Abstract
Kinetoplast DNA of Crithidia fasciculata is a network containing several thousand topologically interlocked DNA minicircles. In the prereplicative Form I network, each of the 5000 minicircles is intact and linked to an average of three neighbors (i.e. the minicircle valence is 3). Replication involves the release of minicircles from the interior of the network, the synthesis of nicked or gapped progeny minicircles and the attachment of the progeny to the network periphery. The ultimate result is a Form II network of 10,000 nicked or gapped minicircles. Our measurements of minicircle valence and density, and the network's surface area, revealed striking changes in network topology during replication. During the S phase, the peripheral newly replicated minicircles have a density twice that of minicircles in Form I networks, which suggests that the valence might be as high as 6. Most of the holes in the central region that occur from the removal of intact minicircles are repaired so that the central density and valence remain the same, as in prereplicative networks. When minicircle replication is complete at the end of the S phase, the isolated network has the surface area of a prereplicative network, despite having twice the number of minicircles. During the G2 phase, the Form II network undergoes a remodeling in which the area doubles and the valence is reduced to 3. Finally, the interruptions in the minicircles are repaired and the double-sized network splits in two.
Full text
PDF
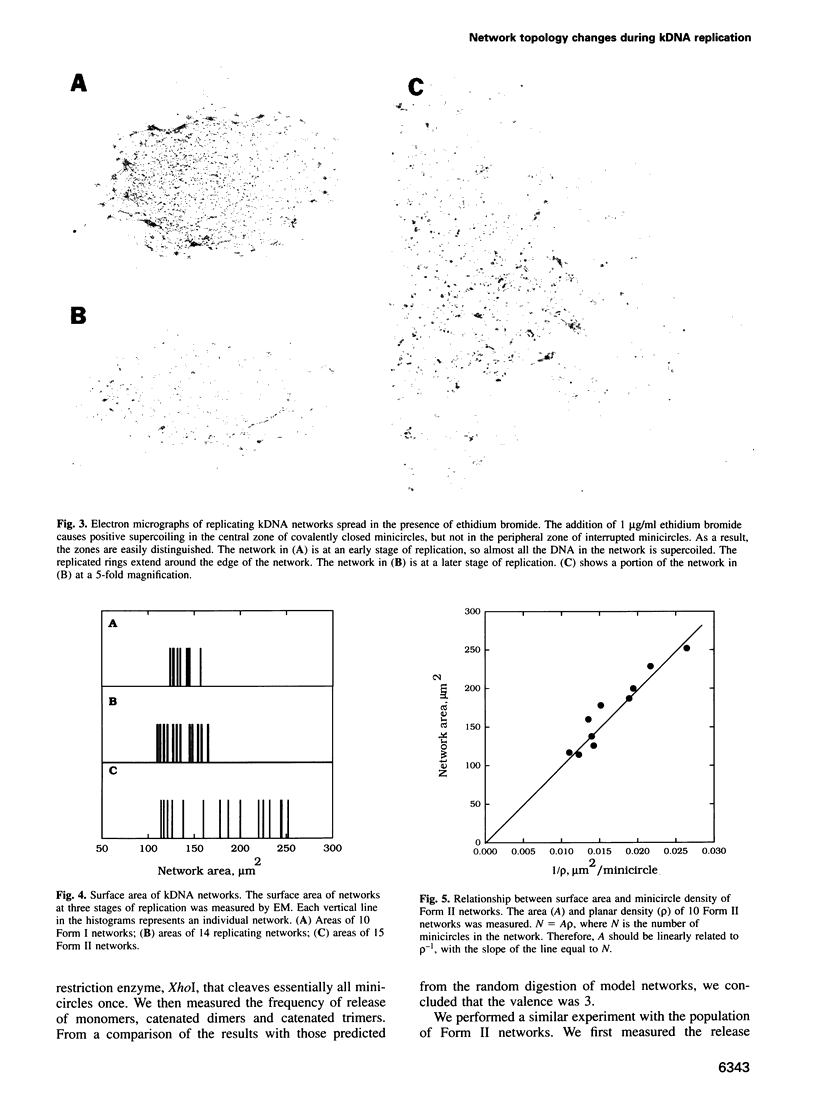
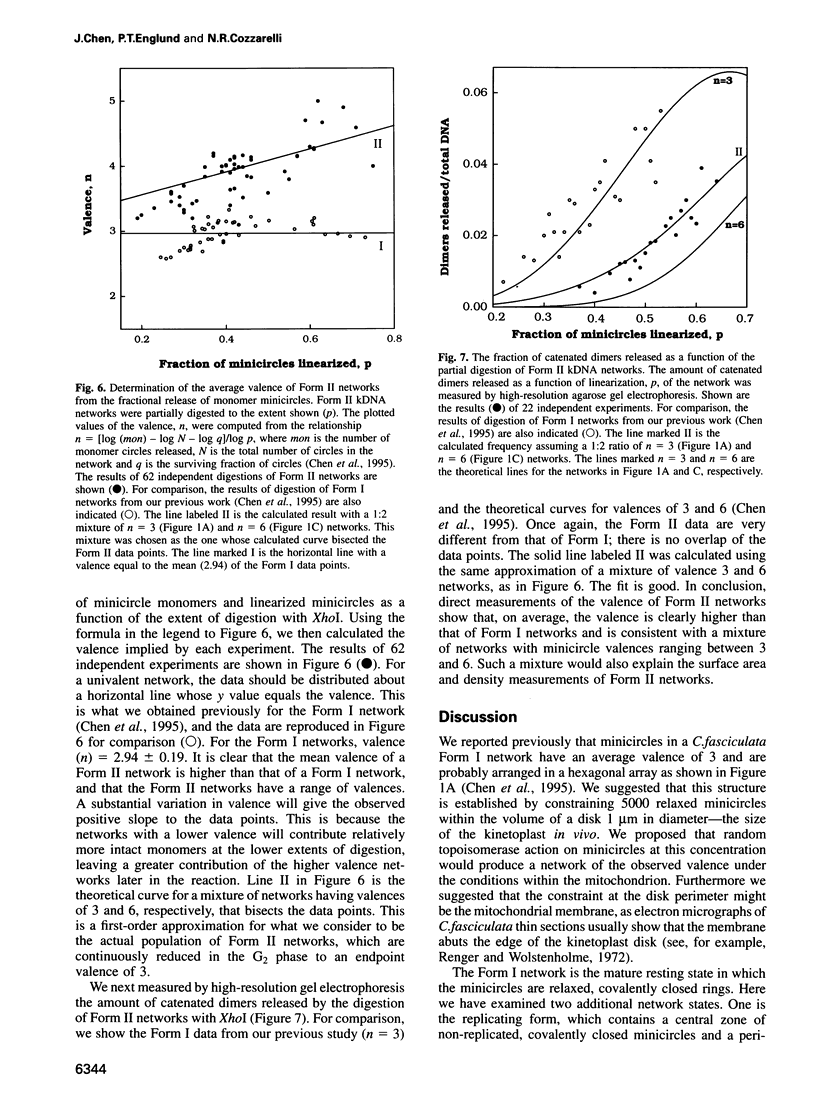
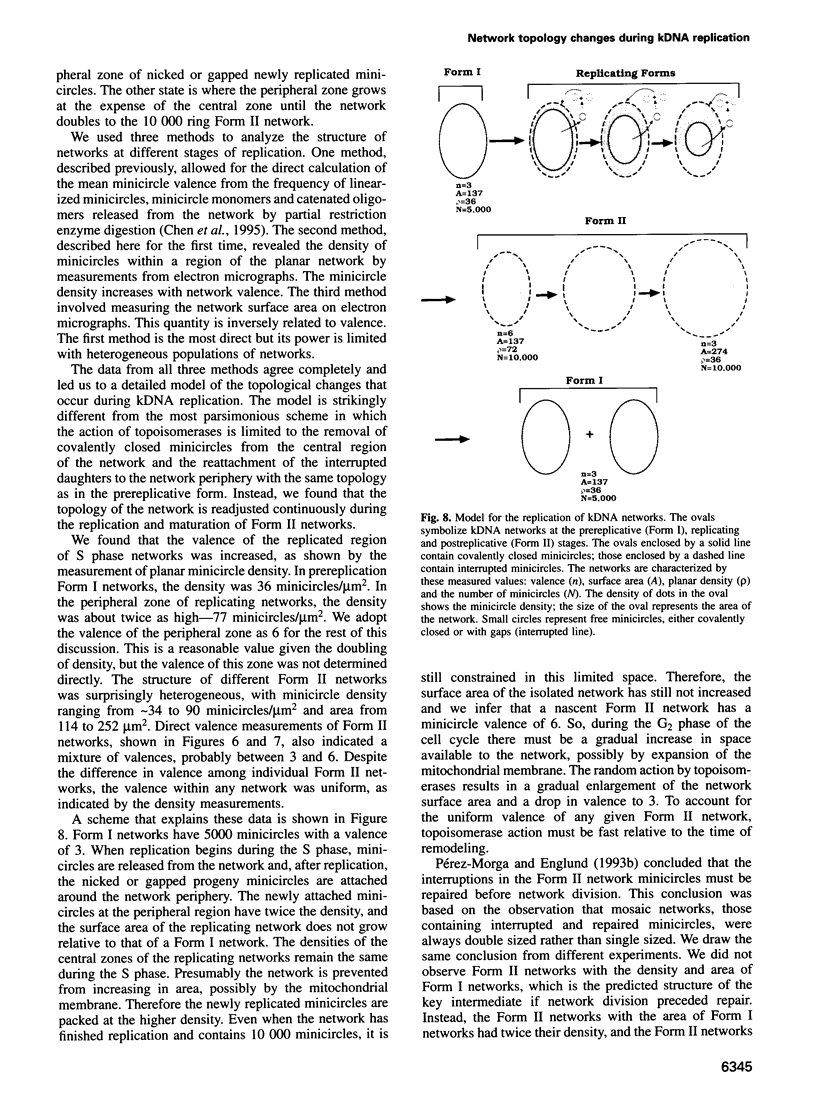
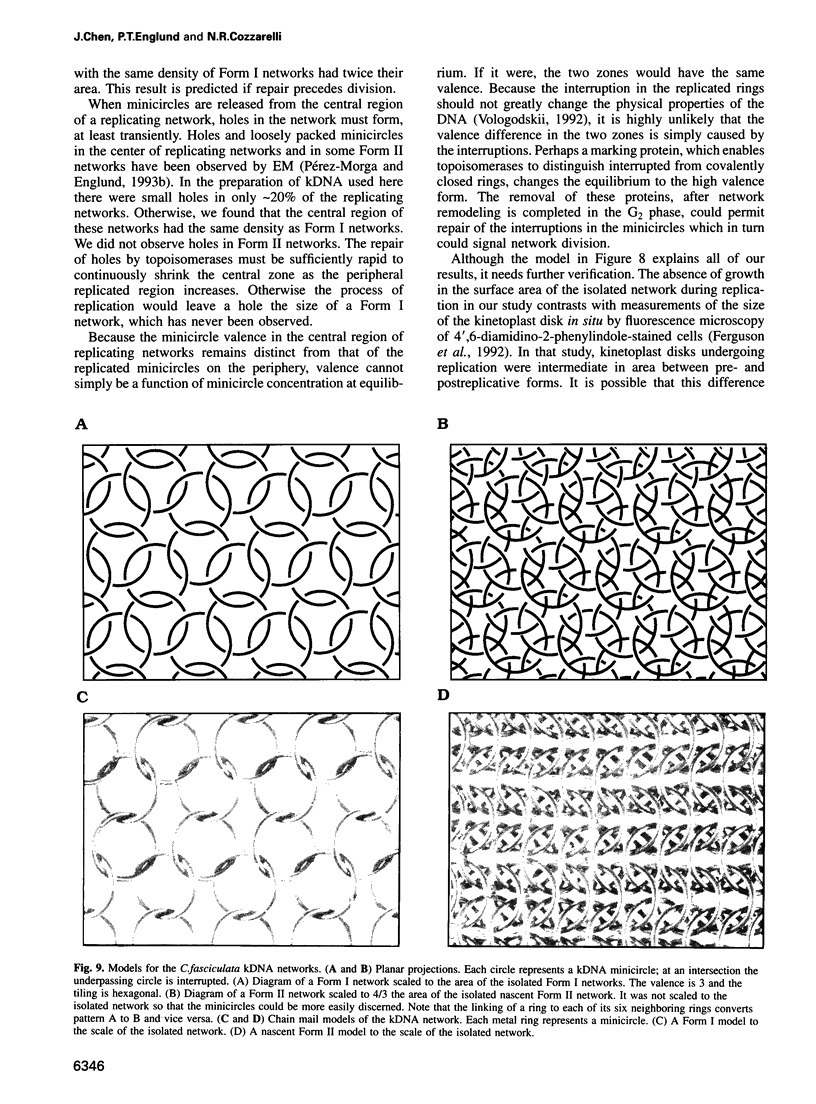

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benne R. RNA editing in trypanosomes. Eur J Biochem. 1994 Apr 1;221(1):9–23. doi: 10.1111/j.1432-1033.1994.tb18710.x. [DOI] [PubMed] [Google Scholar]
- Chen J., Rauch C. A., White J. H., Englund P. T., Cozzarelli N. R. The topology of the kinetoplast DNA network. Cell. 1995 Jan 13;80(1):61–69. doi: 10.1016/0092-8674(95)90451-4. [DOI] [PubMed] [Google Scholar]
- Cozzarelli N. R. The structure and function of DNA supercoiling and catenanes. Harvey Lect. 1991 1992;87:35–55. [PubMed] [Google Scholar]
- Englund P. T. The replication of kinetoplast DNA networks in Crithidia fasciculata. Cell. 1978 May;14(1):157–168. doi: 10.1016/0092-8674(78)90310-0. [DOI] [PubMed] [Google Scholar]
