Abstract
Objective
BMP-2 is approved for fracture non-union and spine fusion. We aimed to further dissect its downstream signaling events in chondrocytes with the ultimate goal to develop novel therapeutics that can mimic BMP-2 effect but have less complications.
Methods
BMP-2 effect on COX-2 expression was examined using RT-PCR and Western blot analysis. Genetic approach was used to identify the signaling pathway mediating the BMP-2 effect. Similarly, the pathway transducing the PGE2 effect on ATF4 was investigated. Immunoprecipitation was performed to assess the complex formation after PGE2 binding.
Results
BMP-2 increased COX-2 expression in primary mouse costosternal chondrocytes (PMCSC). The results from the C9 Tet-off system demonstrated that endogenous BMP-2 also upregulated COX-2 expression. Genetic approaches using PMCSC from ALK2fx/fx, ALK3fx/fx, ALK6−/−, and Smad1fx/fx mice established that BMP-2 regulated COX-2 through activation of ALK3-Smad1 signaling. PGE-2 EIA showed that BMP-2 increased PGE2 production in PMCSC. ATF4 is a transcription factor that regulates bone formation. While PGE2 did not have significant effect on ATF4 expression, it induced ATF4 phosphorylation. In addition to stimulating COX-2 expression, BMP-2 also induced phosphorylation of ATF4. Using COX-2 deficient chondrocytes, we demonstrated that the BMP-2 effect on ATF4 was COX-2-dependent. Tibial fracture samples from COX-2−/− mice showed reduced phospho-ATF4 immunoreactivity compared to WT ones. PGE2 mediated ATF4 phosphorylation involved signaling primarily through the EP2 and EP4 receptors and PGE2 induced an EP4-ERK1/2-RSK2 complex formation.
Conclusions
BMP-2 regulates COX-2 expression through ALK3-Smad1 signaling, and PGE2 induces ATF4 phosphorylation via EP4-ERK1/2-RSK2 axis.
Introduction
BMP-2 regulates cellular function by binding serine/threonine kinase receptors composed of a complex of type I and type II receptors with subsequent phosphorylation/activation of Smad1/5/8. Three different type I BMP receptors (ALKs 2, 3, and 6) have been identified1,2. Recombinant BMP-2 is FDA approved to stimulate bone formation in spine fusion surgery and for the treatment of non-union of tibial fractures3,4. Recent clinical reports have documented adverse events related to the use of BMP-2 that include ectopic bone formation, osteolysis and soft tissue inflammation5–7. More clearly defining the downstream signaling events that are stimulated by BMP-2 may improve safety as well as lead to the development of more selective agents for skeletal diseases.
A classic Smad signaling pathway mediates BMP-2 receptor signaling. Smads 1, 5 and 8 associate with the type I receptors and are phosphorylated and released into the cytoplasm following ligand binding to the receptor. The receptor associated Smads form a complex with Smad4 in the cytoplasm, and then translocate to the nucleus and modulate gene transcription. In addition, BMP receptors also activate the MAP kinase (MAPK) signaling pathway. TAK1 is a MAP-kinase-kinase-kinase that associates with the receptor complex and initiates signaling through the MAPK pathways. Recent reports show that TAK1 is an important mediator of BMP-2 effects in chondrocytes8–10. We have also shown that the classic Smad and a non-classic TAK1-p38-ATF2 pathways are both involved in maintenance of articular cartilage and have a role in the pathogenesis of osteoarthritis (OA)11.
In addition to its effect on osteo-chondral specific transcriptional factors, BMP-2 also increases the expression of COX-2 in osteoblasts12. COX-2 is a critical factor in the skeletal repair process as COX-2 deletion mutant (COX-2−/−) mice have delayed fracture union13. PGE2 is a major metabolite downstream of COX-2. PGE2 exerts its effect through binding to G-protein coupled cell receptors, EP1, EP2, EP3, and EP414. Our previous study indicates that PGE2 inhibits the differentiation of chicken growth plate chondrocytes15. However, the reports from other groups demonstrate a complex interaction of BMP-2 and COX-2. PGE2 can upregulate BMP-2 expression in human mesenchymal stem cells via its EP4 receptor16,17. We also previously demonstrate that systemic administration of EP4 agonist accelerates fracture healing in COX-2−/− mice18. The findings from other groups have shown that both EP2 and EP4 agonists enhance fracture healing19, 20. In contrast, our previous study demonstrates that EP1 receptor is a negative regulator of fracture healing21.
The EP4 receptor has a unique long intracellular domain that can be internalized upon ligand binding and forms complex in cytoplasm with other molecules22. For examples, PGE2 can activate ERK1/2 pathway through EP4 and induce ATF4 phosphorylation in cancer cells23, 24. Previous studies have established that ERK1/2 can phosphorylate and activate RSK-225. ATF4 plays a critical role in skeletal development and maintenance. ATF4 null mutant mice exhibit an osteoporotic phenotype due to a reduced bone formation rate. Enzymatic activity assay revealed that a 90KD ribosomal S6 kinase 2 (RSK2) is a direct upstream kinase able to phosphorylate ATF4. RSK2 mutation has been found in the Coffin-Lowry Syndrome, an X-linked disorder characterized by skeletal anomalies and mental retardation. RSK2 deficient mice display a similar phenotype as ATF4 mutant mice26,27. Interestingly, ATF4 also promotes osteoclast differentiation and bone resorption via direct and indirect mechanisms28,29. Further, ATF4 may regulate differentiation of osteochondal progenitors in an Ihh dependent mechanism30,31.
Based on these observations, we hypothesized that BMP-2 regulates ATF4 expression and activity in chondrocytes in a COX-2 dependent manner. Our current study aimed to establish a molecular cascade linking these pathways. Our results show that BMP-2 increases COX-2 expression via a classic ALK3-Smad1 pathway. PGE2 induces ATF4 phosphorylation mainly through EP4 receptor with downstream signaling involving ERK1/2-RSK2 activation.
Materials and methods
Cells
Primary mouse costosternal chondrocytes (PMCSC) were isolated from the mice with different genetic backgrounds as previously described32,33. Briefly, the rib cages including sterna were dissected from 3-days-old pups. Soft tissue was removed after serial incubation with pronase (Roche Laboratory, Nutley, NJ, USA, 2mg/ml in PBS) at 37°C shaker for 45 min and then in collagenase D (R oche, 3mg/ ml in full medium) at 37°C for 60 min. After thorough wash, the rib cages were further digested with collagenase D in a petri dish for 5 hours at 37°C incubator. Then the cells were filtered through 40μm mesh to remove tissue debris. After centrifuge, the cell pellet was washed and re-suspended with DMEM containing 10% FBS.
