Abstract
Elevated shear stress within the skeletal muscle microvasculature is implicated in the induction of a longitudinal splitting form of angiogenesis, which is characterized by the lack of basement membrane breakage. We investigated whether the transcriptional regulator, Ets-1, is responsive to changes in hemodynamic forces and if so, whether Ets-1 controls microvascular endothelial cell integrity by inducing the expression of inhibitors of matrix degrading proteases. Rats were treated with prazosin for 2, 4 and 7 days to increase in microvascular shear stress in hindlimb skeletal muscles. In complimentary in vitro experiments, rat microvascular skeletal muscle endothelial cells were exposed to laminar shear stress (15 dyne/cm2) for 0.5, 2, and 24 hours. TaqMan PCR analysis of laser microdissected capillaries isolated from EDL muscles demonstrated transient (after 2 days) induction of Ets-1 gene expression. In cultured cells, a transient up-regulation of Ets-1 mRNA was observed after 2hr shear stimulation, accompanied by increased phosphorylation of Ets-1 and enhanced Ets-1 DNA binding activity. This response was modulated by ERK1/2 and p38 MAP kinases, but was not dependent on NOS or COX-2 activity. PAI-1, TIMP-1 and TIMP-3 mRNA were elevated significantly in prazosin treated EDL, and in response to shear stimulation in vitro. In cultured endothelial cells, Ets-1 RNA interference abolished the shear-induced increases in Ets-1, PAI-1, TIMP-1 and TIMP-3 mRNA expression. These results suggest that enhanced laminar shear stress may act to preserve the integrity of microvascular walls in part through Ets-1-dependent induction of protease inhibitors.
Keywords: Angiogenesis, Proteases, Mechanotransduction, Transcription Factors, Laser Capture Microdissection
Introduction
Shear stress plays an important role in vascular homeostasis and remodeling. The onset of shear stress initiates multiple intracellular events (Davies, 1995). Endothelial cells adapt to the shear forces and most of the initial events are restrained under constant exposure to laminar shear. Numerous studies in both micro- and macrovasculature have demonstrated that constant laminar shear maintains the vascular permeability barrier, controls nitric oxide bioavailability and promotes vasculoprotection via inhibition of coagulation, leukocyte adhesion and matrix metalloproteinase production (Gimbrone et al, 2000;Busse and Fleming, 1998;Parmar et al, 2006;Milkiewicz et al, 2006). Conversely, oscillatory flow or low shear promotes sustained changes that contribute to the inflammatory and proliferative responses during development of atherosclerosis (Gimbrone et al, 2000).
Within the microcirculation, persistent elevations in wall shear stress induce outward remodeling of arterioles (Skalak and Price, 1996) and angiogenesis within capillary networks (Hudlicka, 1998). While it is clear that specific patterns of gene expression underlie the shear-induced angiogenesis response, the transcriptional regulation of this form of vascular remodeling is not understood. The ETS family of transcription factors is credited with transcriptional regulation of greater than 500 genes (Turner et al, 2007), and Ets-1 specifically is associated with processes of cellular growth, differentiation, organ development and in angiogenesis of various tissues, including tumors (Sharrocks, 2001;Sementchenko and Watson, 2000). Over-expression of Ets-1 induced an invasive phenotype of endothelial cells and upregulated expression of angiogenic growth factors including VEGF and HGF in human vascular smooth muscle cells and in ischemic rat hind limb (Hashiya et al, 2004). Dominant-negative Ets-1 gene delivery blocked migration and reduced HGF–and bFGF-induced angiogenesis in vascular endothelial cells (Hashiya et al, 2004). Despite accumulated knowledge of the involvement of Ets-1 in vascular remodeling and angiogenesis in diverse tissues, the regulation of this transcription factor in response to shear stress stimulation presently is unknown.
Shear stress induced capillary growth occurs through luminal division and is characterized by an absence of proliferation or the basement membrane degradation typically seen during branching angiogenesis (Egginton et al, 2001). The integrity of the vascular basement membrane is maintained by finely controlled and self-limited physiological processes of matrix protein synthesis and breakdown. Matrix metalloproteinases (MMPs) and serine proteases (tissue plasminogen activator [t-PA] and urokinase-plasminogen activator [u-PA]) are key mediators of this balance. MMPs, a family of zinc-dependent endopeptidases, regulate arterial remodeling, wound healing and angiogenesis, through proteolysis of extracellular matrix and cell surface receptors, activation of other MMPs and release of matrix-sequestered growth factor (Birkedal-Hansen et al, 1993). This family includes both soluble and membrane-associated MMPs (MT-MMPs). Serine proteases (u-PA and t-PA) activate zymogen plasminogen into the broadly acting protease plasmin, and are reported to modify angiogenesis in various physiological and pathological conditions (Pepper, 2001). Consistent with the morphological phenotype of shear stress-induced angiogenesis, exposure of endothelial cells to laminar shear stress downregulates the production of MMPs (Yun et al, 2002;Milkiewicz et al, 2006) and uPA (Sokabe et al, 2004). Further restraint of MMP and PA activity occurs through production of exclusive inhibitors designated as tissue inhibitors of metalloproteinases (TIMPs) and plasminogen activator inhibitors (PAIs). The effect of shear stress on protease inhibitor expression has not been established. We hypothesized that shear stress exposure would increase protease inhibitor expression, in conjunction with the established decrease in protease production.
Aside from the established role of Ets-1 in regulating the expression of MMP-1, -3 and -9 (Sementchenko and Watson, 2000), it also has been reported that plasminogen activator inhibitor-1 is a target gene for Ets-1 (Kaneko et al, 2002). Similarly, the TIMP-1 promoter is activated by Ets-1 (Logan et al, 1996). Although TIMP-3 regulation by Ets-1 has not been reported, its promoter contains multiple consensus Ets binding sequences (Sun et al, 1995) and was shown to be regulated by the Ets family member ELF3 (Jobling et al, 2002). Therefore, we examined the modulation of Ets-1 and these protease inhibitors in response to enhanced shear stress and we investigated the role of Ets-1 in the shear stress-dependent regulation of PAI-1, TIMP-1 and TIMP-3 production.
Methods
Materials
Chemicals were purchased from Sigma Aldrich unless stated otherwise. Cell culture components were purchased from Invitrogen Canada. The inhibitors U0126, SB203580, PD98059 and NS 398 were obtained from Calbiochem.
Rat studies
Male Sprague Dawley rats (200–250 gram; Charles River Labs, Quebec) were subject to chronic vasodilation of skeletal muscle vasculature using the alpha1 adrenergic antagonist prazosin (50 mg/L in drinking water) (Dawson and Hudlicka, 1989). Surgical procedures were carried out under anesthesia (intraperitoneal injection of ketamine (80 mg/kg) and xylazine (10mg/kg)), in accordance with Animal Care Procedures at York University and in conformity with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85-23, revised 1996). Rats were provided with regular drinking water (n=4), or drinking water containing prazosin (50 mg/L). After 2, 4, or 7 days (n=4 for each group), extensor digitalis longus (EDL) muscles were removed and frozen in isopentane precooled in liquid nitrogen prior to further analyses.
