Abstract
In the current study we show the dissociation and tumor accumulation dynamics of dual labeled near infrared (NIR) quantum dot core self-assembled lipidic nanoparticles (SALNPs) in a mouse model upon intravenous administration. Using advanced in vivo fluorescence energy transfer imaging techniques, we observed swift exchange with plasma protein components in the blood and progressive SALNP dissociation and subsequent trafficking of individual SALNP components following tumor accumulation. Our results suggest that upon intravenous administration SALNPs quickly transform, which may affect their functionality. The presented technology provides a modular in vivo tool to visualize SALNP behavior in real time and may contribute to improving the therapeutic outcome or molecular imaging signature of SALNPs.
Keywords: Quantum dots, Förster resonance energy transfer, in vivo imaging, tumor-bearing mice, lipidic nanoparticles
In the last two decades self-assembled lipidic nanoparticles (SALNPs) have been shown to be broadly applicable as intravenously injectable agents for biomedical purposes.1–5 SALNPs can serve as delivery vehicles for a wide variety of drugs, ranging from cytostatic agents to small interfering RNAs (siRNA) and proteins, and as molecular imaging probes.
Since their introduction by Dubertret and colleagues,6 hybrid SALNPs that consist of a nanocrystal core covered by a self-assembled lipid-coating have been widely explored as imaging agents as many nanocrystals exhibit unique diagnostic features.1, 7 These hybrid SALNPs possess unprecedented possibilities with respect to their multifunctionality, potential for derivatization and biocompatibility, as well as to serve as drug targeting vehicles.5, 8
The flexibility and versatility of SALNPs derive from their self-assembled nature, which allows facile inclusion and exchange of functional components as well as fine-tuning of composition. Despite their widespread application in in vivo studies, primarily for preclinical cancer diagnosis and therapy, 5, 9 studies that address the dissociation kinetics of self-assembled nanoparticles, including SALNPs, after intravenous administration are scarce.10 However, in order to maintain their functionality and fulfill their targeting purpose, the integrity of the assembled nanoparticle structure is crucial. Upon intravenous administration (Figure 1a, I), SALNPs are initially exposed to plasma proteins, lipoproteins and circulating cells (Figure 1a, II).10–12 In addition, they are exposed to the mononuclear phagocyte system (MPS), i.e. splenic phagocytic cells and the Kupffer cells of the liver (Figure 1a, III).13 After extravasation from the vasculature into e.g. the tumor interstitium (Figure 1a, IV), facilitated by the highly permeable tumor vasculature, nanoparticles may interact with components of the extracellular matrix, tumor associated macrophages and/or tumor cells.14 Finally, upon their dissociation and draining into the lymphatic system, nanoparticles or nanoparticle components may be retained by lymphocytes (Figure 1a, V).15, 16
Figure 1. Schematic of nanoparticle trafficking and fate upon intravenous administration.
a, Schematic illustration of the blood circulation, dissociation dynamics, tumor accumulation, and trafficking of self-assembled nanoparticles upon intravenous administration(I) in a tumor-bearing mouse. Several distinct compartments include the blood (II), the tumor (IV, interstitium), lymphatics and lymph nodes (V), and clearance organs of the mononuclear phagocyte system (III). b, Schematic structure of a self-assembled lipid-nanoparticle that consists of a near infrared quantum dot core covered by a self-assembled lipid-coating that is composed of Cy7-labeled and PEGylated lipids (QD710-Cy7-PEG). c, TEM images of QD710-Cy7-PEG with (lower) and without negative staining (upper). Both scale bars are 20 nm. d, Emission spectra of QD710-Cy7-PEG nanoparticles in PBS with different percentages of Cy7-lipids in the lipid corona. λExc = 500 nm. At increasing content of Cy7-lipids, the QD emission decreased dramatically, while Cy7 emission increased correspondingly, confirming the occurrence of FRET from the QD cores to the Cy7 dyes in the corona.
In a previous study, we have successfully studied the dynamics of lipoprotein interactions in vitro using quantum dot (QD) and Cy5.5 dual labeled nanoparticles using Förster resonance energy transfer (FRET) principles.17 In the current study we further developed this technology to monitor these processes in real time by in vivo fluorescence imaging techniques. To that end we advanced the design of our dual labeled nanoparticle by tuning its optical features to the near infrared (NIR). In combination with various advanced florescence imaging technologies, this nanoparticle allowed us to investigate the dynamics of nanoparticle accumulation and dissociation in a tumor mouse model.
RESULTS AND DISCUSSION
Highly efficient and air-stable CdTe/CdSe/CdS/ZnS core/multi-shell QDs were synthesized to serve as a FRET donor. Their emission band was tuned to centre at 710 nm (see Supporting Information, SI, Methods and TEM images in Figure S1). These QDs were coated by a PEGylated self-assembled lipid monolayer,6 and the dye-lipids incorporated in this nanoparticle corona functioned as 800 nm emitting FRET acceptors. The resulting nanoparticle (QD710-Cy7-PEG) is schematically presented in Figure 1b. Negative staining transmission electron microscopy (TEM) images confirmed a lipid corona covering a single QD nanocrystal (Figure 1c). The occurrence of FRET was confirmed by measuring emission spectra of a series of these particles containing varying amounts of Cy7-lipids. As plotted in Figure 1d, with increasing Cy7-lipid, the QD emission at 710 nm decreased, while the dye emission at 800 nm increased correspondingly. We further measured the QD emission lifetime of these samples and observed a decrease in lifetime, which corroborated that the above intensity changes were due to FRET (SI Figure S3).18, 19
The large spectral separation between the QD and Cy7-lipid enables us to trace the individual nanoparticle components simultaneously, while FRET between the QD core and the Cy7-lipid allows sensitive and semi-quantitative monitoring of the dissociation of the lipid corona from the QD core. To test the possibility of studying this FRET principle in an in vivo pilot experiment, QD710-Cy7-PEG was subcutaneously injected into the dorsal side of nude mice. Similar to in vitro conditions,17 dissociation of Cy7-lipids could be directly detected as an increase in QD intensity and simultaneous decrease of FRET intensity (SI Figure S4).
