Abstract
Background
Postoperative outcomes of patients undergoing laparoscopic-assisted colectomy (LAC) have shown modest improvements in recovery but only minimal differences in quality of life (QOL) compared with open colectomy. We therefore sought to assess the effect of LAC on QOL in the short and long term, using individual item analysis of multi-item QOL assessments.
Methods
QOL variables were analyzed in 449 randomized patients from the COST trial 93-46-53 (INT 0146). Both cross-sectional single-time and change from baseline assessments were run at day 2, week 2, month 2, and month 18 postoperatively in an intention-to-treat analysis using Wilcoxon rank-sum tests. Stepwise regression models were used to determine predictors of QOL.
Results
Of 449 colon cancer patients, 230 underwent LAC and 219 underwent open colectomy. Subdomain analysis revealed a clinically moderate improvement from baseline for LAC in total QOL index at 18 months (P = 0.02) as well as other small symptomatic improvements. Poor preoperative QOL as indicated by a rating scale of ≤ 50 was an independent predictor of poor QOL at 2 months postoperatively. QOL variables related to survival were baseline support (P = 0.001) and baseline outlook (P = 0.01).
Conclusions
Eighteen months after surgery, any differences in quality of life between patients randomized to LAC or open colectomy favored LAC. However, the magnitude of the benefits was small. Patients with poor preoperative QOL appear to be at higher risk for difficult postoperative courses, and may be candidates for enhanced ancillary services to address their particular needs.
The vastly expanded field of laparoscopic surgery now applies to a broad range of diseases including colon cancer, and several large randomized trials have shown no difference in recurrence rates and survival for laparoscopic-assisted colectomy (LAC) compared with open colectomy.1–3 In addition, these studies have demonstrated more rapid postoperative recovery after LAC, leading to modest reductions in length of stay.1,4–6 The extent to which these differences translate into meaningful differences in QOL is less clear.
The Clinical Outcomes of Surgical Therapy (COST) study was a randomized controlled trial initiated by the North Central Cancer Treatment Group and funded by the National Cancer Institute.1,7 This study addressed the risks, benefits, and oncologic outcome of LAC for colon cancer, including QOL as well as clinical endpoints.1,7 In the primary analysis of the COST trial, recurrence and overall survival did not differ between open colectomy versus LAC, and LAC was associated with a statistically significant reduction in the duration of postoperative in-hospital analgesia and length of stay.1
An analysis of the QOL outcomes in the COST study through 2 months of follow-up showed no difference in change scores for pain intensity, a summary symptom scale, a summary QOL index, and a global rating of QOL (with one exception: the global rating change from baseline score favored LAC at the 2-week time point).7 Other than pain, individual items from the symptom scale and QOL index were not evaluated in that analysis, raising the question of whether differences in specific domains could have been missed.8 Because of the short follow-up at the time of publication, outcomes at 18 months were not included. While other studies have reported longer-term QOL outcomes, longitudinal assessments that control for baseline QOL are not available.9,10
The primary analysis of the COST study QOL results focused on treatment comparison, but recent studies have explored and validated the prognostic value of pretreatment patient self-reported QOL scores in several advanced malignancies including colorectal cancer.11–13 Other groups have studied the impact of low baseline QOL on survival, and validated QOL cutoff scores considered clinically deficient, at or below which predicted survival decreased.14–16
We therefore undertook an analysis of the QOL data from the COST trial to complete the long-term QOL outcomes of patients randomized to LAC versus open colectomy. We also sought to determine whether single-item assessments might be more sensitive to differences between treatment groups than the multi-item scales reported in the original QOL analysis, to assess factors associated with QOL outcomes, and to evaluate the effect of baseline QOL on subsequent QOL and survival.8
METHODS
Original Analytical Considerations
The QOL assessment performed during the COST trial 93-46-43 (INT 0146) was conducted by surveying patients with standardized instruments for assessing symptoms and QOL of cancer patients including the Symptom Distress Scale (SDS) and QOL index (QLI) plus a global QOL rating scale after approval by each Institutional Review Board participating in the study.7 The SDS measures symptom frequency and distress in 11 domains (total of 13 items) on a severity scale of 1 through 5 (higher score indicating poorer QOL).17,18 The QLI measures QOL in five domains (again with higher score indicating poorer QOL), while the global rating scale is based on respondents’ quantification of their health in the past 2 weeks on a scale of 0 to 100 (lower score indicating poorer QOL).19 Throughout the COST trial as well as this study, the change from baseline scores for the SDS pain scale, the SDS summary score, the QLI summary score, and the global rating scale values were compared between treatment groups using Wilcoxon rank-sum tests separately for each time point. To remain consistent with the original analysis, all comparisons were performed using intention-to-treat analyses in which patients were analyzed according to assigned treatment group, either LAC or open colectomy. Patient characteristics were compared between treatment groups using Fisher’s exact tests for discrete variables and Wilcoxon rank-sum tests for continuous variables.
Individual Item Analysis and Supplementary Longitudinal Data
The individual item analysis was carried out for each item of the SDS and QLI using the same comparative hypothesis testing as the summated scales (Wilcoxon procedures comparing treatment group QOL variable scores at each time point and change from baseline scores).
