eisai, Genentech, GSK, Merck/Schering-Plough, Novartis, Kyowa Hakko Kirin Pharma, Keryx, Sigma Tau
Renee M. McGovern: funding from Merck for PK analysis
Jihong Xu: none reported
Serena Desideri: none reported
Xiabu Ye: none reported
Matthew M. Ames: funding from Merck for PK analysis
Igor Espinoza-Delgado: none reported, Michael D. Prados: none reported
Patrick Y. Wen: Consultant for Novartis, Merck. Paid Speaker for Merck. Research Support from Amgen, AstraZeneca, Esai, Sanofi-Aventis, Genentech, Genzyme, Novartis, Medimmune, Vascular Biogenics. Royalties from UpToDate, DEMOS Publishers.
References
- 1.Lane AA, Chabner BA. Histone deacetylase inhibitors in cancer therapy. J Clin Oncol. 2009;27:5459–68. doi: 10.1200/JCO.2009.22.1291. [DOI] [PubMed] [Google Scholar]
- 2.Siegel D, Hussein M, Belani C, Robert F, Galanis E, Richon VM, et al. Vorinostat in solid and hematologic malignancies. J Hematol Oncol. 2009;2:31. doi: 10.1186/1756-8722-2-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eyupoglu IY, Hahnen E, Buslei R, Siebzehnrubl FA, Savaskan NE, Luders M, et al. Suberoylanilide hydroxamic acid (SAHA) has potent anti-glioma properties in vitro, ex vivo and in vivo. J Neurochem. 2005;93:992–9. doi: 10.1111/j.1471-4159.2005.03098.x. [DOI] [PubMed] [Google Scholar]
- 4.Ugur HC, Ramakrishna N, Bello L, Menon LG, Kim SK, Black PM, et al. Continuous intracranial administration of suberoylanilide hydroxamic acid (SAHA) inhibits tumor growth in an orthotopic glioma model. J Neurooncol. 2007;83:267–75. doi: 10.1007/s11060-007-9337-z. [DOI] [PubMed] [Google Scholar]
- 5.Yin D, Ong JM, Hu J, Desmond JC, Kawamata N, Konda BM, et al. Suberoylanilide hydroxamic acid, a histone deacetylase inhibitor: effects on gene expression and growth of glioma cells in vitro and in vivo. Clin Cancer Res. 2007;13:1045–52. doi: 10.1158/1078-0432.CCR-06-1261. [DOI] [PubMed] [Google Scholar]
- 6.An Z, Gluck CB, Choy ML, Kaufman LJ. Suberoylanilide hydroxamic acid limits migration and invasion of glioma cells in two and three dimensional culture. Cancer Lett. 2010;292:215–27. doi: 10.1016/j.canlet.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 7.Galanis E, Jaeckle KA, Maurer MJ, Reid JM, Ames MM, Hardwick JS, et al. Phase II trial of vorinostat in recurrent glioblastoma multiforme: a north central cancer treatment group study. J Clin Oncol. 2009;27:2052–8. doi: 10.1200/JCO.2008.19.0694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stupp R, Hegi ME, Mason WP, van den Bent MJ, Taphoorn MJ, Janzer RC, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10:459–66. doi: 10.1016/S1470-2045(09)70025-7. [DOI] [PubMed] [Google Scholar]
- 9.Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. The New England journal of medicine. 2005;352:987–96. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 10.Wen P, Puduvalli VK, Kuhn J, Reid JM, Cloughesy T, Yung WK, et al. Phase I study of vorinostat (suberoylanilide hydroxamic acid) in combination with temozolomide (TMZ) in patients with malignant gliomas (NABTC 04–03) J Clin Oncol. 2007;25 abstr 2039. [Google Scholar]
- 11.Schold SC, Jr, Kuhn JG, Chang SM, Bosik ME, Robins HI, Mehta MP, et al. A phase I trial of 1,3-bis(2-chloroethyl)-1-nitrosourea plus temozolomide: a North American Brain Tumor Consortium study. Neuro Oncol. 2000;2:34–9. doi: 10.1093/neuonc/2.1.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Parise RA, Holleran JL, Beumer JH, Ramalingam S, Egorin MJ. A liquid chromatography-electrospray ionization tandem mass spectrometric assay for quantitation of the histone deacetylase inhibitor, vorinostat (suberoylanilide hydroxamicacid, SAHA), and its metabolites in human serum. J Chromatogr B Analyt Technol Biomed Life Sci. 2006;840:108–15. doi: 10.1016/j.jchromb.2006.04.044. [DOI] [PubMed] [Google Scholar]
- 13.Xu J, Sampath D, Lang FF, Prabhu S, Rao G, Fuller GN, et al. Vorinostat modulates cell cycle regulatory proteins in glioma cells and human glioma slice cultures. J Neurooncol. 2011;105:241–51. doi: 10.1007/s11060-011-0604-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dhodapkar M, Rubin J, Reid JM, Burch PA, Pitot HC, Buckner JC, et al. Phase I trial of temozolomide (NSC 362856) in patients with advanced cancer. Clin Cancer Res. 1997;3:1093–100. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.