Abstract
Flow cytometric (FC) enumeration of abnormal plasma cells (APCs) for diagnosis and prognostication of plasma cell dyscrasias (PCD) is challenging. We studied antigen expression in normal plasma cells (NPC) (N=34) and APC in a series of unselected PCD (N=59). NPC subpopulations often demonstrated CD19(−), CD20(+), CD45(−) or dim and CD56(+), an immunophenotype observed in PCD. However abnormal CD81 was only observed in APCs (APC detection sensitivity 95%; specificity 100%). We evaluated differences in antigen expression patterns among MGUS (N=14), SMM (N=35) and MM (N=10), finding the combination of CD45 and CD56 helpful in differentiating MGUS from SMM and MM (p=0.0002).
Keywords: monoclonal gammopathy of uncertain significance, smoldering multiple myeloma, multiple myeloma, normal plasma cell immunophenotype, CD81
Introduction
Plasma cell dyscrasias (PCD) are a heterogeneous group of disorders with a spectrum of clinical presentations from asymptomatic monoclonal gammopathy of uncertain significance (MGUS) and smoldering multiple myeloma (SMM) to the symptomatic multiple myeloma (MM) with its high morbidity and diminished quality of life. Although diagnosis is based upon serum M spike, extent of plasma cell (PC) involvement of the BM and presence of end organ damage, flow cytometric (FC) characterization and quantification of abnormal plasma cells (APCs) has been used in the diagnosis, prognostication and monitoring of PCD (1–5). FC determination of the percentage of total bone marrow PCs that are phenotypically aberrant (% APC) versus the percent of total PCs with a normal immunophenotype (NPC) allows risk stratification of progression of MGUS and SMM patients to overt MM and can be used for prognostication in MM (6–12). FC studies can also help predict response to autologous stem cell transplantation (12–14).
All of the FC studies in PCD rely on an accurate differentiation of APC from NPC. FC analysis provides a consistent and stringent method for differentiating APCs from their normal counterpart based on the expression of a variety of surface antigens and demonstration of intracellular light chain restriction. Aberrant surface antigen expression can be demonstrated in the majority of MGUS, SMM and MM cases, with typical aberrant antigen profiles including expression of CD56, CD20 or CD117, diminished CD38, and complete absence of CD19 and/or CD45 (6,15,16). The simultaneous analysis of CD38, CD56, CD19, CD45 and CD138 expression has been reported to detect a significant APC population in most patients with myeloma, even in the absence of intracytoplasmic immunoglobulin detection (4,5,15–17). The precise enumeration of APCs in the presence of NPCs, however, remains challenging, especially in specimens with low numbers of PCs (e.g. MGUS). Panels with greater specificity for differentiating APCs from NPCs are needed to be able to accurately assess prognosis and minimal residual disease. CD81 is strongly expressed on the surface of NPCs but MM cell lines are shown to underexpress CD81, making it a potentially useful marker in differentiating APCs from NPCs (18). Furthermore, levels of CD81 expression may correlate with prognosis in myeloma (19). However, little information is available regarding its expression in the APCs of MM, SMM and MGUS patients. In the current study we evaluated the expression of CD19, CD20, CD38, CD45, CD56, CD81 and CD138 as well as intracellular immunoglobulin light chain (kappa/lambda) in APCs and NPCs in patients with MM, SMM and MGUS to determine their sensitivity and specificity in detecting PCD. Furthermore, we evaluated the role of these markers in an attempt to differentiate the early stage disease of MGUS from SMM and MM.
Materials and Methods
Patients
Bone marrow aspirates from 59 untreated patients with PCD (14 MGUS, 35 SMM, and 10 MM) were submitted for diagnostic FC evaluation as part of screening for a prospective clinical natural history study (NCT01109407) of myeloma precursor disease and in some cases to determine eligibility for several research protocols. In addition bone marrow was evaluated in 5 patients referred to our institution to rule out MGUS, primarily due to anemia and reported mildly increased plasma cells in bone marrow evaluations performed at other institutions. These 5 patients were determined to have no evidence of a PCD (including absence of M spike, less than 5% PC on BM biopsy, polyclonal PCs by IHC and FC) or other neoplastic process and normal bone marrow specimen. All patients signed institutional review board approved informed consent to be screened for eligibility. The diagnosis of MGUS, SMM and MM was based on bone marrow PC infiltration, serum M-protein levels and radiological and clinical findings as per the criteria defined by International Myeloma Working Group (20). The patterns of antigen expression of all markers were evaluated in the APCs (as defined by monoclonal light chain expression) in 59 cases and in NPCs from 5 normal bone marrows. 29 cases with PCD had greater than 5% NPCs (as defined by polyclonal intracellular light chain expression) (14 MGUS, 14 SMM, and 1 MM) and the patterns of antigen expression were evaluated in these normal polyclonal plasma cells. Therefore normal plasma cells were evaluated in 34 bone marrows (5 normal and 29 cases with co-existent PCD).
