Abstract
Previous studies in Long-Evans rats demonstrated a significant relationship between variation in pup licking/grooming and arched-back nursing (LG-ABN) and offspring development. However, maternal care is dynamic and exhibits significant temporal variation. In the current study, we assessed temporal variation in LG and ABN in lactating rats across the circadian cycle and determined the impact of these behaviors for the prediction of offspring hypothalamic gene expression, anxiety-like behavior, and responsiveness to high fat diet (HFD). We find that distinguishing between dams that engage in stable individual differences in maternal behavior (Low, Mid, High) requires assessment across the light-dark phases of the light cycle and across multiple postpartum days. Amongst juvenile female offspring, we find a positive correlation between maternal LG and mRNA levels of estrogen receptor alpha and beta and the oxytocin receptor (when LG is assessed across the light-dark cycle or in the dark phase). In young adults, we find sex-specific effects, with female High LG offspring exhibiting increased exploration of a novel environment and increased latency to approach HFD and male High LG offspring displaying increased activity in a novel environment and reduced HFD consumption. Importantly, these effects on behavior were primarily evident when LG was assessed across the light-dark cycle and ABN was not associated with these measures. Overall, our findings illustrate the dissociation between the effects of LG and ABN on offspring development and provide critical insights into the temporal characteristics of maternal behavior that have methodological implications for the study of maternal effects.
Keywords: medial preoptic area, open-field test, high fat diet, circadian, Long Evans rats
Introduction
Variation in maternal behavior can influence multiple aspects of infant development (Meaney, 2001). In laboratory rats, stable individual differences in maternal postpartum care, particularly frequency of pup licking/grooming (LG), are associated with brain region-specific changes in gene expression, receptor density, and a broad range of behavioral phenotypes. Adult male offspring reared by dams that engage in low levels of LG (Low LG) are found to have reduced hippocampal plasticity, heightened hypothalamic-pituitary-adrenal (HPA) response to stress, behavioral inhibition, and impairments in learning and memory (Liu et al., 1997; Caldji et al., 1998; Liu et al., 2000; Champagne et al., 2008) compared to High LG offspring. Amongst adult female offspring, the experience of Low LG compared to High LG is associated with decreased hypothalamic oxytocin receptor and estrogen receptor alpha levels with consequences for estrogen sensitivity and maternal behavior (Champagne et al., 2001; Champagne et al., 2003b). Moreover, cross-fostering studies indicate that the frequency of maternal LG experienced during postnatal development is predictive of these maternally-induced effects (Francis et al., 1999; Champagne et al., 2006).
The study of individual differences in maternal behavior as a moderator of offspring development has typically examined the overall frequency of maternal care occurring during the early postpartum period. However, there is evidence indicating significant temporal variation in maternal behavior during this period. Mother-litter contact in Norway rats increases during the light cycle and is dependent upon diurnal temperature cycles (Leon et al., 1984). Nursing (the primary form on mother-infant contact) has also been observed to be more frequent during the light compared to dark phase of the cycle (Grota & Ader, 1969; Toki et al., 2007; Ivy et al., 2008). Disturbances to mother-infant interactions, through use of postnatal handling, maternal separation, or variable foraging manipulations results in temporal shifts in maternal behavior rather than changes to overall frequency and it has been proposed that this change to the temporal dynamic of care may underlie observed variation in offspring stress-induced neuroendocrine and behavioral responses (Macri et al., 2004; Macri & Wurbel, 2007). Thus, consideration of the temporal characteristics of mother-infant interactions may be important methodological considerations within the study of maternal effects on development.
Despite the potential influence of timing of observation in the assessment of maternal care and prediction of offspring outcomes, a wide range of methodologies have been used to assess frequency of home-cage maternal behavior (Champagne et al., 2003a; Champagne et al., 2003b; Macri et al., 2004; Bosch & Neumann, 2008; Curley et al., 2009; D'Amato et al., 2011). We hypothesized that the strategy for characterizing postpartum maternal care received by offspring would have a significant impact on establishing the relationship between variation in maternal behavior and offspring neurobiological and behavioral outcomes. Thus, the aims of the current study were to determine: 1) temporal dynamics of home-cage maternal behavior; 2) the effect of temporal sampling on establishing variation in maternal care; and 3) the impact of temporal sampling on the prediction of offspring outcomes. To address the third aim we tested outcomes that have been previously linked to variation in maternal care, including offspring hormone receptor gene expression within the brain and open-field behavior, as well as approach and consumption of a high fat diet reward as a variable not yet explored within the context of the effects of maternal care.
Materials and methods
Animals & husbandry
Forty female and twenty male Long Evans rats (purchased from Charles River) were maintained on a 12:12hr light-dark schedule with white lights on at 0800h and off at 2000h and housed 2 per cage (same-sex) in 26 × 50 × 22cm polycarbonate cages in the animal facility at the Department of Psychology, Columbia University. Food and water were available ad libitum and replenished daily by animal care staff at 0900. Animals were habituated to the facility for 2 weeks prior to mating. Pair-housed virgin females were mated for one week. Pregnant females were single-housed 1–2 days before parturition. 34 females became pregnant and gave birth and were included in the study. Cages were cleaned at birth and every 7 days until weaning at 21 days, but were otherwise unmanipulated. Day of birth is considered postnatal day 0 (PN0), and observations began at the first dark-period observation (2100h) after birth. Consistent with previous studies, litters were sexed but not culled. Litter size and male/female pup ratio for litters included in this study are reported in Table 1. Weaned offspring were pair-housed by sex. All procedures were performed in accordance with guidelines of the NIH regarding the Guide for the Care and Use of Laboratory Animals and with the approval of the Institutional Animal Care and Use Committee (IACUC) at Columbia University.
Table 1.
Litter size and sex ratio (mean & range) of Low, Mid, & High LG litters
| Maternal LG Status | Litter Size | Litter Sex Ratio | ||
|---|---|---|---|---|
| mean | range | mean | range | |
| Low | 11.20 | 7–15 | 1.54 | .86–2.33 |
| Mid | 11.65 | 5–16 | 1.24 | .22–3.0 |
| High | 11.00 | 5–14 | 1.56 | .43–3.0 |
Study Design
Offspring were either sacrificed at weaning (PN21) for gene expression analysis or tested in two behavioral tests in the late juvenile/early adult period (see Figure 1 for summary of experimental design). 124 offspring (54 male, 70 female) from 34 dams were tested in the open-field apparatus at PN50–54. All animals were exposed to high fat diet in the home cage overnight on PN56. Half of the males and half of the females were then tested at PN58–60 for home cage activity, while the remaining offspring were tested for high fat diet approach and consumption.
FIGURE 1.
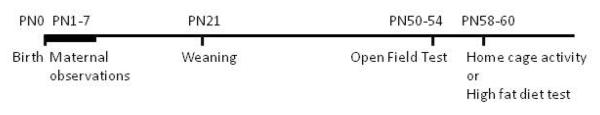
Illustration of study experimental design.
Home-cage maternal behavior
Home-cage maternal behavior was scored as previously described (Champagne et al., 2003a). Maternal behavior was observed for ten 60-minute observation periods daily during postnatal days (PN) 1–6. Observations took place during both the dark (2100h, 2300h, 0100h, 0300h, 0400h, 0600h) and light phases (1000h, 1300h, 1500h, 1700h) of the circadian cycle. During the dark phase, dim red lights were used to permit observations. During observation sessions, behavior was recorded every 3 minutes, resulting in a total of 1200 observations for each dam. Behaviors scored included contact with pups, nursing posture, pup licking/grooming (LG; dam licking the anogenital region or head/body of any pup in the litter), nest-building, eating, drinking, and self-grooming. Nursing postures have been described previously (Champagne et al., 2003a) and included passive nursing (dam is on her side or back), flat-back nursing, crouched nursing, and arched-back nursing (ABN; dam engaged in a kyphosis posture over the litter). Combinations of behaviors were also possible (e.g. ABN and LG can co-occur). Observers were trained to quickly mark codes for each behavior or combination of behaviors on a score sheet as soon as each cage was observed in every 3-minute period. Frequency of a behavior was calculated as the number of observations of the behavior divided by the total observations, resulting in a value that represents the percentage of observations in which that behavior was observed.
Selection of dams as Low, Mid, & High in maternal behavior
Dams were designated as being Low, Mid, or High in LG and in ABN. For LG, this designation was based either on 1) overall LG across all observations (Total), 2) LG occurring during the light phase of the light/dark cycle (Light), and 3) LG occurring during the dark phase of the light/dark cycle (Dark). Low LG status indicated that the frequency of LG that the dam was observed to engage in during the period of sampling (Total, Light, or Dark) was one standard deviation or more below the cohort mean frequency; Mid LG indicated that the dam engaged in a frequency of LG that was within one standard deviation of the cohort mean; High LG indicated the female was observed to display a frequency of LG that was one standard deviation or more above the cohort mean. For ABN, status (Low, Mid, High) was based on frequency of ABN across total observations conducted for each female.
Divergent classification of maternal behavior status was calculated in order to determine whether observing dams in a more restricted time frame would accurately capture “total” levels of maternal behavior. Divergent classification was determined by comparing the LG group of a dam (Low, Mid, or High LG) based on total observations to group classification based on a subset of observations (Light, Dark, or from a single day) as described above. There was considered to be a classification error if a dam was classified into a group other than the group based on total observations. For example, an error would be counted if a High LG dam based on total observations was calculated to be a Mid LG dam when only light-period observations were used. Determining divergent classification of maternal behavior in this manner enables identification of best and worst behavioral sampling strategies on these measures. To supplement this classification strategy, we calculated correlation coefficients between LG based on total observations and LG calculated from a subset of observations (Light, Dark, or from a single day).
