Abstract
Endothelin-1 has been implicated in atherosclerotic and ischemic heart disease. No population-based studies have examined the association of endothelin-1 with coronary heart disease (CHD). We performed a cross-sectional analysis of 961 older women and men. CHD was defined as a history of myocardial infarction, coronary surgery, angina, or major Q-wave abnormality on electrocardiogram. We examined the association of endothelin-1 with CHD after adjusting for known risk factors and atherosclerosis measures. A total of 248 women and 156 men had CHD. Median endothelin-1 levels were similar by sex and higher among those with vs. those without CHD (3.3 vs. 3.1 pg/ml, p<0.001). After adjusting for age, smoking, low density lipoproteincholesterol, high density lipoprotein-cholesterol, hypertension, diabetes, alcohol use, exercise, aspirin, cholesterol-lowering medication and hormone therapy use, endothelin-1 had a stronger association with CHD in women (OR 3.02, 1.43-6.37) than in men (OR 1.82, 0.74-4.51). Age modified the effect of endothelin-1 with CHD in men (OR 0.47 for age <75 years vs. 3.84 in men ≥75 years, p-for-interaction=0.05). Further adjustment for ankle brachial index and carotid intima media thickness did not alter these results. In conclusion, higher endothelin-1 levels are independently associated with CHD in women of all ages, and among older men only.
Keywords: coronary disease, endothelin, risk factors, epidemiology
Introduction
We report here the association of endothelin-1 with the prevalence of coronary heart disease (CHD) in a large population of community-dwelling white, middle or upper-middle class, older adults enrolled in the Rancho Bernardo Study. We were able to control for all traditional cardiac risk factors and two measures of subclinical cardiovascular disease (ankle-brachial index and carotid atherosclerosis). We also determined whether the endothelin-1 associations varied by age, sex, smoking status, hypertension, or diabetes mellitus.
Methods
We examined data from older men and women enrolled in the Rancho Bernardo Study who participated in a clinical research evaluation during 1997-1999. The Rancho Bernardo Study is a population-based study of adult residents in a southern California community. The study was approved by the University of California, San Diego institutional review committee; participants gave written informed consent.
Of 1,096 participants who attended this clinical visit, we excluded 135 (12%) who were missing endothelin-1 measurements. Those with missing endothelin-1 levels were younger (58 vs. 77 years), had less hypertension (26 vs. 51%), a lower prevalence of diabetes (4 vs. 12%), higher ankle brachial index values (1.16 vs. 1.11), and lower carotid intima media thickness measurements (0.84 vs. 0.98 cm for the common carotid artery, 1.26 vs. 1.54 cm for internal carotid artery) (p<0.05 for each comparison). They did not differ with respect to sex, body mass index, blood pressure measurements, or lipoprotein levels.
Data Collection
Age, sex, health-related behaviors (smoking, alcohol use and exercise), personal and family medical history, and medication use were assessed. Weight and height were measured using standard protocols, and body mass index was calculated (kg/m2). Waist circumference was measured with a flexible tape measure at the level of minimum abdominal circumference. Two resting systolic and diastolic blood pressure measurements were obtained for each participant using a mercury sphygmomanometer; mean blood pressure was used for analyses. Hypertension was defined as systolic blood pressure ≥140 mmHg and/or diastolic blood pressure ≥90 mmHg and/or by the use of anti-hypertensive medication.
Morning venous blood was obtained after a requested 12 hour fast, and again 2 hours after a 75-g oral glucose load. Plasma glucose was measured using a glucose oxidase method. Diabetes was classified if a participant reported a physician diagnosis of diabetes, or had a fasting plasma glucose ≥126 mg/dl or a 2-hour post-challenge glucose of ≥200 mg/dl1. Fasting plasma cholesterol and triglycerides were measured by enzymatic methods with an ABA-200 biochromatic analyzer (Abbott); high density lipoprotein-cholesterol was assayed by precipitation, and low density lipoprotein-cholesterol was calculated using the Friedewald formula2.
