Abstract
The pituitary gonadotropin hormones, FSH and LH, are essential for fertility. Containing an identical α-subunit (CGA), they are comprised of unique β-subunits, FSHβ and LHβ, respectively. These two hormones are regulated by the hypothalamic decapeptide, GnRH, which is released in a pulsatile manner from GnRH neurons located in the hypothalamus. Varying frequencies of pulsatile GnRH stimulate distinct signaling pathways and transcriptional machinery after binding to the receptor, GnRHR, on the cell surface of anterior pituitary gonadotropes. This ligand-receptor binding and activation orchestrates the synthesis and release of FSH and LH, in synergy with other effectors of gonadotropin production, such as activin, inhibin and steroids. Current research efforts aim to discover the mechanisms responsible for the decoding of the GnRH pulse signal by the gonadotrope. Modulating the response to GnRH has the potential to lead to new therapies for patients with altered gonadotropin secretion, such as those with hypothalamic amenorrhea or polycystic ovarian syndrome.
Keywords: Gonadotrope, Gonadotropins, GnRH, FSH, LH
INTRODUCTION
FSH and LH secretion from the gonadotrope is controlled by the hypothalamic decapeptide, GnRH (Belchetz et al., 1978). Acting primarily in the anterior pituitary, GnRH binds to its native high-affinity seven-transmembrane receptor (GnRHR) on the cell surface of the gonadotrope, stimulating signaling cascades that confer the production of these gonadotropins. FSH and LH exert their effects on the ovaries and testes, leading to steroidogenesis and gametogenesis, highlighting their critical role in reproductive function (Burger et al., 2004). Released in a pulsatile manner from the hypothalamus, differential GnRH pulse frequencies and amplitudes alter the secretion patterns of FSH and LH (Savoy-Moore and Swartz, 1987; Wildt et al., 1981), with increasing frequencies resulting in preferential secretion of LH, whereas decreasing frequencies result in greater FSH release. Although considerable research has been dedicated to elucidating the mechanisms by which GnRH controls the production and secretion of FSH and LH, less is known about how the gonadotrope decodes the pulsatile GnRH signal.
Three members of the GnRHR family have been identified in vertebrates (type I, II and III) (Millar, 2005). In mammalian gonadotropes, Type I GnRHR (throughout this review referred to as GnRHR) shares 85% sequence homology amongst human, rat, sheep, cow and pig species (as reviewed in detail (Sealfon et al., 1997)). Although type II GnRHR is present in both the pig and monkey, it is notably absent from the mouse, rat, sheep and cow, as well as silenced, in both the human and chimpanzee genomes (Hapgood et al., 2005; Millar, 2003). Upon stimulation, GnRHR does not desensitize, a result of an absent C-terminus (Davidson et al., 1994; McArdle et al., 1995; McArdle et al., 1996; Willars et al., 1999). Therefore, other potential mechanisms modulating the cellular response to pulsatile GnRH include ligand-mediated receptor internalization, changes in receptor number, or changes in the activity of signaling pathways downstream of the GnRHR. Indeed, lack of GnRHR desensitization, atypical compared to most other G protein-coupled receptors, may contribute to the ability of the gonadotrope to respond differentially to varying GnRH pulse frequencies. It has been demonstrated previously in perifused rat pituitary cultures that Gnrhr mRNA is expressed in a pulsatile GnRH dependent manner (Kaiser et al., 1997). GnRH pulses increase Gnrhr mRNA levels compared to untreated controls, with levels greater at fast than at slow frequencies (Kaiser et al., 1997). Therefore, gonadotropes could potentially respond differentially to pulsatile GnRH by changes in the numbers of cell surface receptor numbers (Kaiser et al., 1995).
The control of FSH and LH synthesis is closely linked to the transcription of the distinct β-subunits, Fshb and Lhb respectively. Both FSH and LH contain a common α-subunit (CGA); therefore, it is FSHβ and LHβ that confer the specific actions of the gonadotropins (Gharib et al., 1990). Like FSH and LH secretion, the transcription of the gonadotropin subunits is also dependent on GnRH pulse frequency (Dalkin et al., 1989; Haisenleder et al., 1991; Jakubowiak et al., 1989; Kaiser et al., 1997). A decreased frequency of pulsatile GnRH favors Fshb transcription, whilst an increased frequency favors Lhb transcription. Although levels of Cga mRNA do respond to pulsatile GnRH, the regulation in response to varying frequencies of pulsatile GnRH is less important for overall FSH and LH production, since Cga is produced in excess over Lhb and Fshb at both fast and slow GnRH pulse frequencies (Landy et al., 1991; Weiss et al., 1990). Continuous exposure to GnRH downregulates both mRNA levels and secretion of gonadotropins, compared to pulsatile GnRH; therefore, biosynthesis of both FSH and LH is critically dependent on the pulsatile nature of the GnRH signal (Belchetz et al., 1978; Burger et al., 2004; Ferris and Shupnik, 2006; Gharib et al., 1990; Haisenleder et al., 1991).
The importance of the differential control of FSH and LH secretion is highlighted by disorders associated with dysregulation of their release from the pituitary. Patients with low levels of GnRH, FSH and LH, for example in association with idiopathic hypogonadotropic hypogonadism or Kallmann syndrome, are infertile (Seminara et al., 1998). Conversely, accelerated GnRH pulse frequency, associated with increased levels of LH over FSH, is associated with polycystic ovarian syndrome (PCOS). This disorder affects 5-15 % of the female population within reproductive age, and is also linked to obesity, insulin resistance, as well as other metabolic and cardiovascular abnormalities (Blank et al., 2007; Dunaif, 1997; Hoffman and Ehrmann, 2008). Therefore, it is evident that highly controlled interactions between the hypothalamic GnRH neurons and pituitary gonadotropes are critical for appropriate control of FSH and LH release and subsequent gonadal stimulation and reproductive function.
CURRENT CELLULAR MODELS
Gonadotropes comprise 5-15% of the total anterior pituitary cell population (Ooi et al., 2004), which can make studies of gonadotropin regulation using primary cultures difficult. Several factors need to be considered whilst investigating the regulation of FSH and LH synthesis in response to GnRH stimulation of the gonadotrope, such as paracrine effects of factors secreted from gonadotropes or other pituitary cell types, as well as the effects of factors secreted from folliculostellate cells (Denef, 2008; Fallest and Schwartz, 1991; Kawakami et al., 2002; Thackray et al., 2010). Numerous in vivo animal models have been employed to examine gonadotrope biology, including gonadectomized rats (Dalkin et al., 1989; Haisenleder et al., 1991) and rhesus-monkeys (Wildt et al., 1981). The generation of genetic mouse models, such as gonadotrope-specific ERK1/2 knock-out mice (Bliss et al., 2009), provide an opportunity to elucidate the effects of abrogating signaling pathways implicated in the regulation of Fshb and Lhb transcription in vivo. It is also worth highlighting two transgenic mouse models that allow for cell sorting and subsequent isolation and purification of primary gonadotropes (Wen et al., 2008; Wu et al., 2004). However, other limitations present themselves, such as acquiring enough purified gonadotropes to carry out significant characterization studies. Heterologous cell models such as HeLa cells have recently been published (Armstrong et al., 2009a, 2010), requiring relatively fewer cell numbers and utilizing techniques such as live cell imaging.
The emergence of two murine gonadotrope-derived cell lines, αT3-1 and LβT2 cells, have allowed researchers to study homogeneous gonadotropic cell populations (Alarid et al., 1996; Thomas et al., 1996; Turgeon et al., 1996; Windle et al., 1990). Representing an immature gonadotrope at an earlier stage of differentiation, αT3-1 cells express CGA, GnRHR, and SF1, although they lack expression of Fshb and Lhb subunits (Windle et al., 1990). In comparison, the generation of LβT2 cells provided a significant advance (Alarid et al., 1996). LβT2 cells have been shown to produce Fshb in response to activin A (Graham et al., 1999), coupled with other studies that demonstrated that these cells express Fshb and Lhb subunits, as well as secrete both FSH and LH (Graham et al., 1999; Pernasetti et al., 2001; Turgeon et al., 1996). LβT2 cells remain the only homologous cell line available for the study of FSH and LH synthesis and secretion; therefore this model heavily influences the material covered in this review, with comparison to the in vivo murine models mentioned above. As a result of being a homologous cell line, studies conducted with LβT2 cells may lack the effects of paracrine factors produced by other pituitary cell types that may influence GnRH signaling in the gonadotrope. However, these cells express activin and follistatin, two autocrine effectors of Fshb transcription (Takeda et al., 2007). Another potential limitation of this cell line is the relatively low levels of FSH production in LβT2 cells compared to primary gonadotropes. While it is not clear if the regulatory pathways identified in LβT2 cells accurately reflect those used in primary gonadotropes, they reflect the best in vitro gonadotrope-derived cell line currently available.