Gene expression
Female offspring of a representative sample of dams (Low, Mid, & High LG – only 1 pup per litter, n=13) were sacrificed by rapid decapitation at PN21. Whole brains were immediately removed, snap-frozen, and stored at −80°C until further processing. The anterior ventral medial region of the hypothalamus, corresponding to the medial preoptic area (MPOA), was carefully dissected in a cryostat cooled to −20°C. Samples were weighed and then homogenized for 15 seconds in 700ul lysis buffer RLTplus (Qiagen) with 0.1% beta mercaptoethanol. RNA and DNA were extracted using a dual RNA-DNA extraction kit (Qiagen) according to manufacturer's instructions. cDNA was created from RNA using a reverse transcription kit (Applied Biosystems), according to manufacturer's instructions. Samples were stored at −20°C until further processing. Relative gene expression was measured by real-time quantitative PCR on a 96-well 7500Fast qPCR thermocycler using SybrFast (Applied Biosystems) with standard amplification and Ct calculation protocols by Applied Biosystems. All primers were designed to span exons and were tested for specificity (single melt curve peak) and efficiency (87–105%). Primer pairs are included in Table 2. Calculations of relative gene expression of estrogen receptor alpha (ERα), estrogen receptor beta (ERβ), and oxytocin receptor (OTR) were done using the 2ΔΔCT method (Schmittgen & Livak, 2008) using cyclophilin-A and beta-actin as internal control genes. By this method, each target is additionally normalized to one group, here to Low offspring (in each method of classification), to obtain relative rather than absolute increases or decreases in expression.
Table 2.
Rat RT-PCR primer sequences
| Gene name | Forward primer | Reverse primer |
|---|---|---|
| Cyclophillin-A | ATGGTCAACCCCACCGTGTTCTTC | ATCCTTTCTCCCCAGTGCTCAGAG |
| Beta-actin | ATGGATGACGATATCGCTGCG | GGTGACAATGCCGTGTTCAAT |
| ERα | GCCTTCTACAGGTCCAATTCTGAC | ACAGCACAGTAGCGAGTCTCC |
| ERβ | GCAGAACCTCAAAAGAGTCCTTGG | ACGCCGTAATGATACCCAGATG |
| OTR | TTCTTCGTGCAGATGTGGAG | GAGCATGTAGATCCACGGGT |
Open-field test
Male (Low LG: n=8; Mid LG: n=34; High LG: n=12) and female (Low LG: n=16; Mid LG: n=42; High LG: n=12) offspring (1–2 per sex per litter) were tested for exploratory behavior in a novel arena at PN50–54. At the time of testing, animals were placed in an open-field apparatus (90 × 90 × 60cm) for 10 minutes. Following the testing session, fecal boli were counted, animals were returned to their home-cage, and the apparatus was cleaned with an ethanol solution. Testing sessions were recorded and quantified by an ANY-maze tracking system (Stoeling). Animals were tested under a bright light to maximize anxiety-like behaviors and testing occurred in the first half of the light cycle to avoid disrupting circadian patterns. Analysis included 3 measures: 1) time spent in the center area of the open-field (exploration), 2) total distance travelled during testing (activity), and 3) number of fecal boli emitted. The center of the open-field was defined as the inner 70% of the square, leaving a 13.5cm wide perimeter (outer area).
Home-cage activity
Male (Low LG: n=5; Mid LG: n=16; High LG: n=6) and female (Low LG: n=9; Mid LG: n=20; High LG: n=6) offspring (1–2 per sex per litter) were tested for home-cage activity as adults (PN58–60). Animals were placed in a clean cage mixed with some of their own home-cage bedding, with food and water, starting at 2000h for 24 hours. Movements were recorded from above by a video tracking system and quantified by ANY-maze. Parameters were set within the ANY-maze program to ensure continuous movement tracking even when the animal was below the water bottle and out of direct view of the video recorder. Distances traveled within the cage (in total, by light or dark phase, or by hour) were analyzed as a measure of activity.
High fat diet approach & consumption
In order to test whether variation in maternal care influenced offspring motivated behavior, male (Low LG: n=5; Mid LG: n=16; High LG: n=6) and female (Low LG: n=7; Mid LG: n=20; High LG: n=8) adult offspring (1–2 per sex per litter) were tested for high fat diet (HFD) consumption at PN58–60. HFD pellets have been shown to be rewarding for rodents with no training necessary to induce exploration and consumption of HFD (Teegarden & Bale, 2007). HFD (D12492; Research Diets) contained 60% kcal from fat, 20% from protein, and 20% from carbohydrates, and essential vitamins and minerals. Animals were previously familiarized with the diet and were not food-restricted during testing to ensure that measures reflected motivational drives rather than the establishment of normal homeostasis. Animals remained in their home-cage during testing. In order to familiarize animals with the novel chow, all animals received one half pellet of HFD (approximately 2 grams) in their home-cage 2 days prior to testing. All animals were observed to consume the entire portion within 24 hours. On the day of testing, four HFD pellets (approximately 13g) were weighed and placed in the cage 4h after the start of the light phase of the light-dark cycle. Latency to approach, number of sniffs, and latency to bite the food pellet were recorded within the 6-minute period following introduction of the pellets. Pellets were weighed after 2 hours and after 4 hours, at which time the remaining HFD was removed.
Statistical analysis
All statistics were performed using SPSS (IBM, Version 19). Analysis of maternal behavior over time (days or hours) was conducted with repeated measures ANOVA. Correlations between maternal behaviors, between LG total and LG derived from other sampling strategies, or between maternal behavior and offspring outcomes were done with two-tailed Pearson correlation. Comparison of correlation coefficients (Total LG with Light LG, Dark LG, or LG from a single day) was conducted using Fisher's z transformation. Analysis of offspring behavior was conducted using maternal care as the fixed factor and litter (dam), litter size, and male/female pup ratio (individually or together as indicated) as covariates in a univariate general linear model. Significant main effects of maternal behavior on offspring behavior were further elucidated using Tukey's HSD post-hoc tests. Significance was set at the p<0.05 level.
Results
Temporal dynamics of maternal behavior
1. Variation in maternal behavior as a function of postpartum day and time
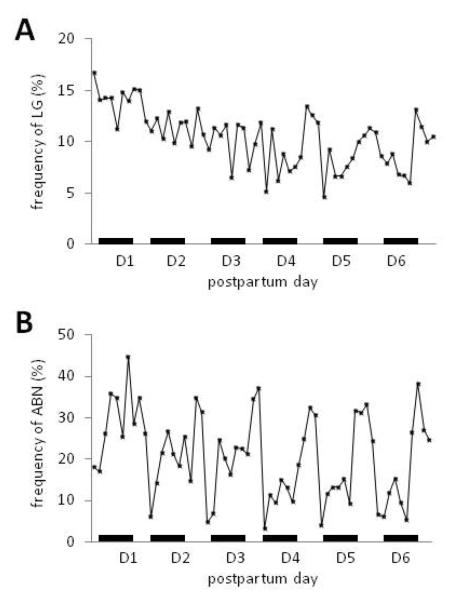
In order to establish the temporal dynamics of early postpartum maternal behavior, we first examined total frequencies of maternal LG and ABN by all dams across the first week postpartum. There was a main effect of day [F(1,6) = 14.99, p<0.001 Figure 2A] and time [F(1,9) = 4.09, p<0.001] , as well as a day by time interaction [F(1,46) = 1.41, p<0.05] on the frequency of LG and on the frequency of ABN [day: F(1,6) = 11.41, p<0.001, time: F(1,9) = 21.08, p<0.001; interaction: F(1,46) = 2.80, p<0.01 Figure 2B]. Levels of LG did not begin to show substantial diurnal variation until the fourth day postpartum (see Figure 2A), whereas diurnal patterning in ABN frequency were readily apparent starting the first day postpartum (see Figure 2B). LG frequency declined across the week such that by postpartum days 4–6 LG levels were 60% of their initial frequency. There was an overall difference in LG between light and dark phases [t(33)=−21.00, p<0.001] such that mean LG during the light was elevated compared to the dark [light=11.52%±0.38, dark=9.65%±0.41]. In contrast, ABN frequency was relatively stable across the week but showed strong within-day patterning. ABN levels were nearly 50% greater in the light than in the dark [light=29.1%±0.87, dark=15.1%±0.53; t(33)=10.78, p<0.001].
FIGURE 2.
Temporal variation in home-cage maternal licking/grooming (LG) and arched back nursing (ABN). Frequency (mean) of pup-directed (A) LG and (B) ABN across the first 6 days postpartum (n=34 dams). Black bars indicate dark phase of the light-dark cycle.
