Abstract
Objective
To describe the rationale, timeline, study design, and protocol overview of the Therapeutic Hypothermia after Pediatric Cardiac Arrest trials.
Design
Multicenter randomized controlled trials.
Setting
Pediatric intensive care and cardiac ICUs in the United States and Canada.
Patients
Children from 48 hours to 18 years old, who have return of circulation after cardiac arrest, who meet trial eligibility criteria, and whose guardians provide written consent.
Interventions
Therapeutic hypothermia or therapeutic normothermia.
Measurements and Main Results
From concept inception in 2002 until trial initiation in 2009, 7 years were required to plan and operationalize the Therapeutic Hypothermia after Pediatric Cardiac Arrest trials. Two National Institute of Child Health and Human Development clinical trial planning grants (R21 and R34) supported feasibility assessment and protocol development. Two clinical research networks, Pediatric Emergency Care Applied Research Network and Collaborative Pediatric Critical Care Research Network, provided infrastructure resources. Two National Heart Lung Blood Institute U01 awards provided funding to conduct separate trials of in-hospital and out-of-hospital cardiac arrest. A pilot vanguard phase that included half the clinical sites began on March 9, 2009, and this was followed by full trial funding through 2015.
Conclusions
Over a decade will have been required to plan, design, operationalize, and conduct the Therapeutic Hypothermia after Pediatric Cardiac Arrest trials. Details described in this report, such as participation of clinical research networks and clinical trial planning grants utilization, may be of utility for individuals who are planning investigator-initiated, federally supported clinical trials.
Keywords: cardiac arrest, in hospital, mortality, multicenter, outcome, out of hospital, pediatric, randomized controlled trial, targeted temperature control, therapeutic hypothermia, therapeutic normothermia
Cardiac arrest (CA) is a tragic event that is often associated with high mortality and poor quality of life outcome in all age groups. Children who survive CA commonly sustain neurologic injury that may result in a lifetime of dependency for all aspects of care. The pathophysiology and outcome of pediatric CA differ greatly between those that occur out-of-hospital , commonly in healthy children, and those that occur in-hospital, typically in children with complex underlying disorders. There is a great need for neuroprotective therapies for both populations of pediatric CA survivors, and future randomized controlled trials (RCTs) must distinguish between cases occurring out-of-hospital and in-hospital (1).
In 2002, landmark RCTs in adults with out-of-hospital ventricular fibrillation or tachycardia (VF/VT) associated CA (2, 3) demonstrated improved survival with good neurologic outcome after therapeutic hypothermia (TH). In 2005, newborns with birth-associated hypoxic ischemic encephalopathy (HIE) (4–6) were reported to have improved survival and neurobehavioral outcome following TH initiated within 6 hours of birth. There are, however, major differences in the etiology and pathophysiology of CA across age groups, and results in neonates and adults should not be extrapolated to children. Furthermore, a recent pediatric traumatic brain injury (TBI) RCT reported a strong trend for worse outcome in those receiving TH (7). No adequately powered RCT of TH has been conducted in the pediatric (nonnewborn) CA population, and such trials are urgently needed to guide current and future practice.
Our investigative team, which has worked together since 2002, has brought together two federally funded pediatric clinical research networks with a common data coordinating center (DCC) to conduct the Therapeutic Hypothermia After Pediatric Cardiac Arrest (THAPCA) trials. The primary objective of these trials is to determine whether TH improves survival with good neurobehavioral outcome in children who have been resuscitated after CA in the out-of-hospital (THAPCA-out-of-hospital trial) and in-hospital (THAPCA-in-hospital trial) settings. This report describes the rationale, timeline, study design, and protocol overview of the THAPCA trials.
MATERIALS AND METHODS
Timeline: Key Milestones in the Development of the THAPCA Trials
Background
The simultaneous publication in 2002 of the landmark adult trials of TH for out-of-hospital CA provided the impetus for planning the THAPCA trials (2, 3) (Table 1). There are key differences between the typical out-of-hospital adult and pediatric CA events: VF/VT shockable arrests in adults versus asystole/pulseless electrical activity (PEA) nonshockable arrests in children, emergency medical services resuscitation quality issues, impact of hypoxia and ischemic injury in the developing versus mature brain, and others. Such major differences provide a very strong argument that pediatric trials are urgently needed to determine if TH is effective in this age group.
Table 1.
Timeline for Therapeutic Hypothermia After Pediatric Cardiac Arrest Trials
| Year | Key Events |
|---|---|
| 2002 | PECARN originates and adult therapeutic hypothermia RCTs published New England Journal of Medicine |
| 2003 | National Institute of Child Health and Human Development R21 Request for Applications Pediatric Cardiac |
| Arrest, Hypothermia RCT planning grant (HD044955) (July 2003) awarded | |
| 2004–2006 | Cohort study conducted at 15 PECARN sites |
| 2006 | R34 (HD050531) awarded to support writing MOO (July 2006) |
| 2006 | Collaborative Pediatric Critical Care Research Network sites join PECARN sites to operationalize trials |
| Protocol planning meeting in Washington, DC | |
| Other planning meetings in Toronto and Salt Lake City | |
| 2006 | Prospective yield study at select sites using inclusion and exclusion criteria |
| 2007 | Draft MOO with “refined” protocol |
| NHLBI contacted to submit application > ppg limit (1.5 M) | |
| Letter (5-page study overview) to directors NHLBI— approved | |
| 2008 | R01s submitted (February 2008) |
| Study section review and council approved | |
| Budget and study design modifications by NHLBI (November 2008) | |
| 2009 | HL094345 and HL094339 awards received (March 2009) |
| Institutional Review Board materials and contracts to sites (April 2009) | |
| Vanguard (half of sites) selection (June 2009) | |
| Training (August 2009) | |
| Enrollment begins per schedule (September 1, 2009) | |
| Data Safety and Monitoring Board created | |
| 2009 | Vanguard site enrollment (September 2009–August 31, 2010) |
| Exceeds feasibility (minimum) goal 50 cases and expected goal 75 cases | |
| Actual enrolled: 90 cases | |
| 2010 | Funding approved to add second cohort of sites (September 2010) |
| 2012 | 37 total sites and enrollment of 389 cases as of May 29, 2012 |
PECARN = Pediatric Emergency Care Applied Research Network, RCT = randomized controlled trials.
R21 Award: Preclinical Trial Cohort Study
To determine the feasibility of a multicenter RCT of TH after pediatric CA, we planned a preclinical trial cohort study in conjunction with the Pediatric Emergency Care Applied Research Network (PECARN) http://www.pecarn.org/ (8). Our application, in response to a National Institute of Child Health and Human Development (NICHD) Request for Applications (RFA) to support RCT planning related to CA (RFA-HD-02-026), resulted in an award (HD044955) to conduct the preclinical trial cohort study.
The R21 cohort study, conducted over 18 months at 15 PECARN sites, included 491 in-hospital and out-of-hospital CAs with return of spontaneous circulation (ROSC)/return of circulation (ROC) with extracorporeal membrane oxygenation (ECMO) (1, 9, 10). Approximately one third of the CAs were out-of-hospital and two thirds of the CAs were in-hospital cases; over half the children died during their hospitalization in each cohort (9, 10). When the in-hospital and out-of-hospital cohorts were compared, striking differences were observed (1). The most important finding was the unexpected difference in the cause of death; death was attributed to a neurologic indication in nearly 70% of out-of-hospital cases and only 20% of in-hospital cases. This remarkable difference made it imperative to conduct separate RCTs in these two populations. Sample size estimates, based primarily on information from this cohort, indicated that about twice as many sites as participated in the cohort study would be needed to conduct RCTs in 4 years of enrollment, with an additional fifth year for follow-up.