SIGNALING PATHWAYS ACTIVATED BY PULSATILE GNRH
The GnRHR, a member of the seven-transmembrane G protein-coupled receptor family, and the signaling pathways that are stimulated by the receptor upon activation by GnRH, have been studied extensively. However, it still remains elusive as to how the gonadotrope decodes the pulsatile GnRH signal to preferentially produce either FSH or LH.
The GnRHR has been shown to couple with Gαq/11, Gαi and Gαs (Krsmanovic et al., 2003; Liu et al., 2002b) in hypothalamic cell lines and LβT2 cells, implicating a wide range of pathways that may potentially mediate the pulsatile GnRH response. On the other hand, some studies, such as those investigating G protein coupling to GnRHR in other cell types, including αT3-1 (Grosse et al., 2000; Hsieh and Martin, 1992), CHO-K1, and COS-7 (Grosse et al., 2000) cells support preferential or even exclusive interaction with Gαq/11. Several studies (Han and Conn, 1999; Lin and Conn, 1998; Stanislaus et al., 1998) provide yet another perspective, identifying GnRHR coupling to multiple G protein subunits in the heterologous rat somatolactotropic GGH3 cell line and in primary rat gonadotropes. Therefore an important consideration when investigating the role of G proteins in GnRHR signaling is the cell model being used.
MAPK Pathways
It has been demonstrated that several mitogen-activated protein kinase (MAPK) cascades, including extracellular signal related kinase (ERK), jun N-terminal kinase (JNK), and p38 are stimulated by GnRH (Ben-Menahem and Naor, 1994; Benard et al., 2001; Bonfil et al., 2004; Harris et al., 2002; Levi et al., 1998; Liu et al., 2002b; Mulvaney and Roberson, 2000). These MAPK cascades have been implicated in playing a role to mediate the control of FSH and LH synthesis in response to pulsatile GnRH (Kanasaki et al., 2005).
Rapid and sustained ERK1/2 phosphorylation and activation following slow GnRH pulse frequencies, coupled with higher levels of ERK1/2 phosphorylation versus fast frequency GnRH, implies that distinct patterns of ERK activation/inactivation are regulated by GnRH pulse frequency (Kanasaki et al., 2005). Therefore, the difference in ERK activation in response to varying GnRH pulse frequency could be responsible for the differential expression of Fshb and Lhb in the gonadotrope (Kanasaki et al., 2005). The dependence of Lhb transcription on ERK activation has been well characterized, mediated through the early growth response-1 protein (EGR1) (Dorn et al., 1999; Fortin et al., 2009; Lawson et al., 2007; Lee et al., 1996; Wolfe and Call, 1999). As previously reviewed (Bliss et al., 2010), studies involving male gonadotrope-specific ERK knock-out mice demonstrated little change in the regulation of Fshb expression by GnRH (Bliss et al., 2009). However, in female mice, the increase in Fshb mRNA following ovariectomy was impaired, suggesting an impaired induction by GnRH (Bliss et al., 2009). The direct induction of Fshb and Lhb in gonadotropes by pulsatile GnRH has yet to be assessed in this gonadotrope-specific ERK knock-out model.
A recent study examined the potential for ERK1/2 to be the GnRH pulse frequency signal decoder (Armstrong et al., 2010). Using live-cell imaging to track ERK2-GFP translocation in HeLa cells, this group demonstrated that in response to both fast and slow GnRH pulse frequencies, ERK2-GFP translocated into the nucleus, a mark of both activation and involvement in transcription events. Based on mathematical modeling predictions, they argue that a lack of desensitization of this response, at either pulse frequency, suggests that ERK is not the decoder of the GnRH signal (Armstrong et al., 2010). However, downstream effects of ERK translocation, which may take longer to return to the basal state after each pulse, may provide a mechanism by which the gonadotrope differentially senses fast and slow GnRH pulse frequencies.
We have previously suggested (Ciccone and Kaiser, 2009) that MAPK phosphatases (MKP) may be responsible for the differential ERK1/2 phosphorylation patterns observed after pulsatile GnRH treatment (Kanasaki et al., 2005). This hypothesis is supported by data demonstrating an increase in MKP1 and MKP2 expression in response to GnRH, both in gonadotrope cell lines and in vivo (Zhang and Roberson, 2006). However, two related studies in both static and perifused cultures argue against this possibility. Although LβT2 cells treated with continuous GnRH demonstrated augmented pERK levels after MKP knock-down (Armstrong et al., 2009b; Caunt et al., 2008), perifused HeLa cells (transfected with GnRHR and ERK2-GFP) demonstrated only a 10-20% increase in MKP's compared to cells treated with continuous GnRH (Armstrong et al., 2010). Coupled with data demonstrating that mutation of the site which governs ERK MKP binding affects ERK2-GFP translocation kinetics in response to continuous, but not pulsatile, GnRH (Armstrong et al., 2010), the findings from this group argue against a major role for MKP's in mediating the decoding of the pulsatile GnRH response. Lastly, studies conducted in LβT2 cells demonstrated a significant increase in dual-specificity kinase 1 (DUSP1) after fast GnRH pulse frequencies compared to control and slow frequency stimulated samples (Purwana et al., 2011). In these LβT2 cells, overexpression of MAP3K1 induced both Fshb and Lhb subunit promoter activities, which was inhibited by cotransfection with DUSP1 expression vectors (Purwana et al., 2011). DUSP1 overexpression also prevented the induction of Fshb and Lhb by pulsatile GnRH, suggesting a role for this phosphatase, and therefore MKPs, in the regulation of gonadotropin transcription.
Calcium Signaling
Calcium signaling has been shown to contribute to the gonadotrope response to GnRH. Rapid gonadotropin secretion and activation of CamK1/2 (Ca2+/calmodulin-dependent kinases) have been attributed to GnRH dependent calcium mobilization (Haisenleder et al., 2003a; Haisenleder et al., 2003b; Lim et al., 2007). Importantly, perifusion studies have demonstrated a GnRH pulse frequency dependent effect of calcium on FSH and LH (Burger et al., 2008; Haisenleder et al., 2001). Rat primary cells perifused with BayK8644, a calcium channel agonist, demonstrated increased expression of gonadotropin genes. A slow pulse frequency induced Fshb transcription, whilst conversely, fast frequency pulsatile BayK8644 treatment preferentially stimulated Cga and Lhb subunit transcription (Haisenleder et al., 1997). These findings immediately draw parallels with the actions of pulsatile GnRH on Cga, Lhb and Fshb transcription. It has been demonstrated that calmodulin activation by calcium is required for ERK signaling in the gonadotrope (Roberson et al., 2005), also leading to calcium/calmodulin-dependent kinase II (CamKII) activation (Haisenleder et al., 2003a; Haisenleder et al., 2003b). On the other hand, it was demonstrated that CamKII activation is not regulated by GnRH frequency (Burger et al., 2008; Haisenleder et al., 2003a; Haisenleder et al., 2003b). However, due to the rapid kinetics of CamKII inactivation, faster GnRH pulses may favor prolonged activity and subsequently greater Lhb transcription than Fshb. This model favors calcium signaling as a mechanism by which the gonadotrope decodes GnRH pulse frequency.
