Abstract
OBJECTIVES
A recent large-scale randomized controlled trial (RCT) demonstrated that rectal indomethacin administration is effective in addition to pancreatic stent placement (PSP) for preventing post-endoscopic retrograde cholangiopancreatography (ERCP) pancreatitis (PEP) in high-risk cases. We performed a post hoc analysis of this RCT to explore whether rectal indomethacin can replace PSP in the prevention of PEP and to estimate the potential cost savings of such an approach.
METHODS
We retrospectively classified RCT subjects into four prevention groups: (1) no prophylaxis, (2) PSP alone, (3) rectal indomethacin alone, and (4) the combination of PSP and indomethacin. Multivariable logistic regression was used to adjust for imbalances in the prevalence of risk factors for PEP between the groups. Based on these adjusted PEP rates, we conducted an economic analysis comparing the costs associated with PEP prevention strategies employing rectal indomethacin alone, PSP alone, or the combination of both.
RESULTS
After adjusting for risk using two different logistic regression models, rectal indomethacin alone appeared to be more effective for preventing PEP than no prophylaxis, PSP alone, and the combination of indomethacin and PSP. Economic analysis revealed that indomethacin alone was a cost-saving strategy in 96% of Monte Carlo trials. A prevention strategy employing rectal indomethacin alone could save approximately $150 million annually in the United States compared with a strategy of PSP alone, and $85 million compared with a strategy of indomethacin and PSP.
CONCLUSIONS
This hypothesis-generating study suggests that prophylactic rectal indomethacin could replace PSP in patients undergoing high-risk ERCP, potentially improving clinical outcomes and reducing healthcare costs. A RCT comparing rectal indomethacin alone vs. indomethacin plus PSP is needed.
INTRODUCTION
Preventing pancreatitis after endoscopic retrograde cholangiopancreatography (ERCP) remains an important clinical and research priority. Progress in the last two decades has led to substantive reductions in post-ERCP pancreatitis (PEP) rates due to more appropriate patient selection (1–3), improved procedural techniques (4–6), and the adoption of prophylactic pancreatic duct (PD) stent placement (PSP) in high-risk cases.
PSP, introduced in the late 1990s, has become common clinical practice in the United States and is widely regarded as an effective means of preventing PEP in high-risk cases (7,8). While PD stent placement clearly reduces risk (9,10), it remains technically challenging, time consuming, and costly (11–14). Moreover, attempting to place a PD stent with subsequent failure actually increases the risk of PEP above baseline by inducing injury to the pancreatic orifice (15,16).
Recently, our group published a randomized controlled trial (RCT) demonstrating that rectal indomethacin, a non-steroidal anti-inflammatory drug, reduced the risk of PEP in high-risk patients, most of whom (>80%) had undergone pancreatic stent placement (PSP) (17). While this RCT found that indomethacin confers protection in addition to PSP, there are no studies examining whether indomethacin is effective when administered instead of PSP.
If indomethacin were to obviate the need for PSP, major clinical and cost benefits in ERCP practice could be realized. Therefore, we performed a post hoc analysis of our recently reported indomethacin pharmacoprevention RCT comparing the risk-adjusted benefits conferred by indomethacin alone vs. prophylactic PSP alone, vs. the combination of both. We also performed a cost-benefit analysis of these three prevention strategies.
METHODS
Patient data
This is a post hoc analysis of prospectively collected data obtained during a previously reported multi-center placebo-controlled trial of rectal indomethacin for preventing PEP (17). Briefly, after approval by the institutional review committees of the involved institutions, patients at elevated risk for PEP were randomized to 100 mg of rectal indomethacin or placebo suppositories administered immediately after ERCP. All other procedural and clinical decisions were deferred to the treating physicians. Placement of a PD stent was not mandated, however >80% of subjects received a stent as the study enrolled patients at elevated risk for PEP.