Laser capture microdissection, RNA isolation and cDNA production
Muscle capillaries were isolated from cryosections using the Arcturus PixCell II laser capture microdissection system (LCM; Arcturus Bioscience, USA)(Milkiewicz and Haas, 2005). Prior to microdissection, capillaries were visualized by staining with isolectin GS-IB4 from Griffonia simplicifolia- Alexa Fluor 488 conjugate. Cell lysis and RNA purification were carried out using the PicoPure RNA Isolation Kit (Arcturus Bioscience, USA). First-strand cDNAs were synthesized by reverse transcription using the Superscript II First-Strand Synthesis Kit (Invitrogen) primed with oligo(dT)12–18 and random decamers.
Cell culture and cDNA production
Skeletal muscle endothelial cells (SMEC) were isolated from EDL muscles of male Sprague Dawley rats and propagated in culture up to 9 passages (Han et al, 2003). In control experiments, cells were treated with prazosin (1 μM) for 24 hours and processed for protein analysis. Shear stress experiments were conducted as described previously (Milkiewicz et al, 2006). The shear stress used in vitro (15 dyne/cm2) is comparable to the calculated shear stress present within capillaries of the EDL during prazosin-induced vasodilation (Milkiewicz et al, 2001). For drug treatments, cells were pretreated with drugs at final concentrations of 30 μM for LNNA, 40 μM for SB203580, 0.3 μM for U0126, 50 μM for NS 398, and 50 μM for PD98059. After specific time intervals of shear exposure, cells were processed for RNA or protein analysis. Cells were lysed and first-strand cDNA were produced using Cells-to-cDNA™ (Ambion). Total cellular protein was extracted in RIPA buffer (Tris-HCl, 50 mM, pH 7.4; NP-40, 1%; sodium deoxycholate, 0.25%; NaCl,150 mM; EDTA, 1 mM; PMSF, 1 mM) in the presence of 0.1% EDTA-free protease inhibitor cocktail, 1 mM sodium orthovanadate and 1 mM sodium fluoride. Protein was quantified using the bichoninic acid assay (BCA; Pierce) according to the manufacturer’s instructions.
Real-time RT-PCR
Quantitative real-time PCR (q-PCR) assays for Ets-1, PAI-1, TIMP-1, TIMP-3, and control GAPDH or ribosomal RNA transcripts were carried out using gene-specific TaqMan® FAM- or VIC-labeled probes obtained from Applied Biosystems (Ets1, Rn00561167_m1; PAI-1, Rn00561717_m1; TIMP-1, Rn00587558_m1; TIMP-3, Rn00441826_m1; 18s rRNA, P/N4308329; GAPDH, Rn99999916_s1). Real time PCR analysis was conducted using a ABI PRISM 7700 Sequence Detector System (Applied Biosystems, USA), or Stratagene MX3005P (Stratagene, Inc) as described previously (Milkiewicz and Haas, 2005). The comparative Ct method was used to determine relative quantification of mRNA expression, using 18s or GAPDH to normalize for amount of RNA per sample.
DNA binding assay
Biotinylated double-stranded oligonucleotides containing a consensus (5′GATCTCGAGCCGGAAGTTCGA) (Fisher et al, 1991) or mutated (5′GATCTCGAGCAGGCAGTTCGA) Ets binding site were obtained from Genosys. Ets-1 binding to DNA was assessed using the TransFactor Universal Chemiluminescent system (Clontech), according to manufacturer’s directions. Protein extracts were produced from static or shear stimulated (2 or 4 hr) cells and quantified by bichoninic acid assay (Pierce). 5 μg of cell extract was mixed with 0.5 μg polydIdC and 2 pmol biotinylated oligonucleotide, and incubated in streptavidin-coated wells. Following washes, bound Ets-1 was detected by addition of primary antibody, followed by HRP-secondary antibody. Chemiluminescence was measured using a Victor3 plate reader (Wallac). For each condition, background luminescence (obtained from cell extract incubated with the mutated oligonucleotide) was subtracted from that obtained with the consensus oligonucleotide, then expressed as fold change from static condition.
Immunostaining
Cells were exposed to shear stress for 2 hrs or maintained as static controls. For ERK inhibition, cells were treated with 50 μM PD98059. Some cells were treated with prazosin (1 μM) for 24 hours. Following shear stress, cells were fixed with 3.7% paraformaldehyde, then blocked and permeabilized using 0.05% Triton X-100 and 5% normal goat serum diluted in phosphate-buffered saline. Primary antibody (Ets-1[pT38]; Biosource or total Ets-1; Santa Cruz) was diluted 1:100 in blocking solution. Secondary antibody (Alexa 568-conjugated goat anti-rabbit secondary antibody, Molecular Probes) was diluted 1:400 in blocking solution. Cells were counterstained with DAPI (1:1500), mounted with Immunofluor (ICN) and viewed using a Zeiss Axiovert 200M inverted microscope equipped with Quantix 57 digital cooled CCD camera. All conditions were imaged using identical exposure parameters. Nuclear pixel intensities were quantified using MetaMorph (Universal Imaging Corp.). A 25 × 23 pixel box was placed over individual nuclei to obtain an averaged pixel intensity measurement. At least 10 measurements were used to calculate the average intensity of nuclear Ets-1 under each experimental condition, and experiments were repeated 3 times.
Immunoblotting
Proteins isolated from cultured SMEC (10–30 μg) were separated by SDS-PAGE and transferred onto PVDF membranes (Immobilon P; Millipore). Membranes were incubated with primary antibodies (Cell Signaling: anti-β-actin (#4967), 1:2000; anti-tubulin (#2148), 1:1000; Santa Cruz: rabbit polyclonal anti-Ets-1, 1:200, rabbit polyclonal anti-TIMP-1, 1:200 (sc5538), rabbit polyclonal anti-TIMP-3, 1:200 (sc30075)) and detected using horseradish peroxidase-conjugated secondary antibodies (GE Biosciences) and chemiluminescence (SuperWest Pico; Pierce, USA) with exposure to autoradiography film (Hyperfilm; GE Biosciences). Bands were quantified using AlphaEase software (Alphainnotech, Inc), and normalized for loading to corresponding band intensities for β-actin or tubulin.
siRNA
siRNA targeting Ets-1 (Rn_Ets1_5HP and Rn_Ets1_2HP) and negative control-fluorescein labeled siRNA (#1027282) were purchased from Qiagen and transfected into skeletal muscle microvascular endothelial cells using HiPerfect (Qiagen). Fluorescence imaging of negative control siRNA 4 hrs post-transfection was used to confirm that siRNA was incorporated into >80% of cells. Cells were plated onto coverslips on day 1, transfected with siRNA on day 2, and subjected to 2 hr static or 15 dyne/cm2 shear stress conditions on day 3. Cells were lysed and analyzed for mRNA expression, using Cells- to-cDNA kit (Ambion), followed by real time PCR analysis, as described earlier.
