Abstract
The discovery of a 2-aryl-3-aroyl indole-based small-molecule inhibitor of tubulin assembly (referred to as OXi8006) inspired the design, synthesis, and biological evaluation of a series of diversely functionalized analogues. In the majority of examples, the pendant 2-aryl ring contained a 3-hydroxy-4-methoxy substitution pattern, and the fused aryl ring featured a 6-methoxy group. Most of the variability was in the 3-aroyl moiety, which was modified to incorporate methoxy (33–36), nitro (25–27), halogen (28–29), trifluoromethyl (30), or trifluoromethoxy (31–32) functionalities. In two analogues (34 and 36), the methoxy substitution pattern in the fused aryl ring varied, while in another derivative (35) the phenolic moiety was translocated from the pendant 2-aryl ring to position-7 of the fused aryl ring. Each of the compounds were evaluated for their cytotoxicity (in vitro) against the SK-OV-3 (ovarian), NCI-H460 (lung), and DU-145 (prostate) human cancer cell lines and for their ability to inhibit tubulin assembly. Four of the compounds (30, 31, 35, 36) proved to be potent inhibitors of tubulin assembly (IC50 < 5 µM), and three of these compounds (31, 35, 36) were strongly cytotoxic against the three cancer cell lines. The most active compound (36) in this series, which incorporated a methoxy group at position-7, was comparable in terms of inhibition of tubulin assembly and cytotoxicity to the lead compound OXi8006.
Keywords: Vascular disrupting agent (VDA), inhibitor of tubulin assembly, functionalized indole, combretastatin
1. Introduction
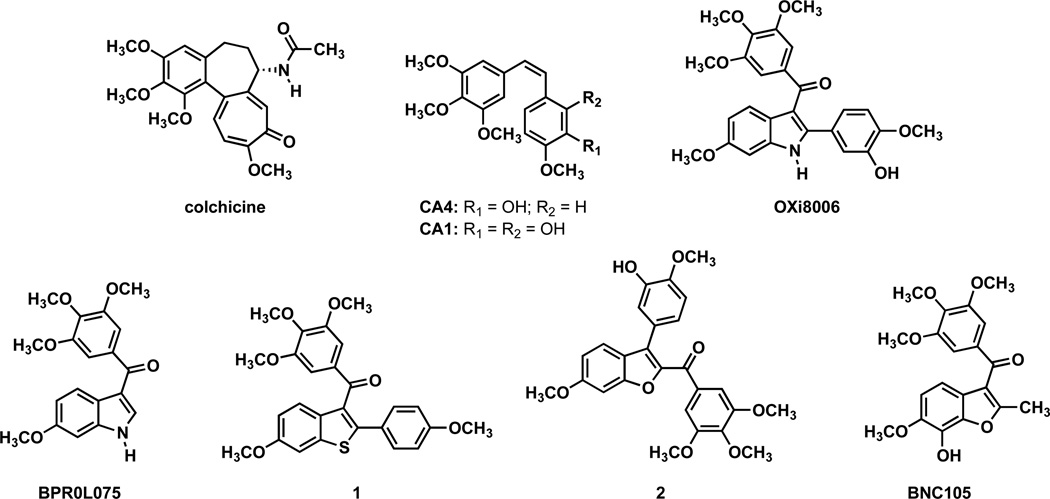
The exploration and assessment of the tumor microenvironment and its physiology have revealed a number of prospective molecular targets for selective therapeutic intervention by small-molecule anti-cancer agents. A well-established target is the dynamic tubulin-microtubule protein system. Microtubules are structurally characterized as biopolymers composed of αβ-tubulin heterodimers.1–5 The dynamic assembly and disassembly of microtubules is linked to a variety of cellular functions, including cell shape, intracellular motility, cellular division, and apoptosis.1–5 More recently, certain small-molecule inhibitors of tubulin assembly have been identified as vascular disrupting agents (VDAs).6 These compounds selectively disrupt tumor vasculature by interfering with the tubulin-microtubule protein system of the endothelial cells lining tumor microvessels, which sets in motion a cascade of cell signaling events leading to morphology changes (rounding up) of these endothelial cells. This results in the occlusion of the vessels, which limits tumor blood flow. This in turn restricts the oxygen and nutrients vital for tumor survival. The vascular network feeding tumors is distinct from normal tissue vasculature and incorporates branching that is often unsystematic and convoluted.7–9 In addition, increased rates of tumor cell proliferation coupled with underdeveloped endothelium, in contrast to normal tissue vasculature, has established tumor vasculature as a selective therapeutic target for anti-cancer agents.9 This approach has led to the development of a class of therapeutics referred to as vascular targeting agents (VTAs). This class is further subdivided into two discrete sub-classes centered upon distinct mechanism(s) of action: vascular disrupting agents (VDAs) and angiogenesis inhibiting agents (AIAs).10 VDAs damage existing tumor vasculature while AIAs impede new tumor vessel formation.10–12 VDAs can be further divided into two distinct groups: biologics and small-molecules. One strategy focuses on the development of indole-based small-molecule VDAs that bind at the colchicine site, named after the natural product originally described as binding at the site (Figure 1)13 and whose interaction with tubulin led to the original isolation of the protein.14 Synthetic and biological studies with indole-based, colchicine site VDAs were originally prompted by the discovery of the potent natural products combretastatin A-4 (CA4) and combretastatin A-1 (CA1) that were isolated from the African bush willow tree, Combretum caffrum, by Pettit and co-workers (Figure 1).15–16 CA4 emerged as a benchmark VDA, and its corresponding prodrug salt CA4P (Zybrestat™) was the first small-molecule tubulin binding VDA to enter clinical trials.17–19 Although no VDA is yet in routine clinical use, several small-molecule VDAs interacting at the colchicine site are in clinical trials.17–21
Figure 1.
Selected colchicine site tubulin binding agents.
VDAs derived from the combretastatin family demonstrate potent antiproliferative activity in various human cancer cell lines in vitro through the inhibition of tubulin polymerization.22–31 These findings led us and others to explore indole-based compounds for potential VDA and antitubulin activities by incorporating into their design structural similarities to the combretastatin series. Our work led to the potent compound 2-(3′-hydroxy-4′-methoxyphenyl)-3-(3″,4″,5″-trimethoxybenzoyl)-6-methoxyindole (referred to as OXi8006),21, 32–34 and Flynn35 has subsequently pursued this compound (through a separate synthetic route) and structurally similar, highly active compounds. Because OXi8006 potently inhibits tubulin assembly (IC50 = 1.1 µM) and cell growth (for example, GI50 = 3.45 nM against SK-OV-3 cells), we initiated further structural studies. As an initial finding, a water-soluble, disodium phosphate prodrug salt, OXi8007, demonstrated distinct in vivo VDA activity in a study employing a SCID mouse model bearing an orthotopic PC-3 (prostate) tumor as imaged by color Doppler ultrasound.36
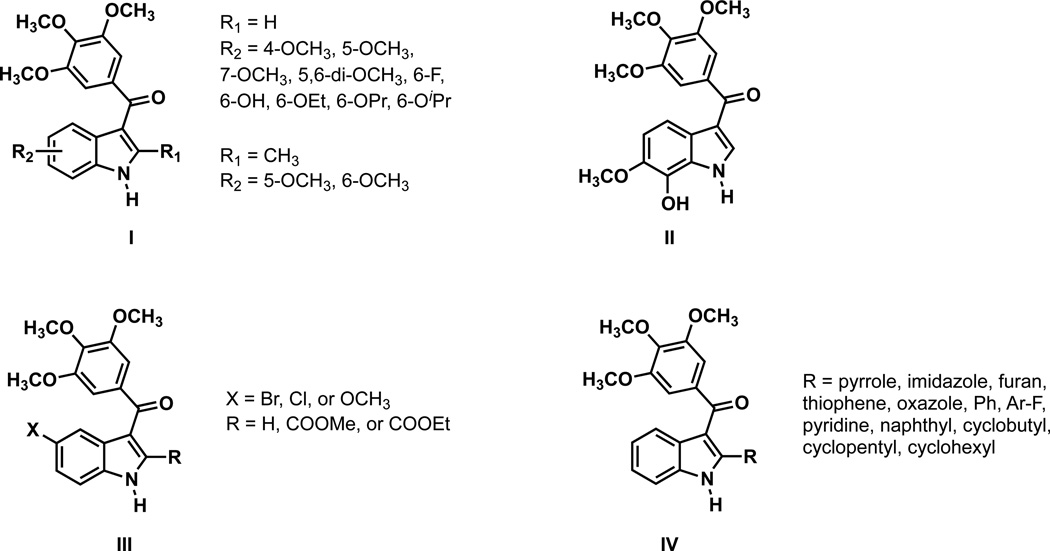
Herein, we report the synthesis and biological evaluation of a series of functionalized analogues of OXi8006 in an effort to further explore the molecular space inherent to 2-aryl-3-aroyl indole-based anti-cancer agents. Our finding32,21,33,34 that OXi8006 is a potent tubulin binding agent combined with the work of Hseih37 with BPR0L075 (Figure 1) provided preliminary structural parallels defining distinct associations between the stilbene aryl rings of CA4 and the aryl and aroyl rings of OXi8006 and BPR0L075. These correlations were further expanded by our previous identification of benzo[b]thiophene 1 and benzo[b]furan 2 as tubulin interacting compounds38–41 and the subsequent studies by Flynn leading to the benzofuran-based BNC105 (Figure 1), a VDA currently undergoing clinical trials.42–44 A narrow but focused literature survey of inhibitors of tubulin assembly that incorporate the indole molecular template confirms the importance of the 3-(3′, 4′, 5′-trimethoxybenzoyl)indole functionality while allowing for structural diversity within the indole core (Figure 2). This is exemplified by structures that include variation in alkoxy substitution (structure I, Fig. 2),37 halogen incorporation (structures I and III),37,45 heterocyclic substitution at the 2-position (structure IV),46 and derivatives of BPR0L075 (such as compound II).47
Figure 2.
Structural diversity within the 3-(3′, 4′, 5′-trimethoxybenzoyl)indole molecular space
The potent inhibition of tubulin assembly and cytotoxicity of OXi8006 and BPR0L075, in addition to the previous studies with benzo[b]thiophene and benzo[b]furan derivatives, led to the present study, which investigates a small collection of diversely modified 2-aryl-3-aroyl indole-based analogues to gain further insight into the structural features of OXi8006 that are most important for biological activity (inhibition of tubulin assembly and cytotoxicity).
2. Results and discussion
2.1 Chemistry
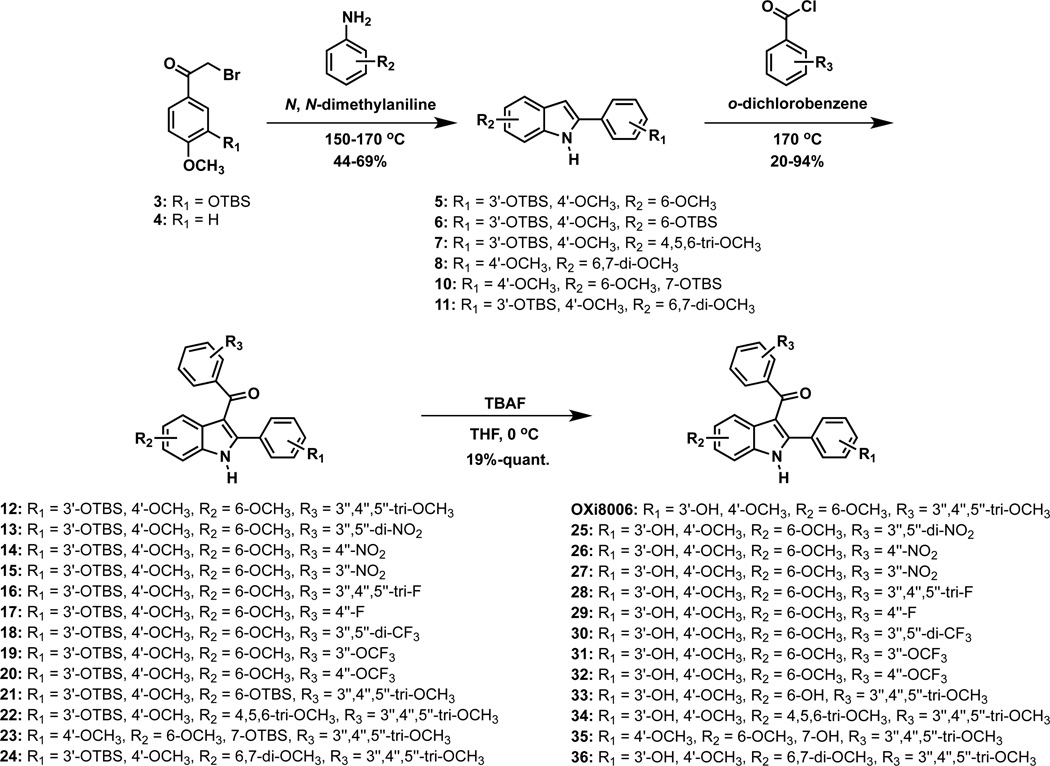
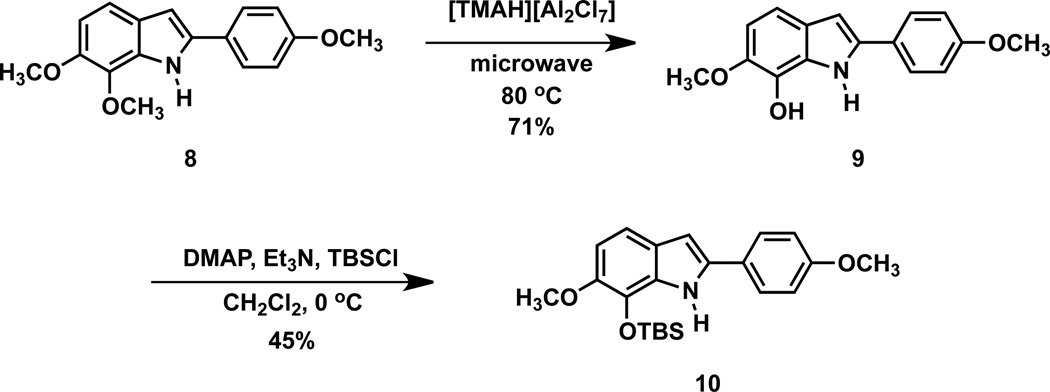
The synthetic route to derivatized OXi8006 analogues 25–36 involved the previously described bromoacetophenone 336 and commercially available bromoacetophenone 4 as key intermediates. 2-Aryl substituted indoles 5–11 were prepared by condensation of bromoacetophenone 3 or 4 with suitable anilines under Bischler-Mohlau conditions48,49 (Scheme 1). Further modification of 2-aryl indole 8 by selective demethoxylation in the presence of ionic liquid [TMAH][Al2Cl7]50 (generated from AlCl3 and trimethylamine hydrochloride (TMAH)) and microwave irradiation yielded the phenolic 2-aryl indole 9, which was subsequently protected as its corresponding TBS derivative 10 (Scheme 2). The regioselectivity of the demethylation reaction was confirmed by X-ray crystallographic analysis of TBS indole 10 (see Supplementary data). Treatment of 2-aryl indoles 5–11 with appropriate functionalized benzoyl chloride derivatives resulted in 2-aryl-3-aroyl indole analogues 12–24 through a benzoylation reaction. Final desilylation with TBAF provided the parent 2-aryl-3-aroyl free phenol indole analogues 25–36.