Atherosclerosis measures performed at this clinical visit included ankle-brachial index (ABI) and carotid intima media thickness (IMT). ABI was measured with the participant in supine position after five minutes of rest. A trained nurse used a mercury sphygmomanometer to measure the blood pressure in each arm over the brachial artery and in each leg over the posterior tibial artery. The ABI was calculated separately for each leg by taking the higher systolic blood pressure of the lower extremity and dividing by the higher systolic blood pressure in the arms. The lower of these 2 ABI measures was used. B-mode ultrasonography of the left and right common and internal carotid artery was performed, and IMT was determined, as described by O’Leary3. The common carotid artery IMT score was calculated as the mean of the left and right measurements. The internal carotid artery IMT score was determined as the mean of the six internal carotid IMT measurements. A total of 229 (24%) of participants had missing or unreadable common carotid or internal carotid IMT measurements.
In 2002, serum samples collected and frozen at -70°C during the 1997-1999 examination were mailed to the Sidney Shaw laboratory in Bern, Switzerland where endothelin-1 levels were measured using radioimmunoassay methods optimizing antibody binding parameters as previously described4. The intra- and interassay coefficients of variation were 8.6% and 13.6%, respectively.
We defined prevalent CHD based on participant reported prior myocardial infarction, or coronary artery bypass graft surgery, or percutaneous transluminal coronary angioplasty, or if there was a definite major Q-wave abnormality by Minnesota code on electrocardiogram. We also included participants who self-reported severe angina by Rose questionnaire criteria5. Of the 961 participants included in this analysis, 2 women and 4 men were missing data on self-reported CHD or electrocardiographic measures.
In preliminary analyses, age-adjusted associations of baseline covariates with prevalent CHD were assessed using chi-square, t-, and Wilcoxon tests as appropriate. To meet linearity assumptions, we log transformed high density lipoprotein-cholesterol, ABI, common carotid artery IMT, internal carotid artery IMT, and endothelin-1 values. We also assessed associations of endothelin-1 with CHD using quartiles of endothelin-1. To assess whether the correlation between endothelin-1 and other cardiovascular variables varied by sex, we computed male and female partial Spearman correlations adjusting for age.
To examine adjusted associations of endothelin-1, we used multivariable logistic regression for prevalent CHD. In nested sequences of models using log-transformed endothelin-1, we adjusted first for age. We then added all potential covariates of CHD and confounders, obtaining an estimate of the overall independent effect of endothelin-1. Next, we added measures of subclinical atherosclerosis, ABI and carotid IMT measures to our final model. This final model was considered exploratory in nature due to a large proportion of participants (24%) who were missing carotid measurements.
Lastly, we checked whether the sex-specific associations of endothelin-1 with prevalent CHD differed by age group (≥75 years vs. <75 years), smoking status (current vs. former or never smoker), hypertension, diabetes, or current hormone use status in women. We used SAS Version 8.2 (SAS Institute, Cary NC) for our analyses.
Results
Approximately 42% of men and 43% of women had CHD. Table 1 displays participant characteristics separately by sex and CHD status. Neither measure of adiposity varied by CHD status in either sex. Both men and women with CHD were about 4 years older than men and women without. As expected, traditional cardiac risk factors were more prevalent among those with CHD than those without. There were small but statistically significant differences in ABI and carotid IMT between those with and without CHD. Median endothelin-1 levels did not differ significantly by sex (3.25 pg/ml in men and 3.18 pg/ml in women, p= 0.09) and were significantly higher among men and women with CHD than those without. While levels of endothelin-1 were lower among women using hormone therapy compared with those not taking hormones (3.10 vs. 3.30 pg/ml, p=0.001), endothelin-1 levels were significantly higher in women using hormones who had CHD compared with those taking hormones but without evidence of CHD (3.20 vs. 2.97 pg/ml, p=0.02).