The C9 tet-off cells were developed from mouse mesenchymal C3H10T1/2 cells after transfection with the BMP-2 gene and antibiotic selection with hygromycin. C9 cells are under the control of the inducible Tet-Off system, e.g. no BMP-2 overproduction in the presence of doxycycline (1μg/ml, Sigma, St. Louis, MO). After the withdrawal of doxycycline, C9 cells overexpress BMP-2 and secrete a large amount of BMP-234.
Rat chondrosarcoma cells (RSC) were used in some experiments including reporter luciferase assay.
In some experiments, cells were pre-treated with BMP-2 type I receptor (BMPRI, ALK2, 3, 6) inhibitor Dorsomorphin (10nM, Sigma) or a TAK1 inhibitor (5Z)-7-Oxozeaenol (10nM, EMD Millipore, Billerica, CA) for 1 hour followed by other treatments.
Mice
The following genetically modified mice have been used in this study: global knock-out (KO) mice: ALK6−/− mice were from Dr. Karen Lyons35, EP1−/− mice from Dr. Matthew Breyer36, EP2−/− mice from Dr. Richard Breyer37; conditional gene floxed mice: ALK2fx/fx mice were from Dr. Vesa Kaartinen38; ALK3fx/fx from Dr. Yuji Mishina39, Smad1fx/fx mice from NIH40; EP4fx/fx mice from Dr. Matthew Breyer41, and TAK1fx/fx mice from Dr. Michael Schneider42.
Luciferase Assay
COX-2 gene promoter reporter was purchased from Panomics (Santa Clara, CA) and transient transfection was performed in RCS using Lipofectamine 2000 from Invitrogen (Grand Island, NY). Cell culture medium containing Lipofectamine 2000 was removed after 12 hours and BMP-2 (100ng/ml, R&D Systems, Minneapolis, MN) was added to culture medium for 48 hours with vehicle as a control. Reporter luciferase assay was done in triplicate with a luminometer (Opticom 1, Promega, Madison, WI).
Real time quantitative PCR (RT-PCR)
Total RNA was harvested using an RNeasy Mini kit (Qiagen, Valencia, CA). Reverse transcription was done with a SuperScript First Strand Synthesis System (Invitrogen). RT-PCR was performed with QuantiTech SYBR Green PCR kit (Qiagen) in Rotor-Gene Real-Time DNA amplification system (Corbett Research, New South Wales, Australia) using the COX-2 primers: forward: 5′-AGAAGGAAATGGCTGCAGAA-3′, reverse: 3′-GCTCGGCTTCCAGTATTGAG-5′; Atf4 primers: forward: 5′-TCGATGCTCTGTTTCGAATG-3′, reverse: 3′-GGCAACCTGGTCGACTTTTA-5′. The expression levels of the genes of interest (GOI) were analyzed with the software from Qiagen. For each GOI, a standard curve was established and a value of each treatment group was obtained. Similarly, a standard curve was established for β-actin and its value in each group was calculated. RT-PCR was done in quadruplicate and each GOI value was normalized by β-actin values. The mean and SD were generated with software from these normalized values.
EIA for PGE2
Prior to BMP-2 treatment, PMCSC were starved for 24 hours in serum-free medium. Then, cells were treated with BMP-2 (100ng/ml) for 12 hours and supernatants were collected. PGE2 concentration was measured using an Enzyme Immunoassay (EIA) kit from Cayman Chemical (Ann Arbor, MI), and the results were represented as the means values from 6 wells and standard deviations.
Construction of EP4-V5 Plasmid
The plasmid was constructed using the Gateway Technology system (Invitrogen). Briefly, mouse EP4 receptor cDNA was produced by PCR with high fidelity polymerase (Invitrogen). A Gateway® entry clone from an attB-flanked PCR product was created with a TOPO® cloning reaction. Through an LR reaction, the product was subcloned to the destination vector pcDNA™3.2/V5-DEST. After sequencing, the correct clone was amplified for future study.
Western blotting and immunoprecipitation
The nonprostanoid EP4 receptor agonist CP-734432 was obtained from Pfizer Global Research and Development (Groton, CT). For in vitro studies, it was used at a final concentration of 10−6 M. Cells were lysed with golden lysis buffer (20 mM Tris, 137 mM NaCl, 5 mM Na2EDTA, 10% glycerol, 1% Triton X-100, 1 mM phenylmethylsulfonyl fluoride, 1 mM aprotinin, 1 mM leupeptin, 1 μM pepstatin A, 10 mM NaF, 1 mM Na3VO4, 1 mM EGTA, 1 mM tetrasodium PP1, and 100 μM P-glycerophosphate). Western blot was performed with the invitrogen system. Chemiluminescence was done with West Femto reagent (Thermo Fisher Scientific, Rockford, IL). The following antibodies were used in the experiments: COX-2 (Cayman Chemicals), ATF4 and phospho ATF4 (Abcam, Cambridge, MA), phospho ERK1/2 and phospho RSK-2 (Cell Signaling Technology, Boston, MA). The experiments were usually repeated for three times. After establishing the trends of protein expression, the clear results with less variation were chosen and represented. Immunoprecipitation (IP) was performed using the Catch and Release Reversible IP System (EMD Millipore). The protein was pulled down with V5 antibody (Invitrogen) and GST antibody (Sigma) was used as antibody control. IP was performed using antibodies against phospho-EKR1/2 and RSK-2.
In vitro gene deletion
PMCSC were isolated and cultured in DMEM containing 10% FBS. After starvation for 24 hours in serum-free medium, the cells were infected with adenovirus (multiplicity of infection of 10) encoding either Cre or GFP (Baylor Collage, TX, website: http://www.bcm.edu/vector/instock-av) for 3 days. After recovering in the standard culture medium containing 10% FBS for 2 days, these cells were subjected to different treatments.
Immunohistochemistry (IHC)
The Day 7 fracture tibial callus samples harvested from WT and COX-2−/− mice were fixed, decalcified and embedded. Paraffin sections were cut and immunostained using an avidin-biotin peroxidase detection system (Vector Labs, Burlingame, CA). After quenching endogenous peroxidase and blocking non-specific signals, the sections were incubated overnight at 4°C with the primary antibody against phospho-ATF4 (1:100, Abcam). The sections were thoroughly washed and then incubated with a biotinylated secondary antibody (Vector) for 2 hours at room temperature. Color reactions were developed with diaminobenzidine (DAB) as substrate (Vector).
Statistical Analysis
The PASW software was used in this study. After testing the homogeneity of variances, the log-transformed values were used for ANOVA. Following analysis of overall group differences on the mean values at 0.05 alpha levels were adjusted using the Turkey correction for multiple comparisons.