2. Temporal variation of individual differences in maternal behavior
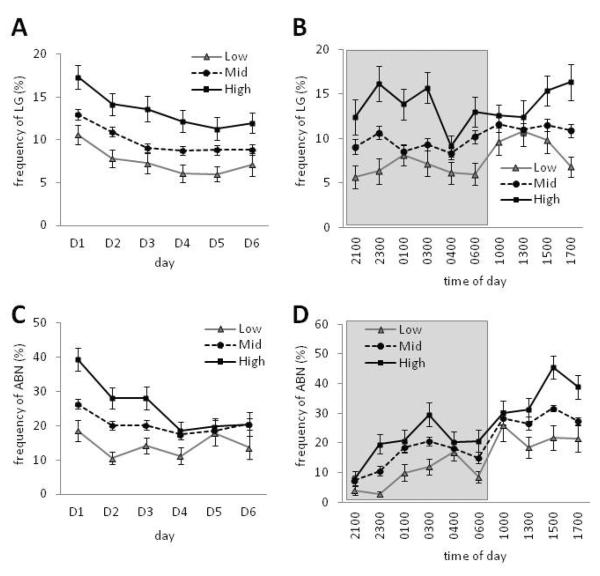
Based on the total LG frequencies calculated with data sampled across the early postpartum period (PN1–6), we characterized 5 Low-, 23 Mid-, and 6 High-LG dams in this cohort. The range and average litter size did not significantly differ among groups, nor did the male/female pup ratio within the litter (Table 1; ANOVA, p>0.4; Levene's F statistic for homogeneity of variance, p>0.17). There was no interaction of LG or ABN with either litter size or male/female pup ratio (p>0.6). Though parental investment has been reported to vary dependent on number and gender ratio of offspring (Schino et al., 1999; Koskela et al., 2009; de Medieros et al., 2010), our findings are consistent with previous studies of Long Evans rats (Champagne et al., 2003a). Here we sought to determine whether individual variation in maternal behavior (Low, Mid, High) varied as a function of day and time. Repeated measures ANOVA with postnatal day as within- and maternal LG as between-group factors indicated a main effect of LG [F(2,31)=18.39, p<0.001] and a main effect of day [F(2,31)=9.16, p<0.001] on pup LG frequency (Figure 3A). Subsequent analysis indicated that High LG dams had significantly elevated levels of LG on each of the first 6 days postpartum compared to Low LG dams (p<0.05) and LG frequency was found to decrease across the postpartum period amongst Low, Mid, and High LG dams. Repeated measures analysis of LG and time of day indicated a main effect of time [F(2,31)=2.94, p<0.01] and a main effect of LG status [F(2,31)=75.42, p<0.001] on pup LG frequency (Figure 3B). Though there was not a significant time by LG status interaction [F(2,31)=1.31, p=0.18], as is evident from Figure 3B, there are observation time periods during which LG status differences are marginal or not-significant, particularly during the observation sessions occurring following the transition from the dark to light phase (1000 & 1300). The temporal characteristics of individual differences in ABN (Low, Mid, High) were likewise examined. Repeated measures ANOVA with postnatal day as within- and ABN status as between-group factors indicated a main effect of day [F(2,31)=4.21, p<0.001] and main effect of ABN status [F(2,31)=19.04 p<0.001] as well as a day by status interaction [F(2,31)=2.12, p<0.05; Figure 3C]. Subsequent analysis indicated that High ABN dams nursed at significantly elevated levels compared to Mid and Low ABN dams on PN0–3 (p<0.05). ABN frequency was stable over this period amongst Low and Mid ABN dams and decreased over the postpartum period amongst High ABN dams. Within days there is a main effect of time [F(2,31)=2.44, p<0.001] and of ABN status [F(2,31)=34.63, p<0.001; Figure 3D] but not an interaction between these variables. [F(2,31)=1.20, p=0.26]. Low, Mid, and High ABN dams all nursed at elevated levels during the light compared to the dark periods of the circadian cycle (see Figure 3D).
FIGURE 3.
Individual differences in postpartum maternal LG and ABN within and across postpartum days. Frequency (mean ± SEM) of LG by Low (n=5), Mid (n=23), and High LG (n=6) dams across (A) the first postnatal week and (B) averaged for each time of day. Frequency (mean ± SEM) of ABN by Low (n=4), Mid (n=24), and High ABN (n=6) dams across (C) the first postnatal week and (D) averaged for each time of day. Shaded box indicates dark phase of the light-dark cycle.
3. Impact of temporal variation in maternal behavior on classification of Low, Mid, and High LG dams
In the previous analyses, LG status was determined using data collected across the entire circadian period from PN1–6. However, strategies for assessing maternal behavior typically focus on a far more limited observational sampling, restricted to either dark phase or light phase observations or determined through observations obtained on a single postpartum day. Within our dataset we determined the accuracy of these reduced sampling strategies for predicting the LG group status that was obtained through use of the more comprehensive sample of observations.
To examine the accuracy of using postpartum maternal care observed on a single day to predict the overall LG status of dams, LG status was recalculated based on 10 hours of observations done within one day. We found a high degree of divergent classification of LG status (e.g. designating females as Mid LG rather than High LG) for each postpartum day when compared to the LG status of dams determined from the total observational dataset (Table 3). Divergent classification on all days was due to females shifting between Mid and Low LG or Mid and High LG groups in both directions; no females shifted between Low and High LG groups on any day.
Table 3.
Divergent classification of LG status when using observations conducted on a single postpartum day
| All Observations Included | Total Error | ||||
|---|---|---|---|---|---|
| Low | Mid | High | |||
| Day1 | Low | 3 | 4 | 0 | 8 |
| Mid | 2 | 18 | 1 | ||
| High | 0 | 1 | 5 | ||
|
| |||||
| Day 2 | Low | 2 | 4 | 0 | 14 |
| Mid | 3 | 16 | 4 | ||
| High | 0 | 3 | 2 | ||
|
| |||||
| Day 3 | Low | 1 | 2 | 0 | 8 |
| Mid | 4 | 21 | 2 | ||
| High | 0 | 0 | 4 | ||
|
| |||||
| Day 4 | Low | 2 | 2 | 0 | 9 |
| Mid | 3 | 20 | 3 | ||
| High | 0 | 1 | 3 | ||
|
| |||||
| Day 5 | Low | 3 | 2 | 0 | 11 |
| Mid | 2 | 18 | 4 | ||
| High | 0 | 3 | 2 | ||
|
| |||||
| Day 6 | Low | 2 | 4 | 0 | 15 |
| Mid | 3 | 16 | 5 | ||
| High | 0 | 3 | 1 | ||
For analysis of time of day sampling effects, LG status was recalculated based on LG frequency occurring exclusively in the light or dark period. We found that divergent classifications of LG status occurred when status was determined using light-only or dark-only observations (Table 4). Moreover, using light-only observations resulted in a greater number of errors (9 dams) when compared to dark-only observations (2 dams). This effect was observed even when adjusting for the number of light vs. dark observations: when only 4 dark observations are used to calculate LG status there were 6 dams misclassified in contrast to the 9 divergent classifications using the 4 light observations. This finding is consistent with the analysis in the previous section indicating that group differences in LG are suppressed during 2 of the 4 light phase observations.
Table 4.
Divergent classification of LG status when using observations conducted exclusively in the light or dark phase of the circadian cycle.
| All Observations Included | Total Error | ||||
|---|---|---|---|---|---|
| Low | Mid | High | |||
| Light Observations | Low | 2 | 1 | 0 | 9 |
| Only (4) | Mid | 3 | 19 | 2 | |
| High | 0 | 3 | 4 | ||
|
| |||||
| Dark Observations | Low | 4 | 0 | 0 | 2 |
| Only (6) | Mid | 1 | 23 | 1 | |
| High | 0 | 0 | 5 | ||
|
| |||||
| Dark Observations | Low | 4 | 3 | 0 | 6 |
| Only (4) | Mid | 1 | 19 | 1 | |
| High | 0 | 1 | 5 | ||
This divergent classification strategy is further supported by correlation analyses in which the correlation coefficients between LG calculated based on total observations and LG based on other sampling strategies are compared. Frequency of LG based on total observations of each of the dams in this study was most highly correlated with LG based on dark observations (Dark: r=0.91) and this correlation coefficient was significantly higher (p<0.01) than that achieved when using light-only or single postpartum day observations (Light: r=0.72, Day 1:r=0.65, Day 2:r=0.51, Day 3: r=0.70, Day 4, r=0.69, Day 5: r=0.52, Day 6: r=0.56). This same pattern is observed when adjusting for the number of light vs. dark observations (Dark-only (n=4 observation sessions/day/dam): r=0.85).
4. Correlation between LG and ABN
To determine the influence of temporal dynamics in maternal care on the relationship between LG and ABN, we examined the correlation between the two behaviors across the postpartum period, during each postpartum day, and during light-only or dark-only observations. Pearson correlation (two-tailed) revealed a significant relationship between LG and ABN when all observations were included [r(32)=0.45, p<0.01; Figure 4]. This positive relationship between LG and ABN frequency within dams was found when including observations from individual postnatal days 3 [r(32)=0.34, p<0.05] and 4 [r(32)=0.52, p<0.01]. No correlation existed between dam LG and ABN frequency on PN 1, 2, 5, or 6. The correlation between LG and ABN was maintained when including light-only observations [r(32)=0.48, p<0.01] or dark-only observations [r(32)=0.49, p<0.01].
FIGURE 4.
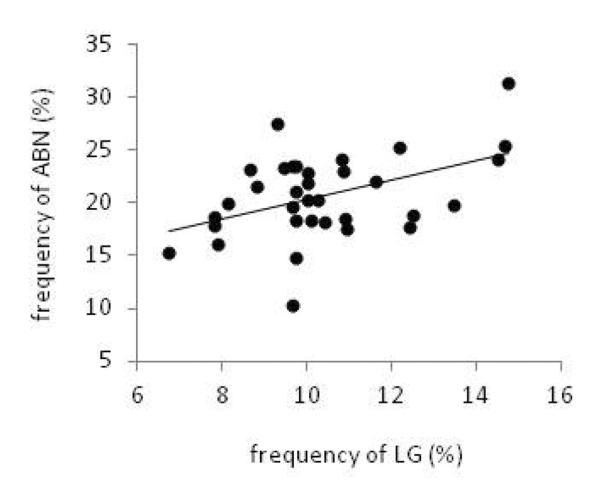
Correlation between postpartum maternal licking/grooming and arched back nursing. Percent of total observations in which a dam was engaged in LG or ABN is significantly correlated (r=0.45, p<0.01).