Subsequently, nude mice bearing HCT116 colon carcinoma on their flank were intravenously administered with either FRET QD710-Cy7-PEG or control QD710-PEG, and subjected to NIR fluorescence imaging. FRET QD710-Cy7-PEG accumulation in the tumor was observed as early as 30 minutes post administration (Figure 2a). Complete image datasets and control groups are included in SI Figure S5–8. Region of interest (ROI) analyses of the tumors revealed different dynamics for the different components (Figure 2b). For control QDs (QD710-PEG), the signal kept increasing for about 2 h, and then slowly declined over 48 h. However, for the dual labelled FRET QD710-Cy7-PEG, the QD signal increased much faster in the initial 2 h and kept rising even past 24 h. This initial faster increase was indicative of the disassociation of Cy7-lipids from the nanoparticle, which caused dequenching of QD emission. The subsequent gradual elevation of QD emission from the tumor by far exceeded the blood presence of the nanoparticles, which we established to have about a one-hour circulation half-life (SI Figure S9). Conversely, after an initial increase, the Cy7 signal started to decrease after 2 h and gradually vanished over 48 h. A similar pattern was observed for the FRET intensity (Figure 2a, b), which confirmed the Cy7-lipid dissociation from the nanoparticles after tumor accumulation.
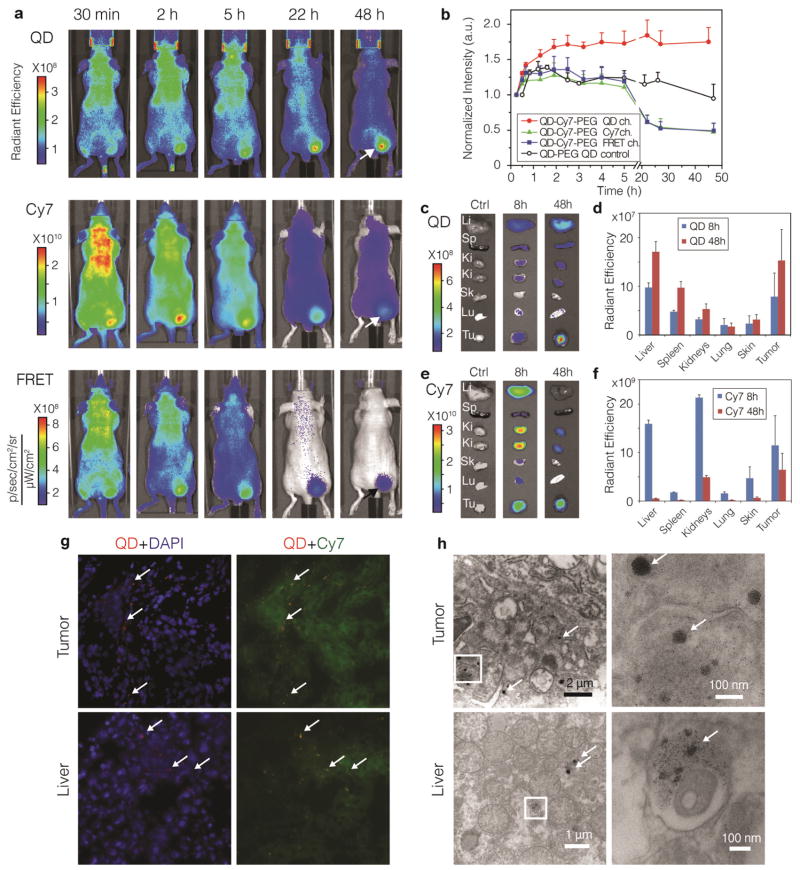
Figure 2. Accumulation and dissociation of QD710-Cy7-PEG in xenograft tumor mouse model upon intravenous administration.
a, Representative in vivo NIR fluorescence images of a tumor bearing mouse injected with 100 pmol/g FRET particles QD710-Cy7-PEG at 30 min, and 2, 5, 22, and 48 h post-injection. Tumor mice injected with 100 pmol/g QD710-PEG nanoparticles containing no Cy7-lipids were used as QD control. Fluorescent signal was collected using three optical filters settings: QD (λExc = 605 ± 18 nm, λEm = 720 ± 10 nm), Cy7 (λExc =745± 18 nm, λEm = 800 ± 10 nm) and FRET (λExc= 605± 18 nm, λEm= 800 ± 10 nm). b. The mean intensities (n=5 mice per group) from the tumor area (indicatad with arrows in a) plotted against post-injection time. QD, Cy7 and FRET intensities were from mice injected with QD710-Cy7-PEG, and QD control were from mice injected with QD710-PEG. Mice were sacrificed at 8 h (n = 3) and 48 h (n = 5) after injection and major organs were subjected to ex vivo fluorescence imaging. Representative images and mean intensities from the organs are presented in c and d for the QD channel and in e and f for the Cy7 channel. Li, liver; Sp, spleen; Ki, kidney; Sk, skin; Lu, lung; Tu, tumor. g, Fluorescence microscopy images of frozen sections of tumor (upper row) and liver (lower row) tissue sections at 48 h post-injection. Signal from QD (λExc = 620 ± 30 nm, λEm= 700 ± 35 nm) is red, DAPI for nucleus staining (λExc = 350 ± 30 nm, λEm= 460 ± 22 nm) is blue and Cy7 (λExc =710 ± 35 nm, λEm= 810 ± 40 nm) is green. Aggregates of QD cores are indicated by arrows. h, Stained transmission electron microscopy (TEM) images of tumor (upper row) and liver (lower row) tissues at 48 h after injection. Insets are magnified on the right. Aggregates of QD cores are indicated by arrows.
