Abstract
Many types of injury such as seizure, ischemia, and oxidative stress cause upregulation of the p75 neurotrophin receptor (p75NTR) in brain neurons, where it promotes apoptosis, however the mechanism by which p75NTR is regulated under these conditions is not well understood. Proinflammatory cytokines such as interleukin-1β (IL-1β) are highly produced under these injury conditions and, in particular, are expressed rapidly in the rat hippocampus after seizure. IL-1β is known to increase neuronal vulnerability under many conditions, although it does not directly induce neuronal death. Recently, we have shown that these cytokines regulate p75NTR induction both in neurons and astrocytes in vitro. Here, we show that IL-1β infusion into the brain induces p75NTR in neurons of the CA1 area of the hippocampus. While IL-1β induction of p75NTR is not sufficient to induce cell death, we demonstrate that IL-1β primes the neurons by recruiting p75NTR and its coreceptor sortilin to the cell surface, making the neurons more vulnerable to subsequent challenge by proNGF. These results suggest a mechanism by which IL-1β exacerbates neuronal death following injury.
Keywords: interleukin-1, neurotrophin, brain injury, apoptosis, NGF, p75
IL-1β is a major proinflammatory cytokine released under conditions of injury, infection, or disease, and is known to be involved in diverse actions in the central nervous system. Although many studies have shown that IL-1β alone does not induce neuronal death either in vitro (Thornton et al., 2006) or in vivo (Lawrence et al., 1998), it has synergistic effects on neuronal damage when provided with other cytokines (Chao et al., 1995, Hu et al., 1997). Moreover, release of IL-1β exacerbates traumatic, ischemic or excitotoxic stimulated neurotoxicity (Yamasaki et al., 1995, Patel et al., 2003), and blocking IL-1β with the receptor antagonist (IL-1ra) attenuates neuronal loss (Allan et al., 2005), suggesting that IL-1β indirectly contributes to neuronal injury.
Our lab has recently reported that proinflammatory cytokines such as IL-1β and TNFα regulate expression of the p75 neurotrophin receptor (p75NTR) both in neurons and astrocytes in vitro (Choi and Friedman, 2009). The p75NTR has diverse roles in regulating neuronal survival, death and axonal growth (Greene and Rukenstein, 1981, Rabizadeh et al., 1993, Frade et al., 1996, Maggirwar et al., 1998, Friedman, 2000). This multifunctional receptor is abundantly expressed in the brain during development, however its expression is limited in the adult brain (Yan and Johnson, 1988). p75NTR is upregulated following many types of brain injury such as traumatic brain injury, seizure, ischemia, oxidative stress and axonal injury (Kokaia et al., 1998, Roux et al., 1999, Casha et al., 2001, Ramos et al., 2007) as well as in CNS neurodegenerative diseases such as Alzheimer’s disease (Hu et al., 2002). The upregulated p75NTR in these pathological conditions has been suggested to be directly involved in neurodegeneration. p75NTR is highly expressed in the hippocampus after pilocarpine-induced seizure (Roux et al., 1999) and induces neuronal cell death by activating the intrinsic caspase cascade (Troy et al., 2002). Furthermore, the unprocessed NGF precursor, proNGF, which is a potent ligand for p75NTR, is also released after injury and induces neuronal apoptosis (Beattie et al., 2002, Volosin et al., 2008).
IL-1β is also known to regulate NGF mRNA expression (Spranger et al., 1990, Friedman et al., 1991), although the form of the NGF protein that is produced has not been identified. Therefore, IL-1β may be involved in neurodegeneration by regulating the p75NTR as well as ligands that promote neuronal death.
In this study, IL-1β infusion into brain increased p75NTR expression but did not induce cell death in vivo. We therefore investigated the functional role of p75NTR upregulation after IL-1β treatment and show that IL-1β specifically exacerbated proNGF induced hippocampal neuronal death by recruiting the p75NTR and sortilin receptors to the cell surface. This study, therefore, identifies a mechanism by which IL-1β may enhance neuronal vulnerability following brain injury.
Experimental procedures
Materials
Human recombinant IL-1β was a gift from Dr. Ron Hart, (Rutgers University, Piscataway, NJ) and human NGF was provided by Genentech, Inc. (South San Francisco). Furin-resistant proNGF was generously provided by Dr. Barbara Hempstead. Eagle’s MEM, Ham’s F12, and penicillin-streptomycin were purchased from Invitrogen (Carlsbad, CA). All other materials were obtained from Sigma (St.Louis, MO).
Stereotaxic cannulation of the hippocampus
Male Sprague Dawley rats (250–275g) were anaesthetized with ketamine (50mg/kg)/xylazine (10mg/kg) and placed in a stereotaxic frame for bilateral implantation of cannula into dorsal hippocampus. The following coordinates were used: anterior-posterior = −3.1mm from bregma, lateral = ±2 mm from midline, dorsoventral = −3mm from skull (Paxinos et al., 1985). Skull holes were made with a dental drill and the guide cannula and support screw were fixed with dental cement. After 7 days, 10ng (in 0.5μl) of IL-1β was infused unilaterally into the hippocampus via the guide cannula at a rate of 0.5μl/min. Animals found to have an incorrectly placed cannula were excluded.
All animal studies were conducted using the NIH (National Institutes of Health) guidelines for the ethical treatment of animals with approval of the Rutgers Institutional Animal Care and Facilities Committee.