NFAT
The nuclear factor of activated T-cells (NFAT) transcription factor has been linked to mediating the GnRH control of transcription upon activation by calcineurin, a protein phosphatase (Armstrong et al., 2009a; Gardner and Pawson, 2009; Lim et al., 2007; Oosterom et al., 2005). It has been demonstrated that emerald fluorescent protein tagged NFAT2 (NFAT2-EFP) translocates into the nucleus upon GnRH stimulation (Armstrong et al., 2009a), and the response is reversible. This mimics studies with ERK2-GFP, demonstrating reversibility of the ERK translocation between GnRH pulses (Armstrong et al., 2010), although the reversibility observed with NFAT2-EFP is markedly slower than that of ERK2-GFP, suggestive of effectors of GnRH signaling further downstream (Armstrong et al., 2009a). NFAT2-EFP undergoes desensitization regardless of GnRH pulse frequency, which challenges mathematical models (Washington et al., 2004) and the hypothesis that calcium/NFAT signaling is responsible for decoding the pulsatile GnRH signal. The two studies investigating ERK and NFAT translocation were carried out in HeLa cells (Armstrong et al., 2009a, 2010), raising the possibility that these effects were cell specific. However, further examination of NFAT2-EFP translocation in LβT2 cells produced results similar to those observed in HeLa cells (Armstrong et al., 2009a).
PKA
GnRH stimulation of PKA in the gonadotrope has been reported previously (Duan et al., 2002; Garrel et al., 2010; Grafer et al., 2009; Thompson et al., 2013; Tsutsumi et al., 2010), alongside elevations in cAMP following GnRH stimulation (Lariviere et al., 2007; Liu et al., 2002b; Tsutsumi et al., 2010). Studies investigating the role of PKA activity in modulating the response to pulsatile GnRH, however, are limited. Using phosphorylated cAMP response element binding protein (CREB) levels, Fshb LUC activity, and Fshb mRNA quantification, it has recently been shown that PKA can mediate stimulation of Fshb, but not Lhb, transcription in gonadotrope cells in response to GnRH at both fast and slow pulse frequencies (Thompson et al., 2013). Coupled with these observations, PKA activity was significantly increased in response to slow pulse frequencies. Interestingly, others have also described increases in PKA activity in response to pulsatile GnRH, although not always in a frequency dependent fashion (Tsutsumi et al., 2010). These two studies implicate PKA in the gonadotrope response to pulsatile GnRH. Differences in the level of activation of PKA measured at both pulse frequencies (Thompson et al., 2013; Tsutsumi et al., 2010) could be due to the greater duration of pulsatile GnRH stimulation (Thompson et al., 2013), or induction of other unknown factors to limit adenylyl cyclase activity, cAMP accumulation, or PKA activity.
It is clear that the pathways regulating the transcription of Fshb and Lhb in the gonadotrope upon stimulation by pulsatile GnRH are complex. Careful consideration must be given to the model used in conducting such pathway studies. Ultimately, the physiological relevance of data generated in cell line models should be investigated using in vivo animal models. The search for the GnRH pulse frequency decoder continues. Techniques such as live-cell imaging have been utilized to investigate the activation and translocation of various kinases and phosphatases upon pulsatile GnRH treatment, yet current focuses on specific signaling pathways have not definitively yielded the decoder. A combination of existing data and further investigation based on mathematic modeling predictions of pulsatile GnRH signaling will lead to a broader understanding of the key signaling pathways involved. Considering the current evidence, it appears that the GnRHR differentially activates multiple distinct signaling pathways in response to either fast or slow GnRH pulse frequencies, potentially as a result of changes in associations with Gαq/11, Gαi and Gαs.
TRANSCRIPTIONAL REGULATION OF FSH AND LH SUBUNITS
The signaling pathways described in this review culminate to mediate an effect of pulsatile GnRH stimulation on three gonadotropin subunit genes: CGA, FSHB and LHB. CGA combines with either FSHB or LHB to form the heterodimeric glycoprotein hormones, FSH and LH, respectively (Gharib et al., 1990). Mediators of β-subunit transcription that are the focus of this review include CREB, ICER, c-Fos, c-Jun, EGR1 and activating transcription factors (ATFs). These transcription factors have been studied extensively, although the mechanisms driving their control by pulsatile GnRH are still to be fully elucidated.
FSH and LH production and release follow distinct pathways in the gonadotrope. Fshb transcription is the rate limiting step of FSH synthesis. Once GnRH signaling pathways are initiated, synthesis of FSH is directly coupled to release through the constitutive secretory pathway (Farnworth, 1995; McNeilly et al., 2003; Nicol et al., 2004). Conversely, LH release upon GnRH signaling is controlled through the regulated signaling pathway, with LH stored in secretory granules until stimulation of secretion (Crawford and McNeilly, 2002; Crawford et al., 2002; Watanabe et al., 1991). Both Fshb and Lhb transcription rates respond differentially to pulsatile GnRH; the signaling pathways responsible for these effects have been studied extensively, with the goal to elucidate the role of transcription factors in decoding this oscillatory signal.
Fshb
Fshb transcription has been reviewed previously in detail (Bernard et al., 2010). Several studies have demonstrated that activation of MAPKs, including pERK1/2, JNK and p38, result in activation of transcription factors, including CREB, c-Fos, c-Jun and ATF's (Ciccone et al., 2008; Liu et al., 2002a; Xie et al., 2005). Using the LβT2 cell line, our group (Ciccone et al., 2008) and others (Coss et al., 2004; Wang et al., 2008) have identified a GnRH responsive element in the Fshb promoter region, which is conserved in humans (Wang et al., 2008) and contains a partial cAMP response element (CRE)/AP1 site. Having established that upstream stimulating factor (USF)1 and USF2 are involved in basal rat Fshb transcription in LβT2 cells and that CREB is involved in the response to continuous GnRH in static culture (Ciccone et al., 2008), subsequent investigations explored the role of CREB further under the perifusion paradigm. It was demonstrated that in LβT2 cells, pulsatile GnRH stimulation of rat Fshb transcription, which occurs preferentially at slow GnRH pulse frequencies, is dependent on CREB binding to the rat Fshb promoter (Ciccone et al., 2010). Coupled with previous data implicating CREB binding protein (CBP) in binding to CREB to stimulate Fshb transcription (Ciccone et al., 2008), this site appears to be important for the response to pulsatile GnRH. CREB promotes Fshb transcription by recruiting CBP when phosphorylated at position Ser133, an event also controlled by pulsatile GnRH, occurring preferentially at slow frequencies and mediated by PKA activity (Ciccone et al., 2008; Ciccone et al., 2010; Thompson et al., 2013). Furthermore, the inducible cAMP early repressor (ICER) has been implicated in regulating the response to pulsatile GnRH. In contrast to CREB phosphorylation, ICER expression and synthesis occurs preferentially at faster GnRH pulse frequencies. ICER protein subsequently competes with CREB at the CRE site on the Fshb promoter to reduce GnRH-stimulated transcription (Ciccone et al., 2010). The signaling pathways that regulate ICER synthesis are yet to be elucidated; however, it is worth noting that ICER phosphorylation at Ser41 marks it for ubiquitination and proteasomal degradation (Yehia et al., 2001). As previously discussed in this review and others (Bernard et al., 2010; Bliss et al., 2010; Ciccone and Kaiser, 2009; Gharib et al., 1990; Naor, 2009; Thackray et al., 2010), ERK and calcium signaling pathways both respond differentially to GnRH frequency and are potential candidates for ICER regulation.
AP1 homo- and hetero-dimers, comprised of a combination of Jun and Fos intermediate-early gene family members, are induced by GnRH (Coss et al., 2004; Kakar et al., 2003; Wurmbach et al., 2001). A recent study demonstrated that pulsatile GnRH increased c-Fos and c-Jun at both slow and fast GnRH pulse frequencies (Mistry et al., 2011). Intriguingly, this group demonstrated that both c-Fos and c-Jun proteins were expressed to a greater extent at fast GnRH pulse frequencies, initially surprising since these factors are enhancers of Fshb transcription. However, they present a model showing that at fast GnRH frequencies, negative effectors of Fshb transcription, namely SKIL and TGIF1, are also induced. These bind to the Fshb promoter and repress any potential action of c-Fos and c-Jun (Mistry et al., 2011). MAPK proteins such as pERK, JNK and p38 can also lead to increased expression of these AP1 proteins (Coss et al., 2004; Liu et al., 2002a). It is not yet understood how GnRH stimulates signaling pathways to induce expression of CREB, ICER and AP1 proteins in an integrated manner to decode pulse frequency and control FSH synthesis (see Figure 1). It is also worth noting that other pathways contribute to regulation of Fshb transcription, including those stimulated by activin and gonadal steroids, although these are not the focus of this review.
