Abstract
Clinically aggressive prostate cancer (PCa) is linked to androgen resistance, metastasis, and expression of neuroendocrine markers. To understand mechanism(s) of neuroendocrine differentiation (NED) of PCa epithelia, we compared neuronal differentiation occurring during embryogenesis, in primary cultures of neural crest (NC) cells, and NED in PCa cell lines (LNCaP and PC3). We demonstrate, hypoxia promotes neuronal and neuroendocrine differentiation of NC cells and PCa cells, respectively, by inducing the miR-106b~25 cluster. In turn, miR-106b~25 comprised of miR-106b, miR-93 and miR-25, down-regulates the transcriptional repressor REST, which represses neuron-specific protein-coding and miRNA genes. In prostate tumors of high Gleason score (≥ 8), an inverse trend was observed between REST and miR-106b~25 induction. Employing miRNA PCR arrays, we identified miRNAs up-regulated by hypoxia in LNCaP cells and REST-knockdown in NC cells. Significantly, a subset of miRNAs (miR-9, miR-25, miR-30d and miR302b) is up-regulated in high Gleason score (≥ 8) PCa, suggesting a mechanism by which NED contributes to PCa malignancy. We propose that loss of REST and induction of this set of microRNAs can serve as potential novel clinical markers of advanced PCa.
Cancer cells, similar to embryonic stem cells, undergo cellular reprogramming generating altered cellular phenotypes. Examples include epithelial cancer cells of the lung, breast and prostate, transdifferentiating to neuroendocrine (NE)-like phenotypes (1-3). The advantage of this neuroendocrine differentiation (NED) for the cancer cell is not fully understood, although secreted neuropeptides can serve as paracrine, proliferative signals (4). In prostate cancer (PCa), NED is associated with clinically aggressive tumors. Advanced prostate tumors (Gleason scores 8 to 10 and some Gleason 7=4+3) are metastatic, androgen-resistant and express high levels of chromograninA (ChgA) and neuron-specific enolase (NSE) (4-6). ChgA and NSE are markers of neuroendocrine cells and also serve as markers of metastatic PCa, associated with hormone-refractory cancer growth, resistance to radiation therapy, and poor prognosis (1-6). In vitro and in vivo evidence supports that the origin of NE-like cells in prostate tumors is fromtransdifferentiation of cancerous luminal secretory cells (7, 8). However, mechanism(s) underlying the cellular reprogramming of luminal epithelial PCa cells to NE-like are not well understood.
It is well established that mechanisms regulating embryonic development provide a context for understanding disease/cancer pathogenesis. According to this principle, in this study, we are investigating and comparing mechanisms of neuronal differentiation in embryogenesis with NED of PCa epithelia. The cellular model of primary cultures of neural crest (NC) cells has been extensively used to understand mechanisms of neuronal differentiation. The NC, a transient, embryonic cell population located between surface ectoderm and neural tube is comprised of pluripotent stem-like cells that migrate along defined routes in the embryo giving rise to various cell types. NC cells from the trunk region of the embryo differentiate to sympathoadrenal and sensory neurons, and non-neuronal melanocytes and glia (9). Micro-environmental factors inducing neuronal differentiation of NC cells include bone morphogenetic proteins (BMPs) (10) and hypoxia (11). BMPs activate expression of pro-neural transcription factor achaete-scute complex homolog 1 (ASH-1) (12) and the downstream paired-like homeobox 2a (Phox2a) (13), essential for sympathoadrenal neuron differentiation (14). However, the mechanism mediating hypoxia-induced neuronal differentiation is unknown.
Various aspects of the mechanism of neuronal differentiation of embryonic NC cells are relevant to NED of PCa. First, expression of neuronal genes occurs both during neuronal differentiation of NC cells (12-14) and NED of PCa cells (1). Second, the embryonic NC is an invaluable model for understanding mechanisms of epithelial-mesenchymal-transition (EMT) and migratory potential. In neurulation, the migratory and cell specification potential of NC cells is orchestrated by a gene regulatory network that includes transcriptional regulators Snail and Twist1 (15). Importantly, the migratory potential of PC3 cells, a human PCa cell line, is linked to these genes (16, 17). Third, hypoxia is a physiologic inducer in embryonic development (18). Primary NC cultures exposed to hypoxia (2% O2) nearly all differentiate to neurons (11). Importantly, hypoxia characterizes poorly vascularized regions of solid tumors and is associated with high Gleason score prostate adenocarcinoma (19). Accordingly, we hypothesize that a common mechanism mediates neuronal differentiation of NC cells and NED of PCa cells.
Hypoxia, a well-recognized micro-environmental factor in embryonic development, closely correlates with PCa disease stage and NED (20). Cellular adaptation to hypoxia involves stabilization of hypoxia-inducible transcription factors (HIFs), HIF-1α and HIF-2α (21). HIFs are considered as prognostic indicators for cancer relapse, metastasis and resistance to treatment (21), and were detected in high Gleason prostate tumors (16, 22). However, the mechanism by which hypoxia induces NED of PCa epithelia cells or SA neuronal differentiation of NC stem cells (11) is not yet understood.
Hypoxia via the action of HIFs induces expression of protein coding genes and also of microRNAs (miRNA) (23). miRNAs repress gene expression post-transcriptionally by interfering with target mRNA stability and/or translation (24). Each miRNA down-regulates a cascade of genes and thus miRNAs regulate over 60% of mammalian genes, many of which play critical roles in tumorigenesis and embryonic development (25). Hypoxia-inducible miR-93 (23) belongs to the miRNA cluster miR-106b~25, encoding miR-106b, miR-93 and miR-25. Interestingly, miR-106b~25 is proto-oncogenic in PCa; all three members of this miRNA cluster are upregulated in PCa, targeting down-regulation of tumor suppressor PTEN (26). Furthermore, miR-106b~25 modulates EMT during cellular reprogramming (27) and promotes neuronal differentiation of neural stem/progenitor cells in adults (28). These findings imply potential roles of miR-106b~25 in NED of PCa cells and neuronal differentiation of NC cells.
A putative target of miR-106b~25 predicted by comparative sequence analysis (29) is the RE-1 silencing transcription factor (REST). In neurulation, REST silences in non-neuronal cells expression of neuron-specific protein coding genes and miRNAs (30, 31). REST is widely expressed in non-neural tissues and neural precursors, regulating chromatin transitions from pluripotent neural progenitors to differentiated neurons (31). Also, REST has been identified by a genetic screen as a tumor suppressor deleted in epithelial tumors and antagonizing the PI3K/Akt pathway (32). Importantly, the PI3K/Akt pathway is essential in NED of PCa (33). In prostate tumors, PTEN, the negative regulator of the PI3K pathway, is down-regulated by miR106~25 (26). Hypoxia induces expression of miR-93 encoded by miR-106b~25 (23) suggesting that hypoxia down-regulates both PTEN and REST in PCa epithelia, thereby inducing expression of neuronal genes and miRNAs allowing neuroendocrine reprogramming.
Herein, employing primary cultures of NC cells and human PCa cell lines (LNCaP and PC3), we demonstrate the role of hypoxia and the significance of REST down-regulation in neuronal/neuroendocrine differentiation. Importantly, down-regulation of REST occurs in human prostate tumors with increasing Gleason score. Since REST represses expression of neuron-specific protein and miRNA genes, employing miRNA PCR arrays we have identified miRNAs induced in NC cells after REST-knockdown and in LNCaP cells by hypoxia. Interestingly, some of these miRNAs are upregulated in prostate tumors with high Gleason score (≥ 8). We propose, these clinically relevant miRNAs are mechanistically linked to advanced PCa pathogenesis and may serve as prognostic indicators of aggressive PCa.