Ex vivo NIR fluorescence imaging of organs revealed FRET QD710-Cy7-PEG to be accumulated in the tumor and major organs (liver, spleen, and kidneys). QD intensities were higher at 48 h than 8 h (Figure 2c, d), suggesting that the dequenching effect due to the dissociation of Cy7-lipid also occurred in these organs. As opposed to the QD intensities, Cy7 intensities in all the organs decreased from 8 h to 48 h (Figure 2e, f), which indicated that the Cy7-lipid had a clearly different bio-distribution compared to QDs. The presence of Cy7-lipids in the kidneys suggests trafficking of this component to the kidneys and subsequent renal clearance. Fluorescence microscopy (FM) of tumor and liver tissues revealed the QDs to be mainly present as clusters, while the Cy7-lipids were found diffusely throughout the cytoplasm (Figure 2g and SI Figure S10, S11). TEM images confirmed the presence of dense QD aggregates, and additionally demonstrated that the QDs had entered cells and were mainly localized inside vesicles of tumor and liver cells (Figure 2h). Altogether, the ex vivo microscopy data corroborated the dissociation of the coating lipids from the QDs.
Tumors grown in dorsal window chambers (SI Figure S12) allowed us to study the dissociation and nanoparticle kinetics in the tumor blood vessels and in the tumor interstitium with intravital confocal laser scanning microscopy (CLSM).14, 20 To that end 610 nm emitting QD610 and Cy5.5-lipids labelled nanoparticles (QD610-Cy5.5-PEG) were employed (see emission spectra in SI Figure S13). Extravasation of QDs and Cy5.5-lipids from the vasculature into the tumor interstitium was clearly observed within 2 h post administration (Figure 3a, SI Movie S1–3). ROI analyses of the vascular and extravascular space revealed different dynamics for the QDs and Cy5.5-lipids in both these compartments (Figure 3b–d). In blood vessels the Cy5.5 and FRET signal decreased more rapidly than the QD signal, indicative of nanoparticle dissociation in circulation (Figure 3b and SI Figure S14). Conversely, extravascularly the QD signal kept increasing in the first 2 hours, while the Cy5.5 and FRET intensities started to decrease after 1h. This behaviour is similar to what we observed with whole body NIR imaging (Figure 2b). In Figure 3d, an overall decrease of FRET/QD ratio confirmed the dissociation of Cy5.5-lipids from the lipid-coated QDs within the first 2 h after administration, in both the vascular and extravascular space. The lipid dissociation constant was derived from fitting the vascular FRET/QD ratio with a monoexponential decay function.21 We found this constant to be 2.7×10−4 s−1 and the dissociation half-life to be around 42 min, indicating that the majority of the Cy5.5-lipids were dissociated from the QD after the first 2 hours. At 48 h a distinct dissociation of QD cores and Cy5.5-lipids was observed in the tumor interstitium (Figure 3a and SI Figure S15).
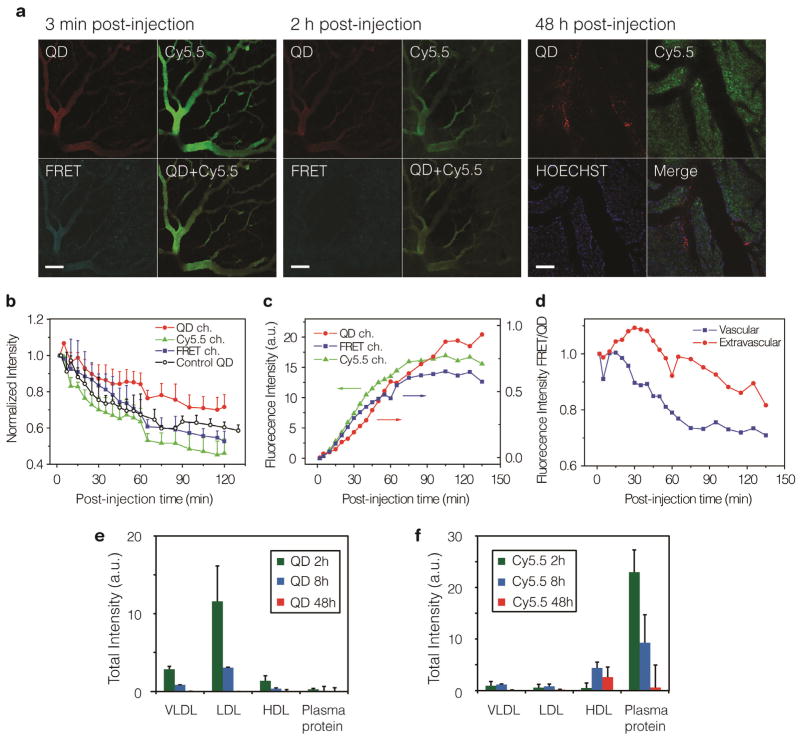
Figure 3. Intravital microscopy of tumors grown in a window chamber and FPLC investigation of the dynamics of lipid-coated nanocrystal.