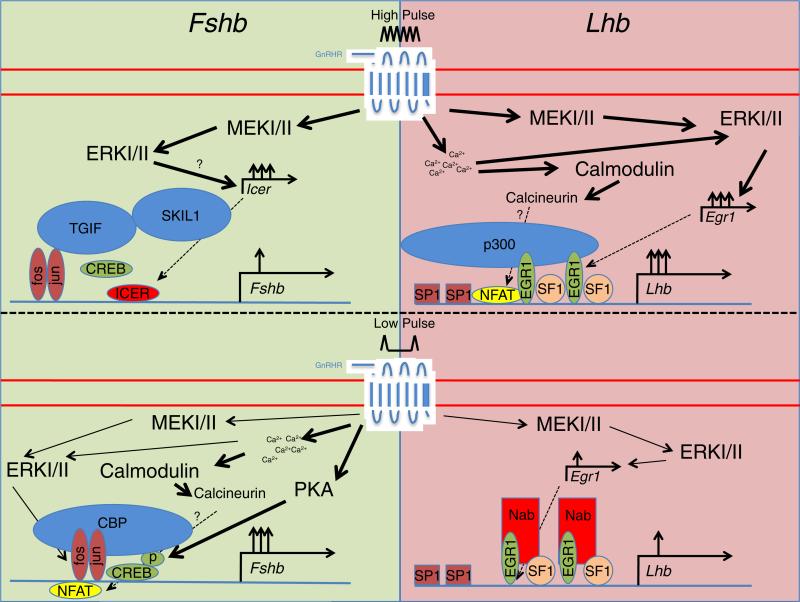
FIG. 1.
Model for the regulation of Fshb and Lhb transcription by pulsatile GnRH. Fast and slow frequency pulsatile GnRH stimulates signaling cascades that mediate the activity and synthesis of transcription factors controlling gonadotropin subunit gene transcription. The pathways stimulated by GnRH can vary in magnitude and duration (as indicated by weighted arrows) in a manner dependent on pulse frequency and lead to the induction of distinct transcription factor networks.
Lhb
In comparison, Lhb transcription has been characterized to a greater extent than its Fshb counterpart. Increased Lhb transcription at fast GnRH pulse frequencies corresponds to elevated EGR1 levels in LβT2 cells (Kanasaki et al., 2005), a key factor in gonadotropin regulation. Two EGR1, two SF1, and a homeodomain element exist in the proximal Lhb promoter (Halvorson et al., 1996; Quirk et al., 2001). EGR1/2 and inhibitors of the EGR family, NAB1/2 (Ngfi-A binding proteins) respond to pulsatile GnRH to a greater extent at fast and slow frequencies, respectively (Kaiser et al., 2000; Lawson et al., 2007). Pharmacologic blockade of ERK reduces both Fshb and Lhb transcription (Kanasaki et al., 2005), in correlation with studies demonstrating that Egr1 transcription is dependent on ERK (Dorn et al., 1999; Fortin et al., 2009; Lawson et al., 2007; Lee et al., 1996; Wolfe and Call, 1999). The more rapid and sustained phosphorylation of ERK at slow GnRH pulse frequencies could be a mediator of NAB1/2 induction, since an increase in EGR1 would still be expected, although this needs to be challenged further in perifusion paradigms. Alternatively, or in addition, GnRH pulsatility has been shown to induce proteasome function. Ubiquitination of EGR1 (as well as SF1) corresponds to GnRH pulse frequency and binding of these transcription factors to the Lhb promoter (Walsh and Shupnik, 2009). In order to differentiate between GnRH pulses, NAB1/2 expression at slow frequencies may serve as a mechanism to reduce (relative to fast GnRH pulse frequencies) Lhb transcription (Lawson et al., 2007).
EGR1 contributes to the induction of MKP2 (Zhang et al., 2001a; Zhang et al., 2001b), providing a potential mechanism by which a classical regulator of Lhb could also affect Fshb transcription. Increased EGR1 and subsequent MKP2 expression at fast GnRH pulse frequencies may decrease phosphorylated ERK levels, followed by reduction in the activity of inducers of Fshb transcription, such as AP1 proteins.
The differential activation of signaling pathways dependent on GnRH pulse frequency underpins the expression or activation of the transcription factors that modulate Fshb and Lhb transcription (see Figure 1). Signaling pathways that are stimulated at both fast and slow GnRH pulse frequencies have been identified; therefore, these cascades are not unique to either pulse frequency condition. This raises the possibility that the magnitude and duration, in addition to the frequency of activation of these pathways, are important in decoding pulsatile GnRH. This is highlighted when we consider the role of ERK, whereby changes in the pattern of ERK activation due to pulsatile GnRH signaling have been observed. Multiple transcription factors are involved in the response to the pulsatile GnRH signal. This represents an apparent sensing by the gonadotrope of the frequency of the GnRH signal. Considering that our example, ERK, also has a fundamental role in Lhb synthesis, further understanding of these signaling mechanisms is required to ultimately reveal how the gonadotrope decodes the pulsatile GnRH signal.
CONCLUSIONS AND FUTURE DIRECTIONS
The most significant question remains unanswered, how do gonadotropes respond differentially to the same ligand? The control of ovulatory and menstrual cycles is extremely complex, so it is not surprising that the mechanisms required to orchestrate these are equally so. A network of signaling pathways have been implicated in both FSH and LH synthesis at both slow and fast GnRH frequencies. In order to decode the GnRH signal, further insight into the kinase cascades and regulation of phosphatase activity and other pathways involved in the inactivation of kinases is necessary. Furthermore, the responses to GnRH could be further mediated or modulated by other pathways such as inhibins and activins, sex steroid feedback, or epigenetic regulation, which have not been discussed here.
HIGHLIGHTS.
The pituitary gonadotropin hormones, FSH and LH, are essential for fertility
GnRH regulates FSH and LH synthesis and secretion from gonadotropes
Preferential Fshḅ or Lhḅ subuniṭ transcription is dependent on GnRH pulse frequency
Varying frequencies of pulsatile GnRH activate multiple distinct signaling pathways
Both stimulatory and repressive transcription factors are activated by pulsatile GnRH
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure Statement: The authors have nothing to disclose
REFERENCES
- Alarid ET, Windle JJ, Whyte DB, Mellon PL. Immortalization of pituitary cells at discrete stages of development by directed oncogenesis in transgenic mice. Development. 1996;122:3319–3329. doi: 10.1242/dev.122.10.3319. [DOI] [PubMed] [Google Scholar]
- Armstrong SP, Caunt CJ, Fowkes RC, Tsaneva-Atanasova K, McArdle CA. Pulsatile and sustained gonadotropin-releasing hormone (GnRH) receptor signaling: does the Ca2+/NFAT signaling pathway decode GnRH pulse frequency? J Biol Chem. 2009a;284:35746–35757. doi: 10.1074/jbc.M109.063917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong SP, Caunt CJ, Fowkes RC, Tsaneva-Atanasova K, McArdle CA. Pulsatile and sustained gonadotropin-releasing hormone (GnRH) receptor signaling: does the ERK signaling pathway decode GnRH pulse frequency? J Biol Chem. 2010;285:24360–24371. doi: 10.1074/jbc.M110.115964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong SP, Caunt CJ, McArdle CA. Gonadotropin-releasing hormone and protein kinase C signaling to ERK: spatiotemporal regulation of ERK by docking domains and dual-specificity phosphatases. Mol Endocrinol. 2009b;23:510–519. doi: 10.1210/me.2008-0333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belchetz PE, Plant TM, Nakai Y, Keogh EJ, Knobil E. Hypophysial responses to continuous and intermittent delivery of hypopthalamic gonadotropin-releasing hormone. Science. 1978;202:631–633. doi: 10.1126/science.100883. [DOI] [PubMed] [Google Scholar]
- Ben-Menahem D, Naor Z. Regulation of gonadotropin mRNA levels in cultured rat pituitary cells by gonadotropin-releasing hormone (GnRH): role for Ca2+ and protein kinase C. Biochemistry. 1994;33:3698–3704. doi: 10.1021/bi00178a029. [DOI] [PubMed] [Google Scholar]
- Benard O, Naor Z, Seger R. Role of dynamin, Src, and Ras in the protein kinase C-mediated activation of ERK by gonadotropin-releasing hormone. J Biol Chem. 2001;276:4554–4563. doi: 10.1074/jbc.M006995200. [DOI] [PubMed] [Google Scholar]