Results
BMP-2 increases COX-2 promoter activity and COX-2 protein expression
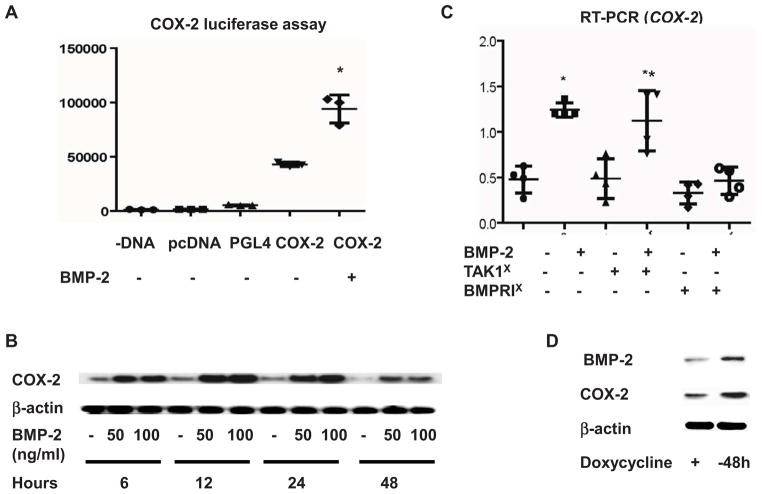
RSCs were transiently transfected with the COX-2 promoter construct for 12 hours and then treated with BMP-2 for 48 hours. Luciferase assay showed that BMP-2 treatment increased COX-2 promoter activity (Fig. 1A). A time-course study was performed to examine the effect of BMP-2 on COX-2 protein expression in PMCSC. BMP-2 treatment increased COX-2 protein levels at 6 hours and the peak effect occurred at 12 hours (Fig. 1B). The COX-2 protein levels remained elevated in the BMP-2 treated cultures over the 48 hour culture period. Subsequent experiments were designed to determine the signaling pathway through which BMP-2 stimulates COX-2 expression. PMCSC were first incubated with either BMPRI inhibitor or TAK1 inhibitor for 1 hour and then treated with BMP-2 for 12 hours. Treatment with BMP-2 in control cultures caused approximately a 3-fold up-regulation of COX-2 mRNA expression. This induction in COX-2 was abolished when the cells were pretreated with the BMPRI inhibitor. In contrast, pre-treatment of the cell cultures with TAK1 inhibitor resulted in a normal induction of COX-2 expression by BMP-2 (Fig. 1C). These findings suggest that BMP-2 regulates COX-2 expression mainly through the classic Smad signaling pathway. The effect of the endogenous BMP-2 on COX-2 expression was examined using C9 Tet-off cells. These cells have normal levels of BMP-2 expression in the presence of doxycycline, but have activation of a BMP-2 transgene upon the withdrawal of doxycycline and thus over-expression of BMP-2. BMP-2 was increased 48 hours after removal of doxycycline compared to C9 cells maintained in doxycycline containing medium. The cultures with over-expression of endogenous BMP-2 also had upregulated COX-2 expression (Fig. 1D).
Figure 1.
Luciferase assay was performed in triplicate after transient transfection of human COX-2 gene promoter reporter construct to RCS. BMP-2 treatment for 48 hours resulted in approximately 2-fold increase of COX-2 luciferase activity (1A). Western blotting analysis demonstrated that BMP-2 mediated COX-2 protein upregulation occurred at 6 hours and peaked at 12 hours after treatment (1B). PMCSC were pretreated with BMPRI or TAK1 inhibitor and followed by a BMP-2 treatment for 12 hours. RT-PCR was performed in quadruplicate and the results showed that TAK1 inhibitor did not have significant effect on the BMP-2 induced COX-2 expression. In contrast, BMPR1 inhibitor almost completely abrogated BMP-2 effect on COX-2 mRNA expression (1C). TAK1X: TAK1 inhibitor, BMPRIX inhibitor, * significant difference between lane 1 and 2 (p=0.01), **significant difference between lane 3 and 4 (p=0.03). The effect of endogenous BMP-2 on COX-2 expression was investigated in Tet-off C9 cells. Western blotting analysis showed that 48 hours after removal of doxycycline, BMP-2 expression was upregulated with a subsequent increase in COX-2 protein expression (1D).
BMP-2 regulates COX-2 expression in a classic ALK3-Smad pathway
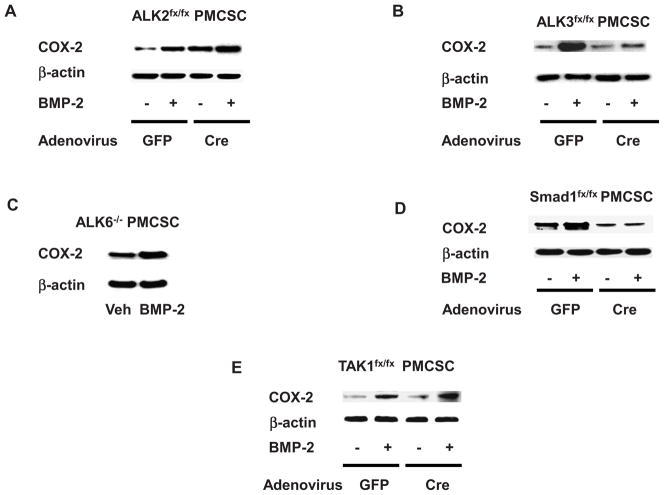
PMCSC were isolated from Alk2fx/fx, Alk3fx/fx, and Alk6−/− mice. The floxed chondrocytes were infected with Ad-GFP or Ad-Cre for 3 days to induce Cre-mediated gene recombination. Then, PMCSC were treated with BMP-2 for 48 hours and protein samples were harvested. Western blotting analysis showed that the Alk2fx/fx PMCSC infected with Ad-GFP were responsive to BMP-2 and had increased COX-2 protein expression in BMP-2 treated cultures. Deletion of the Alk2 gene in Ad-Cre treated cultures resulted in a slight increase in the basal level of COX-2 protein, and the cells continued to respond to BMP-2 with an increase in COX-2 expression (Fig. 2A). Alk3 deficiency resulted in essentially a complete loss of the BMP-2 response of COX-2 induction (Fig. 2B). Similar to the observations in Alk2 deficient mice, The Alk6 −/− PMCSC had an elevated basal level of COX-2 and continued to respond to BMP-2 with induction of COX-2 expression (Fig. 2C). Using a similar approach, we have confirmed that regulation of COX-2 by BMP-2 is mediated through BMPR/Smad signaling pathway. PMCSC isolated from Smad1fx/fx and Tak1fx/fx mice were infected with either Ad-GFP or Ad-Cre. In vitro deletion of the Smad1 gene resulted in a loss of COX-2 induction by BMP-2 (Fig. 2D). In contrast, deletion of the Tak1 gene did not alter the stimulatory effect of BMP-2 on COX-2 expression (Fig. 2E). These findings suggest that BMP-2 regulates COX-2 expression primarily through the classic ALK3-Smad1 signaling pathway.
Figure 2.
