Abstract
Glucagon is unstable and undergoes degradation and aggregation in aqueous solution. For this reason, its use in portable pumps for closed loop management of diabetes is limited to very short periods. In this study, we sought to identify the degradation mechanisms and the bioactivity of specific degradation products. We studied degradation in the alkaline range, a range at which aggregation is minimized. Native glucagon and analogs identical to glucagon degradation products were synthesized. To quantify biological activity in glucagon and in the degradation peptides, a protein kinase A-based bioassay was used. Aged, fresh, and modified peptides were analyzed by liquid chromatography with mass spectrometry (LCMS). Oxidation of glucagon at the Met residue was common but did not reduce bioactivity. Deamidation and isomerization were also common and were more prevalent at pH 10 than 9. The biological effects of deamidation and isomerization were unpredictable; deamidation at some sites did not reduce bioactivity. Deamidation of Gln 3, isomerization of Asp 9, and deamidation with isomerization at Asn 28 all caused marked potency loss. Studies with molecular-weight-cutoff membranes and LCMS revealed much greater fibrillation at pH 9 than 10. Further work is necessary to determine formulations of glucagon that minimize degradation and fibrillation.
Keywords: Glucagon, Degradation, Deamidation, Oxidation, Diabetes mellitus, Alkaline
INTRODUCTION
Glucagon is a 29-amino acid polypeptide whose major function is to increase plasma glucose by promoting glycogenolysis and gluconeogenesis [13]. Early studies with somatostatin established the role of glucagon in the minute-to-minute physiologic regulation of glucose homeostasis [7]. Its current therapeutic role is limited to rescue therapy for hypoglycemia [20].
Several recent investigations have shown the value of automated administration of glucagon with insulin in the setting of closed-loop glycemic control in persons with type 1 diabetes [4, 5, 10, 11, 29]. Because of its potency, glucagon (unlike glucose) offers the potential for delivery from a small portable pump worn on the body. However, such an application is hampered by its intrinsic physical instability and chemical degradation in aqueous solution. In aqueous environments, glucagon forms polymerized gels and fibrils, a process affected by temperature, concentration, and ionic strength [2, 8, 25, 26]. While fibrillation occurs rapidly at acidic pH, it is greatly minimized in alkaline solutions [14, 33]. Although fibrillation is minimized at a pH of 10, degradative processes such as deamidation tend to be prevalent at alkaline pH [15, 22]. Other degradative processes include oxidation at Met residues, a process to which glucagon is susceptible [21].
Joshi and Kirsch extensively studied the mechanisms of glucagon degradation at acid pH and found that deamidation was caused by direct hydrolysis of the amide side-chain by water [19]. In addition, they found unexpectedly that Gln deamidation occurred more commonly than Asn deamidation [16, 18]. In the current study, we explore the mechanisms of glucagon degradation in the alkaline pH range, a promising range for glucagon stability due to its low degree of fibrillation. Since the effect of specific biochemical changes such as deamidation or oxidation on receptor binding and biological activity are not always clear, we used a cell-based bioassay in the current study to measure the consequences of the aging-induced modifications. In addition, aged native glucagon was characterized by liquid chromatography coupled with mass spectrometry (LCMS). To better understand the effects of specific degradation products, the results for aged glucagon were compared to synthesized peptides that were identical to known glucagon degradation products.
1. RESEARCH DESIGN AND METHODS
1.1 Glucagon and degradation peptide synthesis
Native human glucagon and modified amino acid glucagon analogs were synthesized by AmbioPharm, Inc. (Beech Island, SC). The peptides were synthesized using standard stepwise N-9-fluorenylmethoxycarbonyl solid-phase peptide synthesis chemistry (Fmoc) and assembled in a linear fashion on Fmoc-Thr(tBu)-Wang resin [1]. The peptide was purified to >99% and shipped as a lyophilized solid.
1.2 Sample formulation and aging
Glycine buffer was prepared from solid glycine (Sigma-Aldrich, Saint Louis, MO) and brought to pH 9 or 10 with NaOH. Glucagon was added to yield a concentration of 1 mg/ml. The solutions were then passed through a 0.2 μm filter into a glass vial, sealed, and placed into a 37° C incubator for the desired aging period. Unaged samples were immediately placed into a −20° C freezer. After aging was completed, the samples were frozen at −20° C until analysis.
1.3 Green fluorescent protein-protein kinase A bioassay
The glucagon bioassay employed an engineered cell line expressing the human glucagon receptor and a fluorescent protein kinase A (PKA) catalytic subunit reporter molecule. The cAMP generated from activation of the glucagon receptor redistributes fluorescent PKA from a concentrated pattern to a diffuse, cytoplasmic pattern with a reduction in fluorescent signal. CHO-K1 cells stably overexpressing the human glucagon receptor and the catalytic domain of human PKA fused to the N-terminus of enhanced green fluorescent protein (EGFP) [35] were obtained from Thermo Scientific (Pittsburgh, PA). Cells were seeded at 20,000 cells per well in a 96-well plate and cultured overnight at 37°C/5% CO2 in Ham’s F-12 medium supplemented with 1% penicillin-streptomycin, 100 μg/ml amphotericin B, 0.5 mg/ml G418, 1 mg/ml Zeocin and 10% FBS. After overnight culture, the cells were exposed to glucagon (serially diluted in culture medium without antibiotics) for 30 min at 37°C/5% CO2. Cells were then fixed in 10% formalin for 20 minutes, washed, and subsequently labeled with 1 μM Hoechst DAPI in PBS for 30 minutes.
Cells were then imaged with a Marianas fluorescence microscope (Intelligent Imaging Innovations, Inc; Denver, CO) to quantify the DAPI and EGFP signals at 10×. Images were analyzed and quantified for fluorescence using Slidebook 5.0 (Intelligent Imagining Innovations). The fluorescence data measures the cellular response to glucagon as a GFP/DAPI ratio. The data was normalized against a zero glucagon control (designated as 100% fluorescence) then plotted as a dose response curve (x = dose of glucagon in pg/ml; y = % of bioactivity loss). The loss of fluorescence is the measure of the cellular response to glucagon. Using XLfit software program V. 5.3.1.3 (IDBS, Guildford, U.K.), a fitted curve was created using a sigmoidal Boltzman equation . Using the fitted curve, the 50% bioactivity level (EC50, a metric of potency, in pg/ml) was determined as the midpoint between the high and low data plateaus.
