Abstract
While families at increased risk for familial breast/ovarian cancer continue to overestimate their cancer risk with increased cancer worries about the future, few studies have examined factors that affect inherited cancer risk perception and cancer worries in both survivors and unaffected female relatives. The purpose of this study was to examine variables that may affect cancer worries and risk perceptions from a family-based perspective in a racially diverse, community-based, random sample of 146 dyads consisting of adult female breast and/or ovarian cancer survivors and their unaffected female relatives (N = 292). Results indicated that coping style, self-efficacy, partner’s income, family role relationship, and cancer risk perception were significant contributors to the survivors’ and their unaffected relatives’ cancer worries. Significant variables for perception of cancer risk for both survivors and relatives included income, race, family history of cancer, and cancer worries. Relatives had a higher perception of cancer risk, whereas survivors had more cancer worries. Additionally, the level of cancer worries reported by one member of the dyad was related to the amount of worries reported by the other. The results from this study underscore the importance of clinicians addressing concerns of both affected and unaffected members of families at increased risk of cancer to assist them in managing cancer worries and having realistic risk appraisals to make informed decisions about their own and their family’s health surveillance options.
Keywords: cancer, oncology, risk perception, cancer worries, family
Introduction
When cancer runs in families, there is an ever-present concern and worry regarding the risk of future cancer for an individual diagnosed with cancer, as well as for other family members. Currently, there are over 2.3 million breast cancer survivors and 174,000 ovarian cancer survivors [1], and in 2007, there will be an estimated 180,510 newly diagnosed cases of breast cancer and 22,430 newly diagnosed cases of ovarian cancer in the US [2]. Of these cases, approximately 5–10% will be attributed to inherited mutations in cancer susceptibility genes, such as BRCA1 and BRCA2 [3, 4]. First-degree female relatives of these individuals will be at a 50% risk for carrying these mutations, placing them at a greatly increased lifetime risk of developing breast or ovarian cancer, compared with members of the general population.
Overestimation of personal cancer risk is frequently reported by individuals with cancer [5–9]. Additionally, cancer worries are a critical concern for survivors and their family members and have been linked to increased anxiety, emotional distress, and impaired role functioning [10–12]. However, information is lacking on factors that are associated with cancer worries and risk perception within the context of the family at increased risk of breast/ovarian cancer and on how survivors and family members influence each other’s worry and perception of cancer risk. Further research on cancer risk perception and cancer worries is essential for clinicians to help families at increased risk manage their fears and worries and make informed decisions about their health and surveillance behaviors.
Using a family stress appraisal model, the purpose of this study was to: (1) identify factors associated with cancer worries and perception of cancer risk in a racially diverse, community-based sample of breast and/or ovarian cancer survivors and their unaffected female relatives; (2) assess differences in the predictors of cancer worries and perception of cancer risk between survivors and relatives; and (3) determine the interrelationship between survivors’ and relatives’ cancer worries and risk perceptions.
Background
Studies on risk perception and cancer worries
Perception of cancer risk has consistently been cited as a major factor influencing the decision to undergo genetic counseling and testing by women at increased risk of inherited breast or ovarian cancer [5–9]. Overestimation of an individual’s risk status has been associated with several negative outcomes, including increased anxiety and distress for one’s self and family members [9, 13], lower perception of control over cancer [9], depression [9], and excessive hyper-vigilance in screening practices [6]. Research also suggests that women interpret genetic risk information based on their personal experience within their families [14, 15], and may continue to perceive their risk as being high, despite information that indicates that it is actually lower than expected [16, 17]. A heightened perception of risk can have further adverse effects upon women who continue to report cancer-specific distress even after undergoing counseling, genetic testing, or prophylactic surgery [11, 18, 19].
Cancer-specific worries have also been found to influence genetic testing decisions [16, 18] or adherence to breast mammography and screening practices [20–22]. While a moderate level of cancer worry appears to be positively related to improved mammography screening and adherence [10, 20, 21], excessive amounts have been associated with increased anxiety [12, 23], impairment in role functioning [12], and hyper-vigilant breast examinations [24]. Additionally, there are inconsistent reports in the literature regarding the relationship between cancer worry and perception of risk, and both appear to be critical factors in decision-making processes regarding health screening and genetic uptake options. Some studies report a positive relationship between cancer worries and risk perceptions [11, 24–26], whereas others have found no association [9, 27]. Given the significant effects that excessive worry and heightened risk perception have on women at increased risk of inherited cancer and their follow-up with surveillance and disease management options, further research is needed to determine the factors that may influence both survivors’ and their family members’ worries and risk perceptions.
Factors affecting perception of risk and cancer worries
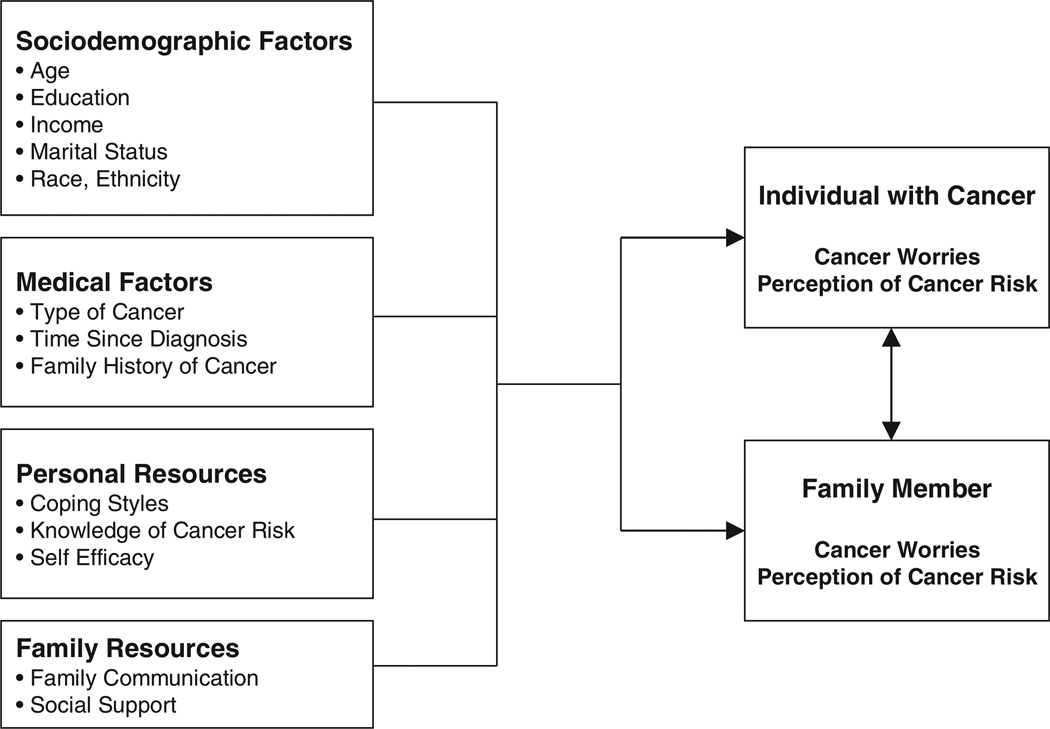
A family stress appraisal model, based on the family resiliency model [28], was used to guide this study to identify factors that may be associated with cancer risk perception and cancer worries. The current study is part of a larger investigation of family decision making utilizing inherited cancer risk information. In Figure 1, stress appraisal is the outcome, measured by cancer worries and perception of cancer risk of both the cancer survivors and their unaffected female relatives. The family’s stress appraisal is influenced both by sociodemographic and medical factors, as well as by personal and family resources. The model also suggests that there is an interrelationship between the perception of risk and cancer worries of the cancer survivor and that of her unaffected female relative.
