Abstract
Brain amyloid can be measured using positron emission tomography. There are mixed reports as to whether amyloid measures are correlated with measures of cognition (in particular memory) depending on the cohorts and cognitive domains assessed. In Alzheimer's disease (AD) patients and those at heightened risk for AD, cognitive performance may be related to the level and extent of classical AD pathology (amyloid plaques and neurofibrillary angles), but it is also influenced by neurodegeneration, neurocognitive reserve, and vascular health. We discuss what recent neuroimaging research has discovered about cognitive deficits in AD, and offer suggestions for future research.
Keywords: dementia, mild cognitive impairment, MRI, PET, memory, executive function
Introduction
In recent years, there has been a vast increase of brain imaging studies focusing on Alzheimer's disease (AD). Some neuroimaging studies evaluate disease progression; others attempt to predict or better understand cognitive decline. Here we review what brain imaging has revealed about cognitive deficits in AD, focusing on developments from the last five years.
Structural magnetic resonance imaging (MRI) is the mainstay of AD imaging research. Many studies explore how regional brain volumes relate to various cognitive functions in control, mild cognitive impairment (MCI) and AD cohorts. Correlations between brain structure and cognition are easiest to detect in cohorts that include AD and MCI, as the disease process promotes brain atrophy as well as cognitive decline. In such cohorts, memory performance is highly correlated with measures of temporal lobe structures affected early in the disease process, including the hippocampus and entorhinal cortex. Executive function and global cognition typically show significant associations with broader measures: whole brain atrophy, ventricular enlargement, and cortical thickness across multiple brain regions 57-60. Performance on executive function tasks and the brain regions that support them may also contribute to episodic memory ability 61.
The hallmarks of AD pathology include amyloid plaques and neurofibrillary tangles, which spread throughout the brain in stereotypical patterns 5. Related changes in MRI and PET signal mark AD progression, but active debate exists about which neuroimaging biomarkers best detect the earliest changes 62, 63. A correlation between cognition and imaging biomarkers should be strongest when, during the disease stage assessed, the biomarker shows decline-related variability in a region essential to the cognitive function being evaluated. Cognitive performance depends on the level of neurodegeneration due to AD pathology, as well as co-morbid conditions and lifestyle choices. Additionally, the link between disease burden and cognition depends on how well the brain can successfully compensate for that degeneration (based on redundancy, plasticity, or other mechanisms promoting neurocognitive reserve).
Here, we discuss how brain imaging elucidates relationships between cognitive changes and AD-related pathological changes. We focus on brain biomarkers that relate most directly to AD processes, either through links to known pathology, or through examination of longitudinal cognitive changes. Where possible, we emphasize prodromal AD, when specific types of cognitive changes may be easier to isolate from others. Many papers explore brain structure and cognition in those with certain AD risk factors (such as apolipoprotein E allele 4 (APOE4). As brain differences associated with genotype may arise from factors unrelated to AD risk, we focus here on amyloid biomarkers and longitudinal changes to identify increased AD risk.
Direct relationships between brain amyloid and cognition
Brain amyloid - assessed using PET scanning (Box 1) - may relate directly to cognition in AD patients and those at increased risk for AD. Some studies reported significant relationships between amyloid PET signal and episodic memory or a composite measure that includes episodic memory 7, 42, 49, 64-66. Others focused on global cognition, relating amyloid signal to well-known global cognition measures 40, 43, 67. A few did examine how amyloid signal relates to episodic memory, but only detected significant relationships between amyloid signal and other cognitive measures 44, 68. Others did not detect a significant relationship between amyloid signal and cognition 38, 69, 70. These differing findings may be influenced by differences in methods, sample sizes, and cohort choices. In particular, studies that did not detect a direct relationship between amyloid signal and cognition largely focused on normal healthy older controls 38, 69, 70, while nearly all studies in which amyloid signal related to cognition also included cognitively impaired subjects. In one notable exception in controls 65, rather than relating amyloid signal to cognitive measures selected a priori, the authors used a discriminant function that weighted cognitive scores to best distinguish older adults with high versus low levels of PIB PET signal 65.
Box 1. Amyloid imaging.
Previously, a definitive AD diagnosis was possible only at autopsy by assessing AD neuropathological hallmarks - amyloid plaques and neurofibrillary tangles 5. The unpredictable interval between the patients' last clinical assessments and their deaths made it difficult to relate pre-mortem cognition to the pattern of plaques and tangles found at autopsy. Recently, new methods have allowed us to evaluate brain amyloid levels in the living brain using positron emission tomography (PET). This has greatly advanced our ability to understand cognitive deficits in AD and disease progression 6. Indeed, time-lapse movies that track the disease trajectory based on the expected amyloid signal at different disease stages have been created using regression models that relate PET measures to cognitive impairment or disease severity 7 (Figure 1). Additionally, non-demented adults with higher brain amyloid PET signal are more likely to decline cognitively over time, while those with low levels are less likely to decline cognitively in the short term 8,9.
Brain amyloid levels can be measured in vivo using various radiotracers sensitive to brain amyloid. Proof of concept for amyloid PET in live humans was established in 2002, using 2-(1-(6-[(2-[18F]fluoroethyl)(methyl)amino]-2-naphthyl)ethylidene)malononitrile (also known as FDDNP) 10. FDDNP PET is the only commonly used amyloid probe that also highlights neurofibrillary tangles in living humans. The PET probe, N-methyl-[11C]2-(4-methylaminophenyl)-6-hydroxybenzothiazole (known more commonly as “Pittsburgh Compound B” or PIB) was introduced in 2004 11. PIB binds with high specificity to senile amyloid plaques, but does not highlight neurofibrillary tangles. It has a higher signal to noise ratio than FDDNP, but the [11C] radiotracer used to label PIB has a shorter radioactive half-life than the [18F] tracer associated with FDDNP, so scans must be performed more rapidly after probe creation. Newer amyloid PET probes include [(18)F]3′-F-PiB (flutemetamol) 12, (18)F-AV-45 (florbetapir) 13, and (18)F-AV-1 (florbetaben) 14. Like PIB, these tracers are specific to amyloid, but like FDDNP, they offer a longer half-life, improving practicality of use. All of these PET tracers are in relatively wide use, as are fluorodeoxyglucose (FDG) and perfusion PET. Along with Magnetic Resonance Imaging (MRI) and Computerized Tomography (CT) scans, these have been used to evaluate AD for two decades.