- Bernard DJ, Fortin J, Wang Y, Lamba P. Mechanisms of FSH synthesis: what we know, what we don't, and why you should care. Fertil Steril. 2010;93:2465–2485. doi: 10.1016/j.fertnstert.2010.03.034. [DOI] [PubMed] [Google Scholar]
- Blank SK, McCartney CR, Helm KD, Marshall JC. Neuroendocrine effects of androgens in adult polycystic ovary syndrome and female puberty. Semin Reprod Med. 2007;25:352–359. doi: 10.1055/s-2007-984741. [DOI] [PubMed] [Google Scholar]
- Bliss SP, Miller A, Navratil AM, Xie J, McDonough SP, Fisher PJ, Landreth GE, Roberson MS. ERK signaling in the pituitary is required for female but not male fertility. Mol Endocrinol. 2009;23:1092–1101. doi: 10.1210/me.2009-0030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliss SP, Navratil AM, Xie J, Roberson MS. GnRH signaling, the gonadotrope and endocrine control of fertility. Front Neuroendocrinol. 2010;31:322–340. doi: 10.1016/j.yfrne.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonfil D, Chuderland D, Kraus S, Shahbazian D, Friedberg I, Seger R, Naor Z. Extracellular signal-regulated kinase, Jun N-terminal kinase, p38, and c-Src are involved in gonadotropin-releasing hormone-stimulated activity of the glycoprotein hormone follicle-stimulating hormone beta-subunit promoter. Endocrinology. 2004;145:2228–2244. doi: 10.1210/en.2003-1418. [DOI] [PubMed] [Google Scholar]
- Burger LL, Haisenleder DJ, Aylor KW, Marshall JC. Regulation of intracellular signaling cascades by GNRH pulse frequency in the rat pituitary: roles for CaMK II, ERK, and JNK activation. Biol Reprod. 2008;79:947–953. doi: 10.1095/biolreprod.108.070987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger LL, Haisenleder DJ, Dalkin AC, Marshall JC. Regulation of gonadotropin subunit gene transcription. J Mol Endocrinol. 2004;33:559–584. doi: 10.1677/jme.1.01600. [DOI] [PubMed] [Google Scholar]
- Caunt CJ, Armstrong SP, Rivers CA, Norman MR, McArdle CA. Spatiotemporal regulation of ERK2 by dual specificity phosphatases. J Biol Chem. 2008;283:26612–26623. doi: 10.1074/jbc.M801500200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciccone NA, Kaiser UB. The biology of gonadotroph regulation. Curr Opin Endocrinol Diabetes Obes. 2009;16:321–327. doi: 10.1097/MED.0b013e32832d88fb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciccone NA, Lacza CT, Hou MY, Gregory SJ, Kam KY, Xu S, Kaiser UB. A composite element that binds basic helix loop helix and basic leucine zipper transcription factors is important for gonadotropin-releasing hormone regulation of the follicle-stimulating hormone beta gene. Mol Endocrinol. 2008;22:1908–1923. doi: 10.1210/me.2007-0455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciccone NA, Xu S, Lacza CT, Carroll RS, Kaiser UB. Frequency-dependent regulation of follicle-stimulating hormone beta by pulsatile gonadotropin-releasing hormone is mediated by functional antagonism of bZIP transcription factors. Mol Cell Biol. 2010;30:1028–1040. doi: 10.1128/MCB.00848-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coss D, Jacobs SB, Bender CE, Mellon PL. A novel AP-1 site is critical for maximal induction of the follicle-stimulating hormone beta gene by gonadotropin-releasing hormone. J Biol Chem. 2004;279:152–162. doi: 10.1074/jbc.M304697200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford JL, McNeilly AS. Co-localisation of gonadotrophins and granins in gonadotrophs at different stages of the oestrous cycle in sheep. J Endocrinol. 2002;174:179–194. doi: 10.1677/joe.0.1740179. [DOI] [PubMed] [Google Scholar]
- Crawford JL, McNeilly JR, Nicol L, McNeilly AS. Promotion of intragranular co-aggregation with LH by enhancement of secretogranin II storage resulted in increased intracellular granule storage in gonadotrophs of GnRH-deprived male mice. Reproduction. 2002;124:267–277. doi: 10.1530/rep.0.1240267. [DOI] [PubMed] [Google Scholar]
- Dalkin AC, Haisenleder DJ, Ortolano GA, Ellis TR, Marshall JC. The frequency of gonadotropin-releasing-hormone stimulation differentially regulates gonadotropin subunit messenger ribonucleic acid expression. Endocrinology. 1989;125:917–924. doi: 10.1210/endo-125-2-917. [DOI] [PubMed] [Google Scholar]
- Davidson JS, Wakefield IK, Millar RP. Absence of rapid desensitization of the mouse gonadotropin-releasing hormone receptor. Biochem J. 1994;300(Pt 2):299–302. doi: 10.1042/bj3000299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denef C. Paracrinicity: the story of 30 years of cellular pituitary crosstalk. J Neuroendocrinol. 2008;20:1–70. doi: 10.1111/j.1365-2826.2007.01616.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorn C, Ou Q, Svaren J, Crawford PA, Sadovsky Y. Activation of luteinizing hormone beta gene by gonadotropin-releasing hormone requires the synergy of early growth response-1 and steroidogenic factor-1. J Biol Chem. 1999;274:13870–13876. doi: 10.1074/jbc.274.20.13870. [DOI] [PubMed] [Google Scholar]
- Duan WR, Ito M, Park Y, Maizels ET, Hunzicker-Dunn M, Jameson JL. GnRH regulates early growth response protein 1 transcription through multiple promoter elements. Mol Endocrinol. 2002;16:221–233. doi: 10.1210/mend.16.2.0779. [DOI] [PubMed] [Google Scholar]
- Dunaif A. Insulin resistance and the polycystic ovary syndrome: mechanism and implications for pathogenesis. Endocr Rev. 1997;18:774–800. doi: 10.1210/edrv.18.6.0318. [DOI] [PubMed] [Google Scholar]
- Fallest PC, Schwartz NB. Acute inhibitory effects of 17 beta-estradiol are observed on gonadotropin secretion from perifused pituitary fragments of metestrous, but not proestrous, rats. Endocrinology. 1991;128:273–279. doi: 10.1210/endo-128-1-273. [DOI] [PubMed] [Google Scholar]
- Farnworth PG. Gonadotrophin secretion revisited. How many ways can FSH leave a gonadotroph? J Endocrinol. 1995;145:387–395. doi: 10.1677/joe.0.1450387. [DOI] [PubMed] [Google Scholar]
- Ferris HA, Shupnik MA. Mechanisms for pulsatile regulation of the gonadotropin subunit genes by GNRH1. Biol Reprod. 2006;74:993–998. doi: 10.1095/biolreprod.105.049049. [DOI] [PubMed] [Google Scholar]
- Fortin J, Lamba P, Wang Y, Bernard DJ. Conservation of mechanisms mediating gonadotrophin-releasing hormone 1 stimulation of human luteinizing hormone beta subunit transcription. Mol Hum Reprod. 2009;15:77–87. doi: 10.1093/molehr/gan079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner S, Pawson AJ. Emerging targets of the GnRH receptor: novel interactions with Wnt signalling mediators. Neuroendocrinology. 2009;89:241–251. doi: 10.1159/000165377. [DOI] [PubMed] [Google Scholar]
- Garrel G, Simon V, Thieulant ML, Cayla X, Garcia A, Counis R, Cohen-Tannoudji J. Sustained gonadotropin-releasing hormone stimulation mobilizes the cAMP/PKA pathway to induce nitric oxide synthase type 1 expression in rat pituitary cells in vitro and in vivo at proestrus. Biol Reprod. 2010;82:1170–1179. doi: 10.1095/biolreprod.109.082925. [DOI] [PubMed] [Google Scholar]
- Gharib SD, Wierman ME, Shupnik MA, Chin WW. Molecular biology of the pituitary gonadotropins. Endocr Rev. 1990;11:177–199. doi: 10.1210/edrv-11-1-177. [DOI] [PubMed] [Google Scholar]