Figure 1.
Family model of stress appraisal regarding inherited cancer risk based on McCubbin and McCubbin Resiliency Model [28]
Multiple studies have demonstrated the interrelationships that exist between cancer patients and their spouses/caregivers/family members in distress, depression, adjustment, or quality of life [29–34]. However, little systematic study of relatives facing possible familial cancer risk has been conducted from a community-based perspective, with most reported studies on high-risk individual family members attending genetic clinics. Research to date indicates that information on inherited cancer risk and genetic uptake options have significant effects upon families, including distant and problematic communication patterns [35, 36], family conflict and relationship problems [37, 38], adverse psychological distress in children [13, 39], distress in handling other family issues [40], and the burden of family ‘guilt’ [14, 36]. Further research is essential to determine what factors are important to consider in order for clinicians to assist families in managing their ongoing worries and risk concerns and help them make appropriate and informed decisions about their health.
Methods
Sample
A racially diverse, population-based, random sample of adult female breast and/or ovarian cancer survivors was identified through the Metropolitan Detroit Cancer Surveillance System (MDCSS), which is part of the National Cancer Institute’s Surveillance, Epidemiology and End Results Program. Potential participants were stratified by race (Caucasian vs African-American) and diagnosis (breast cancer only vs ovarian cancer with or without breast cancer). Power calculations indicated that a final sample size of 140 survivors (plus an equal number of their unaffected relatives) was required to have a power of 80% at an α level of 0.05 to detect a medium effect size. Using National Comprehensive Cancer Network criteria for increased risk of genetic/familial cancer susceptibility, the survivor had to be diagnosed with (i) a single breast cancer by 50 years of age, (ii) multiple breast cancers by 80 years of age, (iii) ovarian cancer by 80 years of age, or (iv) breast and ovarian cancer by 80 years of age. In addition, survivors had to be at least 18 years of age at diagnosis, with at least one breast or ovarian cancer diagnosis between January 1, 1999, and December 31, 2002, and when initially contacted, willing to confirm the cancer diagnosis and the availability of an eligible family member to participate. Eligible family members were defined as first-, second-, or third-degree female relatives over the age of 17 years who had never been diagnosed with cancer and who were available to a complete in-person interview. To recruit the required study population, 674 randomly selected patients were identified through the MDCSS. Of those identified, 386 were determined to be eligible (physician permission, living, able to be contacted). Initially, 213 patients (55%) agreed to participate. During final accrual, 24 additional patients became ineligible due to unavailability of a relative to participate. The final community-based sample, after refusals, was 146 dyads (N = 292), with a participation rate of 38%. Women who declined participation cited the following reasons: currently in a research study, not interested, too ill or undergoing treatment, lack of time, unwillingness to contact family members, and sensitivity to talking about the cancer diagnosis. We compared survivors who participated with eligible nonparticipating survivors and found only one difference, which occurred with ovarian cancer survivors. Non-participants were significantly older (mean age at diagnosis = 55 years) than participating ovarian cancer survivors (mean age at diagnosis = 49 years, p = 0.007).
Procedures
Institutional review board approval was obtained prior to the start of the study. The MDCSS staff notified the cancer survivor’s physician by letter that she was eligible to be contacted for the study and requested that the physician inform them if there was any reason the patient should not be contacted. If no response was received within 3 weeks, the survivor was sent an introduction letter describing the study along with a telephone number and response sheet to return if she did not want to be contacted. If the survivor did not decline contact after 2 weeks, a member of the MDCSS staff contacted her by telephone to determine her level of interest in participating and to establish if there was an eligible relative to join her. Once a survivor was selected for the study, her name and contact information was forwarded to the investigative team with her permission to schedule an interview, which took place in the survivor’s home, another site selected by the family, or in the investigator’s offices. Prior to participating, both the survivor and her relative gave written informed consent. Although both members of the family dyad were present during the interview, each person completed the self-report questionnaires independently while a research nurse or genetic counselor was present to facilitate the process. All participants received a $25 honorarium for their participation.
Measures
Sociodemographic factors
Personal demographic and medical information was collected. Family history of cancer was quantified using the Family History Assessment Tool (FHAT) [41]. The FHAT was designed as a resource tool for primary-care physicians to identify individuals at increased risk for inherited cancer susceptibility that would warrant a referral for genetic counseling. A scoring system based on the family history of cancer (breast, ovarian, prostate, colon) assigns weighted point values based on risk factors (age of onset, type of cancer, degree of relationship to the affected relative), with a score of 10 or greater triggering a genetic counseling referral. Since the FHAT was originally developed for unaffected individuals, we adapted the scoring to accommodate both survivors’ and female relatives’ family histories. Each individual’s score was calculated and the average score for the dyad was used to describe the family’s risk.
Personal resources
Coping style was measured by the Miller Behavioral Style Scale (MBSS) [42]. The scale is composed of 32 items, with two subscales to reflect monitoring and blunting coping styles. The MBSS has been shown to have adequate test–retest reliability and predictive validity [42], with a clear two-factor solution [43]. The scale provides a monitoring score, a blunting score, and a total score. For this study, Cronbach’s α coefficient for the monitoring score was 0.67 and for the blunting score was 0.64. Knowledge of cancer risk was measured by a questionnaire from Lerman et al. [44]. Eleven true/false statements were used to assess knowledge of inherited cancer risk, specifically as it relates to hereditary breast and ovarian cancer syndrome. The questionnaire was modified to reflect the reading ability of our population-based sample. The measure has a reported high internal consistency (α = 0.74), with the higher the summary score, the greater the knowledge base about genetic cancer risk. In our study, internal reliability of the scale was not demonstrated, and we subsequently dropped the instrument from the main analysis. Self-efficacy, or the degree of confidence and belief that an individual has in his/her capability to handle situations, was measured by the General Self-Efficacy Scale [45]. This scale is composed of 23 items and contains two subscales. For our study, we used only the general self-efficacy subscale of 17 items. The higher the score, the higher are the self-efficacy expectations of the individual. Evidence of construct and criterion validity and reliability has been reported [45], and for our study the α coefficient was 0.81.
Family resources
Two variables measured family resources: family communication and social support. Family communication was measured by the Family Relationship Inventory (FRI) [46]. The FRI is part of the Family Environment Scale (FES), which consists of 90 items that measure 10 different dimensions of family relationships. The FES has been found to have acceptable test–retest reliability with numerous studies supporting both construct and discriminate validity [47]. The 18-item FRI consists of 3 subscales of cohesion, conflict, and expressiveness and was used as a global measure of family interaction for this study. Cronbach’s α reliability for survivors and family members was 0.79. Social support was measured using the Personal Resource Questionnaire [48]. The instrument consists of 25 items that measure the amount of general social support that people perceive they receive from others (notably from the family and other significant others). Responses range from ‘strongly disagree’ to ‘strongly agree’ with higher scores indicating more support. Evidence of internal consistency has been reported, along with construct validity [48]. For this study, the α coefficient was 0.80.