Offspring outcomes as a function of maternal behavior
Assessment of maternal behavior has been used to elucidate the environmental, hormonal, neurobiological, and genetic contributions to the emergence of this critical aspect of reproduction. However, maternal behavior also serves to shape the development of offspring, promoting growth, survival, and long-term biobehavioral characteristics. We therefore examined several outcome measures in offspring to determine 1) the predictive value of group differences in maternal behavior and 2) the effect of time of day of behavior sampling on the association between maternal LG and offspring phenotype.
1. Hypothalamic gene expression amongst juvenile females
Levels of ERα, ERβ, and OTR were examined in the MPOA of PN21 female offspring. There was no main effect of litter size or sex ratio (p>0.2) on offspring gene expression and these measures were therefore not included in further analysis. The expression pattern of one animal was determined to be more than 2-times the standard deviation greater than the group average (in Total LG) and was not used in analyses, resulting in a sample size of 12. We found a positive linear correlation between overall maternal LG (Total LG) and offspring preoptic mRNA levels of ERα [r(12)=0.71, p<0.01], ERβ [r(12)=0.61, p<0.05], and OTR [r(12)=0.64, p<0.05]. These correlations remained significant when using dark-observation maternal LG (Dark LG) for all three hormone receptors: ERα [r(12)=0.74, p<0.01], ERβ [r(12)=0.59, p<0.05], and OTR [r(12)=0.59, p<0.05]. However, when using Light LG observations, a significant correlation between frequency of maternal care and receptor mRNA levels was only observed for OTR mRNA [r(12)=0.65, p<0.05] and only for ERα mRNA when using ABN observations [r(12)=0.68, p<0.05].
2. Exploration of a novel environment
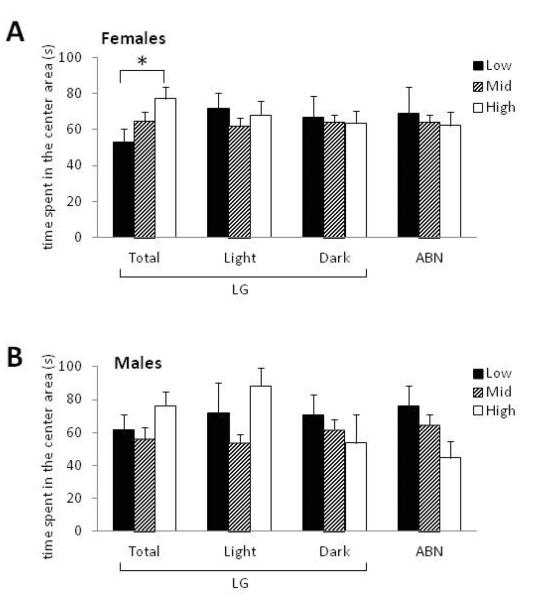
Exploration of a novel open-field, a standard measure of anxiety-like behavior (Belzung & Griebel, 2001), was examined in female and male offspring using litter (dam) as a covariate. We did not find a significant linear correlation between Total LG (continuous variable) and any measure of open-field behavior (p>0.20). There was an effect of male/female ratio [F(1,53)=3.81, p<0.01], but not litter size (p>0.23), on time spent in the center of the open-field for male offspring but not for female offspring (p>0.60) and thus sex ratio was used as a covariate and males and females were analyzed separately. Though the ANOVA only indicated a trend for a main effect of dam LG status on time female offspring spent in the center of the open-field when total maternal observations were used, when only High LG and Low LG female offspring were included in the analysis (consistent with previous analytic approaches; Champagne & Meaney, 2007), we confirm the finding of a significant increase in center exploration amongst High LG compared to Low LG females [F(1,26)=7.38, p<0.05; Figure 5A]. There was no effect of dam LG status as determined by Light or Dark, or of ABN status on time females spent in the center of the open-field. There was no significant effect of maternal behavior on distance traveled during open-field testing or on the amount of fecal boli emitted by female offspring when LG status was determined using Total, Light, or Dark observations and no effect of ABN on these measures. Amongst male offspring (controlling for litter sex ratio and litter), there was no effect of maternal LG status on center time when Total or Dark observations were used as predictors. There was a significant main effect of Light-observation LG status on time male offspring spent in the center area of the open-field [F(2,53)=4.21, p<0.05; Figure 5B]. Post-hoc comparison by Light-LG status indicated significant increases in center time amongst High compared to Mid LG male offspring (p<0.05), but not amongst other groups. Similarly, there was only an effect of Light-observation maternal LG status (but not Total, Dark, or ABN) on distance traveled in the open-field [F(2, 53)=3.42, p<0.05], that was driven by significant increases in distance traveled by High LG compared to Mid LG male offspring (High LG: 56.60±3.07 meters; Mid LG: 46.58±2.50). Fecal boli count was not different amongst males grouped by any determination of maternal care. However, amongst individual male offspring, boli production was significantly negatively correlated with center time [r(52)=−0.39, p<0.01].
FIGURE 5.
Offspring open-field behavior. Time (seconds) spent in the center of a novel open-field by (A) female and (B) male offspring of Low, Mid, and High LG or ABN dams. Dam LG status was determined based on Total observations, Light phase observations, or Dark phase observations. Dam ABN status was determined based on total observations. *p<0.05.
3. Home-cage activity
Exploratory behavior in a novel open-field may be the consequence of differences in general activity. To determine whether maternal care predicts general activity we examined offspring home-cage activity (distance traveled) over 24 hours. We did not find a significant linear correlation between Total LG (continuous variable) and home-cage activity (p>0.2). There was no main effect of sex on this outcome and males and females were subsequently analyzed together. Repeated measures ANOVA revealed a significant main effect of time [F(2, 23)=106.58, p<0.001] but not of maternal care (using Total, Light, Dark, or ABN observations), and there was no interaction between these variables. Subsequent analysis controlling for litter, litter size, and male/female pup ratio, confirmed a significant main effect of lights on/off on distance traveled [F(1,123)=231.62, p<0.001], with greater average distance traveled during the dark phase (39.84±16.64 meters) compared to the light phase (6.74±4.43 meters). This finding is consistent with previous reports of diurnal variation in activity (Toki et al., 2007).
4. Approach and consumption of a high fat diet
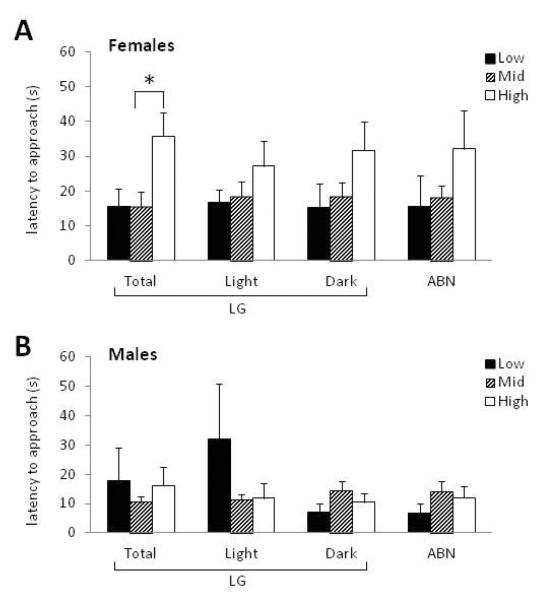
Latency to approach and amount of high fat diet eaten in 4 hours were measured in female and male offspring. We found a trend for significant linear correlation between Total LG (continuous variable) and latency to approach HFD in female offspring [r(61)=0.29, p=.09] but did not find a significant correlation between this variable and any other measure within HFD testing. Analyses indicated a main effect of offspring sex on latency to approach HFD [F(1,60)=11.58, p<0.001] and amount eaten [F(1,60)=12.23, p<0.001] and so subsequent analyses examined each sex separately. There was no main effect of litter size (p>0.50) or sex ratio (p>0.10) on latency to approach or amount of HFD eaten amongst males or females. In all analyses, litter (dam) was included as a covariate. When dam LG status was determined using the complete observation dataset there was a main effect of Total LG status on female offspring latency to approach the HFD [F(2,34)=3.95, p<0.05; Figure 6A]. Post-hoc comparisons indicated significantly increased latency to approach amongst female offspring of High compared to Mid LG dams (p<0.05). There was no main effect of maternal care on female offspring approach when dam status was determined by Light, Dark, or ABN observations. No differences were found in the amount of HFD consumed amongst females, regardless of the method by which maternal care was determined (Table 5).
FIGURE 6.
Offspring latency to approach a high fat food reward. Latency (seconds) for (A) female and (B) male offspring to first approach HFD pellets placed in the home cage. Dam LG status was determined based on Total observations, Light phase observations, or Dark phase observations. Dam ABN status was determined based on total observations.*p<0.05
Table 5.