1.4 Liquid chromatography-mass spectrometry
Unaged, aged, and modified peptide samples were analyzed by LCMS in the OHSU Proteomics Shared Resource. Samples were diluted to 1 μM in 1% formic acid from 287 μM (equivalent to 1 mg/ml) and placed into Agilent autosampler vials, which were held at 4°C until analysis. Approximately 10 picomoles of protein was injected into an Agilent 1100 high performance liquid chromatography (HPLC) system fitted with a reverse-phase Agilent ZORBAX SB-C18 capillary column (Agilent Technologies, Santa Clara, CA). A mobile phase of 1% formic acid and a gradient of acetonitrile from 7.5-45% over 60 min were used. Mass spectrometry analysis was performed in a Thermo linear ion trap Velos instrument (Thermo Fisher Scientific, Inc., San Jose, CA). Peptide MS analysis and identification was performed using Xcalibur 2.2 (ThermoFisher Scientific Inc., San Jose, CA).
Unmodified glucagon has a monoisotopic molecular weight (MW) of 3,480.62 Da. Detection of oxidized or deamidated glucagon was carried out by examining species that had a MW increase of +16 or +1 Da, respectively. Other modifications were determined from the mass spectrum using a software program (Xcalibur 2.0, Thermo-Scientific) and web based proteomics tools.
1.5 Molecular weight fractionation
Size fractionation was performed by taking 100 μl of sample and applying it to an Amicon filter (Millipore Corp, Billerica, MA) with a nominal MW limit of 100 kDa. Material was centrifuged at 14,000 RPM for 5 min to separate the low and high-MW complexes. The filtrates were analyzed by LCMS as described above. The high-MW complexes (retentates) were resolubilized using 6 M urea and shaken for 30 min. After the incubation period, the filter tube was inverted into a collection tube, briefly centrifuged, and the material sent for LCMS.
1.6 Synthesis of peptide modifications
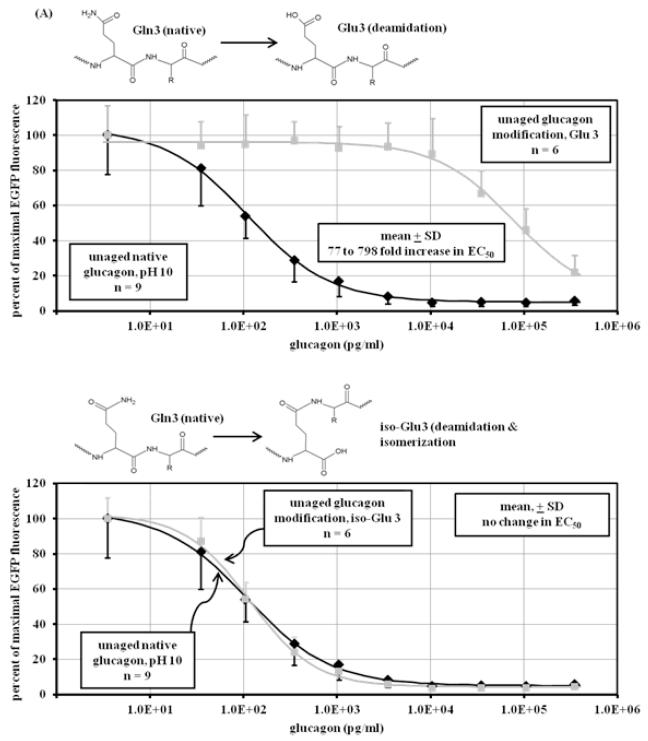
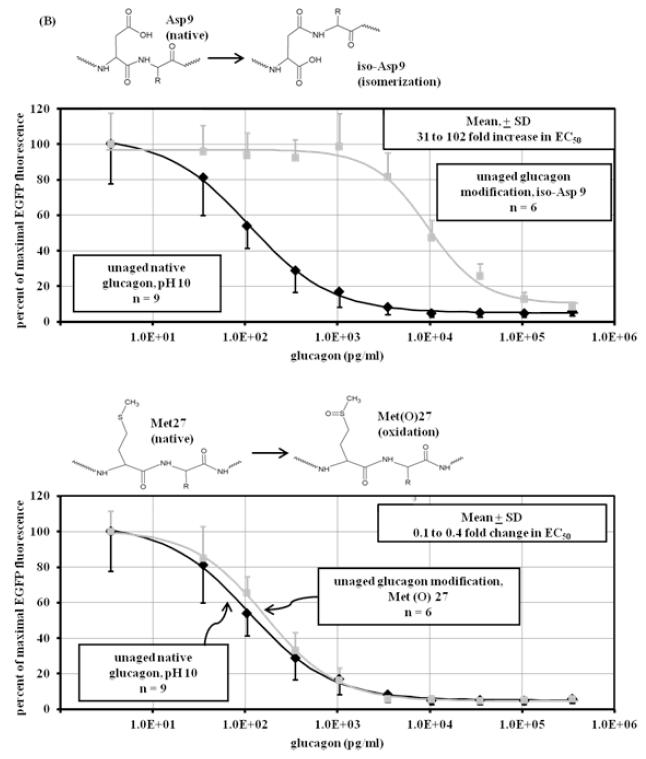
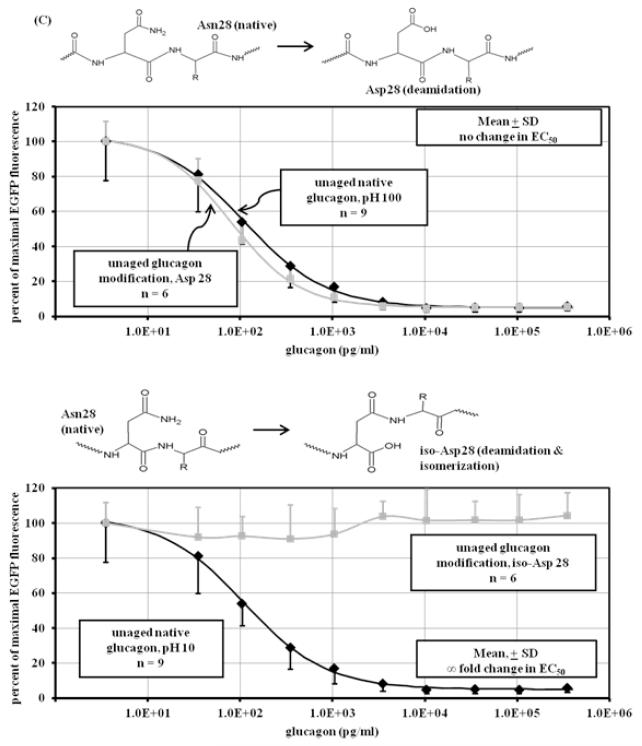
Although LCMS and bioassay data provide clues as to the identity of glucagon degradation products, the specific nature of those products can be elusive. For this reason, we synthesized modifications of glucagon and subjected those modified peptides to the same analyses (LCMS and cell-based bioassay) as for aged native glucagon. Six modifications were chosen, including deamidation, deamidation with isomerization, isomerization alone, and oxidation. The sites of these modifications encompassed much of the glucagon sequence, with two modifications occurring at the N terminus, one more centrally located and three at the C terminus. The modifications were: Gln 3 deamidated to Glu 3; Gln 3 deamidated and isomerized (rearranged) to iso-Glu 3; Asp 9 isomerized to iso-Asp 9; Met 27 oxidized to Met oxide 27; Asn 28 deamidated to Asp 28; and Asn 28 deamidated and isomerized to iso-Asp 28. Reaction diagrams for each can be found in Figure 4 A-C and are referred to as Glu3, iso-Glu3, iso-Asp9, Met(O)27, Asp28, and iso-Asp28. Like native glucagon samples, the solutions of modified glucagon analogs were created to a concentration of 1 mg/m before being analyzed by the glucagon bioassay and by LCMS. These samples were resuspended immediately before analysis to avoid aging in the aqueous state.