Statistical Analysis
Statistical significance was determined by ANOVA and Fisher’s or Tukey post-hoc tests using StatView software version 5.0 or Graphpad Prism version 4. Results are expressed as mean ± SE for at least three separate experiments. P < 0.05 was considered significant.
Results
Ets-1 expression in response to shear stress
Previous studies demonstrated that chronic administration of prazosin enhances shear stress within rat skeletal muscle microvasculature and induces angiogenesis, with detectable increases in capillary to fibre ratio (C:F) after 7 days (Dawson and Hudlicka, 1989;Milkiewicz et al, 2001). We utilized laser microdissected capillaries from rat EDL for RNA analysis (Figure 1A,B). Ets-1 mRNA level within capillaries from prazosin-treated EDL muscles showed an increase after 2 days, but returned to control by 4 and 7 days of elevated shear stress (Figure 1C).
Figure 1. Chronic vasodilation causes transient increases in Ets-1 expression in skeletal muscle capillaries isolated by laser capture microdissection.
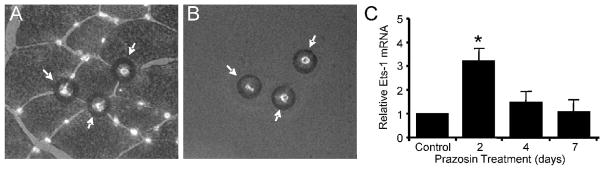
Following treatment of rats for 2 to 7 days with the α1 adrenergic antagonist prazosin, EDL muscles were removed for analysis. Skeletal muscle capillaries were detected by lectin-Alexa488 staining of frozen muscle sections to facilitate the process of isolation by laser microdissection. (A) The black rings denote areas of melting of the cap membrane associated with individual laser pulses. (B) Detection of capillaries on the cap subsequent to their capture confirmed successful lifting of the capillaries from the heterogeneous tissue sample. (C) Subsequent to capture, mRNA was isolated from the capillaries and utilized for real time q-PCR detection of mRNA encoding Ets-1. Chronic vasodilatation resulted in a transient large increase in Ets-1 mRNA in capillary-enriched samples from rat EDL muscles *p<0.05 compared to control, n=4 per condition.
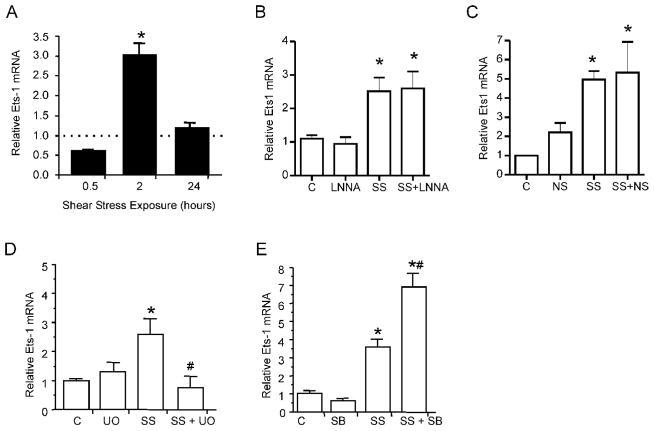
In cultured microvascular endothelial cells, application of shear at 15 dyne/cm2 for 2 hours induced a transient elevation in Ets-1 mRNA that returned to control by 24 hours of shear stress exposure (Figure 2A). Interestingly, we did not observe changes in Ets-1 mRNA levels in cultured endothelial cells exposed to 10% static stretch for the same duration (1.3 ± 0.15 fold above control; p>0.05), suggesting differential responsiveness of Ets-1 dependent on the mechanical stress. We next examined the involvement of various intracellular signaling pathways in modulating shear stress-induced Ets-1 expression. Laminar shear stress increases NO production via transcriptional upregulation and the prolonged stability of eNOS mRNA (Fleming and Busse, 2003). However, the nitric oxide synthase inhibitor N-nitro-L-arginine (LNNA) did not attenuate Ets-1 mRNA levels in shear stress stimulated endothelial cells (Figure 2B). We tested if cyclooxygenase-2 (COX2) activity contributed to shear stress induced Ets-1 expression in endothelial cells, as COX2 activity is shear-sensitive (Okahara et al, 1998) and an association between COX2 activity and Ets-1 expression was demonstrated in cancer cells (Ito et al, 2004). Application of a selective COX2 inhibitor, NS 398, did not modify the shear stress responsiveness of Ets-1 mRNA (Figure 2C). Previous studies showed links between ERK1/2 and p38 in the regulation of Ets-1 (Dittmer, 2003). Given that both ERK1/2 and p38 are phosphorylated after 2 hours of shear stress exposure (Milkiewicz et al, 2006), we examined their involvement in shear-induced Ets-1 production. Inhibition of ERK1/2 activation using U0126 abolished the shear stress-induced increase in Ets-1 expression (Figure 2D). Conversely, SB203580 treatment augmented the shear stress-induced expression of Ets-1 (Figure 2E).
Figure 2. Shear stress exposure induces changes in Ets-1 expression in cultured skeletal muscle microvascular endothelial cells.
Endothelial cells were exposed to laminar shear stress (SS) (15 dynes/cm2) or static conditions for 0.5, 2 or 24 hours.(A) Real time q-PCR analysis of Ets-1 mRNA revealed an enhanced gene expression after 2hr of stimulation. For each time point, mRNA level in sheared samples were expressed as a ratio to their respective static control (set to 1, dotted line). (B) In cells subjected to 2 h laminar shear stress, the NOS inhibitor LNNA did not prevent the shear induction in Ets-1 mRNA. (C) The COX-2 inhibitor NS 398 also did not suppress the induction of Ets-1 mRNA observed in response 2 hours of shear stimulation. (D) Induction of Ets-1 mRNA by 2 hr shear stress exposure was abolished in cells pretreated with the MEK1/2 inhibitor U0126. (E) Inhibition of p38 activity with SB203580 augmented the shear stress induction of Ets-1 mRNA. *p<0.05 compared to control, #p<0.05 compared to shear stress; n=3–4 per condition. C - static control; SS- Shear stress; NS – NS 398; U0-U0126; SB-SB203580.