Scheme 1.
Synthetic route to OXi8006 analogues from bromoacetophenones 3 and 4.
Scheme 2.
Synthetic route to modified indole 10
2.2 Biological Evaluation
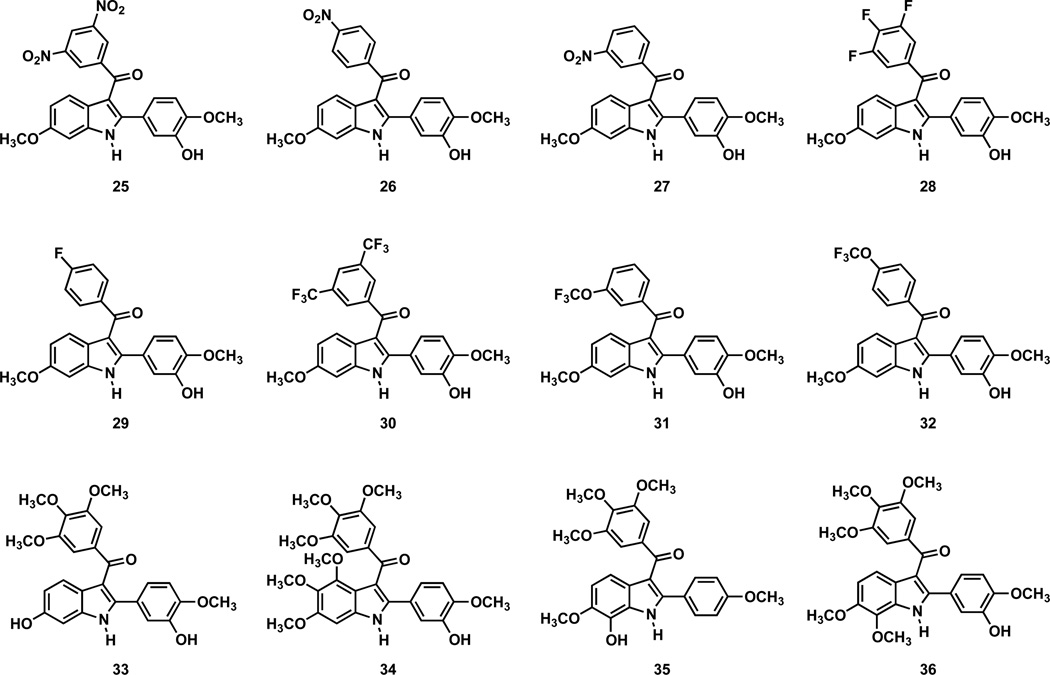
The series of 2-aryl-3-aroyl indole analogues (Fig. 3) were evaluated for their cytotoxicity against the SK-OV-3, NCI-H460, and DU-145 human cancer cell lines (Table 1) and for their ability to inhibit tubulin assembly (Table 2). The two most active compounds (35 and 36) in the series featured substitution at position-7 in the fused aryl ring. Compound 36 (7-methoxy) was comparable to OXi8006 in terms of both cytotoxicity (sub-micromolar) and inhibition of tubulin assembly (IC50 = 1.1 µM), and analogue 35, in which the hydroxyl group was transposed from the pendant 2-aryl ring to position-7 of the fused aryl ring, was nearly equipotent. Replacement of the 6-methoxy group with a 6-hydroxy moiety (analogue 33) resulted in a loss of antitubulin activity (> 20 µM) and a significant decrease in cytotoxicity. All structural modifications in the 3-aroyl moiety that replaced the 3,4,5-trimethoxy motif (inherent to OXi8006) with a different functionality resulted in a decrease in cytotoxicity (compared to OXi8006 and the reference stilbene compound, CA4). However, the 3,5-bis-trifluoromethyl analogue (30) and the 3-trifluoromethoxy derivative (31) remained relatively good inhibitors of tubulin assembly (3.1 and 3.7 µM, respectively). Although selective fluorine substitution in CA4 analogues has been generally well-tolerated,51 this trend did not carry forward to this indole series of compounds. A 3,4,5-trifluoro analogue (28) demonstrated only modest inhibition of tubulin assembly (7.5 µM), while a 3-fluoro derivative (29) was inactive (> 20 µM) in this assay. Nitro-bearing analogues (25–27) were similarly inactive. These results suggest the importance of the 3,4,5-trimethoxy substitution pattern in the 3-aroyl moiety and appropriate substitution at positions 6 and 7 of the fused aryl ring for maintaining potent cytotoxicity and inhibition of tubulin assembly in this series of compounds.
Figure 3.
Molecular structures of synthesized 2-aryl-3-aroylindole analogues 25–36.
Table 1.
Cytotoxicity against human cancer cell lines SK-OV-3, NCI-H460, and DU-145
| GI50 (µM) ± SD Sulforhodamine B assaya | |||
|---|---|---|---|
| Compound | SK-OV-3 | NCI-H460 | DU-145 |
| CA4 | 0.00533 ± 0.00180 | 0.00449 ± 0.0000648b | 0.00484 ± 0.000848b |
| OXi8006 | 0.00345 ± 0.000409 | 0.0379 ± 0.00182 | 0.0356 ± 0.00107 |
| 25 | 4.35 ± 0.290 | 4.08 ± 0.119 | 5.52 ± 0.106 |
| 26 | 21.1 ± 2.45 | 16.8 ± 2.13 | 8.16 ± 4.41 |
| 27 | 2.65 ± 1.94 | 15.7 ± 1.64 | 7.11 ± 2.16 |
| 28 | 19.8 ± 3.95 | 5.54 ± 0.505 | 10.0 ± 9.83 |
| 29 | 15.0 ± 9.60 | 49.6 ± 3.20 | 10.0 ± 4.21 |
| 30 | 1.45 ± 0.365 | 2.86 ± 0.104 | 3.01 ± 0.0829 |
| 31 | 0.283 ± 0.0395 | 3.57 ± 0.508 | 2.99 ± 0.235 |
| 32 | 3.05 ± 0.895 | 2.35 ± 0.159 | 3.21 ± 0.294 |
| 33 | 2.167 ± 0.3657 | 2.910 ± 0.4833 | 3.401 ± 1.471 |
| 34 | 25.1 ± 0.262 | 41.8 ± 2.52 | 28.3 ± 18.0 |
| 35 | 0.264 ± 0.0418 | 0.177 ± 0.0245 | 0.309 ± 0.0143 |
| 36 | 0.0119 ± 0.00422 | 0.992 ± 0.320 | 0.0181 ± 0.000772 |
Table 2.
Inhibition of tubulin polymerization and colchicine binding
| Inhibition of colchicine binding (%) ±SD |
|||
|---|---|---|---|
| Compound | Inhibition of tubulin polymerization IC50 (µM)±SD |
1 µM | 5µM |
| CA4 | 1.3±0.07 | 88±2 | 98±0.5 |
| OXi8006 | 1.1±0.04 | 40±0.2 | 75±0.2 |
| 25 | > 20 | nda | nd |
| 26 | > 20 | nd | nd |
| 27 | 19±0.8 | nd | 21±1 |
| 28 | 7.5±2 | nd | 26±2 |
| 29 | > 20 | nd | nd |
| 30 | 3.1±0.2 | nd | 26±2 |
| 31 | 3.7±0.4 | nd | 19±4 |
| 32 | > 20 | nd | nd |
| 33 | > 20 | nd | nd |
| 34 | > 20 | nd | nd |
| 35 | 1.0±0.1 | 51±0.4 | 85±0.7 |
| 36 | 1.1±0.4 | 31±4 | 67±3 |
nd = not determined in this study.
3. Conclusion
In summary, the results of this study have significantly extended our knowledge of functional group tolerability for 2-aryl-3-aroyl indole analogues. The most promising new analogues (35, 36) demonstrated inhibition of tubulin assembly comparable to the reference compounds OXi8006 and CA4, and future studies will evaluate these compounds (as their corresponding water-soluble phosphate prodrug salts) for their potential to function as VDAs.
4. Experimental
4.1 Chemistry
4.1.1 Materials and instrumentation
CH2Cl2 and tetrahydrofuran (THF) were used in their anhydrous forms, as obtained from the chemical suppliers. Reactions were performed under an inert atmosphere using nitrogen gas, unless specified otherwise. Thin-layer chromatography (TLC) plates (precoated glass plates with silica gel 60 F254, 0.25 mm thickness) were used to monitor reactions. Purification of intermediates and products was carried out with a flash purification system (Biotage Isolera 1 or 4) using silica gel (200–400 mesh, 60 Å) prepacked columns. Reactions carried out under microwave irradiation were performed with a Biotage Initiator Microwave Synthesizer. Intermediates and products synthesized were characterized on the basis of their 1H NMR (500 MHz), 13C NMR (125 MHz), and 19F NMR (470 MHz) spectroscopic data using a Varian VNMRS 500 MHz instrument. Spectra were recorded in CDCl3, (CD3)2SO, or (CD3)2CO. All of the chemical shifts are expressed in ppm (δ), coupling constants (J) are presented in Hz, and peak patterns are reported as broad (br), singlet (s), doublet (d), triplet (t), double doublet (dd), double triplet (dt), triplet of triplets (tt), doublet of doublets of doublets (ddd), and multiplet (m). Purity of the final compounds was further analyzed at 25 °C using an Agilent 1200 HPLC system with a diode-array detector (λ = 190–400 nm), a Zorbax XDB-C18 HPLC column (4.6 mm – 150 mm, 5 µm), and a Zorbax reliance cartridge guard-column; solvent A: acetonitrile, solvent B: H2O; gradient: 10%A / 90%B to 100%A / 0%B over 0 to 40 min; post-time 10 min; flow rate 1.0 mL/min; injection volume 20 µL; monitored at wavelengths of 210, 254, 230, 280, and 360 nm. Mass spectrometry was carried out under positive ESI (electrospray ionization) using a Thermo Scientific LTQ Orbitrap Discovery instrument.
4.1.2. 2-(3′-tert-Butyldimethylsilyloxy-4′-methoxyphenyl)-6-methoxyindole (5)36
To a solution of m-anisidine (2.05 mL, 18.4 mmol) dissolved in N,N-dimethylaniline (20 mL) at 170 °C was added dropwise bromoacetophenone 3 (2.0 g, 5.6 mmol) in EtOAc (5 mL). The reaction mixture was stirred at 170 °C for 12 h. Upon completion of the reaction, the reaction mixture was cooled to room temperature and extracted with EtOAc (3 × 50 mL). The combined organic extract was dried over Na2SO4 and concentrated under reduced pressure. Purification by flash chromatography using a prepacked 25 g silica column [solvent A: EtOAc; solvent B: hexanes; gradient: 12%A / 88%B (4 CV), 12%A / 88%B → 100%A / 0%B (10 CV), 100%A / 0%B (2.6 CV); flow rate: 25 mL/min; monitored at 254 and 280 nm] resulted in the desired 2-phenylindole derivative 5 (1.49 g, 3.88 mmol, 69%, Rf = 0.48 (50:50 hexanes:EtOAc)) as light tan crystals. 1H NMR (CDCl3, 500 MHz): δ 8.11 (br s, 1H, NH), 7.47 (d, J = 8.5 Hz, 1H, ArH), 7.16 (dd, J = 8.5 Hz, 2.0 Hz, 1H, ArH), 7.13 (d, J = 2.5 Hz, 1H, ArH), 6.90 (d, J = 8.5 Hz, 1H, ArH), 6.89 (d, J = 2.5 Hz, 1H, ArH), 6.79 (dd, J = 8.5 Hz, 2.5 Hz, 1H, ArH), 6.64 (dd, J = 2.0 Hz, 1.0 Hz, 1H, ArH), 3.86 (s, 3H, OCH3), 3.84 (s, 3H, OCH3), 1.04 (s, 9H, C(CH3)3), 0.21 (s, 6H, Si(CH3)2). 13C NMR (CDCl3, 125 MHz): δ 156.3, 150.5, 145.4, 137.4, 136.9, 125.8, 123.7, 120.9, 118.2, 117.8, 112.4, 109.9, 98.6, 94.5, 55.6, 55.4, 25.7, 18.5, −4.6.
4.1.3. 2-(3′-tert-Butyldimethylsilyloxy-4′-methoxyphenyl)-6-tert-butyldimethylsilyloxyindole (6)
To a solution of 3-(tert-butyldimethylsilyloxy)aniline (2.06 g, 9.21 mmol) in N,N-dimethylaniline (20 mL) at 170 °C was added compound 3 (1.00 g, 2.79 mmol) dropwise in EtOAc (5 mL). The reaction mixture was stirred at 170 °C for 12 h. Upon completion of the reaction, the reaction mixture was cooled to room temperature and extracted with EtOAc (3 × 50 mL). The combined organic extract was dried over Na2SO4 and concentrated under reduced pressure. Purification by flash chromatography using a prepacked 100 g silica column [solvent A: EtOAc; solvent B: hexanes; gradient: 2%A / 98%B (4 CV), 2%A / 98%B → 20%A / 80%B (10 CV), 20%A / 80%B (2 CV); flow rate: 40 mL/min; monitored at 254 and 280 nm] resulted in the desired 2-phenylindole 6 (0.59 g, 1.23 mmol, 44%, Rf = 0.39 (90:10 hexanes:EtOAc)) as a brown solid. 1H NMR (CDCl3, 500 MHz): δ 8.09 (br s, 1H, NH), 7.37 (d, J = 8.5 Hz, 1H, ArH), 7.10 (d, J = 1.5 Hz, 1H, ArH), 7.01 (dd, J = 8.5 Hz, 1.5 Hz, 1H, ArH), 6.82 (s, 1H, ArH), 6.72 (d, J = 8.5 Hz, 1H, ArH), 6.67 (dd, J = 8.5 Hz, 1.5 Hz, 1H, ArH), 6.54 (s, 1H, ArH), 3.70 (s, 3H, OCH3), 1.02 (s, 9H, C(CH3)3), 1.01 (s, 9H, C(CH3)3), 0.20 (s, 6H, Si(CH3)2), 0.18 (s, 6H, Si(CH3)2). 13C NMR (CDCl3, 125 MHz): δ 150.7, 150.6, 145.4, 137.7, 137.3, 126.0, 124.5, 120.6, 118.5, 118.0, 114.6, 112.4, 101.8, 98.7, 55.4, 25.93, 25.89, 18.6, 18.4, −4.3, −4.5. HPLC: 25.45 min., purity at 254 nm >99%. HRMS (ESI+): m/z calculated for C27H42NO3Si2 [M+H]+ 484.2698, found 484.2698.