a, Mice with tumor grown in a window chamber were injected with 130 pmol/g QD610-Cy5.5-PEG and continuously observed for 2 h, and discretely observed until 48 h post administration. Representative fluorescence images at 30 min, 2 h and 48 h are shown in a. The scale bar represents 100 μm. Four optical channels were used: QD (λExc = 488 nm, λEm= 612–655 nm) is shown in red, Cy5.5 (λExc = 633 nm, λEm= 698–719 nm) is shown in green, FRET (λExc = 488 nm, λEm= 698–719 nm) in cyan and HOECHST for nucleus staining (λExc = 780 nm, λEm= 435–485 nm) in blue. The normalized fluorescence intensities in three channels (QD in filled circles, Cy5.5 in filled triangles and FRET in filled square) are plotted against post-injection time for the vascular in b, and for extravascular space in c. QD610-PEG nanoparticles containing non Cy5.5-lipids were used as QD control (empty circles). d, The FRET/QD intensity ratios derived from the graphs in b, c represent the relative extent of FRET per QD and are plotted against time. Plasma collected from mice at 2 h, 8 h and 48 h after injected with 100 pm/g QD-Cy5.5-PEG (n=3 animals for each time point) separated in different fractions using fast protein liquid chromatography (FPLC). The total fluorescent intensities from four different protein fractions: VLDL (25–90 nm), LDL (18–25 nm), HDL (5–15 nm) and small plasma proteins (< 5 nm) are summarized in e, for QD signal (λExc = 430 ± 18 nm, λEm= 620 ± 10 nm), and in f, for Cy5.5 signal (λExc = 640 ± 18 nm, λEm= 700 ± 10 nm).
The interaction between nanoparticles and serum proteins has recently been investigated by us and others.10, 11, 17, 22 The afore-presented results indicated that the lipid-coated nanocrystals also vividly exchange coating lipids with blood proteins. Fast protein liquid chromatography (FPLC) 23 was employed to study different plasma fractions collected at 2, 8 and 48 h after intravenously administration of QD610-Cy5.5-PEG. At 2 h post-injection, the dominant QDs and Cy5.5-lipid intensities were found in different fractions (Figure 3e), indicating that the nanoparticle remained in blood had already dissociated with their lipid coating through lipid exchange while in circulation, which consists with the dissociation rate determined above. Although at early time points the majority of Cy5.5-lipids were found associated with small plasma proteins (<5 nm), at 48 h the main Cy5.5 intensity was observed in the HDL fraction (5–10 nm). This observation was in agreement with the clearance data for the Cy7/Cy5.5-labeled lipids where Cy5.5-lipids associated with small plasma proteins could be cleared renally, while labelled lipids that were associated with HDL could be retained as a result of the renal clearance threshold of around 5.5 nm.24, 25 The latter caused Cy5.5-lipids to be mainly present in the HDL fractions at later time points.
To better understand lymphatic drainage dynamics of the nanoparticles in the sentinel lymph node (SLN), we isolated this process by injecting QD710-Cy7-PEG in the periphery of solid tumors in nude mice. 15, 16 A representative NIR fluorescence image of this process is presented in Figure 4a. We observed the nanoparticles drained from the tumor and migrated to the inguinal lymph nodes (as SLN) within minutes. ROI analyses on the SLN revealed different dynamics for QDs and Cy7-lipids (Figure 4b), suggesting that after the nanoparticles reached SLN, the QD cores were retained and Cy7-lipids were moved away. Ex vivo fluorescence imaging of SLN and organs was performed at 5 h and 45 h post administration to mimic a situation where nanoparticles are first allowed to accumulate in the tumor for 3 h after intravenous administration. Here, we also observed a difference in biodistribution between QDs and Cy7-lipids. The QDs were mostly retained in SLN and their intensity increased from 5 h to 45 h, due to dissociation of Cy7-lipids (Figure 4c, d). Conversely, the intensity of the Cy7-lipid in SLN decreased over time, and its presence in the liver and kidneys was observed (Figure 4e, f), implying trafficking of this component to these organs and subsequent renal clearance (Figure 1a). FM (Figure 4g) and TEM images (Figure 4h) of excised SLN revealed the QD to be aggregated inside phagocytes and not to be co-localized with the Cy7-lipids.
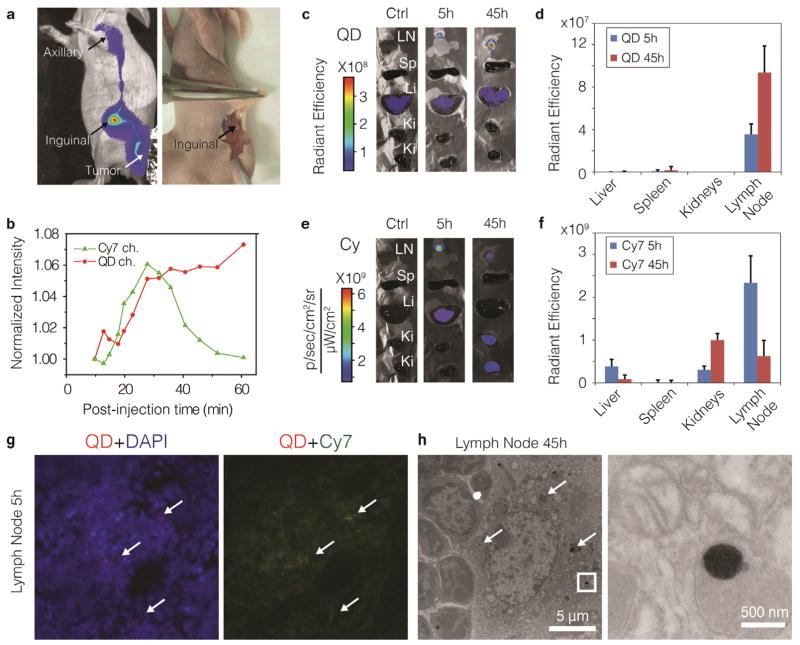
Figure 4. Peri-tumoral administration of lipid-coated nanocrystals.