High fat diet consumption in 4 hrs (g ± SEM) amongst offspring reared by Low, Mid, or High LG or ABN dams
| Female | Male | |||||
|---|---|---|---|---|---|---|
| Low | Mid | High | Low | Mid | High | |
| LG Total | 4.5 ± 0.4 | 5.4 ± 0.4 | 4.8 ± 0.5 | 7.7 ± 1.2 | 7.3 ± 0.5 | 4.8 ± 1.0* |
| LG Light | 4.5 ± 1.0 | 5.2 ± 0.3 | 5.2 ± 0.5 | 5.9 ± 0.5 | 7.0 ± 0.5 | 6.6 ± 1.2 |
| LG Dark | 5.0 ± 0.3 | 5.2 ± 0.3 | 4.6 ± 0.6 | 8.1 ± 1.6 | 7.1 ± 0.5 | 4.8 ± 1.2 |
| ABN | 4.9 ± 0.3 | 5.3 ± 0.3 | 4.3 ± 0.5 | 6.3 ± 1.0 | 7.3 ± 0.5 | 5.6 ± 1.2 |
p<.05
There were no differences amongst male offspring in latency to approach HFD based on any measure of maternal care (Figure 6B), though it should be noted that there was a significant difference in variability between Low LG and Mid/High LG, with Low LG offspring exhibiting a heightened degree of variability on this measure when LG status was determined through light-only observations (p<0.05). Analysis indicated a main effect of Total dam LG status on HFD consumed [F(2,26)=3.44, p<0.05; Table 5]. Post-hoc analysis indicated significantly lower consumption by male offspring of High compared to Mid LG dams (p<0.05). There was no effect of maternal Dark LG, Light LG or ABN status on male offspring food consumption.
Discussion
Early life experiences can shape offspring development and in the current study we illustrate the critical importance of considering the temporal variance of these experiences. Mother-infant interactions during the postpartum period are dynamic, variable, and dependent on the circadian cycle. Here we demonstrate that in laboratory rats there is variation within days and across days in the frequency of two behaviors which have previously been predictive of long-term neurobiological outcomes in offspring: licking/grooming (LG) and arched-back nursing (ABN). Consistent with previous studies (Grota & Ader, 1969; Toki et al., 2007; Ivy et al., 2008) we find that LG and ABN are generally elevated in frequency during the light phase of the circadian cycle. Moreover, these forms of mother-infant interaction decrease significantly across the postpartum period. Within these overall patterns, we find that individual differences in maternal care emerge and it is evident that the classification of maternal behavior (Low, Mid, High) is significantly influenced by the timing of behavioral sampling. Consequently, the prediction of offspring outcomes is influenced by the strategy used to determine the frequency of maternal care. Thus, variation in hypothalamic gene expression, exploratory behavior, and responsiveness to a salient food reward can be observed in offspring who experience variation in maternal care. However, the threshold to detect this relationship is significantly influenced by the type of maternal behavior assessed and the way in which that behavior is quantified relative to the light-dark cycle.
Temporal variation and individual differences in maternal behavior
Maternal care is responsive to the changing developmental needs of offspring and varies in response to environmental factors such as temperature, stress, and social contact (Reisbick et al., 1975; Woodside & Leon, 1980; Jans & Leon, 1983; Leon et al., 1984; Novakov & Fleming, 2005; Champagne & Meaney, 2007; Farrell & Alberts, 2007). However, even amongst the stable conditions present in laboratory housing, it is evident that there is dynamic temporal variation in care and individual differences in the pattern of this variation. Overall, we find that pup LG is elevated in the early postpartum period, decreasing across the first postpartum week, and is relatively stable within each day. In contrast, frequency of ABN is relatively stable across the first week and exhibits defined daily patterning such that ABN levels are almost two-fold higher during the light compared to dark period of the circadian cycle. This effect is consistent with previous reports of elevated nursing during the day (Toki et al., 2007; Ivy et al., 2008). When we consider individual differences in maternal care (Low, Mid, High), we find that across days differences in LG status are maintained despite the reduction in LG that occurs in all groups across the postpartum period. Within days, though group differences are generally maintained, there are time periods during which there is no significant difference in LG between Low, Mid, and High LG dams – a phenomenon that has implications for predicting offspring outcomes. In contrast, ABN decreases significantly amongst High ABN dams across the postpartum period whereas amongst Low and Mid ABN dams the frequency of this behavior is relatively stable across days. Within days, it is apparent that group differences in ABN are less evident at particular times of day, particularly after the transition from light to dark or dark to light periods.
We find that temporal variability in individual differences in maternal behavior accounts for a subset of dams to be divergently classified in terms of LG status (Low, Mid, High) when behavioral sampling is restricted by day or time of day. The degree of divergent classification is most pronounced when behavioral sampling is restricted to only one postnatal day or based on observations conducted exclusively during the light phase of the light-dark cycle. Thus, if the extensive collection of behavioral data described here is not feasible, our data suggest that an observation schedule including data collection over multiple postpartum days (rather than any one postnatal day; see Table 3) and involving dark cycle observations (rather than light cycle observations; see Table 4) would generate a data set that most closely resembles a more comprehensive data set. This conclusion is likewise supported by the high linear correlation between average frequency of LG derived from dark cycle observations and average frequency LG derived from total observations (which may account for the significant correlation between dark-only observations and offspring mRNA levels). It is perhaps not surprising that fewer observations lead to less accurate determination of maternal care. However, the effect of light-only observations on divergent classification was not the consequence of having fewer light compared to dark observations in the overall sampling. Group differences in LG were found to be less pronounced during light compared to dark observations and it may be that the temporal dynamics of maternal care interacting with individual differences in LG leads to periods of enhanced and reduced LG amongst Low, Mid, and High LG dams. The suppression of group differences in LG following the transition from the dark to the light period of the light cycle may also be influenced by daily animal husbandry. Though cage changes did not occur during the observational sampling period, food and water were replenished and room maintenance conducted by animal facilities staff following the transition to lights-on. It is therefore possible that this daily disruption led to reduced mother-infant contact during this period. This hypothesis was not tested directly in the current study, but is supported by data from Macri and Wurbel (2007) finding increased off-nest bouts independent of total maternal care when food is manipulated. Thus, the interaction between individual differences in maternal care and the temporal and environmental cues that may influence these differences are likely critical methodological issues in the study of maternal care and offspring outcomes.
Association and Dissociation Between LG and ABN
Though both LG and ABN are critical for promoting offspring survival, it is clear that there is dissociation in the patterns of these behaviors, suggesting unique neurobiological mechanisms underlying each of these behaviors. The medial preoptic area (MPOA) of the hypothalamus and its projections to the mesolimbic dopamine system, including the ventral tegmental area (VTA) and nucleus accumbens (NAc), are associated with active oral maternal behaviors such as nest building, pup retrieval, and licking (Jacobson et al., 1980; Numan & Smith, 1984; Lee & Brown, 2002; Li & Fleming, 2003). Lesions damaging the MPOA or its projections to the VTA inhibit pup retrieval and licking but not nursing (Numan & Smith, 1984). These active maternal behaviors are dependent upon oxytocin acting upon its receptor: oxytocin receptor antagonists delivered to the MPOA or to the VTA increase the latency of lactating dams to retrieve pups to a nest (Ahdieh et al., 1987; Pedersen et al., 1994). In tests of pup retrieval, the retrieval of pups to a nest is necessary in order for crouching and nursing to commence. As such, few studies have found impairments to nursing behavior separate from pup retrieval. However, blockade of dopamine receptors in the nucleus accumbens of lactating dams inhibits maternal retrieval and LG but simultaneously enhances nursing (Keer & Stern, 1999), further indicating dissociations between the underlying neurobiology of these facets of maternal behavior.
The known functions of LG and ABN also advocate for separate analysis of these behaviors. Gathering of pups in a nest and pup LG typically precedes and facilitates nursing (Stern & Johnson, 1989), and both behaviors increase mother-pup and pup-pup contact, important for thermoregulation, arousal, and survival (Stone et al., 1976; Hofer et al., 1976; Rosenblatt & Lehrman 1963). Nursing serves primarily to nourish offspring (Lynch, 1976) as well as regulate pup heart and respiratory rates (Hofer, 1973). LG is a form of tactile stimulation that serves to clean pups, alert pups to nursing opportunities, and facilitates pup urination and defecation, and sexual development when directed at the anogenital region (Rosenblatt & Lehrman, 1963; Friedman et al., 1981; Pedersen et al., 1982; Moore, 1984; Birke & Sadler, 1987; Stern & Johnson, 1989). While the two maternal behaviors often co-occur, previous studies have found them to vary independently. Female rats unable to nurse will still crouch and groom pups, and undernourished pups elicit increased LG from dams regardless of the dam's ability or frequency of nursing (Lynch, 1976). Likewise, dams with reduced oral feedback have been found to groom pups less frequently but are faster to crouch over young pups to nurse (Hofer et al., 1976; Stern & Johnson, 1989), demonstrating the ability to alter each behavior independently. Thus LG and ABN have distinct developmental roles and underlying neurobiological mechanisms, and this dissociation may account for the distinct effects of these behaviors on offspring biobehavioral outcomes that we observe in the current study.
Impact of maternal behavior on offspring outcomes
Individual variation in maternal care received in infancy has been found associated with variation in multiple aspects of offspring brain and behavior. In particular, levels of maternal care have been linked to variation in offspring response to stress (Liu et al., 1997; Caldji et al., 1998; Liu et al., 2000; Champagne et al., 2008), hippocampal plasticity (Liu et al., 2000; Champagne et al., 2008), and variation in neuroendocrine systems involved in maternal and reproductive behaviors (Champagne et al., 2001; Champagne et al., 2003a; Champagne et al., 2006; Champagne & Meaney, 2007; Cameron et al., 2008). The current study indicates that the method of assessing maternal behavior influences the capacity to predict offspring outcomes. In most cases, significant differences were found among offspring only when dam behavior was determined using a behavioral sampling that included both light and dark cycle observations (Total LG). Moreover, though previous studies (Liu et al., 1997) and our own analyses indicate a correlation between LG and ABN behavior amongst lactating dams, ABN was typically not a significant predictor of offspring outcomes. Thus we illustrate specificity of the prediction of offspring neurobiological and behavioral outcomes which regard to the method of early life assessment and the aspect of maternal care which characterizes the postnatal period.