4.1.4. 2-(3′-tert-Butyldimethylsilyloxy-4′-methoxyphenyl)-4,5,6-trimethoxyindole (7)
To a solution of 3,4,5-trimethoxyaniline (0.336 g, 1.84 mmol) in N,N-dimethylaniline (20 mL) at 170 °C was added compound 3 (0.20 g, 0.56 mmol) dropwise in EtOAc (5 mL). The reaction mixture was stirred at 170 °C for 12 h. Upon completion of the reaction, the reaction mixture was cooled to room temperature and extracted with EtOAc (3 × 50 mL). The combined organic extract was dried over Na2SO4 and concentrated under reduced pressure. Purification by flash chromatography using a prepacked 50 g silica column [solvent A: EtOAc; solvent B: hexanes; gradient: 7%A / 93%B (4 CV), 7%A / 93%B → 60%A / 40%B (10 CV), 60%A / 40%B (2 CV); flow rate: 40 mL/min; monitored at 254 and 280 nm] resulted in the desired 2-phenylindole 7 (0.14 g, 0.32 mmol, 58%, Rf = 0.31 (70:30 hexanes:EtOAc)) as colorless crystals. 1H NMR (CDCl3, 500 MHz): δ 8.06 (br s, 1H, NH), 7.15 (dd, J = 8.5 Hz, 2.0 Hz, 1H, ArH), 7.10 (d, J = 2.0 Hz, 1H, ArH), 6.90 (d, J = 8.5 Hz, 1H, ArH), 6.70 (dd, J = 2.0 Hz, 1.0 Hz, 1H, ArH), 6.66 (d, J = 0.5 Hz, 1H, ArH), 4.13 (s, 3H, OCH3), 3.90 (s, 3H, OCH3), 3.88 (s, 3H, OCH3), 3.84 (s, 3H, OCH3), 1.03 (s, 9H, C(CH3)3), 0.19 (s, 6H, Si(CH3)2). 13C NMR (CDCl3, 125 MHz): δ 151.0, 150.6, 145.6, 145.5, 136.4, 135.8, 133.9, 125.8, 118.3, 117.9, 116.6, 112.5, 96.3, 89.8, 61.6, 60.9, 56.2, 55.2, 25.9, 18.5, −4.6. HPLC: 20.17 min., purity at 254 nm 94.2%. HRMS (ESI+): m/z calculated for C24H34NO5Si [M+H]+ 444.2201, found 444.2200.
4.1.5. 2-(4′-Methoxyphenyl)-6,7-dimethoxyindole (8)
To a solution of 2,3-dimethoxyaniline (0.92 mL, 6.85 mmol) dissolved in N,N-dimethylaniline (10 mL) was added 4-methoxybromoacetophenone 4 (0.79 g, 3.43 mmol). The solution was heated to reflux and stirred at 150 °C for 12 h. Upon completion of the reaction, the reaction mixture was cooled to room temperature and extracted with EtOAc (3 × 50 mL). The combined organic extract was dried over Na2SO4 and concentrated under reduced pressure. Purification by flash chromatography using a prepacked 100 g silica column [solvent A: EtOAc; solvent B: hexanes; gradient: 5%A / 95%B (4 CV), 5%A / 95%B → 40%A / 60%B (10 CV), 40%A / 60%B (4 CV); flow rate: 40 mL/min; monitored at 254 and 280 nm] resulted in the desired 6,7-dimethoxy-2-phenylindole 8 (0.50 g, 1.76 mmol, 51%, Rf = 0.35 (80:20 hexanes:EtOAc)) as a tan solid. 1H NMR (CDCl3, 500 MHz): δ 8.61 (br s, 1H, NH), 7.61 (d, J = 8.7 Hz, 2H, ArH), 7.28 (d, J = 8.5 Hz, 1H, ArH), 6.97 (d, J = 8.7 Hz, 2H, ArH), 6.87 (d, J = 8.6 Hz, 1H, ArH), 6.66 (d, J = 2.1 Hz, 1H, ArH), 4.09 (s, 3H, OCH3), 3.97 (s, 3H, OCH3), 3.85 (s, 3H, OCH3). 13C NMR (CDCl3, 125 MHz): δ 159.2, 147.1, 138.0, 134.2, 131.3, 126.4, 126.0, 125.3, 115.3, 114.5, 108.5, 98.8, 61.1, 57.4, 55.4. HPLC: 15.30 min., purity at 254 nm 90.6%. HRMS (ESI+): m/z calculated for C17H18NO3 [M+H]+ 284.1281, found 284.1282.
4.1.6. 2-(4′-Methoxyphenyl)-6-methoxy-7-hydroxyindole (9)
Trimethoxyindole 8 (0.61 g, 2.16 mmol) was dissolved in a solution of [Al2Cl7][TMAH] (6.3 mL, 3.13 mmol, 0.496 M in CH2Cl2). The reaction mixture was sealed and subjected to microwave irradiation at 80 °C for 1 h. Upon completion of the reaction, the reaction mixture was diluted with NaHCO3 and extracted with CH2Cl2 (3 × 20 mL). The combined organic extract was dried over Na2SO4 and concentrated under reduced pressure. Purification by flash chromatography using a prepacked 100 g silica column [solvent A: EtOAc; solvent B: hexanes; gradient: 7%A / 93%B (4 CV), 7%A / 93%B → 60%A / 40%B (10 CV), 60%A / 40%B (2 CV); flow rate: 40 mL/min; monitored at 254 and 280 nm] resulted in the desired 6-methoxy-7-hydroxy-2-phenylindole 9 (0.42 g, 1.55 mmol, 71%, Rf = 0.36 (70:30 hexanes:EtOAc)) as a tan solid. 1H NMR ((CD3)2CO, 500 MHz): δ 10.11 (br s, 1H, NH), 7.85 (d, J = 8.7 Hz, 2H, ArH), 7.66 (s, 1H, OH), 6.98 (m, 3H, ArH), 6.81 (d, J = 8.5 Hz, 1H, ArH), 6.66 (d, J = 2.2 Hz, 1H, ArH), 3.83 (s, 3H, OCH3), 3.81 (s, 3H, OCH3). 13C NMR ((CD3)2CO, 125 MHz): δ 159.9, 142.5, 138.8, 133.1, 128.4, 127.2, 127.1, 126.5, 115.0, 111.3, 108.9, 99.0, 58.3, 55.7. HPLC: 13.47 min., purity at 254 nm 85.8%. HRMS (ESI+): m/z calculated for C16H16NO3 [M+H]+ 270.1125, found 270.1129.
4.1.7. 2-(4′-Methoxyphenyl)-6-methoxy-7-tert-butyldimethylsilyloxyindole (10)
To a solution of free phenol indole 9 (0.08 g, 0.28 mmol) in CH2Cl2 (5 mL) at 0 °C was added Et3N (0.04 mL, 0.31 mmol) and DMAP (0.01 g, 0.11 mmol). The reaction mixture was stirred for 10 min, and TBSCl (0.05 g, 0.31 mmol) was added gradually. The solution was allowed to warm to room temperature over 12 h. Upon completion of the reaction, water (10 mL) was added, and the reaction mixture was extracted with CH2Cl2 (3 × 50 mL). The combined organic extract was dried over Na2SO4 and concentrated under reduced pressure. Purification by flash chromatography using a prepacked 25 g silica column [solvent A: EtOAc; solvent B: hexanes; gradient: 2%A / 98%B (4 CV), 2%A / 98%B → 20%A / 80%B (10 CV), 20%A / 80%B (5.2 CV); flow rate: 35 mL/min; monitored at 254 and 280 nm] resulted in the TBS indole product 10 (0.05 g, 0.02 mmol, 45%, Rf = 0.64 (70:30 hexanes:EtOAc)) as a light tan solid. 1H NMR (CDCl3, 500 MHz): δ 8.03 (br s, 1H, NH), 7.53 (d, J = 8.7 Hz, 2H, ArH ), 7.13 (d, J = 8.5 Hz, 1H, ArH), 6.98 (d, J = 8.7 Hz, 2H, ArH), 6.80 (d, J = 8.5 Hz, 1H, ArH), 6.61 (d, J = 2.2 Hz, 1H, ArH), 3.86 (s, 6H, OCH3), 1.11 (s, 9H, C(CH3)3), 0.24 (s, 6H, Si(CH3)2). 13C NMR (CDCl3, 125 MHz): δ 159.3, 145.2, 137.5, 131.2, 130.2, 126.2, 125.9, 125.6, 114.6, 112.9, 108.5, 99.0, 57.0, 55.5, 26.3, 18.8, −4.2. HPLC: 21.73 min., purity at 254 nm 93.7%. HRMS (ESI+): m/z calculated for C22H30NO3Si [M+H]+ 384.1989, found 384.1990.
4.1.8. 2-(3′-tert-Butyldimethylsilyloxy-4′-methoxyphenyl)-6,7-dimethoxyindole (11)
To a solution of 2,3-dimethoxyaniline (2.19 mL, 16.3 mmol) dissolved in N,N-dimethylaniline (20 mL) was added bromoacetophenone 3 (2.93 g, 8.16 mmol). The solution was heated to reflux and stirred at 150 °C for 12 h. Upon completion of the reaction, the reaction mixture was cooled to room temperature and extracted with EtOAc (3 × 50 mL). The combined organic extract was dried over Na2SO4 and concentrated under reduced pressure. Purification by flash chromatography using a prepacked 50 g silica column [solvent A: EtOAc; solvent B: hexanes; gradient: 5%A / 95%B (4 CV), 5%A / 95%B → 40%A / 60%B (10 CV), 40%A / 60%B (2 CV); flow rate: 50 mL/min; monitored at 254 and 280 nm] resulted in the desired 6,7-dimethoxy-2-phenylindole 11 (1.43 g, 3.47 mmol, 43%, Rf = 0.40 (80:20 hexanes:EtOAc)) as a tan solid. 1H NMR (CDCl3, 500 MHz): δ 8.63 (br s, 1H, NH), 7.33 (d, J = 8.5 Hz, 1H, ArH), 7.28 (d, J = 2.2 Hz, 1H, ArH), 7.26 (dd, J = 8.5 Hz, 2.2 Hz 1H, ArH), 6.92 (dd, J = 8.4 Hz, 1.4 Hz, 2H, ArH), 6.71 (d, J = 2.2 Hz, 1H, ArH), 4.15 (s, 3H, OCH3), 4.01 (s, 3H, OCH3), 3.88 (s, 3H, OCH3), 1.15 (s, 9H, C(CH3)3), 0.31 (s, 6H, Si(CH3)2). 13C NMR (CDCl3, 125 MHz): δ 150.7, 147.1, 145.4, 137.9, 134.2, 131.2, 126.0, 125.7, 118.4, 118.1, 115.2, 112.3, 108.7, 99.0, 60.9, 57.3, 55.4, 25.8, 18.5, −4.5. HPLC: 21.28 min., purity at 254 nm >99%. HRMS (ESI+): m/z calculated for C23H32NO4Si [M+H]+ 414.2095, found 414.2095.
4.1.9. 2-(3′-tert-Butyldimethylsiloxy-4′-methoxyphenyl)-3-(3″,5″dinitrobenzoyl)-6-methoxyindole (13)
To a solution of compound 5 (0.50 g, 1.30 mmol) in o-dichlorobenzene (20 mL) was added 3,5-dinitrobenzoylchloride (0.45 g, 1.90 mmol). The reaction mixture was heated to reflux at 160 °C for 12 h. The o-dichlorobenzene was removed by simple distillation, and the resulting dark colored solid was subjected to flash chromatography using a prepacked 50 g silica column [solvent A: EtOAc; solvent B: hexanes; gradient: 7%A / 93%B (4 CV), 7%A / 93%B → 60%A / 40%B (11 CV), 60%A / 40%B (2 CV); flow rate: 40 mL/min; monitored at 254 and 280 nm] resulting in TBS-indole 13 as a pale yellow powder (0.40 g, 0.69 mmol, 53%, Rf = 0.59 (70:30 hexanes:EtOAc)). 1H NMR (CDCl3, 500 MHz): δ 8.85 (t, J = 2.0 Hz, 1H, ArH), 8.62 (d, J = 2.0 Hz, 2H, ArH), 8.60 (br s, 1H, NH) 8.15 (d, J = 8.5 Hz, 1H, ArH), 7.00 (dd, J = 8.5 Hz, 2.0 Hz, 1H, ArH), 6.95 (d, 2.0 Hz, 1H, ArH), 6.87 (dd, J = 8.0 Hz, 2.0 Hz, 1H, ArH), 6.61 (d, J = 8.5 Hz, 1H, ArH), 6.56 (d, J = 2.5 Hz, 1H, ArH), 3.90 (s, 3H, OCH3), 3.70 (s, 3H, OCH3), 0.89 (s, 9H, C(CH3)3), 0.00 (s, 6H, Si(CH3)2). 13C NMR (CDCl3, 125 MHz): δ 187.3, 158.1, 152.1, 147.8, 145.3, 145.1, 143.1, 136.5, 129.1, 123.5, 123.4, 122.7, 122.4, 122.3, 120.1, 112.8, 112.5, 111.8, 95.0, 55.9, 55.5, 25.6, 18.4, −4.8. HPLC: 20.28 min., purity at 254 nm 93.1%. HRMS (ESI+): m/z calculated for C29H32N3O8Si [M+H]+ 578.1953, found 578.1950.