Immunocytochemistry
Two days after IL-1β infusion, rats were anesthetized with ketamine/xylazine and perfused transcardially with saline followed by 4% paraformaldehyde. The brains were removed and postfixed in 4% paraformaldehyde before being cryoprotected in 30% sucrose for 2 d. Brains were then sectioned on a cryostat (Leica), and mounted onto charged slides for immunostaining. Frozen brain sections (12 μm) were warmed at 37°C for 1 min, washed with PBS, blocked in 10% goat serum with PBS plus 0.3% Triton X-100, and then incubated with anti-p75NTR (Upstate; 1:500) and NeuN (Chemicon; 1:500) overnight at 4°C. The sections were then washed three times with PBS for 15 min, followed by incubation with the secondary antibodies, Fluor 488-conjugated donkey anti-rabbit and texas red-conjugated goat anti-mouse (Jackson; 1:500) at room temp in the dark for 1 h, washed three times with PBS for 15 min. Hoechst 33342 dye (1 μg/ml; Sigma) was added into PBS during the last wash to label nuclei. Cell death was examined by terminal deoxynucleotidyl transferase (TdT)-mediated dUTP nick end labeling (TUNEL) staining following manufacturer’s manual (Roche, Mannheim, Germany). Sections were mounted with anti-fading medium (ProLong Gold; Invitrogen) and were examined by fluorescence microscopy (Nikon). Images were obtained using MetaMorph and figures were assembled in Adobe Photoshop.
Analysis of Cerebrospinal fluid (CSF)
Rats were anesthetized with ketamine/xylazine and placed in a stereotaxic frame for collecting CSF from cisterna magna using a 25 gauge needle. Only CSF samples that did not contain blood contamination were mixed with protease inhibitors, flash frozen, and stored at −80°C until analysis.
Western blot analysis
Cells were lysed in RIPA buffer (50mM Tris-HCl, pH7.5 150mM NaCl, 5mM EDTA, 1% Nonidet P-40, 0.5% deoxycholic acid, 0.5% SDS) supplemented with a protease inhibitor mixture (Roche Products, Welwyn Garden City, UK), 1mM sodium vanadate, and 5mM sodium fluoride. Proteins were quantified by Bradford assay (Bio-Rad, Hercules, CA), equal amounts of proteins were run on 10 % polyacrylamide gel, and transferred to a nitrocellulose membrane. Membranes were blocked in 5% non-fat milk in TBST and then probed with antibodies to p75NTR (Upstate Biotechnology, Inc., Lake Placid, NY), and actin (Sigma, St. Louis, MO), NGF (Sigma). Bands were visualized by enhanced chemical luminescence (Pierce, Rockford, IL).
Quantitative real-time reverse transcription PCR
Dorsal hippocampus was freshly homogenized, mRNA and proteins were isolated using TRIZOL reagent (Invitrogen, Carlsbad, CA). cDNA was generated using SuperScript™ II Reverse Transcriptase with random hexamers (Invitrogen), and SYBR-green based quantitative real-time PCR was performed using primers specific for p75NTR (rat, forward: 5′-CTGATGCTGAATGCGAAGAG-3′, reverse: 5′-TCACCATATCCGCCACTGTA-3′), NGF (rat, forward: 5′-CAAGGACGCAGCTTTCTATCCTG-3′, reverse: 5′-CTTCAGGGACAGAGTCTCCCTCT-3′), or actin (forward:5′-TCATGAAGTGTGACGTTGACATCCGT-3′, reverse:5′-CTTAGAAGCATTTGCGGTG CACGATG-3′) with the comparative CT method (ΔΔCT)(ABI).
Neuronal cultures
Hippocampal neuronal cultures were prepared as described previously (Farinelli et al., 1998, Friedman, 2000). Rat hippocampi were dissected from embryonic day 18, dissociated, plated on poly-D-lysine (0.1mg/ml)-coated dishes, and maintained in a serum-free environment. The medium consisted of a 1:1 mixture of Eagle’s MEM and Ham’s F12 supplemented with glucose (6mg/ml), insulin (25μg/ml), putrescine (60μM), progesterone (20nM), transferrin (100μg/ml), selenium (30nM), penicillin (0.5U/ml), and streptomycin (0.5μg/ml). Cultures were maintained in 5% CO2 at 37°C for 5 days and subjected to IL-1β treatment for the times indicated.
Survival assay
Survival of cultured hippocampal neurons was assayed by a method we have described previously (Farinelli et al., 1998; Maroney et al., 1999; Friedman, 2000). After removal of the medium, cultured cells were lysed, and intact nuclei were counted using a hemocytometer. Nuclei of dead cells either disintegrate or, if in the process of dying, appear pyknotic and irregularly shaped. In contrast, nuclei of healthy cells are phase bright and have clearly defined limiting membranes. Cell counts were performed in triplicate wells.
Biotinylation of cell surface proteins
Cells were treated with IL-1β for 6 h and washed with pre-chilled PBS once and with PBS++ (PBS containing 1mM MgCl2 and 2.5 mM CaCl2) twice. Cell surface proteins were biotinylated with sulfo-NHS-SS-Biotin (Pierce) at 4°C for 1 h, quenched with glycine, and washed with PBS++ twice. Biotinylated cells were lysed in buffer containing 50mM Tris, 150mM NaCl, 1mM EDTA, 1% Nonidet P40, 0.5% deoxycholate, protease inhibitor mixture, 1mM sodium vanadate and 5mM sodium fluoride, and lysates were incubated with streptavidin-agarose (Pierce) overnight at 4°C. After centrifugation (4500g for 3min at 4°C), supernatants were saved and pellets were washed with lysis buffer three times. Pellets (biotinylated surface protein) and supernatants (intracellular pool) were analyzed by Western blot for p75NTR (Upstate), sortilin (BD), and transferrin receptor (Invitrogen).
In Situ Zymography
One day following IL-1β infusion, rats were anesthetized with ketamine/xylazine, and their brains were flash frozen, sectioned on a cryostat (Leica), and mounted onto charged slides for in situ zymography. Fresh frozen sections (12μm) were covered with a buffer containing 1% low-melting point agarose (BioRad, Hercules, CA), 0.1 M Tris, pH 7.5, 2.5% milk and 20 μg/mL plasminogen. Areas of tPA activity were detected as black spots on dark field of views, where the endogenous enzyme degraded the substrate. Quantification is expressed as the surface area of black spots, indicating enzymatic activity, relative to the total surface area of the hippocampus.