Flow Cytometric Immunophenotyping
Specimens were processed within 12 h of collection by washing twice with phosphate buffered saline (PBS) to remove cytophilic antibodies and staining for 30 min at room temperature with a panel of antibodies against surface and intracellular kappa and lambda (Polyclonal Rabbit Anti-Human, F(ab′)2, (Dako) and surface CD19APC (clone SJ25C1), CD20APC-H7 (clone L27), CD38v450 (clone HB7), CD45v500 (clone HI30), CD56PE-Cy7 (clone NCAM16.2), CD81FITC (clone JS-81), and CD138 PerCP-Cy5.5 (clone MI15) (BD Biosciences, San Jose, CA) as previously described (21). For intracellular light chain evaluation, cells were stained with antibodies against surface antigens and then permeabilized with Fix and Perm (Invitrogen, Frederick, MD) followed by incubation with anti-kappa and anti-lambda antibodies or isotype controls. All cells were fixed in 1.0% paraformaldehyde and stored at 4°C for up to 12 h before acquisition. Specimens were acquired using an 8-color multiparametric approach on a 3-laser FACS Canto II (BD Biosciences, San Jose, CA) with DiVa 6.1.1 software and analyzed by FCS Express 3 software (DeNovo Software, Los Angeles, CA). Approximately 2 – 5 million cells were acquired for each cocktail.
Definition of APC and NPC
PCs were identified by gating on cells with CD138 positivity and strong CD38 expression. CD45 and light scatter properties were also examined to exclude debris, doublets and lymphocytes. NPCs were defined based upon polyclonal intracellular light chain expression. APCs were defined based upon a cluster (antigen expression profile) of plasma cells with monoclonal intracellular light chain expression. The pattern of CD81 and CD19, CD20, CD45, CD56 and intracellular light chain expression was studied in NPCs and APCs. The levels of antigen expression were categorized as follows: negative (N) when there was no staining demonstrated; partial positive (PP) when there was partial expression; weak positive (WP) when all the cells had dim expression; moderate positive (MP) when the cells had moderate expression; and strong positive (SP) when the cells had strong expression.
Bone Marrow Biopsy
Sections from decalcified B5 fixed core biopsies were stained with CD138 antibody using a Dako (Carpinteria, CA) or a Ventana (Tucson, AZ) autostaining system. The percent plasma cells was estimated based upon CD138 expression by immunohistochemistry.
Biochemistry
Serum M-protein quantitation was performed by electrophoresis with the Sebia Hydrasys® (Norcross, GA 30071) automated system utilizing protein Hydragels™. The protein gels were then scanned using a HYRYS™ densitometer and the serum proteins including M-proteins calculated using Sebia Phoresis™ software. Identification of the M-proteins was carried out on a Sebia Hydrasys® system utilizing IF Hydragels™. Serum free light chain (sFLC) analysis was performed with a Siemens BN™ II nephelometric system (Siemens USA) coupled with Freelite® reagents from The Binding Site Group Ltd. (Birmingham, UK).
Results
Patient Characteristics
We studied 59 specimens of PCD (14 MGUS, 35 SMM, and 10 MM) and 5 normal bone marrow specimens with no evidence of PCD. Clinical and laboratory details are listed in Table 1. The mean age of patient groups were as follows: MGUS 59.4 years; SMM 59.6 years; and MM 57.9 years). The male to female ratio differed little between the MGUS, SMM and MM categories, except for a slight male predominance (20/15) in the SMM category only. The mean percent of BM plasma cells and M protein were lowest in MGUS (7.8%, 0.6 g/dL), intermediate in SMM (20.9%, 1,95 g/dL) and highest in MM (40.8%, 2.7 g/dL).
Table 1.
Characteristics of Control, MGUS, SMM and MM Patients.