Neuropathological amyloid deposition should be distinguished from the PET measure of amyloid, which may highlight only a fraction of actual brain pathology 15. Here, “amyloid signal” refers to the PET signal designed primarily to measure brain amyloid.
Even when higher levels of amyloid signal did not relate directly cognitive performance 40, 71, some found that they still correlated with future cognitive decline, including in controls 9, 38, 40, 71. The relationship between amyloid and cognition may also vary by diagnosis. Landau and colleagues found that PIB signal was related to current global cognition in MCI, but only to future memory decline in controls 40. Similarly, others found that amyloid signal related to episodic memory in MCI, but not in AD patients or controls 66. Mormino and colleagues likewise found that PIB signal related to episodic memory in MCI, but only in one cohort (out of two) of controls 49. We examine why higher brain amyloid may relate inconsistently to cognitive performance - a key question for clinical trials where higher PIB signal is sometimes used as an enrollment criterion.
Neurodegeneration may influence how brain amyloid and cognition relate
Mormino and colleagues found that in MCI and controls, global PIB signal was related to hippocampal volume. When both the PIB index and hippocampal volume were included in the same model to predict episodic memory, hippocampal volume remained a significant predictor, but the PIB index did not. This suggests that rather than having a direct relationship with cognition, the effect of brain amyloid on cognition may be mediated by hippocampal volume 49. High amyloid signal is among the earliest AD indications, while brain atrophy and detectable cognitive changes are more dynamic later 62. This would explain why amyloid signal is more commonly related to cognition in people with MCI 40, 49, 66. In MCI, greater AD-related neurodegeneration is expected than in controls. By the time AD processes have progressed enough to cause cognitive decline, amyloid may have promoted other mechanisms that influence cognition, such as toxicity caused by neurofibrillary tangles, inflammation, or Aβ oligomers 46. The concept of neurodegeneration mediating the amyloid-cognition relationship also explains why some studies do not find a significant relationship between amyloid signal and cognition in AD patients 40, 66. Brain amyloid rises rapidly early in AD, but may plateau as cognitive decline progresses 62. Cognitive decline and other AD biomarkers such as hippocampal atrophy may continue to progress while the deposition of brain amyloid may not, resulting in a disconnection between amyloid and cognitive performance later in AD.
Several other studies also support the idea that brain amyloid in non-demented older adults exerts its effect on cognition via neurodegeneration 38, 69, 70, 72. Dore and colleagues did not find that amyloid signal correlated directly with cognition, but greater amyloid signal was associated with thinner cortex in the posterior parietal and temporal lobes, and hippocampal region only in older controls who were PIB+ (had suprathreshold levels of global PIB signal). Thinner cortex in the precuneus and hippocampus was in turn associated with poorer episodic memory performance, even after controlling for amyloid signal 69. Others likewise found significant associations in cognitively intact older adults between higher global PIB signal and lower cortical volume, in regions that included the hippocampus, temporal cortex, and anterior and posterior cingulate. Regional brain volume in those regions was also associated with memory performance 38. In two other studies of older controls, high global PIB indices were not directly associated with cognition, or with measures of neurodegeneration such as structural MRI or FDG PET. However, brain amyloid still modulated the relationships between neurodegeneration and cognition. PIB+ controls had stronger relationships between greater neurodegeneration and cognition (specifically worse memory and executive function 70 and faster memory decline 71) than PIB- controls.
Neurocognitive reserve may influence how AD pathology and cognition relate
Neurocognitive reserve is defined as the variability in clinical symptoms across people for a given a level of brain pathology. The term includes both brain structural health and the ability to compensate actively for pathological insult 73. Reserve may be influenced by baseline intelligence, education, and lifetime cognitive engagement.
One large study related amyloid signal, brain structure, and cognitive performance in controls with and without memory complaints. PIB+ subjects without memory complaints had larger temporal lobes and better verbal learning performance 74 than PIB-controls. The larger temporal lobes may have been necessary for PIB+ adults to maintain their cognitive ability. In contrast, PIB+ controls with subjective memory complaints had smaller regional brain volumes and worse global cognition and visual memory than PIB-controls with memory complaints. There was a trend toward complaint-free PIB+ adults having greater brain gray matter volume than PIB+ adults with memory complaint 74. Possibly, PIB+ controls with memory complaints originally had less gray matter than those without memory complaints, and therefore had less neurocognitive reserve. Alternatively, perhaps PIB+ controls with memory complaints had more atrophy than PIB+ controls without memory complaints because of larger amounts or a longer duration of brain amyloid.
The notion of neurocognitive reserve suggests that improving brain structure and connectivity through cognitive or physical activity may help compensate for pathological insult to the brain. Intriguingly, cognitive activity may even modify amyloid deposition, rather than just compensating for it. In older controls, those who were the most cognitively active in their lifetimes had brain amyloid levels similar to young controls, but those who were least active cognitively had amyloid signal profiles that resembled AD patients 75. Similarly, the longitudinal decline in FDG PET metabolism was slower in AD patients with higher premorbid intelligence, and therefore cognitive reserve 76.
Vascular health may influence cognition in older adults
At autopsy more than 60% of AD patients have deep white matter lesions thought to relate to cardiovascular risk 77. These lesions are visible as white matter hyperintensities (WMH) on T2-weighted brain MRI scans. They contribute to the presentation of AD-like symptoms in older adults with higher brain amyloid levels 78. Baseline WMH volume 79, 80 and extension of existing WMH, although not the appearance of new WMHs 45, were both associated with future cognitive decline, including global cognition 79, episodic memory 45, and executive function 45, 80. WMHs in the temporal lobe are associated with memory impairment and those in the frontal lobe relate to slower mental speed 81. WMH are related to FDG PET hypometabolism and executive function decline, but they are not correlated with CSF Aβ levels 82, an amyloid measure that correlates well with amyloid PET signal 83. This suggests that WMHs do not affect amyloid; instead, their effect is additive to that of AD-specific pathology 82.