The primary end point of the study was the development of PEP and the secondary end point was the severity of PEP, both defined according to standard consensus criteria (18). Patient demographics, risk factors for PEP, ERCP procedural elements, and follow-up data were recorded on standardized data collection forms by a member of the study team who was blinded to the treatment received. All data were subsequently entered into a web-based database and managed by an independent data management service. The final database contained information on 602 study subjects. The clinical and procedural characteristics collected for each subject are listed in online Supplementary Appendix A online.
Clinical comparison of prevention strategies
Eighteen percent of study subjects did not receive a prophylactic pancreatic stent as the endoscopist did not deem the case high-risk enough to merit PSP (e.g., difficult cannulation not requiring a precut sphincterotomy) or because placement was not technically feasible (failed pancreatic access). Fifty-one percent of subjects did not receive indomethacin as the randomization process assigned them to placebo. We, therefore, retrospectively classified study subjects into four groups: (1) no prophylaxis (randomized to placebo, PSP deferred by endoscopist), (2) PD stent alone (randomized to placebo), (3) rectal indomethacin alone (randomized to indomethacin, PSP deferred by endoscopist), and (4) the combination of PD stent and indomethacin. Groups 1 and 2 comprised the control arm of the RCT while groups 3 and 4 comprised the indomethacin arm.
Comparing unadjusted PEP rates among these four groups would have yielded biased conclusions about the effi cacy of the various interventions as these study groups differed significantly regarding their underlying risk for the primary outcome (PEP). Because the decision to place a PD stent was left to the discretion of the endoscopist, patients at higher risk for PEP were more likely to receive a stent, as they elicited more concern on the part of the endoscopist.
To correct this potential confounding, we adjusted for subjects’ underlying risk of PEP using two analytic approaches. In the first analysis, we included patient's ‘PEP risk score’ as a covariate in a logistic regression model with PEP (dichotomous) as the dependent variable and PD stent placement and indomethacin treatment as predictor variables. The risk score was calculated for each subject by assigning one point for each major RCT inclusion criterion and 0.5 points for each minor RCT inclusion criterion (17). This resulted in a continuous risk score ranging from 0.5 to 5.5. For example, a patient included in our study for a clinical suspicion of sphincter of Oddi dysfunction (major inclusion criterion) who experienced pancreatic acinarization (minor inclusion criterion) and underwent a pancreatic sphincterotomy (major inclusion criterion) had a risk score of 2.5. The criteria used to calculate the risk score are listed in Table 1.
Table 1.
Major and minor study inclusion criteria used to calculate post-ERCP pancreatitis risk score
| Major criteria | Minor criteria |
|---|---|
| Clinical suspicion of sphincter of Oddi dysfunction | Age < 50 years old and female gender |
| History of post-ERCP pancreatitis | History of recurrent pancreatitis |
| Pancreatic sphincterotomy | ≥3 Pancreatic injections, with at least one injection to tail |
| Pre-cut (access) sphincterotomy | Pancreatic acinarization |
| >8 Cannulation attempts | Pancreatic brush cytology |
| Pneumatic dilation of intact biliary sphincter | |
| Ampullectomy |
ERCP, endoscopic retrograde cholangiopancreatography.
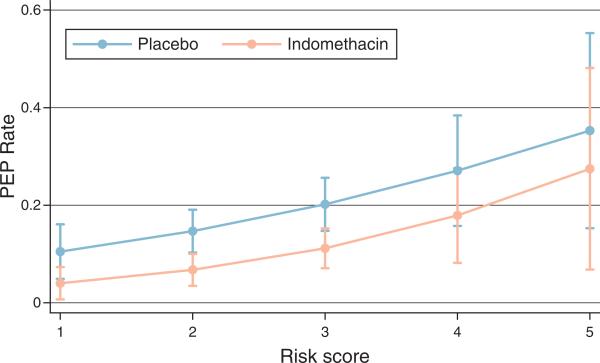
While an increasing risk score did predict a higher likelihood of PEP in our clinical study, this relationship did not appear linear, likely due to the synergistic nature of PEP risk factors (19) (Figure 1). Moreover, every major inclusion criterion contributed equally to our risk score, even though certain inclusion criteria are known to confer more risk for PEP than others. Thus, the PEP risk score may not have resulted in an adequate adjustment, and we, therefore, also attempted to control for risk using a second approach. In this second analysis, we calculated a propensity score for every subject's probability of receiving a pancreatic stent. This propensity score predicts a subject's conditional probability of receiving a PD stent based on all of the other observed characteristics of that subject, including the criteria used to generate the risk score and other factors such as age, gender, biliary interventions, study site, and trainee involvement in the ERCP. As patients at higher risk for PEP were more likely to receive a stent, we included the propensity score as a surrogate marker for PEP risk (along with PD stent placement and indomethacin treatment) in a second logistic regression model.