Table 1.
Characteristics of Rancho Bernardo Study participants, 1997-1999
| Variable | WOMEN | MEN | ||||
|---|---|---|---|---|---|---|
| CHD (n=248) | No CHD (n=334) | p † | CHD (n=156) | No CHD (n=217) | p † | |
| Age (years) | 79 ± 8 | 75 ± 8 | <0.001 | 78 ± 8 | 74 ± 8 | <0.001 |
| Current smoker | 7 (3%) | 16 (5%) | 0.23 | 3 (2%) | 12 (5%) | 0.08 |
| Alcohol ≥ 1 drink/day | 201 (81%) | 283 (85%) | 0.28 | 135 (86%) | 198 (91%) | 0.20 |
| Exercise 3 times/week | 163 (66%) | 244 (73%) | 0.07 | 125 (80%) | 171 (79%) | 0.66 |
| Body mass index (kg/m2) | 24.5 ± 4.2 | 25.1 ± 4.3 | 0.15 | 26.1 ± 3.4 | 26.6 ± 3.8 | 0.26 |
| Waist circumference (cm) | 80 ± 11 | 80 ± 12 | 0.58 | 95 ± 10 | 95 ± 10 | 0.95 |
| Systolic blood pressure (mmHg) | 143 ± 20 | 136 ± 21 | <0.001 | 141 ± 21 | 133 ± 19 | <0.001 |
| Diastolic blood pressure (mmHg) | 71 ± 9 | 73 ± 11 | 0.03 | 74 ± 9 | 76 ± 9 | 0.04 |
| Hypertension | 148 (60%) | 149 (45%) | <0.001 | 88 (56%) | 101 (46%) | 0.06 |
| Diabetes | 30 (12%) | 23 (7%) | 0.03 | 35 (22%) | 32 (15%) | 0.06 |
| Low density lipoprotein (mg/dL) | 122 ± 33 | 123 ± 34 | 0.75 | 113 ± 33 | 122 ± 28 | 0.01 |
| High density lipoprotein* (mg/dL) | 66 (24) | 65 (24) | 0.93 | 46 (19) | 50 (15) | 0.07 |
| Aspirin use | 92 (37%) | 79 (24%) | <0.001 | 93 (60%) | 95 (44%) | 0.002 |
| Cholesterol lowering therapy | 50 (20%) | 46 (14%) | 0.04 | 48 (31%) | 43 (20%) | 0.01 |
| Current hormone therapy use | 108 (44%) | 159 (48%) | 0.33 | -- | -- | -- |
| Ankle-Brachial index* | 1.14 (0.05) | 1.14 (0.05) | 0.02 | 1.13 (0.06) | 1.14 (0.03) | <0.001 |
| Common carotid artery intima media thickness* (mm) | 0.96 (0.20) | 0.91 (0.21) | <0.001 | 1.00 (0.26) | 0.94 (0.23) | 0.01 |
| Internal carotid artery intima media thickness* (mm) | 1.48 (1.16) | 1.08 (0.93) | <0.001 | 1.68 (1.25) | 1.43 (1.02) | 0.12 |
| Endothelin-1* (pg/ml) | 3.30 (1.05) | 3.07 (0.80) | <0.001 | 3.36 (0.88) | 3.2 (0.93) | 0.007 |
Values represent n (%) or mean ± SD unless otherwise footnoted.
value represents median (IQR)
p-values by chi-square, t-test, or Wilcoxon test as appropriate
Of all covariates included in our analyses, endothelin-1 was most strongly correlated with age in both men (r=0.30, p<0.001) and women (r=0.24, p<0.001). After age-adjustment, sex-specific endothelin-1 levels were correlated with waist circumference, systolic blood pressure, fasting plasma glucose, ABI, and common and internal carotid IMT (Table 2). These correlations were stronger in men than in women.
Table 2.