Subsequent to the initial report, supplementary data became available for evaluations conducted at 18 months postoperatively for the QLI assessment and global QOL rating scale (SDS was not collected at the 18-month time point), and the same Wilcoxon methods used in the initial report were carried out to compare the 18-month time point and change from baseline scores.
Clinical Significance
For this report, to put the various tests of statistical significance in a clinically relevant context, the clinical significance of each comparison was determined by the effect size method as described by Cohen.20,21 This method defines clinically significant changes in QOL in terms of the standard deviation (SD) of baseline QOL scores, with small clinical significance correlating with effect size of 0.2 to <0.5 SD, medium clinical significance correlating with effect size of 0.5 to <0.8 SD, and large clinical significance correlating with effect size ≥0.8 SD.
Factors Influencing Postoperative QOL
A subsequent analysis using stepwise linear regression modeling and stepwise logistic modeling was undertaken to identify factors influencing postoperative overall QOL and complications at each time point. Linear regression models were used for predicting the various continuous QOL variables, while logistic regression modeling was used for predicting the presence/absence of clinically deficient overall QOL as defined below. Complications were graded as previously defined in the COST trial.1,22 Each of the individual items in the SDS, QLI, and global rating scale were used as dependent variables in separate stepwise linear regression model processes to select baseline variables associated with assigned treatment group. Variables were included in the model if their entry significance level was at least 0.10. Separate models were then run for each endpoint and for values at 2 days, 2 weeks, 2 months, and 18 months from surgery.
QOL Factors Influencing Overall Survival
A survival analysis involving stepwise Cox proportional hazards models was carried out to estimate the influence of low baseline QOL on overall survival in the COST patients in the presence of other baseline covariates. Recent studies have explored and validated the prognostic value of pre-treatment patient self-reported QOL scores in several advanced malignancies including colorectal cancer, as well as the impact of low baseline QOL on survival.11–13 Other studies validated QOL cutoff scores considered clinically deficient, at or below which predicted survival decreased.14–16 In this analysis, a clinically deficient quality of life score was defined as QOL ≤ 50 (on a 0–100 point scale) as described by Qi et al.16
RESULTS
Patient Characteristics
A total of 449 patients in the COST trial (including all patients for whom QOL data were collected) were included in this study; 230 patients were assigned to the LAC group, while 219 patients underwent open colectomy by assignment. Table 1 provides patient demographics by assigned treatment group. There were no statistically significant differences between groups in any of the patient or tumor characteristics assessed. The only statistically significant difference between groups was that a higher percentage of LAC patients had a clinically deficient baseline QOL (QOL ≤ 50, 18% versus 11% in the open colectomy group, P = 0.045). Given the large number of tests done in making the pretreatment comparisons, this single result is likely spurious.
TABLE 1.
Patient demographics by treatment arm
| LAC (N = 230) | OPEN (N = 219) | Total (N = 449) | P-value | |
|---|---|---|---|---|
| Gender | 0.5417 | |||
| Female | 111 (48%) | 112 (51%) | 223 (50%) | |
| Male | 119 (52%) | 107 (49%) | 226 (50%) | |
| Race | 0.1678 | |||
| Asian | 1 (0%) | 6 (3%) | 7 (2%) | |
| Black | 17 (7%) | 19 (9%) | 36 (8%) | |
| Hispanic | 10 (4%) | 5 (2%) | 15 (3%) | |
| Other | 1 (0%) | 0 (0%) | 1 (0%) | |
| White | 201 (88%) | 189 (86%) | 390 (87%) | |
| Age (years) | 0.3785 | |||
| Mean (SD) | 68.2 (11.84) | 69.5 (10.59) | 68.8 (11.25) | |
| Range | (28–96) | (38–95) | (28–96) | |
| Stage | 0.0634 | |||
| 1 | 89 (39%) | 68 (31%) | 157 (35%) | |
| 2 | 77 (34%) | 78 (36%) | 155 (35%) | |
| 3 | 58 (26%) | 61 (28%) | 119 (27%) | |
| 4 | 3 (1%) | 11 (5%) | 14 (3%) | |
| ASA | 0.8323 | |||
| I and II | 198 (86%) | 187 (85%) | 385 (86%) | |
| III | 32 (14%) | 32 (15%) | 64 (14%) | |
| Any postoperative complication | 0.1763 | |||
| No | 183 (80%) | 185 (84%) | 368 (82%) | |
| Yes | 47 (20%) | 34 (16%) | 81 (18%) | |
| Postoperative complication grade | 0.5039 | |||
| 0 | 183 (80%) | 185 (85%) | 368 (82%) | |
| 1 | 30 (13%) | 19 (9%) | 49 (11%) | |
| 2 | 16 (7%) | 14 (6%) | 30 (7%) | |
| 4 | 1 (0%) | 1 (0%) | 2 (0%) | |
| Overall QOL ≤ 50 | 0.0448 | |||
| No | 171 (82%) | 174 (89%) | 345 (86%) | |
| Yes | 37 (18%) | 21 (11%) | 58 (14%) |
ASA American Society of Anesthesiologists
Effect of Treatment Assignment on QOL at 18 Months
By 18 months after surgery, 31 patients had died. Of the 418 patients remaining, 188 (45%) patients responded to the QOL index and global rating scale questionnaires. Cross-sectional comparison of demographics between responders and nonresponders was performed. Patient characteristics were similar except that patients with stage 3 or 4 disease were less likely to respond (P = 0.04).