Multimodal imaging and cognitive deficits
Multi-modal brain imaging allows researchers to evaluate the effects on cognition of one specific biomarker (such as glucose metabolism) while controlling for the effects of others (such as hippocampal volume), to estimate the relative contribution of each. Several papers have evaluated the relative contributions of structural connectivity, assessed using diffusion tensor imaging (DTI), and regional brain volume or cortical thickness.
In two large studies, even after controlling for hippocampal volume, DTI mean diffusivity (MD) in the hippocampus was lower (more intact) in those with better episodic memory 84, 85. Others found that in non-demented older adults, DTI measures in the fornix 86 and parahippocampal cingulum 87 correlated with memory performance better than hippocampal volume did. Lee and colleagues also found that in AD patients and non-demented adults, better performance specific to episodic memory and executive function tasks was related to higher fractional anisotropy (FA; more intact white matter) in the fornix body 88, whose fibers originate in the hippocampus. However, in contrast with the other studies 84, 85, when both DTI measures and hippocampal volumes were included in the same model, only hippocampal volume continued to be significantly associated with memory performance 88. Unlike the other studies 84, 85, the study by Lee and colleagues included AD patients 88. These differing results could be explained by a model in which myelin degenerates early as has been proposed previously 89. Hippocampal degeneration may be apparent on MRI later than deficits in fornix microstructure on DTI 87. However, once hippocampal degeneration becomes more extensive, it begins to limit memory ability more than white matter integrity does. This notion is supported by another study in which the relationship between MD and memory was strongest in those with normal hippocampal volumes 84.
Correlating biomarkers with cognition
AD results in massive brain degeneration and eventually includes deficits in most cognitive functions. Therefore, in AD case-control studies, differences in any given cognitive domain may appear to relate to a wide range of biomarker differences, simply because the various markers all change with disease. Here, we selected papers that related imaging biomarkers to cognition in prodromal AD as well as patients. We focused on papers that either correlated longitudinal changes in imaging measures with longitudinal changes in cognitive measures, or those that included correlations with CSF biomarkers for AD.
The hippocampus is important for episodic memory and degenerates early in AD. Some studies have related hippocampal health to cognition. One found that global cognition and delayed episodic memory were both associated with hippocampal radial distance - the distance from each point on the hippocampal surface to the medial core of the hippocampus - in AD and MCI, but not in controls 90. This again emphasizes that AD-related changes in hippocampal structure are likely to become detectable once cognitive decline is evident. After controlling for baseline hippocampal volume, memory performance at follow-up was correlated with hippocampal radial distance predominantly in CA1 of the hippocampus 90, a region with early deposition of neurofibrillary tangles in incipient AD 91. Additionally, in MCI, greater longitudinal declines in resting state functional MRI (rs-fMRI) synchronicity - between the hippocampus and the left posterior cingulate - were associated with larger declines in episodic memory performance 92.
When analyses included the neocortex, higher medial temporal lobe atrophy rates in controls have been associated with a greater decline in verbal episodic memory performance and a baseline AD-related CSF biomarker 93. In MCI, higher temporal lobe atrophy rates also correlated with greater declines in global cognition and episodic memory 94. Also in MCI, memory decline has been specifically linked to longitudinal atrophy in the entorhinal cortex, while frontal lobe atrophy was associated with declines in executive function. Declines in semantic (category) fluency were related to atrophy rates in a broader network that included bilateral temporal lobe, left frontal lobe, and left anterior cingulate 95.
Lateral ventricle structure offers one of the highest effect sizes of all neuroimaging measures for distinguishing AD patients from contro96. In AD patients and non-demented older adults, differences in the ventricular surface were related to global cognition, longitudinal cognitive decline, and CSF levels of Aβ42 protein levels 39, which are reduced in AD patients 97. Declines in executive function have been associated with right frontal and left temporal horn enlargement of the lateral ventricles 98.
Concluding remarks
In AD patients and those at increased risk, brain amyloid may influence cognition indirectly, through neurodegenerative processes. Other measures of brain health including vascular health, and individual differences in brain structure and connectivity throughout a lifetime, may influence the extent to which insult from AD pathology results in detectable cognitive changes. Longitudinal studies and studies with imaging biomarkers from multiple modalities provide valuable insights into cognitive deficits in AD.
Figure 1.
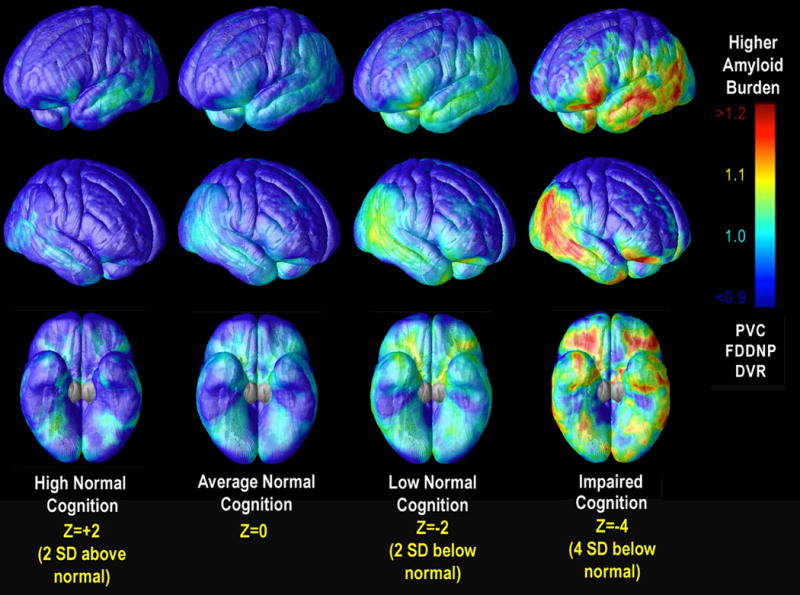
Brain Pathology at Different Stages of Cognitive Decline. Here we show the projected mean [18F]FDDNP signal, based on cross-sectional data from people with a broad range of impairment, and calculated for various levels of cognitive performance in AD and MCI patients and cognitively intact older adults. Red regions are those in which greater predicted [18F]FDDNP signal was associated with lower cognitive Z scores at each cortical voxel. Adapted from 7 and reprinted with the permission of the journal and authors.