4.1.10. 2-(3′-tert-Butyldimethylsiloxy-4′-methoxyphenyl)-3-(4″-nitrobenzoyl)-6-methoxyindole (14)
To a solution of compound 5 (0.20 g, 0.52 mmol) in o-dichlorobenzene (10 mL) was added 3-nitrobenzoylchloride (0.15 g, 0.78 mmol). The reaction mixture was heated to reflux at 160 °C for 12 h. The o-dichlorobenzene was removed by simple distillation, and the resulting dark colored solid was subjected to flash chromatography using a prepacked 100 g silica column [solvent A: EtOAc; solvent B: hexanes; gradient: 7%A / 93%B (4 CV), 7%A / 93%B → 60%A / 40%B (10 CV), 60%A / 40%B (8.8 CV); flow rate: 40 mL/min; monitored at 254 and 280 nm] resulting in TBS-indole 14 as a yellow powder (0.14 g, 0.28 mmol, 51%, Rf = 0.36 (70:30 hexanes:EtOAc)). 1H NMR (CDCl3, 500 MHz): δ 8.37 (br s, 1H, NH), 8.06 (d, J = 8.5 Hz, 1H, ArH), 7.97 (d, J = 8.5 Hz, 2H, ArH) 7.69 (d, J = 8.5 Hz, 2H, ArH), 6.97 (dd, J = 8.5 Hz, 2.0 Hz, 1H, ArH), 6.93 (d, J = 2.5 Hz, 1H, ArH), 6.75 (m, 2H, ArH), 6.58 (d, J = 8.0 Hz, 1H, ArH), 3.89 (s, 3H, OCH3), 3.71 (s, 3H, OCH3), 0.97 (s, 9H, C(CH3)3), 0.07 (s, 6H, Si(CH3)2). 13C NMR (CDCl3, 125 MHz): δ 190.9, 157.8, 152.1, 148.9, 145.6, 145.2, 144.4, 136.5, 130.3, 124.1, 123.7, 123.0, 122.69, 122.67, 121.7, 113.0, 112.3, 111.7, 94.9, 55.9, 55.6, 25.8, 18.6, −4.6. HPLC: 20.23 min., purity at 254 nm >99%. HRMS (ESI+): m/z calculated for C29H33N2O6Si [M+H]+ 533.2102, found 533.2100.
4.1.11. 2-(3′-tert-Butyldimethylsiloxy-4′-methoxyphenyl)-3-(3″-nitrobenzoyl)-6-methoxyindole (15)
To a solution of compound 5 (0.10 g, 0.26 mmol) in o-dichlorobenzene (10 mL) was added 3-nitrobenzoylchloride (0.07 g, 0.39 mmol). The reaction mixture was heated to reflux at 160 °C for 12 h. The o-dichlorobenzene was removed by simple distillation, and the resulting dark colored solid was subjected to flash chromatography using a prepacked 25 g silica column [solvent A: EtOAc; solvent B: hexanes; gradient: 12%A / 88%B (4 CV), 12%A / 88%B → 100%A / 0%B (10 CV), 100%A / 0%B (2.8 CV); flow rate: 25 mL/min; monitored at 254 and 280 nm] resulting in TBS-indole 15 as pale yellow crystals (0.13 g, 0.25 mmol, 94%, Rf = 0.63 (50:50 hexanes:EtOAc)). 1H NMR (CDCl3, 500 MHz): δ 9.15 (br s, 1H, NH), 8.30 (t, J = 2.0 Hz, 1H, ArH), 8.06 (ddd, J = 8.0 Hz, 2.0 Hz, 1.0 Hz, 1H, ArH) 8.03 (d J = 9.5 Hz, 1H, ArH), 7.88 (dt, J = 8.0 Hz, 1.0 Hz, 1H, ArH), 7.29 (t, J = 8.0 Hz, 1H, ArH), 6.92 (dd, J = 7.0 Hz, 2.0 Hz, 1H, ArH), 6.91 (d, J = 2.0 Hz, 1H, ArH), 6.78 (dd, J = 8.0 Hz, 2.0 Hz, 1H, ArH), 6.66 (d, J = 2.5 Hz, 1H, ArH), 6.50 (d, J = 8.5 Hz, 1H, ArH), 3.82 (s, 3H, OCH3), 3.65 (s, 3H, OCH3), 0.91 (s, 9H, C(CH3)3), 0.00 (s, 6H, Si(CH3)2). 13C NMR (CDCl3, 125 MHz): δ 190.4, 157.6, 151.7, 147.4, 144.9, 144.6, 141.3, 136.6, 135.0, 128.9, 125.4, 124.7, 124.1, 123.4, 122.7, 122.3, 122.0, 112.4, 112.3, 111.6, 94.9, 55.7, 55.4, 25.7, 18.4, −4.7. HPLC: 20.13 min., purity at 254 nm >99%. HRMS (ESI+): m/z calculated for C29H33N2O6Si [M+H]+ 533.2102, found 533.2100.
4.1.12. 2-(3′-tert-Butyldimethylsiloxy-4′-methoxyphenyl)-3-(3″,4″,5″-trifluorobenzoyl)-6-methoxyindole (16)
To a solution of compound 5 (0.61 g, 1.59 mmol) in o-dichlorobenzene (10 mL) was added 3,4,5-trifluorobenzoylchloride (3.12 mL, 2.38 mmol). The reaction mixture was heated to reflux at 160 °C for 12 h. The o-dichlorobenzene was removed by simple distillation, and the resulting dark colored solid was subjected to flash chromatography using a prepacked 100 g silica column [solvent A: EtOAc; solvent B: hexanes; gradient: 7%A / 93%B (4 CV), 7%A / 97%B → 60%A / 40%B (10 CV), 60%A / 40%B (5.5 CV); flow rate: 40 mL/min; monitored at 254 and 280 nm] resulting in TBS-indole 16 as a white powder (0.70 g, 1.29 mmol, 81%, Rf = 0.48 (50:50 hexanes:EtOAc)). 1H NMR (CDCl3, 500 MHz): δ 8.44 (br s, 1H, NH), 7.95 (d, J = 9.0 Hz, 1H, ArH), 7.25 (m, 2H, ArH) 6.94 (dd, J = 8.5 Hz, 2.5 Hz, 1H, ArH), 6.91 (d, J = 2.0 Hz, 1H, ArH), 6.83 (dd, J = 8.0 Hz, 2.0 Hz, 1H, ArH), 6.79 (d, J = 2.0 Hz, 1H, ArH), 6.70 (d, J = 8.5 Hz, 1H, ArH), 3.87 (s, 3H, OCH3), 3.78 (s, 3H, OCH3), 0.97 (s, 9H, C(CH3)3), 0.09 (s, 6H, Si(CH3)2). 13C NMR (CDCl3, 125 MHz): δ 189.1, 157.7, 152.1, 150.6 (ddd, JC-F = 250.0 Hz, 10.0 Hz, 3.1 Hz), 145.4, 143.5, 141.8 (dt, JC-F = 255.9 Hz, 15.5 Hz), 136.5, 135.6 (d, JC-F = 3.9 Hz), 124.4, 123.2, 122.8, 121.4, 121.6, 114.1 (dd, JC-F = 16.9 Hz, 5.1 Hz), 112.3, 112.2, 112.0, 94.8, 55.9, 55.6, 25.7, 18.5, −4.8. 19F NMR (CDCl3, 470 MHz): δ −134.0 (dd, J = 21.2 Hz, 7.5 Hz, 2F, ArF), −155.6 (tt, J = 20.2 Hz, 6.6 Hz, 1F, ArF). HPLC: 21.32 min., purity at 254 nm >99%. HRMS (ESI+): m/z calculated for C29H30F3NNaO4Si [M+Na]+ 564.1788, found 564.1786.
4.1.13. 2-(3′-tert-Butyldimethylsiloxy-4′-methoxyphenyl)-3-(4″-fluorobenzoyl)-6-methoxyindole (17)
To a solution of compound 5 (0.10 g, 0.26 mmol) in o-dichlorobenzene (10 mL) was added 4-fluorobenzoylchloride (0.05 mL, 0.39 mmol). The reaction mixture was heated to reflux at 160 °C for 12 h. The o-dichlorobenzene was removed by simple distillation, and the resulting dark colored solid was subjected to flash chromatography using a prepacked 25 g silica column [solvent A: EtOAc; solvent B: hexanes; gradient: 12%A / 88%B (4 CV), 12%A / 88%B → 100%A / 0%B (10 CV), 100%A / 0%B (2 CV); flow rate: 25 mL/min; monitored at 254 and 280 nm] resulting in TBS-indole 17 as pale yellow crystals (0.09 g, 0.18 mmol, 69%, Rf = 0.73 (50:50 hexanes:EtOAc)). 1H NMR (CDCl3, 500 MHz): δ 8.59 (br s, 1H, NH), 7.88 (d, J = 9.5 Hz, 1H, ArH), 7.65 (m, 2H, ArH), 6.89 (m, 2H, ArH), 6.82 (m, 4H, ArH), 6.60 (d, J = 8.0 Hz, 1H, ArH), 3.84 (s, 3H, OCH3), 3.73 (s, 3H, OCH3), 0.96 (s, 9H, C(CH3)3), 0.08 (s, 6H, Si(CH3)2). 13C NMR (CDCl3, 125 MHz): δ 191.7, 164.8 (d, JC-F = 251 Hz), 157.4, 151.7, 145.1, 142.8, 136.4, 136.09, 136.06, 132.2 (d, JC-F= 9 Hz), 124.6, 123.4, 123.1, 122.4, 121.6, 114.9 (d, JC-F = 22 Hz), 113.0, 111.8, 94.7, 55.8, 55.5, 25.8, 18.6, −4.6. 19F NMR (CDCl3, 470 MHz): δ −108.1 (m, 1F, ArF). HPLC: 20.59 min., purity at 254 nm >99%. HRMS (ESI+): m/z calculated for C29H33FNO4Si [M+H]+ 506.2157, found 506.2155.
4.1.14. 2-(3′-tert-Butyldimethylsiloxy-4′-methoxyphenyl)-3-(3″,5″-bis-trifluoromethylbenzoyl)-6-methoxyindole (18)
To a solution of compound 5 (0.20 g, 0.52 mmol) in o-dichlorobenzene (15 mL) was added 3,5-bis-trifluoromethylbenzoyl chloride (0.14 mL, 0.78 mmol). The reaction mixture was heated to reflux at 160 °C for 12 h. The o-dichlorobenzene was removed by simple distillation, and the resulting dark colored solid was subjected to flash chromatography using a prepacked 50 g silica column [solvent A: EtOAc; solvent B: hexanes; gradient: 7%A / 93%B (4 CV), 7%A / 93%B → 60%A / 40%B (10 CV), 60%A / 40%B (2 CV); flow rate: 40 mL/min; monitored at 254 and 280 nm] resulting in TBS-indole 18 as pale orange crystals (0.26 g, 0.42 mmol, 81%, Rf = 0.47 (70:30 hexanes:EtOAc)). 1H NMR (CDCl3, 500 MHz): δ 8.70 (br s, 1H, NH), 8.07 (d, J = 9.0 Hz, 1H, ArH), 8.03 (s, 2H, ArH) 7.74 (s, 1H, ArH), 6.96 (dd, J = 9.0 Hz, 2.5 Hz, 1H, ArH), 6.92 (d, J = 2.0 Hz, 1H, ArH), 6.85 (dd, J = 8.0 Hz, 2.0 Hz, 1H, ArH), 6.65 (d, J = 8.5 Hz, 1H, ArH), 6.62 (d, J = 2.5 Hz, 1H, ArH), 3.86 (s, 3H, OCH3), 3.71 (s, 3H, OCH3), 0.91 (s, 9H, C(CH3)3), 0.00 (s, 6H, Si(CH3)2). 13C NMR (CDCl3, 125 MHz): δ 189.5, 157.8, 152.0, 145.3, 144.5, 141.5, 136.6, 131.3 (q, JC-F = 33 Hz), 129.7 (m), 124.47 (q, Jc-f = 7 Hz), 124.46, 124.0, 123.1 (q, JC-f = 275 Hz), 122.9, 122.7, 122.5, 122.2, 112.4, 112.1, 94.9, 55.8, 55.5, 25.6, 18.4, −4.8. 19F NMR (CDCl3, 470 MHz): δ −62.8 (s, 6F, CF3). HPLC: 22.22 min., purity at 254 nm 96.3%. HRMS (ESI+): m/z calculated for C31H32F6NO4Si [M+H]+ 624.1999, found 624.1997.
4.1.15. (2-(3′-tert-Butyldimethylsilyloxy-4′-methoxyphenyl)-3-(3″-trifluoromethoxybenzoyl)-6-methoxyindole (19)
To a solution of compound 5 (1.14 g, 2.97 mmol) in o-dichlorobenzene (15 mL) was added 3-trifluoromethoxybenzoyl chloride (0.70 mL, 4.45 mmol). The reaction mixture was heated to reflux at 170 °C for 12 h. The o-dichlorobenzene was removed by simple distillation, and the resulting dark green colored solid was subjected to flash chromatography using a prepacked 50 g silica column [solvent A: EtOAc; solvent B: hexanes; gradient: 7%A / 93%B (4 CV), 7%A / 93%B → 60%A / 40%B (10 CV), 60%A / 40%B (2 CV); flow rate: 40 mL/min; monitored at 254 and 280 nm] resulting in TBS-indole 19 as a yellow powder (1.21 g, 2.11 mmol, 71%, Rf = 0.43 (70:30 hexanes:EtOAc)). 1H NMR (CDCl3, 500 MHz): δ 8.31 (br s, 1H, NH), 7.96 (d, J = 8.4 Hz, 1H, ArH), 7.52 (dt, J = 7.5 Hz, 1.3 Hz, 1H, ArH) 7.48 (s, 1H, ArH), 7.17 (t, J = 7.8 Hz, 1H, ArH), 7.13 (d, J = 8.2 Hz, 1H, ArH), 6.93 (m, 2H, ArH), 6.81 (m, 2H, ArH), 6.62 (d, J = 9.0 Hz, 1H, ArH), 3.88 (s, 3H, OCH3), 3.73 (s, 3H, OCH3), 0.97 (s, 9H, C(CH3)3), 0.08 (s, 6H, Si(CH3)2). 13C NMR (CDCl3, 125 MHz): δ 191.8, 157.4, 151.6, 148.8, 144.9, 144.1, 142.1, 136.6, 129.2, 128.0, 124.3, 123.6, 123.5, 122.9, 122.2, 122.0, 121.4, 120.4 (q, JC-F = 256 Hz), 112.6, 112.0, 111.6, 94.8, 55.7, 55.3, 25.7, 18.4, −4.7. 19F NMR (CDCl3, 470 MHz): δ −57.8 (s, 3F, OCF3). HPLC: 21.53 min., purity at 254 nm >99%. HRMS (ESI+): m/z calculated for C30H33F3NO5Si [M+H]+ 572.2075, found 572.2071.