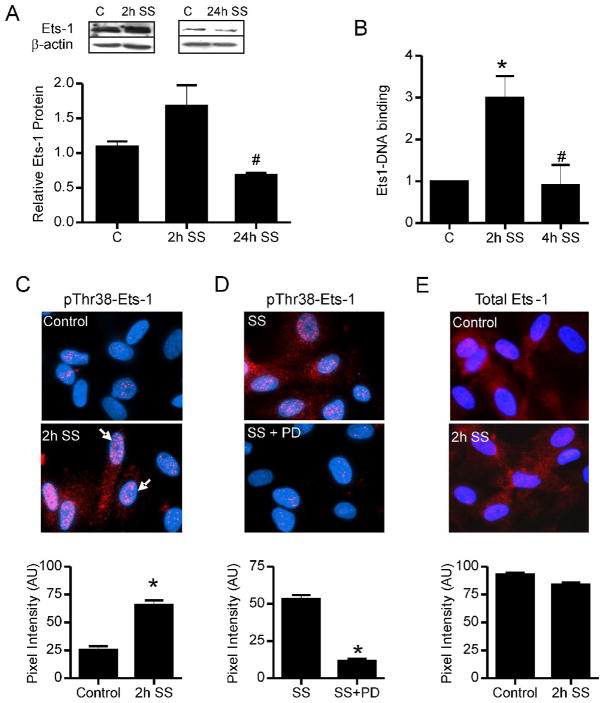
2 hr shear stress induced a small increase in Ets-1 protein, which was significantly reduced by 24 hr of continuous shear stress (Figure 3A). Interestingly, DNA binding assays detected a significant increase in the binding of Ets-1 to a consensus DNA sequence in extracts from 2 hr shear stress stimulated cells, which returned to basal levels in cells stimulated by 4 hr shear stress (Figure 3B). Because researchers have established that ERK1/2 phosphorylation of Ets-1 protein on Thr38 (Callaway et al, 2006) increases Ets-1 transcriptional activity (Yang et al, 1996), we examined phosphorylation levels of Ets-1. Shear stress significantly increased nuclear levels of Thr38 phosphorylated Ets-1 (Figure 3C). The increased phosphorylation of Ets-1 in shear stimulated cells was reduced by concomitant inhibition of ERK1/2 activation (Figure 3D). Shear stress stimulation caused a moderate decrease in nuclear levels of total Ets-1 protein (Figure 3E). Treatment of cultured microvascular cells with prazosin did not affect phospho-Ets1 or total Ets1 levels (Supplementary Figure 1).
Figure 3. Shear stress induced modifications in Ets-1 protein.
(A) Western blotting of total cell extracts showed an insignificant increase in Ets-1 protein level in cell subjected to 2 hr of shear stimulation (2h SS). Ets-1 protein was reduced after 24 hr-exposure (24h SS). β-actin was used as a loading control. #p<0.05 vs. control and 2h SS. (B) DNA binding assays were performed using Ets1 consensus sequence oligonucleotides and cellular extracts isolated from control cells or cells subjected to shear stress for 2 and 4 hours (2h and 4h SS). *p<0.05 compared to control, n=3–4 per condition. #p<0.05 compared to 2h shear stress. (C) Immunostaining using a α-phosphoThr38-Ets-1 antibody (red) was performed on static control and 2h SS-stimulated cells. Cells were counterstained with DAPI (blue) to demarcate nuclei. Arrows point to clusters of phospho-ets1 staining within the nucleus. Pixel intensities of nuclear Ets-1 staining were quantified using MetaMorph software. *p<0.05 compared to control. (D) Pretreatment of cells with the MEK1/2 inhibitor, PD98059, prevented the shear stress-induced increase in nuclear phospho-Ets1 (red, phospho Thr38-Ets-1; blue, DAPI). (E) Nuclear levels of total Ets-1 levels were reduced slightly in response to 2h SS (red, Ets-1; blue, DAPI).
Flow –induced changes in expression of protease inhibitors
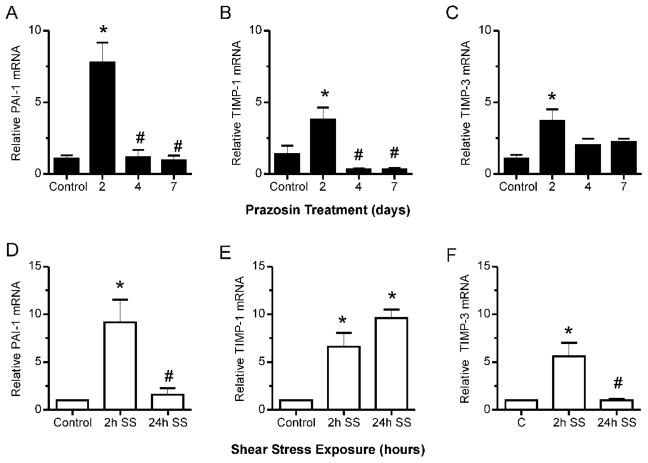
Considering that shear stress induced capillary growth occurs in the absence of basement membrane degradation or elevated proteolytic activity (Zhou et al, 1998;Rivilis et al, 2002), we were interested specifically in Ets1-dependent regulation of protease inhibitors. We examined the shear stress responsiveness of 3 protease inhibitors, PAI-1, TIMP-1 and TIMP-3, as each was reported to contain Ets consensus binding sequences in its promoter. Analysis of muscle samples from prazosin treated rats indicated that PAI-1, TIMP-1 and TIMP-3 mRNA increased significantly in response to prazosin treatment (Figure 4A,B,C). Notably, the pattern of expression of these protease inhibitors in response to prazosin stimulation correlated with that observed for Ets-1 mRNA (Figure 1C). Similarly, expression of PAI-1, TIMP-1 and TIMP-3 mRNA increased significantly in cultured endothelial cells after 2 hours of shear stimulation by 9-, 6.5- and 5-fold respectively in comparison to static conditions (Figure 4 D,E,F). However, only TIMP-1 mRNA level remained elevated after 24 hours of shear exposure (Figure 4E). PAI-1 protein was elevated significantly in cellular extracts following 2 hours of shear stress stimulation (Figure 5A), as was TIMP-1 protein (Figure 5B), while TIMP-3 protein was not detectable.
Figure 4. Regulation of PAI-1, TIMP-1 and TIMP-3 mRNA by enhanced shear stress.
mRNA levels of PAI-1 (A), TIMP-1 (B) and TIMP-3 (C) were detectable in EDL muscles. In muscles from prazosin treated rats, mRNA levels of each were significantly increased. *p<0.05 compared to control, #p<0.05 compared to 2h shear stress, n=3–4 per condition. Shear stress exposure of microvascular endothelial cells stimulated significant increases in PAI-1 (D), TIMP-1 (E) and TIMP-3 (F) mRNA. While PAI-1 and TIMP-3 exhibited transient increases, TIMP-1 mRNA remained elevated after 24h shear exposure. *p<0.05 compared to control, #p<0.05 compared to 2h shear stress, n=4–7 per condition.
Figure 5. Protein analysis of PAI-1 and TIMP-1 in response to shear stress.
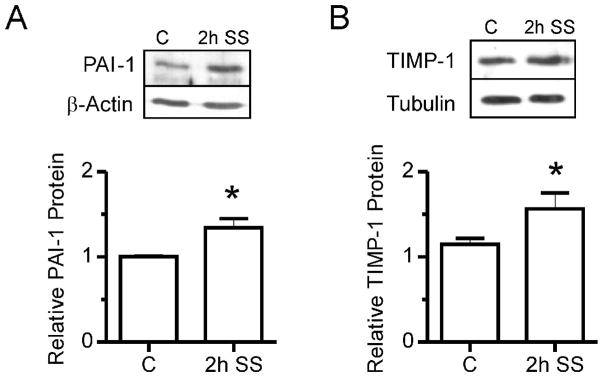
(A) Western blotting of total cell extracts showed a significant increase in PAI-1 protein level in cell subjected to 2 hr of shear stimulation (2h SS). β-actin was used as a loading control. *p<0.05 compared to control, n=4. (B) TIMP-1 protein also increased significantly in response to 2h shear stress exposure. Tubulin was used as a loading control. *p<0.05 compared to control, n=6.