Figure 4.
Dose-response curve of unaged modified peptides. Shown above each graph are the chemical modifications of glucagon that are represented by the synthetic peptide. Response was measured in CHO cells overexpressing GFP-PKA by the diminution of GFP fluorescence expressed as percent of the maximum. (A) upper panel: Deamidation of glutamine at position 3 (Glu3) of glucagon (■) at pH 10 (n=6) decreases potency, showing a significant (p<0.001) loss of glucagon potency (78 to 798 fold) compared to unaged native glucagon (◆) at pH 10 (n=9); lower panel: deamidation and isomerization of glutamine at position 3 (iso-Glu3) of glucagon (■) at pH 10 (n=6) does not significantly (p=0.77) affect potency, showing no loss of potency compared to unaged native glucagon (◆) at pH 10 (n=9); (B) upper panel: isomerization of aspartate at position 9 (iso-Asp9) of glucagon (■) at pH 10.0 (n=6) decreases potency, showing a significant (p<0.001) loss of glucagon potency (31 to 102 fold) compared to unaged native glucagon (◆) at pH 10 (n=9); lower panel: oxidation of methionine at position 27 (Met(O)27) of glucagon (■) at pH 10 (n=6) does not significantly decreases potency (p=0.37), showing 0 to 0.5 fold loss of potency compared to unaged native glucagon (◆) at pH 10 (n=9); (C) upper panel: deamidation of asparagine at position 28 of glucagon (■) at pH 10 (n=6) does not significantly (p=0.13) affect potency, showing no loss of potency compared to unaged native glucagon (◆) at pH 10 (n=9); lower panel: deamidation and isomerization of asparagine at position 28 of glucagon (■) at pH 10 (n=6) leads to complete loss of potency and power compared to unaged native glucagon (◆) at pH 10 (n=9).
1.7 Statistical analysis
Comparisons were analyzed by a two-sided Student’s t-test with significance defined as p < 0.05. Results are presented as mean ± SD unless otherwise specified.
2. RESULTS
2.1 Bioassay shows loss of glucagon potency after aging
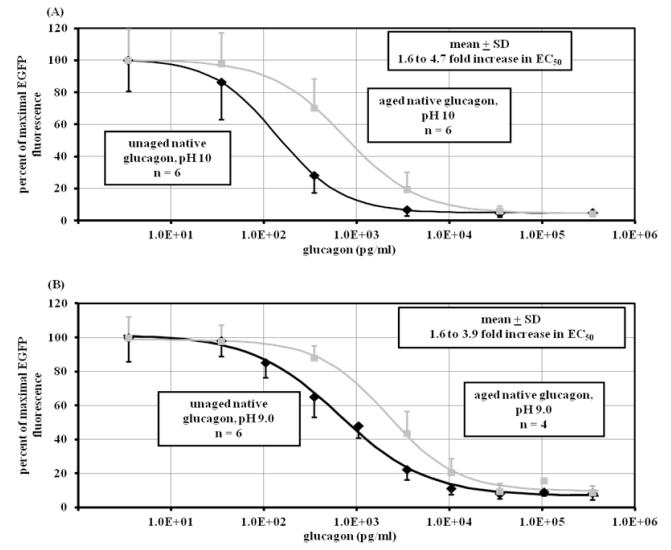
The bioassay was used to compare fresh vs aged glucagon. Figure 1A shows that after aging glucagon at pH 10 for 7 days at 37° C, potency declined as measured by an increase of EC50 by 1.6 to 4.7-fold vs fresh glucagon (p < 0.001, n = 6 for aged and fresh). Figure 1B shows a similar increase of EC50 (1.6 to 3.9-fold) for glucagon aged at pH 9 for 7 days vs fresh glucagon (p < 0.001, n = 6 for fresh, 4 for aged).
Figure 1.
Dose-response curve of aged and unaged native glucagon. Response was measured in CHO cell line by diminution of EGFP-PKA fluorescence expressed as percent of the maximum. (A) Native glucagon aged (■) for seven days at pH 10 (n=6) significantly decreases potency (p<0.001), showing a 1.6 to 4.8 increase in EC50 compared to unaged native glucagon (◆) at pH 10 (n=6). (B) Native glucagon aged (■) for seven days at pH 9.0 (n=6) significantly decreases potency (p<0.001), showing a 1.6 to 3.9 increase in EC50 compared to unaged native glucagon (◆) at pH 9 (n=4).