Outcome variables
Perception of cancer risk was measured by a scale developed by Schwartz et al. [25]. The scale used in this study was modified to address perception of risk of inherited breast and/or ovarian cancer for individuals and their family members with slight revisions in three items to determine self-perception of developing breast and/or ovarian cancer. Two additional items were also added to address the focus of this study related to perception of risk for other family members, rather than only for the respondent. An example of an item added was, ‘compared to women with a family history of breast and/or ovarian cancer, what are the chances that other family members will develop cancer?’ Two versions of the scale were used to reflect the perception of risk from both the viewpoints of the cancer survivor and the unaffected family member. The instrument uses a five-point Likert scale ranging from ‘much lower’ to ‘much higher’ to generate a composite score. For this study, the internal reliability coefficient was α=0.75.
Cancer worry was measured by a scale that has been used in multiple other studies by Lerman and colleagues on inherited cancer risk [10, 22, 49]. The four-item scale measures frequency of worry and effect of worry on mood and in performing daily tasks. Test–retest reliability and internal consistency have been reported [49], and for this study Cronbach’s α coefficient was 0.86.
Statistical analysis
To identify the factors associated with cancer worries and perception of cancer risk, while taking into account the effects of survivors and unaffected female relatives on one another, we used the Actor–Partner Interdependence Model (APIM) with SPSS MIXED MODELS version 14 [50–54]. The multilevel approach of the APIM allowed us to examine both individual effects as well as the effects of both members of the dyad on each other at the same time. To assess differences in predictors of cancer worries and perception of cancer risk between survivors and relatives, interaction terms for each potential predictor and the role (survivor vs relative) of the individual were also examined in the model. A backward stepwise elimination procedure was employed using a criteria of p⩽0.05. After the final model of main effect predictors was determined, interactions significant at p⩽0.20 along with the main effect for the variable (if not already included in the model) were entered into the model. Backward elimination of these effects following the above criteria was then performed to arrive at the final model.
The APIM approach assesses both actor and partner effects. Actor effects occur when an individual’s score on a predictor variable affects his/her own outcome (survivor or relative). However, partner effects in a dyad occur when an individual’s score on the predictor variable affects the outcome of the other member of the dyad. In this sample, the survivor influences her relative or vice versa [50, 54]. Actor and partner effects are only assessed on variables where the score can differ both within and between the dyads (i.e. age, education, self-efficacy, coping style, cancer worries, perception of cancer risk). Thus, when information is collected on dyads and the goal is to assess both the effects of the individual and their dyad partner on each other, the APIM approach is ideal.
Prior to analysis, all variables were examined for accuracy, distributional assumptions of the analyses, and missing values. Owing to the high correlation between the family history of cancer for the survivors and relatives (r=0.83, p<0.001), the average family history of cancer score for each dyad was used in the analysis instead of separate scores for the survivor and relative. The average family history of cancer score was positively skewed and a base 10 logarithm transformation was applied. After removal of three multivariate outliers and imputation of missing values, the final data set consisted of 289 cases (143 dyads with complete data and three dyads with a single case). Because interaction terms were modeled and to expand the interpretability of the regression coefficients, variables that did not have a meaningful value of 0 were centered [55] using the mean from the combined data.
Results
Sample characteristics
The demographic and medical characteristics of the sample are listed in Table 1. The majority of family members in the study were daughters of cancer survivors (n = 64), followed by sisters (n = 52) and a smaller number of mothers (n = 19) and other types of relatives (n = 11). Since we stratified the sample on race, there were 78 Caucasian and 68 African-American dyads. The majority of the survivors had breast cancer (n = 81), over one-third had ovarian cancer (n = 50), and 15 women had 2 or more primary cancers (2 breast primaries, breast and ovarian cancer, or a third primary cancer diagnosis). Of the women in this study, 22 had experienced a recurrence, and 8 were currently undergoing treatment. In this population potentially at an increased risk for inherited cancer, only 15 survivors (10.3%) had genetic counseling due to a family history of cancer and 11 (7.5%) had genetic testing for cancer predisposition.
Table 1.
Demographic and medical characteristics of the study sample (N = 292)
| Characteristics | Cancer survivor (n = 146) |
Female relative (n = 146) |
|---|---|---|
| Cancer site (%) | ||
| Breast | 81 (55.5) | |
| Ovarian | 50 (34.2) | |
| Multiple | 15 (10.3) | |
| Race (%) | ||
| Caucasian | 78 (53.4) | 78 (53.4) |
| African-American | 68 (46.6) | 68 (46.6) |
| Relationship of family member (%) | ||
| Mother | 19 (13.0) | |
| Sister | 52 (35.6) | |
| Daughter | 64 (43.8) | |
| Other (half-sister, aunt, cousin) | 11 (7.6) | |
| Age (years) | ||
| Mean | 51 | 41 |
| Range | 25–78 | 18–85 |
| Education (%) | ||
| <High school | 10 (6.9) | 12 (8.2) |
| High school | 36 (24.7) | 34 (23.3) |
| Some college | 45 (31.0) | 56 (38.4) |
| College or post-grad | 54 (37.5) | 44 (30.1) |
| Marital status (%) | ||
| Married | 83 (57.7) | 58 (39.7) |
| Separated | 2 (1.4) | 4 (2.7) |
| Widowed | 11 (7.5) | 13 (8.9) |
| Divorced | 23 (15.8) | 16 (11.0) |
| Single | 25 (17.1) | 53 (36.3) |
| Other | 1 (.7) | 2 (1.4) |
| Family annual income (%) | ||
| <$5000 | 7 (4.8) | 8 (5.5) |
| $5001–15 000 | 9 (6.2) | 13 (8.9) |
| $15 001–30 000 | 19 (13.0) | 40 (27.4) |
| $30 001–50 000 | 40 (27.4) | 32 (21.9) |
| $50 001–75 000 | 39 (26.7) | 24 (16.4) |
| >$75 000 | 32 (21.9) | 27 (18.5) |
| Employment status (%) | ||
| Employed | 94 (64.4) | 103 (70.5) |
| Retired | 22 (15.1) | 15 (10.3) |
| Homemaker | 12 (8.2) | 15 (10.3) |
| Not employed | 18 (12.3) | 13 (8.9) |
Results for appraisal of cancer worries
Using the APIM model, we initially examined the relationship of each factor and appraisal of cancer worries separately. The results are presented in Table 2. When the unaffected relative in the dyad was the mother, the dyad had higher levels of cancer worries. Survivors also had more cancer worries than their relatives. There were significant actor effects for education, marital status, self-efficacy, coping style, and perception of cancer risk. These effects occurred for both survivors and unaffected female relatives. Married women had more worries than non-married women. Women with higher levels of education and higher self-efficacy had fewer worries. Additionally, women with a higher monitoring coping style and a higher perception of cancer risk had more worries. However, at this first level of analysis in the APIM model, no significant partner effects (i.e. the effect of one member of the dyad on the other) were detected.