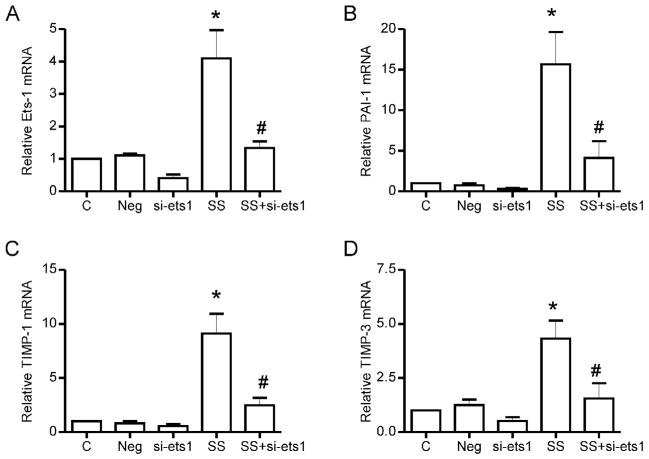
To determine whether shear stress-mediated PAI-1, TIMP-1 and TIMP-3 expression is Ets-1 dependent, we suppressed Ets-1 expression using RNA interference. Ets-1 siRNA reduced both basal and shear stress induced increases in Ets-1 mRNA (Figure 6A). Likewise, the basal and shear stress-stimulated increases in mRNA levels of PAI-1, TIMP-1 and TIMP-3 were suppressed significantly, compared to untreated samples and those treated with non-specific (negative) siRNA (Figure 6 B,C,D).
Figure 6. The effect of Ets-1 gene silencing on shear stress-mediated PAI-1, TIMP-1 and TIMP-3 gene expression in microvascular endothelial cells.
siRNA targeting Ets-1 was used to downregulate endogenous Ets-1 in microvascular endothelial cells. Non-binding negative control siRNA was used in all experiments. Ets-1 siRNA treatment resulted in significant attenuation of shear stress induced increases in Ets-1 mRNA itself (A), as well as the increases in PAI-1 (B), TIMP-1 (C) and TIMP-3 (D). *p<0.05 versus control untreated, and versus the negative siRNA treated cells; #p<0.05 compared to 2h shear stress; n=3–4 per condition.
Discussion
This study is the first to report shear stress-induced increases in Ets-1 expression and DNA binding activity in microvascular endothelial cells. Further, these findings provide evidence that Ets-1 may contribute to the protective effects of laminar shear stress as a result of Ets-1 dependent upregulation of protease inhibitors.
Using LCM to extract capillaries from skeletal muscle, we measured a significant increase in Ets-1 mRNA following 2 days of prazosin treatment. Prazosin treatment was previously reported to elicit a 3-fold increase in capillary shear stress (from 5 dynes/cm2 to 15 dynes/cm2) (Milkiewicz et al, 2001). We found that stimulation of cultured microvascular endothelial cells with a similar magnitude of laminar shear stress produced a substantial increase in Ets-1 mRNA that occurred independently of NOS or COX2 activity, but was dependent on ERK1/2 activation. This observation corresponds with the findings that shear stress induced phosphorylation of ERK1/2 was not attenuated by either NOS inhibition (Milkiewicz et al, 2006) or COX2 inhibition (current study). While the present analysis did not identify an upstream pathway leading to ERK1/2 phosphorylation, previous reports indicate that integrin β1 contributes to shear stress activation of ERK1/2 (Ishida et al, 1996) via FAK, Src and PKCε activation (Jalali et al, 1998;Ni et al, 2003).
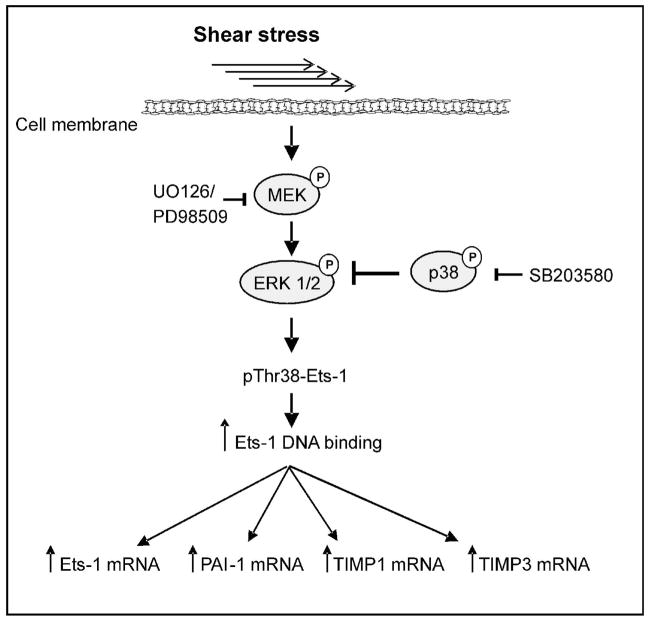
Laminar shear stress stimulation induced the phosphorylation of Ets-1 on Thr-38 and an increase in Ets-1 mRNA, both of which were dependent on ERK1/2 activity. The data are consistent with reports showing that ERK2 forms a transient signalling complex with Ets-1 subsequent to ERK2 activation by MEK, resulting in phosphorylation of Thr38 on Ets-1 (Callaway et al, 2006) and that this phosphorylation is associated with increased transcriptional activity of Ets-1 (Yang et al, 1996;Foulds et al, 2004). Additionally, the Ets-1 promoter itself contains Ets consensus sequences, enabling positive autoregulation by the phosphorylated transcription factor (Majerus et al, 1992). We propose that the increased ERK1/2 phosphorylation in response to shear stress (as previously observed in microvascular endothelial cells-Milkiewicz et al.2006) induces phosphorylation of Thr38 on Ets-1, increasing its transcriptional activation of downstream targets including Ets-1 itself, PAI-1, and TIMP-1 and -3 (Figure 7).
Figure 7. Schematic of proposed Ets-1 signal pathway in response to shear stress stimulation.
Shear stress sensors transduce signals leading to ERK1/2 phosphorylation and Thr38 phosphorylation of Ets-1. Increased transcriptional activity of Ets-1 leads to expression of target genes, including Ets-1 itself, PAI-1, TIMP-1 and TIMP-3.