Western blot analysis revealed that the BMP-2 mediated COX-2 induction was partially preserved in Alk2fx/fx PMCSC infected with Ad-Cre (2A). In contrast, In vitro deletion of Alk3 by infecting Alk3fx/fx PMCSC with Ad-Cre resulted in a significant loss of BMP-2 responsiveness regarding COX-2 expression (2B). The ALK6−/− PMCSC showed a similar response to BMP-2 as WT cells regarding COX-2 protein induction (2C). In vitro deletion of Smad1 in PMCSC significantly abrogated the BMP-2 effect on COX-2 expression (2D). In contrast, deletion of Tak1 by infecting the Tak1fx/fx PMCSC with Ad-Cre had a minimal effect on the BMP-2 mediated COX-2 expression (2E). Veh: Vehicle control.
BMP-2 increases PGE-2 production
PGE-2 EIA revealed that BMP-2 treatment resulted in an enhanced production of PGE2 in mouse articular chondrocytes. PGE2 concentration was measured 12 hours after treatment and represented as the mean and standard deviation (BMP-2 treatment: 487.08 ± 57.99; vehicle control: 211.07 ± 31.16).
BMP-2 induces ATF4 phosphorylation through COX-2/PGE2 signaling
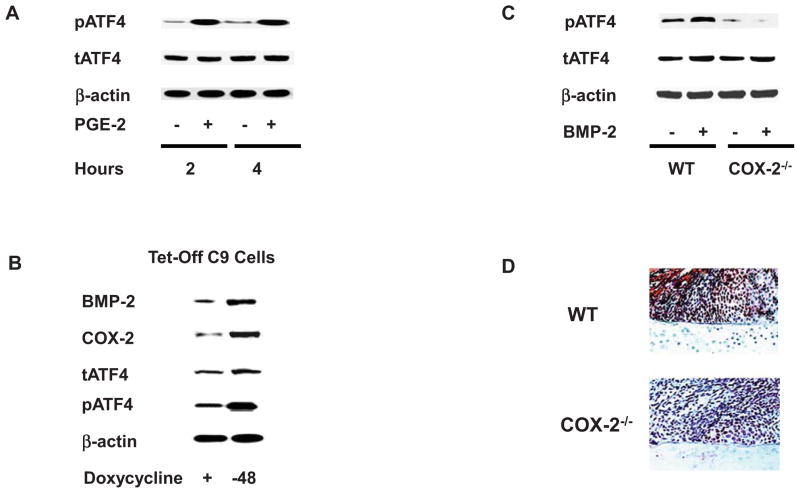
As PGE2, the major metabolite of COX-2, has been shown to stimulate ATF4 phosphorylation in prostate cancer cells24, we subsequently conducted experiments to determine whether PGE2 regulates ATF4 phosphorylation in chondrocytes. Treatment of PMCSC with PGE2 did not result in changes in Atf4 mRNA expression (data not shown). However, PGE2 strongly stimulated ATF4 phosphorylation after 2 and 4 hours of treatment (Fig. 3A). The Tet-off C9 cells were used to examine if endogenous BMP-2 regulates ATF4 phosphorylation. Doxycycline was removed from culture medium and western blotting analysis was performed with the antibodies against phospho-ATF4, total ATF4, and BMP-2. Overexpression of BMP-2 was detected 48 hours after the withdrawal of doxycycline, at which point increased expression of ATF4 protein and enhanced ATF4 phosphorylation was observed (Fig. 3B). To determine if BMP-2 regulates ATF4 phosphorylation in a COX-2 dependent mechanism, PMCSC were isolated from WT and COX-2−/− mice. While BMP-2 induced ATF4 phosphorylation in WT PMCSC, the induction of ATF4 phosphorylation was absent in COX-2−/− PMCSC (Fig. 3C). Consistent with these findings, callus samples from tibia fracture of COX-2−/− mice harvested at day 7 post-surgery showed much weaker immunostaining to phospho-ATF4 compared to the staining present in WT mice (Fig. 3D).
Figure 3.
PMCSC isolated from WT mice were treated with PGE2 (10−6 M) for 2 or 4 hours and Western blotting analysis showed that PGE2 induced ATF4 phosphorylation (3A). The effect of endogenous BMP-2 on ATF4 phosphorylation was examined in the Tef-off C9 cells. The results indicated that BMP-2 was overexpressed 48 hours after doxycycline withdrawal with a slight increase of total ATF4 protein. ATF4 phosphorylation then became evident (3B). PMCSC from both WT and COX-2−/− mice were treated with BMP-2 and western blotting analysis was performed with an antibody for phospho-ATF4. While BMP-2 induced ATF4 phosphorylation in WT PMCSC, its effect was largely lost in COX-2−/− PMCSC (3C). Day 7 fracture callus samples from COX-2−/− mice showed a much weaker immunoreactivity to phospho-ATF4 than that in WT samples (3D).
PGE2 mediates ATF4 phosphorylation through EP4 receptor
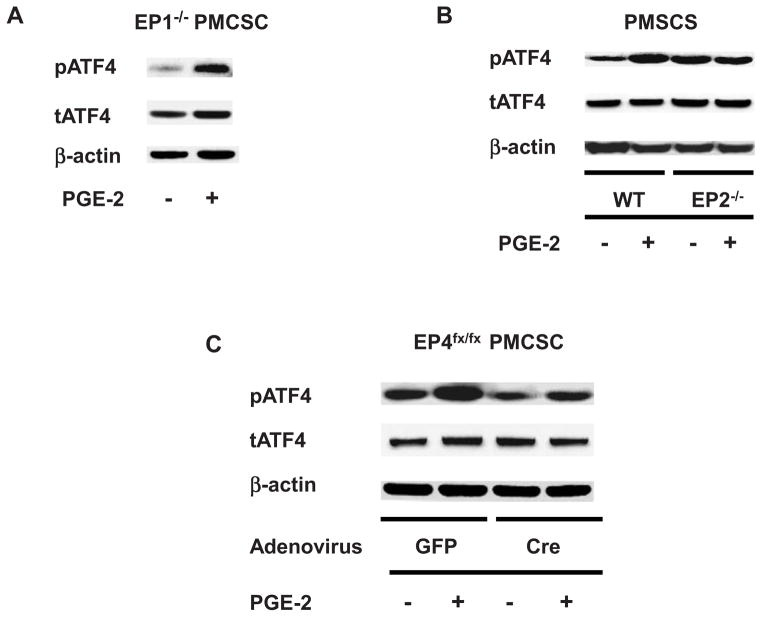
As COX-2-PGE2 mediated signaling is necessary for ATF4 phosphorylation, experiments were performed to examine the relative importance of the EP receptors in the PMCSC population. PMCSC were isolated from EP1−/−, EP2−/− and EP4fx/fx mice. PMCSC from EP4fx/fx mice underwent Cre recombination in vitro following infection with Ad-Cre. Western blot analysis was performed using cell lysates extracted from cultures with 2-hours treatment with PGE2. PGE2 stimulated ATF4 phosphorylation in EP1−/− PMCSC (Fig. 4A), indicating that the EP1 receptor is not involved in the phosphorylation ATF4. EP2−/− PMCSC had an elevated basal level of phospho-ATF4. In contrast to EP1−/− PMCSC, no increase in ATF4 phosphorylation occurred following PGE2 treatment in EP2−/− PMCSC cultures (Fig. 4B). PMCSC isolated from EP4fx/fx mice were infected with Ad-Cre or Ad-GFP. Deletion of the EP4 gene reduced the basal level of phospho-ATF4 and blocked the induction of ATF4 phosphorylation by PGE2 (Fig. 4C).