| Control | MGUS | SMM | MM | |
|---|---|---|---|---|
|
| ||||
| Age: Mean/Median (Range) | 62.5/64 (35–83) | 59.4/62 (37–70) | 59.6/57 (48–76) | 57.9/57.5 (42–74) |
| N=5 | N=14 | N= 35 | N= 10 | |
|
| ||||
| Sex | ||||
| Male | 2 | 7 | 20 | 5 |
| Female | 3 | 7 | 15 | 5 |
| N=5 | N= 14 | N= 35 | N=10 | |
|
| ||||
| Morphology: % PC | 3.8/5 (1–5) | 7.8/8 (5–9) | 20.9/13 (10–75) | 40.8/32.5 (10–95) |
| Mean/Median (Range) | N=5 | N=14 | N= 34 | N=10 |
|
| ||||
| FC: % of cells that are PC | 0.03/0.02 (0.01–0.07) | 0.15/0.07 (0.01–0.6) | 1.8/0.5 (0.01–19) | 10.5/0.35 (0.2–38) |
| Mean/Median (Range) | N=5 | N=14 | N= 35 | N=10 |
|
| ||||
| % APC by FC | 0/0 (0-0) | 64.5/72 (15–92) | 92/97.5 (31–100) | 97.7/99 (88–100) |
| Mean/Median (Range) | N=5 | N=14 | N= 35 | N=10 |
|
| ||||
| M protein g/dL | 0 0/0 |
0.7/0.6 (0.2–1.5) | 1.7/1.5 (0.1–5.4) | 3.4/2.2 (1.2–8.6) |
| Mean/Median (Range) | N=5 | N=12* | N= 29^ | N=7@ |
|
| ||||
| Type of Serum Ig | ||||
| IgG kappa | 7 | 15 | 4 | |
| IgG lambda | 3 | 8 | 1 | |
| IgA kappa | - | 3 | 1 | |
| IgA lambda | 2 | 5 | - | |
| IgM kappa | 1 | 1 | - | |
| IgM lambda | - | - | - | |
| Serum light chains only | ||||
| Kappa | 1 | 1 | 2 | |
| Lambda | - | 2 | 1 | |
N= number of patients. BM: bone marrow. Morphology: % BM replacement by PC: percent of bone marrow cellularity occupied by plasma cells based on CD138 immunoperoxidase of bone marrow core biopsy. FC: % of cells that are PC: flow cytometry enumeration of plasma cells in second aspirate as determined by CD38 and CD138 co-expression and exclusion of debris. % APC by FC: percent of the plasma cells that have an abnormal immunophenotype as determined by flow cytometry.
MGUS M- Protein: M-Protein was too low to quantify in one patient and one patient had light chain only.
SMM M-Protein: M-protein could not be quantified due to beta region migration in three patients and three patients had light chain only.
MM M-Protein: Three patients had light chain only.
Immunophenotypic Profile of NPC Versus APC
The antigenic expression patterns for CD19, CD20, CD45, CD56 and CD81 were studied in APCs (59 BM specimens) and NPCs (34 BM specimens) (Tables 2 and 3, Figure 1). This combination of antibodies allowed ready identification of APCs in all cases based on an aberrant pattern of expression of CD19, CD20, CD45, CD56 and CD81. Loss of CD19 was found to be a highly sensitive marker in detecting APCs; it was negative in all APCs (100%) but was also negative in a small subset of polyclonal NPCs in 17 of 34 specimens (50%) (Table 3). In these 17 cases a mean 24% of normal plasma cells were CD19 negative (S.D.: 15, range 7–50) (Table 3, Figure 1). Aberrant expression of CD56 was present in the APCs in 41 of 59 specimens (69%) but also found to be expressed by a small subset of polyclonal NPCs in 9 of 34 NPC specimens (mean 10% of NPCs CD56+, S.D.:5, range: 5–22%). CD45 expression was abnormal (negative, partial or aberrantly weak positive) in 53 (90%) of 59 APC cases. Intermediate (normal) CD45 expression was observed in the APCs in only 6 of 59 cases (10%). Interestingly, there was dim CD45 expression in a subset of polyclonal NPCs in 14 of 34 NPC specimens (mean 86% of NPCs CD45 dim, S.D.:15, range: 63–100%). Aberrant CD20 expression was found in APCs in 20 of 59 specimens (34%) but was also found to be positive in a subset of polyclonal NPCs in 3 of 34 NPC specimens (mean 21% of NPCs CD20+, S.D.:14, range: 13–37%). On evaluation of CD81 expression, we found that it was homogeneously brightly expressed by NPCs in all specimens (100%) (Table 3, Figure 1). However CD81 was negative or dim in the APCs in 56 of 59 cases (95%). No NPC populations with dim or negative CD81 were identified.
Table 2.