Age-adjusted correlations (p-values) of cardiovascular risk factors with log endothelin-1
| Variable | Overall (n=961) | Women (n=584) | Men (n=377) |
|---|---|---|---|
| Body mass index (kg/m2) | 0.02 (0.58) | 0.04 (0.37) | −0.08 (0.15) |
| Waist circumference (cm) | 0.07 (0.03) | 0.07 (0.09) | −0.03 (0.57) |
| Systolic blood pressure (mmHg) | 0.11 (<0.001) | 0.05 (0.22) | 0.21 (<0.001) |
| Diastolic blood pressure (mmHg) | 0.05 (0.15) | 0.02 (0.71) | 0.07 (0.19) |
| Low density lipoprotein-cholesterol (mg/dL) | −0.02 (0.47) | −0.02 (0.65) | −0.02 (0.70) |
| High density lipoprotein-cholesterol (mg/dL) | −0.04 (0.20) | −0.04 (0.38) | 0.03 (0.53) |
| Fasting plasma glucose (mg/dL) | 0.08 (0.01) | 0.08 (0.07) | 0.07 (0.19) |
| Ankle-Brachial Index | −0.11 (<0.001) | −0.08 (0.08) | −0.20 (<0.001) |
| Common carotid artery intima media thickness (mm) | 0.09 (0.01) | 0.05 (0.31) | 0.12 (0.03) |
| Internal carotid artery intima media thickness (mm) | 0.11 (0.002) | 0.09 (0.07) | 0.13 (0.03) |
We created a multivariate model for all participants that combined all variables related to CHD whether or not they were related to endothelin-1. In this model adjusted for age, sex, current smoking, alcohol use, exercise, hypertension, diabetes mellitus, low density lipoproteincholesterol, high density lipoprotein-cholesterol, current aspirin use, cholesterol medication use, and hormone therapy use, log endothelin-1 was associated with a increased odds of CHD (OR 2.47 95% CI 1.37 – 4.42). The association of endothelin-1 with CHD appeared stronger among women (OR 3.02, 1.43-6.37) than in men (OR 1.82, 0.74-4.51), but the sex difference was not statistically significant (p-for-interaction 0.39). Because the magnitude of the effect was substantially different between sexes despite the lack of significant interaction, subsequent analyses were sex-specific.
Among men, adjusting for age alone attenuated the effect of endothelin-1 with CHD (unadjusted OR 3.11, 95% CI 1.32-7.34 to age-adjusted OR 1.83, 95% CI 0.75-4.46). Sequential adjustment for traditional cardiac risk factors and potential confounders did not materially alter this result (OR 1.77, 95% CI 0.68-4.62), and further addition of the ankle brachial index and carotid IMT measures did not change the association (Table 3). In women, the age-adjusted estimate for log endothelin-1 with CHD was OR 2.56, 95% CI 1.25-5.22. In a model adjusted for age, smoking, alcohol use, exercise, hypertension, diabetes, low density lipoprotein-cholesterol, high density lipoprotein-cholesterol, aspirin use, cholesterol-lowering medication use, and hormone therapy, log endothelin-1 was still associated with significantly increased odds of CHD (OR 3.11, 95% CI 1.46-6.65). Addition of ankle brachial index and carotid IMT measures strengthened this association (OR 4.94, 95% CI 1.84-13.26).
Table 3.