2.2 LCMS demonstrates the structural changes that contribute to potency loss during aging
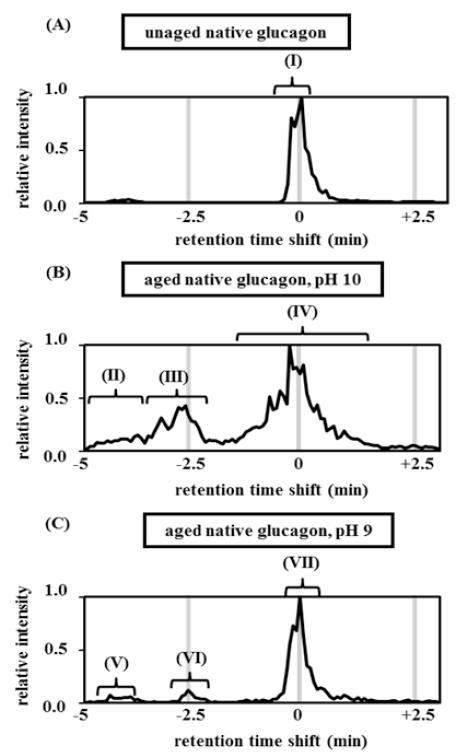
Unaged, native glucagon elutes as a single peak, as shown in Figure 2A, peak I. This peak was designated as having a retention time shift of zero. The +4 m/z peak (871.16 Da) was the largest mass-to-charge peak (data not shown). Figure 2B shows glucagon’s structural changes when aged at pH 10 for 7 days. With this preparation, the chromatogram reveals several secondary peaks (peaks II, III) that elute before the main peak. In addition, the main peak broadens substantially (3 min vs 1 min for fresh glucagon). Mass spectrometry analysis indicates that region II of Figure 2B is composed of oxidized glucagon (+16 Da). The oxidized glucagon becomes more hydrophilic and thus elutes earlier than the parent. In an attempt to verify the origin of this peak, the LCMS chromatogram was mass filtered using the mass of oxidized glucagon (+16). With mass filtration, oxidation was verified (region II was rich in oxidation while region III and IV show a minimal number of +16 ions, (see Supplementary Figure 1A).
Figure 2.
Reverse-phase LCMS chromatograms of unaged and aged native glucagon at 2 different pH values. The peak identification and retention time shifts were identified from the mass/ charge ratio for the +4 ion using mass spectrometry. Samples were separated using rpHPLC under the following conditions (A) unaged native glucagon at pH 10, (B) aged native glucagon at pH 9, (C) aged native glucagon at pH 10. Region I: +0 Da, unmodified; region II, V: +16 Da, oxidation at pH 10; region III, VI: +1 Da; deamidation with or without isomerization; region IV, VII: +0 Da, unmodified or deamidation with or without isomerization (cannot specifically detect deamidation due to isotopic distribution masking deamidated m/z spectrum).
An additional degradation product of the glucagon aged at pH 10 is shown in region III of Figure 2B and consists primarily of deamidated glucagon (+1 Da). This region may include isomerized products (no change in mass). Region IV of Figure 2B shows substantial broadening of the parent peak (3 min vs 1 min), with no major difference in MW. Deamidated glucagon is only one Dalton heavier than native glucagon and can be found within the isotopic distribution of the m/z spectrum for native glucagon, thus masking deamidation if native glucagon is present in large quantities. In addition, isomerization cannot be excluded as a contributor to peak broadening.
To better separate non-deamidated, singly deamidated, and doubly deamidated glucagon fractions, we also examined the isotopic distribution of the m/z spectrum. Least-squares fitting of a mixed model of atomic isotopic distributions showed that glucagon aged at pH 10 has more deamidation events than pH 9 (see Supplementary Figure 2).
Figure 2C shows glucagon in pH 9 buffer aged for 7 days at 37° C. The LCMS peaks are generally similar to those of the glucagon aged at pH 10, though with much less degradation (compare regions II, III, and IV from Figure 2B with regions V, VI and VII in Figure 2C). The m/z spectrum shown in Figure 2C is similar to the spectrum shown in Figure 2B, with oxidation at −4 minutes (V) and deamidation at −2.5 minutes (VI). Region V contained high amounts of oxidized glucagon as found by filtering for the oxidized glucagon mass (see Supplementary Figure 1B). The parent peak at pH 9 (VII) is only slightly broadened, further indicating that deamidation and isomerization were reduced at lower pH. The level of deamidation was also reduced when examined as the isotopic deconvolution distribution, as shown in Supplementary Figure 2. In addition to oxidation, deamidation, and isomerization, we found evidence of very minor degrees of chain scission at various points in the molecule, including near the N-terminus, near the C-terminus, and toward the mid portion (data not shown).
2.3 Fibrillation/Polymerization is pH dependent
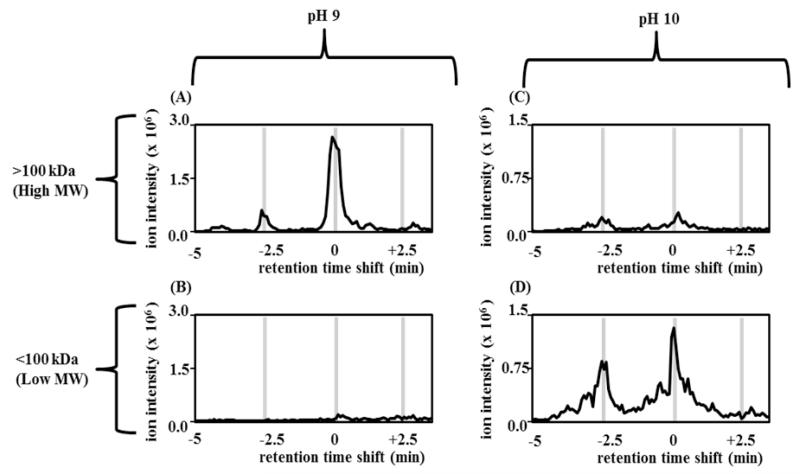
In order to compare the amounts of high versus low-MW complexes formed during aging at pH 10 and 9, molecular weight fractionation experiments were undertaken. Proteins were separated using a threshold of 100 kDa, equivalent to a multimer of 30 glucagon units. The two fractions for each pH value were then analyzed via LCMS. Absolute amounts of peptide from each pH were compared by quantifying the amount of peptide ions detected by MS.