R34 Award: Preparation of Investigator-Initiated Clinical Trials Application to NIH
To complete planning for the THAPCA trials, we obtained a R34 (PA-04–008) award (HD050531) to support the development of the manual of operations and related materials. In 2006, a new PICU clinical research network, the Collaborative Pediatric Critical Care Research Network (CPCCRN) (http://www.cpccrn.org/), joined the existing PECARN sites in planning the THAPCA trials (11). Both networks use the same DCC (8, 11). Following protocol development meetings, we conducted a prospective examination of inclusion and exclusion criteria at clinical sites to obtain an improved estimate of the available sample sizes and site numbers required to conduct future RCTs. A major topic of discussion during initial protocol meetings was temperature management in the control group; there was consensus that there should be active temperature management to achieve normothermia in this group.
NIH/NHLBI Special Application Process
NHLBI requires that investigators, proposing multisite clinical trials with direct costs of $500,000 or more, obtain permission before submitting an application (http://www.nhlbi.nih.gov/funding/policies/500kweb.htm). As required, we met with NHLBI Program and Review staff to discuss the project and subsequently requested permission to proceed with an application. NHLBI granted approval to submit two linked R01 application in February 2008 cycle, one to support a DCC and a separate application to support 30 clinical sites to perform two separate parallel RCTs for in-hospital and out-of-hospital pediatric CA (THAPCA-in-hospital and THAPCA-out-of-hospital).
NIH/NHLBI Review and Funding
After review, the THAPCA clinical trials were funded (HL094345 and HL094339) and included some study design changes, budget modifcations, and the implementation of a pilot Vanguard Phase, described elsewhere in detail (12). Funding began March 9, 2009, to allow 6 months to complete study launch activities followed by initiation of patient screening and enrollment on September 1, 2009.
Study Design Overview
Specific Aims and Hypotheses
The primary objective of the THAPCA trials is to determine the efficacy of TH to improve survival with good neurobehavioral outcome in children who are resuscitated after CA in the in-hospital and out-of-hospital settings as separate RCTs. Specifically, the primary hypotheses of the THAPCA-out-of-hospital and THAPCA-in-hospital are as follows:
Hypothesis 1: Pediatric patients with out-of-hospital CA who are treated with TH will have higher survival with good neurobehavioral outcome at 12 months following resuscitation, compared with children who receive the normothermia control therapy.
Hypothesis 2: Pediatric patients with in-hospital CA who are treated with TH will have higher survival with- good neurobehavioral outcome at 12 months following resuscitation, compared with children who receive the normothermia control therapy.
Key safety outcomes for both trials include all-cause 28-day mortality, risk of infection, arrhythmias, and bleeding.
Study Sites and Organization
THAPCA encompasses two separate phase III RCTs that investigate the efficacy of TH to improve survival and neurobehavioral outcome of children after CA in the in-hospital and out-of-hospital setting. THAPCA was planned to include 30 or more clinical sites in the United States and Canada with the majority of sites associated with the PECARN and CPCCRN research networks. Both networks used a common DCC at the University of Utah (Principal Investigator [PI], J. Michael Dean, MD, MBA). Additional PICUs at medical centers with strong interest in the project were successfully recruited (Fig. 1). The final study protocol was developed in partnership with PECARN and CPC-CRN site investigators and consultants with special experience and interest in pediatric RCTs associated with TH.
Figure 1.
Site distribution map of the United States and Canada. See Appendix 1 for site with site investigator listing.
Patient Inclusion/Exclusion Criteria
Children older than 48 hours old and up to age 18 years, who sustain a CA requiring chest compressions for at least 2 minutes, are eligible. Other inclusion criteria are need for mechanical ventilation following resuscitation and that CA is unplanned (not part of surgical procedure). Major exclusions are Glasgow Coma Scale motor component score of 5 or 6, preexisting terminal illness or lack of commitment to full support, CA associated with trauma, severe bleeding, and inability to obtain informed consent within 6 hours of ROSC. The full inclusion and exclusion list is provided in Table 2. Use of TH in children who are eligible for THAPCA but not randomized is discouraged; occurrence of such “off-study” hypothermia is monitored at all participating hospitals.
Table 2.
Therapeutic Hypothermia after Pediatric Cardiac Arrest Trials Inclusion and Exclusion Criteria
| Inclusion Criteria | Exclusion Criteria |
|---|---|
| Patients will be eligible for enrollment if they meet all of the following inclusion criteria | Patients will be ineligible for enrollment if any of the following exclusion criteria are met |
| Suffered CA requiring chest compressions for at least 2 min (1 20 s) with ROSC/return of circulation | The parent or legal guardian does not speak English or Spanish |
| Age > 48 hr (with a corrected gestational age of at least 38 wk) and < 1 8 yr | Randomization is impossible within 6 hr of ROSC |
| Patient requires continuous mechanical ventilation | Patient is on extracorporeal membrane oxygenation when arrest occurs |
| The CA was unplanned (i.e., not part of cardiac surgical procedure) | Continuous infusion of epinephrine or norepinephrine at very high doses (≥ 2µ/kg/min) received immediately prior to randomization |
| Glasgow Coma Scale motor response of five (localizing pain or for infants less than 2 yr, withdraws to touch) or six (obeys commands, or for infants, normal spontaneous movement) prior to randomization | |
| History of a prior CA with chest compressions for at least 2 min during the current hospitalization but outside the 6-hr window for randomization | |
| Preexisting terminal illness with life expectancy < 1 2 mo | |
| Lack of commitment to aggressive intensive care therapies including do not resuscitate orders and other limitations to care | |
| CA was associated with severe brain, thoracic, or abdominal trauma | |
| Active and refractory severe bleeding prior to randomization | |
| Near drowning in ice water with patient core temperature ≥ 32°C on presentation | |
| Patient is pregnant | |
| Patient participation in a concurrent interventional trial whose protocol, in the judgment of the THAPCA investigators, prevents effective application of one or both THAPCA therapeutic treatment arms, or otherwise significantly interferes with carrying out the THAPCA protocol | |
| Patient is newborn with acute birth asphyxia | |
| Patient cared for in a neonatal ICU after arrest | |
| Patient has sickle cell anemia | |
| Patient known to have preexisting cryoglobulinemia | |
| Central nervous system tumor with ongoing chemotherapy or radiation therapy | |
| Progressive degenerative encephalopathy | |
| Chronic hypothermia secondary to hypothalamic, pituitary, or related condition for which body temperature is consistently below 37°C | |
| Any condition in which direct skin surface cooling would be contraindicated, such as large burns, decubitus ulcers, cellulitis, or other conditions with disrupted skin integrity | |
| Previous enrollment in the THAPCA trials |
CA = cardiac arrest, ROSC = return of spontaneous circulation, THAPCA = Therapeutic Hypothermia after Pediatric Cardiac Arrest.
Enrollment/Randomization Plans
Site institutional review board (IRB) approved written informed consent is required and obtained from guardians of all subjects. For international readers who may have questions about indemnity in U.S. trials like THAPCA, all site IRBs have their own consent form description related to how each site handles this issue. The DCC has site Pls who sign statements related to conduct of the study and the lead site (University of Michigan) subcontracts to each site describes the site’s trial responsibility related to screening, enrollment, maintenance of equipment and execution of the study protocol and manual of operations.