Figure 1.
Risk of post-endoscopic retrograde cholangiopancreatography pancreatitis according to study subjects’ risk score.
Cost-benefit analysis
Utilizing the analyses outlined above, published literature, and publicly available cost data, we estimated the cumulative costs of care associated with three PEP prevention strategies: (1) rectal indomethacin alone, (2) PSP alone, and (3) PSP plus indomethacin from the perspective of a 3rd party payer. As indomethacin monotherapy was less costly and more effective than either PSP-based strategy (see results below), we did not perform a typical cost-effectiveness analysis (which presumes that one strategy is both more effective and more costly than another). Rather, we performed a cost-benefit analysis, with the primary goal of projecting the potential cost savings associated with indomethacin alone prophylaxis in individuals undergoing high-risk ERCP.
The clinical probabilities and assumptions used in this analysis are listed in Table 2. The probabilities of developing PEP associated with each of the 3 strategies were calculated in the first portion of this study using the methodology described above. We assumed that all ERCPs were performed by expert endoscopists who would be frequently successful in PD stent placement (12–14), and that attempted but unsuccessful placement of a PD stent would result in an elevated risk of PEP (15,16). We also assumed that an abdominal radiograph would be obtained in all patients undergoing PSP to document spontaneous stent passage, and endoscopic retrieval would be performed in those with retained stents (14–16). We assumed that no complications occurred during follow-up upper endoscopy (biasing our results in favor of PSP-based strategies).
Table 2.
Clinical probability estimates utilized in the cost-benefit analysis
| Clinical outcome | Probability | Range | Reference |
|---|---|---|---|
| PEP if receiving indomethacin alone | 0.071 | 0.02–0.23 (B)a | This study |
| PEP if receiving PD stent alone | 0.157 | 0.08–0.27 (B) | This study |
| PEP if receiving combination of indomethacin and PD stent | 0.095 | 0.04–0.18 (B) | This study |
| Death as a result of PEP | 0.005 | 0.001–0.01 (B) | 25 |
| Successful PD stent placement | 0.90 | 0.7–1.0 (B) | 12–14 |
| Probability of post-ERCP pancreatitis after failed attempt at PD stent placement | 0.65 | 0.2–0.8 (B) | 15–16 |
| Retained pancreatic stent | 0.15 | 0.05–0.25 (B) | 14–16 |
ERCP, endoscopic retrograde cholangiopancreatography; PD, pancreatic duct; PEP, post-ERCP pancreatitis.
Beta distribution used to model this parameter in probabilistic sensitivity analysis.
Cost estimates for the endoscopic procedures and abdominal radiographs were obtained from 2012 regional Medicare reimbursement data for southeast Michigan (20). The cost of hospitalization for PEP was obtained from the Healthcare Cost and Utilization Project through the Agency for Healthcare Research and Quality (21). The cost of generic indomethacin supposi-tories was obtained from a national vendor (G&W Laboratories, South Plainwell, NJ). Costs estimates are summarized in Table 3.
Table 3.