- Grafer CM, Thomas R, Lambrakos L, Montoya I, White S, Halvorson LM. GnRH stimulates expression of PACAP in the pituitary gonadotropes via both the PKA and PKC signaling systems. Mol Endocrinol. 2009;23:1022–1032. doi: 10.1210/me.2008-0477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham KE, Nusser KD, Low MJ. LbetaT2 gonadotroph cells secrete follicle stimulating hormone (FSH) in response to active A. J Endocrinol. 1999;162:R1–5. doi: 10.1677/joe.0.162r001. [DOI] [PubMed] [Google Scholar]
- Grosse R, Schmid A, Schoneberg T, Herrlich A, Muhn P, Schultz G, Gudermann T. Gonadotropin-releasing hormone receptor initiates multiple signaling pathways by exclusively coupling to G(q/11) proteins. J Biol Chem. 2000;275:9193–9200. doi: 10.1074/jbc.275.13.9193. [DOI] [PubMed] [Google Scholar]
- Haisenleder DJ, Burger LL, Aylor KW, Dalkin AC, Marshall JC. Gonadotropin-releasing hormone stimulation of gonadotropin subunit transcription: evidence for the involvement of calcium/calmodulin-dependent kinase II (Ca/CAMK II) activation in rat pituitaries. Endocrinology. 2003a;144:2768–2774. doi: 10.1210/en.2002-0168. [DOI] [PubMed] [Google Scholar]
- Haisenleder DJ, Dalkin AC, Ortolano GA, Marshall JC, Shupnik MA. A pulsatile gonadotropin-releasing hormone stimulus is required to increase transcription of the gonadotropin subunit genes: evidence for differential regulation of transcription by pulse frequency in vivo. Endocrinology. 1991;128:509–517. doi: 10.1210/endo-128-1-509. [DOI] [PubMed] [Google Scholar]
- Haisenleder DJ, Ferris HA, Shupnik MA. The calcium component of gonadotropin-releasing hormone-stimulated luteinizing hormone subunit gene transcription is mediated by calcium/calmodulin-dependent protein kinase type II. Endocrinology. 2003b;144:2409–2416. doi: 10.1210/en.2002-0013. [DOI] [PubMed] [Google Scholar]
- Haisenleder DJ, Workman LJ, Burger LL, Aylor KW, Dalkin AC, Marshall JC. Gonadotropin subunit transcriptional responses to calcium signals in the rat: evidence for regulation by pulse frequency. Biol Reprod. 2001;65:1789–1793. doi: 10.1095/biolreprod65.6.1789. [DOI] [PubMed] [Google Scholar]
- Haisenleder DJ, Yasin M, Marshall JC. Gonadotropin subunit and gonadotropin-releasing hormone receptor gene expression are regulated by alterations in the frequency of calcium pulsatile signals. Endocrinology. 1997;138:5227–5230. doi: 10.1210/endo.138.12.5611. [DOI] [PubMed] [Google Scholar]
- Halvorson LM, Kaiser UB, Chin WW. Stimulation of luteinizing hormone beta gene promoter activity by the orphan nuclear receptor, steroidogenic factor-1. J Biol Chem. 1996;271:6645–6650. doi: 10.1074/jbc.271.12.6645. [DOI] [PubMed] [Google Scholar]
- Han XB, Conn PM. The role of protein kinases A and C pathways in the regulation of mitogen-activated protein kinase activation in response to gonadotropin-releasing hormone receptor activation. Endocrinology. 1999;140:2241–2251. doi: 10.1210/endo.140.5.6707. [DOI] [PubMed] [Google Scholar]
- Hapgood JP, Sadie H, van Biljon W, Ronacher K. Regulation of expression of mammalian gonadotrophin-releasing hormone receptor genes. J Neuroendocrinol. 2005;17:619–638. doi: 10.1111/j.1365-2826.2005.01353.x. [DOI] [PubMed] [Google Scholar]
- Harris D, Bonfil D, Chuderland D, Kraus S, Seger R, Naor Z. Activation of MAPK cascades by GnRH: ERK and Jun N-terminal kinase are involved in basal and GnRH-stimulated activity of the glycoprotein hormone LHbeta-subunit promoter. Endocrinology. 2002;143:1018–1025. doi: 10.1210/endo.143.3.8675. [DOI] [PubMed] [Google Scholar]
- Hoffman LK, Ehrmann DA. Cardiometabolic features of polycystic ovary syndrome. Nat Clin Pract Endocrinol Metab. 2008;4:215–222. doi: 10.1038/ncpendmet0755. [DOI] [PubMed] [Google Scholar]
- Hsieh KP, Martin TF. Thyrotropin-releasing hormone and gonadotropin-releasing hormone receptors activate phospholipase C by coupling to the guanosine triphosphate-binding proteins Gq and G11. Mol Endocrinol. 1992;6:1673–1681. doi: 10.1210/mend.6.10.1333052. [DOI] [PubMed] [Google Scholar]
- Jakubowiak A, Janecki A, Steinberger A. Similar effects of inhibin and cycloheximide on gonadotropin release in superfused pituitary cell cultures. Biol Reprod. 1989;41:454–463. doi: 10.1095/biolreprod41.3.454. [DOI] [PubMed] [Google Scholar]
- Kaiser UB, Halvorson LM, Chen MT. Sp1, steroidogenic factor 1 (SF-1), and early growth response protein 1 (egr-1) binding sites form a tripartite gonadotropin-releasing hormone response element in the rat luteinizing hormone-beta gene promoter: an integral role for SF-1. Mol Endocrinol. 2000;14:1235–1245. doi: 10.1210/mend.14.8.0507. [DOI] [PubMed] [Google Scholar]
- Kaiser UB, Jakubowiak A, Steinberger A, Chin WW. Differential effects of gonadotropin-releasing hormone (GnRH) pulse frequency on gonadotropin subunit and GnRH receptor messenger ribonucleic acid levels in vitro. Endocrinology. 1997;138:1224–1231. doi: 10.1210/endo.138.3.4968. [DOI] [PubMed] [Google Scholar]
- Kaiser UB, Sabbagh E, Katzenellenbogen RA, Conn PM, Chin WW. A mechanism for the differential regulation of gonadotropin subunit gene expression by gonadotropin-releasing hormone. Proc Natl Acad Sci U S A. 1995;92:12280–12284. doi: 10.1073/pnas.92.26.12280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakar SS, Winters SJ, Zacharias W, Miller DM, Flynn S. Identification of distinct gene expression profiles associated with treatment of LbetaT2 cells with gonadotropin-releasing hormone agonist using microarray analysis. Gene. 2003;308:67–77. doi: 10.1016/s0378-1119(03)00446-3. [DOI] [PubMed] [Google Scholar]
- Kanasaki H, Bedecarrats GY, Kam KY, Xu S, Kaiser UB. Gonadotropin-releasing hormone pulse frequency-dependent activation of extracellular signal-regulated kinase pathways in perifused LbetaT2 cells. Endocrinology. 2005;146:5503–5513. doi: 10.1210/en.2004-1317. [DOI] [PubMed] [Google Scholar]
- Kawakami S, Fujii Y, Okada Y, Winters SJ. Paracrine regulation of FSH by follistatin in folliculostellate cell-enriched primate pituitary cell cultures. Endocrinology. 2002;143:2250–2258. doi: 10.1210/endo.143.6.8857. [DOI] [PubMed] [Google Scholar]
- Krsmanovic LZ, Mores N, Navarro CE, Arora KK, Catt KJ. An agonist-induced switch in G protein coupling of the gonadotropin-releasing hormone receptor regulates pulsatile neuropeptide secretion. Proc Natl Acad Sci U S A. 2003;100:2969–2974. doi: 10.1073/pnas.0535708100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landy H, Boepple PA, Mansfield MJ, Whitcomb RW, Schneyer AL, Crawford JD, Crigler JF, Jr., Crowley WF., Jr. Altered patterns of pituitary secretion and renal excretion of free alpha-subunit during gonadotropin-releasing hormone agonist-induced pituitary desensitization. J Clin Endocrinol Metab. 1991;72:711–717. doi: 10.1210/jcem-72-3-711. [DOI] [PubMed] [Google Scholar]