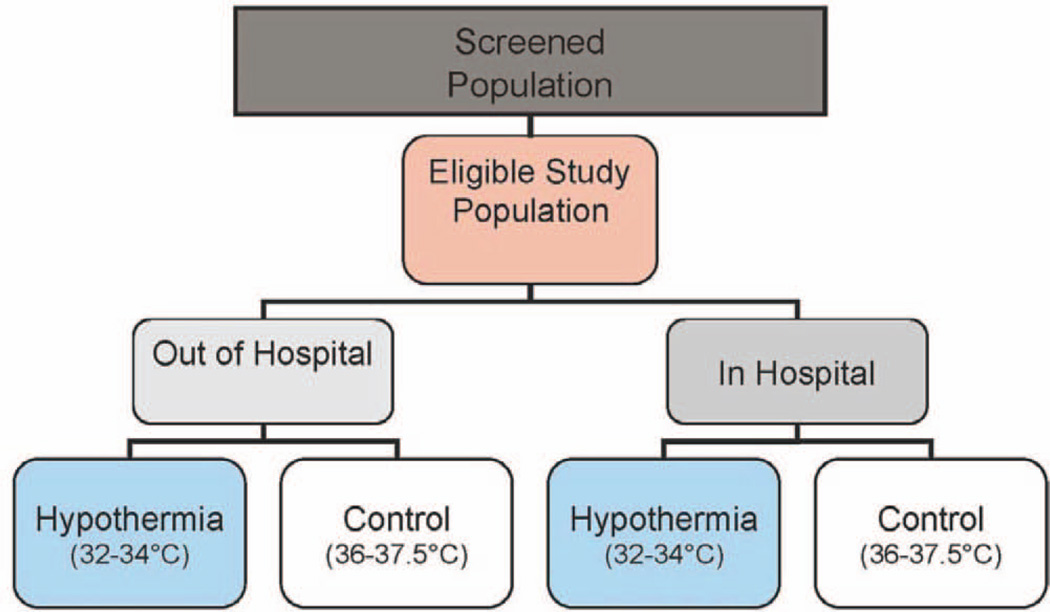
Subjects are enrolled separately in the out-of-hospital and in-hospital settings (Fig. 2). Within each trial, randomization to TH or therapeutic normothermia (TN) occurs in a 1:1 ratio using randomized blocks stratified by clinical center and age at entry (younger than 2 yr, 2–11 yr, and 12 yr and older). Randomization is carried out using a telephone-based system. An Internet-based backup randomization system is available; each center is additionally provided with an envelope-based backup randomization to be used in “emergency” settings when neither remote system is available. Randomization is required within 6 hours of CA ROSC/ROC. Target total enrollments are 250 evaluable cases for THAPCA-out-of-hospital and 504 evaluable cases for THAPCA-in-hospital. It is expected that over 800 total patients will be enrolled, as patients with suboptimal pre-arrest neurobehavioral status (discussed below) will not be included in the primary efficacy analyses.
Figure 2.
Overview of Therapeutic Hypothermia after Pediatric Cardiac Arrest trials.
In the planning of THAPCA, extensive discussions related to use of an Exception from Informed Consent occurred. It was concluded that informed consent with a 6-hour therapeutic window was necessary as animal studies had demonstrated a therapeutic window for neuroprotection after CA exists and also because U.S. neonatal hypothermia for HIE trials used a 6-hour window for eligibility. If enrollment had been poor, it was planned to approach our Data Safety and Monitoring Board (DSMB) for permission to use the Exception from Informed Consent.
Clinical Protocol Overview
With the exception of temperature-related management for each arm, all other monitoring and interventions described below are considered standard of care for the post-CA patient meeting THAPCA inclusion and exclusion criteria. Other therapies, administered during the times corresponding to the entire 120-hour intervention period, are in accordance with each site’s clinical practice.
Interventions and Temperature Monitoring
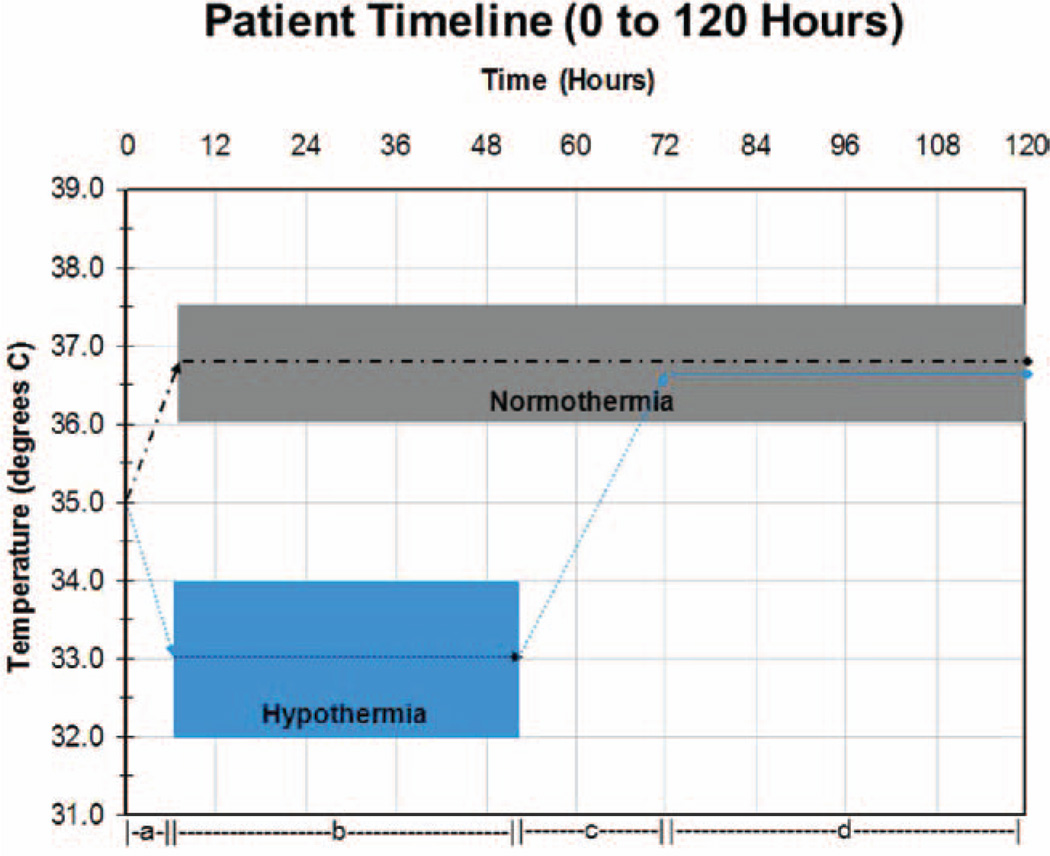
Following study enrollment and randomization to TH, children are initially paralyzed, sedated, and cooled (or warmed if indicated) by surface cooling using a Blanketrol III cooling unit with two appropriate-sized mattresses (Cincinnati SubZero, Cincinnati, out-of-hospital) applied anteriorly and posteriorly, to achieve and maintain a core temperature of 32–34°C for 48 hours. After 48 hours, the TH group is slowly rewarmed over 16 hours or longer to a target temperature range of 36–37.5°C, and this temperature is maintained through the remainder of the 120-hour intervention period. Following study enrollment and randomization to TN, children receive identical care except core temperature is actively maintained in a range of 36–37.5°C for 120 hours (Fig. 3). A servo-control mode (auto control or gradient variable mode) is used uniformly.