Figure 4.
The PGE2 effect on the phosphorylation of ATF4 was well-reserved in the EP1−/− PMCSC (4A). Although the basal level of phospho-ATF4 was slightly higher in the EP2−/− PMCSC, such cells lost their responsiveness to the PGE2 regarding ATF4 phosphorylation (4B). In vitro deletion of EP4 receptor by infecting EP4fx/fx PMCSC with Ad-Cre significantly abrogated the PGE2 effect on ATF4 phosphorylation (4C).
EP4, ERK1/2 and RSK2 form a protein complex
ATF4 is a member of the cAMP response element binding proteins (CREB) and is phosphorylated by PKA signaling. EP2 and EP4 both are known activators of the PKA signaling pathway. In addition, ATF4 phosphorylation is mediated by ERK1/2-RSK2 signaling. We performed further experiments to determine whether EP4 activates ERK1/2 signaling in PMCSC. Treatment of PGE2 for 30 minutes resulted in a robust stimulation of ERK1/2 phosphorylation, and EP4 agonist, less a lesser extent, also induced ERK1/2 phosphorylation (Fig. 5A). We then constructed a plasmid consisting of EP4 receptor fused to the V5 tag and transfected this plasmid into RCS cells. The cells were treated with PGE2 for 1 hour and the cell lysates were subjected to IP using either GST or V5 antibody, followed by immunoblotting with antibodies against phospho-ERK1/2 and phospho-RSK2. Our results suggested that EP4, phospho-ERK1/2 and phospho-RSK2 formed a protein-protein complex (Fig. 5B), consistent with the regulation of ATF4 phosphorylation. Such complex formation was not detectible in the lysates from untreated cells.
Figure 5.
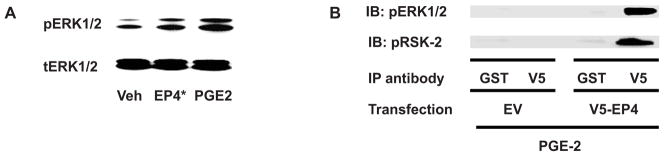
The lysates from the PMCSC treated with PGE2 or EP4 agonist were subjected to western blotting analysis with antibodies for total and phospho-ERK1/2. Both PGE2 and EP4 agonist induced the phosphorylation of ERK1/2 (5A). RCS cells were transfected with either V5-tagged EP4 receptor (V5-EP4) or the emptor vector (EV) and lysates were immunoprecipitated with the antibodies for either V5 or GST. Western blotting was performed with the antibodies against phospho-ERK1/2 and phospho-RSK-2. The result suggested that upon PGE2 treatment, EP4 formed the complex with both phospho-ERK1/2 and phospho-RSK2 (5B). Such complex formation was not observed in untreated cells. EP4*: EP4 agonist, Veh: vehicle control, EV: empty vector.
Discussion
Previous studies have established that both BMP-2 and COX-2 gene expressions are important signals in bone regeneration (4,13) The current study shows that COX-2 expression in PMCSC is regulated by BMP-2 signaling. Pharmacologic and genetic approaches were subsequently used to define the signaling pathways involved in the induction of COX-2 by BMP-2, and to examine subsequent signaling events downstream of COX-2 and its major metabolite PGE2. The experiments establish that BMP-2 induces COX-2 through a pathway that involves ALK3/Smad1 signaling with a subsequent increased PGE2 production. We further establish that induction of ATF4 phosphorylation by BMP-2 is dependent on COX-2 expression and is mediated by a PGE2 through EP2/EP4 receptor signaling events. ATF4 is a key transcription factor in chondrocytes. Since one of the mechanisms for ATF4 phosphorylation involves RSK2/ERK1/2 signaling, we examined the potential for EP4 to activate this pathway in chondrocytes. We demonstrated that PGE2 and an EP4 agonist stimulate ERK1/2 phosphorylation. Furthermore, the IP experiments established that the EP4 receptor co-precipitates with RSK2 and ERK1/2. Altogether the experiments show that BMP-2 stimulates important regulatory pathways in chondrocytes through the induction of COX-2 and subsequent downstream signaling events.
Both BMP-2 and COX-2/PGE2 are critical factors in the early responses to bone injury and are required for normal bone regeneration (4,13). BMP-2 has been shown to be expressed in early fracture repair in mesenchyme and in immature chondrocytes. Because global deletion of BMP-2 results in early embryonic death, the effects of BMP-2 deletion in bone regeneration has been examined in mice with constitutive gene deletion using a Prx1-Cre that results in loss of gene expression in limb mesenchyme derived cell populations. Fractures in mice with constitutive deletion of BMP-2 have marked inhibition of fracture healing with reduced proliferation of periosteal cells along the bone surface, minimal accumulation of callus tissues, and delayed differentiation of cartilage and bone43. In contrast, deletion of BMP-4 has minimal effects44, suggesting that BMP-2 is particularly important in bone regeneration and that other factors do not compensate for absence of BMP-2.
The COX-2 gene is also expressed early in fractures and its expression corresponds primarily with the endochondral phase of bone repair. Peak COX-2 expression has been observed at the time when an immature cartilage callus has developed repair. In situ hybridization of murine fracture callus has shown expression of COX-2 primarily in chondro-progenitors that co-express col2a1 and in immature chondrocytes, with reduced expression occurring as chondrocytes mature. Prior work in our laboratory and by others has shown that COX-2 is critical for the early events in fracture healing mature13, 45, 46. The fracture healing phenotype observed with deletion of COX-2 is similar, although not as severe, as that observed with conditional deletion of the BMP-2. COX-2 gene deletion results in reduced proliferation of the periosteal cell population, decreased accumulation of fracture callus, and delayed differentiation of bone and cartilage. The similarities, but less severe phenotype observed in COX-2 compared to BMP-2 gene deletion suggests that these signals may be linked. The current findings demonstrate that BMP-2 regulates the expression of COX-2 in chondrocytes and that COX-2 is involved in the activation of important signals in cartilage.