Cost estimates (in 2012 US dollars) utilized in the cost-benefit analysis
| Clinical intervention | Cost | Range | Reference no. |
|---|---|---|---|
| ERCP (CPT code 43260) | $2,630.30 | $1,315–$5,260 (G)a | 19 |
| ERCP with stent placement (CPT code 43268) | $3,138.26 | $1,569–$6,276 (G) | 19 |
| Abdominal radiograph (CPT code 74000) | $75.23 | $37–$150 (G) | 19 |
| Upper endoscopy with foreign body removal (CPT code 43247) | $991.27 | $495–$1,982 (G) | 19 |
| Reimbursement for post-ERCP pancreatitis | $5,700.00 | $2,850-$11,400 (G) | 20 |
| Indomethacin (1 dose, generic) | $8.00 | $4–$16 (G) | Information from vendor |
CPT, current procedural terminology; ERCP, endoscopic retrograde cholangiopancreatography.
Gamma distribution was used to model this parameter in probabilistic sensitivity analysis.
Sensitivity analysis was used to identify variables that had important effects on cost. One-way sensitivity analyses were performed on each variable in the model. Two-way sensitivity analyses were performed using variables identified in one-way analyses. Probabilistic sensitivity analysis (2nd order Monte Carlo analysis) was also performed, where 13 variables were simultaneously varied over their sensitivity analysis ranges according to specified probability distributions (100,000 Monte Carlo trials) (Tables 2 and 3). Beta and gamma distributions were assumed for proportions and costs, respectively (22). For each distribution, we assumed that the mean was equal to the point estimate and that the s.d. was equal to the sensitivity analysis range/(2×1.96). Results were used to calculate 95% confidence intervals around base-case cost estimates. We also evaluated cost-effectiveness and generated acceptability curves for each strategy using net benefits calculations. As our model focused on a discrete event (ERCP and its immediate complications), discounting of costs and life-years was not performed.
Statistical analysis
All statistical analyses were performed using the STATA 12 statistical package (StataCorp LP, College Station, TX). Propensity score calculation was conducted using the psmatch2 command (23). Predicted marginal probabilities for PEP after logistic regression were calculated using the margins command. The cost-benefit analysis was performed by constructing a simple mathematical model in the TreeAge Pro decision modeling software (TreeAge Software, Williamstown, MA).
RESULTS
Risk of PEP
The unadjusted PEP rates in the four groups are listed in Table 4. The group of subjects who received no prophylaxis (n = 58) had a PEP rate of 20.7%. Those who received a pancreatic stent alone (n = 249) had a PEP rate of 16.1%. Those who received both indomethacin and a PD stent (n = 247) had a PEP rate of 9.7%, and the group who received indomethacin alone (n = 48) had a PEP rate of 6.3%.
Table 4.
Unadjusted and adjusted risk of post-ERCP pancreatitis, by group
| Study group | Unadjusted risk (%) | Risk after risk score adjustment (%) | Risk after propensity score adjustment (%) |
|---|---|---|---|
| No prophylaxis (n=58) | 20.7 | 25.7 | 23.1 |
| PD stent alone (n=249) | 16.1 | 15.3 | 15.7 |
| Indomethacin and PD stent (n=247) | 9.7 | 9.4 | 9.5 |
| Indomethacin alone (n=48) | 6.3 | 7.8 | 7.1 |
ERCP, endoscopic retrograde cholangiopancreatography; PD, pancreatic duct.
Patients who received a PD stent were indeed at higher risk for PEP, evidenced by a higher mean risk score (2.50 vs. 1.84, P < 0.001) and a higher likelihood of having a clinical suspicion of sphincter of Oddi dysfunction (87 vs. 59%) or having undergone a pancreatic sphincterotomy (68 vs. 6%).
Risk adjustment using PEP risk score
After adjusting for risk by including subject's risk score in the multivariable logistic regression model, the rates of PEP in the four groups were as follows (Table 4): 25.7%. for subjects receiving no prophylaxis, 15.3% for subjects receiving stent alone, 9.4% for subjects receiving the combination of indomethacin and PSP, and 7.8% for those who received indomethacin alone.
Risk adjustment using propensity score to receive a pancreatic stent
After adjusting PEP rates according to subject's propensity score to receive a pancreatic stent in the multivariable logistic regression model, the rates of PEP in the four groups were as follows (Table 4): 23.1% for subjects receiving no prophylaxis, 15.7% for subjects receiving stent alone, 9.5% for subjects receiving the combination of indomethacin and PSP, and 7.1% for those receiving indomethacin alone.