Figure 3 shows the results of size fractionation for glucagon aged for 7 days at 37° C. The ion intensity of high substances at pH 9 was significantly greater than at pH 10. The chromatograms shows a much greater amount of high-MW compounds at pH 9 vs 10, suggesting that the amount of polymerized (fibrillated) protein is much greater at pH 9. The finding of similar retention times of the main degradation products in the retentate at pH 9 (3A) and in the filtrate at pH 10 (3C) suggest similar degradation mechanisms at the two pH values.
Figure 3.
Chromatograms of Size fractionation of aged glucagon at pH 9.0 and 10.0. Size fractionation was performed using 100 kDa NMWCO spin filters and analyzed by reverse-phase LCMS. (A) retentate (high MW complexes) of aged glucagon at pH 9; (B) filtrate (low MW complexes) of aged glucagon at pH 9; (C) retentate of aged glucagon at pH 10; (D) filtrate of aged glucagon at pH 10. The finding of a much greater amount of high MW compounds at pH 9 vs 10 suggests that the amount of fibrillated protein is much greater at pH 9. The finding of similar retention times of the main degradation products in the retentate at pH 9 and in the filtrate at pH 10 suggest similar degradation mechanisms at the two pH values.
2.4 The effect of peptide modifications on glucagon potency
Results for the modified glucagon analogs are shown in Figure 4 A-C. The Glu3 synthetic peptide shown in Figure 4A (upper panel) shows a marked increase (77 to 798-fold) of EC50 , indicating marked loss of potency compared to fresh native glucagon (p < 0.001). Interestingly, the rearrangement of Glu3 to iso-Glu3 (Figure 4A, lower panel) did not increase EC50 despite the change in peptide structure (p = 0.77). When Asp was isomerized to iso-Asp (without deamidation) at position 9 (Figure 4B, upper panel), there was a marked (31 to 102-fold) rise of EC50 (p < 0.001). Oxidation of Met at position 27 did not change EC50, as seen in Figure 4B, lower panel. The EC50 rise for Met(O)27 was very minor, and ranged from 0.1 to 0.4 fold (p = 0.37). Bioactivity of Asn deamidated to Asp at position 28 is shown in Figure 4C, upper panel. Compared to native glucagon, there was no rise in EC50 with this modification (p = 0.13). In contrast, when deamidation at position 28 was accompanied by isomerization, there was a complete loss of potency and power (Figure 4C, lower panel); no response to glucagon was detected even at its highest concentration.
2.5 LCMS analysis of modified peptides
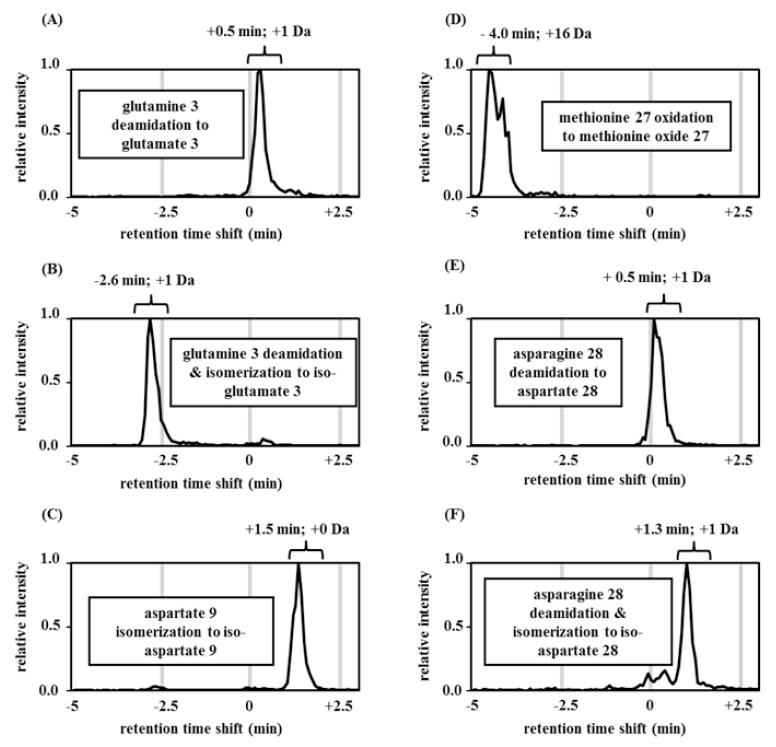
LCMS analyses of the modified glucagon peptides are shown in Figure 5 A-F. The Glu3 modification and the Asp28 modifications have prolonged retention times (approximately +0.5 min). Two of the three isomerizations also show prolonged retention times. The pure isomerization of Asp9 to iso-Asp9 shifted the parent peak by +1.5 min. The deamidation with isomerization to iso-Asp28 shifted the parent peak approximately +1.3 min. The retention time of iso-Glu3 was shifted by −2.6 minutes, which was reminiscent of the time shift for region III and VI for aged glucagon (Figure 2).
Figure 5.
Reverse-phase LCMS chromatograms of synthetic glucagon peptides. (A) Deamidation of glutamine at position 3 (Glu3) of glucagon shows +0.5 minute retention time shift; (B) deamidation and isomerization of glutamine at position 3 (iso-Glu3) of glucagon shows −2.6 minute retention time shift; (C) pure isomerization of aspartate at position 9 (iso-Asp9) of glucagon shows +1.5 minute retention time shift; (D) oxidation of methionine at position 27 (Met(O)27) of glucagon shows −4.0 minute retention time shift; (E) deamidation of asparagine at position 28 of glucagon shows +0.5 minute retention time shift; (F) deamidation and isomerization of asparagine at position 28 of glucagon shows +1.3 minute retention time shift.
We also found evidence of less frequent degradation pathways, including formation of glucagon adducts that likely arise from the addition of reactive molecules (hydrolyzed amino acids from glucagon, glycine buffer molecules, or other reactive molecules) to reactive sites of glucagon. Though uncommon, we found evidence of chain cleavage during aging, resulting in physical breakage of the peptide backbone into two parts.