Pattern of Antigen Expression by Abnormal Plasma Cells (APCs) in MGUS, SMM and MM specimens.
| Markers | CD19 | CD20 | CD45 | CD56 | CD81 | |
|---|---|---|---|---|---|---|
|
| ||||||
| Diagnosis (cases) | Patterns of expression | No (%) | No (%) | No (%) | No (%) | No (%) |
| MGUS (14) | ||||||
| N | 14 (100) | 7 (50) | 1 (7) | 7 (50) | 7 (50) | |
| PP | 6 (43) | 1 (7) | 6 (43) | 2 (14) | ||
| WP | 10 (71) | 3 (21) | ||||
| MP | 1 (7) | 2 (14) | ||||
| SP | 1 (7) | 2 (14) | ||||
|
| ||||||
| SMM (35) | ||||||
| N | 35 (100) | 24 (69) | 7 (20) | 9 (26) | 17 (49) | |
| PP | 10 (29) | 6 (17) | 2 (6) | 5 (14) | ||
| WP | 18(51) | 12 (34) | ||||
| MP | 1 (3) | 4 (11) | ||||
| SP | 24 (69) | 1 (3) | ||||
|
| ||||||
| MM (10) | ||||||
| N | 10 (100) | 8 (80) | 6 (60) | 2 (20) | 5 (50) | |
| PP | 1 (10) | 1 (10) | ||||
| WP | 4 (40) | 5 (50) | ||||
| MP | 1 (10) | |||||
| SP | 7 (70) | |||||
N: negative, no expression demonstrated. PP: partial expression. WP: weak positive, all the cells had dim expression. MP: moderate expression by all cells. SP:strong positive expression by all cells.
Table 3.
Atypical Antigen Expression Patterns in NPC.
| Total number of cases | CD19 negative | CD45 dim or negative* | CD56 positive^ | CD20 Positive^ | CD81 dim or negative* | CD19negative and CD81 dim or negative* | CD19 negative, CD45 dim/negative and CD56 positive | |
|---|---|---|---|---|---|---|---|---|
| MGUS | 14 | 14 | 12 | 7 | 7 | 12 | 12 | 6 |
| SMM | 35 | 35 | 31 | 26 | 11 | 34 | 34 | 23 |
| MM | 10 | 10 | 10 | 8 | 2 | 10 | 10 | 8 |
| Total | 59 | 59 (100%) | 53 (90%) | 41 (69%) | 20 (34%) | 56 (95%) | 56 (95%) | 37 (63%) |
| NPCs with Subset Demonstrating Antigen Expression Similar to APCs | ||||||||
| Total number of cases | CD19 negative | CD45 dim or negative | CD56 positive | CD20 positive | CD81 dim or negative | CD19 negative and CD81 dim or negative | CD19 negative, CD45 dim/negative and/or CD56 positive | |
| 34 | 17 (50%) | 14 (41%) | 9 (26%) | 3 (9%) | 0 (0%) | 0 (0%) | 4 (12%) | |
Includes partial negative;
includes partial positive.
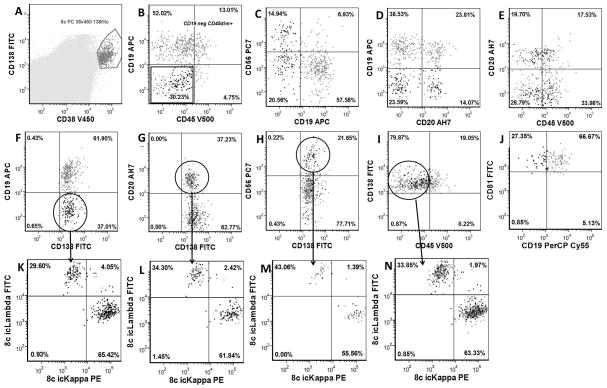
Figure 1.
Immunophenotypic Analysis of Normal Plasma Cells: A: Plasma cell analysis gate based upon bright CD38 and CD138 expression and utilized in B through J. B: CD45 vs. CD19 demonstrates plasma cell population that is CD19 and CD45 negative. C: CD19 vs. CD56 demonstrates a plasma cell population that is CD19 negative and CD56 positive. D: CD20 vs. CD19 demonstrates a plasma cell population that is CD20 positive and CD19 negative. E: CD45 vs. CD20 demonstrates plasma cell population that is CD20 positive and CD45 negative. F: CD19 negative plasma cell gate (in oval) demonstrated in K. G: CD20 positive plasma cell gate (in oval) demonstrated in L. H: CD56 positive plasma cell gate (in oval) demonstrated in M. I: CD45 negative plasma cell gate (in oval) demonstrated in N. J: CD19 vrs CD81: All plasma cells are CD81 bright. K: kappa vs. lambda in CD19 negative normal plasma cells are polyclonal. L: kappa vs. lambda in CD20 positive normal plasma cells are polyclonal. M: kappa vs. lambda in CD56 positive normal plasma cells are polyclonal. N: kappa vs. lambda in CD45 negative normal plasma cells are polyclonal.