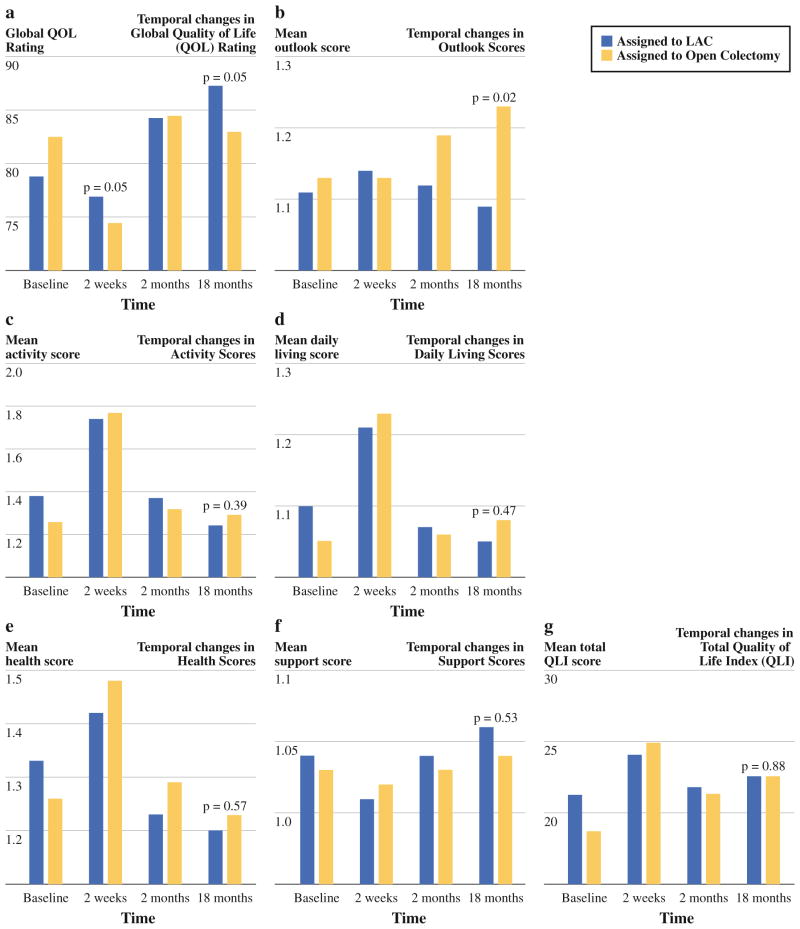
Quality of life analysis was performed using cross-sectional assessments (single-time and change from baseline) at day 2, week 2, month 2, and month 18. At the 18-month single time point, the only statistically significant improvements in LAC patients compared with the open colectomy group were in the global QOL rating and the QLI subdomain of outlook (Fig. 1a, b). There were no differences between treatment arms in the subdomains of activity (P = 0.39), daily living (P = 0.47), health (P = 0.57), support (0.53) or total QLI (P = 0.88) at the 18-month time point (Fig. 1c–g). LAC patients had significantly greater improvement from baseline to 18 months in global QOL rating (P = 0.02), daily living (P = 0.03), health (P = 0.02), and total QLI (P = 0.02).
FIG. 1.
a Temporal changes in global quality of life (QOL) rating. b Temporal changes in outlook scores. c Temporal changes in activity scores. d Temporal changes in daily living scores. e Temporal changes in health scores. f Temporal changes in support scores. g Temporal changes in total quality of life index (QLI)
Single-Item Analysis
The intention-to-treat subdomain analysis demonstrated significant differences favoring LAC in multiple individual items. Table 2 demonstrates the mean QOL subdomain score with standard deviation, the clinical effect, and the statistical significance at each time point and change from baseline. The analysis of early postoperative symptoms was not different between the two groups, with the exception of bowel function which showed a statistically significant improvement from baseline at 2 days and 2 weeks after surgery for the LAC group. However, this improvement corresponded to only a small level of clinical significance at 2 days (effect size of 0.21 SD) but no clinically meaningful significance at 2 weeks (effect size of 0.18 SD). Other small clinical benefits were seen in nausea frequency, pain frequency, and concentration.
TABLE 2.