3. DISCUSSION
3.1 Implications of degradation
Glucagon spontaneously degrades in aqueous solution. To better understand the mechanism of this degradation, we studied aged native glucagon and a number of peptides that were synthesized to mimic glucagon degradation products. An understanding of mechanisms underlying the loss of glucagon bioactivity is important for several reasons. For example, if glucagon is indwelled for several days in a portable pump, e.g., in the setting of an artificial endocrine pancreas system, it must retain bioactivity. In this study, we explored mechanisms of degradation at pH 9-10, an attractive range given its relative protection against amyloid fibrillation [33]. Since the effect of specific biochemical alterations on biological activity cannot be predicted, the use of a cellular bioassay was critical in determining the consequences of these modifications.
3.2 Deamidation and isomerization
During analysis with mass spectrometry, glucagon ionizes into a charged species; there are eight functional groups that can add a proton during the positive ionization step. By using LCMS, we found that the most commonly occurring modifications at alkaline pH are deamidation, isomerization, and oxidation. Deamidation is an important modification that readily occurs upon protein aging. Deamidation of human proteins is common in vivo and has been postulated to be a regulatory mechanism serving as a molecular clock [28]. Deamidation of Asp or Gln can also result in isomerization. In fact, Asp can isomerize without deamidation [34]. Isomerization causes a physical shift of the peptide backbone to the R group without a change in molecular weight [34].
Deamidation of Asn at position 28 caused very little loss of bioactivity. Even the combination of deamidation and isomerization of Gln at position 3 did not substantially decrease bioactivity despite rearrangement of the peptide backbone and increasing overall peptide length. In contrast, deamidation and isomerization of Asn at position 28 caused a marked loss of potency, as did deamidation of Gln at position 3 and isomerization of Asp at position 9. Clearly, in addition to the type of modification that occurs during degradation, the location of the modification is very important regarding the effect on bioactivity.
The glucagon molecule provides numerous sites for deamidation, including one Asn (position 28) and three Gln residues (positions 3, 20, and 24). Because Asn 28 is adjacent to a small residue, the terminal Thr, it is susceptible to deamidation [27]. The Gln residues at positions 3 and 20 also have compact amino acids at their C-terminal poles (Gly and Asp, respectively), favoring deamidation, though Gln residues are less likely to undergo deamidation than Asn [27]. Four of the five deamidated/isomerized glucagon peptides tested, with the exception of deamidation and isomerization at position 3, elute after the parent glucagon peak. This shift of 0.5-1 minute is likely caused by the R-group transforming from an amide to a carboxyl group, which in the presence of the acidic mobile phase is not ionized and thus increases its hydrophobicity. During aging, deamidation/isomerization appears to be present at both pH 10 and 9, as suggested by the broadening of the parent peak. The degree of modification, however, is markedly different at the two pH values. We found much less deamidation/isomerization at pH 9 as compared to pH 10, a finding that may have implications for development of a stable glucagon formulation. Joshi and Kirsch addressed glucagon degradation mechanisms at acid pH and determined that deamidation was caused by direct hydrolysis of the amide side-chain by water [19]. This group also found that in the pH range of 1-3, there was no evidence of isomerization [17].
3.3 Oxidation of glucagon and biological effects of degradation
Due likely to acquisition of a negative charge, glucagon oxidized at the Met residue underwent a retention time shift of approximately −4 min vs native glucagon. A similar retention time shift was also seen in aged native glucagon (regions II and V of Figure 2 and Supplemental Figure 1). It is therefore likely that Met is the residue oxidized during aging of native glucagon, though His and aromatic residues can also undergo oxidation. Previous reports regarding the bioactivity studies of glucagon oxidized at the Met residue have been conflicting. In terms of cAMP generation in cultured hepatocytes, oxidized glucagon was found to be slightly less potent than glucagon by one group [12] and much less potent by another [23]. Another group found that the potency of oxidized glucagon in causing glycogenolysis was normal [3]. In our study, rather than oxidizing native glucagon oxidation, we synthesized Met sulfoxide de novo and found that its potency and power to be essentially normal. Methodological differences in the cited studies may have accounted for the reported differences in bioactivity.
The biological effects of the different molecular alterations in glucagon were unpredictable. For example, deamidation did not uniformly reduce bioactivity, regardless of whether it was or was not accompanied by isomerization, and regardless of whether it occurred in an Asn or a Gln residue. For this reason, in the case of glucagon, it is not possible to generalize about the effect of deamidation on bioactivity without first knowing the location of the alteration.
3.4 Suitability of subcutaneous drugs formulated at an alkaline pH
Although most drugs are formulated at or near neutral pH, an alkaline formulation of the common diuretic, furosemide, was tolerated well when given via the subcutaneous route[31]. In our earlier study, we found that glucagon formulated at an alkaline pH was much less prone to aggregation than glucagon prepared at acid pH [33]. Because of glucagon’s isoelectric point of approximately 7, it is very difficult to achieve soluble preparations of glucagon at neutral pH. In light of these findings, we addressed the question of whether a subcutaneously-administered alkaline protein drug would be tolerable. Because of our interest in drugs stabilized at alkaline pH, we carried out a double blinded study in which normal volunteers were given albumin formulated at pH 7.4 and at pH 10. There was very little difference in discomfort between the two formulations [32]. Although we concede that alkaline glucagon has not been studied in humans, the study with alkaline albumin suggests that a glucagon formulation at pH 9-10 might also be tolerable in humans.
3.5 Aggregation and fibrillation of glucagon
Commercially-available glucagon preparations are acidic after reconstitution with sterile water, with a pH of 2.5-3. Although these preparations are well-suited for emergency treatment of hypoglycemia after reconstitution of the lyophilized powder, if maintained in aqueous solution over time, they form fibrillar amyloid aggregates which are potentially cytotoxic [24]. Glucagon formulated at pH 10 is largely free from these aggregates during aging, but the present study found that there was marked bioactivity loss of glucagon at pH 10 and at pH 9. The reason for the reduction in bioactivity at pH 9 could be the modifications that were found with LCMS, or fibrillation, which is greater at pH 9 than at 10. In this study, it is not possible to determine the degree to which fibrillation vs degradation reduced bioactivity in the cell-based assay. Prior studies from our laboratory [33] and from El Khatib et al. [9] found that fibrillated glucagon retains much of its bioactivity when given to animals, though in our study, we found a slightly delayed effect compared to fresh glucagon. Based on the nearly normal bioactivity in animal studies, we believe that most of the bioactivity loss of the glucagon preparations in the current study was due to molecular degradation.