Table 2.
Relationship of each of the model variables and appraisal of cancer worries using the APIM
| 95% Confi- dence interval for b |
||||||
|---|---|---|---|---|---|---|
| Effect | b | SE | t | p | Lower | Upper |
| Cancer type | 0.89a | |||||
| Ovarian vs breast | − 0.06 | 0.11 | −0.48 | 0.63 | −0.28 | 0.17 |
| Multiple vs breast | −0.01 | 0.19 | −0.05 | 0.96 | −0.38 | 0.36 |
| Years since diagnosis | −0.02 | 0.02 | −1.22 | 0.23 | −0.06 | 0.01 |
| Relationship of relativeb | 0.02a | |||||
| Other vs mother | −0.71 | 0.25 | −2.89 | 0.01 | −1.21 | −0.21 |
| Daughter vs mother | −0.38 | 0.16 | −2.32 | 0.02 | −0.71 | −0.06 |
| Sister vs mother | −0.46 | 0.17 | −2.75 | 0.01 | −0.79 | −0.13 |
| Relationship role | ||||||
| Survivor vs relative | 0.22 | 0.09 | 2.45 | 0.02 | 0.04 | 0.39 |
| Age | ||||||
| Actor effect | −0.005 | 0.004 | −1.40 | 0.16 | −0.01 | 0.002 |
| Partner effect | −0.01 | 0.004 | −1.77 | 0.08 | −0.01 | 0.001 |
| Education | ||||||
| Actor effect | −0.09 | 0.04 | −2.20 | 0.03 | −0.17 | −0.01 |
| Partner effect | 0.01 | 0.04 | 0.23 | 0.82 | −0.07 | 0.10 |
| Income | ||||||
| Actor effect | 0.02 | 0.04 | 0.43 | 0.67 | −0.05 | 0.08 |
| Partner effect | −0.02 | 0.04 | −0.47 | 0.64 | −0.09 | 0.05 |
| Marital status (married vs not married) | ||||||
| Actor effect | 0.11 | 0.05 | 2.24 | 0.03 | 0.01 | 0.20 |
| Partner effect | −0.02 | 0.05 | −0.35 | 0.72 | −0.11 | 0.08 |
| Race | ||||||
| Caucasian vs African-American | 0.14 | 0.11 | 1.37 | 0.17 | −0.06 | 0.35 |
| Family history of cancer | 0.03 | 0.24 | 0.13 | 0.90 | −0.44 | 0.50 |
| General self-efficacy | ||||||
| Actor effect | −0.01 | 0.01 | −2.39 | 0.02 | −0.02 | −0.002 |
| Partner effect | 0.004 | 0.01 | 0.83 | 0.41 | −0.01 | 0.01 |
| Family communication | ||||||
| Actor effect | −0.01 | 0.01 | −0.75 | 0.46 | −0.03 | 0.01 |
| Partner effect | −0.01 | 0.01 | −0.75 | 0.45 | −0.03 | 0.01 |
| Coping style | ||||||
| Actor effect | 0.03 | 0.01 | 2.10 | 0.04 | 0.002 | 0.05 |
| Partner effect | 0.01 | 0.02 | 0.60 | 0.55 | −0.02 | 0.04 |
| Perceived social support | ||||||
| Actor effect | 0.001 | 0.004 | 0.40 | 0.69 | −0.01 | 0.01 |
| Partner effect | −0.002 | 0.004 | −0.63 | 0.53 | −0.01 | 0.005 |
| Perception of cancer risk | ||||||
| Actor effect | 0.22 | 0.07 | 3.45 | 0.001 | 0.09 | 0.35 |
| Partner effect | 0.04 | 0.07 | 0.58 | 0.56 | −0.09 | 0.17 |
Significance of overall effect.
Interaction effect.
The final model for cancer worries contains the main effects and interactions and is presented in Table 3. In the final model the dyad-level effect of the relationship of the partner remained significant. When the mother was interviewed, the dyad had higher levels of cancer worries. Statistically significant actor effects included perception of cancer risk, coping style, and self-efficacy. Women with a higher monitoring coping score and higher perception of cancer risk had more worries, whereas women with higher self-efficacy had fewer worries. This occurred for both survivors and their unaffected female relatives. There was one significant partner effect, for income, which was moderated by the role of the women (both survivors and relatives). The association of partner’s income and cancer worries was not the same for survivors and relatives. For survivors there was a positive association between their relative’s income and their own cancer worries (β = 0.09, p = 0.048). For relatives there was a negative association between the survivor’s income and their cancer worries (β = −0.14, p = 0.004). This interaction also indicated that the difference in cancer worries between survivors and relatives depended on the income of the partner in the relationship. When the partner’s income is low there is no difference in worries between survivors and relatives. When the partner’s income is high, survivors have more worries than relatives.
Table 3.
Parameter estimates of fixed effects predicting appraisal of cancer worries in final model
| 95% Confidence interval |
|||||||
|---|---|---|---|---|---|---|---|
| Effect Intercept | Parameter estimate | Standard error | Approx. df | t | p-Value | Lower bound | Upper bound |
| Intercept | 0.458 | 0.187 | 220 | 2.45 | 0.015 | 0.090 | 0.825 |
| Age | −0.006 | 0.004 | 267 | −1.57 | 0.118 | −0.013 | 0.001 |
| Level of schooling | −0.073 | 0.041 | 270 | −1.78 | 0.076 | −0.153 | 0.008 |
| General self-efficacy | −0.014 | 0.005 | 264 | −2.75 | 0.006 | −0.024 | −0.004 |
| Coping style | 0.027 | 0.012 | 267 | 2.26 | 0.024 | 0.004 | 0.051 |
| Perception of cancer risk | 0.238 | 0.062 | 273 | 3.85 | 0.000 | 0.116 | 0.359 |
| Relationship role | 0.314 | 0.091 | 167 | 3.45 | 0.001 | 0.134 | 0.494 |
| Relationship of relative | 0.028a | ||||||
| Other vs mother | −0.634 | 0.239 | 139 | −2.65 | 0.009 | −1.107 | −0.161 |
| Daughter vs mother | −0.399 | 0.161 | 149 | −2.48 | 0.014 | −0.718 | −0.081 |
| Sister vs mother | −0.415 | 0.159 | 139 | −2.61 | 0.010 | −0.730 | −0.100 |
| Income of partner | 0.087 | 0.044 | 266 | 1.98 | 0.048 | 0.001 | 0.173 |
| Role by partner incomeb | 0.225 | 0.061 | 211 | 3.67 | 0.000 | 0.104 | 0.346 |
Significance of overall effect.
Interaction effect.
The interrelationship between survivors’ and relatives’ cancer worries was assessed with the partial intraclass correlation for the final model. The partial intraclass correlation was 0.19 (p = <0.01), indicating that after controlling for all the predictors, there was a significant and fairly strong correlation between survivors’ and relatives’ appraisal of cancer worries. This suggests that survivors and relatives mutually affected each other’s cancer worries.