Figure 3.
Therapeutic hypothermia and therapeutic normothermia temperature timeline 0–120 hr. Dashed and dotted line = normothermia group; dotted line = hypothermia group. Intervals: a = time from randomization to assigned temperature range; b = 48hr of assigned temperature; c = rewarming of hypothermia group; d = interval of controlled normothermia in both groups.
Dual central temperature monitoring is required during active temperature management. A primary central temperature probe is placed into the distal third of the esophagus, although other central sites (rectal or bladder) may be substituted. This probe is connected to the Blanketrol III unit for servo temperature control. A secondary central temperature probe is placed per rectum or via a temperature sensing Foley catheter into the bladder; this provides safety backup monitoring if the primary probe does not reflect central temperature. A difference between the primary and secondary probes of ± 1°C is considered acceptable.
Vascular Access
Central venous access is required to safely administer fluids and other medications, such as inotrope/ vasopressor infusions, and an arterial catheter is required for blood pressure monitoring. Use of central venous access and arterial catheters was considered standard of care in this population.
Management of Sedation, Analgesia, and Neuromuscular Blockade
The recommended sedatives and analgesia agents are benzodiazepines and opioids. Suggested agents are midazolam and fentanyl for both groups, as were used in the adult RCTs (2,3). Midazolam and fentanyl administration by continuous infusion, supplemented by intermittent dosing, is suggested. After 72 hours, sedation and analgesia use is at the discretion of the primary clinical team. Vecuronium is the recommended, but not required, neuromuscular blocker to facilitate control of shivering. Other nondepolarizing agents to control shivering and facilitate temperature control in both the TH and TN groups may be used.
Special Issues of ECMO
Surface cooling is often not required for temperature regulation of children on ECMO. The desired temperature is titrated by the bedside ECMO specialist by changing the heat exchanger temperature. Only a single central temperature probe is required.
Laboratory Testing
Table 3 describes the minimum or required laboratory testing conducted throughout the intervention period (0–120 hr). Electrolytes, blood urea nitrogen, creatinine, and glucose are measured at least every 6 hours during the periods corresponding to active cooling and rewarming in the hypothermia group and at least every 12 hours during the remaining intervention period (up to 120 hr). Other blood monitoring during the intervention period includes daily complete blood count, liver function tests (specifically, alanine aminotransferase, aspartate aminotransferase, prothrombin time, partial thromboplastin time), amylase, lipase, magnesium, calcium, and phosphorus. Arterial blood gas and lactate are measured daily for the first 3 days. Blood cultures are obtained at the time of randomization and on days 1,2, and 3. Urine cultures are obtained at the time of randomization and on days 2 and 4. Chest radiographs are done daily during the intervention period to confirm esophageal temperature probe position.
Table 3.
Summary of Monitoring Required for Hypothermia After Pediatric Cardiac Arrest Trials
| Physiologic Studies |
| Arterial Line |
| Central venous catheter (or equivalent) |
| Temperature (central) |
| Esophageal (primary) |
| Chest radiograph (esophageal probe placement) |
| Rectal or foley (secondary) |
| Extracorporeal membrane oxygenation exception: 1 central temperature, any site |
| Laboratory Studies |
| Electrolytes (Na, K, CI, HC03, blood urea nitrogen, creatinine, glucose) |
| q 6 hr during cooling and rewarming |
| q 12 hr other times |
| Mg, P04, Ca (q 24 hr) |
| Liver function tests (bilirubin, alanine transaminase, and aspartate transaminase) amylase, lipase (q 24 hr) |
| Coagulation (prothrombin time/international normalized ratio, partial thromboplastin time) (q 24 hr) |
| Complete blood count (q 24 hr) |
| Arterial blood gas (q 24 hr), lactate (q 24 hr) × 3 d |
| Cultures |
| Blood (d 0,1,2,3) |
| Urine (d 0,2,4) |
| Respiratory and other (as clinically indicated) |
Minimal monitoring required for patients enrolled
Other Monitoring During and After Intervention
The total volume of fluids received and total urine output are recorded daily for the first 5 days. Surgical procedures to treat bleeding postrandomization and concomitant medications and other procedures or therapies administered during the first 10 days are recorded.
There are no study-specific interventions after the 120-hour treatment period. Subsequent temperature management is according to the PICU clinical practice at each site; minimum and maximum daily temperatures, including the measurement site, are recorded through day 10. The dates of PICU and hospital discharge are recorded. All other therapies administered during the times corresponding to the entire intervention period are in accordance with each site’s clinical practice.
Data Collection and Confidentiality
Clinical site personnel enter study data into a secure Web-based repository maintained at the DCC. All study data are identified solely via coded study identification number to maintain patient confidentiality.
Study Monitoring
Monitoring of Safety Endpoints
Monitoring of infection events associated with these trials is accomplished by examination of all cultures obtained up to 7 days (168 hr) after the CA. Status of all cultures up to 10 days (240 hr) post-CA is recorded. All arrhythmias occurring during the first 7 days are also recorded. Blood products (RBCs, platelets, fresh frozen plasma, and cryoprecipitate) received during the first 7 days are recorded. Mortality, a key safety outcome, is recorded at 28 days post arrest for all enrolled patients (Table 4).
Table 4.
Planned Outcomes of the Therapeutic Hypothermia After Pediatric Cardiac Arrest Trials
| Primary outcome |
| VABS ≥ 70 at 12 mo post CA |
| Secondary outcomes |
| All cases |
| Survival at 1 2 mo following CA |
| Change in neurobehavioral function from pre-CA baseline to 1 2-mo measurement (delta VABS-I I) |
| Tertiary outcomes |
| Survivors only |
| Neuropsychological battery score at 1 2-mo evaluation |
| Neurologic abnormality scores at 1 2-mo evaluation (modified Pediatric Resuscitation after Cardiac Arrest Neurologii Outcome Measure pediatric stroke scale) |
| Safety outcomes |
| All-cause 28-d mortality |
| Prevalence of culture-proven infection within 7 d |
| Blood |
| Urine |
| Respiratory |
| Other |
| Blood product requirement within 7 d post CA |
| Arrhythmias within 7 d |
VABS = Vineland Adaptive Behavior Scales, CA = cardiac arrest.
Adverse Event Reporting
Adverse events are recorded for 14 days after randomization or until hospital discharge, whichever occurs earlier. All adverse events are tabulated by study arm for review by the DSMB at their meetings. Serious adverse events are reviewed by the medical monitor of the THAPCA trials within 24 hours of notification of the DCC.
Site Monitoring
Trained site monitors were sent to clinical centers after the first three subjects were enrolled, and subsequently, they are sent for every five enrollments. Site monitors review regulatory documents, consent forms, and do selected source verification. Remote monitoring of selected data is done by DCC staff to complement the physical site visits.
Study Outcomes
Primary Outcome
The primary efficacy endpoint is survival with good neurobehavioral outcome at 12-month follow-up, defined as an age-corrected standard score of 70 or greater on the Vineland Adaptive Behavior Scales-Second Edition (VABS-II) (13). The VABS-II is a caregiver report measure of functional skills, examining communication, daily living, socialization, and motor skills. The VABS-II is being collected via telephone by a trained interviewer blinded to assigned treatment from a central location. Enrolled children, whose reported prearrest VABS-II is less than 70 (based on data obtained within 24 hr of enrollment), will not be included in the primary efficacy analysis. Treatment effect will be assessed separately for the out-of-hospital and in-hospital trials.