3.5 Conclusions and other approaches to stabilization
To the extent that a stable liquid glucagon formulation can be developed, its use as a rescue medication for the treatment of severe hypoglycemia would not require mixing of water with a lyophilized powder. In addition, a stable liquid form of glucagon could be incorporated into a portable pump as part of an artificial endocrine pancreas for home use. There are several research groups addressing alternative formulations of glucagon to achieve stability, some of which involve creation of glucagon analogs [6]. Other approaches include the use of non-aqueous solvents (Xeris Pharmaceuticals) and the use of surfactants to increase solubility in the neutral pH range [30].
We conclude that during aging of glucagon at pH 9 and 10, peptide degradation mechanisms include oxidation, which did not substantially impair bioactivity. The occurrence of deamidation, with or without isomerization, at some sites, though not others, did lead to bioactivity loss. A much lower amount of high MW polymers developed during aging at pH 10 compared to pH 9, but the quantity of degradation was greater at pH 10.
Supplementary Material
ACKNOWLEDGMENTS
We thank the Juvenile Diabetes Research Foundation (#17-2012-15) for supporting this research. Mass spectrometric analysis was performed by the OHSU Proteomics Shared Resource (PSR) with partial support from NIH core grants P30EY010572 and P30CA069533. GFP-PKA bioassay imaging was performed at the OHSU Imaging and Morphology Core supported by NIH core grant P51RR000163. Special thanks to Dr. Philip Wilmarth of the OHSU PSR for providing the deamidation deconvolution template and training, Dr. Oleg Varlamov from the ONPRC for troubleshooting the GFP-PKA bioassay, Ashley E. White from ONPRC for GFP-PKA imaging and data analysis, Dr. Anda Cornea of the ONPRC Imaging Core, and Dr. Bruce Frank from Thermalin for consultation. Authors Caputo and Ward are the guarantors of this work and, as such, had full access to all the data in the study. They take full responsibility for the integrity of the data and the accuracy of the data analysis. WKW, JRC, MAJ, and CR conceived of, and planned the project. NC, CB, PB, MAJ, LD, and JMC researched and collected data; NC, CB, JRC, LD, CR, JMC, and WKW performed data analysis; NC, CB, JRC, and WKW wrote the manuscript.
Abbreviations
- HPLC
high performance liquid chromatography
- MS
mass spectrometry
- RT
retention time
- EGFP
enhanced green fluorescent protein
- PKA
protein kinase A
- MW
molecular weight
- Da
Dalton
- Fmoc
N-9-fluorenylmethoxycarbonyl
- rp
reverse phase
- CHO-K1
Chinese hamster ovary cells, K1 line
- EC50
effective concentration for 50% maximal activity
REFERENCES
- [1].Amblard M, Fehrentz JA, Martinez J, Subra G. Methods and protocols of modern solid phase Peptide synthesis. Mol Biotechnol. 2006;33:239–54. doi: 10.1385/MB:33:3:239. [DOI] [PubMed] [Google Scholar]
- [2].Beaven GH, Gratzer WB, Davies HG. Formation and structure of gels and fibrils from glucagon. Eur J Biochem. 1969;11:37–42. doi: 10.1111/j.1432-1033.1969.tb00735.x. [DOI] [PubMed] [Google Scholar]
- [3].Bromer WW. Springer-Verlag; New York, NY: 1983. Chemical Characteristics of Glucagon. [Google Scholar]
- [4].Castle JR, Engle JM, El Youssef J, Massoud RG, Ward WK. Factors influencing the effectiveness of glucagon for preventing hypoglycemia. J Diabetes Sci Technol. 2010;4:1305–10. doi: 10.1177/193229681000400603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Castle JR, Engle JM, El Youssef J, Massoud RG, Yuen KC, Kagan R, et al. Novel use of glucagon in a closed-loop system for prevention of hypoglycemia in type 1 diabetes. Diabetes Care. 2010;33:1282–7. doi: 10.2337/dc09-2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Chabenne JR, DiMarchi MA, Gelfanov VM, DiMarchi RD. Optimization of the native glucagon sequence for medicinal purposes. J Diabetes Sci Technol. 2010;4:1322–31. doi: 10.1177/193229681000400605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Cherrington AD, Chiasson JL, Liljenquist JE, Jennings AS, Keller U, Lacy WW. The role of insulin and glucagon in the regulation of basal glucose production in the postabsorptive dog. J Clin Invest. 1976;58:1407–18. doi: 10.1172/JCI108596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Christensen PA, Pedersen JS, Christiansen G, Otzen DE. Spectroscopic evidence for the existence of an obligate pre-fibrillar oligomer during glucagon fibrillation. FEBS Lett. 2008;582:1341–5. doi: 10.1016/j.febslet.2008.03.017. [DOI] [PubMed] [Google Scholar]
- [9].El-Khatib FH, Jiang J, Gerrity RG, Damiano ER. Pharmacodynamics and stability of subcutaneously infused glucagon in a type 1 diabetic Swine model in vivo. Diabetes Technol Ther. 2007;9:135–44. doi: 10.1089/dia.2006.0006. [DOI] [PubMed] [Google Scholar]
- [10].El-Khatib FH, Russell SJ, Nathan DM, Sutherlin RG, Damiano ER. A bihormonal closed-loop artificial pancreas for type 1 diabetes. Sci Transl Med. 2010;2:27ra. doi: 10.1126/scitranslmed.3000619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Haidar A, Legault L, Dallaire M, Alkhateeb A, Coriati A, Messier V, et al. Glucose-responsive insulin and glucagon delivery (dual-hormone artificial pancreas) in adults with type 1 diabetes: a randomized crossover controlled trial. CMAJ. doi: 10.1503/cmaj.121265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Ichiba H, Ogawa T, Yajima T, Fukushima T. Analysis of hydroxyl radical-induced oxidation process of glucagon by reversed-phase HPLC and ESI-MS/MS. Biomed Chromatogr. 2009;23:1051–8. doi: 10.1002/bmc.1222. [DOI] [PubMed] [Google Scholar]