Sex-specific odds ratios for risk factors and coronary heart disease in multivariate analyses*
| Variable | WOMEN OR (95% CI) | MEN OR (95% CI) |
|---|---|---|
| Age, per year | 1.04 (1.00-1.07) | 1.06 (1.02-1.10) |
| Current smoker | 0.67 (0.18-2.49) | 0.23 (0.04-1.44) |
| Alcohol use | 0.74 (0.40-1.38) | 0.56 (0.23-1.31) |
| Hypertension | 1.47 (0.92-2.35) | 1.23 (0.70-2.16) |
| Diabetes mellitus | 1.17 (0.53-2.57) | 1.19 (0.56-2.54) |
| Low density lipoprotein-cholesterol (per mg/dL) | 1.00 (0.99-1.01) | 0.99 (0.98-0.99) |
| Log High density lipoprotein cholesterol | 1.59 (0.63-4.06) | 0.45 (0.15-1.36) |
| Current aspirin use | 2.48 (1.53-4.02) | 1.61 (0.92-2.79) |
| Current statin use | 1.70 (0.94-3.09) | 1.94 (0.99-3.80) |
| Current hormone therapy use | 1.05 (0.64-1.71) | -- |
| Log ankle brachial index | 0.21 (0.02-2.95) | <0.001 (<0.001-0.04) |
| Log common carotid artery intima media thickness | 1.37 (0.37-5.02) | 0.72 (0.18-2.90) |
| Log internal carotid artery intima media thickness | 1.48 (0.85-2.58) | 0.68 (0.36-1.31) |
| Log endothelin-1 | 4.94 (1.84-13.26) | 1.67 (0.53-5.29) |
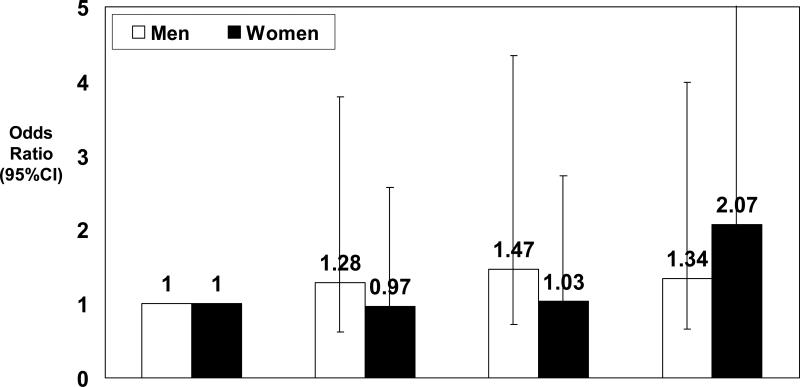
We examined the association between quartiles of endothelin-1 and CHD in models adjusted for all the above traditional risk factors and confounders. In women, a higher odds of CHD was apparent only in the fourth quartile for endothelin-1 (>3.7 pg/ml: OR 2.07, 95% CI 1.23-3.50), whereas the highest quartile of endothelin-1 was not associated with CHD among men (> 3.7 pg/ml: OR 1.34, 95% CI 0.68-2.64). (Figure 1)
Figure 1.
Association between increasing quartile of endothelin-1 with CHD among men and women after adjusting for age, smoking, alcohol use, hypertension, diabetes, low density lipoproteincholesterol, high density lipoprotein-cholesterol, aspirin use, cholesterol-lowering medication use, and hormone therapy use.
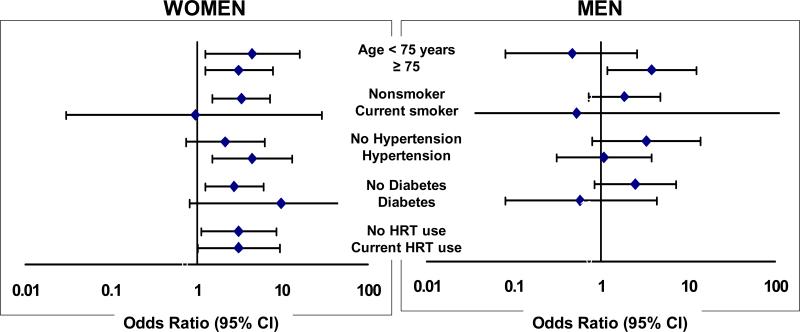
Next, we checked whether the sex-specific association of endothelin-1 with CHD varied in selected subgroups. (Figure 2) The association of endothelin-1 with CHD did not differ by smoking status, hypertension, diabetes, or use of female hormone therapy. However, in men the association of endothelin-1 with CHD varied by age group: in men aged 75 years or older endothelin-1 was associated higher odds of CHD (OR 3.84, 1.18-12.47) compared with no association in younger men (OR 0.47, 0.08-2.62 for age <75; p-for-interaction=0.05). Indeed, in multivariate models that were performed separately by age, endothelin-1 was associated with a similarly increased risk in older men and women (OR 3.29, 1.04 – 10.44 in men and OR 2.43, 0.96-6.12 in women, p-for-interaction=0.68). In men and women less than 75 years of age, the association between endothelin-1 and CHD was stronger in women (OR 4.88, 1.28 – 18.52) than men (OR 0.40, 0.07 – 2.28, p-for-interaction=0.02).