Secondary, Tertiary, and Safety Outcomes
A list of secondary, tertiary, and safety outcomes is summarized in Table 4. Outcomes assessed only among survivors have been designated tertiary, as the comparability between treatment arms provided by randomization will not be guaranteed in the patients who survive to 1 year.
Outcome Follow-up Overview
At 3 and 12 months, the VABS-II is obtained via telephone interview by a trained clinician blind to treatment group at a single-designated site (Kennedy Krieger Institute, Baltimore, MD). Mortality status is also determined at 12 months. Additionally, 1 year after CA, all survivors participate in an onsite neurologic evaluation. All survivors under the age of 6 years at the 1-year follow-up also participate in an onsite neurobehavioral evaluation. For survivors 6 years old and older, only those who are sufficiently responsive (based on specific criteria obtained in the VABS-II 12-month interview) are scheduled to participate in a neurobehavioral assessment. Children who are not determined to be sufficiently responsive are assigned the lowest possible scores for the test battery.
Sample Size Calculation
For power calculations, rates of the primary outcome are estimated to be 15% to 35% for out-of-hospital and 35% to 55% in in-hospital CA (in the TN arm). The target sample sizes (subjects available for the primary analysis) of 250 out-of-hospital and 504 in-hospital will provide 80% to 90% power to detect an absolute treatment benefit of hypothermia, with respect to the binary primary outcome above, in the range of 15% (hypothesized magnitude of benefit in in-hospital setting) to 20% (hypothesized benefit in out-of-hospital setting) at an alpha level of 0.05 for each study, accounting for interim efficacy monitoring by the DSMB. Total enrollment projections are higher to account for loss to follow-up and exclusion from the primary analysis of children with a baseline neurologic deficit (VABS-II below 70). In five neonatal and adult TH RCTs for CA or HIE conducted so far, observed absolute treatment effects ranged from 16% to 32% and total study enrollment ranged from 70 to 300 cases.
Interim Monitoring
An independent NHLBI-appointed DSMB meets twice yearly to assess study safety, efficacy, and performance issues. The DSMB operates according to a charter that mandates regular interim review of study efficacy outcomes, with prespecified, conservative O’Brien-Fleming boundaries for early stopping in the setting of observed treatment superiority (14). Per the DSMB charter, early trial termination in the setting of futility may also be considered if conditional power (chance of detecting a significant treatment effect if the trial were continued) is very low.
Efficacy Analysis Plan
The primary analysis, performed separately within each trial, will use a chi-square testing approach to compare proportions of patients with good neurobehavioral outcome (alive with VABS-II at least 70) at 12 months following resuscitation, among enrolled patients with prearrest VABS-II of at least 70. Two secondary within-trial analyses will compare proportions of all enrolled patients alive at 12 months following resuscitation, as well as compare change in VABS-II from prearrest to 12 months among all patients. The latter analysis, performed using nonparametric rank-based approaches, will treat patients deceased at 12 months as having the worst possible deterioration from their prearrest state. For each trial, the primary analysis will use a two-sided chi-square test with alpha level of 0.05. The two secondary outcomes will be analyzed by chi-square test (survival) and Mann-Whitney test (change in neurobehavioral status), with a joint alpha level of 0.05. The statistical tests above will be stratified using the age categories that were used as randomization strata. A statistical analysis plan (SAP) is in place and will be adhered to for all study analyses; any analyses not specified a priori in the SAP will be explicitly described as exploratory in study reports.
Trial Organization including Research networks
Research Network Resources
The PECARN network has supported this project since 2002 with its DCC, subcommittees, and steering committee to support conduct of RCTs. The NICHD-sponsored CPCCRN uses the same DCC and has supported and participated in the development of the trial since 2006. Both networks have experience in multicenter RCTs. The investigative team is multidisciplinary and includes experts in Pediatric Critical Care Medicine, Neonatology, Neuropsychology, Emergency Medicine, Physical and Rehabilitative Medicine, Neurology, Cardiology, TH, and conduct of RCTs in children.
Data Safety and Monitoring Board
THAPCA has an independent DSMB, which was created according to NHLBI guidelines. The DSMB meets at regular intervals to assess study safety, efficacy, and performance issues.
Executive Committee
An Executive Committee oversees the conduct of the THAPCA trials. Members also evaluate proposals for ancillary studies that are linked to the parent trials and review and make recommendations concerning trial issues that arise (see Appendix 2 for membership composition).
Steering Committee
Steering committee meetings of the site PIs occur monthly via conference calls and in-person at an annual 3-day training meeting. During the monthly meetings, screening and enrollment by site are reviewed, updates on amendments and other information are shared, best practices by sites are presented, and case discussions occur. The DCC and PI have weekly conference calls to overview study progress and planning of all phases of study.
Site Information
As of May 29, 2012, the THAPCA trials had 35 active sites (two sites inactive) in the United States and Canada (Fig. 1). Three additional sites were pending activation.
DISCUSSION
TH improves survival with better functional outcome in a wide range of animal models including CA (15–17), stroke (18, 19), TBI (20, 21), birth asphyxia (22, 23), and other conditions (24). In 2002, landmark RCTs in adults with out-of-hospital VF/VT CA were the first reports showing improved outcomes for a TH intervention in a specific human condition (2, 3). However, after nearly a decade, no additional RCTs of TH for adult CA have been reported, and important questions remain concerning the generalizability of the original trials’ findings. One key unanswered question concerns the efficacy of TH in nonshockable CA rhythms, such as PEA or asystole; such cases were excluded in the initial RCTs. Recently, a French registry report described improved outcome associated with TH for out-of-hospital VF/VT CA; however, patients with initial asystole or PEA CA did not benefit from TH (25). Another vital question concerns whether TH benefit is generalizable to in-hospital CA cases; no trials have been conducted in this population. Another issue is the optimal duration of TH; no trials have investigated TH for adult CA longer than 24 hours.
The only nonneonatal pediatric condition in which TH has been studied in adequately powered RCTs is TBI. Although small preliminary studies showed promise for TH improving TBI outcomes (26–28), subsequent larger RCTs in adults and children have not demonstrated efficacy (7, 29). The largest pediatric trial to date (Hypothermia Paediatric Head Injury Trial [HYP-HIT]) reported not only a lack of efficacy of TH for TBI but also a very strong trend for higher mortality in the TH-treated group (7). A subsequent NIH-sponsored multicenter RCT (Cool KIDS trial) of TH for TBI was designed to address some potential shortcomings in the HYP-HIT trial and was recently terminated because of futility to demonstrate a difference in the primary study outcome (30).
A major shortcoming of both the adult out-of-hospital VF/VT trials and neonatal HIE trials reported so far concerns the control group temperature management; fever was often not successfully prevented in controls. In post-RCT publications from both the adult CA and neonatal HIE trials, fever commonly occurred in control cases and was associated with much worse outcomes (31, 32). This trend supports both experimental findings in animal models and studies of human brain injury that report a strong association of fever with worse neurologic outcomes (33). International resuscitation guidelines for both pediatric and adult CA strongly recommend aggressive management to avoid fever (34). However, TN requires similar interventions to those required for TH (35), and THAPCA is the first RCT that compares the efficacy of TH and TN in survivors of CA or HIE. An adult RCT currently registered on clinicaltrials.gov is examining whether TH is superior to TN in adult CA survivors (36).