Cost-benefit analysis
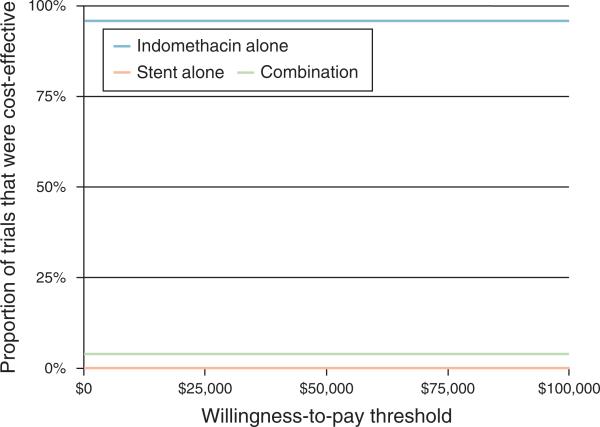
In our economic analysis, the base-case cost of care for one high-risk ERCP patient who receives indomethacin alone is $3,042.70 compared with $3,834.54 for the combination of indomethacin and PSP, and $4,515.04 for PSP alone. In sensitivity analysis, the variables with the largest impact on total cost were the cost of ERCP and the cost of PEP. However, no single variable resulted in indomethacin mono-prevention becoming more costly than either PSP-based strategy. Monte Carlo analysis revealed that indomethacin mono-prevention results in a mean per-patient savings of $793.80 (95% confidence interval: − 112.60–1619.00) over the combination of indomethacin and PSP, and $1,472.40 (95% confidence interval: 491.00–2804.10) over PSP alone. In ~4% of trials, the combination of indomethacin and PSP was less costly than indomethacin alone. Using net benefits calculations, indomethacin alone was the most cost-effective strategy in ~96% of trials regardless of willingness-to-pay, up to a willingness-to-pay threshold of $100,000 per life-year gained (Figure 2).
Figure 2.
Acceptability curves for indomethacin alone, stent alone, and combination of indomethacin and stent.
Assuming that 20% (100,000) of the 500,000 ERCPs that occur annually in the United States are high-risk cases, then the indomethacin alone strategy would save approximately $150 million per year compared with the PSP alone strategy, and $85 million per year compared with the indomethacin plus PSP strategy (assuming base-case values).
DISCUSSION
This post hoc analysis of a prospective RCT found that, after adjusting for differences in underlying risk using two different approaches, patients who received prophylactic rectal indomethacin alone appeared to have a lower risk of PEP than those who received a prophylactic pancreatic stent alone or the combination of both preventive interventions. The associated economic analysis suggests that a prevention strategy of indomethacin alone could substantially reduce healthcare costs associated with high-risk ERCP.
Prophylactic PSP is thought to reduce the risk of PEP by relieving pancreatic ductal hypertension that develops because of procedure-induced edema and stenosis of the pancreatic orifice (12,13,24). PD stent placement, however, is not completely effective as orifice edema is only one of several relevant pathophysiologic mechanisms in PEP. Other factors, such as chemical, allergic, enzymatic, and infectious injury are also though to contribute (25), and may be induced or potentiated by the process of placing a PD stent. Indeed, indomethacin alone may be more effective than any strategy involving PSP as it avoids manipulation of the pancreatic orifice and instrumentation of the PD.
In addition to the potential reduction in PEP, an indomethacin alone strategy would avoid the phenomenon of attempted but failed PSP, which is associated with a high rate of PEP by activating aforementioned pathogenic factors but providing no ductal decompression. It would also avoid the 4% of cases that result in significant non-pancreatitis complications induced by PSP, such as stent migration and duct perforation (10), as well as the rare complications that occur during follow-up upper endoscopy to remove retained stents.