Results
IL-1β increases p75NTR in vivo
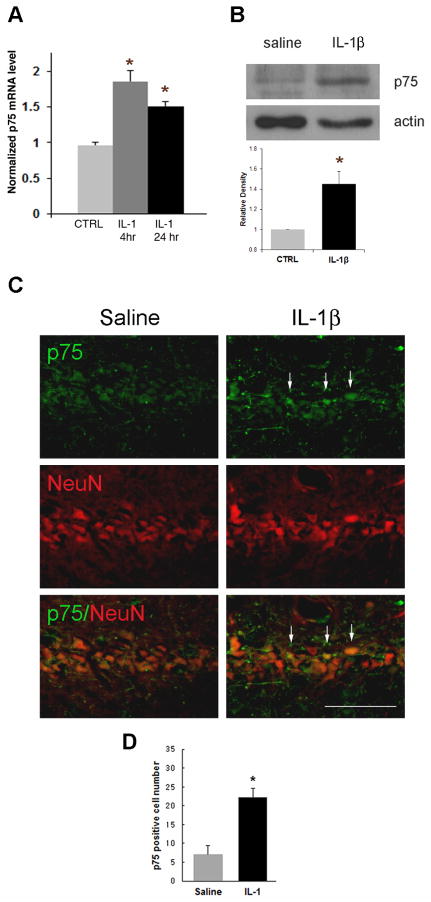
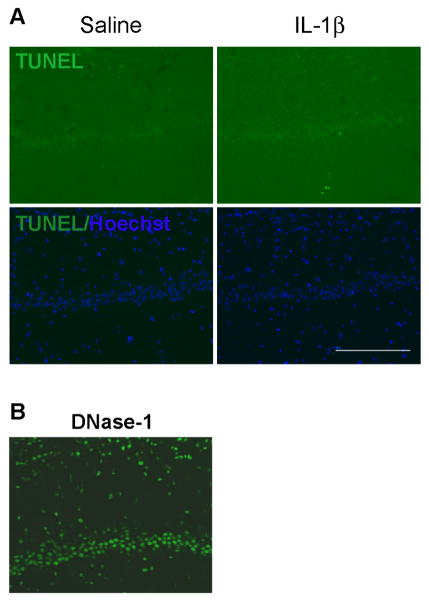
To investigate whether IL-1β induces p75NTR expression in vivo, IL-1β (10ng) was directly infused into one hippocampal hemisphere through a cannula. As a control, saline was infused into the contralateral hippocampus. p75NTR mRNA was increased in the hippocampus that had been infused with IL-1β compared to the contralateral side with saline infusion (Fig. 1A). Western blot analysis of tissue samples taken from dorsal hippocampus shows that p75NTR protein was also elevated by IL-1β (Fig. 1B), and cell counts indicate that the number of cells expressing p75NTR was increased by IL-1β infusion (figure 1D). Since IL-1β can affect multiple cell types, sections were double labeled with anti-p75NTR and a neuronal marker, NeuN to examine where p75NTR was regulated following IL-1β infusion. In addition to non-neuronal cells, p75NTR expression was found in hippocampal neurons (Fig. 1C), consistent with our previous in vitro data (Choi and Friedman, 2009). In light of previous work showing cell death mediated by increased p75NTR expression, we examined whether IL-1β induced cell death as a result of p75NTR induction. Neither saline nor IL-1β infusion induced TUNEL positive cells (Fig. 2), which is consistent with many studies demonstrating that IL-1β alone does not induce neuronal cell death (Lawrence et al., 1998). The positive control of DNase I treatment showed that the assay would detect apoptotic neurons (figure 2B). Since IL-1β infusion induced neuronal expression of p75NTR but no apoptosis, these findings suggest that p75NTR induction alone is not sufficient to mediate cell death.
Figure 1. Unilateral IL-1β infusion increases p75NTR expression in vivo.
Rats were cannulated 7 days before infusion with IL-1β (10ng). A. p75NTR mRNA was induced by IL-1β. Tissue was taken after 4 hr or 1d treatment with IL-1β (mean±SEM, n = 3). Asterisk denotes difference from saline (p<0.05). B. 2 days after the infusion, each hippocampus was taken for Western blot assay. p75NTR expression was increased with IL-1β infusion. Quantification of blots from three different experiments and densitometric values were normalized to actin and are expressed relative to the saline control (CTRL). Error bars represent SEM. * P<0.05 relative to CTRL, two-tailed t test. C. 2 days after the IL-1β infusion, brains were perfused, sectioned through the hippocampus, immunostained with anti-p75NTR (green) and anti-NeuN (red). IL-1β infusion increased p75NTR expression (arrows) in the CA1 region of the hippocampus (right column) compared to saline infusion (left column). Scale bars = 50μm. n=6. D. Counts of p75NTR-positive cells indicated a 3-fold increase following IL-1β infusion.
Figure 2. IL-1β is not sufficient to mediate cell death in vivo.
A. TUNEL staining showed negative labeling with both saline (left column) and IL-1β (right column) infusion, indicating that IL-1β itself does not directly elicit cell death. Double staining for TUNEL (green) and Hoechst (blue). Images are representative of two independent experiments. B. Positive control TUNEL assay with DNase-I. Scale bars = 50μm.