Significant Symptoms Distress Scale mean values by treatment arma
| Variable | LAC (SD) | OPEN (SD) | Total (SD) | Effect size (SD) | P-value |
|---|---|---|---|---|---|
| Nausea distress | |||||
| Baseline | 1.4 (0.75) | 1.3 (0.67) | 1.3 (0.71) | 0.0561 | |
| Day 2 | 1.6 (0.91) | 1.6 (0.92) | 1.6 (0.91) | 0.2946 | |
| Week 2 | 1.3 (0.66) | 1.4 (0.80) | 1.3 (0.73) | 0.3039 | |
| Month 2 | 1.2 (0.47) | 1.3 (0.69) | 1.2 (0.59) | 0.25 | 0.0303 |
| Δ (2 months-baseline) | −0.2 (0.75) | 0.1 (0.88) | −0.1 (0.82) | 0.29 | 0.0048 |
| Pain frequency | |||||
| Baseline | 1.8 (1.05) | 1.8 (1.08) | 1.8 (1.07) | 0.5815 | |
| Day 2 | 2.4 (1.05) | 2.5 (1.19) | 2.5 (1.12) | 0.7939 | |
| Week 2 | 1.8 (0.95) | 1.9 (0.93) | 1.9 (0.94) | 0.4458 | |
| Month 2 | 1.4 (0.76) | 1.6 (0.88) | 1.5 (0.82) | 0.21 | 0.0284 |
| Δ (2 months-baseline) | −0.4 (1.16) | −0.2 (1.18) | −0.3 (1.17) | 0.19 | 0.0611 |
| Bowel function | |||||
| Baseline | 1.9 (1.23) | 1.8 (1.13) | 1.9 (1.19) | 0.1083 | |
| Day 2 | 3.4 (1.68) | 3.7 (1.64) | 3.5 (1.66) | 0.0638 | |
| Week 2 | 1.9 (1.19) | 2.0 (1.25) | 2.0 (1.22) | 0.3647 | |
| Month 2 | 1.7 (0.97) | 1.7 (1.17) | 1.7 (1.07) | 0.4950 | |
| Δ (2 weeks-baseline) | 1.5 (1.84) | 1.8 (1.87) | 1.6 (1.86) | 0.21 | 0.0421 |
| Δ (2 weeks-baseline) | −0.1 (1.58) | 0.2 (1.45) | 0.1 (1.52) | 0.18 | 0.0387 |
| Δ (2 months–baseline) | −0.2 (1.36) | 0.0 (1.57) | −0.1 (1.46) | 0.2046 | |
| Concentration | |||||
| Baseline | 1.4 (0.71) | 1.4 (0.68) | 1.4 (0.69) | 0.7143 | |
| Day 2 | 1.7 (1.06) | 1.7 (0.99) | 1.7 (1.02) | 0.8472 | |
| Week 2 | 1.4 (0.77) | 1.3 (0.55) | 1.3 (0.67) | 0.0353 | |
| Month 2 | 1.3 (0.55) | 1.3 (0.76) | 1.3 (0.66) | 0.9089 | |
| Δ (2 weeks-baseline) | 0.1 (0.84) | −0.1 (0.68) | 0.0 (0.77) | 0.23 | 0.0128 |
| Δ (2 months-baseline) | 0.0 (0.69) | 0.0 (0.86) | 0.0 (0.77) | 0.2407 | |
| Cough | |||||
| Baseline | 1.4 (0.58) | 1.6 (0.70) | 1.5 (0.65) | 0.0003 | |
| Day 2 | 1.6 (0.64) | 1.6 (0.67) | 1.6 (0.65) | 0.6509 | |
| Week 2 | 1.4 (0.58) | 1.4 (0.55) | 1.4 (0.57) | 0.4050 | |
| Month 2 | 1.3 (0.48) | 1.3 (0.54) | 1.3 (0.51) | 0.5813 | |
| Δ (2 months-baseline) | −0.1 (0.59) | −0.2 (0.68) | −0.2 (0.64) | 0.0157 | |
Effect size (clinical significance): small = 0.2 to < 0.5 SD; medium = 0.5 to <0.8 SD; large ≥0.8 SD
SD standard deviation, Δ change
Higher scores indicate poorer QOL
Assignment to the LAC arm was associated with improved scores in several subdomains of the QLI and in the global rating scale at various time points and change from baseline (Table 3). At single time points, scores for outlook at month 18 and global rating scale at week 2 and month 18 all favored LAC. The most clinically significant item favoring LAC was the change from baseline to 18 months in total QLI (effect size of 0.77 SD). All other significant changes from baseline to 18 months demonstrated small clinical significance. As described in the original COST study publication, the change from baseline to 2 weeks in global rating scale was also significant in our analysis but not reported in Table 3.7 No single domain in the SDS, QLI or global QOL rating scale favored open colectomy.
TABLE 3.