Materials and Methods
Cell Culture
Primary cultures of trunk neural crest (NC) cells were prepared from Japanese quail (Coturnix coturnix) embryos as described (34). LNCaP cells were purchased from ATCC and cultured in RPMI 1640 (Invitrogen) containing 2.0g/L NaHCO3 and 10% fetal bovine serum (Atlanta Biologicals). PC-3 cells were from ATCC and cultured in F-12K Nutrient Mixture (Invitrogen) with 10% fetal bovine serum.
Hypoxia Treatment
A hypoxia chamber (Billups-Rothenberg, Inc.) was used for hypoxia treatment of cells. Mixed gas containing 5% O2, 5% CO2 and 90% N2 was delivered into the chamber at 15 liters per minute for 90 seconds, once a day during culture period.
RNA Interference
siRNA duplexes were designed and synthesized with Stealth RNAi siRNA Technology (Invitrogen). Stealth RNAi siRNA Negative Control (Invitrogen) was used as a transfection control. Three different siRNA duplexes targeting the same gene were mixed as a siRNA pool. The siRNA pool (200 pmol) was transfected with 10μl Lipofectamine 2000 Reagent (Invitrogen) per 60mm dish. OPTI-MEM I Reduced Serum Medium (Invitrogen) without antibiotics was used to dilute siRNA and Lipofectamine 2000 Reagent. Final concentration of siRNA pool was 40 nM in a 60 mm dish. Primary NC cultures were transfected 24h before replating. See supplementary Table S1 for siRNA sequences.
Real-time PCR
Total RNA was isolated by the TRIZOL (Invitrogen) method. cDNA was synthesized from total RNA with iScript cDNA Synthesis Kit (Bio-Rad) for protein coding genes and RT2 miRNA First Strand Kit (SABiosciences) for miRNAs. PCR Arrays were carried out using RT2 miRNA PCR Array System (SABiosciences). Real-time PCR was performed on ABI7300 real-time PCR system (Applied Biosystems). See Supplementary Table S2 for primer sequences.
Immunoblotting and Immunofluorescence Microscopy performed as described (34).
Results
In NC cells, hypoxia induces expression of miR-106b~25 and neuronal genes while repressing expression of REST
Earlier studies with murine NC stem cells have shown that hypoxia greatly enhances differentiation of the neuronal sympathoadrenal lineage by an unknown mechanism (11). We employed primary cultures of avian NC cells grown in hypoxia for 24hrs, from day 0 to day 1 after replating (34), followed by incubation in normoxia. Under these conditions, we observed enhanced number of cells immunostaining for tyrosine hydroxylase (TH), a marker of sympathoadrenal neurons, and significantly reduced number of pigmented melanocytes (Fig. 1A). The enhanced number of TH-positive cells and the reduced number of melanocytes in the same culture, due to hypoxia treatment, supports our previous results of a dynamic balance between the neuronal vs. melanocyte lineages (34).
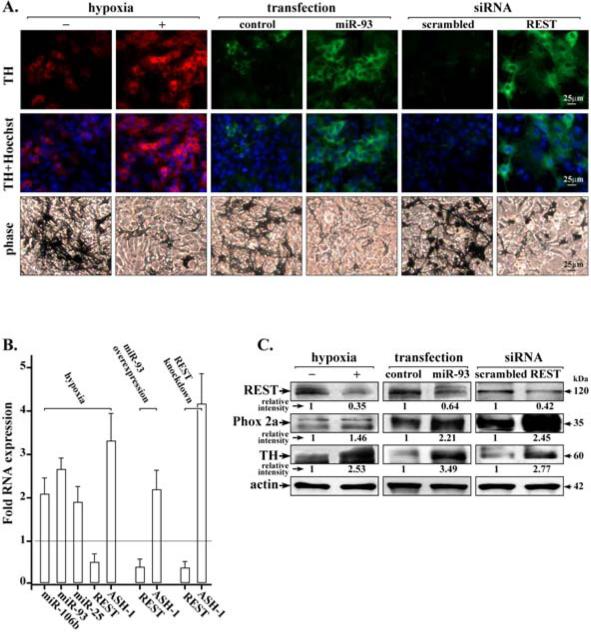
Figure 1. Hypoxia promotes neuronal differentiation of NC cells by induction of miR106b~25 and down-regulation of REST.
A. Immunofluorescence microscopy of neuronal marker tyrosine hydroxylase (TH) in NC cells grown in normoxia or hypoxia, with over-expression of miR-93, or REST knockdown by siRNA transfection. Phase contrast images of NC cultures show pigmented melanocytes. B. Real-time PCR quantification of miR-106b, miR-93 and miR-25 levels, REST and ASH-1 under the indicated conditions. Fold RNA expression under indicated conditions is relative to control, i.e., normoxia, scrambled miR or siRNA. Data shown are the average from three independent RNA preparations, each RNA preparation analyzed by real-time PCR using identical triplicates. Quantification is relative to GAPDH. Error bars represent +/− standard error of the mean. C. Immunoblots of REST, Phox2a and TH using whole cell extracts (WCE) isolated from NC cells grown in normoxia or hypoxia, with over-expression of miR-93, and REST knockdown by siRNA transfection, as indicated. Actin serves as internal control. A representative assay is shown from at least 3 independent experiments.
Since hypoxia induces miR-93 (11) whose putative target is the neuron-specific transcriptional repressor REST (29), we investigated in NC cultures the effect of miR-93 over-expression as well as the effect of REST knockdown by siRNA transfection on neuronal differentiation. Enhanced numbers of TH-positive cells and reduced numbers of melanocytes were observed both with miR-93 over-expression and REST knockdown (Fig. 1A). Importantly, hypoxia treatment of NC cell cultures enhanced expression of all members of the miR-106b~25 cluster (miR-106b, miR-93 and miR-25) by nearly 2-fold (Fig. 1B). By contrast, in hypoxia the mRNA level of REST was reduced by nearly 50% compared to its level in cells grown in normoxia. Under these conditions mRNA levels of pro-neural transcription factor ASH-1 increased by 3-fold (Fig. 1B). Similarly, over-expression of miR-93 or REST knockdown significantly decreased REST expression levels, while ASH-1 mRNA exhibited a 2-fold and 4-fold increase, respectively.
Immunoblots confirmed at the protein level the down-regulation of REST by hypoxia, miR-93 over-expression, and REST knockdown by transfection of REST siRNA. Conversely, under conditions of REST down-regulation, protein levels of neuronal markers Phox2a and TH were increased (Fig. 1C). Thus, exposure of NC cultures to hypoxia induced expression of miR-106b~25, down-regulation of REST, increased expression of pro-neural transcription factors ASH-1 and Phox2a, and neuronal differentiation.
In human PCa cell lines, hypoxia induces expression of miR-106b~25 and neuronal markers while repressing expression of REST
In PCa, tumor hypoxia and expression of neuronal markers are associated with advanced clinical stage, therapy resistance and poor prognosis (1, 35). To understand the effect of hypoxia on NED and tumor progression, we employed the human PCa LNCaP cell line, a well-established cellular model of NED, and the metastatic PC3 cell line. LNCaP cells exposed to hypoxia were analyzed for expression of the proto-oncogenic miR-106b~25 cluster, the neuron-specific transcriptional repressor REST, and neuronal NSE (Fig. 2A). All three members of miR-106b~25 were induced by hypoxia, the induction ranging from 1.8 to 2.7-fold. By contrast, in hypoxia, REST mRNA level was reduced by 40% compared to normoxia. A significant 12-fold increase was observed in the expression of NSE mRNA. The protein level of HIF1α was increased while REST protein level began to decrease 3hrs after exposure to hypoxia (Fig. 2B).