Results for perception of cancer risk
The separate relationships between each of the predictor variables and perception of cancer risk using the APIM are presented in Table 4. Dyad-level predictors that were significant included cancer type, relationship role, race, and family history of cancer. The perception of risk was higher for dyads where the cancer type was ovarian compared with breast. For race, Caucasian dyads had higher perceptions of risk than African-American dyads. Those family dyads with a higher reported family history of cancer also had higher perception of risk. Additionally, survivors had lower perception of risk than their female relatives. There were also several actor effects for both survivors and female relatives that included age, income, marital status, and appraisal of cancer worries. Older women had less perception of risk than younger women, and women who were married and with higher income also had a higher perception of risk. Additionally, the greater the cancer worries of both survivors and female relatives, the greater was their perception of cancer risk. The only two significant partner effects were income and marital status. When the partner’s income was higher, the actor (either the survivor or relative) had more perception of risk. The perception of risk was also greater when the partner was married than when she was not married.
Table 4.
Relationship of each of the model variables and perception of cancer risk using the APIM
| 95% Confidence interval for b |
||||||
|---|---|---|---|---|---|---|
| Effect | b | SE | t | p | Lower | Upper |
| Cancer type | 0.04a | |||||
| Ovarian vs breast | 0.25 | 0.10 | 2.54 | 0.01 | 0.06 | 0.45 |
| Multiple vs breast | 0.19 | 0.16 | 1.15 | 0.25 | −0.13 | 0.51 |
| Years since diagnosis | 0.02 | 0.02 | 1.01 | 0.31 | −0.02 | 0.05 |
| Relationship of partner | 0.74a | |||||
| Other vs mother | −0.07 | 0.23 | −0.32 | 0.75 | −0.53 | 0.38 |
| Daughter vs mother | 0.04 | 0.15 | 0.25 | 0.80 | −0.26 | 0.33 |
| Sister vs mother | −0.07 | 0.15 | −0.49 | 0.63 | −0.40 | 0.23 |
| Relationship role | ||||||
| Survivor vs relative | −0.17 | 0.08 | −2.14 | 0.03 | −0.32 | −0.01 |
| Age | ||||||
| Actor effect | −0.01 | 0.003 | −2.35 | 0.02 | −0.01 | −0.001 |
| Partner effect | 0.004 | 0.003 | −1.45 | 0.15 | −0.001 | 0.01 |
| Education | ||||||
| Actor effect | 0.05 | 0.04 | 1.241 | 0.22 | −0.03 | 0.12 |
| Partner effect | 0.06 | 0.04 | 1.51 | 0.13 | −0.02 | 0.13 |
| Income | ||||||
| Actor effect | 0.10 | 0.03 | 3.28 | 0.001 | 0.04 | 0.16 |
| Partner effect | 0.06 | 0.03 | 1.969 | 0.05 | −0.0002 | 0.12 |
| Marital status (married vs not married) | ||||||
| Actor effect | 0.14 | 0.04 | 3.21 | 0.001 | 0.05 | 0.22 |
| Partner effect | 0.13 | 0.04 | 3.13 | 0.002 | 0.05 | 0.22 |
| Race | ||||||
| Caucasian vs African-American | 0.49 | 0.09 | 5.79 | o0.001 | 0.33 | 0.66 |
| Family history of cancer | 0.74 | 0.20 | 3.68 | o0.001 | 0.34 | 1.13 |
| General self-efficacy | ||||||
| Actor effect | 0.004 | 0.005 | 0.85 | 0.40 | −0.005 | 0.01 |
| Partner effect | 0.002 | 0.005 | 0.50 | 0.62 | −0.007 | 0.01 |
| Family communication | ||||||
| Actor effect | 0.02 | 0.01 | 1.81 | 0.07 | −0.002 | 0.04 |
| Partner effect | 0.02 | 0.01 | 1.56 | 0.12 | −0.004 | 0.04 |
| Monitor/blunting | ||||||
| Actor effect | −0.01 | 0.01 | −0.52 | 0.61 | −0.03 | 0.02 |
| Partner effect | 0.001 | 0.02 | 0.07 | 0.94 | −0.03 | 0.03 |
| Perceived social support | ||||||
| Actor effect | 0.004 | 0.003 | 1.17 | 0.24 | −0.002 | 0.01 |
| Partner effect | 0.003 | 0.003 | 0.77 | 0.44 | −0.004 | 0.01 |
| Appraisal of cancer worries | ||||||
| Actor effect | 0.18 | 0.05 | 3.42 | 0.001 | 0.08 | 0.28 |
| Partner effect | 0.04 | 0.05 | 0.72 | 0.47 | −0.07 | 0.14 |
Significance of overall effect.
The final model of the predictors of the perception of cancer risk is presented in Table 5. After controlling for other variables, there were three dyad-level predictors. Caucasian dyads and dyads with a higher family history of cancer had a higher perception of cancer risk. Unaffected female relatives in the dyads also reported a higher perception of risk than their family members who were cancer survivors. In the final model, two actor effects were significant, namely income and appraisal of cancer worries. Women with higher income and higher cancer worries had greater perception of cancer risk. In the final model, when testing for interactions, no significant partner effects were found, nor differences in the predictors between survivors and relatives.
Table 5.
Parameter estimates of fixed effects predicting perception of cancer risk in the final model
| 95% Confidence interval |
|||||||
|---|---|---|---|---|---|---|---|
| Effect | Parameter estimate | Standard error | Approx. df | t | p-Value | Lower bound | Upper bound |
| Intercept | −0.066 | 0.073 | 247 | −0.91 | 0.365 | −0.210 | 0.078 |
| Relationship role | −0.242 | 0.078 | 152 | −3.10 | 0.002 | −0.397 | −0.088 |
| Income | 0.088 | 0.030 | 268 | 2.95 | 0.003 | 0.029 | 0.147 |
| Race | 0.337 | 0.086 | 160 | 3.92 | 0.000 | 0.167 | 0.507 |
| Family history of cancer | 0.563 | 0.179 | 144 | 3.15 | 0.002 | 0.210 | 0.916 |
| Appraisal of cancer worries | 0.184 | 0.048 | 278 | 3.85 | 0.000 | 0.090 | 0.278 |
The partial intraclass correlation for the final model was 0.04, which was not significant. The small magnitude of this correlation points to a weak interrelationship between the survivors’ and relatives’ perception of risk, unlike their appraisal of cancer worries.
Discussion
Several sociodemographic variables, coping style, self-efficacy, and family history of cancer, were significant contributors to the survivors’ and their unaffected relatives’ appraisal of risk or cancer worries. Using the APIM model, we found more similarities than differences between cancer survivors and their unaffected female relatives. We also found more ‘actor’ effects for both the survivor’s and the relative’s worries and perceptions of cancer risk than effects of the partner’s influence on subjects’ perceptions and cancer worries.
Coping style, self-efficacy, and perception of cancer risk accounted for the most variance in both the survivor’s and the female relative’s cancer worries. Having a coping style that is vigilant in scanning risk and health information and having heightened risk perceptions contributed to more cancer worries, which is a finding also supported in other research [24–26, 56, 57]. These factors are important to consider for both members of the dyad in facing familial cancer risk and in determining how to manage these worries while seeking risk information. Concurrently, a higher sense of self-efficacy contributed to lower cancer worries. Thus, feelings of self-efficacy may be important protective factors in helping to allay cancer worries.