| Variable | LAC (SD) | OPEN (SD) | Total (SD) | Effect size (SD) | P-value |
|---|---|---|---|---|---|
| Daily living: | |||||
| Baseline | 1.1 (0.35) | 1.0 (0.22) | 1.1 (0.29) | 0.0987 | |
| Week 2 | 1.2 (0.51) | 1.2 (0.50) | 1.2 (0.50) | 0.5402 | |
| Month 2 | 1.1 (0.31) | 1.1 (0.30) | 1.1 (0.30) | 0.4652 | |
| Month 18 | 1.1 (0.27) | 1.1 (0.31) | 1.1 (0.29) | 0.4665 | |
| Δ (18 months-baseline) | −0.1 (0.47) | 0.0 (0.34) | 0.0 (0.42) | 0.31 | 0.0264 |
| Health | |||||
| Baseline | 1.3 (0.55) | 1.3 (0.50) | 1.3 (0.53) | 0.1664 | |
| Week 2 | 1.4 (0.59) | 1.5 (0.57) | 1.4 (0.58) | 0.1953 | |
| Month 2 | 1.2 (0.43) | 1.3 (0.52) | 1.3 (0.47) | 0.2783 | |
| Month 18 | 1.2 (0.45) | 1.2 (0.47) | 1.2 (0.46) | 0.5665 | |
| Δ (18 months-baseline) | −0.1 (0.62) | 0.0 (0.56) | 0.0 (0.60) | 0.31 | 0.0218 |
| Outlook | |||||
| Baseline | 1.1 (0.32) | 1.1 (0.35) | 1.1 (0.34) | 0.7235 | |
| Week 2 | 1.1 (0.36) | 1.1 (0.35) | 1.1 (0.36) | 0.7067 | |
| Month 2 | 1.1 (0.32) | 1.2 (0.42) | 1.2 (0.37) | 0.0690 | |
| Month 18 | 1.1 (0.29) | 1.2 (0.45) | 1.2 (0.38) | 0.36 | 0.0182 |
| Δ (18 months-baseline) | 0.0 (0.33) | 0.1 (0.48) | 0.0 (0.41) | 0.0730 | |
| Total QLI | |||||
| Baseline | 21.3 (14.82) | 18.7 (10.70) | 20.1 (13.19) | 0.5156 | |
| Week 2 | 24.1 (14.98) | 24.9 (15.65) | 24.5 (15.29) | 0.7184 | |
| Month 2 | 21.8 (13.58) | 21.3 (14.13) | 21.6 (13.80) | 0.7498 | |
| Month 18 | 22.6 (18.52) | 22.6 (17.21) | 22.6 (17.64) | 0.8786 | |
| Δ (18 months-baseline) | −6.3 (13.42) | 5.5 (17.01) | −0.3 (16.30) | 0.77 | 0.0191 |
| Global rating scale | |||||
| Baseline | 78.8 (18.68) | 82.5 (15.99) | 80.6 (17.51) | 0.0759 | |
| Week 2 | 76.9 (18.37) | 74.4 (17.64) | 75.7 (18.04) | 0.14 | 0.0473 |
| Month 2 | 84.3 (16.27) | 84.5 (14.40) | 84.4 (15.40) | 0.4638 | |
| Month 18 | 87.3 (14.89) | 83.0 (18.63) | 85.2 (16.93) | 0.26 | 0.0528 |
| Δ (18 months–baseline) | 8.0 (18.35) | 1.4 (18.50) | 4.9 (18.66) | 0.36 | 0.0190 |
SD standard deviation, Δ change, QLI quality of life index
Higher scores indicate poorer QOL
Higher scores indicate better QOL
Effect size (clinical significance): small = 0.2 to < 0.5 SD; medium = 0.5 to <0.8 SD; large ≥0.8 SD
Regression Models
Stepwise linear and logistic regressions were performed on the variables previously listed to determine association with better QOL outcomes. Baseline values were the strongest predictors of all outcomes at each time point through postoperative month 2. LAC treatment arm and complications were predictors of several outcomes as shown in Table 4. In the early postoperative period, complications (overall and grade of complication) were particularly influential in the QOL scores. Interestingly, at 18 months after surgery, complications were no longer a significant prediction of QOL or any individual subdomain. As expected, baseline QOL ≤ 50 was associated with significantly worse QOL symptoms such as nausea frequency, insomnia, fatigue, activity, and overall health at 2 weeks and 2 months. At 18 months, clinically deficient baseline QOL did not significantly predict low overall QOL (Table 4). While other variables such as individual patient and tumor characteristics were found to be significant in predicting postoperative QOL, none of these were as consistently apparent as baseline QOL values. Interestingly, higher disease stage was associated with poorer findings for several symptoms including health, outlook, and overall QOL at 2 months, but these associations did not remain at 18 months, which may be due to the low response rate of stage 3 and 4 patients.
TABLE 4.