TIMP-1, TIMP-3 and PAI-1 mRNA were induced in skeletal muscle microvessels subjected to sustained increases in shear stress, and in vitro, in endothelial cells exposed to laminar shear stress. In cultured endothelial cells, this induction was reduced significantly in cells treated with Ets-1 siRNA, providing novel evidence that the shear-dependent regulation of these genes is mediated by Ets-1. In support of our findings, Ets-1 deficiency was reported to reduce fibroblast cell production of some MMPs and also TIMPs 1 and 3, in response to inflammatory or growth factor stimuli (Hahne et al, 2006). Ets-1−/− mice also were characterized to have impaired aortic remodeling in response to Angiotensin II, which correlated with downregulation of the Ets-1 target gene, PAI-1 (Zhan et al, 2005). PAI-1 promoter regulation by coordinated Ets-1 and Sp-1 interactions was reported in hepatocytes (Nakatsuka et al, 2006). While the activation of Sp-1 in response to shear stress has been observed (Yun et al, 2002), additional studies are needed to determine whether Ets-1 – Sp-1 interactions cooperate to activate transcription of PAI-1, TIMP-1 and TIMP-3 in endothelial cells.
Numerous studies have established that Ets-1 plays a role in promoting angiogenesis. However, the ETS family typically is associated with transcriptional activation of proteases involved in extracellular matrix degradation and cellular invasion (Iwasaka et al, 1996). Our findings indicate a divergent role for Ets-1 in response to shear stress stimulation, as illustrated by the upregulation of protease inhibitors. While there is precedence for the shear stress dependent transcriptional regulation and increased DNA binding activity of Ets-1, which were observed in rheumatic synovial cells (Sun and Yokota, 2001) and cultured hepatocytes (Nakatsuka et al, 2006), the effect of fluid shear stress on Ets-1 expression in endothelial cells has not been reported previously.
Previous studies indicate that shear stress downregulates expression of MMPs and uPA in endothelial cells (Yun et al, 2002;Milkiewicz et al, 2006;Sokabe et al, 2004). The coincident upregulation of PAI-1 and TIMPs-1 and -3 in endothelial cells exposed to increased shear stress provides an additional mechanism by which the cells are able to fine tune their proteolytic state. Thus, increased levels of these protease inhibitors may contribute to the maintained structural integrity of the capillary. While substantial elevations in PAI-1 correlate with cardiovascular diseases and increased risk of thrombosis, physiological levels of PAI-1 contribute to the balance between angiogenesis and capillary stability (Balsara et al, 2006;Devy et al, 2002). Deficiency of PAI-1 enhances smooth muscle cell migration under flow stimulation (Cullen et al, 2004). PAI-1, in addition to its protease inhibitor function, modulates cell adhesion and migration by competing with u-PA receptor (u-PAR) for binding to vitronectin (Zhou et al, 2003;Czekay et al, 2003). PAI-1 also downregulates Akt signaling through inactivation of PTEN and acts as a negative regulator of cell proliferation (Balsara et al, 2006). A limited number of studies have examined the effects of shear stress on TIMPs. Shear dependent upregulation of TIMP-1 was observed in rheumatic synovial cells (Sun and Yokota, 2001). Recently, studies have revealed new roles for TIMPs in regulation of apoptosis and proliferation that are independent of their MMP inhibitory activities (Chirco et al, 2006). TIMP-1 induces anti-apoptotic signals that include Akt activation and Bcl-2 expression (Guedez et al, 1998). However, TIMP-1 also triggers cell cycle arrest by downregulation of cyclinD1 and upregulation of p27KIP1(Taube et al, 2006). TIMP-3 negatively regulates cell proliferation and angiogenesis by directly blocking tyrosine kinase receptor signaling pathways through interactions with VEGFR2 (Qi et al, 2003). These reports, together with the current data showing shear stress induction of PAI-1, TIMP-1 and TIMP-3 in microvascular endothelial cells, are consistent with earlier observations of the lack of endothelial cell abluminal migration or proliferation during shear stress-stimulated angiogenesis (Egginton et al, 2001).
In summary, the current study expands our understanding of the effects of shear stress on endothelial cell gene expression through demonstrating the shear sensitivity of the transcriptional regulator, Ets-1. Further, Ets-1 contributes substantially to the shear stress stimulated increases in expression of protease inhibitors PAI-1, TIMP-1 and TIMP-3. In coordination with events that downregulate protease expression, this signal cascade may be a mechanism by which capillary integrity and endothelial cell quiescence are maintained during exposure to elevated shear stress and may contribute to the phenotypic characteristics of shear stress induced angiogenesis.
Supplementary Material
Acknowledgments
Funding: Canadian Institutes of Health Research MOP-53272 and Heart and Stroke Foundation of Canada NA 6089 to T.L.H.; Natural Sciences and Engineering Research Council of Canada PostGraduate Scholarship to E.G.
Reference List
- Balsara RD, Castellino FJ, Ploplis VA. A novel function of plasminogen activator inhibitor-1 in modulation of the AKT pathway in wild-type and plasminogen activator inhibitor-1-deficient endothelial cells. J Biol Chem. 2006;281:22527–22536. doi: 10.1074/jbc.M512819200. [DOI] [PubMed] [Google Scholar]
- Birkedal-Hansen H, Moore WG, Bodden MK, Windsor LJ, Birkedal-Hansen B, DeCarlo A, Engler JA. Matrix metalloproteinases: a review. Crit Rev Oral Biol Med. 1993;4:197–250. doi: 10.1177/10454411930040020401. [DOI] [PubMed] [Google Scholar]
- Busse R, Fleming I. Pulsatile stretch and shear stress: physical stimuli determining the production of endothelium-derived relaxing factors. J Vasc Res. 1998;35:73–84. doi: 10.1159/000025568. [DOI] [PubMed] [Google Scholar]
- Callaway KA, Rainey MA, Riggs AF, Abramczyk O, Dalby KN. Properties and regulation of a transiently assembled ERK2.Ets-1 signaling complex. Biochemistry. 2006;45:13719–13733. doi: 10.1021/bi0610451. [DOI] [PubMed] [Google Scholar]