Another ‘actor’ effect for perception of cancer risk was cancer worries. Interestingly, both cancer worries and perception of cancer risk each made independent positive contributions to the other in the multilevel modeling. While there have been inconsistencies reported in the literature regarding a positive or negative relationship between these two factors [24–27], this study supported the interdependence in risk appraisal influencing cancer worries and vice versa. This suggests that attention by clinicians to both cancer worry and to overestimation of perceived cancer risk is critical in assisting individuals and families in managing their fears and making more realistic risk appraisals.
Results also indicate that there are other sociodemographic factors important to consider within families at risk. Race was a factor that significantly influenced perception of risk with Caucasian dyads having a higher perception of risk than African-American dyads. Although there is limited research on ethnic and racial differences, this result further supports the finding by Hughes et al. [58] that African-American women were less likely to report increased personal risk perceptions than White women.
The only ‘partner’ effect that was significant for cancer worries was income, which was moderated by the role relationship of the survivor or female relative. When the relative’s income was high, survivors had significantly more worries. Income was also significant as an actor effect for perception of cancer risk. A possible explanation may be that higher income levels may be associated with higher educational levels that indicate a more realistic threat appraisal of cancer in the future.
Several significant within-dyad effects were also found, particularly in role and family relationships. When cancer survivors and female relatives were mothers, they had significantly more cancer worries than their dyad partner in the study. A possible explanation is that mothers who are survivors are concerned about possible cancer in the future for their children or for themselves about the threat this poses to their role as caregivers. For mothers who are unaffected relatives, they may have increased worry for their daughters who are cancer survivors. From this study, it is evident that the mother–daughter relationship appears to be an important factor in cancer worries and risk perceptions and has clinical implications for addressing concerns mothers may have about cancer worry. Further role differences were noted between survivors and female relatives, with survivors having more cancer worries than their relatives, whereas at the same time relatives reported a significantly higher perception of risk. Additionally, family history of cancer contributed to increased risk perceptions of both survivors and female relatives, a finding supported in other research [8, 10, 13]. This study further underscores the prevalence of increased risk perceptions among various family members and the importance of addressing these concerns in clinical practice.
Another important finding from this study was the interdependence between the survivors’ and female relatives’ cancer worries. When one member’s cancer worries increased, the other’s worry also was elevated. While the interrelationship between family members’ adjustment to cancer has been well documented in previous research with other cancer populations [14, 30, 31, 59], the current study supports the mutual and partner relationship that family members at risk for breast/ovarian cancer have regarding each other’s cancer worries. In this study, families that participated may also have had a higher level of closeness in their relationship, reflected in more interaction and a mutual increase in cancer worries.
While many factors were supported in the model, others were not. Sociodemographic characteristics, select medical factors, and personal resources contributed to either the family members’ cancer worries or perception of risk. However, unexpectedly, social support and family communication did not correlate with either cancer worries or risk perceptions of survivors and their relatives. A possible explanation is that since survivors had self-selected their relatives to participate, they were already talking more and the measures of social support and family communication we used were capturing broader processes of support and communication. Based upon our findings, there is also a need in the future to further develop and test the theoretical model. Other variables that may have applicability to cancer worries and risk perception could be included, such as cancer-specific distress or anxiety [11, 18] and other concurrent stressors that families face that may influence risk perception and cancer worries [60].
This study had several limitations. The retrospective, cross-sectional design should be viewed with caution. For relatives dealing with familial cancer risk, prospective studies are needed to determine if survivors’ and their family members’ worries or risk perceptions change over time. A second limitation is that this study included only female relative dyads. Other family role relationships, most notably spouses, would be important to include to capture other perspectives that factor into an entire family’s response to their shared cancer risk. A third limitation was the response rate of only 38%. Although a population-based sample, those who refused may have been either unwilling to contact relatives or felt uncomfortable to discuss cancer risk with family members. Another limitation was that this community-based sample included a small number of individuals who reportedly had genetic testing, but the design of the study did not allow confirmation of this nor determination of the genetic test results, which may have influenced their current perceptions of cancer risk. The low percentage of participants who did have genetic counseling may have contributed to the seeming interdependence of cancer worry and risk perception between survivors and relatives. Future research is needed to evaluate whether the interdependence we observed, especially in regard to cancer worries, might be different in groups where most of the subjects had undergone genetic counseling. A final limitation was the knowledge questionnaire used in this study. The previously reported psychometric properties were not observed in this population-based sample; thus, we were unable to ascertain the sample’s current understanding and knowledge regarding inherited cancer risk. Future studies are needed to assess community knowledge of inherited cancer risk and how this impacts on outcomes for members of a family.
Conclusion
Overall, the results of this study indicate that there are important individual and family factors that are related to both breast and ovarian cancer survivors’ and their female relatives’ perceptions of cancer risk and cancer worries. Income, race, family cancer history, and cancer worries were significant actor effects related to cancer risk perception. Coping style, self-efficacy, family role relationship, and cancer risk perception were significantly related to cancer worries. Additionally, the level of cancer worries reported by one member of the dyad was related to the amount of worries reported by the other. The income of the partner was also associated with differences in cancer worries between survivors and their relatives. These findings underscore the importance of clinicians addressing concerns of both affected and unaffected family members at increased risk of cancer to assist them in managing cancer worries and having realistic risk appraisals to make informed decisions about their surveillance options.
Acknowledgements
This study was supported by a grant from NIH, NINR #R21 NR008584-01.