Figure 2.
The association of log endothelin-1 with CHD within select subgroups is shown separately by sex. The models are adjusted for age, current smoking, alcohol use, physical activity, hypertension, diabetes, low density lipoprotein-cholesterol, high density lipoprotein-cholesterol, aspirin use, cholesterol lowering medication use, and hormone therapy use, excluding the variable for the subgroup of interest. P-for-interaction >0.20 for all subgroups except for age in men (p=0.05).
Discussion
In this cohort, endothelin-1 levels were higher in men and women with prevalent CHD. Among multiple CHD risk factors, endothelin-1 was significantly correlated only with age, systolic blood pressure, and fasting plasma glucose levels. Endothelin-1 was also associated with 2 measures of atherosclerosis, ABI and carotid intima media thickness. This cross-sectional endothelin-1 association with CHD was independent of traditional cardiac risk factors and ABI and IMT, 2 measures of atherosclerosis. This association was present in younger and older women and only in older men (age ≥ 75 years), and did not vary by smoking, hypertension, diabetes status or postmenopausal hormone therapy.
In this older cohort, the median levels of endothelin-1 were similar in both sexes. Sex differences in circulating endothelin-1 levels have not been studied in large populations and results have been inconsistent in prior reports from small studies6,7. In a study of endothelin-1 levels in healthy men, premenopausal women, pregnant women, and transsexual patients receiving sex-hormone treatment, investigators found that endothelin-1 levels were higher in men than in women and that exogenous testosterone raised levels and estradiol lowered levels of endothelin-16. Although we did not find sex differences in absolute levels of circulating endothelin-1, we did find that endothelin-1 was not associated with CHD in men <75 years. This observation coupled with the sex-hormone associations described above raises the possibility that endogenous sex hormones modulate the activity of endothelin-1. Younger men with higher levels of bioavailable testosterone may have different biological mechanisms for CHD that do not involve endothelin-1 than older men and women. While women taking hormone replacement therapy had lower median endothelin-1 levels than women not using hormones, the associations between endothelin-1 and CHD were similar for hormone users and non-users, making it unlikely that estrogen plays a major role in modulating the endothelin-1 association with heart disease.
Potential explanations for our finding that endothelin-1 was not associated with CHD in younger men only could reflect a distinct biologic mechanism for protection against endothelin-1 in younger men, or this finding could be due to chance, bias, or confounding, Since we did not find a statistically significant interaction by sex in our analysis of the entire cohort, our positive results observed only after age stratification may be due to chance alone. The significant interaction by sex in our analysis of men and women < 75 years of age (OR 4.88, 1.28 – 18.52 in women vs. OR 0.40, 0.07 – 2.28 in men, p-for-interaction=0.02) decreases the likelihood of chance explaining this sex difference. Since the Rancho Bernardo Study population included older adults with a mean age of 77 years, there is a real possibility of survivor bias. However, survivor bias would not explain why we found no significant association between endothelin-1 and CHD in younger men. Finally, the possibility of unmeasured confounding always exists in observational studies, although we did adjust our models for all traditional cardiac risk factors and other potential confounders including cholesterol-lowering medications and aspirin.