IL-1β releases NGF into the CSF
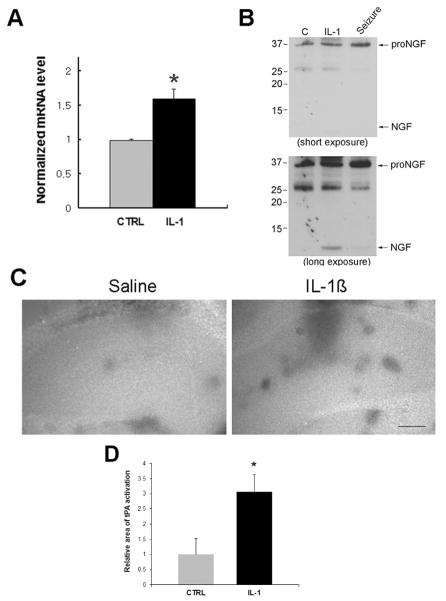
IL-1β is also known to regulate NGF expression in the brain (Spranger et al., 1990, Yasuda et al., 2007), however those studies did not investigate which form of the NGF protein was secreted. We confirmed the in vivo regulation of NGF mRNA by IL-1β in tissue samples taken from the dorsal hippocampus after IL-1β or saline infusion analyzed by quantitative RT-PCR. Consistent with previous studies, IL-1β increased NGF mRNA levels compared to saline (Fig 3A). Since proNGF has been shown to be involved in cell death as a ligand for p75NTR following injury (Beattie et al., 2002, Volosin et al., 2008), we examined which forms of NGF were secreted following infusion of IL-1β compared to untreated animals and to rats following pilocarpine-induced seizures. The cerebrospinal fluid (CSF) was collected and analyzed by Western blot. The CSF sample taken from IL-1β infused rats showed no change in proNGF and increased levels of mature NGF compared to the CSF samples from control rats (Fig. 3B), while the seizure rats showed increased proNGF without cleavage to mature NGF in the CSF. Basal levels of proNGF were detected in control and IL-1β-infused rats, suggesting that IL-1β, in the absence of injury, increased NGF expression and the subsequent increased secretion of mature NGF, but not proNGF which is induced by seizures (Volosin et al., 2008) and other types of injury (Beattie et al., 2002).
Figure 3. IL-1β induces increased secretion of mature NGF.
A. Bars indicate NGF mRNA level (mean±SEM, n = 3) by qPCR after 4 h treatment with IL-1β. Asterisk denotes difference from saline (p<0.05). B. Western blots show cerebrospinal fluid (CSF) collected from IL-1β infused rats compared to saline-infused controls or rats after pilocarpine-induced seizures. A short exposure shows the seizure-induced increase in proNGF without being cleaved, while the longer exposure shows the IL-1β-induced increase in mature NGF in the CSF. Blot is representative of three independent experiments. C. In situ zymogram shows increased tPA activity with IL-1β infusion. Fresh frozen sections were covered with an in situ zymogram assay buffer containing 20 μg/mL plasminogen. Areas of tPA activity are indicated by black spots on a bright background. Images are representative of two independent experiments. Scale bars = 250μm. D. Quantification of the area showing tPA activity relative to the entire hippocampus in the control and IL-1β infused hippocampus. Asterisk indicates significantly different from control by Student’s t-test, p < 0.01.
We have recently demonstrated that enzymes such as MMP7 and tPA that process proNGF cleavage are reduced following seizures, leading to increased extracellular proNGF (Le and Friedman, 2012). To investigate whether IL-1β may alter proNGF processing enzyme activity to result in increased mature NGF rather than proNGF, in situ zymography for MMP7 and tPA was performed. In contrast to the seizure-induced injury, IL-1β infusion showed no change in MMP7 activity (not shown), and increased tPA activity compared to the saline infused side (Figure 3C), suggesting that IL-1β promotes tPA-mediated cleavage of proNGF to NGF. Taken together, these data suggest that IL-1β induces p75NTR and NGF expression, but does not prevent proNGF cleavage in vivo and therefore by itself is not sufficient to induce cell death.
IL-1β exacerbates proNGF mediated hippocampal neuron death
IL-1β increased the activity of tPA, a proNGF-cleaving enzyme, in vivo, preventing the ability to co-infuse IL-1β and proNGF into the brain. Therefore, we used cultured neurons to investigate the functional consequences of IL-1β-mediated p75NTR upregulation. Hippocampal neurons express the p75NTR and sortillin receptors (figure 4A), both of which are necessary for proNGF-induced apoptosis {Volosin, 2006 #1137}. Neurons were pretreated with IL-1β for 4–6 hours to allow p75NTR induction, and then subjected to either NGF or proNGF treatment overnight. Both NGF (100ng/mL) and proNGF (1–10ng/mL) caused hippocampal neuronal death (Fig. 4), consistent with previous studies (Friedman, 2000, Volosin et al., 2008). Interestingly, whereas IL-1β did not affect NGF mediated cell death (Fig. 4B), IL-1β pretreatment exacerbated proNGF induced cell death (Fig. 4C), suggesting that this cytokine may make neurons selectively more vulnerable to proNGF.
Figure 4. IL-1β primed neurons are more vulnerable to proNGF than NGF.
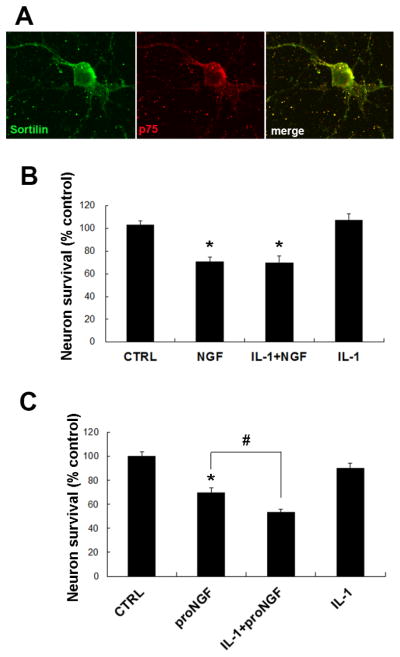
A. Immunostaining of hippocampal neurons for sortilin and p75NTR demonstrating colocalization of the receptors. B. Bars show relative cell number (mean±SEM, n = 4 independent experiments) in hippocampal neuronal cultures. Cells were treated for 4–6 h with IL-1β (10ng/mL), followed by overnight treatment with NGF (100ng/mL). IL-1β did not exacerbate NGF-mediated neuronal death. Asterisks denote difference from untreated control (p < 0.05). C. Bars indicate relative cell number (mean±SEM, n = 4 independent experiments) in hippocampal neuronal cultures treated with IL-1β (10 ng/mL) for 4–6 hrs, followed by proNGF (1–10ng/mL). ProNGF elicited more cell death in IL-1β primed neurons. * denotes difference from control and # indicates difference from proNGF alone (p<0.05; one-way ANOVA and Tukey’s post hoc analysis).