a, NIR fluorescence image (left, laid over bright field image) show in both the QD and the Cy7 channel (Cy7 channel shown here), that the QD710-Cy7-PEG migrated through lymphatic draining from the periphery of the tumor to the inguinal node (as sentinel lymph node (SLN)) and further to the axillary node. After re-injection with 1% Evans blue, QD710-Cy7-PEG and Evans blue were found co-localized in the same lymph node, as indicated by the arrows (a, right, color image). b, Normalized total fluorescent intensities of QD710 (squares) and Cy7 (triangles) from the SLN are plotted against post-injection time, showing their different dynamic behaviours. Mice were sacrificed at 5 h and 45 h post-injection time (n=3 for each time point). Subsequently, the inguinal node and major organs were subject to ex vivo fluorescence imaging. Representative images and mean intensities are depicted in c and d for the QD channel and in e and f for the Cy7 channel. LN, lymph node. g, Fluorescence microscopy images of SLN tissue at 5 h post-injection. Merged images are shown with signal from QD (red), Cy7 (green) and DAPI (blue). Spots of QD accumulates are indicated with arrows. h, TEM images of SLN tissues at 45h after injection. Aggregates of QD cores inside the phagocyte are indicated by arrows. Insets are enhanced on the right.
In summary, SALNPs are dynamic structures that progressively disintegrate due to a lipid exchange process after intravenous administration (Figure 1a I, II). Upon vascular extravasation and accumulation in the tumor interstitium this process continues (Figure 1a IV). In case of the QD core SALNPs used in the current study, cellular internalization causes the QD cores to sequester in the tumor, lymph nodes and MPS organs (Figure 1a III, V), while their coating lipids partially follow different clearance kinetics in the circulatory system and can also be cleared renally.
Although the in vivo dissociation behaviour and the nanoparticle stability found in the current study is valid only for the particular type of SALNPs studied, 1, 6 the multi-faceted strategy we developed to assess the in vivo stability by FRET is flexible and applicable to a wide variety of SALNPs, including lipid-polymer hybrid nanoparticles26 and lipoprotein-derived nanoparticles,27 Moreover, it can be used to evaluate differently formulated SALNPs and screen for compositions with improved stability.17
CONCLUSION
Our approach represents a modular in vivo optical imaging tool to visualize the behaviour of self-assembled nanoparticles in real time and may contribute to enhancing the therapeutic outcome or improving the molecular imaging signature of this widely used class of nanoparticles. The in vivo dissociation behaviour of SALNPs may influence their drug delivery efficiency, and may also have implications for other types of self-assembled nanoparticles, such as nanoparticles comprised of block copolymers.28, 29 Our study also provides a framework to improve the specificity of self-assembled diagnostic nanoparticles since the in vivo integrity of such systems can now be carefully monitored. At the same time the exchange phenomenon may be exploited to transfer amphiphilic agents, such as cholesterol derivitized siRNAs30 or diagnostically active amphiphiles,1 from SALNPs to lipoproteins in the body.
METHODS
Synthesis of Cy7-DMPE-labeled PEG-DSPE-coated NIR quantum dot micelles (QD710-Cy7-PEG)
Synthesis of the NIR QD is described in SI method. For synthesis of QD710-Cy7-PEG, a typical process is described below. 20 μmol of DSPE-PEG2000, and 1 nmol NIR-QDs were dispersed in 0.5 ml chloroform. Cy7 labeled lipid was added to this mixture at various mass percentages of 0%, 0.1%, 0.2 %, 0.5 % and 1 %. The dispersion was dripped into 2 ml heated (over 80°C) deionized water under vigorous stirring. After all the organic solvent was evaporated, this water dispersion was centrifuged at 2000 rpm for 10 min to remove uncoated QDs and large aggregates. The QD micelle dispersion was then purified by centrifuging 200 μL on top of 1 ml of 30% w/w KBr solution at 14,500 rpm for 2 h using a microcentrifuge tube. Centrifugation transferred the QD micelles into the KBr layer, and the top 250 μL containing empty micelles and free lipids was taken off and discarded. The remaining solution was collected (precipitation on the bottom of tube was discarded), and washed/desalted three times with PBS using a Vivaspin 30,000 MWCO tube. The nanoparticle solution was finally enriched to desired concentration by Vivaspin centrifugation.
Cell culture and tumor model
HCT-116 cells were obtained from the Tumor Cell Biorepository of the Department of Oncological Sciences at the Icahn School of Medicine at Mount Sinai, and were cultured at 37°C under 5% CO2 in DMEM culture medium, supplemented with 10% FBS.
Six week old female Swiss nude mice were obtained from Taconic (Albany, NY) and were supplied with water and a standard rodent chow ad libitum. Five days before NIR fluorescent imaging, diet was changed to AIN-93M maintenance purified diet (TestDiet, Richmond, IN) in order to reduce the autofluorescent background. All animal handling was approved by the Icahn School of Medicine at Mount Sinai Institutional Animal Care and Use Committee. The tumor model was established by inoculation of 2.5 million HCT-116 cells, suspended in 50 μl serum-free DMEM, on the right side flank of the mice. Tumors were grown for 2 weeks to an average volume of 400 mm3, as measured with a digital caliper and by applying the formula V = 0.52 × a2 × b, where a is the smallest measured diameter and b is the largest measured diameter.
In vivo fluorescence imaging
Tumor-bearing mice were injected intravenously by tail vein injection with QD710-Cy7-PEG or QD710-PEG at a dose of 100 pmol/g (n=5). For each experimental group, NIR fluorescence images of the mice were recorded at various time points after injection (t=0.25, 0.5, 0.66, 1.3, 1.9, 2.5, 3.2, 4, 5, 22, 27, 45h) using a Xenogen IVIS Spectrum imaging system (Alemeda, CA). Mice were anesthetized using vaporized isoflurane, with a 4% induction dose, and 5 mice at a time were positioned in the IVIS with isoflurane administered at 1.5% via a nose cone. Three optical channels were recorded with selected excitation and emission band pass filters: QD (λExc = 605 ± 18 nm, λEm = 720 ± 10 nm), Cy7 (λExc =745± 18 nm, λEm = 800 ± 10 nm) and FRET (λExc= 605 ± 18 nm, λEm= 800 ± 10 nm). Overall acquisition time was 20s and all settings were kept the same for each time point, enabling comparison of intensity values. Results were processed and analyzed using Living Image software by drawing a region of interest (ROI) in the tumor area.