- Ferguson M. L., Torri A. F., Pérez-Morga D., Ward D. C., Englund P. T. Kinetoplast DNA replication: mechanistic differences between Trypanosoma brucei and Crithidia fasciculata. J Cell Biol. 1994 Aug;126(3):631–639. doi: 10.1083/jcb.126.3.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson M., Torri A. F., Ward D. C., Englund P. T. In situ hybridization to the Crithidia fasciculata kinetoplast reveals two antipodal sites involved in kinetoplast DNA replication. Cell. 1992 Aug 21;70(4):621–629. doi: 10.1016/0092-8674(92)90431-b. [DOI] [PubMed] [Google Scholar]
- Hajduk S. L., Harris M. E., Pollard V. W. RNA editing in kinetoplastid mitochondria. FASEB J. 1993 Jan;7(1):54–63. doi: 10.1096/fasebj.7.1.8422975. [DOI] [PubMed] [Google Scholar]
- Hajduk S. L., Klein V. A., Englund P. T. Replication of kinetoplast DNA maxicircles. Cell. 1984 Feb;36(2):483–492. doi: 10.1016/0092-8674(84)90241-1. [DOI] [PubMed] [Google Scholar]
- Hoeijmakers J. H., Weijers P. J. The segregation of kinetoplast DNA networks in Trypanosoma brucei. Plasmid. 1980 Jul;4(1):97–116. doi: 10.1016/0147-619x(80)90086-4. [DOI] [PubMed] [Google Scholar]
- Melendy T., Sheline C., Ray D. S. Localization of a type II DNA topoisomerase to two sites at the periphery of the kinetoplast DNA of Crithidia fasciculata. Cell. 1988 Dec 23;55(6):1083–1088. doi: 10.1016/0092-8674(88)90252-8. [DOI] [PubMed] [Google Scholar]