IL-1β recruits p75NTR and sortilin to the plasma membrane
Since sortilin is a required coreceptor for p75NTR in proNGF-mediated neuronal cell death (Nykjaer et al., 2004), we examined the level of sortilin and p75NTR at the cell surface following IL-1β treatment. Biotinylation assays showed increased sortilin and p75NTR on the cell surface in IL-1β primed neurons (Fig. 5), while transferrin receptor levels were unchanged by IL-1β treatment. These results suggest that IL-1β elicits selective recruitment of sortilin and p75NTR to the plasma membrane, which increases vulnerability to proNGF treatment.
Figure 5. IL-1β recruits sortilin and p75NTR receptors to the plasma membrane.
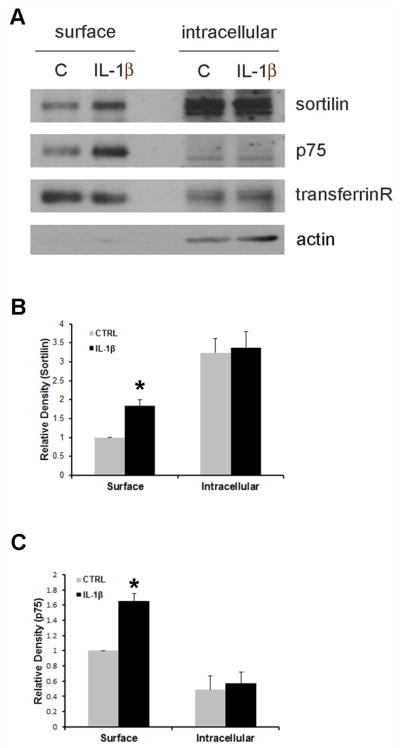
A. Cultured hippocampal neurons were treated with IL-1β for 8h, incubated with biotin for 1h, and lysates were precipitated with streptavidin. Total biotinylated cell surface protein and 20μg of non-biotinylated intracellular proteins were analyzed by Western blot for sortilin, p75NTR and transferrin receptor. Blots were stripped and re-probed for actin, which was only present in the intracellular fraction. Quantification of sortilin (B) and p75NTR (C) from three independent blots. Asterisk denotes difference from control (p<0.05; one-way ANOVA and Tukey’s post hoc analysis).
Discussion
Many changes occur in the brain following injury or in neurodegenerative disease that contribute to neuronal loss, including the induction of proinflammatory cytokines. In this study we have specifically investigated the effects of IL-1β in the absence of the numerous other changes associated with brain injury. Recently, our lab has demonstrated that IL-1β increased the expression of p75NTR in hippocampal neurons in culture, and our previous studies demonstrated that induction of p75NTR in hippocampal neurons contributes to neuronal loss following seizure-induced injury (Troy et al., 2002). Moreover, seizures caused an alteration in the activity of the enzymes that regulate proNGF cleavage, leading to the stabilization of proNGF in the extracellular environment, which was crucial for neuronal death to occur (Le and Friedman, 2012). Additional studies have demonstrated that p75NTR induced after many types of injury or in pathological disease may play a role in neuronal degeneration (Armstrong et al., 1991, Syroid et al., 2000, Casha et al., 2001, Beattie et al., 2002, Yan and Feng, 2004), however the underlying mechanisms by which p75NTR is regulated have not been extensively studied. Since the induction of proinflammatory cytokines, specifically IL-1β, is a key feature of the injury response, we investigated whether this cytokine was sufficient to mediate the changes in the brain that lead to neuronal loss. In the present study, we have shown that IL-1β infusion directly into the hippocampus in vivo in the absence of injury induced p75NTR expression but failed to mediate cell death, leading to an investigation of the functional role of p75NTR after elevation by IL-1β. We found that IL-1β recruited both p75NTR and sortilin to the plasma membrane, making the neurons more vulnerable to cell death upon proNGF stimulation.
IL-1β increases p75NTR and NGF in vivo
The induction of proinflammatory cytokines following injury is responsible for various cellular functions such as inflammation, the production of other cytokines, growth factors, and neurotrophins. The current study has shown increased levels of p75NTR following direct infusion of IL-1β into the hippocampus without injury, suggesting that increased p75NTR in response to seizure or in disease may be due to a direct effect of proinflammatory cytokines that are elevated. We also found that IL-1β itself was not sufficient to mediate cell death in vivo even with the elevated p75NTR expression, suggesting that p75NTR induction itself is insufficient to induce cell death. IL-1β has been shown to induce NGF mRNA in the brain, but the form of the NGF protein induced had not previously been determined. We confirmed the increase in NGF mRNA, and demonstrated that increased mature NGF, but not proNGF, was secreted following IL-1β treatment. Moreover, IL-1β alone was not sufficient to elicit the changes in the activity of proNGF cleaving enzymes seen following seizure that are necessary to stabilize proNGF in the extracellular environment (Le and Friedman, 2012). We had previously demonstrated that seizures caused a decrease in MMP7 activity, leading to decreased proNGF processing. In contrast, IL-1β alone evoked no change in MMP7 activity, and actually increased tPA activity, which can also cleave proNGF (Lee et al., 2007). Since IL-1β alone, in the absence of injury, did not inhibit the activity of the extracellular proNGF cleaving enzymes, proNGF could not be administered to the brain in vivo to assess the consequences of IL-1β-mediated p75NTR induction, therefore this analysis was performed on cultured hippocampal neurons.