Though the investigation of the impact of mother-infant interactions has primarily been correlational, there are experimental approaches which help to elucidate the causal role of maternal LG in offspring development. Offspring born to High LG mothers but cross-fostered to and reared by Low LG mothers at birth spend less time exploring the center of an open arena compared to offspring reared by High LG mothers (Francis et al., 1999), suggesting that the postnatal experience of LG, rather than prenatal or genetic factors, contributes to open-field performance. Similar conclusions are derived from analysis of cross-fostering effects on the expression of ERα (Champagne et al., 2006). Artificial rearing of pups with specific amounts of licking-like tactile stimulation delivered via paintbrush has also illustrated the impact of this experience on measures of sexual dimorphism in males (Lenz et al., 2008), dopamine levels within the nucleus accumbens (Afonso et al., 2011), and attention/impulsivity (Lovic et al., 2011). These manipulations of the rearing environment provide compelling support for the hypothesis that postnatal maternal LG is causal in these outcome measures. However, it should be noted, that these strategies have limitations. Maternal behavior of foster dams can be altered by pup characteristics, which may reduce or increase levels of LG (Curley et al., 2010). Artificial rearing is a dramatically different rearing condition from control rearing, and also involves reduced peer interactions with littermates. Though further exploration of the consequences of LG for development can implement these methodologies, the control obtained through these approaches is at the cost of disruption to the natural dynamic of mother-infant/social interactions.
Variation in maternal behavior as a predictor of hypothalamic gene expression
Estrogen and oxytocin signaling in the hypothalamus are necessary for active maternal behavior including licking/grooming, nest building, and pup retrieval (Pedersen & Prange, 1979; Ahdieh et al., 1987; van Leengoed et al., 1987). Oxytocin and estrogen signaling can be modulated by environment and experience, particularly early in life, as demonstrated in studies of brief maternal separation (Winkelmann-Duarte et al., 2007; Todeschin et al., 2009), artificial rearing (Novakov & Fleming, 2005), and juvenile social isolation (Ruscio et al., 2009). Variation in OTR binding and ERα mRNA and protein levels in the MPOA have also been linked to variation in maternal LG in lactating dams, and with experience of maternal LG in adult virgin female offspring (Champagne et al., 2003a; Champagne & Meaney, 2006; 2007). Analysis in previous studies examining the impact of maternal LG on offspring OTR, ERα, and ERβ in the MPOA compared only offspring of Low and High LG dams (Champagne et al., 2003a; Champagne & Meaney, 2006; Champagne et al., 2006; Champagne & Meaney, 2007), and in the case of OTR, quantification of receptor density rather than receptor expression was the focus of these analyses. Our findings are consistent with this earlier work and indicate that amongst juvenile female offspring there is a positive linear correlation between postnatal LG and levels of OTR and ERα mRNA in the MPOA. Thus, the variation in these neuroendocrine systems which mediates variation in maternal behavior of adult female offspring (Champagne et al., 2001; Champagne et al., 2003b) emerges early in development. However, this maternal effect is primarily observed when the assessment of maternal behavior includes both light and dark cycle or dark cycle-only maternal observations and ABN is only predictive of ERα mRNA. Interestingly, previous analyses had not indicated significant group differences in ERβ mRNA in response to Low vs. High LG in adult female offspring (Champagne et al., 2003a). There could be multiple methodological explanations to account for this discrepancy between the current and previous studies, including the age of assessment (juvenile vs. adulthood) and method of assessment (RT-qPCR vs. in situ hybridization). In addition, it is important to note that in previous studies, LG status was determined primarily from light period observations (Champagne et al., 2003a; Champagne & Meaney, 2006; Champagne et al., 2006) and we have established that light phase observations may have a reduced capacity to differentiate stable individual differences in LG. The detailed characterization of LG behavior that we have undertaken in the current study may thus provide an effective strategy for elucidating subtle yet behaviorally meaningful neurobiological differences in offspring.
Impact of maternal behavior on offspring exploratory and reward-directed behaviors
Variation in postnatal LG was found to be a significant predictor of behavioral responses in a novel environment (open-field test) and of approach and consumption of a high fat diet. However, these behavioral outcomes were found to be highly sex-specific. Female offspring reared by High LG dams were found to engage in higher levels of exploration in the open-field compared to female offspring of Low LG dams. The influence of maternal LG on exploration by male offspring was only observed when using light phase observations of LG behavior and this effect was based primarily on the elevated exploration of High LG compared to Mid LG males. Though these differential effects in males and females and the discrepancy between the findings of the current study and previous reports of elevated exploration amongst High LG compared to Low LG males (Champagne & Meaney, 2006; 2007) may be due to methodological differences in the assessment of LG (i.e. 3–4 light period and 2 dark-period observations in previous studies vs. 4 light-period and 6 dark-period observations in the current study), as well as differences in the age at testing (i.e. day 90 vs. day 50–60), and method of open-field coding (i.e. manual vs. automated tracking), recently there have been several reports suggesting female-specific effects of early maternal environment. Amongst C57BL/6J mice, Low vs. High maternal LG was found to differentiate female but not male offspring on several measures of anxiety-like behavior (Pedersen et al., 2011). In a study of outbred Harlan mice, no differences in open-field behavior were found amongst male offspring raised by one dam vs. two dams (D'Amato et al., 2011). A sex-specific effect of within-litter vs. between-litter LG on offspring anxiety-like behavior has also been found only amongst female offspring (Cavigelli et al., 2010). Though the long-term effects of LG on the anxiety-like behavior of both males and females have been reported, observations of maternal care during the postpartum period suggest that male pups receive more frequent anogenital licking than females (Moore & Morelli, 1979; Moore, 1984) and this differential care may account for the sex-specific outcomes associated with overall LG frequency.
Though the effect size of the behavioral changes induced by variation in maternal LG is relatively modest, this is both expected and in keeping with the effect size induced by early life manipulations of mother-infant interactions. Female mice that experience maternal separation display a similarly modest but significant decrease in time spent in the center area of an open-field apparatus (maternally separated females spent approximately 40s less time in the center area compared to controls; Tsude & Ogawa, 2012). Likewise, a small but significant reduction in center time (12s vs. 5s during a 5-minute test) has been reported in female rats isolated at weaning (Hermes et al., 2011). Within the life history of the organism, the effects of these early life experiences are likely to be meaningful for predicting response to challenges in the environment. We propose that in a more naturalistic context, where individuals are exposed to threats, such as predators and reduced food availability, these modest behavioral changes could have a significant impact on reproduction and survival.
The responsiveness of offspring to food reward cues has not previously been explored in the context of studies on the effects of natural variation in maternal care. High fat diet was used as stimulus known to be rewarding to rodents, that is preferable even over high-carbohydrate diets, and which motivates exploration and consumption shortly after initial exposure (Teegarden & Bale, 2007). It is possible that the relative novelty and palatability of the food, rather than its high fat content, are the factors motivating rats to explore and consume the HFD. However, the HFD was not a completely novel food, as all offspring in the current study were exposed to HFD two days prior to testing, and offspring were not food-deprived prior to testing so as to focus on rewarding aspects of the HFD above and beyond stress and hunger.
The HFD food reward allowed testing of both reward approach and consumption behaviors as a function of early life maternal care. Despite engaging in increased exploration of a novel environment, female offspring of High LG dams exhibit increased latencies to approach the HFD, potentially indicating decreased “liking”, “wanting”, or motivation toward HFD (Ikemoto & Panksepp, 1996) rather than increased anxiety. Appetitive and consummatory reward responses are known be to dissociated (Ikemoto & Panksepp, 1996) and consistent with this dissociation, we find that though female offspring of High LG dams are slower to approach the HFD, High and Low LG female offspring do not differ in the amount of HFD consumed, suggesting group similarities hedonic liking. Thus, it may be the case that initial motivation toward this particular food reward is altered by the experience of variation in postnatal maternal LG. Our finding of decreased HFD consumption by High LG compared to Low LG male offspring was somewhat surprising in light of female-specific findings after handling manipulations (McIntosh et al., 1999), and further indicates a distinction between early life handling/separation experiments and natural variations in maternal care (Macri et al., 2004; Macri & Wurbel, 2007). Both stress and eating behavior are regulated by the HPA axis, and our findings of differential approach and consumption of a high fat diet may be a consequence of maternal LG effects on the HPA response to stress (Dallman et al., 1995; Leal & Moreira, 1996). Consistent with other outcome measures, significant group differences in both approach and consumption were only evident when maternal LG was determined from total observations, and was unrelated to maternal ABN, further emphasizing the distinct influences of these early life experiences.
Implications & Conclusions
Our findings demonstrate that though the frequency of maternal care can significantly predict variation in hypothalamic gene expression, behavioral response to novelty, and appetitive/consummatory behavior, many of these effects are sex-specific and the timing of maternal observations can affect the capacity to characterize maternal behavior and predict offspring outcomes. Moreover, the current study illustrates the specificity of developmental effects in response to a particular form of maternal behavior – LG – and highlights the dissociation between LG and ABN both in terms of the temporal dynamics of these behaviors in lactating dams and in shaping biobehavioral outcomes in offspring. The study of natural variations in maternal behavior can be a very powerful strategy for elucidating the effects of early life environmental experience, however, the implementation of this strategy requires careful methodological consideration. Though discrete and less time consuming behavioral assessment strategies are often preferable and logistically a better fit with experimental designs, in the case of the assessment of individual differences in behavior, these strategies may likely be insufficient to account for the dynamic temporal variations in behavior – even within the stable conditions of a laboratory setting. Future analyses of the long-term neurobiological and behavioral consequence of maternal care should consider this issue, particularly when exploring novel outcome measures.