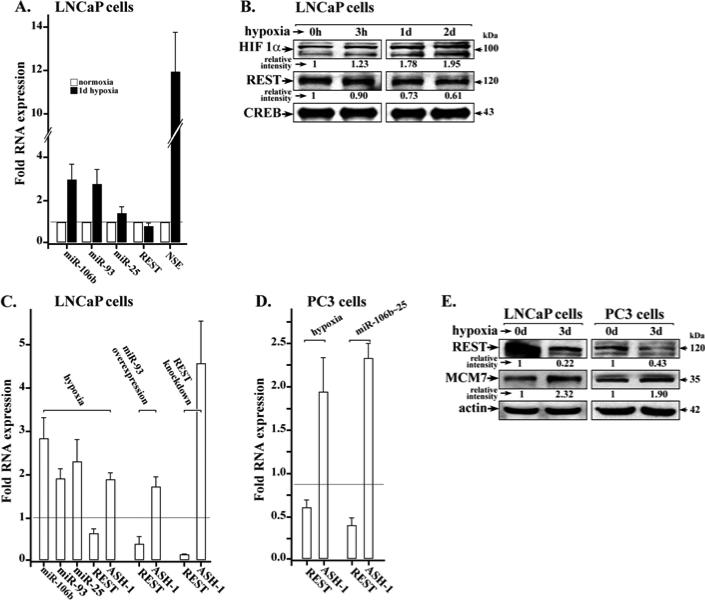
Figure 2. Hypoxia induces miR-106b~25 and decreases REST expression.
A. Real-time PCR quantification of miR-106b, miR-93, miR-25, REST, and NSE levels using total RNA isolated from LNCaP cultures grown in normoxia or hypoxia for 1 day (1d). Fold RNA expression is the ratio of expression in hypoxia vs. normoxia. Data shown are the average from three independent RNA preparations, each RNA preparation analyzed by real-time PCR using identical triplicates. Quantification is relative to GAPDH. Error bars represent +/− standard error of the mean. B. Immunoblots of HIF1α and REST using nuclear extracts isolated from LNCaP cultures grown in hypoxia for 0h, 3h, 1 day and 2 days. CREB serves as internal control. A representative assay is shown from at least 3 independent experiments. C. and D. Real-time PCR quantification of miR-106b, miR-93, miR-25, REST and ASH-1 in LNCaP (C.) and PC3 cells (D.). Conditions include 2-day treatment with hypoxia, miR-93 overexpression and REST knockdown by siRNA transfection. Fold RNA expression under indicated conditions is relative to control, i.e., normoxia, or scrambled miR/siRNA. Data shown are the average from three independent RNA preparations, each RNA preparation analyzed by real-time PCR using identical triplicates. Quantification is relative to GAPDH. Error bars represent +/− standard error of the mean. E. Immunoblots of REST and MCM7 from lysates of LNCaP and PC3 cells grown in normoxia or hypoxia for 3 days (3d). Actin serves as internal control. A representative assay is shown from at least 2 independent experiments.
Furthermore, in conditions of hypoxia relative to normoxia both LNCaP and PC3 cells exhibited increased mRNA levels of proneural ASH-1, while expression of REST was reduced (Fig. 2C and D). Similarly, REST mRNA levels were reduced by over-expression of miR-93 or miR-106b~25, and knockdown of REST by siRNA transfection (Fig. 2C and D). Conversely, pro-neural ASH-1 levels increased. Under these conditions, immunoblots confirmed the reduction of REST at the protein level. Since the miR-106b~25 cluster is encoded in intron-13 of the MCM7 gene (26), we also examined by immunoblots, protein levels of MCM7 in cells treated by hypoxia. Increased MCM7 protein levels are detected in both LNCaP and PC3 cells following 3-day hypoxia treatment (Fig. 2E).
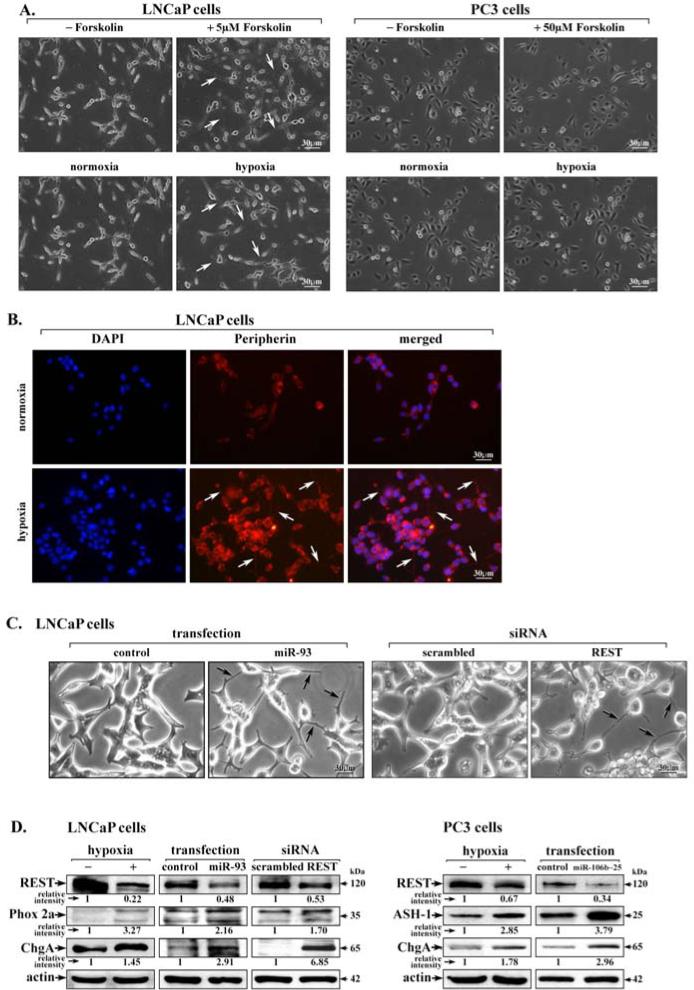
The induction in LNCaP and PC3 cells of proneural ASH-1 by hypoxia or overexpression of miR-106b~25 (Fig. 2C and D) suggests these PCa cell lines assume a neuronal phenotype. Accordingly, we investigated neurite formation in LNCaP and PC3 cells as a function of hypoxia (Fig. 3A). As positive control, we treated these cells with the cAMP inducing agent forskolin, a known inducer of neuronal differentiation of LNCaP cells (4), but not PC3 cells (Fig. 3A). Similarly, hypoxia induced formation of short neurites in LNCaP cells but not PC3 cells (Fig. 3A). Neurite formation by hypoxia in LNCaP cells was further confirmed by peripherin immunostaining (Fig. 3B). Likewise, mir-93 overexpression or REST knockdown by siRNA transfection induced neurite formation in LNCaP cells (Fig. 3C). Interestingly, in both LNCaP and PC3 cells hypoxia, miR-106b~25 overexpression, or REST knockdown induced expression of proneural genes Phox2a, ASH-1, and ChgA (Fig. 3D).
Figure 3. Hypoxia via miR-106b~25 reduces REST expression promoting expression of neuroendocrine markers in human PCa cells.