Economically, this study demonstrated that indomethacin mono-prevention would save approximately $150 million annually in the United States compared with PSP alone, and $85 million annually compared with combination therapy. Moreover, as PSP requires ~10 min to perform (14), indomethacin mono-prevention would save ~1 million procedural minutes (or 16,666 procedure hours) annually, beneficial to patients and endoscopy units alike by allowing unit and physician manpower for delivery of other endoscopic services.
Despite these proposed clinical and cost benefits, this analysis is intended to be hypothesis-generating, and, therefore, the results of this study should not change clinical practice at this time. This post hoc observational study does not produce the same quality of evidence as our RCT, which supports the use of indomethacin in addition to PSP in high-risk cases (17). Moreover, the results of this study must be interpreted in the context of several important limitations. First, underlying subject risk was adjusted by accounting for variations in the observed characteristics of study participants. Unobserved and unrecorded patient and procedural characteristics, however, are also likely to contribute to subject risk and may not have been accounted for in our risk adjustment analyses.
Second, the cost analysis was based on the adjusted PEP pancreatitis rates seen in our indomethacin pharmacoprevention RCT and may not reflect the frequency of this complication in other institutions or regions. Further, the calculations were based on local Medicare reimbursement rates, which vary regionally, and Medicare reimbursement for stent placement may be lower than stated when multiple interventions are performed during the same ERCP. While these factors likely limit the generalizability of the economic analysis results, the cost savings associated with an indomethacin alone strategy remain substantial when clinical probabilities and costs were varied broadly in the sensitivity analysis. Despite these limitations, the hypothesis generated by the findings of this study is of major potential impact and merits further investigation.
In summary, this hypothesis-generating study suggests that prophylactic rectal indomethacin may eliminate the need for PSP in patients undergoing high-risk ERCP. A prevention strategy involving indomethacin alone could improve clinical outcomes and substantially reduce healthcare costs. Therefore, a RCT comparing rectal indomethacin alone vs. indomethacin plus PSP appears justified.
Supplementary Material
Study Highlights.
WHAT IS CURRENT KNOWLEDGE
✓ Rectal indomethacin administration is effective in addition to pancreatic stent placement for preventing PEP in high-risk patients.
✓ There are no studies examining whether rectal indomethacin is effective when administered instead of PSP.
WHAT IS NEW HERE
✓ After adjusting for risk, rectal indomethacin alone appeared to be more effective for preventing PEP than no prophylaxis, PSP alone, and the combination of indomethacin and PSP.
✓ A prevention strategy employing rectal indomethacin alone could save approximately $150 million annually in the United States compared with a strategy of PSP alone, and $85 million compared with a strategy of indomethacin and PSP.
✓ A randomized controlled trial comparing rectal indomethacin alone vs. indomethacin plus pancreatic stent placement appears justified.
Acknowledgments
Financial support: This study was supported by NIH grants 1R21DK090343 and UL1RR024986.
Footnotes
Specific author contributions: Study concept and design; acquisition of data; analysis and interpretation of data; drafting of the manuscript; critical revision of the manuscript for important intellectual content; statistical analysis: B. Joseph Elmunzer; study concept and design; analysis and interpretation of data; critical revision of the manuscript for important intellectual content; statistical analysis: Peter D.R. Higgins; analysis and interpretation of data; critical revision of the manuscript for important intellectual content; statistical analysis: Sameer D. Sain; analysis and interpretation of data; critical revision of the manuscript for important intellectual content: James M. Scheiman; analysis and interpretation of data; critical revision of the manuscript for important intellectual content; statistical analysis: Robert A. Parker; analysis and interpretation of data; critical revision of the manuscript for important intellectual content: Amitabh Chak; analysis and interpretation of data; critical revision of the manuscript for important intellectual content: Joseph Romagnuolo; analysis and interpretation of data; critical revision of the manuscript for important intellectual content: Patrick Mosler; analysis and interpretation of data; critical revision of the manuscript for important intellectual content; statistical analysis: Rodney A. Hayward; analysis and interpretation of data; critical revision of the manuscript for important intellectual content: Grace H. Elta; acquisition of data; critical revision of the manuscript for important intellectual content: Sheryl J. Korsnes; acquisition of data; critical revision of the manuscript for important intellectual content: Suzette E. Schmidt; analysis and interpretation of data; critical revision of the manuscript for important intellectual content: Stuart Sherman; analysis and interpretation of data; critical revision of the manuscript for important intellectual content: Glen A. Lehman; analysis and interpretation of data; critical revision of the manuscript for important intellectual content: Evan L. Fogel.