Box 2. Predicting cognitive decline.
One of the most valuable tasks for Alzheimer's disease (AD) researchers is to identify baseline biomarkers typically associated with AD that will predict cognitive decline over time. Neuroimaging research has contributed considerably to this end. Some of these studies have been performed in cognitively intact older adults, often using longitudinal MRI to evaluate brain regions previously implicated in AD 16-18. Across several studies, the integrity of various regions in the temporal lobe were among the best predictors of cognitive decline in controls e.g. 16, 17, 19, 20. A few found that hippocampal volume specifically 19, 20, but not entorhinal cortex structure 19, 20 predicted cognitive decline.
In MCI too, cognitive decline is heralded by low integrity of medial temporal cortex, especially the entorhinal cortex, hippocampus, and amygdala across many studies e.g. 21-25. In a smaller number of studies, integrity of (or atrophy in) the posterior cingulate cortex and precuneus was also an indicator of future decline 21, 23, 25-27. Some studies found that FDG PET rather than MRI measures best predicted conversion from MCI to AD within a few years 28, 29. However, typically models that combined information from multiple modalities, such as MRI, FDG PET, CSF biomarkers, and cognitive measures, predicted cognitive decline better than any one biomarker alone 28, 30-32
The quest to identify new predictors of cognitive decline now includes high-field structural MRI scanning, diffusion imaging, arterial spin labeling (a form of MRI that measures perfusion), and resting state functional MRI, which measures coherence in brain activity. Other efforts to predict future cognitive or brain decline, or both, are adding information on common and rare genetic variants 33, 34 as well as data on gene expression, proteomics, and epigenetic markers of aging such as methylation. Another key area is how to predict decline most accurately when some patient data is missing; in clinical settings, not all patients will have the same sets of neuroimaging or genetic data collected, and specialized methods are being developed to best predict decline based on the available data 35. Finally, as most people with AD also harbor some vascular pathology, a very active line of work in neuroimaging has related brain integrity and cognitive decline to cardiovascular health and other lifestyle factors, including diet and obesity 36, and physical activity 37.
Box 3. Future directions.
Many large AD studies correlate cross-sectional biomarker measures with longitudinal cognitive decline (e.g.,38-40). This is important for predicting cognitive decline - something vital to personal health and clinical studies 32. However, more studies that correlate longitudinal brain biomarker changes with longitudinal cognitive changes (e.g., 41) would better clarify how these biomarkers relate to specific cognitive deficits.
Many correlations between amyloid PET and cognition use a global index (numeric summary) derived from the PET amyloid probe signal; only a few examine amyloid PET signal using voxelwise analyses 7, 42 or in individual regions of interest analyses 9, 43. Numeric summaries limit the number of statistical comparisons and are simple and practical for clinical studies. However, more voxelwise amyloid PET studies may help relate specific cognitive functions to regional brain amyloid patterns.
To better identify pathological processes that promote specific cognitive symptoms, more researchers should examine the relationship between cognition and an imaging measure while controlling for levels of biomarkers from other modalities. Relationships between biomarkers and cognition are disease stage-dependent, so focusing these studies on specific disease stages is particularly vital for understanding cognitive changes.
Currently, measures of “memory” or “executive function” are often based on composite scores composed of test scores that vary widely from study to study (e.g, composite scores for “executive”, “frontal lobe”, or “non-memory” function might include working memory 44-47, and verbal fluency 45-47, or only mental flexibility and response inhibition 9, 48, 49. Standardizing cognitive measures would help to interpret results in the context of earlier findings.
Large imaging studies in cognitively intact adults have explored how AD risk gene variants relate to brain measures (e.g., 50-52). Future MRI genetics studies in healthy adults should also evaluate amyloid and tau pathology and their correlations to cognition, so that gene effects related and unrelated to AD pathology can be distinguished.
Technological imaging advances may advance our understanding of brain changes related to cognition in AD. Ultra-high field 7 Tesla MRI provides sharper resolution of hippocampal microstructure - revealing hippocampal subregions and molecular layers whose properties can be linked to cognition 53. Connectomics, which measures the organization, efficiency, and speed of brain networks, is beginning to be applied in AD research 51, 54-56. Evaluating connectivity measures over time and in conjunction with specific cognitive tests -particularly in prodromal AD - would provide new insights into the circuitry underlying cognitive deficits in AD.
Highlights.
Amyloid PET signal inconsistently relates to cognition in Alzheimer's disease (AD).
Neurodegeneration may mediate how neuropathology relates to cognition in AD.
Vascular health and neurocognitive reserve may also modify this relationship.
Biomarkers from multiple modalities help us investigate these relationships.
Acknowledgments
This research was funded by NIH grants (R01 AG040060, R01MH097268, and U01 AG024904).
Glossary
- Amyloid signal
We use the term “amyloid signal” throughout this review to mean the positron emission tomography (PET) signal that arises in the brain using a radiotracer designed to bind primarily (or selectively) to amyloid. See Box 1.
- Controls
Unless otherwise stated, “controls” in this review refers to older adults whose cognitive test scores indicate that they are not cognitively impaired relative to their age- and education-matched peers. Most papers do not indicate whether or not these subjects have subjective memory complaints.
- Episodic memory
Memories that can be explicitly stated, such as the recall of specific events and experiences. Episodic memory is impaired in the AD spectrum. It relies heavily on the intact function of the hippocampal formation, which degenerates in AD.
- Executive function
Cognitive processes that regulate and control other processes. These include cognitive abilities such as planning, impulse control, mental flexibility, attention, problem solving. In some cognitive studies, working memory and language tasks such as phonemic and category fluency are included under this umbrella term as well.
- Fluorodeoxyglucose positron emission tomography (FDG PET)
A brain imaging method used to measure glucose metabolism in living humans (or experimental animal models). The tracer molecule is retained more in tissues with high metabolic rates. Signal is associated primarily with synaptic activity. At rest AD patients and those at greater risk for AD show decreased FDG PET signal (hypometabolism) in characteristic brain regions 1.