4.1.16. (2-(3′-tert-Butyldimethylsilyloxy-4′-methoxyphenyl)-3-(4″-trifluoromethoxybenzoyl)-6-methoxyindole (20)
To a solution of compound 5 (1.14 g, 2.97 mmol) in o-dichlorobenzene (15 mL) was added 4-trifluoromethoxybenzoyl chloride (0.70 mL, 4.45 mmol). The reaction mixture was heated to reflux at 170 °C for 12 h. The o-dichlorobenzene was removed by simple distillation, and the resulting dark green colored solid was subjected to flash chromatography using a prepacked 50 g silica column [solvent A: EtOAc; solvent B: hexanes; gradient: 7%A / 93%B (4 CV), 7%A / 93%B → 60%A / 40%B (10 CV), 60%A / 40%B (1.1 CV); flow rate: 40 mL/min; monitored at 254 and 280 nm] resulting in TBS-indole 20 as a yellow powder (0.92 g, 1.61 mmol, 54%, Rf = 0.43 (70:30 hexanes:EtOAc)). 1H NMR (CDCl3, 500 MHz): δ 8.29 (br s, 1H, NH), 8.00 (d, J = 8.6 Hz, 1H, ArH), 7.64 (d, J = 8.7 Hz, 2H, ArH) 6.97 (d, J = 8.0 Hz, 2H, ArH), 6.93 (m, 2H, ArH), 6.83 (d, J = 2.2 Hz, 1H, ArH), 6.73 (dd, J = 8.3 Hz, 2.2 Hz, 1H, ArH), 6.58 (d, J = 8.4 Hz, 1H, ArH), 3.88 (s, 3H, OCH3), 3.73 (s, 3H, OCH3), 0.98 (s, 9H, C(CH3)3), 0.10 (s, 6H, Si(CH3)2). 13C NMR (CDCl3, 125 MHz): δ 191.9, 157.3, 151.6, 151.0, 144.8, 144.1, 138.4, 136.6, 131.3, 124.2, 123.8, 122.8, 122.2, 121.4, 120.3 (q, JC-F = 256 Hz), 119.7, 112.6, 111.9, 111.3, 94.8, 55.6, 55.1, 25.6, 18.4, −4.8. 19F NMR (CDCl3, 470 MHz): δ −57.7 (s, 3F, OCF3). HPLC: 21.61 min., purity at 254 nm >99%. HRMS (ESI+): m/z calculated for C30H33F3NO5Si [M+H]+ 572.2075, found 572.2075.
4.1.17. 2-(3′-tert-Butyldimethylsiloxy-4′-methoxyphenyl)-3-(3″,4″,5″-trimethoxybenzoyl)-6-tert-butyldimethylsiloxyindole (21)
To a solution of compound 6 (0.19 g, 0.39 mmol) in o-dichlorobenzene (20 mL) was added 3,4,5-trimethoxybenzoyl chloride (0.13 g, 0.58 mmol). The reaction mixture was heated to reflux at 160 °C for 12 h. The o-dichlorobenzene was removed by simple distillation, and the resulting dark colored solid was subjected to flash chromatography using a prepacked 50 g silica column [solvent A: EtOAc; solvent B: hexanes; gradient: 7%A / 93%B (4 CV), 7%A / 93%B → 60%A / 40%B (10 CV), 60%A / 40%B (2 CV); flow rate: 40 mL/min; monitored at 254 and 280 nm] resulting in di-TBS-indole 21 as a pale yellow powder (0.04 g, 0.59 mmol, 20%, Rf = 0.33 (70:30 hexanes:EtOAc)). 1H NMR (CDCl3, 500 MHz): δ 8.31 (br s, 1H, NH), 7.90 (d, J = 8.5 Hz, 1H, ArH), 6.99 (s, 2H, ArH) 6.94 (dd, J = 8.5 Hz, 2.0 Hz, 1H, ArH), 6.89 (d, J = 2.0 Hz, 1H, ArH), 6.82 (dd, J = 8.5 Hz, 2.0 Hz, 1H, ArH), 6.76 (d, J = 2.5 Hz, 1H, ArH), 6.70 (d, J = 8.0 Hz, 1H, ArH), 3.79 (s, 3H, OCH3), 3.75 (s, 3H, OCH3), 3.69 (s, 6H, OCH3), 1.01 (s, 9H, C(CH3)3), 0.94 (s, 9H, C(CH3)3), 0.22 (s, 6H, Si(CH3)2), 0.04 (s, 6H, Si(CH3)2). 13C NMR (CDCl3, 125 MHz): δ 191.8, 153.0, 152.6, 151.7, 145.2, 142.3, 141.3, 136.5, 134.6, 125.2, 123.9, 122.3, 121.9, 116.7, 112.9, 111.8, 107.4, 105.1, 101.6, 60.9, 56.1, 55.5, 25.9, 25.8, 18.46, 18.45, −4.2, −4.8. HPLC: 23.31 min., purity at 254 nm 90.6%. HRMS (ESI+): m/z calculated for C37H52NO7Si2 [M+H]+ 678.3277, found 678.3279.
4.1.18. 2-(3′-tert-Butyldimethylsiloxy-4′-methoxyphenyl)-3-(3″,4″,5″-trimethoxybenzoyl)-4,5,6-trimethoxyindole (22)
To a solution of compound 7 (0.05 g, 0.11 mmol) in o-dichlorobenzene (10 mL) was added 3,4,5-trimethoxybenzoyl chloride (0.04 g, 0.17 mmol). The reaction mixture was heated to reflux at 160 °C for 12 h. The o-dichlorobenzene was removed by simple distillation, and the resulting dark green colored solid was subjected to flash chromatography using a prepacked 25 g silica column [solvent A: EtOAc; solvent B: hexanes; gradient: 12%A / 88%B (4 CV), 12%A / 88%B → 100%A / 0%B (10 CV), 100%A / 0%B (2 CV); flow rate: 25 mL/min; monitored at 254 and 280 nm] resulting in TBS-indole 22 as a pale yellow powder (0.03 g, 0.05 mmol, 46%, Rf = 0.50 (50:50 hexanes:EtOAc)). 1H NMR (CDCl3, 500 MHz): δ 8.25 (br s, 1H, NH), 7.19 (s, 2H, ArH), 6.99 (dd, J = 8.5 Hz, 2.5 Hz, 1H, ArH), 6.88 (d, J = 2.5 Hz, 1H, ArH), 6.75 (d, J = 8.0 Hz, 1H, ArH), 6.69 (s, 1H, ArH), 3.90 (s, 3H, OCH3), 3.85 (s, 3H, OCH3), 3.82 (s, 3H, OCH3), 3.76 (s, 3H, OCH3), 3.74 (s, 6H, OCH3), 3.72 (s, 3H, OCH3), 0.93 (s, 9H, C(CH3)3), 0.04 (s, 6H, Si(CH3)2). 13C NMR (CDCl3, 125 MHz): δ 193.4, 152.9, 152.1, 151.4, 146.4, 145.3, 142.2, 137.8, 136.6, 134.2, 132.4, 124.7, 121.1, 120.5, 116.7, 114.4, 112.3, 107.6, 89.8, 61.4, 61.0, 60.8, 56.4, 56.3, 55.6, 25.8, 18.5, −4.7. HPLC: 18.60 min., purity at 254 nm 90.7%. HRMS (ESI+): m/z calculated for C34H44NO9Si [M+H]+ 638.2780, found 638.2780.
4.1.19. 2-(4′-Methoxyphenyl)-3-(3″,4″,5″-trimethoxybenzoyl)-6-methoxy-7-tert-butyldimethylsilyloxyindole (23)
To a solution of compound 10 (0.05 g, 0.13 mmol) in o-dichlorobenzene (10 mL) was added 3,4,5-trimethoxybenzoyl chloride (0.05 g, 0.20 mmol). The reaction mixture was heated to reflux at 160 °C for 12 h. The o-dichlorobenzene was removed by simple distillation, and the resulting dark green colored solid was subjected to flash chromatography using a prepacked 25 g silica column [solvent A: EtOAc; solvent B: hexanes; gradient: 10%A / 90%B (4 CV), 10%A / 90%B → 80%A / 20%B (10 CV), 80%A / 20%B (2 CV); flow rate: 25 mL/min; monitored at 254 and 280 nm] resulting in TBS-indole 23 as a yellow powder (0.06 g, 0.10 mmol, 76%, Rf = 0.36 (60:40 hexanes:EtOAc)). 1H NMR (CDCl3, 500 MHz): δ 8.20 (br s, 1H, NH), 7.56 (d, J = 8.7 Hz, 1H, ArH), 7.26 (d, J = 9.0 Hz, 2H, ArH) 6.96 (s, 2H, ArH), 6.94 (d, J = 8.7 Hz, 1H, ArH), 6.78 (d, J = 8.6 Hz, 2H, ArH), 3.88 (s, 3H, OCH3), 3.80 (s, 3H, OCH3), 3.76 (s, 3H, OCH3), 3.70 (s, 6H, OCH3), 1.09 (s, 9H, C(CH3)3), 0.26 (s, 6H, Si(CH3)2). 13C NMR (CDCl3, 125 MHz): δ 192.0, 160.2, 152.6, 146.0, 142.8, 141.3, 134.9, 130.3, 129.99, 129.95, 125.0, 124.8, 114.4, 114.2, 113.4, 109.9, 107.5, 61.0, 56.7, 56.2, 55.5, 26.3, 18.8, −4.1. HPLC: 20.21 min., purity at 254 nm >99%. HRMS (ESI+): m/z calculated for C32H39NNaO7Si [M+Na]+ 600.2388, found 600.2383.
4.1.20. 2-(3′-tert-Butyldimethylsiloxy-4′-methoxyphenyl)-3-(3″,4″,5″-trimethoxybenzoyl)-6,7-dimethoxyindole (24)
To a solution of compound 11 (0.25 g, 0.60 mmol) in o-dichlorobenzene (10 mL) was added 3,4,5-trimethoxybenzoyl chloride (0.15 g, 0.66 mmol). The reaction mixture was heated to reflux at 160 °C for 12 h. The o-dichlorobenzene was removed by simple distillation, and the resulting dark green colored solid was subjected to flash chromatography using a prepacked 50 g silica column [solvent A: EtOAc; solvent B: hexanes; gradient: 7%A / 93%B (4 CV), 7%A / 93%B → 60%A / 40%B (10 CV), 60%A / 40%B (5.2 CV); flow rate: 50 mL/min; monitored at 254 and 280 nm] resulting in TBS-indole 24 as a yellow powder (0.08 g, 0.14 mmol, 23%, Rf = 0.17 (70:30 hexanes:EtOAc)). 1H NMR (CDCl3, 500 MHz): δ 8.53 (br s, 1H, NH), 7.71 (d, J = 8.5 Hz, 1H, ArH), 6.98 (m, 4H, ArH) 6.77 (d, J = 2.0 Hz, 1H, ArH), 6.73 (d, J = 8.5 Hz, 1H, ArH), 4.06 (s, 3H, OCH3) 3.96 (s, 3H, OCH3), 3.79 (s, 3H, OCH3), 3.76 (s, 3H, OCH3), 3.69 (s, 6H, OCH3), 0.94 (s, 9H, C(CH3)3), 0.03 (s, 6H, Si(CH3)2). 13C NMR (CDCl3, 125 MHz): δ 191.8, 152.6, 151.8, 148.0, 145.2, 142.8, 141.3, 134.6, 134.0, 130.2, 125.2, 125.1, 122.3, 122.2, 116.8, 113.1, 111.8, 110.4, 107.4, 61.3, 60.9, 57.3, 56.1, 55.5, 25.8, 18.5, −4.7. HPLC: 19.24 min., purity at 254 nm >99%. HRMS (ESI+): m/z calculated for C33H41NNaO8Si [M+Na]+ 630.2494, found 630.2491.
4.1.21. 2-(3′-Hydroxy-4′-methoxyphenyl)-3-(3″,5″-dinitrobenzoyl)-6-methoxyindole (25)
To a well-stirred solution of compound 13 (0.40 g, 0.69 mmol) in THF (10 mL) at 0 °C was added tetrabutylammonium fluoride (TBAF) (1.03 mL, 1.03 mmol, 1 M in THF) dropwise. The reaction mixture was stirred for 30 min while warming to room temperature. The reaction mixture was quenched with water (10 mL) and extracted with EtOAc (3 × 10 mL). The combined organic extract was dried over Na2SO4 and concentrated under reduced pressure. Purification by flash column chromatography using a prepacked 50 g silica column [solvent A: EtOAc; solvent B: hexanes; gradient: 7%A / 93%B (4 CV), 7%A / 93%B → 60%A / 40%B (10 CV), 60%A / 40%B (2 CV); flow rate: 40 mL/min; monitored at 254 and 280 nm] afforded the desired indole free phenol ligand 25 (0.15 g, 0.33 mmol, 47%, Rf = 0.12 (70:30 hexanes:EtOAc)) as a yellow powder. 1H NMR ((CD3)2SO, 500 MHz): δ 12.21 (br s, 1H, NH), 9.06 (br s, 1H, OH), 8.68 (t, J = 2.0 Hz, 1H, ArH), 8.41 (d, J = 2.0 Hz, 2H, ArH) 8.05 (d, J = 8.5 Hz, 1H, ArH), 6.97 (dd, J = 2.0 Hz, 1H, ArH), 6.92 (dd, J = 8.5 Hz, 2.0 Hz, 1H, ArH), 6.71 (dd, J = 8.0 Hz, 2.0 Hz, 1H, ArH), 6.68 (d, J = 8.5 Hz, 1H, ArH), 6.54 (d, J = 2.0 Hz, 1H, ArH), 3.83 (s, 3H, OCH3), 3.65 (s, 3H, OCH3. 13C NMR ((CD3)2SO, 125 MHz): δ 186.7, 156.8, 148.2, 147.1, 146.7, 146.1, 142.8, 136.7, 128.5, 123.3, 121.77, 121.75, 121.7, 119.3, 117.2, 112.0, 111.7, 111.1, 95.0, 55.7, 55.3. HPLC: 13.87 min., purity at 254 nm 93.4%. HRMS (ESI+): m/z calculated for C23H18N3O8 [M+H]+ 464.1088, found 464.1087.