Since exact enumeration of APCs is utilized in determining risk in plasma cell dyscrasias, the goal of FC is highly sensitive detection of APCs but strict specificity to allow absolute differentiation of APCs from residual NPCs. Therefore the sensitivity and specificity of detection of APCs by the CD19, CD20, CD45, CD56 and CD81 expression pattern was examined (Table 3). We found that CD19 had the highest sensitivity at 100% but only 50% specificity since a subpopulation of polyclonal CD19 negative PCs was observed in 50% of the NPC cases studied. However; CD81 was the most specific (100%) and second most sensitive (95%) marker for the detection of APC. CD45 had high sensitivity at 90% but only 59% specificity of APC detection. CD20 had high specificity (91%) of APC detection but only 34% sensitivity, while CD56 had modest sensitivity (69%) and specificity (74%). Using a combination of CD19 negative, CD45 dim or negative and CD56 positive, 63% of APC populations fulfilled all three of these characteristics. However, small sub-populations with at least one of these characteristics were observed in 23 out of 34 (68%) NPC cases and in 4 cases all three characteristics (CD19 negative, CD45 dim or negative, CD56 positive) were observed in NPCs (Table 3). In contrast, using a combination of CD19 negative and CD81 dim or negative, 95% sensitivity was attained and since all NPCs had normal bright CD81 this combination also gave 100% specificity (Table 3).
Differences in Immunophenotypic Profile between MGUS, SMM and MM
We evaluated the differences in the pattern of antigen expression among MGUS, SMM and MM (Table 3) and found no significant difference in CD19, CD20 or CD81 expression. Total CD45 negativity (i.e. no CD45 positive or partial positive clonal APC) is associated with MM (60%) compared to SMM a (20%) and MGUS (7%) (p=0.020). Identification of a CD56 negative clonal APC population (total negative or partial negative) is associated with MGUS (93% of cases) compared to SMM (31% of cases) or MM (30%) (P=0.0015). The combination of CD56 negativity (total or partial) and CD45 positivity (weak, partial, or moderate positivity) correlates highly with MGUS (86%) compared to SMM or MM (22%)(p=0.0002).
Discussion
FC enumeration of abnormal plasma cells in the bone marrow of patients with plasma cell dyscrasias has demonstrated prognostic value, assisting in risk stratification of progression of MGUS and SMM patient’s to overt MM (8,22) and in predicting progression-free and overall survival (6,7,9). In addition, FC detection of circulating APCs in myeloma patients is a predictor of overall survival (11,23). Furthermore FC remission is shown to be a relevant prognostic factor for patients undergoing autologous stem cell transplantation (12,13). Since exact enumeration of APCs is clearly of clinical value in MGUS, SMM and MM, differentiating APCs from residual NPCs is crucial. The classic APC immunophenotype has been described as CD38 bright positive (dimmer than normal plasma cells), CD138 positive, CD19 negative, CD45 dim to negative and CD56 positive with CD20 positivity in select cases (24). Although the majority of NPCs are CD19 positive, CD45 moderate, and CD56 negative, small sub-populations of normal, non-neoplastic plasma cells with an immunophenotype similar to APCs are detected. CD19 and CD56 have been reported to have variable expression in NPCs of healthy individuals, with 20–40% of NPCs CD19 negative and 10–47% CD56 positive and in fact, CD19 negative CD56 positive PCs are believed to represent long lived terminal stage NPCs (1,15,25,26). CD45 dim plasma cells can be observed in normal donor bone marrow (25) and its expression has been reported to decline during NPC differentiation (27,28). The presence of CD19 negative, CD45 dim and CD56 positive plasma cells in sub-populations of NPCs may lead to a miscalculation of the percent of APCs in precursor monoclonal gammopathies, which would affect risk assessment (26). It also may have significant impact in determination of MRD in MM.
Our studies have confirmed the low specificity of CD19, CD45 and CD56 in accurate delineation of APCs from NPCs. Although we found that CD19 had the highest sensitivity (100%) of APC detection it only had 50% specificity. CD45 had moderately high sensitivity at 90% but only 59% specificity of APC detection. CD20 had high specificity (91%) of APC detection but only 34% sensitivity while CD56 had modest sensitivity (69%) and specificity (74%). Introduction of CD81 to the plasma cell dyscrasia panel increased the accuracy of APC enumeration. All NPCs show strong expression of CD81, in contrast, 95% of APCs demonstrated either negative or abnormally weak expression. CD81 was the most specific (100%) and second most sensitive (95%) marker for the detection of APC.
We examined multiparametric approaches to differentiate APCs from residual NPCs in all cases to improve upon accuracy of enumeration using assessment of a combination of antigens. Using the combination of aberrant CD19 negative, CD45 dim or negative and/or CD56 positive, 100% of APC populations had one of these three aberrant characteristics, indicating 100% sensitivity. However, small sub-populations with at least one of these aberrant characteristics were observed in 23 out of 34 (68%) NPC cases (Table 3) leading to a specificity of 32%. In contrast, 100% sensitivity and 100% specificity was attained using a combination of CD19 negative and CD81 dim or negative, as all APCs were CD19 negative and all NPCs had normal bright CD81 (Table 3). Although multiparametric evaluation of CD19, CD45, and CD56 allow extreme sensitivity in detecting the presence of a plasma cell dyscrasia, the addition of CD81 provides vastly improved specificity which should be useful in APC enumeration for prognostication in MGUS, SMM and MM and detection of minimal residual disease.