References
- 1.National Cancer Institute. Cancer Survivorship Research, Estimated U.S. Cancer Prevalence. National Cancer Institute; 2007. [accessed 3/9, 2007]. (Available from: http://cancer-control.cancer.gov/ocs/prevalence.html.) [Google Scholar]
- 2.American Cancer Society. Cancer Facts and Figures 2007. Atlanta, GA: American Cancer Society; 2007. [Google Scholar]
- 3.Garber JE, Offit K. Hereditary cancer predisposition syndromes. J Clin Oncol. 2005;23:276–292. doi: 10.1200/JCO.2005.10.042. [DOI] [PubMed] [Google Scholar]
- 4.Robson ME. Clinical considerations in the management of individuals at risk for hereditary breast and ovarian cancer. Cancer Control. 2002;9:457–465. doi: 10.1177/107327480200900602. [DOI] [PubMed] [Google Scholar]
- 5.Bluman LG, Rimer BK, Berry DA, et al. Attitudes, knowledge, and risk perceptions of women with breast and/or ovarian cancer considering testing for BRCA1 and BRCA2. J Clin Oncol. 1999;17:1040–1046. doi: 10.1200/JCO.1999.17.3.1040. [DOI] [PubMed] [Google Scholar]
- 6.Lerman C, Daly M, Masny A, Balshem A, et al. Attitudes about genetic testing for breast-ovarian cancer susceptibility. J Clin Oncol. 1994;12:843–850. doi: 10.1200/JCO.1994.12.4.843. [DOI] [PubMed] [Google Scholar]
- 7.Struewing JP, Lerman C, Kase RG, Giambarresi TR, Tucker MA. Anticipated uptake and impact of genetic testing in hereditary breast and ovarian cancer families. Cancer Epidemiol Biomarkers Prev. 1995;4:169–173. [PubMed] [Google Scholar]
- 8.Neise C, Rauchfuss M, Paepke S, Beier K, Lichtenegger W, et al. Risk perception and psychological strain in women with a family history of breast cancer. Onkologie. 2001;24:470–475. doi: 10.1159/000055128. [DOI] [PubMed] [Google Scholar]
- 9.Audrain J, Schwartz MD, Lerman C, Hughes C, Peshkin BN, Biesecker B, et al. Psychological distress in women seeking genetic counseling for breast-ovarian cancer risk: the contributions of personality and appraisal. Ann Behav Med. 1997;19:370–377. doi: 10.1007/BF02895156. [DOI] [PubMed] [Google Scholar]
- 10.Lerman C, Trock B, Rimer BK, Jepson C, Brody D, Boyce A, et al. Psychological side effects of breast cancer screening. Health Psychol. 1991;10:259–267. doi: 10.1037//0278-6133.10.4.259. [DOI] [PubMed] [Google Scholar]
- 11.van Oostrom I, Meijers-Heijboer H, Lodder LN, et al. Long-term psychological impact of carrying a BRCA1/2 mutation and prophylactic surgery: a 5-year follow-up study. J Clin Oncol. 2003;21:3867–3874. doi: 10.1200/JCO.2003.10.100. [DOI] [PubMed] [Google Scholar]
- 12.Trask PC, Paterson AG, Wang C, et al. Cancer-specific worry interference in women attending a breast and ovarian cancer risk evaluation program: impact on emotional distress and health functioning. Psycho-Oncology. 2001;10:349–360. doi: 10.1002/pon.510. [DOI] [PubMed] [Google Scholar]
- 13.Baider L, Ever-Hadani P, Kaplan De-Nour A. Psychological distress in healthy women with familial breast cancer: like mother, like daughter? Int J Psychiatry Med. 1999;29:411–420. doi: 10.2190/LD2F-ND7R-19JK-WL4G. [DOI] [PubMed] [Google Scholar]
- 14.Mellon S, Berry-Bobovski L, Gold R, Levin N, Tainsky M. Communication and decision-making about seeking inherited cancer risk information: findings from female survivor-relative focus groups. Psycho-Oncology. 2006;15:193–208. doi: 10.1002/pon.935. [DOI] [PubMed] [Google Scholar]
- 15.Emery J, Kumar S, Smith H. Patient understanding of genetic principles and their expectations of genetic services within the NHS: a qualitative study. Community Genet. 1998;1:78–83. doi: 10.1159/000016141. [DOI] [PubMed] [Google Scholar]
- 16.Lerman C, Lustbader E, Rimer BK, et al. Effects of individualized breast cancer risk counseling: a randomized trial. J Natl Cancer Inst. 1995;87:286–292. doi: 10.1093/jnci/87.4.286. [DOI] [PubMed] [Google Scholar]
- 17.Weinstein ND, Atwood K, Puleo E, Fletcher R, Colditz G, Emmons KM. Colon cancer: risk perceptions and communication. J Health Commun. 2004;9:53–65. doi: 10.1080/10810730490271647. [DOI] [PubMed] [Google Scholar]
- 18.Lerman C, Hughes C, Lemon SJ, et al. What you don’t know can hurt you: adverse psychologic effects in members of BRCA1-linked and BRCA2-linked families who decline genetic testing. J Clin Oncol. 1998;16:1650–1654. doi: 10.1200/JCO.1998.16.5.1650. [DOI] [PubMed] [Google Scholar]
- 19.Lodder L, Frets PG, Trijsburg RW, et al. Attitudes and distress levels in women at risk to carry a BRCA1/BRCA2 gene mutation who decline genetic testing. Am J Med Genet A. 2003;119:266–272. doi: 10.1002/ajmg.a.10168. [DOI] [PubMed] [Google Scholar]
- 20.Diefenbach MA, Miller SM, Daly MB. Specific worry about breast cancer predicts mammography use in women at risk for breast and ovarian cancer. Health Psychol. 1999;18:532–536. doi: 10.1037//0278-6133.18.5.532. [DOI] [PubMed] [Google Scholar]
- 21.Stefanek ME, Wilcox P. First degree relatives of breast cancer patients: screening practices and provision of risk information. Cancer Detect Prev. 1991;15:379–384. [PubMed] [Google Scholar]
- 22.McCaul KD, Schroeder DM, Reid PA. Breast cancer worry and screening: some prospective data. Health Psychol. 1996;15:430–433. doi: 10.1037//0278-6133.15.6.430. [DOI] [PubMed] [Google Scholar]
- 23.Kash KM, Holland JC, Halper MS, Miller DG. Psychological distress and surveillance behaviors of women with a family history of breast cancer. J Natl Cancer Inst. 1992;84:24–30. doi: 10.1093/jnci/84.1.24. [DOI] [PubMed] [Google Scholar]
- 24.Brain K, Norman P, Gray J, Mansel R. Anxiety and adherence to breast self-examination in women with a family history of breast cancer. Psychosom Med. 1999;61:181–187. doi: 10.1097/00006842-199903000-00010. [DOI] [PubMed] [Google Scholar]
- 25.Schwartz MD, Lerman C, Miller SM, Daly M, Masny A. Coping disposition, perceived risk, and psychological distress among women at increased risk for ovarian cancer. Health Psychol. 1995;14:232–235. doi: 10.1037//0278-6133.14.3.232. [DOI] [PubMed] [Google Scholar]
- 26.Bish A, Sutton S, Jacobs C, Levene S, Ramirez A, Hodgson S. Changes in psychological distress after cancer genetic counselling: a comparison of affected and unaffected women. Br J Cancer. 2002;86:43–50. doi: 10.1038/sj.bjc.6600030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Loescher LJ. Cancer worry in women with hereditary risk factors for breast cancer. Oncol Nurs Forum. 2003;30:767–772. doi: 10.1188/03.ONF.767-772. [DOI] [PubMed] [Google Scholar]
- 28.McCubbin MA, McCubbin HI. Resiliency in families: a conceptual model of family adjustment and adaptation in response to stress and crises. In: McCubbin HI, Thompson AI, McCubbin MA, editors. Family Assessment: Resiliency, Coping, and Adaptation—Inventories for Research and Practice. Madison, WI: University of Wisconsin System; 1996. pp. 1–64. [Google Scholar]