4.1.22. 2-(3′-Hydroxy-4′-methoxyphenyl)-3-(4″-nitrobenzoyl)-6-methoxyindole (26)
To a well-stirred solution of compound 14 (0.14 g, 0.27 mmol) in THF (10 mL) at 0 °C was added TBAF (0.40 mL, 0.40 mmol, 1 M in THF) dropwise. The reaction mixture was stirred for 30 min while warming to room temperature. The reaction mixture was quenched with water (10 mL) and extracted with EtOAc (3 × 10 mL). The combined organic extract was dried over Na2SO4 and concentrated under reduced pressure. Purification by flash column chromatography using a prepacked 50 g silica column [solvent A: EtOAc; solvent B: hexanes; gradient: 12%A / 88%B (3 CV), 12%A / 88%B → 100%A / 0%B (11 CV), 100%A / 0%B (2 CV); flow rate: 40 mL/min; monitored at 254 and 280 nm] afforded the desired indole phenol ligand 26 (0.02 g, 0.05 mmol, 19%, Rf 0.23 (50:50 hexanes:EtOAc)) as a yellow powder. 1H NMR ((CD3)2SO, 500 MHz): δ 12.04 (br s, 1H, NH), 9.05 (s, 1H, OH), 7.98 (d, J = 8.0 Hz, 2H, ArH), 7.83 (d, J = 8.5 Hz, 1H, ArH), 7.61 (d, J = 8.5 Hz, 2H, ArH), 6.95 (d, J = 1.0 Hz, 1H, ArH), 6.85 (dd, J = 8.5 Hz, 1.5 Hz, 1H, ArH), 6.71 (s, 1H, ArH), 6.68 (d, J = 8.5 Hz, 1H, ArH), 6.63 (d, J = 8.5 Hz, 1H, ArH), 3.81 (s, 3H, OCH3), 3.65 (s, 3H, OCH3). 13C NMR ((CD3)2SO, 125 MHz): δ 190.1, 156.6, 148.3, 148.0, 146.1, 146.0, 145.6, 136.6, 129.8, 123.9, 122.6, 122.0, 121.47, 121.45, 116.8, 111.62, 111.55, 111.4, 94.9, 55.7, 55.3. HPLC: 13.19 min., purity at 254 nm >99%. HRMS (ESI+): m/z calculated for C23H19N2O6 [M+H]+ 419.1238, found 419.1237.
4.1.23.2-(3′-Hydroxy-4′-methoxyphenyl)-3-(3″-nitrobenzoyl)-6-methoxyindole (27)
To a well-stirred solution of compound 15 (0.13 g, 0.25 mmol) in THF (10 mL) at 0 °C was added TBAF (0.38 mL, 0.38 mmol, 1 M in THF) dropwise. The reaction mixture was stirred for 30 min while warming to room temperature. The reaction mixture was quenched with water (10 mL) and extracted with EtOAc (3 × 10 mL). The combined organic extract was dried over Na2SO4 and concentrated under reduced pressure. Purification by flash column chromatography using a prepacked 50 g silica column [solvent A: EtOAc; solvent B: hexanes; gradient: 15%A / 85%B (4.5 CV), 40%A / 60%B (16 CV), 40%A / 60%B → 100%A / 0%B (2 CV), 100%A / 0%B (10.5 CV); flow rate: 40 mL/min; monitored at 254 and 280 nm] afforded the desired indole phenol ligand 27 (0.10 g, 0.25 mmol, Rf = 0.28 (50:50 hexanes:EtOAc)) quantitatively as a yellow powder. 1H NMR ((CD3)2CO, 500 MHz): δ 10.95 (br s, 1H, NH), 8.25 (t, J = 2.0 Hz, 1H, ArH), 8.13 (ddd, J = 8.0 Hz, 2.0 Hz, 1.0 Hz, 1H, ArH), 8.02 (d, J = 8.5 Hz, 1H, ArH), 7.93 (dt, J = 7.5 Hz, 1.0 Hz, 1H, ArH), 7.69 (s, 1H, OH), 7.49 (t, J = 8.0 Hz, 1H, ArH), 7.06 (d, J = 2.0 Hz, 1H, ArH), 6.91 (dd, J = 9.0 Hz, 2.5 Hz, 1H, ArH), 6.81 (d, J = 2.0 Hz, 1H, ArH), 6.78 (dd, J = 8.5 Hz, 2.0 Hz, 1H, ArH), 6.73 (d, J = 8.0 Hz, 1H, ArH), 3.86 (s, 3H, OCH3), 3.75 (s, 3H, OCH3). 13C NMR ((CD3)2CO, 125 MHz): δ 190.5, 158.5, 149.2, 148.5, 147.4, 146.0, 143.1, 138.0, 135.9, 130.1, 125.8, 125.7, 124.8, 123.7, 123.0, 122.9, 117.5, 113.0, 112.7, 112.3, 95.8, 56.5, 56.0. HPLC: 12.97 min., purity at 254 nm >99%. HRMS (ESI+): m/z calculated for C23H19N2O6 [M+H]+ 419.1238, found 419.1236.
4.1.24. 2-(3′-Hydroxy-4′-methoxyphenyl)-3-(3″,4″,5″-trifluorobenzoyl)-6-methoxyindole (28)
To a well-stirred solution of compound 16 (0.70 g, 1.29 mmol) in THF (10 mL) at 0 °C was added TBAF (1.94 mL, 1.94 mmol, 1 M in THF) dropwise. The reaction mixture was stirred for 30 min while warming to room temperature. The reaction mixture was quenched with water (10 mL) and extracted with EtOAc (3 × 10 mL). The combined organic extract was dried over Na2SO4 and concentrated under reduced pressure. Purification by flash column chromatography using a prepacked 50 g silica column [solvent A: EtOAc; solvent B: hexanes; gradient: 12%A / 88%B (4 CV), 12%A / 88%B → 100%A / 0%B (10 CV), 100%A / 0%B (2 CV); flow rate: 40 mL/min; monitored at 254 and 280 nm] afforded the desired indole free phenol ligand 28 (0.35 g, 0.41 mmol, 64%, Rf = 0.18 (50:50 hexanes:EtOAc)) as a tan powder. 1H NMR ((CD3)2SO, 500 MHz): δ 12.02 (br s, 1H, NH), 9.12 (br s, 1H, OH), 7.85 (d, J = 8.5 Hz, 1H, ArH), 7.28 (m, 2H, ArH), 6.94 (d, J = 2.0 Hz, 1H, ArH), 6.86 (dd, J = 9.0 Hz, 2.0 Hz, 1H, ArH), 6.80 (d, J = 8.0 Hz, 1H, ArH), 6.71 (d, J = 2.0 Hz, 1H, ArH), 6.69 (dd, J = 8.0 Hz, 2.0 Hz, 1H, ArH), 3.81 (s, 3H, OCH3), 3.73 (s, 3H, OCH3). 13C NMR ((CD3)2SO, 125 MHz): δ 188.0, 156.6, 149.5 (ddd, JC-f = 247.6 Hz, 10.2 Hz, 3.0 Hz) 148.2, 146.1, 145.5, 140.0 (dt, JC-f = 251.4 Hz, 15.5 Hz), 136.71 (d, JC-f = 5.6 Hz), 136.65, 124.2, 122.0, 121.4, 121.2, 116.8, 113.5 (dd, JC-f = 16.6 Hz, 4.8 Hz), 111.8, 111.5, 110.8, 94.9, 55.8, 55.3. 19F NMR ((CD3)2SO, 470 MHz): δ −135.5 (dd, J = 21.6 Hz, 8.5 Hz, 2F, ArF), −158.6 (tt, J = 21.2 Hz, 6.6 Hz, 1F, ArF). HPLC: 14.33 min., purity at 254 nm >99%. HRMS (ESI+): m/z calculated for C23H17F3NO4 [M+H]+ 428.1104, found 428.1104.
4.1.25. 2-(3′-Hydroxy-4′-methoxyphenyl)-3-(4″-fluorobenzoyl)-6-methoxyindole (29)
To a well-stirred solution of compound 17 (0.09 g, 0.18 mmol) in THF (10 mL) at 0 °C was added TBAF (0.30 mL, 0.30 mmol, 1 M in THF) dropwise. The reaction mixture was stirred for 30 min while warming to room temperature. The reaction mixture was quenched with water (10 mL) and extracted with EtOAc (3 × 10 mL). The combined organic extract was dried over Na2SO4 and concentrated under reduced pressure. Purification by flash column chromatography using a prepacked 50 g silica column [solvent A: EtOAc; solvent B: hexanes; gradient: 35%A / 65%B (5 CV), 35%A / 65%B → 50%A / 50%B (17.5 CV), 100%A / 0%B (7 CV); flow rate: 40 mL/min; monitored at 254 and 280 nm] afforded the desired indole phenol ligand 29 (0.05 g, 0.12 mmol, 64%, Rf = 0.31 (50:50 hexanes:EtOAc)) as a tan powder. 1H NMR ((CD3)2CO, 500 MHz): δ 10.78 (br s, 1H, NH), 7.77 (d, J = 8.5 Hz, 1H, ArH), 7.66 (m, 2H, ArH, 1H, OH) 7.03 (d, J = 2.5 Hz, 1H, ArH), 6.96 (m, 2H, ArH), 6.92 (d, J = 2.0 Hz, 1H, ArH), 6.84 (dd, J = 9.0 Hz, 2.5 Hz, 1H, ArH), 6.81 (dd, J = 8.0 Hz, 2.0 Hz, 1H, ArH), 6.78 (d, J = 8.5 Hz, 1H, ArH), 3.84 (s, 3H, OCH3), 3.79 (s, 3H, OCH3. 13C NMR ((CD3)2CO, 125 MHz): δ 190.6, 164.3 (d, JC-f = 248 Hz), 157.2, 147.9, 146.3, 143.0, 137.00, 136.98, 136.9, 131.8 (d, JC-f = 9 Hz), 125.1, 123.0, 121.7, 121.5, 116.0, 114.4 (d, Jc-f = 22 Hz), 111.17, 111.15, 94.5, 55.4, 55.9. 19F NMR ((CD3)2CO, 470 MHz): δ −110.8 (m, 1F, ArF). HPLC: 12.91 min., purity at 254 nm >99%. HRMS (ESI+): m/z calculated for C23H19FNO4 [M+H]+ 392.1293, found 392.1291.
4.1.26. 2-(3′-Hydroxy-4′-methoxyphenyl)-3-(3″,5″-bis-trifluoromethylbenzoyl)-6-methoxyindole (30)
To a well-stirred solution of compound 18 (0.26 g, 0.42 mmol) in THF (10 mL) at 0 °C was added TBAF (0.63 mL, 0.63 mmol, 1 M in THF) dropwise. The reaction mixture was stirred for 30 min while warming to room temperature. The reaction mixture was quenched with water (10 mL) and extracted with EtOAc (3 × 10 mL). The combined organic extract was dried over Na2SO4 and concentrated under reduced pressure. Purification by flash column chromatography using a prepacked 50 g silica column [solvent A: EtOAc; solvent B: hexanes; gradient: 7%A / 93%B (4 CV), 7%A / 93%B → 60%A / 40%B (10 CV), 60%A / 40%B (5.2 CV); flow rate: 40 mL/min; monitored at 254 and 280 nm] afforded the desired indole free phenol ligand 30 (0.22 g, 0.42 mmol, Rf = 0.21 (70:30 hexanes:EtOAc)) quantitatively as an orange powder. 1H NMR ((CD3)2SO, 500 MHz): δ 12.10 (br s, 1H, NH), 9.00 (br s, 1H, OH), 7.99 (m, 1H, ArH, 1H, OH), 7.90 (s, 2H, ArH) 6.96 (d, J = 2.0 Hz, 1H, ArH), 6.89 (dd, J = 9.0 Hz, 2.5 Hz, 1H, ArH), 6.64 (m, 3H, ArH), 3.82 (s, 3H, OCH3), 3.66 (s, 3H, OCH3). 13C NMR ((CD3)2SO, 125 MHz): δ 188.4, 156.7, 148.2, 146.3, 146.2, 142.2, 136.7, 129.7 (q, JC-F = 33 Hz), 128.9 (m), 123.7 (m), 123.5, 123.0 (q, JC-f = 272 Hz), 122.0, 121.6, 121.4, 116.9, 111.7, 111.6, 111.0, 94.9, 55.6, 55.3. 19F NMR ((CD3)2SO, 470 MHz): δ −61.4 (s, 6F, CF3). HPLC: 16.17 min., purity at 254 nm >99%. HRMS (ESI+): m/z calculated for C25H18F6NO4 [M+H]+ 510.1135, found 510.1134.
4.1.27. 2-(3′-Hydroxy-4′-methoxyphenyl)-3-(3″-trifluoromethoxybenzoyl)-6-methoxyindole (31)
To a well-stirred solution of compound 19 (1.21 g, 2.11 mmol) in THF (10 mL) at 0 °C was added TBAF (3.20 mL, 3.20 mmol, 1 M in THF) dropwise. The reaction mixture was stirred for 30 min while warming to room temperature. The reaction mixture was quenched with water (10 mL) and extracted with EtOAc (3 × 10 mL). The combined organic extract was dried over Na2SO4 and concentrated under reduced pressure. Purification by flash column chromatography using a prepacked 50 g silica column [solvent A: EtOAc; solvent B: hexanes; gradient: 12%A / 88%B (4 CV), 12%A / 88%B → 100%A / 0%B (10 CV), 100%A / 0%B (4 CV); flow rate: 50 mL/min; monitored at 254 and 280 nm] afforded the desired indole free phenol ligand 31 (0.67 g, 1.51 mmol, 71%, Rf = 0.33 (50:50 hexanes:EtOAc)) as an orange powder. 1H NMR ((CD3)2CO, 500 MHz): δ 10.89 (br s, 1H, NH), 7.89 (d, J = 8.7 Hz, 1H, ArH), 7.72 (s, 1H, OH), 7.55 (d, J = 7.4 Hz, 1H, ArH), 7.47 (s, 1H, ArH), 7.30 (t, J = 7.8 Hz, 1H, ArH), 7.26 (d, J = 8.3 Hz, 1H, ArH ), 7.04 (d, J = 2.2 Hz, 1H, ArH), 6.93 (d, J = 2.0 Hz, 1H, ArH), 6.88 (dd, J = 8.7, 2.3 Hz, 1H, ArH), 6.78 (dd, J = 8.3, 2.0 Hz, 1H, ArH ), 6.72 (d, J = 8.3 Hz, 1H, ArH), 3.84 (s, 3H, OCH3), 3.76 (s, 3H, OCH3). 13C NMR ((CD3)2CO, 125 MHz): δ 191.4, 158.1, 149.4 (q, JC-f = 2 Hz), 148.9, 147.2, 145.2, 143.8, 137.8, 130.3, 128.9, 125.6, 123.9, 123.7, 122.63, 122.59, 122.2, 121.3 (q, JC-F = 254.75 Hz), 116.9, 112.8, 112.3, 112.0, 95.6, 56.2, 55.8. 19F NMR ((CD3)2CO, 470 MHz): δ −58.5 (s, 6F, OCF3). HPLC: 14.71 min., purity at 254 nm >99%. HRMS (ESI+): m/z calculated for C24H19F3NO5 [M+H]+ 458.1210, found 458.1210.