Although we were able to evaluate the association of endothelin-1 with CHD independent of traditional risk factors and 2 subclinical measures of atherosclerosis, the nature of cross-sectional data limits interpretation, and we are therefore are unable to determine whether endothelin-1 is a marker of existing disease or is implicated in the pathogenesis of CHD. We also did not have measures of inflammation available at this clinical visit to determine whether the association of endothelin-1 with CHD was independent of or modified by the level of inflammation. CRP has been correlated with endothelin-18, and found to enhance endothelial lectin-like oxidized LDL receptor-1 that plays a pivotal role in oxLDL in promoting endothelial dysfunction9. Lastly, the endothelin-1 assay used had moderately high coefficients of variation which may bias our results towards the null.
Acknowledgments
Grant Support: Alka M. Kanaya was funded in part by K12 AR47659, and P30–AG15272 under the Resource Centers for Minority Aging Research program by the National Institute on Aging, the National Institute of Nursing Research, and The National Center on Minority Health and Health Disparities, National Institutes of Health. Dr. Barrett-Connor is Principal Investigator for the Rancho Bernardo study, funded by the National Institutes on Aging grant NIA 5R01 AG07181 and the National Institute of Diabetes and Digestive and Kidney Diseases grant 5R01 DK31801.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hirai Y, Adachi H, Fujiura Y, Hiratsuka A, Enomoto M, Imaizumi T. Plasma endothelin-1 level is related to renal function and smoking status but not to blood pressure: an epidemiological study. J Hypertens. 2004;22:713–718. doi: 10.1097/00004872-200404000-00013. [DOI] [PubMed] [Google Scholar]
- 2.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 3.O'Leary DH, Polak JF, Kronmal RA, Manolio TA, Burke GL, Wolfson SK., Jr. Carotid-artery intima and media thickness as a risk factor for myocardial infarction and stroke in older adults. Cardiovascular Health Study Collaborative Research Group. N Engl J Med. 1999;340:14–22. doi: 10.1056/NEJM199901073400103. [DOI] [PubMed] [Google Scholar]
- 4.Shaw SG, Schmid M, Casty A. Critical factors in the radioimmunoassay of endothelin-1, endothelin-3, and big endothelin-1 in human plasma. Anal Biochem. 2000;278:143–149. doi: 10.1006/abio.1999.4451. [DOI] [PubMed] [Google Scholar]
- 5.Rose G, Hamilton PS, Keen H, Reid DD, McCartney P, Jarrett RJ. Myocardial ischaemia, risk factors and death from coronary heart-disease. Lancet. 1977;1:105–109. doi: 10.1016/s0140-6736(77)91701-9. [DOI] [PubMed] [Google Scholar]
- 6.Polderman KH, Stehouwer CD, van Kamp GJ, Dekker GA, Verheugt FW, Gooren LJ. Influence of sex hormones on plasma endothelin levels. Ann Intern Med. 1993;118:429–32. doi: 10.7326/0003-4819-118-6-199303150-00006. [DOI] [PubMed] [Google Scholar]
- 7.Evans RR, Phillips BG, Singh G, Bauman JL, Gulati A. Racial and gender differences in endothelin-1. Am J Cardiol. 1996;78:486–488. doi: 10.1016/s0002-9149(96)00344-x. [DOI] [PubMed] [Google Scholar]
- 8.Jialal I, Devaraj S, Venugopal SK. C-reactive protein: risk marker or mediator in atherothrombosis? Hypertension. 2004;44:6–11. doi: 10.1161/01.HYP.0000130484.20501.df. [DOI] [PubMed] [Google Scholar]
- 9.Li L, Roumeliotis N, Sawamura T, Renier G. C-reactive protein enhances LOX-1 expression in human aortic endothelial cells: relevance of LOX-1 to C-reactive protein-induced endothelial dysfunction. Circ Res. 2004;95:877–883. doi: 10.1161/01.RES.0000147309.54227.42. [DOI] [PubMed] [Google Scholar]