A. Phase contrast of LNCaP and PC3 cells treated for 1 day with the indicated concentration of forskolin or hypoxia. Arrows point to neurites. B. Immunofluorescence microscopy of peripherin using LNCap cells grown in normoxia or hypoxia for 1 day. DAPI staining indicates cell nucleus. C. Phase contrast images of LNCaP cells transfected with miR-93, REST siRNA or corresponding scrambled controls, as indicated. Arrows point to neurites. D. Immunoblots of REST, Phox2a, ChgA, and ASH-1, from lysates of LNCaP and PC3 cells grown in normoxia or hypoxia, with over-expression of miR106b~25, or REST knockdown by siRNA transfection, as indicated. Actin serves as internal control. A representative assay is shown from at least 3 independent experiments.
Despite the absence of neurite formation in PC3 cells exposed to hypoxia, in both LNCaP and PC3 cells hypoxia or expression of miR-106b~25 down-regulates REST, promoting expression of pro-neural transcription factors ASH-1 and Phox2a, and neuronal marker ChgA.
Deregulated expression of REST and miR-106b~25 in human prostate tumors
To determine the clinical relevance of the observations derived from cell-based assays of NC cultures (Fig. 1) and human PCa LNCaP and PC3 cell lines (Figs. 2 and 3), we quantified in paired human prostate tissue (tumor vs. benign) expression of markers associated with neuronal differentiation, hypoxia, and expression of miR-106b~25 (Fig. 4). Markers indicative of neuronal differentiation include REST and NR2F2, the latter being the earliest neuronal marker during development (36). Hypoxia was monitored by expression of phosphoglycerate kinase1 (PGK1, 21); expression of miR-106b~25 was monitored by mRNA levels of MCM7, the host gene that encodes miR-106b~25 in intron-13 (26), and by expression of the individual members of the miR-106b~25 cluster.
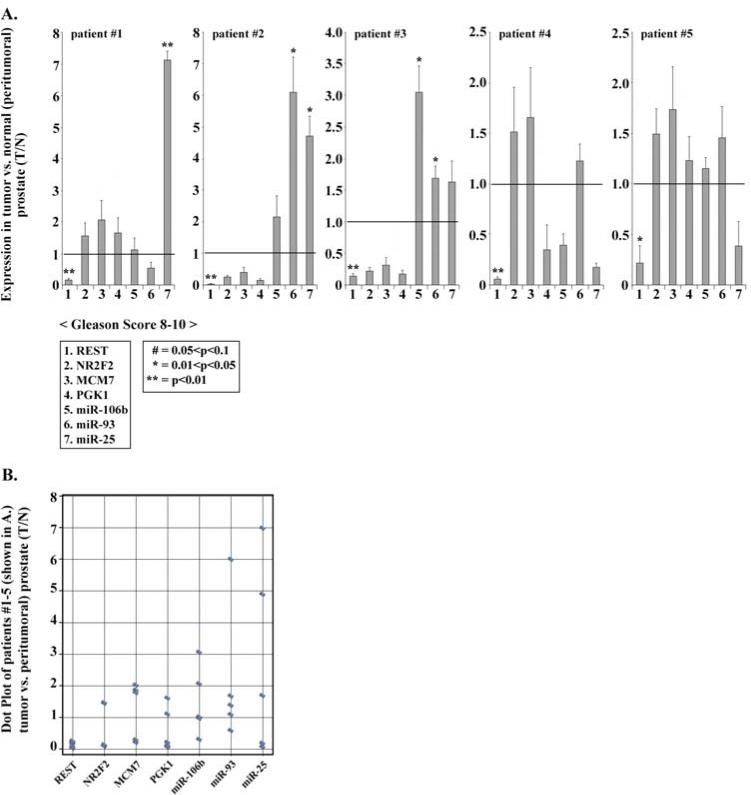
Figure 4. Deregulated expression of REST and miR-106b~25 in human prostate tumors.
A. Real-time PCR quantification of indicated genes employing RNA isolated from human prostate tumors with Gleason score 8-10 and the corresponding peri-tumoral tissue. Data shown are the average from three independent RNA preparations, each RNA preparation analyzed by real-time PCR using identical triplicates. Quantification is relative to GAPDH. Error bars represent +/− standard error of the mean. B. Dot plot analyses of the data from part A.
We analyzed nine prostate tumors having Gleason score ≥ 8 (Fig. 4 and Supplementary Fig. 1). Five of the analyzed tumors (indicated as patient # 1- 5) exhibited reduced expression of REST and up-regulation of at least one of the members of the miR-106b~25 cluster (Fig. 4A). Although the small sample size precludes statistical analyses to link REST downregulation and miR-106b~25 induction, dot plot representation of these data shows an inverse trend between REST and miR-106b~25 expression levels (Fig. 4B).
MiRNAs expressed in high Gleason prostate tumors overlap miRNAs induced by hypoxia in PCa cell lines and REST-knockdown in NC cultures
The transcriptional repressor REST suppresses expression of a broad network of genes involved in neuronal differentiation, including protein coding and miRNA genes (29-30). Furthermore, miRNAs have powerful regulatory potential in gene expression; changes in miRNA expression profiles closely associate with embryonic development as well as initiation and progression of cancers (37-39).
To gain more understanding about the down-regulation of REST in advanced prostate tumors (Fig. 4), we determined the miRNAs up-regulated in prostate tumors with increasing Gleason score. We reasoned that since REST suppresses expression of a broad network of genes involved in neuronal differentiation including miRNA genes (29, 30), REST down-regulation during PCa disease progression will result in expression of REST suppressed miRNAs as during REST-knockdown in NC cells. Likewise, miRNAs induced by hypoxia during PCa progression would overlap miRNAs induced in hypoxia-treated LNCaP cells. Employing miFinder RT2 PCR arrays, we identified miRNAs overexpressed in human prostate tumors with increasing Gleason score (Table I), as well as miRNAs induced by hypoxia in LNCaP cells (Supplementary Table S3), and REST-knockdown in NC cells (Supplementary Table S4). The human miFinder RT2 PCR array (Qiagen) is composed of the most abundant and best characterized miRNAs. RNAs analyzed by this PCR array were isolated from prostate tumors with Gleason scores ranging from 3 to 10. Specifically, RNA from at least five tumors per group, Gleason scores 5 through 10, were pooled for the miRNA PCR array analysis. Pooled RNA from the corresponding benign prostate tissues served as noncancerous control. We also analyzed miRNA expression in RNA samples isolated from LNCaP cells grown in normoxia vs. hypoxia for three days (supplementary Table S3), and from NC cultures following REST-knockdown by REST siRNA transfection vs. transfection of scrambled siRNA (Supplementary Table S4). Each group of RNAs was analyzed by the miRNA PCR array, employing three independent RNA preparations.
Table I.