- [13].Ichihara A, Nakamura T, Tanaka K. Use of hepatocytes in primary culture for biochemical studies on liver functions. Mol Cell Biochem. 1982;43:145–60. doi: 10.1007/BF00223006. [DOI] [PubMed] [Google Scholar]
- [14].Jackson MA, Caputo N, Castle JR, David LL, Roberts CT, Jr., Ward WK. Stable liquid glucagon formulations for rescue treatment and bi-hormonal closed-loop pancreas. Curr Diab Rep. 12:705–10. doi: 10.1007/s11892-012-0320-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Johnson BA, Shirokawa JM, Aswad DW. Deamidation of calmodulin at neutral and alkaline pH: quantitative relationships between ammonia loss and the susceptibility of calmodulin to modification by protein carboxyl methyltransferase. Arch Biochem Biophys. 1989;268:276–86. doi: 10.1016/0003-9861(89)90589-4. [DOI] [PubMed] [Google Scholar]
- [16].Joshi AB, Kirsch LE. The estimation of glutaminyl deamidation and aspartyl cleavage rates in glucagon. Int J Pharm. 2004;273:213–9. doi: 10.1016/j.ijpharm.2004.01.006. [DOI] [PubMed] [Google Scholar]
- [17].Joshi AB, Kirsch LE. The relative rates of glutamine and asparagine deamidation in glucagon fragment 22-29 under acidic conditions. J Pharm Sci. 2002;91:2331–45. doi: 10.1002/jps.10213. [DOI] [PubMed] [Google Scholar]
- [18].Joshi AB, Rus E, Kirsch LE. The degradation pathways of glucagon in acidic solutions. Int J Pharm. 2000;203:115–25. doi: 10.1016/s0378-5173(00)00438-5. [DOI] [PubMed] [Google Scholar]
- [19].Joshi AB, Sawai M, Kearney WR, Kirsch LE. Studies on the mechanism of aspartic acid cleavage and glutamine deamidation in the acidic degradation of glucagon. J Pharm Sci. 2005;94:1912–27. doi: 10.1002/jps.20405. [DOI] [PubMed] [Google Scholar]
- [20].Kedia N. Treatment of severe diabetic hypoglycemia with glucagon: an underutilized therapeutic approach. Diabetes Metab Syndr Obes. 2011;4:337–46. doi: 10.2147/DMSO.S20633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Matilainen L, Maunu SL, Pajander J, Auriola S, Jaaskelainen I, Larsen KL, et al. The stability and dissolution properties of solid glucagon/gamma-cyclodextrin powder. Eur J Pharm Sci. 2008;36:412–20. doi: 10.1016/j.ejps.2008.11.006. [DOI] [PubMed] [Google Scholar]
- [22].Meinwald YC, Stimson ER, Scheraga HA. Deamidation of the asparaginyl-glycyl sequence. Int J Pept Protein Res. 1986;28:79–84. doi: 10.1111/j.1399-3011.1986.tb03231.x. [DOI] [PubMed] [Google Scholar]
- [23].Nooijen WJ, Kempen HJ. Immunogenicity and bioactivity of glucagon, modified at methionine-27. Horm Metab Res. 11:459–63. doi: 10.1055/s-0028-1092761. [DOI] [PubMed] [Google Scholar]
- [24].Onoue S, Ohshima K, Debari K, Koh K, Shioda S, Iwasa S, et al. Mishandling of the therapeutic peptide glucagon generates cytotoxic amyloidogenic fibrils. Pharm Res. 2004;21:1274–83. doi: 10.1023/b:pham.0000033016.36825.2c. [DOI] [PubMed] [Google Scholar]
- [25].Pedersen JS, Dikov D, Flink JL, Hjuler HA, Christiansen G, Otzen DE. The changing face of glucagon fibrillation: structural polymorphism and conformational imprinting. J Mol Biol. 2005;355:501–23. doi: 10.1016/j.jmb.2005.09.100. [DOI] [PubMed] [Google Scholar]
- [26].Pedersen JS, Dikov D, Otzen DE. N- and C-terminal hydrophobic patches are involved in fibrillation of glucagon. Biochemistry. 2006;45:14503–12. doi: 10.1021/bi061228n. [DOI] [PubMed] [Google Scholar]
- [27].Robinson NE. Protein deamidation. Proc Natl Acad Sci U S A. 2002;99:5283–8. doi: 10.1073/pnas.082102799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Robinson NE, Zabrouskov V, Zhang J, Lampi KJ, Robinson AB. Measurement of deamidation of intact proteins by isotopic envelope and mass defect with ion cyclotron resonance Fourier transform mass spectrometry. Rapid Commun Mass Spectrom. 2006;20:3535–41. doi: 10.1002/rcm.2767. [DOI] [PubMed] [Google Scholar]
- [29].Russell SJ, El-Khatib FH, Nathan DM, Damiano ER. Efficacy determinants of subcutaneous microdose glucagon during closed-loop control. J Diabetes Sci Technol. 2010;4:1288–304. doi: 10.1177/193229681000400602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Steiner SS, Li M, Hauser R, Pohl R. Stabilized glucagon formulation for bihormonal pump use. J Diabetes Sci Technol. 2010;4:1332–7. doi: 10.1177/193229681000400606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Verma AK, da Silva JH, Kuhl DR. Diuretic effects of subcutaneous furosemide in human volunteers: a randomized pilot study. Ann Pharmacother. 2004;38:544–9. doi: 10.1345/aph.1D332. [DOI] [PubMed] [Google Scholar]
- [32].Ward WK, Castle JR, Branigan DL, Massoud RG, El Youssef J. Discomfort from an alkaline formulation delivered subcutaneously in humans: albumin at pH 7 versus pH 10. Clin Drug Investig. 32:433–8. doi: 10.2165/11632840-000000000-00000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Ward WK, Massoud RG, Szybala CJ, Engle JM, El Youssef J, Carroll JM, et al. In vitro and in vivo evaluation of native glucagon and glucagon analog (MAR-D28) during aging: lack of cytotoxicity and preservation of hyperglycemic effect. J Diabetes Sci Technol. 2010;4:1311–21. doi: 10.1177/193229681000400604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Yang H, Zubarev RA. Mass spectrometric analysis of asparagine deamidation and aspartate isomerization in polypeptides. Electrophoresis. 2010;31:1764–72. doi: 10.1002/elps.201000027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Zaccolo M, De Giorgi F, Cho CY, Feng L, Knapp T, Negulescu PA, et al. A genetically encoded, fluorescent indicator for cyclic AMP in living cells. Nat Cell Biol. 2:25–9. doi: 10.1038/71345. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.