- Fractional anisotropy (FA)
A diffusion tensor imaging measure. FA measures the extent to which water is directionally constrained. Higher values are generally associated with greater white matter integrity.
- Mean diffusivity (MD)
A diffusion tensor imaging measure. It is an average measure of water diffusivity along the axon and in two directions perpendicular to the axon. Higher values tend to be associated with less intact white matter.
- Mild cognitive impairment (MCI)
a condition marked by cognitive changes beyond those expected for age and education, but not yet large enough to impair activities of daily living or be classified as dementia 2. Having MCI increases the risk of developing Alzheimer's disease (AD) and, in many people with MCI, there already may be AD pathology.
- Resting state functional magnetic resonance imaging (rs-fMRI)
also called “task-free” fMRIrs-fMRI measures regional changes in oxygenated blood in the brain while the subject lies quietly at rest, usually with their eyes open. The “default mode network” (DMN) is a group of brain regions - among them medial temporal lobe, posterior parietal and medial prefrontal frontal cortex - that are more active at rest than during a task. Activity in these network regions is typically correlated, but may become less synchronized in characteristic ways in those who have or are at increased risk for AD and certain other neurological disorders. Lower DMN synchronicity is associated with higher amyloid PET signal, even in cognitively intact older adults 3, 4.
- Semantic memory
memory of meanings and other concept-based knowledge that make up general knowledge about the world.
Footnotes
Conflict of interest: The authors have no actual or potential conflict of interest in writing this work.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Johnson KA, et al. Brain imaging in Alzheimer disease. Cold Spring Harb Perspect Med. 2012;2:a006213. doi: 10.1101/cshperspect.a006213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Petersen RC. Mild cognitive impairment: transition between aging and Alzheimer's disease. Neurologia. 2000;15:93–101. [PubMed] [Google Scholar]
- 3.Ouchi Y, Kikuchi M. A review of the default mode network in aging and dementia based on molecular imaging. Rev Neurosci. 2012;23:263–268. doi: 10.1515/revneuro-2012-0029. [DOI] [PubMed] [Google Scholar]
- 4.Sperling RA, et al. Amyloid deposition is associated with impaired default network function in older persons without dementia. Neuron. 2009;63:178–188. doi: 10.1016/j.neuron.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thompson PM, Vinters HV. Pathologic Lesions in Neurodegenerative Diseases. In: Teplow D, editor. The Molecular Biology of Neurodegenerative Diseases. Elsevier; 2012. pp. 1–40. [DOI] [PubMed] [Google Scholar]
- 6.Braskie MN, et al. Recent advances in imaging Alzheimer's disease. Journal of Alzheimer's Disease. 2013;33(Suppl 1):S313–327. doi: 10.3233/JAD-2012-129016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Braskie MN, et al. Plaque and tangle imaging and cognition in normal aging and Alzheimer's disease. Neurobiology of Aging. 2010;31:1669–1678. doi: 10.1016/j.neurobiolaging.2008.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wolk DA, et al. Amyloid imaging in mild cognitive impairment subtypes. Annals of Neurology. 2009;65:557–568. doi: 10.1002/ana.21598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Small GW, et al. Prediction of cognitive decline by positron emission tomography of brain amyloid and tau. Archives of Neurology. 2012;69:215–222. doi: 10.1001/archneurol.2011.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shoghi-Jadid K, et al. Localization of neurofibrillary tangles and beta-amyloid plaques in the brains of living patients with Alzheimer disease. American Journal of Geriatric Psychiatry. 2002;10:24–35. [PubMed] [Google Scholar]
- 11.Klunk WE, et al. Imaging brain amyloid in Alzheimer's disease with Pittsburgh Compound-B. Annals of Neurology. 2004;55:306–319. doi: 10.1002/ana.20009. [DOI] [PubMed] [Google Scholar]
- 12.Nelissen N, et al. Phase 1 study of the Pittsburgh compound B derivative 18F-flutemetamol in healthy volunteers and patients with probable Alzheimer disease. Journal of Nuclear Medicine. 2009;50:1251–1259. doi: 10.2967/jnumed.109.063305. [DOI] [PubMed] [Google Scholar]
- 13.Choi SR, et al. Preclinical properties of 18F-AV-45: a PET agent for Abeta plaques in the brain. Journal of Nuclear Medicine. 2009;50:1887–1894. doi: 10.2967/jnumed.109.065284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rowe CC, et al. Imaging of amyloid beta in Alzheimer's disease with 18F-BAY94-9172, a novel PET tracer: proof of mechanism. Lancet Neurology. 2008;7:129–135. doi: 10.1016/S1474-4422(08)70001-2. [DOI] [PubMed] [Google Scholar]
- 15.Chetelat G. Reply: The amyloid cascade is not the only pathway to AD. Nature Reviews Neurology. 2013;9:356. doi: 10.1038/nrneurol.2013.21-c2. [DOI] [PubMed] [Google Scholar]
- 16.Murphy EA, et al. Six-month atrophy in MTL structures is associated with subsequent memory decline in elderly controls. NeuroImage. 2010;53:1310–1317. doi: 10.1016/j.neuroimage.2010.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smith CD, et al. Structural brain alterations before mild cognitive impairment in ADNI: validation of volume loss in a predefined antero-temporal region. Journal of Alzheimer's Disease. 2012;31(Suppl 3):S49–58. doi: 10.3233/JAD-2012-120157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dickerson BC, Wolk DA. MRI cortical thickness biomarker predicts AD-like CSF and cognitive decline in normal adults. Neurology. 2012;78:84–90. doi: 10.1212/WNL.0b013e31823efc6c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chiang GC, et al. Identifying cognitively healthy elderly individuals with subsequent memory decline by using automated MR temporoparietal volumes. Radiology. 2011;259:844–851. doi: 10.1148/radiol.11101637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nettiksimmons J, et al. Subtypes based on cerebrospinal fluid and magnetic resonance imaging markers in normal elderly predict cognitive decline. Neurobiology of Aging. 2010;31:1419–1428. doi: 10.1016/j.neurobiolaging.2010.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aksu Y, et al. An MRI-derived definition of MCI-to-AD conversion for long-term, automatic prognosis of MCI patients. PLOS ONE. 2011;6:e25074. doi: 10.1371/journal.pone.0025074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chincarini A, et al. Local MRI analysis approach in the diagnosis of early and prodromal Alzheimer's disease. NeuroImage. 2011;58:469–480. doi: 10.1016/j.neuroimage.2011.05.083. [DOI] [PubMed] [Google Scholar]