Postoperative quality of life symptoms and ratings predicted by LAC, complications, and QOL ≤ 50
| Time | LAC | P-value | Complication | P-value | QOL ≤ 50 | P-value |
|---|---|---|---|---|---|---|
| Day 2 | Bowel habits | 0.07 | Concentration | 0.02 | Nausea frequency | 0.005 |
| Breathing | 0.05 | Appearance (G) | 0.005 | Nausea distress | 0.004 | |
| Breathing (G) | 0.006 | Concentration | 0.06 | |||
| Week 2 | Appetite | 0.03 | Total SDS | 0.0004 | Total SDS | 0.06 |
| Concentration | 0.01 | Nausea frequency | 0.0056 | Nausea frequency | 0.02 | |
| Outlook | 0.04 | Nausea distress (G) | 0.0002 | Nausea distress | 0.06 | |
| Overall QOL | 0.02 | Appetite | 0.01 | Appetite | 0.009 | |
| Insomnia | 0.005 | Insomnia | 0.03 | |||
| Pain frequency | 0.07 | Pain frequency | 0.02 | |||
| Fatigue (G) | 0.009 | Pain distress | 0.02 | |||
| Bowel habits | 0.003 | Fatigue | 0.03 | |||
| Concentration (G) | 0.008 | Daily living | 0.10 | |||
| Appearance (G) | 0.0003 | Health | 0.01 | |||
| Breathing | 0.03 | |||||
| Outlook | 0.03 | |||||
| Cough (G) | 0.07 | |||||
| Total QLI | 0.04 | |||||
| Activity | 0.0045 | |||||
| Daily living (G) | 0.007 | |||||
| Health | 0.002 | |||||
| Outlook (G) | 0.05 | |||||
| Overall QOL | 0.002 | |||||
| Month 2 | Nausea distress | 0.02 | Appetite (G) | 0.005 | Nausea frequency | 0.04 |
| Appetite | 0.04 | Pain frequency | 0.09 | Insomnia | 0.04 | |
| Pain frequency | 0.04 | Pain distress | 0.03 | Fatigue | 0.01 | |
| Outlook | 0.07 | Appearance (G) | 0.009 | Concentration | 0.08 | |
| Cough (G) | 0.02 | Breathing | 0.08 | |||
| Total QLI (G) | 0.06 | Activity | 0.0004 | |||
| Daily living (G) | 0.01 | Health | 0.0001 | |||
| Month 18 | Outlook | 0.007 | Overall QOL | 0.09 | ||
| Overall QOL | 0.05 |
QOL quality of life, QLI quality of life index, (G) significant complication grade
Using stepwise logistic regression to determine factors associated with a QOL score ≤50, only baseline QOL ≤ 50 was significantly associated at 2 weeks and 2 months (P < 0.001) (Table 5). By month 18, only age predicted QOL ≤ 50 (P = 0.03). The patient’s level of support did approach significance for predicting QOL ≤ 50 (P = 0.08) at 2 months, but this resolved by 18 months.
TABLE 5.
Predictive variables for having QOL ≤ 50
| Time | Predictive variable | Odds ratio | P-value |
|---|---|---|---|
| Week 2 | Baseline QOL ≤ 50 | 3.72 | 0.001 |
| Age | 1.04 | 0.03 | |
| White | 3.99 | 0.09 | |
| Pain frequency | 1.33 | 0.05 | |
| Cough | 2.00 | 0.003 | |
| Activity | 1.53 | 0.08 | |
| Outlook | 3.29 | 0.006 | |
| Month 2 | Baseline QOL ≤ 50 | 4.96 | 0.0004 |
| Support | 2.90 | 0.08 | |
| Month 18 | Age | 1.15 | 0.03 |
| Activity | 2.57 | 0.07 |
QOL quality of life
Survival Analysis
Using Cox proportional hazards models, significant predictors of survival were identified. As expected, patient and disease characteristics of increased age, higher stage of disease, and poor overall baseline health were associated with shorter survival. Of the QOL subdomains tested, only baseline outlook [hazard ratio (HR) = 0.58, 95% confidence interval (CI) 0.38–0.88, P = 0.01] and support (HR = 2.85, 95% CI 1.52–5.35, P = 0.001) were significantly associated with overall survival. Interestingly, clinically deficient baseline QOL was not related to overall survival; however, the number of events for those with clinically significant deficits in QOL was relatively small.
All analyses were also repeated after excluding patients with stage 4 disease, and there were no meaningful differences in results.
DISCUSSION
Long-Term Follow-Up
Laparoscopic-assisted colectomy has been shown to result in equivalent oncologic outcome and some benefit with regard to shorter hospital stay, less narcotic use, and better bowel function.1–6,23,24 However, a clinically important effect on patient QOL has not been clearly demonstrated.3,7 Our analysis of the 18-month outcomes of the COST trial as well as a new analysis of single-item scores at early and later time points provides important new, but essentially confirmatory evidence. In particular, we found that any differences from baseline between treatment groups at 18 months favored LAC, but the magnitudes of the effects were predominantly small.
Single-Item Analysis
Subdomain analysis demonstrated significant improvement in both selected short-term postoperative symptoms and long-term QOL ratings for LAC patients. While baseline OQL was significantly better in LAC patients, the true differences or lack thereof were seen in the change from baseline analysis. These differences are of small clinical magnitude (as defined by the effect size), but the fact that no subdomain analysis favored open colectomy supports the previous conclusion that LAC for colon cancer resection is just as suitable, if not more beneficial, than open colectomy in the appropriately chosen patient. The SDS, QOL index, and global QOL rating scale all evaluate items which may be of particular importance to patients within the immediate postoperative time (i.e., pain, nausea, bowel function, daily living, and overall health). Therefore, subdomain analysis of these tools provides some insight into the specific benefits patients might experience from laparoscopic surgery.
Factors Influencing Postoperative QOL
Using both global and subdomain analysis, we were able to gain a better understanding of which variables predict QOL. The impact of complications on overall QOL is somewhat contrary to what would be expected. While the presence of postoperative complications was associated with worse symptoms in the short term, there was no measurable effect on overall QOL. By 18 months, complications were not related to any of the QOL measures, suggesting that the effects of complications resolved over time.