Induction of microRNAs in human prostate tumors
| miRNA |
Gleason score |
Hypoxia-inducedb LNCap cells | REST-knockdownc NC cells | |||
|---|---|---|---|---|---|---|
| 3a | 5a | 7a | 8-10a | |||
| miR-21 | 1.22 ± 0.45 | 1.50 ± 0.15 | 2.50 ± 0.22 | 5.05 ± 0.72 |
|
|
| miR-24 | 0.90 ± 0.36 | 1.36 ± 0.56 | 2.06 ± 0.42 | 13.4 ± 1.18 | ||
| miR-25 | 0.53 ± 0.22 | 1.03 ± 0.46 | 1.96 ± 0.20 | 11.6 ± 1.02 | ||
| miR-30e | 1.37 ± 0.15 | 1.22 ± 0.08 | 2.83 ± 0.09 | 19.0 ± .090 | ||
| miR-125b | 1.22 ± 0.08 | 1.77 ± 0.43 | 2.01 ± 0.15 | 6.70 ± 0.85 | ||
| miR-142-3p | 1.26 ± 0.14 | 1.92 ± 0.26 | 3.78 ± 0.48 | 7.63 ± 0.39 | ||
| miR-151-5p | 0.11 ± 0.02 | 0.78 ± 0.30 | 4.38 ± 0.54 | 10.3 ± 1.35 | ||
| miR-210 | 0.01 ± 0.00 | 1.88 ± 0.30 | 2.25 ± 0.18 | 4.60 ± 0.48 | ||
| miR-223 | 2.28 ± 0.88 | 1.42 ± 0.06 | 1.35 ± 0.13 | 10.8 ± 0.44 | ||
| miR-374a | 0.24 ± 0.10 | 0.80 ± 0.40 | 1.16 ± 0.22 | 20.8 ± 1.48 | ||
| miR-9 | 0.65 ± 0.13 | 1.21 ± 0.35 | 1.74 ± 0.14 | 3.89 ± 0.55 |
|
|
| let-7e | 0.09 ± 0.03 | 1.02 ± 0.12 | 3.46 ± 0.36 | 14.3 ± 0.68 | ||
| miR-302b | 0.01 ± 0.01 | 1.35 ± 0.77 | 3.14 ± 0.86 | 7.63 ± 1.25 | ||
| miR-19b | 0.37 ± 0.11 | 1.41 ± 0.14 | 1.74 ± 0.34 | 4.76 ± 0.84 | ||
| miR-29c | 0.27 ± 0.03 | 1.31 ± 0.07 | 1.44 ± 0.09 | 3.37 ± 0.07 | ||
| miR-30d | 1.51 ± 0.31 | 2.06 ± 0.71 | 2.65 ± 0.71 | 22.7 ± 1.87 | ||
| let-7g | 0.07 ± 0.02 | 0.91 ± 0.06 | 3.58 ± 0.88 | 3.41 ± 0.93 | ||
| miR-30c | 0.37 ± 0.19 | 0.86 ± 0.37 | 2.25 ± 0.41 | 3.74 ± 1.14 |
|
|
| miR-7 | 0.27 ± 0.14 | 1.27 ± 0.10 | 3.36 ± 0.54 | 3.51 ± 1.12 | ||
| miR-27a | 1.59 ± 0.42 | 2.13 ± 0.46 | 2.28 ± 0.06 | 15.9 ± 1.00 | ||
| miR-106b | 0.80 ± 0.14 | 1.73 ± 0.54 | 1.93 ± 0.44 | 2.49 ± 0.54 | ||
| miR-150 | 1.03 ± 0.17 | 1.12 ± 0.04 | 1.65 ± 0.15 | 7.27 ± 0.40 | ||
| miR-155 | 1.42 ± 0.24 | 1.47 ± 0.13 | 1.45 ± 0.13 | 5.58 ± 0.16 | ||
| miR-93 | 0.02 ± 0.02 | 1.87 ± 1.04 | 1.84 ± 0.48 | 2.50 ± 0.22 | ||
| miR-191 | 0.38 ± 0.21 | 1.43 ± 0.31 | 0.91 ± 0.31 | 6.28 ± 1.48 | ||
| miR-424 | 0.10 ± 0.04 | 1.04 ± 0.36 | 1.67 ± 0.11 | 8.28 ± 0.12 | ||
| miR-425 | 1.46 ± 0.54 | 1.22 ± 0.62 | 3.48 ± 0.24 | 4.06 ± 0.94 | ||
The human miFinder RT2 PCR array (Qiagen), composed of the most abundant and best characterized miRNAs, was employed for miRNA expression in RNA samples isolated from human prostate tumors with Gleason scores ranging from 3 to 10. Specifically, RNA from at least five tumors per group, Gleason scores 5 through 10, were pooled for the miRNA PCR array analysis. Pooled RNA from the corresponding benign prostate tissues served as noncancerous control. Results represent the average of three independent RNA preparations +/- standard error of the mean.
miRNA expression employing the human miFinder RT2 PCR array (Qiagen), in RNA isolated from LNCaP cells grown in normoxia vs. hypoxia for three days (Supplementary Table S3).
miRNA expression employing the human miFinder RT2 PCR array (Qiagen), in RNA samples isolated from NC cultures following REST-knockdown by REST siRNA transfection vs. transfection with scrambled siRNA (Supplementary Table S4).
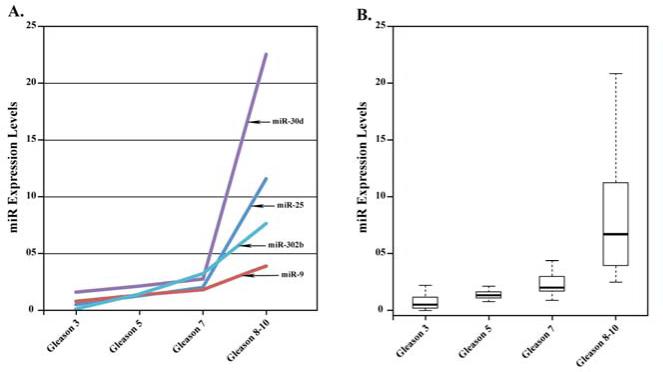
MiRNAs induced in prostate tumors are presented in Table I as groups A-C. Group-A miRNAs elevated in high Gleason prostate tumors, overlap with miRNAs induced by hypoxia in LNCaP cells, including miR-210 a well-documented hypoxia inducible miRNA (23) and miR-25 described herein (Figs.1-2). Group-B miRNAs elevated in high Gleason prostate tumors overlap with miRNAs induced during neuronal differentiation of NC cells by REST-knockdown (Supplementary Table S4). MicroRNAs elevated by REST-knockdown in NC cells include miR-124 and miR-9, both well-documented regulators of neurogenesis (44, 45). By contrast in high Gleason prostate tumors only miR-9 is over-expressed, known to down-regulate expression of E-cadherin (39) and androgen receptor (40). Other miRNAs overlapping with those induced by REST knockdown in NC cells include miR-30d and miR-302b (Fig. 5A). Induction of group-C miRNAs is independent of hypoxia or REST-knockdown. Statistical analysis of the data using ANOVA (Analysis of Variance) with a multiple comparison adjustment shows that microRNA expression in tumors with Gleason score 8-10 is significantly larger than observed in tumors of lower Gleason scores (Fig. 5B). Based on these comparative analyses, we propose that group A and B miRNAs are linked to hypoxia-induced REST down-regulation occurring during clinical progression of PCa to the aggressive form.
Figure 5.
A. Scatter plot analysis showing expression level of indicated microRNAs (induced by REST knockdown) in prostate tumors with increasing Gleason score (data from Table I). B. Box plot representation of microRNA expression in prostate tumors with increasing Gleason score (data from Table I).
Discussion
Herein, we report comparative studies between primary cultures of NC cells and human PCa cell lines, elucidating mechanism(s) of neuronal differentiation in embryogenesis, and NED of advanced PCa, respectively. We demonstrate that hypoxia is a key factor in this neuronal/neuroendocrine reprogramming of pluripotent NC cells and PCa cells in vitro, acting by inducing miR-106b~25; in turn, this microRNA cluster down-regulates the neuron-specific transcriptional repressor REST. Significantly, in high Gleason score (≥ 8) PCa, we observe down-regulation of REST and induction of at least one member of the miR-106b~25 cluster. We also identified groups of miRNAs that are up-regulated by hypoxia and REST knockdown in LNCaP and NC cells, respectively. Significantly, a subset of these miRNAs are also up-regulated in high Gleason score (≥ 8) PCa. We propose that miRNAs mediating aspects of neuronal differentiation of NC cells are also involved in NED of PCa cells, leading to progression of the disease to the clinically aggressive form. Therefore, this study sheds light on potential novel clinical markers and therapy targets for diagnosis and treatment of advanced PCa.