- 29.Northouse LL, Dorris G, Charron-Moore C. Factors affecting couples’ adjustment to recurrent breast cancer. Soc Sci Med. 1995;41:69–76. doi: 10.1016/0277-9536(94)00302-a. [DOI] [PubMed] [Google Scholar]
- 30.Northouse LL, Templin T, Mood D. Couples’ adjustment to breast disease during the first year following diagnosis. J Behav Med. 2001;24:115–136. doi: 10.1023/a:1010772913717. [DOI] [PubMed] [Google Scholar]
- 31.Northouse LL, Mood D, Templin T, Mellon S, George T. Couples’ patterns of adjustment to colon cancer. Soc Sci Med. 2000;50:271–284. doi: 10.1016/s0277-9536(99)00281-6. [DOI] [PubMed] [Google Scholar]
- 32.Mellon S, Northouse LL. Family survivorship and quality of life following a cancer diagnosis. Res Nurs Health. 2001;24:446–459. doi: 10.1002/nur.10004. [DOI] [PubMed] [Google Scholar]
- 33.Compas BE, Worsham NL, Epping-Jordan J, et al. When mom or dad has cancer: markers of psychological distress in cancer patients, spouses, and children. Health Psychol. 1994;13:507–515. [PubMed] [Google Scholar]
- 34.Mellon S, Northouse LL, Weiss LK. A population-based study of the quality of life of cancer survivors and their family caregivers. Cancer Nurs. 2006;29:120–131. doi: 10.1097/00002820-200603000-00007. [DOI] [PubMed] [Google Scholar]
- 35.Green J, Richards M, Murton F, Statham H, Hallowell N. Family communication and genetic counseling: the case of hereditary breast and ovarian cancer. J Genet Couns. 1997;6:45–60. doi: 10.1023/A:1025611818643. [DOI] [PubMed] [Google Scholar]
- 36.Forrest K, Simpson SA, Wilson BJ, et al. To tell or not to tell: barriers and facilitators in family communication about genetic risk. Clin Genet. 2003;64:317–326. doi: 10.1034/j.1399-0004.2003.00142.x. [DOI] [PubMed] [Google Scholar]
- 37.Julian-Reynier C, Welkenhuysen M, Hagoel L, Decruyenaere M, Hopwood P CRISCOM Working Group. Risk communication strategies: state of the art and effectiveness in the context of cancer genetic services. Eur J Hum Genet. 2003;11:725–736. doi: 10.1038/sj.ejhg.5201037. [DOI] [PubMed] [Google Scholar]
- 38.Hughes C, Lerman C, Schwartz M, et al. All in the family: evaluation of the process and content of sisters’ communication about BRCA1 and BRCA2 genetic test results. Am J Med Genet. 2002;107:143–150. doi: 10.1002/ajmg.10110. [DOI] [PubMed] [Google Scholar]
- 39.Tercyak KP, Peshkin BN, Streisand R, Lerman C. Psychological issues among children of hereditary breast cancer gene (BRCA1/2) testing participants. Psycho-Oncology. 2001;10:336–346. doi: 10.1002/pon.531. [DOI] [PubMed] [Google Scholar]
- 40.Halbert CH, Schwartz MD, Wenzel L, et al. Predictors of cognitive appraisals following genetic testing for BRCA1 and BRCA2 mutations. J Behav Med. 2004;27:373–392. doi: 10.1023/b:jobm.0000042411.56032.42. [DOI] [PubMed] [Google Scholar]
- 41.Gilpin CA, Carson N, Hunter AG. A preliminary validation of a family history assessment form to select women at risk for breast or ovarian cancer for referral to a genetics center. Clin Genet. 2000;58:299–308. doi: 10.1034/j.1399-0004.2000.580408.x. [DOI] [PubMed] [Google Scholar]
- 42.Miller SM. Monitoring and blunting: validation of a questionnaire to assess styles of information seeking under threat. J Pers Soc Psychol. 1987;52:345–353. doi: 10.1037//0022-3514.52.2.345. [DOI] [PubMed] [Google Scholar]
- 43.Muris P, Schouten E. Monitoring and blunting: a factor analysis of the miller behavioral style scale. Pers Individ Diff. 1994;17:285–287. [Google Scholar]
- 44.Lerman C, Narod S, Schulman K, et al. BRCA1 testing in families with hereditary breast-ovarian cancer. A prospective study of patient decision making and outcomes. J Am Med Assoc. 1996;275:1885–1892. [PubMed] [Google Scholar]
- 45.Scherer M, Maddux JE, Mercandante B, Prentice-Dunn S, Jacobs B, Rogers RW. The self-efficacy scale: construction and validation. Psychol Rep. 1982;51:663–671. [Google Scholar]
- 46.Moos RH, Moos BS. Family Environment Scale Manual. California: Consulting Psychologists; 1981. [Google Scholar]
- 47.Fredman N, Sherman R, editors. Handbook of Measurements for Marriage and Family Therapy. New York: Brunner Mazal; 1987. [Google Scholar]
- 48.Brandt PA, Weinert C. The PRQ—a social support measure. Nurs Res. 1981;30:277–280. [PubMed] [Google Scholar]
- 49.McCaul KD, Branstetter AD, O’Donnell SM, Jacobson K, Quinlan KB. A descriptive study of breast cancer worry. J Behav Med. 1998;21:565–579. doi: 10.1023/a:1018748712987. [DOI] [PubMed] [Google Scholar]
- 50.Campbell L, Kashy DA. Estimating actor, partner, and interaction effects for dyadic data using PROC MIXED and HLM: a user-friendly guide. Pers Relationships. 2002;9:327–342. [Google Scholar]
- 51.Kashy DA, Kenny DA. The analysis of data from dyads and groups. In: Reis HT, Judd CM, editors. Handbook of Research Methods in Social and Personality Psychology. New York, NY: Cambridge University Press; 2000. pp. 451–477. [Google Scholar]
- 52.Kenny DA. Models of non-independence in dyadic research. J Soc Pers Relationships. 1996;13:279–294. [Google Scholar]
- 53.Kenny DA, Cook W. Partner effects in relationships research: conceptual issues, analytic difficulties, and illustrations. Pers Relationships. 1999;6:433–448. [Google Scholar]
- 54.Rayens MK, Svavarsdottir EK. A new methodological approach in nursing research: an actor, partner, and interaction effect model for family outcomes. Res Nurs Health. 2003;26:409–419. doi: 10.1002/nur.10100. [DOI] [PubMed] [Google Scholar]
- 55.Aiken L, West S. Multiple Regression: Testing and Interpreting Interactions. Newbury Park, CA: Sage; 1991. [Google Scholar]
- 56.Tercyak KP, Lerman C, Peshkin BN, et al. Effects of coping style and BRCA1 and BRCA2 test results on anxiety among women participating in genetic counseling and testing for breast and ovarian cancer risk. Health Psychol. 2001;20:217–222. [PubMed] [Google Scholar]
- 57.Lerman C, Schwartz MD, Miller SM, Daly M, Sands C, Rimer BK. A randomized trial of breast cancer risk counseling: interacting effects of counseling, educational level, and coping style. Health Psychol. 1996;15:75–83. doi: 10.1037//0278-6133.15.2.75. [DOI] [PubMed] [Google Scholar]
- 58.Hughes C, Lerman C, Lustbader E. Ethnic differences in risk perception among women at increased risk for breast cancer. Breast Cancer Res Treat. 1996;40:25–35. doi: 10.1007/BF01806000. [DOI] [PubMed] [Google Scholar]
- 59.Cassileth BR, Lusk EJ, Strouse TB, Miller DS, Brown LL, Cross PA. A psychological analysis of cancer patients and their next-of-kin. Cancer. 1985;55:72–76. doi: 10.1002/1097-0142(19850101)55:1<72::aid-cncr2820550112>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 60.Northouse LL, Mood D, Kershaw T, et al. Quality of life of women with recurrent breast cancer and their family members. J Clin Oncol. 2002;20:4050–4064. doi: 10.1200/JCO.2002.02.054. [DOI] [PubMed] [Google Scholar]