4.1.28. 2-(3′-Hydroxy-4′-methoxyphenyl)-3-(4″-trifluoromethoxybenzoyl)-6-methoxyindole (32)
To a well-stirred solution of compound 20 (0.92 g, 1.60 mmol) in THF (5 mL) at 0 °C was added TBAF (2.5 mL, 2.5 mmol, 1 M in THF) dropwise. The reaction mixture was stirred for 30 min while warming to room temperature. The reaction mixture was quenched with water (10 mL) and extracted with EtOAc (3 × 10 mL). The combined organic extract was dried over Na2SO4 and concentrated under reduced pressure. Purification by flash column chromatography using a prepacked 50 g silica column [solvent A: EtOAc; solvent B: hexanes; gradient: 12%A / 88%B (4 CV), 12%A / 88%B → 100%A / 0%B (10 CV), 100%A / 0%B (4 CV); flow rate: 50 mL/min; monitored at 254 and 280 nm] afforded the desired indole free phenol ligand 32 (0.43 g, 0.96 mmol, 60%, Rf = 0.33 (50:50 hexanes:EtOAc)) as a yellow powder. 1H NMR ((CD3)2CO, 500 MHz): δ 10.86 (br s, 1H, NH), 7.91 (d, J = 8.8 Hz, 1H, ArH), 7.69 (s, 1H, OH), 7.65 (d, J = 8.5 Hz, 2H, ArH), 7.10 (d, J = 8.5 Hz, 2H, ArH), 7.03 (d, J = 2.2 Hz, 1H, ArH), 6.90 (s, 1H, ArH ), 6.87 (dd, J = 8.8 Hz, 2.2 Hz, 1H, ArH), 6.71 (m, 2H, ArH), 3.84 (s, 3H, OCH3), 3.77 (s, 3H, OCH3). 13C NMR ((CD3)2CO, 125 MHz): δ 191.6, 158.1, 151.3 (q, Jc-f = 2 Hz), 148.9, 147.2, 145.1, 140.5, 137.8, 132.0, 125.7, 123.7, 122.72, 122.67, 121.7 (q, JC-F = 254.75 Hz), 120.7, 116.9, 113.0, 112.3, 111.8, 95.5, 55.1, 55.8. 19F NMR ((CD3)2CO, 470 MHz): δ −58.5 (s, 6F, OCF3). HPLC: 14.80 min., purity at 254 nm >99%. HRMS (ESI+): m/z calculated for C24H19F3NO5 [M+H]+ 458.1210, found 458.1210.
4.1.29. 2-(3′-Hydroxy-4′-methoxyphenyl)-3-(3″,4″,5″-trimethoxybenzoyl)-6-hydroxyindole (33)
To a well-stirred solution of compound 21 (0.40 g, 0.59 mmol) in THF (10 mL) at 0 °C was added TBAF (1.00 mL, 1.00 mmol, 1 M in THF) dropwise. The reaction mixture was stirred for 30 min while warming to room temperature. The reaction mixture was quenched with water (10 mL) and extracted using EtOAc (3 × 10 mL). The combined organic extract was dried over Na2SO4 and concentrated under reduced pressure. Purification by flash column chromatography using a prepacked 50 g silica column [solvent A: EtOAc; solvent B: hexanes; gradient: 12%A / 88%B (4 CV), 12%A / 88%B → 100%A / 0%B (10 CV), 100%A / 0%B (1.4 CV); flow rate: 40 mL/min; monitored at 254 and 280 nm] afforded the desired indole free phenol ligand 33 (0.11 g, 0.25 mmol, 42%, Rf = 0.03 (70:30 hexanes:EtOAc)) as a yellow powder. 1H NMR ((CD3)2SO, 500 MHz): δ 11.62 (br s, 1H, NH), 9.19 (br s, 1H, OH), 9.00 (br s, 1H, OH), 7.66 (d, J = 8.5 Hz, 1H, ArH), 6.82 (d, J = 2.0 Hz, 1H, ArH) 6.78 (s, 2H, ArH), 6.74 (d, J = 9.0 Hz, 2H, ArH), 6.67 (t, J = 2.5 Hz, 1H, ArH), 6.65 (t, J = 3.0 Hz, 1H, ArH), 3.69 (s, 3H, OCH3), 3.61 (s, 6H, OCH3), 3.59 (s, 3H, OCH3). 13C NMR ((CD3)2SO, 125 MHz): δ 192.0, 155.2, 153.4, 148.5, 147.0, 143.7, 141.8, 138.0, 136.4, 126.5, 123.4, 122.7, 122.0, 116.9, 113.1, 112.3, 111.8, 106.0, 97.4, 60.4, 56.18, 56.17. HPLC: 8.95 min., purity at 254 nm 90.7%. HRMS (ESI+): m/z calculated for C25H24NO7 [M+H]+ 450.1547, found 450.1547.
4.1.30. 2-(3′-Hydroxy-4′-methoxyphenyl)-3-(3″,4″,5″-trimethoxybenzoyl)-4,5,6-trimethoxyindole (34)
To a well-stirred solution of compound 22 (0.03 g, 0.05 mmol) in THF (10 mL) at 0 °C was added TBAF (0.1 mL, 0.1 mmol, 1 M in THF) dropwise. The reaction mixture was stirred for 30 min while warming to room temperature. The reaction mixture was quenched with water (10 mL) and extracted with EtOAc (3 × 10 mL). The combined organic extract was dried over Na2SO4 and concentrated under reduced pressure. Purification by flash column chromatography using a prepacked 100 g silica column [solvent A: EtOAc; solvent B: hexanes; gradient: 12%A / 88%B (4 CV), 12%A / 88%B → 100%A / 0%B (10 CV), 100%A / 0%B (2 CV); flow rate: 40 mL/min; monitored at 254 and 280 nm] afforded the desired indole phenol ligand 34 (0.02 g, 0.03 mmol, 60%, Rf = 0.10 (50:50 hexanes:EtOAc)) as a yellow powder. 1H NMR (CDCl3, 500 MHz): δ 8.30 (br s, 1H, NH), 7.15 (s, 2H, ArH), 6.99 (d, J = 2.0 Hz, 1H, ArH) 6.89 (dd, J = 8.0 Hz, 2.0 Hz, 1H, ArH), 6.71 (d, J = 8.5 Hz, 1H, ArH), 6.68 (s, 1H, ArH), 5.62 (s, 1H, OH), 3.90 (s, 3H, OCH3), 3.85 (s, 3H, OCH3) 3.84 (s, 3H, OCH3), 3.82 (s, 3H, OCH3), 3.74 (s, 6H, OCH3), 3.69 (s, 3H, OCH3). 13C NMR (CDCl3, 125 MHz): δ 193.4, 152.8, 152.2, 146.8, 146.5, 145.8, 142.1, 137.8, 137.0, 134.6, 132.4, 125.1, 120.5, 116.1, 113.7, 112.6, 110.9, 107.5, 89.7, 61.4, 61.0, 60.8, 56.4, 56.3, 56.1. HPLC: 11.13 min., purity at 254 nm >99%. HRMS (ESI+): m/z calculated for C28H30NO9 [M+H]+ 524.1915, found 524.1912.
4.1.31. 2-(4′-Methoxyphenyl)-3-(3″,4″,5″-trimethoxybenzoyl)-6-methoxy-7-hydroxyindole (35)
To a well-stirred solution of compound 23 (0.008 g, 0.014 mmol) in THF (10 mL) at 0 °C was added TBAF (0.01 mL, 0.01 mmol, 1 M in THF) dropwise. The reaction mixture was stirred for 30 min while warming to room temperature. The reaction mixture was quenched with water (10 mL) and extracted with EtOAc (3 × 10 mL). The combined organic extract was dried over Na2SO4 and concentrated under reduced pressure. Purification by flash column chromatography using a prepacked 10 g silica column [solvent A: EtOAc; solvent B: hexanes; gradient: 7%A / 93%B (4 CV), 7%A / 93%B → 60%A / 40%B (10 CV), 60%A / 40%B (5 CV); flow rate: 12 mL/min; monitored at 254 and 280 nm] resulting in free phenol indole 35 as a dark brown powder (0.006 g, 0.013 mmol, 90%, Rf = 0.22 (50:50 hexanes:EtOAc)). 1H NMR (CDCl3, 500 MHz): δ 8.57 (br s, 1H, NH), 7.52 (d, J = 8.4 Hz, 1H, ArH), 7.29 (d, J = 8.1 Hz, 2H, ArH) 6.95 (br s, 3H, ArH), 6.74 (d, J = 8.1 Hz, 2H, ArH), 5.76 (br s, 1H, OH), 3.97 (s, 3H, OCH3), 3.80 (s, 3H, OCH3), 3.75 (s, 3H, OCH3), 3.69 (s, 6H, OCH3). 13C NMR (CDCl3, 125 MHz): δ 192.2, 160.1, 152.6, 143.7, 142.3, 141.3, 134.8, 131.0, 130.4, 125.5, 125.3, 124.5, 114.0, 113.0, 112.8, 108.7, 107.5, 61.0, 57.6, 56.2, 55.4. HPLC: 11.67 min., purity at 254 nm 96.9%. HRMS (ESI+): m/z calculated for C26H26NO7 [M+H]+ 464.1704, found 464.1704.
4.1.32. 2-(3′-Hydroxy-4′-methoxyphenyl)-3-(3″,4″,5″-trimethoxybenzoyl)-6,7-dimethoxyindole (36)
To a well-stirred solution of compound 24 (0.08 g, 0.14 mmol) in THF (10 mL) at 0 °C was added TBAF (0.21 mL, 0.21 mmol, 1 M in THF) dropwise. The reaction mixture was stirred for 30 min while warming to room temperature. The reaction mixture was quenched with water (10 mL) and extracted with EtOAc (3 × 10 mL). The combined organic extract was dried over Na2SO4 and concentrated under reduced pressure. Purification by flash column chromatography using a prepacked 25 g silica column [solvent A: EtOAc; solvent B: hexanes; gradient: 12%A / 88%B (4 CV), 12%A / 88%B → 100%A / 0%B (10 CV), 100%A / 0%B (2 CV); flow rate: 25 mL/min; monitored at 254 and 280 nm] resulting in free phenol indole 36 as a dark brown powder (0.02 g, 0.04 mmol, 27%, Rf = 0.14 (50:50 hexanes:EtOAc)). 1H NMR (CDCl3, 500 MHz): δ 8.57 (br s, 1H, NH), 7.71 (d, J = 9.0 Hz, 1H, ArH), 6.972 (d, J = 9.0 Hz, 1H, ArH) 6.971 (d, J = 2.0 Hz, 1H, ArH), 6.94 (s, 2H, ArH), 6.79 (dd, J = 8.3 Hz, 2.0 Hz, 1H, ArH), 6.64 (d, J = 8.3 Hz, 1H, ArH), 5.63 (br s, 1H, OH), 4.06 (s, 3H, OCH3), 3.96 (s, 3H, OCH3), 3.83 (s, 3H, OCH3), 3.79 (s, 3H, OCH3), 3.71 (s, 6H, OCH3). 13C NMR (CDCl3, 125 MHz): δ 192.0, 152.6, 148.1, 147.2, 145.7, 143.2, 141.1, 135.0, 134.0, 130.2, 125.4, 125.0, 122.0, 116.8, 114.8, 113.4, 110.52, 110.45, 107.3, 61.3, 60.9, 57.4, 56.17, 56.15. HPLC: 11.45 min., purity at 254 nm >99%. HRMS (ESI+): m/z calculated for C27H27NNaO8 [M+Na]+ 516.1629, found 516.1626.
4.2 Biological evaluation
4.2.1. SRB Assay52,53
We assessed inhibition of human cancer cell growth using the National Cancer Institute’s standard sulforhodamine B assay, as previously described.52 Briefly, cancer cell lines in a 5% fetal bovine serum/RPMI1640 medium, 1% gentamicin solution were plated in 96-well plates and incubated for 24 h. Serial dilutions of the compounds were then added. After 48 h, the cells were fixed with trichloroacetic acid, stained with sulforhodamine B, and read with an automated Biotek plate reader. A growth inhibition of 50% (GI50 or the drug concentration causing a 50% reduction in the net protein increase) was calculated from optical density data.
4.2.2. Colchicine Binding Assay
Inhibition of [3H]colchicine binding was determined using 100 µL reaction mixtures containing 1.0 µM tubulin, 5.0 µM [3H]colchicine (from Perkin-Elmer), 5% (v/v) dimethyl sulfoxide, and potential inhibitors at 1.0 or 5.0 µM. Reaction mixtures also contained components shown to potently stabilize the colchicine binding activity of tubulin:54 1.0 M monosodium glutamate (adjusted to pH 6.6 with HCl in 2.0 M stock solution), 0.5 mg/mL bovine serum albumin, 0.1 M glucose-1-phosphate, 1.0 mM MgCl2, and 1.0 mM GTP. Incubation was for 10 min at 37 °C, a time point at which the reaction in the control is 40–60% complete. Reactions were stopped by adding 2.0 mL of ice-cold water and placing the samples on ice prior to filtration. Each sample was poured onto a stack of two DEAE-cellulose filters, followed immediately by 6 mL of ice-cold water, and the water was aspirated under reduced vacuum. The filters were washed with additional water and placed into vials containing 5 mL of Biosafe II scintillation cocktail. The samples were counted the next day in a Beckman scintillation counter. Samples with potential inhibitors were compared to controls with no inhibitor to determine percent inhibition.