Classic BMP signaling starts with the ligand binding to the type II receptor with subsequent recruitment and phosphorylation of the type I receptors (ALK2, 3, 6). The activated type I BMP receptor phosphorylates the receptor associated Smad3 (Smad 1, 5, 8). In this study, we took advantage of the genetically modified mice to establish the pathway through which BMP-2 regulates COX-2 expression. We first demonstrate that the classic ALK3-Smad1 pathway is the major transducer of the BMP-2 effect on COX-2 expression. In addition to classic Smad pathway, many non-Smad pathways are activated following BMP-2 treatment. We focused on the TAK1/MAPK pathway because this pathway has been studied intensively and mounting evidence suggests a pivotal role of this pathway in chondrocyte differentiation. Our recent publication shows an interdependent relationship between TGF-β-TβRI-Smad3 signaling and TAK1-MAPK pathway11. The report from Dr. Glimcher’s group suggests an essential role of TAK1 in mediating BMP-2 signaling in chondrocytes9. We thus focused on TAK1/MAPK signaling in the BMP-2 mediated COX-2 expression in chondrocytes, and compared the effect of this non-smad pathway to the classic Smad pathway. Our results for the first time establish that classic ALK3-Smad1 pathway plays a major role in the BMP-2 effect on COX-2.
PGE2 is a major end product of COX-2 and it takes effect through its cell surface receptors from EP1 to EP4. While EP1 is a negative regulator of fracture healing21, both EP2 and EP4 signaling accelerate skeletal repair19, 20. These two receptors belong to the G-protein coupled receptor family able to activate the cAMP/PKA signaling. In addition to EP2/4-PKA signaling, other pathways may also mediate PGE2 effect. EP4 is unique because it has a long intracellular domain. Upon ligand binding, EP4 can be internalized to cytoplasm, where it binds to and interacts with different molecules transmitting different PGE2 effect. Previous studies have demonstrated that PGE2 activates ERK1/2 MAPK pathway through EP4, and ERK1/2 can phosphorylate RSK2. In cancer cells, PGE2 induces ATF4 phosphorylation. RSK2 is a direct upstream kinase for ATF4 phosphorylation, and its mutation in human results in insufficient ATF4 phosphorylation, which causes the Coffin-Lowry syndrome characterized by mental retardation and skeletal anomalies. Not surprisingly, RSK2 mutant mice exhibit a bone loss phenotype.
Based on these observations, we postulated that modulation of RSK2 activity may partially mimic BMP-2 effect on cartilage and bone. Our current study aimed to establish a molecular reaction cascade linking COX-2 upregulation, PGE2 overproduction, and ATF4 phosphorylation. Our observations made in genetically engineered mice suggest that both EP2 and EP4 receptors are involved in the PGE2 mediated ATF4 phosphorylation. We focused on EP4 because it has a unique molecular structure and its interaction with other molecules in cytoplasm may transduce different PGE2 effect. Our IP experiment indicates a complex formation of EP4-pERK1/2-pRSK2. Such complex was not detectible in the absence of PGE2. Based on the observations from others and our own, we hypothesized that PGE2 may induce ATF4 phosphorylation via the EP2/4-ERK1/2-RSK2 axis. Noteworthy, different phosphorylation sites in ATF4 molecule may have different effect on ATF4 activity/function. Thus, more studies are needed to generate more detailed information of ATF4 phosphorylation.
Although our current study did not focus on chondrocyte differentiation through these molecular cascades, accumulating data suggest that ATF4 work either alone or together with other transcription factors to regulate differentiation of osteochondral progenitor cells. Recent findings suggest that ATF4 takes its effect through Ihh, an important molecule driving chondrocyte differentiation30, 31. Prior work in our laboratory showed reduced expression of Ihh in fracture callus of COX-2−/− mice. Treatment of these mice with an EP4 agonist markedly increased Ihh expression18. In this study, we demonstrate reduced expression of phospho-ATF4 in fracture callus of COX-2−/− mice. Together, these observations further support a signaling cascade linking BMP-2-COX-2-PGE2-EP4-RSK2-ATF4, with possible involvement of Ihh. Our future work will focus on the relationship between the phosphorylation status of ATF4 and some skeletal diseases.
Conclusions
BMP-2 upregulates COX-2 expression via the classic Smad signaling pathway. In addition, BMP-2 regulates ATF4 phosphorylation in a COX-2 dependent manner. Further, BMP-2 increases PGE2 production and PGE2 induces ATF4 phosphorylation primarily through EP2/4 receptor with the downstream signaling events involving the activation of ERK1/2 and RSK2.
Acknowledgments
This study was supported an NIH grant 5R01AR048681. We thank Ming Xue for her technical assistance, and Dr. Yinglin Xia and Dr. Shaoping Wu for their assistance with statistical analysis.
Role of funding source
The sponsor had not involved in any process of the current study, e.g. the study design, the collection, analysis, and interpretation of data, manuscript preparation and journal submission.
Abbreviation
- BMP
Bone Morphogenic Protein
- COX
Cyclooxygenase
- ATF
Activating Transcription Factor
- ALK
Activin Receptor-like Kinase
- MAPK
Mitogen Activated Protein Kinase
- TGF
Transforming Growth Factor
- TAK
TGF-β Activated Kinase
- PGE
Prostaglandin E
- RSK
The 90 kDa Ribosomal S6 Kinase
- KO
Knock-out
- WT
Wild Type
- Hh
Hedgehog
- ERK
Extracellular Signal Regulated Kinase
- RCS
Rat Chondrosarcoma Cells
- PMCSC
Primary Mouse Costosternal Chondrocytes
Footnotes
Author contribution
TFL: the conception and design, acquisition, analysis and interpretation of data, manuscript preparation, and final approval of the version to be submitted. KY: the study design, acquisition and analysis of data. GY: the acquisition, analysis and interpretation of data. TS: the acquisition, analysis and interpretation of data. TM: the acquisition, analysis and interpretation of data. JHJ: the acquisition, analysis and interpretation of data, manuscript preparation. WS: the conception and design, and critical revision of the manuscript. XZ: The conception and design. GX: preparation and critical revision of the manuscript. YTK: the conception and design, and critical revision of the manuscript. DC: the conception and design, critical revision of the manuscript. RJO: obtaining funding, the conception and design, interpretation of data, manuscript preparation, and final approval of the version to be submitted.