- Pérez-Morga D. L., Englund P. T. The attachment of minicircles to kinetoplast DNA networks during replication. Cell. 1993 Aug 27;74(4):703–711. doi: 10.1016/0092-8674(93)90517-t. [DOI] [PubMed] [Google Scholar]
- Pérez-Morga D., Englund P. T. The structure of replicating kinetoplast DNA networks. J Cell Biol. 1993 Dec;123(5):1069–1079. doi: 10.1083/jcb.123.5.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauch C. A., Perez-Morga D., Cozzarelli N. R., Englund P. T. The absence of supercoiling in kinetoplast DNA minicircles. EMBO J. 1993 Feb;12(2):403–411. doi: 10.1002/j.1460-2075.1993.tb05672.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray D. S. Kinetoplast DNA minicircles: high-copy-number mitochondrial plasmids. Plasmid. 1987 May;17(3):177–190. doi: 10.1016/0147-619x(87)90026-6. [DOI] [PubMed] [Google Scholar]
- Renger H. C., Wolstenholme D. R. The form and structure of kinetoplast DNA of Crithidia. J Cell Biol. 1972 Aug;54(2):346–364. doi: 10.1083/jcb.54.2.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro T. A., Englund P. T. The structure and replication of kinetoplast DNA. Annu Rev Microbiol. 1995;49:117–143. doi: 10.1146/annurev.mi.49.100195.001001. [DOI] [PubMed] [Google Scholar]
- Shlomai J. The assembly of kinetoplast DNA. Parasitol Today. 1994 Sep;10(9):341–346. doi: 10.1016/0169-4758(94)90244-5. [DOI] [PubMed] [Google Scholar]