Acknowledgements
This research was supported by Grant Number DP2OD001674-01 from the Office of the Director, National Institutes of Health.
Footnotes
The authors have no conflicts of interest.
References
- Afonso VM, King SJ, Novakov M, Burton CL, Fleming AS. Accumbal dopamine function in postpartum rats that were raised without their mothers. Horm Behav. 2011;60:632–643. doi: 10.1016/j.yhbeh.2011.08.016. [DOI] [PubMed] [Google Scholar]
- Ahdieh HB, Mayer AD, Rosenblatt JS. Effects of brain antiestrogen implants on maternal behavior and on postpartum estrus in pregnant rats. Neuroendocrinology. 1987;46:522–531. doi: 10.1159/000124875. [DOI] [PubMed] [Google Scholar]
- Belzung C, Griebel G. Measuring normal and pathological anxiety-like behaviour in mice: a review. Behavioural brain research. 2001;125:141–149. doi: 10.1016/s0166-4328(01)00291-1. [DOI] [PubMed] [Google Scholar]
- Birke LI, Sadler D. Differences in maternal behavior of rats and the sociosexual development of the offspring. Dev Psychobiol. 1987;20:85–99. doi: 10.1002/dev.420200111. [DOI] [PubMed] [Google Scholar]
- Bosch OJ, Neumann ID. Brain vasopressin is an important regulator of maternal behavior independent of dams' trait anxiety. Proc Natl Acad Sci U S A. 2008;105:17139–17144. doi: 10.1073/pnas.0807412105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldji C, Tannenbaum B, Sharma S, Francis D, Plotsky PM, Meaney MJ. Maternal care during infancy regulates the development of neural systems mediating the expression of fearfulness in the rat. Proc Natl Acad Sci U S A. 1998;95:5335–5340. doi: 10.1073/pnas.95.9.5335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron N, Del Corpo A, Diorio J, McAllister K, Sharma S, Meaney MJ. Maternal programming of sexual behavior and hypothalamic-pituitary-gonadal function in the female rat. PLoS One. 2008;3:e2210. doi: 10.1371/journal.pone.0002210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavigelli SA, Ragan CM, Barrett CE, Michael KC. Within-litter variance in rat maternal behaviour. Behav Processes. 2010;84:696–704. doi: 10.1016/j.beproc.2010.04.005. [DOI] [PubMed] [Google Scholar]
- Champagne DL, Bagot RC, van Hasselt F, Ramakers G, Meaney MJ, de Kloet ER, Joels M, Krugers H. Maternal care and hippocampal plasticity: evidence for experience-dependent structural plasticity, altered synaptic functioning, and differential responsiveness to glucocorticoids and stress. J Neurosci. 2008;28:6037–6045. doi: 10.1523/JNEUROSCI.0526-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champagne F, Diorio J, Sharma S, Meaney MJ. Naturally occurring variations in maternal behavior in the rat are associated with differences in estrogen-inducible central oxytocin receptors. Proc Natl Acad Sci U S A. 2001;98:12736–12741. doi: 10.1073/pnas.221224598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champagne FA, Francis DD, Mar A, Meaney MJ. Variations in maternal care in the rat as a mediating influence for the effects of environment on development. Physiol Behav. 2003a;79:359–371. doi: 10.1016/s0031-9384(03)00149-5. [DOI] [PubMed] [Google Scholar]
- Champagne FA, Meaney MJ. Stress during gestation alters postpartum maternal care and the development of the offspring in a rodent model. Biol Psychiatry. 2006;59:1227–1235. doi: 10.1016/j.biopsych.2005.10.016. [DOI] [PubMed] [Google Scholar]
- Champagne FA, Meaney MJ. Transgenerational effects of social environment on variations in maternal care and behavioral response to novelty. Behav Neurosci. 2007;121:1353–1363. doi: 10.1037/0735-7044.121.6.1353. [DOI] [PubMed] [Google Scholar]
- Champagne FA, Weaver IC, Diorio J, Dymov S, Szyf M, Meaney MJ. Maternal care associated with methylation of the estrogen receptor-alpha1b promoter and estrogen receptor-alpha expression in the medial preoptic area of female offspring. Endocrinology. 2006;147:2909–2915. doi: 10.1210/en.2005-1119. [DOI] [PubMed] [Google Scholar]
- Champagne FA, Weaver IC, Diorio J, Sharma S, Meaney MJ. Natural variations in maternal care are associated with estrogen receptor alpha expression and estrogen sensitivity in the medial preoptic area. Endocrinology. 2003b;144:4720–4724. doi: 10.1210/en.2003-0564. [DOI] [PubMed] [Google Scholar]
- Curley JP, Davidson S, Bateson P, Champagne FA. Social enrichment during postnatal development induces transgenerational effects on emotional and reproductive behavior in mice. Front Behav Neurosci. 2009;3:25. doi: 10.3389/neuro.08.025.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curley JP, Rock V, Moynihan AM, Bateson P, Keverne EB, Champagne FA. Developmental shifts in the behavioral phenotypes of inbred mice: the role of postnatal and juvenile social experiences. Behav Genet. 2010;40:220–232. doi: 10.1007/s10519-010-9334-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Amato FR, Zanettini C, Sgobio C, Sarli C, Carone V, Moles A, Ammassari-Teule M. Intensification of maternal care by double-mothering boosts cognitive function and hippocampal morphology in the adult offspring. Hippocampus. 2011;21:298–308. doi: 10.1002/hipo.20750. [DOI] [PubMed] [Google Scholar]
- Dallman MF, Akana SF, Strack AM, Hanson ES, Sebastian RJ. The neural network that regulates energy balance is responsive to glucocorticoids and insulin and also regulates HPA axis responsivity at a site proximal to CRF neurons. Ann N Y Acad Sci. 1995;771:730–742. doi: 10.1111/j.1749-6632.1995.tb44724.x. [DOI] [PubMed] [Google Scholar]
- de Medeiros CB, Rees SL, Llinas M, Fleming AS, Crews D. Deconstructing early life experiences: distinguishing the contributions of prenatal and postnatal factors to adult male sexual behavior in the rat. Psychol Sci. 2010;21:1494–501. doi: 10.1177/0956797610382122. [DOI] [PubMed] [Google Scholar]
- Farrell WJ, Alberts JR. Rat behavioral thermoregulation integrates with nonshivering thermogenesis during postnatal development. Behav Neurosci. 2007;121:1333–1341. doi: 10.1037/0735-7044.121.6.1333. [DOI] [PubMed] [Google Scholar]
- Francis D, Diorio J, Liu D, Meaney MJ. Nongenomic transmission across generations of maternal behavior and stress responses in the rat. Science. 1999;286:1155–1158. doi: 10.1126/science.286.5442.1155. [DOI] [PubMed] [Google Scholar]
- Friedman MI, Bruno JP, Alberts JR. Physiological and behavioral consequences in rats of water recycling during lactation. J Comp Physiol Psychol. 1981;95:26–35. doi: 10.1037/h0077753. [DOI] [PubMed] [Google Scholar]
- Grota LJ, Ader R. Effects of litter size on emotionality, adrenocortical reactivity, and susceptibility to gastric erosions in the rat. Psychol Rep. 1969;24:547–549. doi: 10.2466/pr0.1969.24.2.547. [DOI] [PubMed] [Google Scholar]
- Hermes G, Li N, Duman C, Duman R. Post-weaning chronic social isolation produces profound behavioral dysregulation with decreases in prefrontal cortex synaptic-associated protein expression in female rats. Physiol Behav. 2011;104:354–359. doi: 10.1016/j.physbeh.2010.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofer MA. The role of nutrition in the physiological and behavioral effects of early maternal separation on infant rats. Psychosom Med. 1973;35:350–359. doi: 10.1097/00006842-197307000-00009. [DOI] [PubMed] [Google Scholar]
- Hofer MA, Shair H, Singh P. Evidence that maternal ventral skin substances promote suckling in infant rats. Physiol Behav. 1976;17:131–136. doi: 10.1016/0031-9384(76)90279-1. [DOI] [PubMed] [Google Scholar]
- Ikemoto S, Panksepp J. Dissociations between appetitive and consummatory responses by pharmacological manipulations of reward-relevant brain regions. Behav Neurosci. 1996;110:331–345. doi: 10.1037//0735-7044.110.2.331. [DOI] [PubMed] [Google Scholar]
- Ivy AS, Brunson KL, Sandman C, Baram TZ. Dysfunctional nurturing behavior in rat dams with limited access to nesting material: a clinically relevant model for early-life stress. Neuroscience. 2008;154:1132–1142. doi: 10.1016/j.neuroscience.2008.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson CD, Terkel J, Gorski RA, Sawyer CH. Effects of small medial preoptic area lesions on maternal behavior: retrieving and nest building in the rat. Brain Res. 1980;194:471–478. doi: 10.1016/0006-8993(80)91226-3. [DOI] [PubMed] [Google Scholar]
- Jans JE, Leon M. Determinants of mother-young contact in Norway rats. Physiol Behav. 1983;30:919–935. doi: 10.1016/0031-9384(83)90258-5. [DOI] [PubMed] [Google Scholar]
- Keer SE, Stern JM. Dopamine receptor blockade in the nucleus accumbens inhibits maternal retrieval and licking, but enhances nursing behavior in lactating rats. Physiol Behav. 1999;67:659–669. doi: 10.1016/s0031-9384(99)00116-x. [DOI] [PubMed] [Google Scholar]