Conflict of interest
No author in this study has received financial support of any form. None of them has a potential of conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cao X, Chen D. The BMP signaling and in vivo bone formation. Gene. 2005;357(1):1–8. doi: 10.1016/j.gene.2005.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Miyazono K, Kamiya Y, Morikawa M. Bone morphogenetic protein receptors and signal transduction. J Biochem. 2010;147(1):35–51. doi: 10.1093/jb/mvp148. [DOI] [PubMed] [Google Scholar]
- 3.Lad SP, Nathan JK, Boakye M. Trends in the use of bone morphogenetic protein as a substitute to autologous iliac crest bone grafting for spinal fusion procedures in the United States. Spine. 2011;36(4):E274–81. doi: 10.1097/BRS.0b013e3182055a6b. [DOI] [PubMed] [Google Scholar]
- 4.Even J, Eskander M, Kang J. Bone morphogenetic protein in spine surgery: current and future uses. J Am Acad Orthop Surg. 2012;20(9):547–52. doi: 10.5435/JAAOS-20-09-547. [DOI] [PubMed] [Google Scholar]
- 5.Chrastil J, Patel AA. Complications associated with posterior and transforaminal lumbar interbody fusion. J Am Acad Orthop Surg. 2012;20(5):283–91. doi: 10.5435/JAAOS-20-05-283. [DOI] [PubMed] [Google Scholar]
- 6.Argintar E, Edwards S, Delahay J. Bone morphogenetic proteins in orthopaedic trauma surgery. Injury. 2011;42(8):730–4. doi: 10.1016/j.injury.2010.11.016. [DOI] [PubMed] [Google Scholar]
- 7.Lee KB, Taghavi CE, Murray SS, Song KJ, Keorochana G, Wang JC. BMP induced inflammation. J Orthop Res. 2012;30(12):1985–94. doi: 10.1002/jor.22160. [DOI] [PubMed] [Google Scholar]
- 8.Kimura N, Matsuo R, Shibuya H, Nakashima K, Taga T. BMP-2-induced apoptosis is mediated by activation of the TAK1-p38 kinase pathway that is negatively regulated by Smad6. J Biol Chem. 2000;275(23):17647–52. doi: 10.1074/jbc.M908622199. [DOI] [PubMed] [Google Scholar]
- 9.Shim JH, Greenblatt MB, Xie M, Schneider MD, Zou W, Zhai B, et al. TAK1 is an essential regulator of BMP signalling in cartilage. EMBO J. 2009;28(14):2028–41. doi: 10.1038/emboj.2009.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gunnell LM, Jonason JH, Loiselle AE, Kohn A, Schwarz EM, Hilton MJ, et al. TAK1 regulates cartilage and joint development via the MAPK and BMP signaling pathways. J Bone Miner Res. 2010;25(8):1784–97. doi: 10.1002/jbmr.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li TF, Gao L, Sheu TJ, Sampson ER, Flick LM, Konttinen YT, et al. Aberrant hypertrophy in Smad3-deficient murine chondrocytes is rescued by restoring transforming growth factor beta-activated kinase 1/activating transcription factor 2 signaling: a potential clinical implication for osteoarthritis. Arthritis Rheum. 2010;62(8):2359–69. doi: 10.1002/art.27537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chikazu D, Li X, Kawaguchi H, Sakuma Y, Voznesensky OS, Adams DJ, et al. Bone morphogenetic protein 2 induces cyclo-oxygenase 2 in osteoblasts via a Cbfal binding site: role in effects of bone morphogenetic protein 2 in vitro and in vivo. J Bone Miner Res. 2002;17(8):1430–40. doi: 10.1359/jbmr.2002.17.8.1430. [DOI] [PubMed] [Google Scholar]
- 13.Zhang X, Schwarz EM, Young DA, Puzas JE, Rosier RN, O’Keefe RJ. Cyclooxygenase-2 regulates mesenchymal cell differentiation into the osteoblast lineage and is critically involved in bone repair. J Clin Invest. 2002;109(11):1405–15. doi: 10.1172/JCI15681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sugimoto Y, Narumiya S. Prostaglandin E receptors. J Biol Chem. 2007;282(16):11613–7. doi: 10.1074/jbc.R600038200. [DOI] [PubMed] [Google Scholar]
- 15.Li TF, Zuscik MJ, Ionescu AM, Zhang X, Rosier RN, Schwarz EM, et al. PGE2 inhibits chondrocyte differentiation through PKA and PKC signaling. Exp Cell Res. 2004;300(1):159–69. doi: 10.1016/j.yexcr.2004.06.019. [DOI] [PubMed] [Google Scholar]
- 16.Arikawa T, Omura K, Morita I. Regulation of bone morphogenetic protein-2 expression by endogenous prostaglandin E2 in human mesenchymal stem cells. J Cell Physiol. 2004;200(3):400–6. doi: 10.1002/jcp.20031. [DOI] [PubMed] [Google Scholar]
- 17.Welting TJ, Caron MM, Emans PJ, Janssen MP, Sanen K, Coolsen MM, et al. Inhibition of cyclooxygenase-2 impacts chondrocyte hypertrophic differentiation during endochondral ossification. Eur Cell Mater. 2011;22:420–36. doi: 10.22203/ecm.v022a31. [DOI] [PubMed] [Google Scholar]
- 18.Xie C, Liang B, Xue M, Lin AS, Loiselle A, Schwarz EM, et al. Rescue of impaired fracture healing in COX-2−/− mice via activation of prostaglandin E2 receptor subtype 4. Am J Pathol. 2009;175(2):772–85. doi: 10.2353/ajpath.2009.081099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Paralkar VM, Borovecki F, Ke HZ, Cameron KO, Lefker B, Grasser WA, et al. An EP2 receptor-selective prostaglandin E2 agonist induces bone healing. Proc Natl Acad Sci USA. 2003;100(11):6736–40. doi: 10.1073/pnas.1037343100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tanaka M, Sakai A, Uchida S, Tanaka S, Nagashima M, Katayama T, et al. Prostaglandin E2 receptor (EP4) selective agonist (ONO-4819. CD) accelerates bone repair of femoral cortex after drill-hole injury associated with local upregulation of bone turnover in mature rats. Bone. 2004;34(6):940–8. doi: 10.1016/j.bone.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 21.Zhang M, Ho HC, Sheu TJ, Breyer MD, Flick LM, Jonason JH, et al. EP1 (−/−) mice have enhanced osteoblast differentiation and accelerated fracture repair. J Bone Miner Res. 2011;26(4):792–802. doi: 10.1002/jbmr.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Desai S, April H, Nwaneshiudu C, Ashby B. Comparison of agonist-induced internalization of the human EP2 and EP4 prostaglandin receptors: role of the carboxyl terminus in EP4 receptor sequestration. Mol Pharmacol. 2000;58(6):1279–86. doi: 10.1124/mol.58.6.1279. [DOI] [PubMed] [Google Scholar]