- 23.Wee CY, et al. Prediction of Alzheimer's disease and mild cognitive impairment using cortical morphological patterns. Human Brain Mapping. 2012 doi: 10.1002/hbm.22156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eskildsen SF, et al. Prediction of Alzheimer's disease in subjects with mild cognitive impairment from the ADNI cohort using patterns of cortical thinning. NeuroImage. 2013;65:511–521. doi: 10.1016/j.neuroimage.2012.09.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Querbes O, et al. Early diagnosis of Alzheimer's disease using cortical thickness: impact of cognitive reserve. Brain. 2009;132:2036–2047. doi: 10.1093/brain/awp105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Westman E, et al. AddNeuroMed and ADNI: similar patterns of Alzheimer's atrophy and automated MRI classification accuracy in Europe and North America. NeuroImage. 2011;58:818–828. doi: 10.1016/j.neuroimage.2011.06.065. [DOI] [PubMed] [Google Scholar]
- 27.Li Y, et al. Discriminant analysis of longitudinal cortical thickness changes in Alzheimer's disease using dynamic and network features. Neurobiology of Aging. 2012;33:427 e415–430. doi: 10.1016/j.neurobiolaging.2010.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shaffer JL, et al. Predicting cognitive decline in subjects at risk for Alzheimer disease by using combined cerebrospinal fluid, MR imaging, and PET biomarkers. Radiology. 2013;266:583–591. doi: 10.1148/radiol.12120010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Landau SM, et al. Comparing predictors of conversion and decline in mild cognitive impairment. Neurology. 2010;75:230–238. doi: 10.1212/WNL.0b013e3181e8e8b8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang D, Shen D. Multi-modal multi-task learning for joint prediction of multiple regression and classification variables in Alzheimer's disease. NeuroImage. 2012;59:895–907. doi: 10.1016/j.neuroimage.2011.09.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hinrichs C, et al. Predictive markers for AD in a multi-modality framework: an analysis of MCI progression in the ADNI population. NeuroImage. 2011;55:574–589. doi: 10.1016/j.neuroimage.2010.10.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kohannim O, et al. Boosting power for clinical trials using classifiers based on multiple biomarkers. Neurobiology of Aging. 2010;31:1429–1442. doi: 10.1016/j.neurobiolaging.2010.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kohannim O, et al. Multilocus genetic profiling to empower drug trials and predict brain atrophy. NeuroImage. 2013 doi: 10.1016/j.nicl.2013.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rajagopalan P, et al. TREM2 risk variant and loss of brain tissue. New England Journal of Medicine 2013 [Google Scholar]
- 35.Yuan L, et al. Multi-source feature learning for joint analysis of incomplete multiple heterogeneous neuroimaging data. NeuroImage. 2012;61:622–632. doi: 10.1016/j.neuroimage.2012.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jahanshad N, et al. Neuroimaging, nutrition, and iron-related genes. Cellular and Molecular Life Sciences. 2013 doi: 10.1007/s00018-013-1369-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Erickson KI, et al. Exercise training increases size of hippocampus and improves memory. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:3017–3022. doi: 10.1073/pnas.1015950108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Storandt M, et al. Cognitive decline and brain volume loss as signatures of cerebral amyloid-beta peptide deposition identified with Pittsburgh compound B: cognitive decline associated with Abeta deposition. Archives of Neurology. 2009;66:1476–1481. doi: 10.1001/archneurol.2009.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chou YY, et al. Mapping correlations between ventricular expansion and CSF amyloid and tau biomarkers in 240 subjects with Alzheimer's disease, mild cognitive impairment and elderly controls. Neuroimage. 2009;46:394–410. doi: 10.1016/j.neuroimage.2009.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Landau SM, et al. Amyloid deposition, hypometabolism, and longitudinal cognitive decline. Annals of Neurology. 2012;72:578–586. doi: 10.1002/ana.23650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chou YY, et al. Ventricular maps in 804 ADNI subjects: correlations with CSF biomarkers and clinical decline. Neurobiology of Aging. 2010;31:1386–1400. doi: 10.1016/j.neurobiolaging.2010.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chetelat G, et al. Independent contribution of temporal beta-amyloid deposition to memory decline in the pre-dementia phase of Alzheimer's disease. Brain. 2011;134:798–807. doi: 10.1093/brain/awq383. [DOI] [PubMed] [Google Scholar]
- 43.Protas HD, et al. Prediction of cognitive decline based on hemispheric cortical surface maps of FDDNP PET. NeuroImage. 2012;61:749–760. doi: 10.1016/j.neuroimage.2012.02.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ossenkoppele R, et al. Amyloid burden and metabolic function in early-onset Alzheimer's disease: parietal lobe involvement. Brain. 2012;135:2115–2125. doi: 10.1093/brain/aws113. [DOI] [PubMed] [Google Scholar]
- 45.Maillard P, et al. Coevolution of white matter hyperintensities and cognition in the elderly. Neurology. 2012;79:442–448. doi: 10.1212/WNL.0b013e3182617136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Villemagne VL, et al. Longitudinal assessment of Abeta and cognition in aging and Alzheimer disease. Annals of Neurology. 2011;69:181–192. doi: 10.1002/ana.22248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bosch B, et al. Multiple DTI index analysis in normal aging, amnestic MCI and AD. Relationship with neuropsychological performance. Neurobiology of Aging. 2012;33:61–74. doi: 10.1016/j.neurobiolaging.2010.02.004. [DOI] [PubMed] [Google Scholar]