- Lariviere S, Garrel G, Simon V, Soh JW, Laverriere JN, Counis R, Cohen-Tannoudji J. Gonadotropin-releasing hormone couples to 3',5'-cyclic adenosine-5'-monophosphate pathway through novel protein kinase Cdelta and -epsilon in LbetaT2 gonadotrope cells. Endocrinology. 2007;148:1099–1107. doi: 10.1210/en.2006-1473. [DOI] [PubMed] [Google Scholar]
- Lawson MA, Tsutsumi R, Zhang H, Talukdar I, Butler BK, Santos SJ, Mellon PL, Webster NJ. Pulse sensitivity of the luteinizing hormone beta promoter is determined by a negative feedback loop Involving early growth response-1 and Ngfi-A binding protein 1 and 2. Mol Endocrinol. 2007;21:1175–1191. doi: 10.1210/me.2006-0392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SL, Sadovsky Y, Swirnoff AH, Polish JA, Goda P, Gavrilina G, Milbrandt J. Luteinizing hormone deficiency and female infertility in mice lacking the transcription factor NGFI-A (Egr-1). Science. 1996;273:1219–1221. doi: 10.1126/science.273.5279.1219. [DOI] [PubMed] [Google Scholar]
- Levi NL, Hanoch T, Benard O, Rozenblat M, Harris D, Reiss N, Naor Z, Seger R. Stimulation of Jun N-terminal kinase (JNK) by gonadotropin-releasing hormone in pituitary alpha T3-1 cell line is mediated by protein kinase C, c-Src, and CDC42. Mol Endocrinol. 1998;12:815–824. doi: 10.1210/mend.12.6.0120. [DOI] [PubMed] [Google Scholar]
- Lim S, Luo M, Koh M, Yang M, bin Abdul Kadir MN, Tan JH, Ye Z, Wang W, Melamed P. Distinct mechanisms involving diverse histone deacetylases repress expression of the two gonadotropin beta-subunit genes in immature gonadotropes, and their actions are overcome by gonadotropin-releasing hormone. Mol Cell Biol. 2007;27:4105–4120. doi: 10.1128/MCB.00248-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin X, Conn PM. Transcriptional activation of gonadotropin-releasing hormone (GnRH) receptor gene by GnRH and cyclic adenosine monophosphate. Endocrinology. 1998;139:3896–3902. doi: 10.1210/endo.139.9.6214. [DOI] [PubMed] [Google Scholar]
- Liu F, Austin DA, Mellon PL, Olefsky JM, Webster NJ. GnRH activates ERK1/2 leading to the induction of c-fos and LHbeta protein expression in LbetaT2 cells. Mol Endocrinol. 2002a;16:419–434. doi: 10.1210/mend.16.3.0791. [DOI] [PubMed] [Google Scholar]
- Liu F, Usui I, Evans LG, Austin DA, Mellon PL, Olefsky JM, Webster NJ. Involvement of both G(q/11) and G(s) proteins in gonadotropin-releasing hormone receptor-mediated signaling in L beta T2 cells. J Biol Chem. 2002b;277:32099–32108. doi: 10.1074/jbc.M203639200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McArdle CA, Forrest-Owen W, Willars G, Davidson J, Poch A, Kratzmeier M. Desensitization of gonadotropin-releasing hormone action in the gonadotrope-derived alpha T3-1 cell line. Endocrinology. 1995;136:4864–4871. doi: 10.1210/endo.136.11.7588218. [DOI] [PubMed] [Google Scholar]
- McArdle CA, Willars GB, Fowkes RC, Nahorski SR, Davidson JS, Forrest-Owen W. Desensitization of gonadotropin-releasing hormone action in alphaT3-1 cells due to uncoupling of inositol 1,4,5-trisphosphate generation and Ca2+ mobilization. J Biol Chem. 1996;271:23711–23717. doi: 10.1074/jbc.271.39.23711. [DOI] [PubMed] [Google Scholar]
- McNeilly AS, Crawford JL, Taragnat C, Nicol L, McNeilly JR. The differential secretion of FSH and LH: regulation through genes, feedback and packaging. Reproduction. 2003;61:463–476. [PubMed] [Google Scholar]
- Millar RP. GnRH II and type II GnRH receptors. Trends Endocrinol Metab. 2003;14:35–43. doi: 10.1016/s1043-2760(02)00016-4. [DOI] [PubMed] [Google Scholar]
- Millar RP. GnRHs and GnRH receptors. Anim Reprod Sci. 2005;88:5–28. doi: 10.1016/j.anireprosci.2005.05.032. [DOI] [PubMed] [Google Scholar]
- Mistry DS, Tsutsumi R, Fernandez M, Sharma S, Cardenas SA, Lawson MA, Webster NJ. Gonadotropin-releasing hormone pulse sensitivity of follicle-stimulating hormone-beta gene is mediated by differential expression of positive regulatory activator protein 1 factors and corepressors SKIL and TGIF1. Mol Endocrinol. 2011;25:1387–1403. doi: 10.1210/me.2011-0032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulvaney JM, Roberson MS. Divergent signaling pathways requiring discrete calcium signals mediate concurrent activation of two mitogen-activated protein kinases by gonadotropin-releasing hormone. J Biol Chem. 2000;275:14182–14189. doi: 10.1074/jbc.275.19.14182. [DOI] [PubMed] [Google Scholar]
- Naor Z. Signaling by G-protein-coupled receptor (GPCR): studies on the GnRH receptor. Front Neuroendocrinol. 2009;30:10–29. doi: 10.1016/j.yfrne.2008.07.001. [DOI] [PubMed] [Google Scholar]
- Nicol L, McNeilly JR, Stridsberg M, McNeilly AS. Differential secretion of gonadotrophins: investigation of the role of secretogranin II and chromogranin A in the release of LH and FSH in LbetaT2 cells. J Mol Endocrinol. 2004;32:467–480. doi: 10.1677/jme.0.0320467. [DOI] [PubMed] [Google Scholar]
- Ooi GT, Tawadros N, Escalona RM. Pituitary cell lines and their endocrine applications. Mol Cell Endocrinol. 2004;228:1–21. doi: 10.1016/j.mce.2004.07.018. [DOI] [PubMed] [Google Scholar]
- Oosterom J, van Doornmalen EJ, Lobregt S, Blomenrohr M, Zaman GJ. High-throughput screening using beta-lactamase reporter-gene technology for identification of low-molecular-weight antagonists of the human gonadotropin releasing hormone receptor. Assay Drug Dev Technol. 2005;3:143–154. doi: 10.1089/adt.2005.3.143. [DOI] [PubMed] [Google Scholar]
- Pernasetti F, Vasilyev VV, Rosenberg SB, Bailey JS, Huang HJ, Miller WL, Mellon PL. Cell-specific transcriptional regulation of follicle-stimulating hormone-beta by activin and gonadotropin-releasing hormone in the LbetaT2 pituitary gonadotrope cell model. Endocrinology. 2001;142:2284–2295. doi: 10.1210/endo.142.6.8185. [DOI] [PubMed] [Google Scholar]
- Purwana IN, Kanasaki H, Mijiddorj T, Oride A, Miyazaki K. Induction of dual-specificity phosphatase 1 (DUSP1) by pulsatile gonadotropin-releasing hormone stimulation: role for gonadotropin subunit expression in mouse pituitary LbetaT2 cells. Biol Reprod. 2011;84:996–1004. doi: 10.1095/biolreprod.110.088526. [DOI] [PubMed] [Google Scholar]
- Quirk CC, Lozada KL, Keri RA, Nilson JH. A single Pitx1 binding site is essential for activity of the LHbeta promoter in transgenic mice. Mol Endocrinol. 2001;15:734–746. doi: 10.1210/mend.15.5.0628. [DOI] [PubMed] [Google Scholar]
- Roberson MS, Bliss SP, Xie J, Navratil AM, Farmerie TA, Wolfe MW, Clay CM. Gonadotropin-releasing hormone induction of extracellular-signal regulated kinase is blocked by inhibition of calmodulin. Mol Endocrinol. 2005;19:2412–2423. doi: 10.1210/me.2005-0094. [DOI] [PubMed] [Google Scholar]
- Savoy-Moore RT, Swartz KH. Several GnRH stimulation frequencies differentially release FSH and LH from isolated, perfused rat anterior pituitary cells. Adv Exp Med Biol. 1987;219:641–645. doi: 10.1007/978-1-4684-5395-9_35. [DOI] [PubMed] [Google Scholar]
- Sealfon SC, Weinstein H, Millar RP. Molecular mechanisms of ligand interaction with the gonadotropin-releasing hormone receptor. Endocr Rev. 1997;18:180–205. doi: 10.1210/edrv.18.2.0295. [DOI] [PubMed] [Google Scholar]