IL-1β exacerbates proNGF-mediated hippocampal neuron death
IL-1β is known to exacerbate neuronal death after injury, therefore we investigated whether the increase in p75NTR expression might contribute to enhanced cell death in IL-1β primed neurons. Interestingly, priming the neurons with IL-1β increased vulnerability of hippocampal neurons to proNGF, but not NGF.
In order for proNGF to activate p75NTR mediated cell death, sortilin is required as a coreceptor together with p75NTR (Nykjaer et al., 2004). In this study, we showed that IL-1β recruits both sortilin and p75NTR to the cell surface. Sortilin is mostly present in the cytosol (Sarret et al., 2003), and IL-1β induction of sortilin trafficking to the plasma membrane may be a crucial factor for proNGF to cause the p75NTR-mediated cell death observed following injury.
Consequences of p75NTR induction following IL-1β treatment
We confirmed that direct infusion of IL-1β into the hippocampus of the rat brain increased NGF mRNA levels, consistent with previous studies (Spranger et al., 1990). Although many studies have shown that IL-1β elicits induction of NGF mRNA, those studies did not clarify the forms of NGF protein that were produced. Here, we have shown that IL-1β caused and increase in the release of mature NGF, not proNGF, in contrast to what we have observed after seizures and what has been detected in disease. Our previous studies showed that seizure-induced injury caused reduced activity of the proNGF processing enzymes (Le and Friedman, 2012). In contrast, IL-1β increased tPA activity, which activates plasmin, in turn enhancing proNGF cleavage. Since injury induces many changes in the brain in addition to the induction of cytokines, it appears that IL-1β alone is sufficient to induce p75NTR expression, but not the alteration in proNGF processing enzymes required to stabilize proNGF in the extracellular environment. In fact, IL-1β elevated activity of tPA, resulting in the increase of mature NGF, not proNGF, in the extracellular environment. It is likely that the presence of NGF rather than proNGF fails to elicit p75NTR mediated cell death, such as occurs in the hippocampus after injury or in AD. Although this study demonstrated that IL-1β is involved in p75NTR regulation that can occur following injury or in disease, the elevated p75NTR by IL-1β was not sufficient to induce cell death in the uninjured brain. These results indicate that additional mechanisms are likely to be involved in regulating proNGF processing enzymes to stabilize proNGF in injury or disease conditions. Moreover, it has been reported that proNGF isolated from Alzheimer’s patients is highly glycosylated and more stable than proNGF from normal individuals (Pedraza et al., 2005), suggesting possible additional modification mechanisms for proNGF.
Many studies have demonstrated that IL-1β alone does not initiate damage in healthy cells, although IL-1β is known to exacerbate neuronal death with other cytokines (Chao et al., 1995, Hu et al., 1997) or after injury (Yamasaki et al., 1995, Lawrence et al., 1998). However, the mechanisms by which IL-1β contributes to neuronal degeneration have been unknown. Here, we demonstrate that IL-1β may sensitize neurons and make them more vulnerable to proNGF mediated cell death by increasing the availability of the p75NTR/sortilin complex at the plasma membrane. Specifically, recruiting sortilin to cell surface favors proNGF mediated p75NTR activation. Interestingly, this correlates with the environmental changes in AD where increased levels of IL-1β and proNGF have been reported (Griffin et al., 1989, Fahnestock et al., 2001). Thus, IL-1β may serve as a key switch altering the ratio of receptors in the cell surface following injury or in disease; thereby supporting proNGF mediated signaling.
Conclusions
IL-1β is a critical inflammatory cytokine induced in the brain that exacerbates neuronal loss following injury and in disease. Our study suggests that a mechanism by which IL-1β can enhance neuronal vulnerability is by increasing the surface levels of p75NTR and sortilin, thereby creating a cellular environment more vulnerable to proNGF-induced neuronal death following injury or in neurodegenerative disease.
Highlights.
IL-1β induces p75NTR expression in hippocampal neurons in vivo
IL-1β increases tPA activity in vivo
IL-1β promotes secretion of NGF, not proNGF, in vivo
IL-1β enhances surface localization of p75NTR and sortilin in hippocampal neurons
IL-1β increases vulnerability without directly inducing neuronal death
Acknowledgments
This work was supported by NIH Grant NS045556 and the New Jersey Commission for Brain Injury Research Grant 08-3211. The authors would like to thank Barbara Hempstead for generously providing the proNGF, Ron Hart for IL-1β, and Steven Tobia for technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allan SM, Tyrrell PJ, Rothwell NJ. Interleukin-1 and neuronal injury. Nat Rev Immunol. 2005;5:629–640. doi: 10.1038/nri1664. [DOI] [PubMed] [Google Scholar]
- Armstrong DM, Brady R, Hersh LB, Hayes RC, Wiley RG. Expression of choline acetyltransferase and nerve growth factor receptor within hypoglossal motoneurons following nerve injury. The Journal of comparative neurology. 1991;304:596–607. doi: 10.1002/cne.903040407. [DOI] [PubMed] [Google Scholar]