Surprisingly, baseline QOL was the most important predictor of postoperative overall QOL and all QOL subdomains. While baseline QOL was not associated with overall survival, overall QOL, or total QLI, clinically deficient QOL (≤50) was consistently associated with worse individual symptoms in the immediate postoperative period. Baseline QOL ≤ 50 was the strongest predictor of QOL ≤ 50 at week 2 and month 2. These findings raise the question of whether factors such as cancer diagnosis have greater impact on QOL than the actual surgical approach. Indeed, poor patient QOL status is accompanied by the potential for increased cost in time and personnel utilization as patients seek medical intervention for their distress. This suggests that patients with particularly poor preoperative QOL may be at higher risk for difficult postoperative courses, and might be candidates for enhanced ancillary services to address their particular needs, regardless of the surgery they will undergo.
QOL Impact on Survival
Of considerable interest is the finding that baseline outlook and support had a significant impact on overall survival in this patient population. Although lower baseline outlook was associated with decreased survival, we can only speculate on the underlying mechanism. There were only 31 deaths in our study, and unfortunately, our response rate was less than 50% due to patients with higher-stage disease selectively opting out of the 18-month assessment. However, there is no a priori reason to think that the nonresponse rate biased the treatment comparison, and the similarity of our 18-month results to those based on higher response rates at the earlier assessment time points is reassuring.
Nonetheless, these results are provocative. It is possible that patients with poorer outlook are less compliant or less likely to maintain health and promote behaviors such as exercise, thus resulting in poorer survival.25 Alternatively, pre-existing, underlying health problems may affect both outlook and survival. Finally, it is possible that mental attitude has a direct effect on longevity, perhaps through an effect on the immune system. This hypothesis has been tested in several studies with conflicting results.26
These findings must be taken in the context of complexities of overall survival analysis, which include underlying comorbidities and cancer stage. Therefore, a larger analysis needs to be performed taking into account quality of life, comorbidities, and cancer stage.
In summary, the 18-month follow-up demonstrates that single-item analysis gives more insight into the nuances of QOL changes. The new results confirm the results of the first QOL analysis with the addition of some small clinical benefits favoring LAC. In addition, those patients presenting with clinically deficient QOL are at risk for a difficult postoperative course. Identification and early intervention of at-risk patients may result in improved postoperative outcomes.
Supplementary Material
Acknowledgments
Original funding of the Clinical Outcomes of Surgical Therapy trial 93-46-53 was provided by the National Cancer Institute in association with the North Central Cancer Treatment Group.
Footnotes
CONFLICT OF INTEREST We have no commercial interests to disclose in relation to this study.
References
- 1.Clinical Outcomes of Surgical Therapy Study Group et al. A comparison of laparoscopically assisted and open colectomy for colon cancer. N Engl J Med. 2004;20:2050–9. doi: 10.1056/NEJMoa032651. [DOI] [PubMed] [Google Scholar]
- 2.Colon Cancer Laparoscopic or Open Resection Study Group et al. Survival after laparoscopic surgery versus open surgery for colon cancer: long-term outcome of a randomised clinical trial. Lancet Oncol. 2009;10:44–52. doi: 10.1016/S1470-2045(08)70310-3. [DOI] [PubMed] [Google Scholar]
- 3.Jayne DG, Guillou PJ, Thorpe H, et al. Randomized trial of laparoscopic-assisted resection of colorectal carcinoma: 3-year results of the UK MRC CLASICC Trial Group. J Clin Oncol. 2007;25:3061–8. doi: 10.1200/JCO.2006.09.7758. [DOI] [PubMed] [Google Scholar]
- 4.Cera SM, Wexner SD. Minimally invasive treatment of colon cancer. Cancer J. 2005;11:26–35. doi: 10.1097/00130404-200501000-00005. [DOI] [PubMed] [Google Scholar]
- 5.Lacy AM, Garcia-Valdecasas J, Delgado S, Castells A, Taurá P, Piqué JM, et al. Laparoscopy-assisted colectomy versus open colectomy for treatment of non-metastatic colon cancer: a ran-domised trial. Lancet. 2002;359:2224–9. doi: 10.1016/S0140-6736(02)09290-5. [DOI] [PubMed] [Google Scholar]
- 6.Lacy AM, Delgado S, Castells A, Prius HA, Arroyo V, Ibarzabal A, et al. The long term results of a randomized clinical trial of laparoscopy-assisted versus open surgery for colon cancer. Ann Surg. 2008;248:1–7. doi: 10.1097/SLA.0b013e31816a9d65. [DOI] [PubMed] [Google Scholar]