Thus, there is great uncertainty concerning the use of TH in the pediatric CA population until adequately powered RCTs are performed that demonstrate or exclude benefit. The suggestion of lack of benefit in adults with asystole or PEA is extremely concerning, as these arrhythmias are much more common in children with out-of-hospital CA (25). Additional caution is warranted based on findings of pediatric TBI trials that observed a trend of higher mortality with TH (7). TN, as an alternative therapy in contrast to usual temperature management that commonly does not prevent fever, has not been adequately examined.
By the time the results of the current THAPCA trials are reported, over a decade will have elapsed since the original adult clinical trials of TH for out-of-hospital CA were published in 2002. This lag time is attributable to the challenges inherent in determining the feasibility of conducting RCTs in U.S. pediatric hospitals, time required to prepare a successful application for a large NIH-sponsored clinical trial, and complexity inherent in conducting these trials. A unique feature of THAPCA is the concurrent conduct of two separate RCTs (in-hospital and out-of-hospital) with little incremental impact on study cost, as the same protocol and infrastructure resources are being used. To this date, no RCT has been conducted of TH for in-hospital CA in an adult or pediatric population.
After THAPCA findings are reported, gaps in our knowledge will remain. For example, the optimal duration of TH in both of our trials will not be known. The selection of 120 hours of temperature control with 48 hours as duration of TH in THAPCA trials was based on an expert majority consensus of a multidisciplinary group in critical care, emergency medicine, neurology, and others in 2006. Of note, the duration of TH selected for the THAPCA trials was intermediate between the longest administration of TH in adults and neonatal trials available in 2006 (24 and 72 hr). Whether the duration of TH should be titrated (longer or shorter) based on some biomarker(s) or other measurement(s) will not be established during the THAPCA trials. We will only be able to establish whether 48 hours of TH, initiated within 6 hours of ROSC and followed by TN through 120 hours, is superior to 120 hours of TN in two populations. Additionally, our trials will likely be underpowered to determine whether TH has differential effects in subgroups, such as shockable versus nonshockable rhythms. In contrast to out-of-hospital adult CA, children with out-of-hospital CA often have nonshockable rhythms, which have worse prognosis. We anticipate that the majority of our in-hospital CA population will be children with congenital heart disease, and our findings may not be generalizable to all in-hospital CA cases.
We have described some of the key events in the planning and operationalization of our two complex RCTs in a critically ill pediatric population. We anticipate that lessons learned from our experience with THAPCA may be of value to other investigators who are planning complex interventional RCTs in PICU or emergency department settings. Clinical trials in these settings present significant challenges, as described recently (12). Multicenter participation is commonly required to achieve adequate sample size to attain statistical power with reasonable effect size, and multicenter trials may also provide stronger evidence of generalizability of findings compared with single-center studies.
Finally, until the completion of the THAPCA trials, the use of TH should be considered experimental in children after CA in both the in-hospital and out-of-hospital settings, as there have been no RCTs that suggest efficacy and some reports have suggested no benefit or possible harm in other settings (i.e., TBI). During the planning and conduct of THAPCA, many individuals from nonparticipating sites in the United States and international sites have inquired about using our clinical protocol to administer TH. We have denied all requests and explained that the protocol requires extensive training and has not been shown to be safe and efficacious at this time. Our perspective is that this intervention should not routinely be used outside the setting of ongoing clinical trials at this time.
ACKNOWLEDGMENTS
We acknowledge the efforts of the following individuals and groups who have made the THAPCA trials possible:
PECARN: PECARN Steering Committee: N. Kuppermann (Chair), E. Alpern, J. Chamberlain, J. M. Dean, M. Gerardi, J. Goepp, M. Gorelick, J. Hoyle, D. Jaffe, C. Johns, N. Levick, P. Mahajan, R. Maio, K. Melville, S. Miller (deceased), D. Monroe, R. Ruddy, R. Stanley, D. Treloar, M. Tunik, A. Walker. MCHB/ EMSC liaisons: D. Kavanaugh, H. Park. Central Data Management and Coordinating Center. M. Dean, R. Holubkov, S. Knight, A. Donaldson. Data Analysis and Management Subcommittee: J. Chamberlain (Chair), M. Brown, H. Corneli, J. Goepp, R. Holubkov, P. Mahajan, K. Melville, E. Stremski, M. Tunik. Grants and Publications Subcommittee. M. Gorelick (Chair), E. Alpern, J. M. Dean, G. Foltin, J. loseph, S. Miller(deceased), F. Moler, R. Stanley, S. Teach. Protocol Concept Review and Development Subcommittee. D. Jaffe (Chair), K. Brown, A. Cooper, J. M. Dean, C. Johns, R. Maio, N. C. Mann, D. Monroe, K. Shaw, D. Teitelbaum, D. Treloar. Quality Assurance Subcommittee : R. Stanley (Chair), D. Alexander, J. Brown, M. Gerardi, M. Gregor, R. Holubkov, K. Lillis, B. Nordberg, R. Ruddy, M. Shults, A. Walker. Safety and Regulatory Affairs Subcommittee. N. Levick (Chair), J. Brennan, J. Brown, J. M. Dean, J. Hoyle, R. Maio, R. Ruddy, W Schalick, T. Singh, J. Wright.
CPCCRN: C. J. Newth, Department of Anesthesiology and Critical Care Medicine, Children’s Hospital Los Angeles, Los Angeles, CA; R. Harrison, Department of Pediatrics, University of California at Los Angeles, Los Angeles, CA; K. Meert and S. Heidemann, Department of Pediatrics, Children’s Hospital of Michigan, Detroit, MI; T. Shanley and F. Moler, Department of Pediatrics, University of Michigan, Ann Arbor, MI; A. Zuppa and R. Berg, Department of Pediatrics, Children’s Hospital of Philadelphia, Philadelphia, PA; J. Berger and D. Wessel, Department of Pediatrics, Children’s National Medical Center, Washington, DC; H. Dalton and M. Pollack, Department of Pediatrics, Phoenix Children’s Hospital, Phoenix, AZ; M. Bell and J. Carcillo, Department of Critical Care Medicine, Children’s Hospital of Pittsburgh, Pittsburgh, PA; A. Donaldson, R. Holubkov, and J. M. Dean, Department of Pediatrics, University of Utah School of Medicine, Salt Lake City, UT; and C. Nicholson, Eunice Kennedy Shriver National Institute of Child Health and Development, Bethesda, MD.
DSMB: A. Zaritsky, MD (Chair, Pediatric Critical Care), Eastern Virginia University; A. Atz, MD (Pediatric Cardiology and Intensive Care), University of South Carolina; David Glidden, PhD (Biostatistics), UCSF; John Lantos, MD (Bioethics), Children’s Mercy Hospitals; Marianne Gausche-Hill, MD (Pediatric Emergency Medicine), Harbor-UCLA; Greg Holmes, MD (Neurology), Dartmouth University; Richard Ehrenkranz, MD (Neonatology), Yale University.
Supported, in part, by the Pediatric Emergency Care Applied Research Network (PECARN) from cooperative agreements U03MC00001, U03MC00003, U03MC00006, U03MC00007, and U03MC00008 and the Collaborative Pediatric Critical Care Research Network (CPCCRN) from cooperative agreements (U10HD500009, U10HD050096, U10HD049981, U10HD049945, U10HD049983, U10HD050012, and U01HD049934).
Dr. Moler received federal grants HD044955 from the National Institutes of Health (NIH), Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD); HD050531 from NIH, NICHD; and HL094345 from NIH, National Heart Lung Blood Institute (NHLBI). Dr. Dean received federal grant HL094339 from NIH, NHLBI. Drs. Silverstein, Meert, Clark, Holubkov, Browning, Slomine, and Christensen received funding from NIH.