Ex vivo organ fluorescence imaging
The mice that were administered QD710-Cy7-PEG nanoparticles intravenously were sacrificed after 8h (n = 3) or 48h (n = 5) post-injection, and were perfused through the heart with 40ml PBS. Tumors and major organs were harvested and subject to fluorescent imaging immediately in the three optical channels described above. Organs from uninjected mice were used as blank control.
TEM of tumor, lymph node and other tissues
The tumor, lymph node, liver, spleen, and kidney from the above mentioned sacrificed mice were cut into small pieces and fixed in 2.5% glutaraldehyde. The tissue samples were processed by a standard procedure with osmium tetraoxide, and embedded in epoxy resin blocks. The resin blocks were cut into 60 nm sections with a microtome and placed on TEM grids. The sample grids were post stained with 4 % uranyl acetate and lead citrate before imaging. The TEM imaging used the same electron microscopy and conditions as previously described for nanoparticles characterization.
Fluorescence microscopy
Tissue samples of tumor, lymph node, liver, spleen, and kidney from the above mentioned sacrificed mice were embedded in OCT embedding matrix and frozen at −20°C. Five μm sections were cut on a cryostat and transferred to glass microscope slides. Upon use, sections were thawed for 20 minutes and mounted in Vectashield mounting medium with DAPI (Vector Laboratories, Burlingame, CA). Sections were imaged using a Zeiss Axioplan 2 Widefield Microscope (Zeiss, Jena, Germany) equipped with Cy5 and Cy7 filters (Chroma 49000 series, Chroma Technology Corp, Bellows Falls, VT) for imaging of QD710 and Cy7 dye, respectively. Cy5 exposure times were 100, 500, and 1500 milliseconds for liver, lymph node and tumor, respectively. Cy7 exposure times were 2000 milliseconds for liver, and 4000 milliseconds for lymph node and tumor tissues. Cy5 and Cy7 exposure times were kept constant per tissue for QD710-Cy7 and QD710 samples, Magnification was 40 times in all fluorescence microscopy images.
Intravital microscopy
For intravital confocal laser scanning microscopy (CLSM, Zeiss LSM 510 META), tumors grown in dorsal window chambers in mice were used. The mice were anesthetized with a subcutaneous injection of 12 mg/kg midazolam/fentanyl/haloperidol/water (3/3/2/4) and the window chambers (made of polyoxymethylene, build in house) were implanted as previously described in male athymic Balb/c Nu/nu mice of 22 to 24 gram.31 24 h after implanting the chambers, 2–3×106 HCT-116 cells were injected in the center of each chamber. The surgical procedures were performed under sterile conditions. The animals were kept under pathogen-free conditions at a temperature of 19 to 22 °C, 50 to 60% humidity, and 65 air changes per hour, and animals were allowed food and water (which contained 2% sucrose and 67.5 mg/L Baytril (enrofloxacin)) ad libitum. 16–18 days after implantation when the tumors were 0.2 to 0.3 cm thick and filled 30–60% of the window area, the mice were used for experiments.
The mice were anesthetized by subcutaneous injections of 12 mg/kg midazolam/fentanyl/Haldol/water (3/3/2/4), got a catheter (BD Venflon) placed in the tail veins and were placed on a custom build temperature controlled CLSM imaging stage. The tumors were imaged using a long working distance Plan-Neofluor 20x/0.5 objective. DIC contrast was used to focus on tumor vasculature before injection and subsequently either QD610-PEG (n=2) (but only 1 intravascular curve from only one mouse) or QD610-Cy5.5-PEG (n=2) (But extravascular curve from only one) was injected at a dose of 130 pmol/g.
To study nanoparticle and lipid dissociation dynamics, the same tumor region was imaged repeatedly at 3, 5, 10, 15, 20, 25, 30, 35, 40, 45, 50, 55, 60, 70, 80, 90, 100, 110, 120 min post injection. Subsequently, images were obtained throughout the tumor at several time points up to 48 hours post injection. HOECHST 33342 (Sigma) was injected 24 hours post injection of the nanoparticles to visualize cell nuclei facilitating the assessment of nanoparticle integrity and localization. QDs were excited at 488 nm and detected with the META system at 612–655 nm and Cy5.5 was excited at 633 nm and detected with the META system at 698–719 nm. FRET was detected using 488 nm excitation and recording with the META system at 698–719 nm. HOECHST 33342 was excited using two-photon excitation at 780 nm and detected with a bandpass filter at 435–485 nm.
Images were analyzed with ImageJ software. The images obtained in the first 2 hours post injection were aligned and combined into one dynamic series. ROIs were drawn manually in the vasculature and extravascular space from which the fluorescence intensity vs. time curves were obtained. Fluorescence intensities were normalized to the fluorescence intensity observed 3 minutes post injection. The FRET/QD ratio was obtained by dividing the normalized fluorescence intensities.