- 7.Weeks JC, Nelson H, Gelber S, Sargent DJ, Schroeder G Clinical Outcomes of Surgical Therapy (COST) Study Group. Short-term quality-of-life outcomes following laparoscopic-assisted colectomy vs open colectomy for colon cancer. JAMA. 2002;287:321–7. doi: 10.1001/jama.287.3.321. [DOI] [PubMed] [Google Scholar]
- 8.Sloan JA, Aaronson N, Cappelleri JC, Fairclough DL, Varricchio C Clinical Significance Consensus Meeting Group. Assessing the clinical significance of single items relative to summated scores. Mayo Clinic Proc. 2002;77:479–87. [PubMed] [Google Scholar]
- 9.Sokolovic E, Buchmann P, Schlomowitsch F, Szucs TD. Comparison of resource utilization and long-term quality-of-life outcomes between laparoscopic and conventional colorectal surgery. Surg Endosc. 2004;18:1663–7. doi: 10.1007/s00464-003-9168-8. [DOI] [PubMed] [Google Scholar]
- 10.Braga M, Frasson M, Vignali A, Zuliani W, Civelli V, Di Carlo V. Laparoscopic vs. open colectomy in cancer patients: long-term complications, quality of life, and survival. Dis Colon Rectum. 2005;48:2217–23. doi: 10.1007/s10350-005-0185-7. [DOI] [PubMed] [Google Scholar]
- 11.Efficace F, Bottomley A, Coens C, et al. Does a patient’s self-reported health-related quality of life predict survival beyond key biomedical data in advanced colorectal cancer? Eur J Cancer. 2006;42:42–9. doi: 10.1016/j.ejca.2005.07.025. [DOI] [PubMed] [Google Scholar]
- 12.Efficace F, Innominato PF, Bjarnason G, et al. Validation of patient’s self-reported social functioning as an independent prognostic factor for survival in metastatic colorectal cancer patients: results of an international study by the Chronotherapy Group of the European Organisation for Research and Treatment of Cancer. J Clin Oncol. 2008;26:2020–6. doi: 10.1200/JCO.2007.12.3117. [DOI] [PubMed] [Google Scholar]
- 13.Gotay CC, Kawamoto CT, Bottomley A, Efficace F. The prognostic significance of patient-reported outcomes in cancer clinical trials. J Clin Oncol. 2008;26:1355–63. doi: 10.1200/JCO.2007.13.3439. [DOI] [PubMed] [Google Scholar]
- 14.Butt Z, Wagner LI, Beaumont JL, et al. Use of a single-item screening tool to detect clinically significant fatigue, pain, distress, and anorexia in ambulatory cancer practice. J Pain Symptom Manage. 2008;35:20–30. doi: 10.1016/j.jpainsymman.2007.02.040. [DOI] [PubMed] [Google Scholar]
- 15.Temel JS, Pirl WF, Recklitis CJ, Cashavelly B, Lynch TJ. Feasibility and validity of a one-item fatigue screen in a thoracic oncology clinic. J Thorac Oncol. 2006;1:454–9. [PubMed] [Google Scholar]
- 16.Qi Y, Schild SE, Mandrekar SJ, et al. Pretreatment quality of life is an independent prognostic factor for overall survival in patients with advanced stage non-small cell lung cancer. J Thorac Oncol. 2009;4:1075–82. doi: 10.1097/JTO.0b013e3181ae27f5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McCorkle R, Quint-Benoliel J. Symptom distress, current concerns and mood disturbance after diagnosis of life-threatening disease. Soc Sci Med. 1983;17:431–8. doi: 10.1016/0277-9536(83)90348-9. [DOI] [PubMed] [Google Scholar]
- 18.Spitzer WO, Dobson AJ, Hall J, et al. Measuring the quality of life of cancer patients: a concise QL-index for use by physicians. J Chronic Dis. 1981;34:585–97. doi: 10.1016/0021-9681(81)90058-8. [DOI] [PubMed] [Google Scholar]
- 19.Tsevat J, Dawson NV, Matchar DB. Assessing quality of life and preferences in the seriously ill using utility theory. J Clin Epidemiol. 1990;43:735–75. doi: 10.1016/0895-4356(90)90224-d. [DOI] [PubMed] [Google Scholar]
- 20.Cohen J. Statistical power analysis for the behavioral sciences. 2. New Jersey: Lawrence Erlbaum; 1988. [Google Scholar]
- 21.Sloan JA, Dueck A. Issues for statisticians in conducting analyses and translating results for quality of life end points in clinical trials. J Biopharm Stat. 2004;14:73–96. doi: 10.1081/BIP-120028507. [DOI] [PubMed] [Google Scholar]
- 22.Clavien PA, Sanabria JR, Strasberg SM. Proposed classification of complications of surgery with examples of utility in chole-cystectomy. Surgery. 1992;111:518–26. [PubMed] [Google Scholar]
- 23.Veldkamp R, Kuhry E, Hop WC, et al. Laparoscopic surgery versus open surgery for colon cancer: short-term outcomes of a randomised trial. Lancet Oncol. 2005;6:477–84. doi: 10.1016/S1470-2045(05)70221-7. [DOI] [PubMed] [Google Scholar]
- 24.Koopmann MC, Heise CP. Laparoscopic and minimally invasive resection of malignant colorectal disease. Surg Clin North Am. 2008;88:1047–72. doi: 10.1016/j.suc.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 25.Courneya KS, Segal RJ, Reid RD, et al. Three independent factors predicted adherence in a randomized controlled trial of resistance exercise training among prostate cancer survivors. J Clin Epidemiol. 2004;57:571–9. doi: 10.1016/j.jclinepi.2003.11.010. [DOI] [PubMed] [Google Scholar]
- 26.Chida Y, Steptoe A. Positive psychological well-being and mortality: a quantitative review of prospective observational studies. Psychosomatic Med. 2008;70:741–56. doi: 10.1097/PSY.0b013e31818105ba. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.