Hypoxia-induced expression of miR-106b~25
Incomplete angiogenesis during embryogenesis as well as in solid tumors gives rise to hypoxia. During embryonic development, hypoxia promotes neuronal differentiation of NC cells and CNS stem cells (11, 41). In PCa, hypoxia promotes cancer cell survival and proliferation, causing tumor progression, therapy resistance and poor prognosis (21), often accompanied by NED (35). Hypoxia induces expression of a large number of miRNAs through HIFs, including miR-93 (23), a member of the miR-106b~2 cluster, encoded in intron-13 of MCM7 gene (26). The miR-106b~25 is proto-oncogenic and over-expressed in PCa (26), and also mediates neuronal differentiation of adult neural stem/progenitor cells (28). In NC cultures and LNCaP cells hypoxia induces expression of all members of the miR-106b~25 cluster (Figs. 1-2). Interestingly, four putative hypoxia-response-elements (HRE) exist within 2 kb upstream of the transcription start site of the MCM7 gene, although the functionality of these putative HREs remains to be determined. Thus, our finding that hypoxia induces miR-106b~25 provides a plausible mechanism for the observed over-expression of miR-106b~25 in PCa and other solid tumors, and a foundation for the role of this miRNA cluster in regulating NED and neuronal differentiation. This is the first report of hypoxia-mediated induction of the proto-oncogenic miR-106b~25 cluster.
Down-regulation of REST by hypoxia and miR-93
Expression of REST in neural progenitors maintains non-neuronal cell identity, whereas repression of REST allows acquisition of neuronal identity (31). We have shown earlier that expression levels of REST control the switch between neuronal and non-neuronal cell fates, and down-regulation of REST is essential for neuronal differentiation (34). Also, REST is a tumor suppressor involved in various types of cancers (32). Indeed, loss of REST activity during NED of LNCaP cells has been reported at an earlier study, but the mechanism was not explored (42).
Herein, we deciphered a mechanism leading to down-regulation of REST during neuronal differentiation in NC cells. Significantly, we demonstrate that the same mechanism mediates NED of PCa cells. Specifically, in response to hypoxia REST mRNA and protein were reduced in both NC cells and human PCa cell lines. Decreased REST expression was similarly observed by over-expression of miR-93 and miR-106b~25 (Figs. 1-2). Since hypoxia induces expression of all members of the miR-106b~25 cluster (Figs. 1-2), and in agreement with computational predictions that these miRNAs target the 3’UTR of REST mRNA (29), it is reasonable to propose that they mediate the observed down-regulation of REST by hypoxia. Indeed, miR-93 over-expression as well as REST knockdown caused neuronal differentiation of pluripotent NC cells, resulting in up-regulation of pro-neural transcription factors ASH-1 and Phox2a (Fig. 1), and elevated expression of sympathoadrenal lineage marker TH (Fig. 1). Likewise, in both LNCaP and PC3 cells hypoxia, miR-93 over-expression, and REST knockdown significantly elevated expression of pro-neural transcription factors ASH-1 and Phox2a, and NE markers TH and ChgA (Fig. 3). In support of our results, immunohistochemical staining by others showed stabilization of HIF1α in NE-like cells in human prostate tumor (43). Accordingly, we propose that hypoxia induces miR-106b~25 thereby down-regulating REST; in turn, REST down-regulation allows expression of neuron-specific genes in PCa epithelia as in undifferentiated neuroblasts.
Deregulated expression of REST and select microRNAs in human prostate tumors
Our analyses of human prostate tumors demonstrated that the level of REST was reduced in five from nine tumors analyzed, while expression of at least one of the miRNAs of the miR106b~25 cluster, was elevated (Fig. 4). Despite the small sample size in these studies (Fig. 4), our analyses identify an inverse expression pattern between REST and miR-106b~25. Further studies are needed, employing a larger sample of human prostate tumors to statistically establish a link between REST downregulation and miR-106b~25 induction. Importantly, additional support for this suggested inverse relationship between REST and miR-106b~25 is derived from microRNA expression analyses of human tumors with increasing Gleason score (Table I and Fig. 5).
Specifically, studies by others have identified a number of miRNAs repressed by REST, playing a role in neuronal differentiation (29). We reasoned that as during neuronal differentiation of neural progenitors in embryogenesis, NED in PCa will also result in induction of miRNAs. Employing miFinder RT2 PCR arrays, we identified miRNAs induced by REST knockdown in NC cells, by hypoxia in LNCaP cells, and miRNAs overexpressed in human prostate tumors with high Gleason score (Table I). Group-A miRNAs elevated in high Gleason score prostate tumors overlap with miRNAs induced by hypoxia in LNCaP cells (Supplementary Table S3), suggesting that hypoxia is an important parameter in PCa disease progression. Specifically, the hypoxia inducible miR-25 (Figs. 1-2) exhibits robust induction in prostate tumors with Gleason score ≥ 8 (Fig. 5A). Similar to miR-93, miR-25 has putative target sites in the 3’UTR of REST (29). Since over-expression of miR-93 in NC and PCa cell lines down-regulates REST, the elevated expression of miR-25 in high Gleason prostate tumors suggests that it mediates the observed reduction in REST. In turn, we would expect down-regulation of REST in prostate tumors will result in elevated expression of miRNAs characteristic of neuron-specific expression. Indeed, group-B miRNAs overlap with miRNAs induced during normal neuronal differentiation of NC cells (Supplementary Table S4). In NC cells, miRNAs elevated following REST knockdown include miR-124 and miR-9 both well-documented regulators of neurogenesis (44, 45). In high Gleason prostate tumors only miR-9 is over-expressed (Table I). Significantly, miR-9 is a metastasis-promoting miRNA acting by down-regulating E-cadherin (39) and LIFR in E-cadherin-negative breast cancers (46), likely contributing to EMT and metastasis of advanced PCa. On the other hand, recent studies show that expression of the neuron-specific miR-124 is suppressed by DNA methylation in advanced PCa (47). Accordingly, we propose development of the authentic NED phenotype, as during normal neurogenesis, is not the critical event in PCa disease progression; rather it is the expression of select miRNAs occurring simultaneously with aberrant neuronal reprogramming that drive the cancer towards malignancy. This hypothesis is supported by the lack of neurite formation in PC3 cells exposed to hypoxia, despite induction of proneural ASH-1 (Fig. 3D).
In support of this proposal additional miRNAs that exhibit robust expression in high Gleason tumors and up-regulated as a consequence of REST-knockdown include miR-30d and miR-302b. Recent studies have identified miR-30d as an oncomir in cancer, regulating expression of various cancer-linked genes including caspase3 (48, 49). MiR-302b is also a very interesting, playing a role in regulation of pluripotency and neural cell differentiation during embryogenesis (36). Its expression is positively regulated by pluripotency factor Oct4; in turn, miR-302b down-regulates pro-neural transcription factor NR2F2 which suppresses Oct4 expression (36). The significance of this dynamic interplay for the progression of PCa to the advanced stage is not understood. Further studies are needed to understand the role of these miRNAs in PCa disease progression.