- Chirco R, Liu XW, Jung KK, Kim HR. Novel functions of TIMPs in cell signaling. Cancer Metastasis Rev. 2006;25:99–113. doi: 10.1007/s10555-006-7893-x. [DOI] [PubMed] [Google Scholar]
- Cullen JP, Nicholl SM, Sayeed S, Sitzmann JV, Okada SS, Cahill PA, Redmond EM. Plasminogen activator inhibitor-1 deficiency enhances flow-induced smooth muscle cell migration. Thrombosis Research. 2004;114:57–65. doi: 10.1016/j.thromres.2004.05.003. [DOI] [PubMed] [Google Scholar]
- Czekay RP, Aertgeerts K, Curriden SA, Loskutoff DJ. Plasminogen activator inhibitor-1 detaches cells from extracellular matrices by inactivating integrins. J Cell Biol. 2003;160:781–791. doi: 10.1083/jcb.200208117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies PF. Flow-mediated endothelial mechanotransduction. Physiol Rev. 1995;75:519–560. doi: 10.1152/physrev.1995.75.3.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson JM, Hudlicka O. The effects of long term administration of prazosin on the microcirculation in skeletal muscles. Cardiovasc Res. 1989;23:913–920. doi: 10.1093/cvr/23.11.913. [DOI] [PubMed] [Google Scholar]
- Devy L, Blacher S, Grignet-Debrus C, Bajou K, Masson R, Gerard RD, Gils A, Carmeliet G, Carmeliet P, Declerck PJ, Noel A, Foidart JM. The pro- or antiangiogenic effect of plasminogen activator inhibitor 1 is dose dependent. Faseb Journal. 2002;16:147–154. doi: 10.1096/fj.01-0552com. [DOI] [PubMed] [Google Scholar]
- Dittmer J. The biology of the Ets1 proto-oncogene. Mol Cancer. 2003;2:29. doi: 10.1186/1476-4598-2-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egginton S, Zhou AL, Brown MD, Hudlicka O. Unorthodox angiogenesis in skeletal muscle. Cardiovasc Res. 2001;49:634–646. doi: 10.1016/s0008-6363(00)00282-0. [DOI] [PubMed] [Google Scholar]
- Fisher RJ, Mavrothalassitis G, Kondoh A, Papas TS. High-affinity DNA-protein interactions of the cellular ETS1 protein: the determination of the ETS binding motif. Oncogene. 1991;6:2249–2254. [PubMed] [Google Scholar]
- Fleming I, Busse R. Molecular mechanisms involved in the regulation of the endothelial nitric oxide synthase. American Journal of Physiology-Regulatory Integrative and Comparative Physiology. 2003;284:R1–R12. doi: 10.1152/ajpregu.00323.2002. [DOI] [PubMed] [Google Scholar]
- Foulds CE, Nelson ML, Blaszczak AG, Graves BJ. Ras/mitogen-activated protein kinase signaling activates Ets-1 and Ets-2 by CBP/p300 recruitment. Mol Cell Biol. 2004;24:10954–10964. doi: 10.1128/MCB.24.24.10954-10964.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimbrone MA, Topper JN, Nagel T, Anderson KR, Garcia-Cardena G. Endothelial dysfunction, hemodynamic forces, and atherogenesis. Atherosclerosis V: the Fifth Saratoga Conference. 2000;902:230–240. doi: 10.1111/j.1749-6632.2000.tb06318.x. [DOI] [PubMed] [Google Scholar]
- Guedez L, Courtemanch L, Stetler-Stevenson M. Tissue inhibitor of metalloproteinase (TIMP)-1 induces differentiation and an antiapoptotic phenotype in germinal center B Cells. Blood. 1998;92:1342–1349. [PubMed] [Google Scholar]
- Hahne JC, Fuchs T, El Mustapha H, Okuducu AF, Bories JC, Wernert N. Expression pattern of matrix metalloproteinase and TIMP genes in fibroblasts derived from Ets-1 knock-out mice compared to wild-type mouse fibroblasts. Int J Mol Med. 2006;18:153–159. [PubMed] [Google Scholar]
- Han XY, Boyd PJ, Colgan S, Madri JA, Haas TL. Transcriptional up-regulation of endothelial cell matrix metalloproteinase-2 in response to extracellular cues involves GATA-2. J Biol Chem. 2003;278:47785–47791. doi: 10.1074/jbc.M309482200. [DOI] [PubMed] [Google Scholar]
- Hashiya N, Jo N, Aoki M, Matsumoto K, Nakamura T, Sato Y, Ogata N, Ogihara T, Kaneda Y, Morishita R. In vivo evidence of angiogenesis induced by transcription factor ets-1 -Ets-1 is located upstream of angiogenesis cascade. Circulation. 2004;109:3035–3041. doi: 10.1161/01.CIR.0000130643.41587.DB. [DOI] [PubMed] [Google Scholar]
- Hudlicka O. Is physiological angiogenesis in skeletal muscle regulated by changes in microcirculation? Microcirculation. 1998;5:7–23. [PubMed] [Google Scholar]
- Ishida T, Peterson TE, Kovach NL, Berk BC. MAP kinase activation by flow in endothelial cells. Role of beta 1 integrins and tyrosine kinases. Circ Res. 1996;79:310–316. doi: 10.1161/01.res.79.2.310. [DOI] [PubMed] [Google Scholar]
- Ito H, Duxbury M, Benoit E, Clancy TE, Zinner MJ, Ashley SW, Whang EE. Prostaglandin E2 enhances pancreatic cancer invasiveness through an Ets-1-dependent induction of matrix metalloproteinase-2. Cancer Res. 2004;64:7439–7446. doi: 10.1158/0008-5472.CAN-04-1177. [DOI] [PubMed] [Google Scholar]
- Iwasaka C, Tanaka K, Abe M, Sato Y. Ets-1 regulates angiogenesis by inducing the expression of urokinase- type plasminogen activator and matrix metalloproteinase-1 and the migration of vascular endothelial cells. J Cell Physiol. 1996;169:522–531. doi: 10.1002/(SICI)1097-4652(199612)169:3<522::AID-JCP12>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Jalali S, Li YS, Sotoudeh M, Yuan S, Li S, Chien S, Shyy JY. Shear stress activates p60src-Ras-MAPK signaling pathways in vascular endothelial cells. Arterioscler Thromb Vasc Biol. 1998;18:227–234. doi: 10.1161/01.atv.18.2.227. [DOI] [PubMed] [Google Scholar]
- Jobling AI, Fang Z, Koleski D, Tymms MJ. Expression of the ETS Transcription Factor ELF3 in the Retinal Pigment Epithelium. Invest Ophthalmol Vis Sci. 2002;43:3530–3537. [PubMed] [Google Scholar]
- Kaneko T, Fujii S, Matsumoto A, Goto D, Ishimori N, Watano K, Furumoto T, Sugawara T, Sobel BE, Kitabatake A. Induction of Plasminogen Activator Inhibitor-1 in Endothelial Cells by Basic Fibroblast Growth Factor and Its Modulation by Fibric Acid. Arterioscler Thromb Vasc Biol. 2002;22:855–860. doi: 10.1161/01.atv.0000014427.80594.8f. [DOI] [PubMed] [Google Scholar]
- Logan SK, Garabedian MJ, Campbell CE, Werb Z. Synergistic transcriptional activation of the tissue inhibitor of metalloproteinases-1 promoter via functional interaction of AP-1 and Ets-1 transcription factors. J Biol Chem. 1996;271:774–782. doi: 10.1074/jbc.271.2.774. [DOI] [PubMed] [Google Scholar]