- 23.Fujino H, Xu W, Regan JW. Prostaglandin E2 induced functional expression of early growth response factor-1 by EP4, but not EP2, prostanoid receptors via the phosphatidylinositol 3-kinase and extracellular signal-regulated kinases. J Biol Chem. 2003;278(14):12151–6. doi: 10.1074/jbc.M212665200. [DOI] [PubMed] [Google Scholar]
- 24.Jain S, Chakraborty G, Raja R, Kale S, Kundu GC. Prostaglandin E2 regulates tumor angiogenesis in prostate cancer. Cancer Res. 2008;68(19):7750–9. doi: 10.1158/0008-5472.CAN-07-6689. [DOI] [PubMed] [Google Scholar]
- 25.Romeo Y, Zhang X, Roux PP. Regulation and function of the RSK family of protein kinases. Biochem J. 2012;441(2):553–69. doi: 10.1042/BJ20110289. [DOI] [PubMed] [Google Scholar]
- 26.Sassone-Corsi P, Mizzen CA, Cheung P, Crosio C, Monaco L, Jacquot S, et al. Requirement of Rsk-2 for epidermal growth factor-activated phosphorylation of histone H3. Science. 1999;285(5429):886–91. doi: 10.1126/science.285.5429.886. [DOI] [PubMed] [Google Scholar]
- 27.Yang X, Matsuda K, Bialek P, Jacquot S, Masuoka HC, Schinke T, et al. ATF4 is a substrate of RSK2 and an essential regulator of osteoblast biology; implication for Coffin-Lowry Syndrome. Cell. 2004;117(3):387–98. doi: 10.1016/s0092-8674(04)00344-7. [DOI] [PubMed] [Google Scholar]
- 28.Elefteriou F, Ahn JD, Takeda S, Starbuck M, Yang X, Liu X, et al. Leptin regulation of bone resorption by the sympathetic nervous system and CART. Nature. 2005;434(7032):514–20. doi: 10.1038/nature03398. [DOI] [PubMed] [Google Scholar]
- 29.Cao H, Yu S, Yao Z, Galson DL, Jiang Y, Zhang X, et al. Activating transcription factor 4 regulates osteoclast differentiation in mice. J Clin Invest. 2010;120(8):2755–66. doi: 10.1172/JCI42106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang W, Lian N, Li L, Moss HE, Wang W, Perrien DS, et al. Atf4 regulates chondrocyte proliferation and differentiation during endochondral ossification by activating Ihh transcription. Development. 2009;136(24):4143–53. doi: 10.1242/dev.043281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang W, Lian N, Ma Y, Li L, Gallant RC, Elefteriou F, et al. Chondrocytic Atf4 regulates osteoblast differentiation and function via Ihh. Development. 2012;139(3):601–11. doi: 10.1242/dev.069575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li TF, Darowish M, Zuscik MJ, Chen D, Schwarz EM, Rosier RN, et al. Smad3-deficient chondrocytes have enhanced BMP signaling and accelerated differentiation. J Bone Miner Res. 2006;21(1):4–16. doi: 10.1359/JBMR.050911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li TF, Chen D, Wu Q, Chen M, Sheu TJ, Schwarz EM, et al. Transforming growth factor-beta stimulates cyclin D1 expression through activation of beta-catenin signaling in chondrocytes. J Biol Chem. 2006;281(30):21296–304. doi: 10.1074/jbc.M600514200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moutsatsos IK, Turgeman G, Zhou S, Kurkalli BG, Pelled G, Tzur L, et al. Exogenously regulated stem cell-mediated gene therapy for bone regeneration. Mol Ther. 2001;3(4):449–61. doi: 10.1006/mthe.2001.0291. [DOI] [PubMed] [Google Scholar]
- 35.Yi SE, Daluiski A, Pederson R, Rosen V, Lyons KM. The type I BMP receptor BMPRIB is required for chondrogenesis in the mouse limb. Development. 2000;127(3):621–30. doi: 10.1242/dev.127.3.621. [DOI] [PubMed] [Google Scholar]
- 36.Guan Y, Zhang Y, Wu J, Qi Z, Yang G, Dou D, et al. Antihypertensive effects of selective prostaglandin E2 receptor subtype 1 targeting. J Clin Invest. 2007;117(9):2496–505. doi: 10.1172/JCI29838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kennedy CR, Zhang Y, Brandon S, Guan Y, Coffee K, Funk CD, et al. Salt-sensitive hypertension and reduced fertility in mice lacking the prostaglandin EP2 receptor. Nat Med. 1999;5(2):217–20. doi: 10.1038/5583. [DOI] [PubMed] [Google Scholar]
- 38.Dudas M, Sridurongrit S, Nagy A, Okazaki K, Kaartinen V. Craniofacial defects in mice lacking BMP type I receptor Alk2 in neural crest cells. Mech Dev. 2004;121(2):173–82. doi: 10.1016/j.mod.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 39.Mishina Y, Hanks MC, Miura S, Tallquist MD, Behringer RR. Generation of Bmpr/Alk3 conditional knockout mice. Genesis. 2002;32(2):69–72. doi: 10.1002/gene.10038. [DOI] [PubMed] [Google Scholar]
- 40.Huang S, Tang B, Usoskin D, Lechleider RJ, Jamin SP, Li C, et al. Conditional knockout of the Smad1 gene. Genesis. 2002;32(2):76–9. doi: 10.1002/gene.10059. [DOI] [PubMed] [Google Scholar]
- 41.Schneider A, Guan Y, Zhang Y, Magnuson MA, Pettepher C, Loftin CD, et al. Generation of a conditional allele of the mouse prostaglandin EP4 receptor. Genesis. 2004;40(1):7–14. doi: 10.1002/gene.20048. [DOI] [PubMed] [Google Scholar]
- 42.Xie M, Zhang D, Dyck JR, Li Y, Zhang H, Morishima M, et al. A pivotal role for endogenous TGF-beta-activated kinase-1 in the LKB1/AMP-activated protein kinase energy-sensor pathway. Proc Natl Acad Sci U S A. 2006;103(46):17378–83. doi: 10.1073/pnas.0604708103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tsuji K, Bandyopadhyay A, Harfe BD, Cox K, Kakar S, Gerstenfeld L, et al. BMP-2 activity, although dispensable for bone formation, is required for the initiation of fracture healing. Nat Genet. 2006;38(12):1424–9. doi: 10.1038/ng1916. [DOI] [PubMed] [Google Scholar]
- 44.Tsuji K, Cox K, Bandyopadhyay A, Harfe BD, Tabin CJ, Rosen V. BMP4 is dispensable for skeletogenesis and fracture-healing in the limb. J Bone Joint Surg Am. 2008;90 (Suppl 1):14–8. doi: 10.2106/JBJS.G.01109. [DOI] [PubMed] [Google Scholar]
- 45.Lau KH, Kothari V, Das A, Zhang XB, Baylink DJ. Cellular and molecular mechanisms of accelerated fracture healing by COX-2 gene therapy: studies in a mouse model of multiple fractures. Bone. 2013;53:369–81. doi: 10.1016/j.bone.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 46.Naik AA, Xie C, Zuscik MJ, Kingsley P, Schwarz EM, Awad H, et al. Reduced COX-2 expression in aged mice is associated with impaired fracture healing. J Bone Miner Res. 2009;24(2):251–64. doi: 10.1359/jbmr.081002. [DOI] [PMC free article] [PubMed] [Google Scholar]