APPENDIX 1. SITES AND CLINICAL SITE INVESTIGATORS
September 1, 2009
F. Moler, University of Michigan, Ann Arbor, MI
A. Topjian, Children’s Hospital of Philadelphia, Philadelphia, PA
J. Berger, Children’s National Medical Center, Washington, DC
K. Meert, Children’s Hospital of Michigan, Detroit, MI
E. Fink, University of Pittsburgh, Pittsburgh, PA
C. Newth, Children’s Hospital of Los Angeles (USC), Los Angeles, CA
R. Harrison, Mattel Children’s Hospital (UCLA), Los Angeles, CA J. Pineda, Washington University St. Louis Children’s Hospital
D. Wheeler, Children’s Hospital Medical Center, Cincinnati, out-of-hospital
K. Bennett, University of Utah – Primary Children’s Medical Center
C. Schleien, Children’s Hospital of New York, Columbia University, New York, NY
D. Goodman, Children’s Memorial Hospital, Chicago, IL R. Sanders, University of Arkansas, Little Rock, AR
J. Hutchinson, Hospital for Sick Kids, Toronto, ON, Canada
J. Koch, Children’s Hospital, Dallas, TX
L. Loftis, Texas Children’s Hospital (Baylor), Houston, TX
Post April 1, 2010
M. Meyers, Medical College of Wisconsin, Milwaukee, WI
E. Lloyd, Nationwide Children’s Hospital, Columbus, out-of-hospital
H. Dalton, Phoenix Children’s Hospital, Phoenix, AZ Post September 1, 2010
J. Lee-Summers, Johns Hopkins, Baltimore, MD
E. van der Jagt, University of Rochester, Rochester, NY
E. Dobyns, University of Colorado, Denver, CO
J. Zimmerman, University of Washington, Seattle, WA
M. Mathur, Loma Linda University, Loma Linda, CA
J. Alten, University of Alabama, Birmingham, AL
J. Nowak, Minneapolis Children’s Hospital, Minneapolis, MN
A. Theodorou, University of Arizona-Tucson, Tucson, AZ
A. Schwarz, Children’s Hospital of Orange County, Orange, CA
N. Thomas, Penn State University, Hershey, PA
K. Lidsky, Rainbow Babies and Children’s Hospital, Cleveland, OH
S. Shah, University of Tennessee, Memphis, Memphis, TN
N. Pham, Children’s Hospital of Atlanta, Atlanta, GA
M. Porter, University of Louisville, Louisville, KY
Post April 1,2011
A. Stock, All Children’s Hospital (USF), St Petersburg, FL
G. Ofori-Amanfo, Duke, Raleigh, NC
P. McQuillen, UC-SF, San Francisco, CA
T. Wu, UT-San Antonio, TX
APPENDIX 2. EXECUTIVE COMMITTEE
Frank W. Moler, MD, MS, PI (Scientific), University of Michigan
J. Michael Dean, MD, MBA, PI (DCC), University of Utah
Richard Holubkov, PhD, Biostatistician, University of Utah
Kathleen Meert, MD, Critical Care (CPCCRN), Wayne State University
Jamie Hutchison, MD, Critical Care (Canada), University of Toronto
Vinay Nadkarni, MD, Critical Care, University of Pennsylvania
Nate Kuppermann, MD, MPH, Emergency Medicine (PECARN), UC-Davis
Seetha Shankaran, MD, Neonatology, Wayne State University
Faye Silverstein, MD, Neurology, University of Michigan
James Christensen, MD, Rehabilitation Medicine, Kennedy
Krieger Institute
Victoria Pemberton, RNC, MS, NHLBI, NIH
Carol Nicholson, MD, MS, NICHD, CPCCRN, NIH
Tasmeen Singh Weik, DrPH, MCHB EMSC, PECARN, HRSA
REFERENCES
- 1.Moler FW, Meert K, Donaldson AE, et al. Pediatric Emergency Care Applied Research Network: In-hospital versus out-of-hospital pediatric cardiac arrest: A multicenter cohort study. Crit Care Med. 2009;37:2259–2267. doi: 10.1097/CCM.0b013e3181a00a6a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.The Hypothermia After Cardiac Arrest Study Group. Mild therapeutic hypothermia to improve the neurological outcome after cardiac arrest. N Engl J Med. 2002;346:549–556. doi: 10.1056/NEJMoa012689. [DOI] [PubMed] [Google Scholar]
- 3.Bernard SA, Gray TW, Buist MD, et al. Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia. N Engl J Med. 2002;346:557–563. doi: 10.1056/NEJMoa003289. [DOI] [PubMed] [Google Scholar]
- 4.Shankaran S, Laptook AR, Ehrenkranz RA, et al. National Institute of Child Health and Human Development Neonatal Research Network: Whole-body hypothermia for neonates with hypoxic-ischemic encephalopathy. N Engl J Med. 2005;353:1574–1584. doi: 10.1056/NEJMcps050929. [DOI] [PubMed] [Google Scholar]
- 5.Gluckman PD, Wyatt JS, Azzopardi D, et al. Selective head cooling with mild systemic hypothermia after neonatal encephalopathy Multicentre randomised trial. Lancet. 2005;365:663–670. doi: 10.1016/S0140-6736(05)17946-X. [DOI] [PubMed] [Google Scholar]
- 6.Eicher DJ, Wagner CL, Katikaneni LP, et al. Moderate hypothermia in neonatal encephalopathy Efficacy outcomes. Pediatr Neurol. 2005;32:11–17. doi: 10.1016/j.pediatrneurol.2004.06.014. [DOI] [PubMed] [Google Scholar]
- 7.Hutchison JS, Ward RE, Lacroix J, et al. Hypothermia Pediatric Head Injury Trial Investigators and the Canadian Critical Care Trials Group: Hypothermia therapy after traumatic brain injury in children. N Engl J Med. 2008;358:2447–2456. doi: 10.1056/NEJMoa0706930. [DOI] [PubMed] [Google Scholar]
- 8.The Pediatric Emergency Care Applied Research Network: The Pediatric Emergency Care Applied Research Network (PECARN) Rational, development, and first steps. Acad Emerg Med. 2003;10:661–668. [PubMed] [Google Scholar]
- 9.Meert KL, Donaldson A, Nadkarni V, et al. Pediatric Emergency Care Applied Research Network: Multicenter cohort study of in-hospital pediatric cardiac arrest. Pediatr Crit Care Med. 2009;10:544–553. doi: 10.1097/PCC.0b013e3181a7045c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moler FW, Donaldson AE, Meert K, et al. Pediatric Emergency Care Applied Research Network: Multicenter cohort study of out-of-hospital pediatric cardiac arrest. Crit Care Med. 2011;39:141–149. doi: 10.1097/CCM.0b013e3181fa3c17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wilson DF, Dean JM, Newth C, et al. Collaborative Pediatric Critical Care Research Network (CPCCRN) Pediatr Crit Care Med. 2006;7:301–307. doi: 10.1097/01.PCC.0000227106.66902.4F. [DOI] [PubMed] [Google Scholar]
- 12.Pemberton VL, Browning B, Webster A, et al. Therapeutic Hypothermia after Pediatric Cardiac Arrest Trial The Vanguard Phase Experience and Implications for Other Trials. Pediatr Crit Care Med. 2013;14:19–26. doi: 10.1097/PCC.0b013e31825b860b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sparrow SS, Cicchetti DV, Balla DA. Vineland Adaptive Behavior Scales. Second Edition. Circle Pines, MN: AGS Publishing; 2005. [Google Scholar]