Fast protein liquid chromatography (FPLC)
Tumor bearing nude mice (n=9) were anesthetized using isoflurane, and for each mice 100 pmol/g QD610-Cy5.5-PEG was administrated intravenously by tail vein injection. At 2h, 8h and 48h post-injection, 3 mice per time point were sacrificed and 500 μl of blood was drawn from the left ventricle of the heart. Serum was obtained through centrifugation at 10k rpm for 2 × 10 min. Two Superose-6 FPLC columns in series (Shimazu LC solution) were pre-equilibrated with degased filtrated buffer (0.15 M NaCl, 1 mM EDTA). Mouse serum samples were filtrated with a 0.22-micron pore size membrane, and 200μl for each sample were injected to the columns. Samples were then eluted with equilibration buffer while the absorbance of eluted solution was monitored at 280 nm. The eluent was collected and divided into four effective fractions according to predetermined retention time which corresponded to the size of proteins: VLDL (25–90 nm), LDL (18–25 nm), HDL (5–15 nm) and small plasma proteins (< 5 nm). 23 The fluorescence intensities of sample fractions were determined and analyzed by IVIS imaging system. The QD signal in the different fractions was measured with λExc = 430 ± 18 nm, λEm= 620 ± 10 nm and the Cy5.5 signal was measured with λExc = 640 ± 18 nm and λEm= 700 ± 10 nm.
Supplementary Material
Acknowledgments
This work was financially supported by the division of Chemical Sciences (CW) of the Dutch Science Foundation (NWO) under grant number ECHO.06.B.047(A.M.), and the National Heart, Lung, and Blood Institute, National Institutes of Health, as a Program of Excellence in Nanotechnology (PEN) Award, Contract #HHSN268201000045C, the NIH grants R01EB009638 (Z.A.F.), R01CA155432 (W.J.M.M.), and R21CA159075 (A.R.), as well as the Norwegian Cancer Society. We thank Yu Zhou for his help with fluorescence imaging and K. Sæterbø for implanting the dorsal window chambers in mice and culturing cells.
Footnotes
Supporting Information Available: Supporting methods, supporting figures and movies. This material is available free of charge via the Internet at http://pubs.acs.org
References
- 1.Mulder WJM, Strijkers GJ, van Tilborg GAF, Cormode DP, Fayad ZA, Nicolay K. Nanoparticulate Assemblies of Amphiphiles and Diagnostically Active Materials for Multimodality Imaging. Acc Chem Res. 2009;42:904–914. doi: 10.1021/ar800223c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shi J, Xiao Z, Kamaly N, Farokhzad OC. Self-Assembled Targeted Nanoparticles: Evolution of Technologies and Bench to Bedside Translation. Acc Chem Res. 2011;44:1123–1134. doi: 10.1021/ar200054n. [DOI] [PubMed] [Google Scholar]
- 3.Yoo JW, Irvine DJ, Discher DE, Mitragotri S. Bio-Inspired, Bioengineered and Biomimetic Drug Delivery Carriers. Nat Rev Drug Discov. 2011;10:521–535. doi: 10.1038/nrd3499. [DOI] [PubMed] [Google Scholar]
- 4.Whitehead KA, Langer R, Anderson DG. Knocking Down Barriers: Advances in siRNA Delivery. Nat Rev Drug Discov. 2009;8:129–38. doi: 10.1038/nrd2742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peer D, Karp JM, Hong S, Farokhzad OC, Margalit R, Langer R. Nanocarriers as an Emerging Platform for Cancer Therapy. Nat Nanotechnol. 2007;2:751–760. doi: 10.1038/nnano.2007.387. [DOI] [PubMed] [Google Scholar]
- 6.Dubertret B, Skourides P, Norris DJ, Noireaux V, Brivanlou AH, Libchaber A. In Vivo Imaging of Quantum Dots Encapsulated in Phospholipid Micelles. Science. 2002;298:1759–1762. doi: 10.1126/science.1077194. [DOI] [PubMed] [Google Scholar]
- 7.Cormode DP, Sanchez-Gaytan BL, Mieszawska AJ, Fayad ZA, Mulder WJM. Inorganic Nanocrystals as Contrast Agents in MRI: Synthesis, Coating and Introduction of Multifunctionality. NMR Biomed. 2013;26:766–780. doi: 10.1002/nbm.2909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lobatto ME, Fuster V, Fayad ZA, Mulder WJ. Perspectives and Opportunities for Nanomedicine in the Management of Atherosclerosis. Nat Rev Drug Discov. 2011;10:835–852. doi: 10.1038/nrd3578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jain RK, Stylianopoulos T. Delivering Nanomedicine to Solid Tumors. Nat Rev Clin Oncol. 2010;7:653–664. doi: 10.1038/nrclinonc.2010.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li Y, Budamagunta MS, Luo J, Xiao W, Voss JC, Lam KS. Probing of the Assembly Structure and Dynamics Within Nanoparticles During Interaction with Blood Proteins. ACS Nano. 2012;6:9485–9495. doi: 10.1021/nn302317j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nel AE, Madler L, Velegol D, Xia T, Hoek EM, Somasundaran P, Klaessig F, Castranova V, Thompson M. Understanding Biophysicochemical Interactions at The Nano-bio Interface. Nat Mater. 2009;8:543–557. doi: 10.1038/nmat2442. [DOI] [PubMed] [Google Scholar]