Reliance upon morphological estimate of percent of plasma cells in bone marrow as diagnostic criteria for plasma cell myeloma can lead to significant discrepancies. In addition MM is differentiated from SMM based upon detection of end organ disease, which is highly dependent upon the sensitivity of Imaging and other methodologies. As these factors may introduce variability into clinical trials involving these entities, we studied the role of immunophenotypic markers in distinguishing between MGUS, SMM and MM. There were no significant associations of CD19, CD20, and CD81 with diagnosis. There was, however, a strong correlation between identification of a CD56 negative APC population with the diagnosis of MGUS (p=0.0015) compared to SMM and MM. In addition identification of a completely CD45 negative APC correlated with the diagnosis of MM (p=0.020) compared to SMM and MGUS. The combination of CD56 negativity and CD45 positivity in an APC population correlates highly with MGUS compared to SMM or MM (p=0.0002). The observation of a PCD with APC that are homogeneously CD56 positive and CD45 negative should prompt careful review of the evaluation for evidence of end organ disease.
In conclusion, our study indicates that CD81 is a very sensitive and highly specific marker to differentiate APCs from NPCs and in combination with CD19, CD45 and CD56 allows precise quantification of APCs in the presence of a significant NPC population, an important independent variable of prognostication. This combination should also be very helpful in determination of minimal residual disease in MM post therapy as well as post-transplant. In addition, the combination of CD45 and CD56 may assist in differentiating MGUS from SMM and MM. Our results indicate that the inclusion of CD19, CD20, CD38, CD45, CD56, CD81, and CD138 in flow cytometric panels may be extremely useful in evaluation of plasma cell dyscrasia. However, the study size is small and confirmation by larger independent studies is indicated.
Acknowledgments
This research was funded by the intramural program of the National Cancer Institute, National Institutes of Health. The authors wish to acknowledge the technical assistance of Catharine McCoy, Gregory Jasper and Linda Weaver.
Footnotes
Drs. Stetler-Stevenson and Tembhare conceived of and designed the study.
Drs. Yuan, Korde, Manasanch, Zuchlinsky, Calvo, Kurlander, Bhutani, Tageja, Maric, Mulquin, Roschewski, Kwok, Braylan, Landgren, Stetler-Stevenson and Tembhare acquired and interpreted data.
Drs. Venzon and Liewehr analyzed the data.
Drs. Stetler-Stevenson, Tembhare, Braylan and Landgren wrote the manuscript.
The authors do not have any conflict of interest to report.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Paiva B, Almeida J, Perez-Andres M, Mateo G, Lopez A, Rasillo A, Vidriales MB, Lopez-Berges MC, Miguel JF, Orfao A. Utility of flow cytometry immunophenotyping in multiple myeloma and other clonal plasma cell-related disorders. Cytometry B Clin Cytom. 2010;78:239–52. doi: 10.1002/cyto.b.20512. [DOI] [PubMed] [Google Scholar]
- 2.Seegmiller AC, Xu Y, McKenna RW, Karandikar NJ. Immunophenotypic differentiation between neoplastic plasma cells in mature B-cell lymphoma vs plasma cell myeloma. Am J Clin Pathol. 2007;127:176–81. doi: 10.1309/5EL22BH45PHUPM8P. [DOI] [PubMed] [Google Scholar]
- 3.Yuan CM, Stetler-Stevenson M. Role of flow cytometry of peripheral blood and bone marrow aspirates in early myeloma. Semin Hematol. 2011;48:32–8. doi: 10.1053/j.seminhematol.2010.11.005. [DOI] [PubMed] [Google Scholar]
- 4.Witzig TEKT, Stenson M, et al. Syndecan-1 expression on malignant cells from the blood and marrow of patients with plasma cell proliferative disorders and B-cell chronic lymphocytic leukemia. Leukemia and Lymphoma. 1998;31:167–175. doi: 10.3109/10428199809057596. [DOI] [PubMed] [Google Scholar]
- 5.Konoplev S, Medeiros LJ, Bueso-Ramos CE, Jorgensen JL, Lin P. Immunophenotypic profile of lymphoplasmacytic lymphoma/Waldenstrom macroglobulinemia. Am J Clin Pathol. 2005;124:414–420. doi: 10.1309/3G1X-DX0D-VHBN-VKB4. [DOI] [PubMed] [Google Scholar]