- 48.Delano-Wood L, et al. Posterior cingulum white matter disruption and its associations with verbal memory and stroke risk in mild cognitive impairment. Journal of Alzheimer's Disease. 2012;29:589–603. doi: 10.3233/JAD-2012-102103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mormino EC, et al. Episodic memory loss is related to hippocampal-mediated beta-amyloid deposition in elderly subjects. Brain. 2009;132:1310–1323. doi: 10.1093/brain/awn320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Braskie MN, et al. Common Alzheimer's Disease Risk Variant Within the CLU Gene Affects White Matter Microstructure in Young Adults. The Journal of Neuroscience. 2011;31:6764–6770. doi: 10.1523/JNEUROSCI.5794-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jahanshad N, et al. Genome-wide scan of healthy human connectome discovers SPON1 gene variant influencing dementia severity. Proceedings of the National Academy of Sciences USA. 2013;110:4768–4773. doi: 10.1073/pnas.1216206110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Braskie MN, et al. Neuroimaging measures as endophenotypes in Alzheimer's disease. Int J Alzheimers Dis. 2011;2011:490140. doi: 10.4061/2011/490140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kerchner GA, et al. Hippocampal CA1 apical neuropil atrophy and memory performance in Alzheimer's disease. NeuroImage. 2012;63:194–202. doi: 10.1016/j.neuroimage.2012.06.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Daianu M, et al. Breakdown of Brain Connectivity between Normal Aging and Alzheimer's Disease: A Structural k-Core Network Analysis. Brain Connectivity. 2013 doi: 10.1089/brain.2012.0137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang J, et al. Disrupted functional brain connectome in individuals at risk for Alzheimer's disease. Biological Psychiatry. 2013;73:472–481. doi: 10.1016/j.biopsych.2012.03.026. [DOI] [PubMed] [Google Scholar]
- 56.Reijmer YD, et al. Disruption of cerebral networks and cognitive impairment in Alzheimer disease. Neurology. 2013;80:1370–1377. doi: 10.1212/WNL.0b013e31828c2ee5. [DOI] [PubMed] [Google Scholar]
- 57.Stonnington CM, et al. Predicting clinical scores from magnetic resonance scans in Alzheimer's disease. NeuroImage. 2010;51:1405–1413. doi: 10.1016/j.neuroimage.2010.03.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dickerson BC, Wolk DA. Dysexecutive versus amnesic phenotypes of very mild Alzheimer's disease are associated with distinct clinical, genetic and cortical thinning characteristics. Journal of Neurology, Neurosurgery, and Psychiatry. 2011;82:45–51. doi: 10.1136/jnnp.2009.199505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Evans MC, et al. Volume changes in Alzheimer's disease and mild cognitive impairment: cognitive associations. Eur Radiol. 2010;20:674–682. doi: 10.1007/s00330-009-1581-5. [DOI] [PubMed] [Google Scholar]
- 60.Nho K, et al. Voxel and surface-based topography of memory and executive deficits in mild cognitive impairment and Alzheimer's disease. Brain Imaging and Behavior. 2012;6:551–567. doi: 10.1007/s11682-012-9203-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chang YL, et al. Level of executive function influences verbal memory in amnestic mild cognitive impairment and predicts prefrontal and posterior cingulate thickness. Cerebral Cortex. 2010;20:1305–1313. doi: 10.1093/cercor/bhp192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jack CR, Jr, et al. Tracking pathophysiological processes in Alzheimer's disease: an updated hypothetical model of dynamic biomarkers. Lancet Neurology. 2013;12:207–216. doi: 10.1016/S1474-4422(12)70291-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fjell AM, Walhovd KB. New tools for the study of Alzheimer's disease: what are biomarkers and morphometric markers teaching us? Neuroscientist. 2011;17:592–605. doi: 10.1177/1073858410392586. [DOI] [PubMed] [Google Scholar]
- 64.Bahar-Fuchs A, et al. Prediction of amyloid-beta pathology in amnestic mild cognitive impairment with neuropsychological tests. Journal of Alzheimer's Disease. 2013;33:451–462. doi: 10.3233/JAD-2012-121315. [DOI] [PubMed] [Google Scholar]
- 65.Oh H, et al. Effects of age and beta-amyloid on cognitive changes in normal elderly people. Neurobiology of Aging. 2012;33:2746–2755. doi: 10.1016/j.neurobiolaging.2012.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rowe CC, et al. Amyloid imaging results from the Australian Imaging, Biomarkers and Lifestyle (AIBL) study of aging. Neurobiology of Aging. 2010;31:1275–1283. doi: 10.1016/j.neurobiolaging.2010.04.007. [DOI] [PubMed] [Google Scholar]
- 67.Ossenkoppele R, et al. Longitudinal imaging of Alzheimer pathology using [11C]PIB, [18F]FDDNP and [18F]FDG PET. European Journal of Nuclear Medicine and Molecular Imaging. 2012;39:990–1000. doi: 10.1007/s00259-012-2102-3. [DOI] [PubMed] [Google Scholar]
- 68.Mathis CA, et al. In vivo assessment of amyloid-beta deposition in nondemented very elderly subjects. Annals of Neurology. 2012 doi: 10.1002/ana.23797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dore V, et al. Cross-sectional and Longitudinal Analysis of the Relationship Between Abeta Deposition, Cortical Thickness, and Memory in Cognitively Unimpaired Individuals and in Alzheimer Disease. JAMA Neurol. 2013:1–9. doi: 10.1001/jamaneurol.2013.1062. [DOI] [PubMed] [Google Scholar]
- 70.Wirth M, et al. Alzheimer's disease neurodegenerative biomarkers are associated with decreased cognitive function but not beta-amyloid in cognitively normal older individuals. The Journal of Neuroscience. 2013;33:5553–5563. doi: 10.1523/JNEUROSCI.4409-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wirth M, et al. The effect of amyloid beta on cognitive decline is modulated by neural integrity in cognitively normal elderly. Alzheimer's & Dementia. 2013 doi: 10.1016/j.jalz.2012.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ewers M, et al. CSF biomarker and PIB-PET-derived beta-amyloid signature predicts metabolic, gray matter, and cognitive changes in nondemented subjects. Cerebral Cortex. 2012;22:1993–2004. doi: 10.1093/cercor/bhr271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jones RN, et al. Aging, brain disease, and reserve: implications for delirium. The American Journal of Geriatric Psychiatry. 2010;18:117–127. doi: 10.1097/JGP.0b013e3181b972e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chetelat G, et al. Larger temporal volume in elderly with high versus low beta-amyloid deposition. Brain. 2010;133:3349–3358. doi: 10.1093/brain/awq187. [DOI] [PubMed] [Google Scholar]