- Beattie MS, Harrington AW, Lee R, Kim JY, Boyce SL, Longo FM, Bresnahan JC, Hempstead BL, Yoon SO. ProNGF Induces p75-Mediated Death of Oligodendrocytes following Spinal Cord Injury. Neuron. 2002;36:375–386. doi: 10.1016/s0896-6273(02)01005-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casha S, Yu WR, Fehlings MG. Oligodendroglial apoptosis occurs along degenerating axons and is associated with FAS and p75 expression following spinal cord injury in the rat. Neuroscience. 2001;103:203–218. doi: 10.1016/s0306-4522(00)00538-8. [DOI] [PubMed] [Google Scholar]
- Chao CC, Hu S, Ehrlich L, Peterson PK. Interleukin-1 and tumor necrosis factor-alpha synergistically mediate neurotoxicity: involvement of nitric oxide and of N-methyl-D-aspartate receptors. Brain Behav Immun. 1995;9:355–365. doi: 10.1006/brbi.1995.1033. [DOI] [PubMed] [Google Scholar]
- Choi S, Friedman WJ. Inflammatory cytokines IL-1beta and TNF-alpha regulate p75NTR expression in CNS neurons and astrocytes by distinct cell-type-specific signalling mechanisms. ASN Neuro1. 2009;2009(2):e00010. doi: 10.1042/AN20090009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson TJ, Harel S, Arboleda VA, Prunell GF, Shelanski ML, Greene LA, Troy CM. Highly efficient small interfering RNA delivery to primary mammalian neurons induces MicroRNA-like effects before mRNA degradation. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2004;24:10040–10046. doi: 10.1523/JNEUROSCI.3643-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahnestock M, Michalski B, Xu B, Coughlin MD. The precursor pro-nerve growth factor is the predominant form of nerve growth factor in brain and is increased in Alzheimer’s disease. Molecular and cellular neurosciences. 2001;18:210–220. doi: 10.1006/mcne.2001.1016. [DOI] [PubMed] [Google Scholar]
- Farinelli SE, Greene LA, Friedman WJ. Neuroprotective actions of dipyridamole on cultured CNS neurons. J Neurosci. 1998;18:5112–5123. doi: 10.1523/JNEUROSCI.18-14-05112.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frade JM, Rodriguez-Tebar A, Barde YA. Induction of cell death by endogenous nerve growth factor through its p75 receptor. Nature. 1996;383:166–168. doi: 10.1038/383166a0. [DOI] [PubMed] [Google Scholar]
- Friedman WJ. Neurotrophins induce death of hippocampal neurons via the p75 receptor. J Neurosci. 2000;20:6340–6346. doi: 10.1523/JNEUROSCI.20-17-06340.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman WJ, Olson L, Persson H. Temporal and spatial expression of NGF receptor mRNA during postnatal rat brain development analyzed by in situ hybridization. Brain research Developmental brain research. 1991;63:43–51. doi: 10.1016/0165-3806(91)90065-q. [DOI] [PubMed] [Google Scholar]
- Greene LA, Rukenstein A. Regulation of acetylcholinesterase activity by nerve growth factor. Role of transcription and dissociation from effects on proliferation and neurite outgrowth. J Biol Chem. 1981;256:6363–6367. [PubMed] [Google Scholar]
- Griffin WS, Stanley LC, Ling C, White L, MacLeod V, Perrot LJ, White CL, 3rd, Araoz C. Brain interleukin 1 and S-100 immunoreactivity are elevated in Down syndrome and Alzheimer disease. Proc Natl Acad Sci U S A. 1989;86:7611–7615. doi: 10.1073/pnas.86.19.7611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higuchi H, Yamashita T, Yoshikawa H, Tohyama M. PKA phosphorylates the p75 receptor and regulates its localization to lipid rafts. The EMBO journal. 2003;22:1790–1800. doi: 10.1093/emboj/cdg177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu S, Peterson PK, Chao CC. Cytokine-mediated neuronal apoptosis. Neurochem Int. 1997;30:427–431. doi: 10.1016/s0197-0186(96)00078-2. [DOI] [PubMed] [Google Scholar]
- Hu XY, Zhang HY, Qin S, Xu H, Swaab DF, Zhou JN. Increased p75(NTR) expression in hippocampal neurons containing hyperphosphorylated tau in Alzheimer patients. Exp Neurol. 2002;178:104–111. doi: 10.1006/exnr.2002.8018. [DOI] [PubMed] [Google Scholar]
- Kanning KC, Hudson M, Amieux PS, Wiley JC, Bothwell M, Schecterson LC. Proteolytic processing of the p75 neurotrophin receptor and two homologs generates C-terminal fragments with signaling capability. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2003;23:5425–5436. doi: 10.1523/JNEUROSCI.23-13-05425.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim T, Hempstead BL. NRH2 is a trafficking switch to regulate sortilin localization and permit proneurotrophin-induced cell death. The EMBO journal. 2009;28:1612–1623. doi: 10.1038/emboj.2009.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokaia Z, Andsberg G, Martinez-Serrano A, Lindvall O. Focal cerebral ischemia in rats induces expression of P75 neurotrophin receptor in resistant striatal cholinergic neurons. Neuroscience. 1998;84:1113–1125. doi: 10.1016/s0306-4522(97)00579-4. [DOI] [PubMed] [Google Scholar]
- Lawrence CB, Allan SM, Rothwell NJ. Interleukin-1beta and the interleukin-1 receptor antagonist act in the striatum to modify excitotoxic brain damage in the rat. Eur J Neurosci. 1998;10:1188–1195. doi: 10.1046/j.1460-9568.1998.00136.x. [DOI] [PubMed] [Google Scholar]
- Le AP, Friedman WJ. Matrix metalloproteinase-7 regulates cleavage of pro-nerve growth factor and is neuroprotective following kainic acid-induced seizures. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2012;32:703–712. doi: 10.1523/JNEUROSCI.4128-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JY, Kweon HS, Cho E, Byun HR, Kim DH, Kim YH, Han PL, Koh JY. Upregulation of tPA/plasminogen proteolytic system in the periphery of amyloid deposits in the Tg2576 mouse model of Alzheimer’s disease. Neurosci Lett. 2007;423:82–87. doi: 10.1016/j.neulet.2007.06.037. [DOI] [PubMed] [Google Scholar]