- 6.Mateo G, Montalban MA, Vidriales MB, Lahuerta JJ, Mateos MV, Gutierrez N, Rosinol L, Montejano L, Blade J, Martinez R, et al. Prognostic value of immunophenotyping in multiple myeloma: A study by the PETHEMA/GEM cooperative study groups on patients uniformly treated with high-dose therapy. Journal of Clinical Oncology. 2008;26:2737–2744. doi: 10.1200/JCO.2007.15.4120. [DOI] [PubMed] [Google Scholar]
- 7.Paiva B, Vidriales MB, Perez JJ, Mateo G, Montalban MA, Mateos MV, Blade J, Lahuerta JJ, Orfao A, San Miguel JF. Multiparameter flow cytometry quantification of bone marrow plasma cells at diagnosis provides more prognostic information than morphological assessment in myeloma patients. Haematologica. 2009;94:1599–602. doi: 10.3324/haematol.2009.009100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Perez-Persona E, Vidriales MB, Mateo G, Garcia-Sanz R, Mateos MV, de Coca AG, Galende J, Martin-Nunez G, Alonso JM, de Las Heras N, et al. New criteria to identify risk of progression in monoclonal gammopathy of uncertain significance and smoldering multiple myeloma based on multiparameter flow cytometry analysis of bone marrow plasma cells. Blood. 2007;110:2586–92. doi: 10.1182/blood-2007-05-088443. [DOI] [PubMed] [Google Scholar]
- 9.Paiva B, Vidriales MB, Mateo G, Perez JJ, Montalban MA, Sureda A, Montejano L, Gutierrez NC, de Coca AG, de las Heras N, et al. The persistence of immunophenotypically normal residual bone marrow plasma cells at diagnosis identifies a good prognostic subgroup of symptomatic multiple myeloma patients. Blood. 2009;114:4369–4372. doi: 10.1182/blood-2009-05-221689. [DOI] [PubMed] [Google Scholar]
- 10.Rawstron AC, Child JA, Tute RM, Davies FE, Gregory WM, Bell SE, Szubert AJ, Navarro-Coy N, Drayson MT, Feyler S, Ross FM, Cook G, Jackson GH, Morgan GJ, Owen RG. Minimal Residual Disease Assessed by Multiparameter Flow Cytometry in Multiple Myeloma: Impact on Outcome in the Medical Research Council Myeloma IX Study. J Clin Oncol. 2013;31:2540–2547. doi: 10.1200/JCO.2012.46.2119. [DOI] [PubMed] [Google Scholar]
- 11.Nowakowski GS, Witzig TE, Dingli D, Tracz MJ, Gertz MA, Lacy MQ, Lust JA, Dispenzieri A, Greipp PR, Kyle RA, et al. Circulating plasma cells detected by flow cytometry as a predictor of survival in 302 patients with newly diagnosed multiple myeloma. Blood. 2005;106:2276–9. doi: 10.1182/blood-2005-05-1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dingli D, Nowakowski GS, Dispenzieri A, Lacy MQ, Hayman SR, Rajkumar SV, Greipp PR, Litzow MR, Gastineau DA, Witzig TE, et al. Flow cytometric detection of circulating myeloma cells before transplantation in patients with multiple myeloma: a simple risk stratification system. Blood. 2006;107:3384–8. doi: 10.1182/blood-2005-08-3398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Paiva B, Vidriales MB, Cervero J, Mateo G, Perez JJ, Montalban MA, Sureda A, Montejano L, Gutierrez NC, Garcia de Coca A, et al. Multiparameter flow cytometric remission is the most relevant prognostic factor for multiple myeloma patients who undergo autologous stem cell transplantation. Blood. 2008;112:4017–23. doi: 10.1182/blood-2008-05-159624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu H, Yuan C, Heinerich J, Braylan R, Chang M, Wingard J, Moreb J. Flow cytometric minimal residual disease monitoring in patients with multiple myeloma undergoing autologous stem cell transplantation: a retrospective study. Leuk Lymphoma. 2008;49:306–14. doi: 10.1080/10428190701813018. [DOI] [PubMed] [Google Scholar]
- 15.Ocqueteau MOA, Almeida J, et al. Immunophenotypic characterization of plasma cells from monoclonal gammopathy of undetermined significance patients. Implications for the differential diagnosis between MGUS and multiple myeloma. American Journal of Pathology. 1998;152:1655–1665. [PMC free article] [PubMed] [Google Scholar]