CONFLICT OF INTEREST
Guarantor of the article: B. Joseph Elmunzer, MD.
Potential competing interests : The authors declare no conflict of interest and the content is solely the responsibility of the authors and does not necessarily represent the offi cial views of the National Institutes of Health.
SUPPLEMENTARY MATERIAL is linked to the online version of the paper at http://www.nature.com/ajg
REFERENCES
- 1.Petrov MS, Savides TJ. Systematic review of endoscopic ultrasonography versus endoscopic retrograde cholangiopancreatography for suspected choledocholithiasis. Br J Surg. 2009;96:967–74. doi: 10.1002/bjs.6667. [DOI] [PubMed] [Google Scholar]
- 2.Romagnuolo J, Bardou M, Rahme E, et al. Magnetic resonance cholangiopancreatography: a meta-analysis of test performance in suspected biliary disease. Ann Intern Med. 2003;139:547–57. doi: 10.7326/0003-4819-139-7-200310070-00006. [DOI] [PubMed] [Google Scholar]
- 3.Mazen Jamal M, Yoon EJ, Saadi A, et al. Trends in the utilization of endoscopic retrograde cholangiopancreatography in the United States. Am J Gastroenterol. 2007:102;966–75. doi: 10.1111/j.1572-0241.2007.01127.x. [DOI] [PubMed] [Google Scholar]
- 4.Sherman S, Troiano FP, Hawes RH, et al. Sphincter of Oddi manometry: Decreased risk of clinical pancreatitis with use of a modified aspirating catheter. Gastrointest Endosc. 1990;36:462–6. doi: 10.1016/s0016-5107(90)71115-7. [DOI] [PubMed] [Google Scholar]
- 5.Cennamo V, Fuccio L, Zagari RM, et al. Can a wire-guided cannulation technique increase bile duct cannulation rate and prevent post-ERCP pancreatitis?: A meta-analysis of randomized controlled trials. Am J Gastroenterol. 2009;104:2343–50. doi: 10.1038/ajg.2009.269. [DOI] [PubMed] [Google Scholar]
- 6.Cot é GA, Ansstas M, Pawa R, et al. Difficult biliary cannulation: use of physician-controlled wire-guided cannulation over a pancreatic duct stent to reduce the rate of precut sphincterotomy (with video). Gastrointest Endosc. 2010;71:275–9. doi: 10.1016/j.gie.2009.08.028. [DOI] [PubMed] [Google Scholar]
- 7.Freeman ML. Pancreatic stents for prevention of post-endoscopic retrograde cholangiopancreatography pancreatitis. Clin Gastroenterol Hepatol. 2007;5:1354–65. doi: 10.1016/j.cgh.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 8.Brackbill S, Young S, Schoenfeld P, et al. A survey of physician practices on prophylactic pancreatic stents. Gastrointest Endosc. 2006;64:45–52. doi: 10.1016/j.gie.2006.01.058. [DOI] [PubMed] [Google Scholar]
- 9.Choudhary A, Bechtold ML, Arif M, et al. Pancreatic stents for prophylaxis against post-ERCP pancreatitis: a meta-analysis and systematic review. Gastrointest Endosc. 2011;73:275–82. doi: 10.1016/j.gie.2010.10.039. [DOI] [PubMed] [Google Scholar]
- 10.Mazaki T, Masuda H, Takayama T. Prophylactic pancreatic stent placement and post-ERCP pancreatitis: a systematic review and meta-analysis. Endoscopy. 2010;42:842–5. doi: 10.1055/s-0030-1255781. [DOI] [PubMed] [Google Scholar]
- 11.Das A, Singh P, Sivak MV, Jr, et al. Pancreatic-stent placement for prevention of post-ERCP pancreatitis: a cost-effectiveness analysis. Gastrointest Endosc. 2007;65:960–8. doi: 10.1016/j.gie.2006.07.031. [DOI] [PubMed] [Google Scholar]