4.2.3. Inhibition of Tubulin Polymerization
Tubulin assembly experiments were performed with 0.25 mL reaction mixtures (final volume). The mixtures contained 1 mg/mL (10 µM) purified bovine brain tubulin, 0.8 M tubulin monosodium glutamate (adjusted to pH 6.6 with HCl in 2.0 M stock solution), 4% (v/v) dimethyl sulfoxide, 0.4 mM GTP, and varying concentrations of compound. Initially, all components except GTP were preincubated for 15 min at 30 °C in a 0.24 mL volume. After chilling the mixtures on ice, 10 µL of 10 mM GTP was added to each sample. The reaction mixtures were then transferred to cuvettes held at 0 °C in Beckman DU-7400 and DU-7500 spectrophotometers equipped with electronic temperature controllers. The temperature was jumped to 30 °C over about 30 s, and polymerization was followed turbidimetrically at 350 nM for 30 min. Each reaction set included a reaction mixture without compound, and the IC50 was defined as the concentration of compound that inhibited the extent of assembly versus the control after 20 min at 30 °C. These values were obtained by interpolation between the actual experimental values.
Supplementary Material
Acknowledgements
The authors are grateful to the National Cancer Institute of the National Institutes of Health (grant no. 5R01CA140674 to K.G.P. and M.L.T.) and Oxigene Inc. (grant to K.G.P. and M.L.T.) for their financial support of this project, and to the NSF for funding the Varian 500 MHz NMR spectrometer (grant no. CHE- 0420802). The content is solely the responsibility of the authors and does not necessarily reflect the official views of the National Institutes of Health. The authors also thank Dr. James Karban and Dr. Michelle Nemec (Director) for the use of the shared Molecular Biosciences Center at Baylor University, Dr. Alejandro Ramirez (Mass Spectrometry Core Facility, Baylor University), and Dr. Kevin Klausmeyer (X-ray analysis). The authors are grateful to Mr. Robert Harris, Ms. Priscilla Hor, and Ms. Siri Ancha for their valuable contributions to the synthesis of certain analogues.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplementary Data.
Characterization data (1H NMR, 13C NMR, 19F NMR, HPLC, and HRMS) for final compounds and X-ray crystallography for compound 10 are available free of charge via the internet at http://pubs.acs.org.
References and notes
- 1.Jordan MA, Wilson L. Nat. Rev. Cancer. 2004;4:253–265. doi: 10.1038/nrc1317. [DOI] [PubMed] [Google Scholar]
- 2.Lopus M, Yenjerle M, Wilson L. In: In Wiley Encyclopedia of Chemical Biology. Begley TP, editor. Vol. 3. Hoboken, NJ: John Wiley and Sons, Inc.; 2008. pp. 153–160. [Google Scholar]
- 3.Etienne-Manneville S. Curr. Opin. Cell Biol. 2010;22:104–111. doi: 10.1016/j.ceb.2009.11.008. [DOI] [PubMed] [Google Scholar]
- 4.Wade RH. Mol. Biotechnol. 2009;43:177–191. doi: 10.1007/s12033-009-9193-5. [DOI] [PubMed] [Google Scholar]
- 5.Valiron O, Caudron N, Job D. Cell. Mol. Life Sci. 2001;58:2069–2084. doi: 10.1007/PL00000837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mason RP, Zhao D, Liu L, Trawick ML, Pinney KG. Integr. Biol. 2011;3:375–387. doi: 10.1039/c0ib00135j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tozer GM, Kanthou C, Baguley BC. Nat. Rev. Cancer. 2005;5:423–435. doi: 10.1038/nrc1628. [DOI] [PubMed] [Google Scholar]
- 8.Hida K, Hida Y, Shindoh M. Cancer Sci. 2008;99(3):459–466. doi: 10.1111/j.1349-7006.2007.00704.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kanthou C, Tozer GM. Exp. Opin. Ther. Targets. 2007;11(11):1443–1457. doi: 10.1517/14728222.11.11.1443. [DOI] [PubMed] [Google Scholar]
- 10.Siemann DW, Bibby MC, Dark GG, Dicker AP, Eskens FA, Horsman MR, Marme D, Lorusso PM. Clin. Cancer Res. 2005;11:416–420. [PubMed] [Google Scholar]
- 11.Dougherty GJ, Chaplin DJ. In: Vascular Disruptive Agents for the Treatment of Cancer. Meyer T, editor. New York: Springer; 2010. pp. 1–27. ch. 1. [Google Scholar]
- 12.Siemann DWin. In: Vascular-Targeted Therapies in Oncology. Siemann D, editor. London, UK: John Wiley & Sons; 2006. pp. 1–8. ch. 1. [Google Scholar]
- 13.Patil SA, Patil R, Miller DD. Future Med. Chem. 2012;4(16):2085–2115. doi: 10.4155/fmc.12.141. [DOI] [PubMed] [Google Scholar]
- 14.Weisenberg RC, Borisy GG, Taylor W. Biochemistry. 1968;7:4466–4477. doi: 10.1021/bi00852a043. [DOI] [PubMed] [Google Scholar]
- 15.Pettit GR, Singh SB, Hamel E, Lin CM, Alberts DS, Garcia-Kendall D. Experientia. 1989;45(2):209–211. doi: 10.1007/BF01954881. [DOI] [PubMed] [Google Scholar]
- 16.Pettit GR, Singh SB, Niven ML, Hamel E, Schmidt JM. J. Nat. Prod. 1987;50(1):119–131. doi: 10.1021/np50049a016. [DOI] [PubMed] [Google Scholar]
- 17.Hasani A, Leighl N. Clinical Lung Cancer. 2011;12(1):18–25. doi: 10.3816/CLC.2011.n.002. [DOI] [PubMed] [Google Scholar]
- 18.Dowlati A, Robertson K, Cooney M, Petros WP, Stratford M, Jesberger J, Rafie N, Overmoyer B, Makkar V, Stambler B, Taylor A, Waas J, Lewin JS, McCrae KR, Remick SC. J. Clin. Oncol. 2003;62(12):3408–3416. [PubMed] [Google Scholar]
- 19.Rustin GJ, Galbraith SM, Anderson H, Stratford M, Folkes LK, Sena L, Gumbrell L, Price PM. J. Clin. Oncol. 2003;21(15):2815–2822. doi: 10.1200/JCO.2003.05.185. [DOI] [PubMed] [Google Scholar]
- 20.Lee RM, Gewirtz DA. Drug Dev. Res. 2008;69(6):352–358. [Google Scholar]
- 21.Pinney KGin. In: Vascular-Targeted Therapies in Oncology. Siemann D, editor. London, UK: John Wiley & Sons; 2006. pp. 95–121. ch. 6. [Google Scholar]
- 22.Pettit GR, Singh SB, Boyd MR. J. Med. Chem. 1995;38:1666–1672. doi: 10.1021/jm00010a011. [DOI] [PubMed] [Google Scholar]
- 23.Oshumi K, Nakagawa R, Fukuda Y. J. Med. Chem. 1998;41:3022–3032. doi: 10.1021/jm980101w. [DOI] [PubMed] [Google Scholar]
- 24.McGowan AT, Fox BW. Cancer Chemother. Pharmacol. 1990;26:79–81. doi: 10.1007/BF02940301. [DOI] [PubMed] [Google Scholar]
- 25.El-Zayat AAE, Degan D, Drabek S. Anticancer Drugs. 1993;4:19–25. doi: 10.1097/00001813-199302000-00002. [DOI] [PubMed] [Google Scholar]
- 26.Oshumi K, Hatanaka R, Nakagawa R. Anticancer Drug Des. 1999;14:539–548. [PubMed] [Google Scholar]
- 27.Pettit GR, Minardi MD, Boyd MR, Pettit RK. Anticancer Drug Des. 2000;15:397–403. [PubMed] [Google Scholar]
- 28.Pettit GR, Lippert JW., III Anticancer Drug Des. 2000;15:203–216. [PubMed] [Google Scholar]
- 29.Boehle AS, Sipos B, Kliche U, Kalthoff H, Dohrmann P. Ann. Thorac. Surg. 2001;71:1657–1665. doi: 10.1016/s0003-4975(01)02408-0. [DOI] [PubMed] [Google Scholar]
- 30.Pettit GR, Moser BR, Boyd MR. Anticancer Drug Des. 2001;16:185–193. [PubMed] [Google Scholar]
- 31.Ohno T, Kawano K, Tahara K. Gastroenterology. 2001;120:2831. [Google Scholar]
- 32.Pinney KG, Wang F, Hadimani M. US Patent 6849656. 2005 [Google Scholar]
- 33.Hadimani MB, Kessler RJ, Kautz JA, Ghatak A, Shirali R, O’Dell H, Garner CM, Pinney KG. Acta Crystallogr. 2002;C58:330. doi: 10.1107/s0108270102003669. [DOI] [PubMed] [Google Scholar]
- 34.Pinney K, Wang F, Del Pilar Mejia M. From PCT Int. Appl. WO 0119794 A2. 2001 [Google Scholar]
- 35.Flynn BL, Hamel E, Jung MK. J. Med. Chem. 2002;45:2670–2673. doi: 10.1021/jm020077t. [DOI] [PubMed] [Google Scholar]
- 36.Hadimani MB, MacDonough MT, Ghatak A, Strecker TE, Lopez R, Sriram M, Nguyen BL, Hall JJ, Kessler RJ, Shirali AR, Liu L, Garner CM, Pettit GR, Hamel H, Chaplin DJ, Mason RP, Trawick ML, Pinney KG. J. Nat. Prod. 2013 doi: 10.1021/np400374w. http://dx.doi.org/10.1021/np400374w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liou J-P, Chang Y-C, Kuo F-M, Chang C-W, Tseng H-Y, Wang C-C, Yang Y-N, Chang J-Y, Lee S-J, Hsieh HP. J. Med. Chem. 2004;47:4247–4257. doi: 10.1021/jm049802l. [DOI] [PubMed] [Google Scholar]
- 38.Pinney KG, Bounds AD, Dingeman KM, Mocharla VP, Pettit GR, Bai R, Hamel E. Bioorg. Med. Chem. Lett. 1999;9:1081–1086. doi: 10.1016/s0960-894x(99)00143-2. [DOI] [PubMed] [Google Scholar]
- 39.Mullica DF, Pinney KG, Mocharla VP, Dingeman KM, Bounds AD, Sappenfield EL. J. Chem. Crystallogr. 1998;28:289–295. [Google Scholar]
- 40.Kessler RJ, Hadimani B, Pinney KG, Edvardsen K. Poster. Bloomington IN: 38th National Organic Symposium Indiana University; 2003. Jun 8–12, [Google Scholar]
- 41.Kessler RJ. M.S. Thesis. Waco, TX: Baylor University; 2002. Synthesis and Evaluation of New Inhibitors of Tubulin Polymerization and Their Corresponding Prodrugs as Potential Vascular Targeting Agents. [Google Scholar]
- 42.Flynn BL, Flynn GP, Hamel E, Jung MK. Bioorg. Med. Chem. Lett. 2001;11:2341–2343. doi: 10.1016/s0960-894x(01)00436-x. [DOI] [PubMed] [Google Scholar]
- 43.Flynn BL, Gill GS, Grobelny DW, Chaplin JH, Paul D, Leske AF, Lavranos TC, Chalmers DK, Charman SA, Kostewicz E, Shackleford DM, Morizzi J, Hamel E, Jung MK, Kremmidiotis G. J. Med. Chem. 2011;54:6014–6027. doi: 10.1021/jm200454y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kremmidiotis G, Leske AF, Lavranos TC, Beaumont D, Hall A, O’Callaghan M, Matthews CA, Flynn BL. Mol. Cancer Ther. 2010;6:1562–1573. doi: 10.1158/1535-7163.MCT-09-0815. [DOI] [PubMed] [Google Scholar]
- 45.La Regina G, Sarkar T, Bai R, Edler MC, Saletti R, Coluccia A, Piscitelli F, Minelli L, Gatti V, Mazzoccoli C, Palermo V, Mazzoni C, Falcone C, Scovassi AI, Giansanti V, Campiglia P, Porta A, Maresca B, Hamel E, Brancale A, Novellino E, Silvestri R. J. Med. Chem. 2009;52:7512–7527. doi: 10.1021/jm900016t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.La Regina Bai R, Rensen WM, Cesare ED, Coluccia A, Piscitelli F, Famiglini V, Reggio A, Nalli M, Pelliccia S, Pozza ED, Costa B, Granata I, Porta A, Maresca B, Soriani A, Iannitto ML, Santoni A, Li J, Cona MM, Chen F, Ni Y, Brancale A, Dondio G, Vultaggio S, Varasi M, Mercurio C, Martini C, Hamel E, Lavia P, Novellino E, Silvestri R. J. Med. Chem. 2013;56:123–149. doi: 10.1021/jm3013097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ty N, Dupeyre G, Chabot GG, Seguin J, Tillequin F, Scherman D, Michel S, Cachet X. Bioorg. Med. Chem. 2008;16(15):7494–7503. doi: 10.1016/j.bmc.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 48.Bischler A, Brion H. Chem. Ber. 1892;25:2860. [Google Scholar]
- 49.Mohlau H. Chem. Ber. 1881;14:173. [Google Scholar]
- 50.Kemperman GJ, Roeters TA, Hilberink PW. Eur. J. Org. Chem. 2003:1681–1686. [Google Scholar]
- 51.Hall JJ, Sriram M, Strecker TE, Tidmore JK, Jelinek CJ, Kumar GDK, Hadimani MB, Pettit GR, Chaplin DJ, Trawick ML, Pinney KG. Bioorg. Med. Chem. Lett. 2008;18:5146–5149. doi: 10.1016/j.bmcl.2008.07.070. [DOI] [PubMed] [Google Scholar]
- 52.Monks A, Scudiero D, Skehan P, Shoemaker R, Paul K, Vestica D, Hose C, Langley J, Cronise P, Vaigro-Wolf A. J. Natl. Cancer Inst. 1991;83:767–766. doi: 10.1093/jnci/83.11.757. [DOI] [PubMed] [Google Scholar]
- 53.Vichai V, Kirtikara K. Nature Protocols. 2006;1(3):1112–1116. doi: 10.1038/nprot.2006.179. [DOI] [PubMed] [Google Scholar]
- 54.Hamel E, Lin CM. Biochim. Biophys. Acta. 1981;675:226–231. doi: 10.1016/0304-4165(81)90231-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.