- Majerus MA, Bibollet-Ruche F, Telliez JB, Wasylyk B, Bailleul B. Serum, AP-1 and Ets-1 stimulate the human ets-1 promoter. Nucleic Acids Res. 1992;20:2699–2703. doi: 10.1093/nar/20.11.2699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milkiewicz M, Brown MD, Egginton S, Hudlicka O. Association between shear stress, angiogenesis, and VEGF in skeletal muscles in vivo. Microcirculation. 2001;8:229–241. doi: 10.1038/sj/mn/7800074. [DOI] [PubMed] [Google Scholar]
- Milkiewicz M, Haas TL. Effect of mechanical stretch on HIF-1alpha and MMP-2 expression in capillaries isolated from overloaded skeletal muscles: laser capture microdissection study. Am J Physiol Heart Circ Physiol. 2005;289:H1315–H1320. doi: 10.1152/ajpheart.00284.2005. [DOI] [PubMed] [Google Scholar]
- Milkiewicz M, Kelland C, Colgan S, Haas TL. Nitric oxide and p38 MAP Kinase mediate shear stress-dependent inhibition of MMP-2 production in microvascular endothelial cells. J Cell Physiol. 2006;208:229–237. doi: 10.1002/jcp.20658. [DOI] [PubMed] [Google Scholar]
- Nakatsuka H, Sokabe T, Yamamoto K, Sato Y, Hatakeyama K, Kamiya A, Ando J. Shear stress induces hepatocyte PAI-1 gene expression through cooperative Sp1/Ets-1 activation of transcription. Am J Physiol Gastrointest Liver Physiol. 2006;291:G26–G34. doi: 10.1152/ajpgi.00467.2005. [DOI] [PubMed] [Google Scholar]
- Ni CW, Wang DL, Lien SC, Cheng JJ, Chao YJ, Hsieh HJ. Activation of PKC-epsilon, and ERK1/2 participates in shear-induced endothelial MCP-1 expression that is repressed by nitric oxide. Journal of Cellular Physiology. 2003;195:428–434. doi: 10.1002/jcp.10259. [DOI] [PubMed] [Google Scholar]
- Okahara K, Sun B, Kambayashi J. Upregulation of prostacyclin synthesis-related gene expression by shear stress in vascular endothelial cells. Arterioscler Thromb Vasc Biol. 1998;18:1922–1926. doi: 10.1161/01.atv.18.12.1922. [DOI] [PubMed] [Google Scholar]
- Parmar KM, Larman HB, Dai G, Zhang Y, Wang ET, Moorthy SN, Kratz JR, Lin Z, Jain MK, Gimbrone MA, Jr, Garcia-Cardena G. Integration of flow-dependent endothelial phenotypes by Kruppel-like factor 2. Journal of Clinical Investigation. 2006;116:49–58. doi: 10.1172/JCI24787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepper MS. Role of the matrix metalloproteinase and plasminogen activator-plasmin systems in angiogenesis. Arterioscler Thromb Vasc Biol. 2001;21:1104–1117. doi: 10.1161/hq0701.093685. [DOI] [PubMed] [Google Scholar]
- Qi JH, Ebrahem Q, Moore N, Murphy G, Claesson-Welsh L, Bond M, Baker A, Anand-Apte B. A novel function for tissue inhibitor of metalloproteinases-3 (TIMP3): inhibition of angiogenesis by blockage of VEGF binding to VEGF receptor-2. Nat Med. 2003;9:407–415. doi: 10.1038/nm846. [DOI] [PubMed] [Google Scholar]
- Rivilis I, Milkiewicz M, Boyd P, Goldstein J, Brown MD, Egginton S, Hansen FM, Hudlicka O, Haas TL. Differential involvement of MMP-2 and VEGF during muscle stretch-versus shear stress-induced angiogenesis. Am J Physiol Heart Circ Physiol. 2002;283:H1430–H1438. doi: 10.1152/ajpheart.00082.2002. [DOI] [PubMed] [Google Scholar]
- Sementchenko VI, Watson DK. Ets target genes: past, present and future. Oncogene. 2000;19:6533–6548. doi: 10.1038/sj.onc.1204034. [DOI] [PubMed] [Google Scholar]
- Sharrocks AD. The ETS-domain transcription factor family. Nature Reviews Molecular Cell Biology. 2001;2:827–837. doi: 10.1038/35099076. [DOI] [PubMed] [Google Scholar]
- Skalak TC, Price RJ. The role of mechanical stresses in microvascular remodeling. Microcirculation. 1996;3:143–165. doi: 10.3109/10739689609148284. [DOI] [PubMed] [Google Scholar]
- Sokabe T, Yamamoto K, Ohura N, Nakatsuka H, Qin K, Obi S, Kamiya A, Ando J. Differential regulation of urokinase-type plasminogen activator expression by fluid shear stress in human coronary artery endothelial cells. Am J Physiol Heart Circ Physiol. 2004;287:H2027–H2034. doi: 10.1152/ajpheart.00260.2004. [DOI] [PubMed] [Google Scholar]
- Sun HB, Yokota H. Messenger-RNA expression of matrix metalloproteinases, tissue inhibitors of metalloproteinases, and transcription factors in rheumatic synovial cells under mechanical stimuli. Bone. 2001;28:303–309. doi: 10.1016/s8756-3282(00)00454-3. [DOI] [PubMed] [Google Scholar]
- Sun Y, Hegamyer G, Kim H, Sithanandam K, Li H, Watts R, Colburn NH. Molecular Cloning of Mouse Tissue Inhibitor of Metalloproteinases-3 and Its Promoter. J Biol Chem. 1995;270:19312–19319. doi: 10.1074/jbc.270.33.19312. [DOI] [PubMed] [Google Scholar]
- Taube ME, Liu XW, Fridman R, Kim HRC. TIMP-1 regulation of cell cycle in human breast epithelial cells via stabilization of p27(KIP1) protein. Oncogene. 2006;25:3041–3048. doi: 10.1038/sj.onc.1209336. [DOI] [PubMed] [Google Scholar]
- Turner DP, Findlay VJ, Moussa O, Watson DK. Defining ETS transcription regulatory networks and their contribution to breast cancer progression. J Cell Biochem. 2007;102:549–559. doi: 10.1002/jcb.21494. [DOI] [PubMed] [Google Scholar]
- Yang BS, Hauser CA, Henkel G, Colman MS, Van Beveren C, Stacey KJ, Hume DA, Maki RA, Ostrowski MC. Ras-mediated phosphorylation of a conserved threonine residue enhances the transactivation activities of c-Ets1 and c-Ets2. Mol Cell Biol. 1996;16:538–547. doi: 10.1128/mcb.16.2.538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun S, Dardik A, Haga M, Yamashita A, Yamaguchi S, Koh Y, Madri JA, Sumpio BE. Transcription Factor Sp1 Phosphorylation induced by shear stress inhibits membrane type 1-matrix metalloprotinase expression in endothelium. J Biol Chem. 2002;277:34808–34814. doi: 10.1074/jbc.M205417200. [DOI] [PubMed] [Google Scholar]
- Zhan Y, Brown C, Maynard E, Anshelevich A, Ni W, Ho IC, Oettgen P. Ets-1 is a critical regulator of Ang II-mediated vascular inflammation and remodeling. J Clin Invest. 2005;115:2508–2516. doi: 10.1172/JCI24403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou A, Egginton S, Hudlicka O, Brown MD. Internal division of capillaries in rat skeletal muscle in response to chronic vasodilator treatment with alpha1-antagonist prazosin. Cell Tissue Res. 1998;293:293–303. doi: 10.1007/s004410051121. [DOI] [PubMed] [Google Scholar]
- Zhou A, Huntington JA, Pannu NS, Carrell RW, Read RJ. How vitronectin binds PAI-1 to modulate fibrinolysis and cell migration. Nat Struct Biol. 2003;10:541–544. doi: 10.1038/nsb943. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.