- 75.Landau SM, et al. Association of lifetime cognitive engagement and low beta-amyloid deposition. Archives of Neurology. 2012;69:623–629. doi: 10.1001/archneurol.2011.2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lo RY, Jagust WJ. Effect of Cognitive Reserve Markers on Alzheimer Pathologic Progression. Alzheimer Disease and Associated Disorders. 2013 doi: 10.1097/WAD.0b013e3182900b2b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hommet C, et al. Review of cerebral microangiopathy and Alzheimer's disease: relation between white matter hyperintensities and microbleeds. Dementia and Geriatric Cognitive Disorders. 2011;32:367–378. doi: 10.1159/000335568. [DOI] [PubMed] [Google Scholar]
- 78.Provenzano FA, et al. White matter hyperintensities and cerebral amyloidosis: necessary and sufficient for clinical expression of Alzheimer disease? JAMA Neurol. 2013;70:455–461. doi: 10.1001/jamaneurol.2013.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Carmichael O, et al. Longitudinal changes in white matter disease and cognition in the first year of the Alzheimer disease neuroimaging initiative. Archives of Neurology. 2010;67:1370–1378. doi: 10.1001/archneurol.2010.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Marra C, et al. Patterns of cognitive decline and rates of conversion to dementia in patients with degenerative and vascular forms of MCI. Current Alzheimer Research. 2011;8:24–31. doi: 10.2174/156720511794604552. [DOI] [PubMed] [Google Scholar]
- 81.van der Vlies AE, et al. Associations between magnetic resonance imaging measures and neuropsychological impairment in early and late onset alzheimer's disease. Journal of Alzheimer's disease : JAD. 2013;35:169–178. doi: 10.3233/JAD-121291. [DOI] [PubMed] [Google Scholar]
- 82.Lo RY, Jagust WJ. Vascular burden and Alzheimer disease pathologic progression. Neurology. 2012;79:1349–1355. doi: 10.1212/WNL.0b013e31826c1b9d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Landau SM, et al. Comparing PET imaging and CSF measurements of A-beta. Annals of Neurology 2013 [Google Scholar]
- 84.van Norden AG, et al. Diffusion tensor imaging of the hippocampus and verbal memory performance: the RUN DMC study. Human Brain Mapping. 2012;33:542–551. doi: 10.1002/hbm.21231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.den Heijer T, et al. Structural and diffusion MRI measures of the hippocampus and memory performance. NeuroImage. 2012;63:1782–1789. doi: 10.1016/j.neuroimage.2012.08.067. [DOI] [PubMed] [Google Scholar]
- 86.Mielke MM, et al. Fornix integrity and hippocampal volume predict memory decline and progression to Alzheimer's disease. Alzheimer's & Dementia. 2012;8:105–113. doi: 10.1016/j.jalz.2011.05.2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhuang L, et al. Microstructural white matter changes, not hippocampal atrophy, detect early amnestic mild cognitive impairment. PloS one. 2013;8:e58887. doi: 10.1371/journal.pone.0058887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lee DY, et al. Sub-Regional Hippocampal Injury is Associated with Fornix Degeneration in Alzheimer's Disease. Frontiers in Aging Neuroscience. 2012;4:1. doi: 10.3389/fnagi.2012.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bartzokis G. Alzheimer's disease as homeostatic responses to age-related myelin breakdown. Neurobiology of Aging. 2011;32:1341–1347. doi: 10.1016/j.neurobiolaging.2009.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Apostolova LG, et al. Automated 3D mapping of baseline and 12-month associations between three verbal memory measures and hippocampal atrophy in 490 ADNI subjects. Neuroimage. 2010;51:488–499. doi: 10.1016/j.neuroimage.2009.12.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Braak H, Braak E. Staging of Alzheimer's disease-related neurofibrillary changes. Neurobiology of Aging. 1995;16:271–278. doi: 10.1016/0197-4580(95)00021-6. discussion 278-284. [DOI] [PubMed] [Google Scholar]
- 92.Bai F, et al. Aberrant hippocampal subregion networks associated with the classifications of aMCI subjects: a longitudinal resting-state study. PloS One. 2011;6:e29288. doi: 10.1371/journal.pone.0029288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Glodzik L, et al. Phosphorylated tau 231, memory decline and medial temporal atrophy in normal elders. Neurobiology of Aging. 2011;32:2131–2141. doi: 10.1016/j.neurobiolaging.2009.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Leow AD, et al. Alzheimer's disease neuroimaging initiative: a one-year follow up study using tensor-based morphometry correlating degenerative rates, biomarkers and cognition. NeuroImage. 2009;45:645–655. doi: 10.1016/j.neuroimage.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.McDonald CR, et al. Relationship between regional atrophy rates and cognitive decline in mild cognitive impairment. Neurobiology of Aging. 2012;33:242–253. doi: 10.1016/j.neurobiolaging.2010.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gutman BA, et al. Maximizing power to track Alzheimer's disease and MCI progression by LDA-based weighting of longitudinal ventricular surface features. NeuroImage. 2013;70:386–401. doi: 10.1016/j.neuroimage.2012.12.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Andreasen N, et al. CSF markers for Alzheimer's disease: total tau, phospho-tau and Abeta42. The World Journal of Biological Psychiatry. 2003;4:147–155. doi: 10.1080/15622970310029912. [DOI] [PubMed] [Google Scholar]
- 98.Apostolova LG, et al. Ventricular Enlargement and its Clinical Correlates in the Imaging Cohort From the ADCS MCI Donepezil/Vitamin E Study. Alzheimer Disease and Associated Disorders. 2013;27:174–181. doi: 10.1097/WAD.0b013e3182677b3d. [DOI] [PMC free article] [PubMed] [Google Scholar]