- 14.O’Brien PC, Fleming TR. A multiple testing procedure for clinical trials. Biometrics. 1979;35:549–556. [PubMed] [Google Scholar]
- 15.Sterz F, Safar P, Tisherman S, et al. Mild hypothermic cardiopulmonary resuscitation improves outcome after prolonged cardiac arrest in dogs. Crit Care Med. 1991;19:379–389. doi: 10.1097/00003246-199103000-00017. [DOI] [PubMed] [Google Scholar]
- 16.Safar P, Xiao F, Radovsky A, et al. Improved cerebral resuscitation from cardiac arrest in dogs with mild hypothermia plus blood flow promotion. Stroke. 1996;27:105–113. doi: 10.1161/01.str.27.1.105. [DOI] [PubMed] [Google Scholar]
- 17.Corbett D, Nurse S, Colbourne F. Hypothermic neuroprotection. A global ischemia study using 18- to 20-month-old gerbils. Stroke. 1997;28:2238–2242. doi: 10.1161/01.str.28.11.2238. discussion 2243. [DOI] [PubMed] [Google Scholar]
- 18.Maier CM, Ahern Kv, Cheng ML, et al. Optimal depth and duration of mild hypothermia in a focal model of transient cerebral ischemia: Effects on neurologic outcome, infarct size, apoptosis, and inflammation. Stroke. 1998;29:2171–2180. doi: 10.1161/01.str.29.10.2171. [DOI] [PubMed] [Google Scholar]
- 19.Wnfree CJ, Baker CJ, Connolly ES, Jr, et al. Mild hypothermia reduces penumbral glutamate levels in the rat permanent focal cerebral ischemia model. Neurosurgery. 1996;38:1216–1222. doi: 10.1097/00006123-199606000-00034. [DOI] [PubMed] [Google Scholar]
- 20.Dietrich WD, Alonso O, Busto R, et al. Post-traumatic brain hypothermia reduces histopathological damage following concussive brain injury in the rat. Acta Neuropathol. 1994;87:250–258. doi: 10.1007/BF00296740. [DOI] [PubMed] [Google Scholar]
- 21.Clark RS, Kochanek PM, Marion DW, et al. Mild posttraumatic hypothermia reduces mortality after severe controlled cortical impact in rats. J Cereb Blood Flow Metab. 1996;16:253–261. doi: 10.1097/00004647-199603000-00010. [DOI] [PubMed] [Google Scholar]
- 22.Laptook AR, Corbett RJ, Sterett R, et al. Modest hypothermia provides partial neuroprotection when used for immediate resuscitation after brain ischemia. Pediatr Res. 1997;42:17–23. doi: 10.1203/00006450-199707000-00004. [DOI] [PubMed] [Google Scholar]
- 23.Gunn AJ, Gunn TR, Gunning Ml, et al. Neuroprotection with prolonged head cooling started before postischemic seizures in fetal sheep. Pediatrics. 1998;102:1098–1106. doi: 10.1542/peds.102.5.1098. [DOI] [PubMed] [Google Scholar]
- 24.Polderman KH. Application of therapeutic hypothermia in the ICU: Opportunities, pitfalls of a promising treatment modality. Part 1 Indications and evidence. Intensive Care Med. 2004;30:556–575. doi: 10.1007/s00134-003-2152-x. [DOI] [PubMed] [Google Scholar]
- 25.Dumas F, Grimaldi D, Zuber B, et al. Is hypothermia after cardiac arrest effective in both shockable and nonshockable patients? Insights from a large registry. Circulation. 2011;123:877–886. doi: 10.1161/CIRCULATIONAHA.110.987347. [DOI] [PubMed] [Google Scholar]
- 26.Adelson PD, Ragheb J, Kanev P, et al. Phase II clinical trial of moderate hypothermia after severe traumatic brain injury in children. Neurosurgery. 2005;56:740–754. doi: 10.1227/01.neu.0000156471.50726.26. [DOI] [PubMed] [Google Scholar]
- 27.Biswas AK, Bruce DA, Sklar FH, et al. Treatment of acute traumatic brain injury in children with moderate hypothermia improves intracranial hypertension. Crit Care Med. 2002;30:2742–2751. doi: 10.1097/00003246-200212000-00020. [DOI] [PubMed] [Google Scholar]
- 28.Marion DW, Penrod LE, Kelsey SF, et al. Treatment of traumatic brain injury with moderate hypothermia. N Engl J Med. 1997;336:540–546. doi: 10.1056/NEJM199702203360803. [DOI] [PubMed] [Google Scholar]
- 29.Clifton GL, Miller ER, Choi SC, et al. Lack of effect of induction of hypothermia after acute brain injury. N Engl J Med. 2001;344:556–563. doi: 10.1056/NEJM200102223440803. [DOI] [PubMed] [Google Scholar]
- 30.Adelson PD. Hypothermia following pediatric severe traumatic brain inury (pediatric traumatic brain injury consortium) Hypothermia. [Accessed February 24, 2012];J Neurotrauma. 2012 29:A17. doi: 10.1089/neu.2008.0571. Available at: http://www.safar.pitt.edu/content/archive/annualreport/pdf/PNTC_Report_June_2011.pdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Laptook A, Tyson J, Shankaran S, et al. National Institute of Child Health and Human Development Neonatal Research Network: Elevated temperature after hypoxic-ischemic encephalopathy: Risk factor for adverse outcomes. Pediatrics. 2008;122:491–499. doi: 10.1542/peds.2007-1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zeiner A, Holzer M, Sterz F, et al. Hyperthermia after cardiac arrest is associated with an unfavorable neurologic outcome. Arch Intern Med. 2001;161:2007–2012. doi: 10.1001/archinte.161.16.2007. [DOI] [PubMed] [Google Scholar]
- 33.Dietrich WD, Bramlett HM. Hyperthermia and central nervous system injury. Prog Brain Res. 2007;162:201–217. doi: 10.1016/S0079-6123(06)62011-6. [DOI] [PubMed] [Google Scholar]
- 34.Neumar RW, Nolan JP, Adrie C, et al. Post-cardiac arrest syndrome: Epidemiology pathophysiology treatment prognostication A consensus statement from the International Liaison Committee on Resuscitation (American Heart Association, Australian and New Zealand Council on Resuscitation, European Resuscitation Council, Heart and Stroke Foundation of Canada, InterAmerican Heart Foundation Resuscitation Council of Asia, and the Resuscitation Council of Southern Africa); the American Heart Association Emergency Cardiovascular Care Committee; the Council on Cardiovascular Surgery and Anesthesia; the Council on Cardiopulmonary, Perioperative, Critical Care; the Council on Clinical Cardiology and the Stroke Council. Circulation. 2008;118:2452–2483. doi: 10.1161/CIRCULATIONAHA.108.190652. [DOI] [PubMed] [Google Scholar]
- 35.Polderman KH, Herold I. Therapeutic hypothermia controlled normothermia in the intensive care unit Practical considerations, side effects, and cooling methods. Crit Care Med. 2009;37:1101–1120. doi: 10.1097/CCM.0b013e3181962ad5. [DOI] [PubMed] [Google Scholar]
- 36.Target Temperature Management After Cardiac Arrest (TTM) - ClinicalTrials.gov Identifier: NCT01020916