- Maggirwar SB, Sarmiere PD, Dewhurst S, Freeman RS. Nerve growth factor-dependent activation of NF-kappaB contributes to survival of sympathetic neurons. J Neurosci. 1998;18:10356–10365. doi: 10.1523/JNEUROSCI.18-24-10356.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray SS, Perez P, Lee R, Hempstead BL, Chao MV. A novel p75 neurotrophin receptor-related protein, NRH2, regulates nerve growth factor binding to the TrkA receptor. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2004;24:2742–2749. doi: 10.1523/JNEUROSCI.3960-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nykjaer A, Lee R, Teng KK, Jansen P, Madsen P, Nielsen MS, Jacobsen C, Kliemannel M, Schwarz E, Willnow TE, Hempstead BL, Petersen CM. Sortilin is essential for proNGF-induced neuronal cell death. Nature. 2004;427:843–848. doi: 10.1038/nature02319. [DOI] [PubMed] [Google Scholar]
- Patel HC, Boutin H, Allan SM. Interleukin-1 in the brain: mechanisms of action in acute neurodegeneration. Ann N Y Acad Sci. 2003;992:39–47. doi: 10.1111/j.1749-6632.2003.tb03136.x. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C, Pennisi M, Topple A. Bregma, lambda and the interaural midpoint in stereotaxic surgery with rats of different sex, strain and weight. J Neurosci Methods. 1985;13:139–143. doi: 10.1016/0165-0270(85)90026-3. [DOI] [PubMed] [Google Scholar]
- Pedraza CE, Podlesniy P, Vidal N, Arevalo JC, Lee R, Hempstead B, Ferrer I, Iglesias M, Espinet C. Pro-NGF isolated from the human brain affected by Alzheimer’s disease induces neuronal apoptosis mediated by p75NTR. The American journal of pathology. 2005;166:533–543. doi: 10.1016/S0002-9440(10)62275-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabizadeh S, Oh J, Zhong LT, Yang J, Bitler CM, Butcher LL, Bredesen DE. Induction of apoptosis by the low-affinity NGF receptor. Science. 1993;261:345–348. doi: 10.1126/science.8332899. [DOI] [PubMed] [Google Scholar]
- Ramos A, Ho WC, Forte S, Dickson K, Boutilier J, Favell K, Barker PA. Hypo-osmolar stress induces p75NTR expression by activating Sp1-dependent transcription. J Neurosci. 2007;27:1498–1506. doi: 10.1523/JNEUROSCI.4806-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roux PP, Colicos MA, Barker PA, Kennedy TE. p75 neurotrophin receptor expression is induced in apoptotic neurons after seizure. J Neurosci. 1999;19:6887–6896. doi: 10.1523/JNEUROSCI.19-16-06887.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarret P, Krzywkowski P, Segal L, Nielsen MS, Petersen CM, Mazella J, Stroh T, Beaudet A. Distribution of NTS3 receptor/sortilin mRNA and protein in the rat central nervous system. J Comp Neurol. 2003;461:483–505. doi: 10.1002/cne.10708. [DOI] [PubMed] [Google Scholar]
- Spranger M, Lindholm D, Bandtlow C, Heumann R, Gnahn H, Naher-Noe M, Thoenen H. Regulation of Nerve Growth Factor (NGF) Synthesis in the Rat Central Nervous System: Comparison between the Effects of Interleukin-1 and Various Growth Factors in Astrocyte Cultures and in vivo. The European journal of neuroscience. 1990;2:69–76. doi: 10.1111/j.1460-9568.1990.tb00382.x. [DOI] [PubMed] [Google Scholar]
- Syroid DE, Maycox PJ, Soilu-Hanninen M, Petratos S, Bucci T, Burrola P, Murray S, Cheema S, Lee KF, Lemke G, Kilpatrick TJ. Induction of postnatal schwann cell death by the low-affinity neurotrophin receptor in vitro and after axotomy. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2000;20:5741–5747. doi: 10.1523/JNEUROSCI.20-15-05741.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornton P, Pinteaux E, Gibson RM, Allan SM, Rothwell NJ. Interleukin-1-induced neurotoxicity is mediated by glia and requires caspase activation and free radical release. J Neurochem. 2006;98:258–266. doi: 10.1111/j.1471-4159.2006.03872.x. [DOI] [PubMed] [Google Scholar]
- Troy CM, Friedman JE, Friedman WJ. Mechanisms of p75-mediated death of hippocampal neurons. Role of caspases. J Biol Chem. 2002;277:34295–34302. doi: 10.1074/jbc.M205167200. [DOI] [PubMed] [Google Scholar]
- Volosin M, Trotter C, Cragnolini A, Kenchappa RS, Light M, Hempstead BL, Carter BD, Friedman WJ. Induction of proneurotrophins and activation of p75NTR-mediated apoptosis via neurotrophin receptor-interacting factor in hippocampal neurons after seizures. J Neurosci. 2008;28:9870–9879. doi: 10.1523/JNEUROSCI.2841-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamasaki Y, Matsuura N, Shozuhara H, Onodera H, Itoyama Y, Kogure K. Interleukin-1 as a pathogenetic mediator of ischemic brain damage in rats. Stroke. 1995;26:676–680. doi: 10.1161/01.str.26.4.676. discussion 681. [DOI] [PubMed] [Google Scholar]
- Yan Q, Johnson EM., Jr An immunohistochemical study of the nerve growth factor receptor in developing rats. J Neurosci. 1988;8:3481–3498. doi: 10.1523/JNEUROSCI.08-09-03481.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Z, Feng J. Alzheimer’s disease: interactions between cholinergic functions and beta-amyloid. Curr Alzheimer Res. 2004;1:241–248. doi: 10.2174/1567205043331992. [DOI] [PubMed] [Google Scholar]
- Yasuda K, Churchill L, Yasuda T, Blindheim K, Falter M, Krueger JM. Unilateral cortical application of interleukin-1beta (IL1beta) induces asymmetry in fos, IL1beta and nerve growth factor immunoreactivity: implications for sleep regulation. Brain Res. 2007;1131:44–59. doi: 10.1016/j.brainres.2006.11.051. [DOI] [PubMed] [Google Scholar]