Supplementary Material
Highlights.
Hypoxia induces miR-106b~25.
Hypoxia promotes neuronal differentiation of neural crest cells via induction of miR-106b~25 and down-regulation of REST.
Hypoxia promotes neuroendocrine differentiation of PCa cell lines (LNCaP and PC3 cells) via induction of miR-106b~25 and down-regulation of REST.
In high Gleason score prostate tumors an inverse trend was observed between REST and induction of miR-106b~25.
High Gleason score prostate tumors exhibit significant induction of miR-25, miR-9, miR-30d and miR-302b associated with REST-knockdown.
Acknowledgments
Supported by the Purdue University Center for Cancer Research Small Grants Program, The Indiana Clinical and Translational Research CBR/CTR grant # RPO25761 and the specimen storage facility grant # RPO25761 to OMA.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sun Y, Niu J, Huang J. Neuroendocrine differentiation in prostate cancer. Am J Transl Res. 2009;1:148–162. [PMC free article] [PubMed] [Google Scholar]
- 2.Frachon S, Pasquier D, Treilleux I, Seigneurin D, Ringeisen F, Rosier P, et al. Breast carcinoma with predominant neuroendocrine differentiation. Ann Pathol. 2004;24:278–283. doi: 10.1016/s0242-6498(04)93966-1. [DOI] [PubMed] [Google Scholar]
- 3.Garcia-Yuste M, Matilla JM, Gonzalez-Aragoneses F. Neuroendocrine tumors of the lung. Curr Opin Oncol. 2008;20:148–154. doi: 10.1097/CCO.0b013e3282f35ed3. [DOI] [PubMed] [Google Scholar]
- 4.Deeble PD, Cox ME, Frierson HF, Sikes RA, Palmer JB, Davidson E, et al. Androgen-independent growth and tumorigenesis of prostate cancer cells are enhanced by the presence of PKA-differentiated neuroendocrine cells. Cancer Res. 2007;67:3663–3672. doi: 10.1158/0008-5472.CAN-06-2616. [DOI] [PubMed] [Google Scholar]
- 5.May M, Siegsmund M, Hammerman F, Loy V, Gunia S. Prognostic significance of proliferation activity and neuroendocrine differentiation to predict treatment failure after radical prostatectomy. Scand J Urol Nephrol. 2007;41:375–81. doi: 10.1080/00365590701224445. [DOI] [PubMed] [Google Scholar]
- 6.Culine S, El Demery M, Lamy PJ, Iborra F, Avancès C, Pinquet F. Docetaxel and cisplatin in patients with metastatic androgen independent prostate cancer and circulating neuroendocrine markers. J Urol. 2007;178:844–8. doi: 10.1016/j.juro.2007.05.044. [DOI] [PubMed] [Google Scholar]
- 7.Yuan TC, Veeramani S, Lin MF. Neuroendocrine-like prostate cancer cells: neuroendocrine transdifferentiation of prostate adenocarcinoma cells. Endocr Relat Cancer. 2007;14:531–547. doi: 10.1677/ERC-07-0061. [DOI] [PubMed] [Google Scholar]
- 8.Burchardt T, Burchardt M, Chen MW, Cao Y, de la Taille A, Shabsigh A, et al. Transdifferentiation of prostate cancer cells to a neuroendocrine cell phenotype in vitro and in vivo. J Urol. 1999;162:1800–1805. [PubMed] [Google Scholar]
- 9.Le Douarin NM, Kalcheim C. The Neural Crest. 2nd ed. Cambridge University Press; Cambridge, UK: 1999. [Google Scholar]
- 10.Reissmann E, Ernsberger U, Francis-West PH, Rueger D, Brickell PM, Rohrer H. Involvement of bone morphogenetic protein-4 and bone morphogenetic protein-7 in the differentiation of the adrenergic phenotype in developing sympathetic neurons. Development. 1996;122:2079–2088. doi: 10.1242/dev.122.7.2079. [DOI] [PubMed] [Google Scholar]
- 11.Morrison SJ, Csete M, Groves AK, Melega W, Wold B, Anderson DJ. Culture in reduced levels of oxygen promotes clonogenic sympathoadrenal differentiation by isolated neural crest stem cells. J Neurosci. 2000;20:7370–7376. doi: 10.1523/JNEUROSCI.20-19-07370.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shah NM, Groves AK, Anderson DJ. Alternative neural crest cell fates are instructively promoted by TGFbeta superfamily members. Cell. 1996;85:331–343. doi: 10.1016/s0092-8674(00)81112-5. [DOI] [PubMed] [Google Scholar]
- 13.Hirsch MR, Tiveron MC, Guillemot F, Brunet JF, Goridis C. Control of noradrenergic differentiation and Phox2a expression by MASH1 in the central and peripheral nervous system. Development. 1998;125:599–608. doi: 10.1242/dev.125.4.599. [DOI] [PubMed] [Google Scholar]
- 14.Yang C, Kim HS, Seo H, Kim CH, Brunet JF, Kim KS. Paired-like homeodomain proteins, Phox2a and Phox2b, are responsible for noradrenergic cell-specific transcription of the dopamine beta-hydroxylase gene. J Neurochem. 1998;71:1813–1826. doi: 10.1046/j.1471-4159.1998.71051813.x. [DOI] [PubMed] [Google Scholar]
- 15.Acloque H, Adams MS, Fishwick K, Bronner-Fraser M, Nieto MA. Epithelial-mesenchymal transitions: the importance of changing cell state in development and disease. J Clin Invest. 2009;119:1438–1449. doi: 10.1172/JCI38019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Drake JM, Strohbehn G, Bair TB, Moreland JG, Henry MD. ZEB1 enhances transendothelial migration and represses the epithelial phenotype of prostate cancer cells. Mol Biol Cell. 2009;20:2207–2217. doi: 10.1091/mbc.E08-10-1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McKeithen D, Graham T, Chung LW, Odero-Marah V. Snail transcription factor regulates neuroendocrine differentiation in LNCaP prostate cancer cells. Prostate. 2010;70:982–992. doi: 10.1002/pros.21132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Simon MC, Keith B. The role of oxygen availability in embryonic development and stem cell function. Nat Rev Mol Biol. 2008;9:285–96. doi: 10.1038/nrm2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mak P, Leav I, Pursell B, Bae D, Yang X, Tanglienti CA, et al. ERβ Impedes Prostate cancer EMT by Destabilizing HIF-1α and Inhibiting VEGF-Mediated Snail Nuclear Localization: Implications for Gleason Grading. Cancer Cell. 2010;17:319–322. doi: 10.1016/j.ccr.2010.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Movsas B, Chapman JD, Greenberg RE, Hanlon AL, Horwitz EM, Pinover WH, et al. Increasing levels of hypoxia in prostate carcinoma correlate significantly with increasing clinical stage and patient age: an Eppendorf pO(2) study. Cancer. 2000;89:2018–2024. doi: 10.1002/1097-0142(20001101)89:9<2018::aid-cncr19>3.3.co;2-p. [DOI] [PubMed] [Google Scholar]
- 21.Semenza GL. Hypoxia-inducible factors: mediators of cancer progression and targets for cancer therapy. Trends Pharmacol Sci. 2012;33:207–214. doi: 10.1016/j.tips.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nanni S, Benvenuti V, Grasselli AQ, Priolo C, Aiello A, Mattiussi S, et al. Endothelial NOS, estrogen receptor beta, and HIFs cooperate in the activation of a prognostic transcriptional pattern in aggressive human prostate cancer. J Clin Invest. 2009;119:1093–1108. doi: 10.1172/JCI35079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kulshreshtha R, Ferracin M, Wojcik SE, Garzon R, Alder H, Agosto-Perez FJ, et al. A microRNA signature of hypoxia. Mol Cell Biol. 2007;27:1859–1867. doi: 10.1128/MCB.01395-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brodersen P, Voinnet O. Revisiting the principles of microRNA target recognition and mode of action. Nat Rev Mol Cell Biol. 2009;10:141–148. doi: 10.1038/nrm2619. [DOI] [PubMed] [Google Scholar]