- Simpson A. M., Simpson L. Pulse-labeling of kinetoplast DNA: localization of 2 sites of synthesis within the networks and kinetics of labeling of closed minicircles. J Protozool. 1976 Nov;23(4):583–587. doi: 10.1111/j.1550-7408.1976.tb03846.x. [DOI] [PubMed] [Google Scholar]
- Simpson L., Maslov D. A. RNA editing and the evolution of parasites. Science. 1994 Jun 24;264(5167):1870–1871. doi: 10.1126/science.8009214. [DOI] [PubMed] [Google Scholar]
- Simpson L. The mitochondrial genome of kinetoplastid protozoa: genomic organization, transcription, replication, and evolution. Annu Rev Microbiol. 1987;41:363–382. doi: 10.1146/annurev.mi.41.100187.002051. [DOI] [PubMed] [Google Scholar]
- Stuart K., Feagin J. E. Mitochondrial DNA of kinetoplastids. Int Rev Cytol. 1992;141:65–88. doi: 10.1016/s0074-7696(08)62063-x. [DOI] [PubMed] [Google Scholar]
- Sugisaki H., Ray D. S. DNA sequence of Crithidia fasciculata kinetoplast minicircles. Mol Biochem Parasitol. 1987 Apr;23(3):253–263. doi: 10.1016/0166-6851(87)90032-6. [DOI] [PubMed] [Google Scholar]
- Sundin O., Varshavsky A. Terminal stages of SV40 DNA replication proceed via multiply intertwined catenated dimers. Cell. 1980 Aug;21(1):103–114. doi: 10.1016/0092-8674(80)90118-x. [DOI] [PubMed] [Google Scholar]