- 16.Almeida JOA, Ocqueteau M, et al. High-sensitive immunophenotyping and DNA ploidy studies for the investigation of minimal residual disease in multiple myeloma. British Journal of Haematology. 1999;107:121–131. doi: 10.1046/j.1365-2141.1999.01685.x. [DOI] [PubMed] [Google Scholar]
- 17.Lin P, Owens R, Tricot G, Wilson CS. Flow cytometric immunophenotypic analysis of 306 cases of multiple myeloma. Am J Clin Pathol. 2004;121:482–488. doi: 10.1309/74R4-TB90-BUWH-27JX. [DOI] [PubMed] [Google Scholar]
- 18.Tohami T, Drucker L, Shapiro H, Radnay J, Lishner M. Overexpression of tetraspanins affects multiple myeloma cell survival and invasive potential. FASEB J. 2007;21:691–9. doi: 10.1096/fj.06-6610com. [DOI] [PubMed] [Google Scholar]
- 19.Paiva B, Gutierrez NC, Chen X, Vidriales MB, Montalban MA, Rosinol L, Oriol A, Martinez-Lopez J, Mateos MV, Lopez-Corral L, et al. Clinical significance of CD81 expression by clonal plasma cells in high-risk smoldering and symptomatic multiple myeloma patients. Leukemia. 2012;26:1862–9. doi: 10.1038/leu.2012.42. [DOI] [PubMed] [Google Scholar]
- 20.Kyle RA, Durie BG, Rajkumar SV, Landgren O, Blade J, Merlini G, Kroger N, Einsele H, Vesole DH, Dimopoulos M, et al. Monoclonal gammopathy of undetermined significance (MGUS) and smoldering (asymptomatic) multiple myeloma: IMWG consensus perspectives risk factors for progression and guidelines for monitoring and management. Leukemia. 2010;24:1121–7. doi: 10.1038/leu.2010.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jasper GA, Arun I, Venzon D, Kreitman RJ, Wayne AS, Yuan CM, Marti GE, Stetler-Stevenson M. Variables affecting the quantitation of CD22 in neoplastic B cells. Cytometry Part B: Clinical Cytometry. :n/a–n/a. doi: 10.1002/cyto.b.20567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Perez-Persona E, Mateo G, Garcia-Sanz R, Mateos MV, de Las Heras N, de Coca AG, Hernandez JM, Galende J, Martin-Nunez G, Barez A, et al. Risk of progression in smouldering myeloma and monoclonal gammopathies of unknown significance: comparative analysis of the evolution of monoclonal component and multiparameter flow cytometry of bone marrow plasma cells. Br J Haematol. 2010;148:110–4. doi: 10.1111/j.1365-2141.2009.07929.x. [DOI] [PubMed] [Google Scholar]
- 23.Rawstron AC, Owen RG, Davies FE, Johnson RJ, Jones RA, Richards SJ, Evans PA, Child JA, Smith GM, Jack AS, et al. Circulating plasma cells in multiple myeloma: characterization and correlation with disease stage. Br J Haematol. 1997;97:46–55. doi: 10.1046/j.1365-2141.1997.72653.x. [DOI] [PubMed] [Google Scholar]
- 24.Rawstron AC, Orfao A, Beksac M, Bezdickova L, Brooimans RA, Bumbea H, Dalva K, Fuhler G, Gratama J, Hose D, et al. Report of the European Myeloma Network on multiparametric flow cytometry in multiple myeloma and related disorders. Haematologica. 2008;93:431–8. doi: 10.3324/haematol.11080. [DOI] [PubMed] [Google Scholar]
- 25.Cannizzo E, Bellio E, Sohani AR, Hasserjian RP, Ferry JA, Dorn ME, Sadowski C, Bucci JJ, Carulli G, Preffer F. Multiparameter immunophenotyping by flow cytometry in multiple myeloma: The diagnostic utility of defining ranges of normal antigenic expression in comparison to histology. Cytometry B Clin Cytom. 2010;78:231–8. doi: 10.1002/cyto.b.20517. [DOI] [PubMed] [Google Scholar]
- 26.Peceliunas V, Janiulioniene A, Matuzeviciene R, Griskevicius L. Six color flow cytometry detects plasma cells expressing aberrant immunophenotype in bone marrow of healthy donors. Cytometry B Clin Cytom. 2011;80:318–23. doi: 10.1002/cyto.b.20601. [DOI] [PubMed] [Google Scholar]
- 27.Hermiston ML, Xu Z, Weiss A. CD45: A critical regulator of signaling thresholds in immune cells. Annual Review of Immunology. 2003;21:107–137. doi: 10.1146/annurev.immunol.21.120601.140946. [DOI] [PubMed] [Google Scholar]
- 28.Jensen GS, Mant MJ, Belch AJ, Berenson JR, Ruether BA, Pilarski LM. Selective expression of CD45 isoforms defines CALLA+ monoclonal B-lineage cells in peripheral blood from myeloma patients as late stage B cells. Blood. 1991;78:711–9. [PubMed] [Google Scholar]