- 25.Friedman RC, Farh KK, Burge CB, Bartel DP. Most mammalian mRNAs are conserved targets of microRNAs. Genome Res. 2009;19:92–105. doi: 10.1101/gr.082701.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Poliseno L, Salmena L, Riccardi L, Fornari A, Song MS, Hobbs RM, et al. Identification of the miR-106b~25 microRNA cluster as a proto-oncogenic PTEN-targeting intron that cooperates with its host gene MCM7 in transformation. Sci Signal. 2010;3:ra29. doi: 10.1126/scisignal.2000594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li Z, Yang CS, Nakashima K, Rana TM. Small RNA-mediated regulation of iPS cell generation. EMBO J. 2011;30:823–834. doi: 10.1038/emboj.2011.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brett JO, Renault VM, Rafalski VA, Webb AE, Brunet A. The microRNA cluster miR-106b~25 regulates adult neural stem/progenitor cell proliferation and neuronal differentiation. Aging (Albany NY) 2011;3:108–124. doi: 10.18632/aging.100285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu J, Xie X. Comparative sequence analysis reveals an intricate network among REST, CREB and miRNA in mediating neuronal gene expression. Genome Biol. 2006;7:R85. doi: 10.1186/gb-2006-7-9-r85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Otto SJ, McCorkle SR, Hover J, Conaco C, Han JJ, Impey S, et al. A new binding motif for the transcriptional repressor REST uncovers large gene networks devoted to neuronal functions. J Neurosci. 2007;27:6729–39. doi: 10.1523/JNEUROSCI.0091-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ballas N, Grunseich C, Lu DD, Speh JC, Mandel G. REST and its co-repressors mediate plasticity of neuronal gene chromatin throughout neurogenesis. Cell. 2005;121:645–657. doi: 10.1016/j.cell.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 32.Westbrook TF, Martin ES, Schlaback MR, Leng Y, Liang AC, Feng B, et al. A genetic screen for candidate tumor suppressors identifies REST. Cell. 2005;121:837–848. doi: 10.1016/j.cell.2005.03.033. [DOI] [PubMed] [Google Scholar]
- 33.Wu C, Huang J. PI3K-AKT-mTOR is essential for Neuroendocrine Differentiation of PCa. J Biol Chem. 2007;282:3571–3583. doi: 10.1074/jbc.M608487200. [DOI] [PubMed] [Google Scholar]
- 34.Liang H, Fekete DM, Andrisani OM. CtBP2 down-regulation during neural crest specification induces expression of Mitf and REST, resulting in melanocyte differentiation and sympathoadrenal lineage suppression. Mol Cell Biol. 2011;31:955–970. doi: 10.1128/MCB.01062-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mosca A, Berruti A, Russo L, Torta M, Dogliotti L. The neuroendocrine phenotype in prostate cancer: basic and clinical aspects. J Endocrinol Invest. 2005;28:141–145. [PubMed] [Google Scholar]
- 36.Rosa A, Brivanlou AH. A regulatory circuitry comprised of miR-302 and transcription factors OCT4 and NR2F2 regulates human embryonic stem cell differentiation. EMBO J. 2011;30:237–248. doi: 10.1038/emboj.2010.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Smith B, Treadwel J, Zhang D, Ly D, McKinnell I, Walker PR, et al. Large-scale expression analysis reveals distinct microRNA profiles at different stages of human neurodevelopment. PLoS One. 2010;5:e11109. doi: 10.1371/journal.pone.0011109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang B, Pan X, Cobb GP, Anderson TA. microRNAs as oncogenes and tumor suppressors. Dev Biol. 2007;302:1–12. doi: 10.1016/j.ydbio.2006.08.028. [DOI] [PubMed] [Google Scholar]
- 39.Ma L, Young JU, Prabhala H, Pan E, Mestdagh P, et al. miR-9, a MYC/MYCN–activated microRNA regulates E-cadherin and cancer metastasis. Nat Cell Biol. 2010;12:247–256. doi: 10.1038/ncb2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ostling P, Leivonen S- K, Aakula P, Kohonen P, Makela R, et al. Systematic analysis of microRNAs targeting the androgen receptor in cancer cells. Cancer Res. 2011;71:1956–1967. doi: 10.1158/0008-5472.CAN-10-2421. [DOI] [PubMed] [Google Scholar]
- 41.Studer L, Csete M, Lee SH, Kabbani N, Walikonis J, Wold B, et al. Enhanced proliferation, survival, and dopaminergic differentiation of CNS precursors in lowered oxygen. J Neurosci. 2000;20:7377–7383. doi: 10.1523/JNEUROSCI.20-19-07377.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tawadros T, Martin D, Abderrahmani A, Leisinger HJ, Waeber G, Haefliger JA. IB1/JIP-1 controls JNK activation and increased during prostatic LNCaP cells neuroendocrine differentiation. Cell Signal. 2005;17:929–939. doi: 10.1016/j.cellsig.2004.11.013. [DOI] [PubMed] [Google Scholar]
- 43.Monsef N, Helczynski L, Lundwall A, Pahlman S. Localization of immunoreactive HIF-1alpha and HIF-2alpha in neuroendocrine cells of both benign and malignant prostate glands. Prostate. 2007;67:1219–1229. doi: 10.1002/pros.20594. [DOI] [PubMed] [Google Scholar]
- 44.Conaco C, Otto S, Han JJ, Mandel G. Reciprocal actions of REST and microRNA promote neuronal identity. Proc Natl Acad Sci USA. 2006;103:2422–2427. doi: 10.1073/pnas.0511041103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cheng LC, Pastrana E, Tavazoie M, Doetsch F. miR-124 regulates adult neurogenesis in the subventricular zone stem cell niche. Nat Neurosci. 2009;12:399–408. doi: 10.1038/nn.2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen D, Sun Y, Wei Y, Zhang P, Rezaeian AH, Teruya-Feldstein J, et al. LIFR is a breast cancer metastasis suppressor upstream of the HIPO-YAP pathway and a prognostic marker. Nat Med. 2012;18:1511–1519. doi: 10.1038/nm.2940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shi XB, Xue L, Ma AH, Tepper CG, Gandour-Edwards R, Kung JH, et al. Tumor suppressive miR-124 targets androgen receptor and inhibits proliferation of prostate cancer cells. Oncogene. 2012:1–9. doi: 10.1038/onc.2012.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li N, Kaur S, Greshok J, Lassus H, Zhong X, Wang Y, et al. A combined array-based comparative genomic hybridization and functional library screening approach identifies mir-30d an an oncomir in cancer. Cancer Res. 2012;72:154–164. doi: 10.1158/0008-5472.CAN-11-2484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gaziel-Sovran A, Segura MF, Di Micco R, Collins MK, Hanniford D, Vega-Saenz de Miera E, et al. miR-30b/30d regulation of GalNAc transferases enhances invasion and immunosuppression during metastasis. Cancer Cell. 2011:104–118. doi: 10.1016/j.ccr.2011.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.