- 12.Owens DE, 3rd, Peppas NA. Opsonization, Biodistribution, and Pharmacokinetics of Polymeric Nanoparticles. Int J Pharm. 2006;307:93–102. doi: 10.1016/j.ijpharm.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 13.Cormode DP, Skajaa GO, Delshad A, Parker N, Jarzyna PA, Calcagno CG, Merav W, Skajaa T, Briley-Saebo KC, Bell HM, et al. A Versatile and Tunable Coating Strategy Allows Control of Nanocrystal Delivery to Cell Types in the Liver. Bioconjugate Chem. 2011;22:353–361. doi: 10.1021/bc1003179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hak S, Helgesen E, Hektoen HH, Huuse EM, Jarzyna PA, Mulder WJM, Haraldseth O, Davies CDL. The Effect of Nanoparticle Polyethylene Glycol Surface Density on Ligand-Directed Tumor Targeting Studied in Vivo by Dual Modality Imaging. ACS Nano. 2012;6:5648–5658. doi: 10.1021/nn301630n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ballou B, Ernst LA, Andreko S, Harper T, Fitzpatrick JA, Waggoner AS, Bruchez MP. Sentinel Lymph Node Imaging Using Quantum Dots in Mouse Tumor Models. Bioconjug Chem. 2007;18:389–396. doi: 10.1021/bc060261j. [DOI] [PubMed] [Google Scholar]
- 16.Kim S, Lim YT, Soltesz EG, De Grand AM, Lee J, Nakayama A, Parker JA, Mihaljevic T, Laurence RG, Dor DM, et al. Near-Infrared Fluorescent Type II Quantum Dots for Sentinel Lymph Node Mapping. Nat Biotechnol. 2004;22:93–97. doi: 10.1038/nbt920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Skajaa T, Zhao Y, van den Heuvel DJ, Gerritsen HC, Cormode DP, Koole R, Parker JA, Mihaljevic T, Laurence RG, Dor DM. Quantum Dot and Cy5.5 Labeled Nanoparticles to Investigate Lipoprotein Biointeractions via Förster Resonance Energy Transfer. Nano Lett. 2010;10:5131–5138. doi: 10.1021/nl1037903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Medintz IL, Clapp AR, Mattoussi H, Goldman ER, Fisher B, Mauro JM. Self-Assembled Nanoscale Biosensors Based on Quantum Qot FRET donors. Nat Mater. 2003;2:630–638. doi: 10.1038/nmat961. [DOI] [PubMed] [Google Scholar]
- 19.Medintz IL, Mattoussi H. Quantum Dot-Based Resonance Energy Transfer and Its Growing Application in Biology. Phys Chem Chem Phys. 2009;11:17–45. doi: 10.1039/b813919a. [DOI] [PubMed] [Google Scholar]
- 20.Hak S, Reitan NK, Haraldseth O, de Lange Davies C. Intravital Microscopy in Window Chambers: a Unique Tool to Study Tumor Angiogenesis and Delivery of Nanoparticles. Angiogenesis. 2010;13:113–130. doi: 10.1007/s10456-010-9176-y. [DOI] [PubMed] [Google Scholar]
- 21.Reulen SW, Merkx M. Exchange Kinetics of Protein-Functionalized Micelles and Liposomes Studied by Forster Resonance Energy Transfer. Bioconjug Chem. 2010;21:860–866. doi: 10.1021/bc900398p. [DOI] [PubMed] [Google Scholar]
- 22.Salvati A, Pitek AS, Monopoli MP, Prapainop K, Bombelli FB, Hristov DR, Kelly PM, Aberg C, Mahon E, Dawson KA. Transferrin-Functionalized Nanoparticles Lose Their Targeting Capabilities When a Biomolecule Corona Adsorbs on the Surface. Nat Nanotechnol. 2013;8:137–143. doi: 10.1038/nnano.2012.237. [DOI] [PubMed] [Google Scholar]
- 23.Frias JC, Ma Y, Williams KJ, Fayad ZA, Fisher EA. Properties of a Versatile Nanoparticle Platform Contrast Agent To Image and Characterize Atherosclerotic Plaques by Magnetic Resonance Imaging. Nano Lett. 2006;6:2220–2224. doi: 10.1021/nl061498r. [DOI] [PubMed] [Google Scholar]
- 24.Choi HS, Liu W, Misra P, Tanaka E, Zimmer JP, Itty Ipe B, Bawendi MG, Frangioni JV. Renal Clearance of Quantum Dots. Nat Biotechnol. 2007;25:1165–1170. doi: 10.1038/nbt1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Choi HS, Liu W, Liu F, Nasr K, Misra P, Bawendi MG, Frangioni JV. Design Considerations for Tumour-Targeted Nanoparticles. Nat Nanotechnol. 2010;5:42–47. doi: 10.1038/nnano.2009.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Valencia PM, Basto PA, Zhang L, Rhee M, Langer R, Farokhzad OC, Karnik R. Single-Step Assembly of Homogenous Lipid Polymeric and Lipid Quantum Dot Nanoparticles Enabled by Microfluidic Rapid Mixing. ACS Nano. 2010;4:1671–1679. doi: 10.1021/nn901433u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bruns OT, Ittrich H, Peldschus K, Kaul MG, Tromsdorf UI, Lauterwasser J, Nikolic MS, Mollwitz B, Merkel M, Bigall NC, et al. Real-time Magnetic Resonance Imaging and Quantification of Lipoprotein Metabolism in vivo Using Nanocrystals. Nat Nanotechnol. 2009;4:193–201. doi: 10.1038/nnano.2008.405. [DOI] [PubMed] [Google Scholar]
- 28.Euliss LE, Grancharov SG, O’Brien S, Deming TJ, Stucky GD, Murray CB, Held GA. Cooperative Assembly of Magnetic Nanoparticles and Block Copolypeptides in Aqueous Media. Nano Lett. 2003;3:1489–1493. [Google Scholar]
- 29.Berret JF, Schonbeck N, Gazeau F, El Kharrat D, Sandre O, Vacher A, Airiau M. Controlled Clustering of Superparamagnetic Nanoparticles Using Block Copolymers: Design of New Contrast Agents for Magnetic Resonance Imaging. J Am Chem Soc. 2006;128:1755–1761. doi: 10.1021/ja0562999. [DOI] [PubMed] [Google Scholar]
- 30.Schroeder A, Levins CG, Cortez C, Langer R, Anderson DG. Lipid-Based Nanotherapeutics for siRNA Delivery. J Intern Med. 2010;267:9–21. doi: 10.1111/j.1365-2796.2009.02189.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Erikson A, Tufto I, Bjonnum AB, Bruland OS, de Davies CL. The Impact of Enzymatic Degradation on the Uptake of Differently Sized Therapeutic Molecules. Anticancer Res. 2008;28:3557–3566. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.