- Seminara SB, Hayes FJ, Crowley WF., Jr. Gonadotropin-releasing hormone deficiency in the human (idiopathic hypogonadotropic hypogonadism and Kallmann's syndrome): pathophysiological and genetic considerations. Endocr Rev. 1998;19:521–539. doi: 10.1210/edrv.19.5.0344. [DOI] [PubMed] [Google Scholar]
- Stanislaus D, Ponder S, Ji TH, Conn PM. Gonadotropin-releasing hormone receptor couples to multiple G proteins in rat gonadotrophs and in GGH3 cells: evidence from palmitoylation and overexpression of G proteins. Biol Reprod. 1998;59:579–586. doi: 10.1095/biolreprod59.3.579. [DOI] [PubMed] [Google Scholar]
- Takeda M, Otsuka F, Otani H, Inagaki K, Miyoshi T, Suzuki J, Mimura Y, Ogura T, Makino H. Effects of peroxisome proliferator-activated receptor activation on gonadotropin transcription and cell mitosis induced by bone morphogenetic proteins in mouse gonadotrope LbetaT2 cells. J Endocrinol. 2007;194:87–99. doi: 10.1677/JOE-07-0138. [DOI] [PubMed] [Google Scholar]
- Thackray VG, Mellon PL, Coss D. Hormones in synergy: regulation of the pituitary gonadotropin genes. Mol Cell Endocrinol. 2010;314:192–203. doi: 10.1016/j.mce.2009.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P, Mellon PL, Turgeon J, Waring DW. The L beta T2 clonal gonadotrope: a model for single cell studies of endocrine cell secretion. Endocrinology. 1996;137:2979–2989. doi: 10.1210/endo.137.7.8770922. [DOI] [PubMed] [Google Scholar]
- Thompson IR, Ciccone NA, Xu S, Zaytseva S, Carroll RS, Kaiser UB. GnRH Pulse Frequency-Dependent Stimulation of FSHbeta Transcription Is Mediated via Activation of PKA and CREB. Mol Endocrinol. 2013;27:606–618. doi: 10.1210/me.2012-1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsutsumi R, Mistry D, Webster NJ. Signaling responses to pulsatile gonadotropin-releasing hormone in LbetaT2 gonadotrope cells. J Biol Chem. 2010;285:20262–20272. doi: 10.1074/jbc.M110.132662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turgeon JL, Kimura Y, Waring DW, Mellon PL. Steroid and pulsatile gonadotropin-releasing hormone (GnRH) regulation of luteinizing hormone and GnRH receptor in a novel gonadotrope cell line. Mol Endocrinol. 1996;10:439–450. doi: 10.1210/mend.10.4.8721988. [DOI] [PubMed] [Google Scholar]
- Walsh HE, Shupnik MA. Proteasome regulation of dynamic transcription factor occupancy on the GnRH-stimulated luteinizing hormone beta-subunit promoter. Mol Endocrinol. 2009;23:237–250. doi: 10.1210/me.2008-0098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Fortin J, Lamba P, Bonomi M, Persani L, Roberson MS, Bernard DJ. Activator protein-1 and smad proteins synergistically regulate human follicle-stimulating hormone beta-promoter activity. Endocrinology. 2008;149:5577–5591. doi: 10.1210/en.2008-0220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Washington TM, Blum JJ, Reed MC, Conn PM. A mathematical model for LH release in response to continuous and pulsatile exposure of gonadotrophs to GnRH. Theor Biol Med Model. 2004;1:9. doi: 10.1186/1742-4682-1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe T, Uchiyama Y, Grube D. Topology of chromogranin A and secretogranin II in the rat anterior pituitary: potential marker proteins for distinct secretory pathways in gonadotrophs. Histochemistry. 1991;96:285–293. doi: 10.1007/BF00271348. [DOI] [PubMed] [Google Scholar]
- Weiss J, Duca KA, Crowley WF., Jr. Gonadotropin-releasing hormone-induced stimulation and desensitization of free alpha-subunit secretion mirrors luteinizing hormone and follicle-stimulating hormone in perifused rat pituitary cells. Endocrinology. 1990;127:2364–2371. doi: 10.1210/endo-127-5-2364. [DOI] [PubMed] [Google Scholar]
- Wen S, Schwarz JR, Niculescu D, Dinu C, Bauer CK, Hirdes W, Boehm U. Functional characterization of genetically labeled gonadotropes. Endocrinology. 2008;149:2701–2711. doi: 10.1210/en.2007-1502. [DOI] [PubMed] [Google Scholar]
- Wildt L, Hausler A, Marshall G, Hutchison JS, Plant TM, Belchetz PE, Knobil E. Frequency and amplitude of gonadotropin-releasing hormone stimulation and gonadotropin secretion in the rhesus monkey. Endocrinology. 1981;109:376–385. doi: 10.1210/endo-109-2-376. [DOI] [PubMed] [Google Scholar]
- Willars GB, Heding A, Vrecl M, Sellar R, Blomenrohr M, Nahorski SR, Eidne KA. Lack of a C-terminal tail in the mammalian gonadotropin-releasing hormone receptor confers resistance to agonist-dependent phosphorylation and rapid desensitization. J Biol Chem. 1999;274:30146–30153. doi: 10.1074/jbc.274.42.30146. [DOI] [PubMed] [Google Scholar]
- Windle JJ, Weiner RI, Mellon PL. Cell lines of the pituitary gonadotrope lineage derived by targeted oncogenesis in transgenic mice. Mol Endocrinol. 1990;4:597–603. doi: 10.1210/mend-4-4-597. [DOI] [PubMed] [Google Scholar]
- Wolfe MW, Call GB. Early growth response protein 1 binds to the luteinizing hormone-beta promoter and mediates gonadotropin-releasing hormone-stimulated gene expression. Mol Endocrinol. 1999;13:752–763. doi: 10.1210/mend.13.5.0276. [DOI] [PubMed] [Google Scholar]
- Wu JC, Su P, Safwat NW, Sebastian J, Miller WL. Rapid, efficient isolation of murine gonadotropes and their use in revealing control of follicle-stimulating hormone by paracrine pituitary factors. Endocrinology. 2004;145:5832–5839. doi: 10.1210/en.2004-0257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wurmbach E, Yuen T, Ebersole BJ, Sealfon SC. Gonadotropin-releasing hormone receptor-coupled gene network organization. J Biol Chem. 2001;276:47195–47201. doi: 10.1074/jbc.M108716200. [DOI] [PubMed] [Google Scholar]
- Xie J, Bliss SP, Nett TM, Ebersole BJ, Sealfon SC, Roberson MS. Transcript profiling of immediate early genes reveals a unique role for activating transcription factor 3 in mediating activation of the glycoprotein hormone alpha-subunit promoter by gonadotropin-releasing hormone. Mol Endocrinol. 2005;19:2624–2638. doi: 10.1210/me.2005-0056. [DOI] [PubMed] [Google Scholar]
- Yehia G, Schlotter F, Razavi R, Alessandrini A, Molina CA. Mitogen-activated protein kinase phosphorylates and targets inducible cAMP early repressor to ubiquitin-mediated destruction. J Biol Chem. 2001;276:35272–35279. doi: 10.1074/jbc.M105404200. [DOI] [PubMed] [Google Scholar]
- Zhang T, Choy M, Jo M, Roberson MS. Structural organization of the rat mitogen-activated protein kinase phosphatase 2 gene. Gene. 2001a;273:71–79. doi: 10.1016/s0378-1119(01)00574-1. [DOI] [PubMed] [Google Scholar]
- Zhang T, Roberson MS. Role of MAP kinase phosphatases in GnRH-dependent activation of MAP kinases. J Mol Endocrinol. 2006;36:41–50. doi: 10.1677/jme.1.01881. [DOI] [PubMed] [Google Scholar]
- Zhang T, Wolfe MW, Roberson MS. An early growth response protein (Egr) 1 cis-element is required for gonadotropin-releasing hormone-induced mitogen-activated protein kinase phosphatase 2 gene expression. J Biol Chem. 2001b;276:45604–45613. doi: 10.1074/jbc.M107075200. [DOI] [PubMed] [Google Scholar]