- 12.Tarnasky PR, Palesch YY, Cunningham JT, et al. Pancreatic stenting prevents pancreatitis after biliary sphincterotomy in patients with sphincter of Oddi dysfunction. Gastroenterology. 1998;115:1518–24. doi: 10.1016/s0016-5085(98)70031-9. [DOI] [PubMed] [Google Scholar]
- 13.Fazel A, Quadri A, Catalano MF, et al. Does a pancreatic duct stent prevent post-ERCP pancreatitis? A prospective randomized study. Gastrointest Endosc. 2003;57:291–4. doi: 10.1067/mge.2003.124. [DOI] [PubMed] [Google Scholar]
- 14.Zolotarevsky E, Fehmi SM, Anderson MA, et al. Prophylactic 5-Fr pancreatic duct stents are superior to 3-Fr stents: a randomized controlled trial. Endoscopy. 2011;43:325–30. doi: 10.1055/s-0030-1256305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Freeman ML, Overby C, Qi D. Pancreatic stent insertion: consequences of failure and results of a modified technique to maximize success. Gastro intest Endosc. 2004;5:8–14. doi: 10.1016/s0016-5107(03)02530-6. [DOI] [PubMed] [Google Scholar]
- 16.Freeman ML. Role of pancreatic stents in prevention of post-ERCP pancreatitis. JOP. 2004;5:322–7. [PubMed] [Google Scholar]
- 17.Elmunzer BJ, Scheiman JM, Lehman GA, et al. US. Cooperative for Outcomes Research in Endoscopy (USCORE). A randomized trial of rectal indomethacin to prevent post-ERCP pancreatitis. N Engl J Med. 2012;366:1414–22. doi: 10.1056/NEJMoa1111103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cotton PB, Lehman G, Vennes J, et al. Endoscopic sphincterotomy complications and their management: an attempt at consensus. Gastrointest Endosc. 1991;37:383–93. doi: 10.1016/s0016-5107(91)70740-2. [DOI] [PubMed] [Google Scholar]
- 19.Freeman ML, DiSario JA, Nelson DB, et al. Risk factors for post-ERCP pancreatitis: a prospective, multicenter study. Gastrointest Endosc. 2001;54:425–34. doi: 10.1067/mge.2001.117550. [DOI] [PubMed] [Google Scholar]
- 20.2012 Medicare physician fee and hospital schedules for Michigan Loc 01. [15 June 2012];Centers for Medicare and Medicaid Services. ( http://www.cms.gov/Medicare/Medicare.html)
- 21.National inpatient sample . Healthcare Cost and Utilization Project. Agency for Healthcare Research and Quality; Rockville,MD: 2012. [17 June]. http://hcupnet.ahrq.gov. [Google Scholar]
- 22.Briggs AH, Claxton K, Sculpher MJ. Decision Modeling for Health Economic Evaluation. Oxford University Press; New York, NY: 2006. [Google Scholar]
- 23.Leuven E, Sianesi B. PSMATCH2: stata module to perform full Mahalanobis and propensity score matching, common support graphing, and covariate imbalance testing. 2012 http://ideas.repec.org/c/boc/bocode/s432001.html Version 4.0.5 18apr2012.
- 24.Freeman ML, Guda NM. Prevention of post-ERCP pancreatitis;a comprehensive review. Gastrointest Endosc. 2004;59:845–64. doi: 10.1016/s0016-5107(04)00353-0. [DOI] [PubMed] [Google Scholar]
- 25.Cotton PB, Garrow DA, Gallagher J, et al. Risk factors for complications after ERCP: a multivariate analysis of 11,497 procedures over 12 years. Gastrointest Endosc. 2009;70:80–8. doi: 10.1016/j.gie.2008.10.039. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.