- Koskela E, Mappes T, Niskanen T, Rutkowska J. Maternal investment in relation to sex ratio and offspring number in a small mammal - a case for Trivers and Willard theory? J Anim Ecol. 2009;78:1007–1014. doi: 10.1111/j.1365-2656.2009.01574.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leal AM, Moreira AC. Feeding and the diurnal variation of the hypothalamic-pituitary-adrenal axis and its responses to CRH and ACTH in rats. Neuroendocrinology. 1996;64:14–19. doi: 10.1159/000127092. [DOI] [PubMed] [Google Scholar]
- Lee AW, Brown RE. Medial preoptic lesions disrupt parental behavior in both male and female California mice (Peromyscus californicus) Behav Neurosci. 2002;116:968–975. doi: 10.1037//0735-7044.116.6.968. [DOI] [PubMed] [Google Scholar]
- Lenz KM, Graham MD, Parada M, Fleming AS, Sengelaub DR, Monks DA. Tactile stimulation during artificial rearing influences adult function and morphology in a sexually dimorphic neuromuscular system. Dev Neurobiol. 2008;68:542–557. doi: 10.1002/dneu.20608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leon M, Adels L, Coopersmith R, Woodside B. Diurnal cycle of mother-young contact in Norway rats. Physiol Behav. 1984;32:999–1003. doi: 10.1016/0031-9384(84)90292-0. [DOI] [PubMed] [Google Scholar]
- Li M, Fleming AS. The nucleus accumbens shell is critical for normal expression of pup-retrieval in postpartum female rats. Behav Brain Res. 2003;145:99–111. doi: 10.1016/s0166-4328(03)00135-9. [DOI] [PubMed] [Google Scholar]
- Liu D, Diorio J, Day JC, Francis DD, Meaney MJ. Maternal care, hippocampal synaptogenesis and cognitive development in rats. Nat Neurosci. 2000;3:799–806. doi: 10.1038/77702. [DOI] [PubMed] [Google Scholar]
- Liu D, Diorio J, Tannenbaum B, Caldji C, Francis D, Freedman A, Sharma S, Pearson D, Plotsky PM, Meaney MJ. Maternal care, hippocampal glucocorticoid receptors, and hypothalamic-pituitary-adrenal responses to stress. Science. 1997;277:1659–1662. doi: 10.1126/science.277.5332.1659. [DOI] [PubMed] [Google Scholar]
- Lovic V, Keen D, Fletcher PJ, Fleming AS. Early-life maternal separation and social isolation produce an increase in impulsive action but not impulsive choice. Behav Neurosci. 2011;125:481–491. doi: 10.1037/a0024367. [DOI] [PubMed] [Google Scholar]
- Lynch A. Postnatal undernutrition: an alternative method. Dev Psychobiol. 1976;9:39–48. doi: 10.1002/dev.420090107. [DOI] [PubMed] [Google Scholar]
- Macri S, Mason GJ, Wurbel H. Dissociation in the effects of neonatal maternal separations on maternal care and the offspring's HPA and fear responses in rats. Eur J Neurosci. 2004;20:1017–1024. doi: 10.1111/j.1460-9568.2004.03541.x. [DOI] [PubMed] [Google Scholar]
- Macri S, Wurbel H. Effects of variation in postnatal maternal environment on maternal behaviour and fear and stress responses in rats. Animal Behaviour. 2007;73:171–184. [Google Scholar]
- McIntosh J, Anisman H, Merali Z. Short- and long-periods of neonatal maternal separation differentially affect anxiety and feeding in adult rats: gender-dependent effects. Brain Res Dev Brain Res. 1999;113:97–106. doi: 10.1016/s0165-3806(99)00005-x. [DOI] [PubMed] [Google Scholar]
- Meaney MJ. Maternal care, gene expression, and the transmission of individual differences in stress reactivity across generations. Annu Rev Neurosci. 2001;24:1161–1192. doi: 10.1146/annurev.neuro.24.1.1161. [DOI] [PubMed] [Google Scholar]
- Moore CL. Maternal contributions to the development of masculine sexual behavior in laboratory rats. Dev Psychobiol. 1984;17:347–356. doi: 10.1002/dev.420170403. [DOI] [PubMed] [Google Scholar]
- Moore CL, Morelli GA. Mother rats interact differently with male and female offspring. J Comp Physiol Psychol. 1979;93:677–684. doi: 10.1037/h0077599. [DOI] [PubMed] [Google Scholar]
- Novakov M, Fleming AS. The effects of early rearing environment on the hormonal induction of maternal behavior in virgin rats. Horm Behav. 2005;48:528–536. doi: 10.1016/j.yhbeh.2005.04.011. [DOI] [PubMed] [Google Scholar]
- Numan M, Smith HG. Maternal behavior in rats: evidence for the involvement of preoptic projections to the ventral tegmental area. Behav Neurosci. 1984;98:712–727. doi: 10.1037//0735-7044.98.4.712. [DOI] [PubMed] [Google Scholar]
- Pedersen CA, Caldwell JD, Walker C, Ayers G, Mason GA. Oxytocin activates the postpartum onset of rat maternal behavior in the ventral tegmental and medial preoptic areas. Behav Neurosci. 1994;108:1163–1171. doi: 10.1037//0735-7044.108.6.1163. [DOI] [PubMed] [Google Scholar]
- Pedersen CA, Prange AJ., Jr. Induction of maternal behavior in virgin rats after intracerebroventricular administration of oxytocin. Proc Natl Acad Sci U S A. 1979;76:6661–6665. doi: 10.1073/pnas.76.12.6661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen PE, Williams CL, Blass EM. Activation and odor conditioning of suckling behavior in 3-day-old albino rats. J Exp Psychol Anim Behav Process. 1982;8:329–341. [PubMed] [Google Scholar]
- Pedersen CA, Vadlamudi S, Boccia ML, Moy SS. Variations in Maternal Behavior in C57BL/6J Mice: Behavioral Comparisons between Adult Offspring of High and Low Pup-Licking Mothers. Front Psychiatry. 2011;2:42. doi: 10.3389/fpsyt.2011.00042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisbick S, Rosenblatt JS, Mayer AD. Decline of maternal behavior in the virgin and lactating rat. J Comp Physiol Psychol. 1975;89:722–732. doi: 10.1037/h0077059. [DOI] [PubMed] [Google Scholar]
- Rosenblatt JS, Lehrman DS. Maternal behaviour in the laboratory rat. In: Rheingold HL, editor. Maternal behaviour in mammals. Wiley; New York: 1963. [Google Scholar]
- Ruscio MG, Sweeny TD, Gomez A, Parker K, Carter CS. Social environment alters central distribution of estrogen receptor alpha in juvenile prairie voles. Physiol Behav. 2009;98:296–301. doi: 10.1016/j.physbeh.2009.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schino G, Cozzolino R, Troisi A. Social rank and sex-biased maternal investment in captive japanese macaques: behavioural and reproductive data. Folia Primatol (Basel) 1999;70:254–263. doi: 10.1159/000021704. [DOI] [PubMed] [Google Scholar]
- Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008;3:1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- Stern JM, Johnson SK. Perioral somatosensory determinants of nursing behavior in Norway rats (Rattus norvegicus) J Comp Psychol. 1989;103:269–280. doi: 10.1037/0735-7036.103.3.269. [DOI] [PubMed] [Google Scholar]
- Stone EA, Bonnet KA, Hofer MA. Survival and development of maternally deprived rats: role of body temperature. Psychosom Med. 1976;38:242–249. doi: 10.1097/00006842-197607000-00002. [DOI] [PubMed] [Google Scholar]
- Teegarden SL, Bale TL. Decreases in dietary preference produce increased emotionality and risk for dietary relapse. Biol Psychiatry. 2007;61:1021–1029. doi: 10.1016/j.biopsych.2006.09.032. [DOI] [PubMed] [Google Scholar]
- Todeschin AS, Winkelmann-Duarte EC, Jacob MH, Aranda BC, Jacobs S, Fernandes MC, Ribeiro MF, Sanvitto GL, Lucion AB. Effects of neonatal handling on social memory, social interaction, and number of oxytocin and vasopressin neurons in rats. Horm Behav. 2009;56:93–100. doi: 10.1016/j.yhbeh.2009.03.006. [DOI] [PubMed] [Google Scholar]
- Toki S, Morinobu S, Imanaka A, Yamamoto S, Yamawaki S, Honma K. Importance of early lighting conditions in maternal care by dam as well as anxiety and memory later in life of offspring. Eur J Neurosci. 2007;25:815–829. doi: 10.1111/j.1460-9568.2007.05288.x. [DOI] [PubMed] [Google Scholar]
- Tsuda MC, Ogawa S. Long-lasting consequences of neonatal maternal separation on social behaviors in ovariectomized female mice. PLoS One. 2012;7:e33028. doi: 10.1371/journal.pone.0033028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Leengoed E, Kerker E, Swanson HH. Inhibition of post-partum maternal behaviour in the rat by injecting an oxytocin antagonist into the cerebral ventricles. J Endocrinol. 1987;112:275–282. doi: 10.1677/joe.0.1120275. [DOI] [PubMed] [Google Scholar]
- Winkelmann-Duarte EC, Todeschin AS, Fernandes MC, Bittencourt LC, Pereira GA, Samios VN, Schuh AF, Achaval ME, Xavier LL, Sanvitto GL, Mandarim-de-Lacerda CA, Lucion AB. Plastic changes induced by neonatal handling in the hypothalamus of female rats. Brain Res. 2007;1170:20–30. doi: 10.1016/j.brainres.2007.07.030. [DOI] [PubMed] [Google Scholar]
- Woodside B, Leon M. Thermoendocrine influences on maternal nesting behavior in rats. J Comp Physiol Psychol. 1980;94:41–60. doi: 10.1037/h0077652. [DOI] [PubMed] [Google Scholar]