Abstract
This study focuses on the single chain fragment variable (scFv) variant of the original IgA-type antibody, recognizing the α2 C-terminal telopeptide (α2Ct) of human collagen I, designed to inhibit post-traumatic localized fibrosis via blocking the formation of collagen-rich deposits. We have demonstrated that the scFv construct expressed in yeast cells was able to fold into an immunoglobulin-like conformation, but it was prone to forming soluble aggregates. Functional assays, however, indicate that the scFv construct specifically binds to the α2Ct epitope and inhibits collagen fibril formation both in vitro and in a cell culture model representing tissues that undergo post-traumatic fibrosis. Thus, the presented study demonstrates the potential of the scFv variant to serve as an inhibitor of the excessive formation of collagen-rich fibrotic deposits, and it reveals certain limitations associated with the current stage of development of this antibody construct.
Keywords: scFv, fibrosis, tendon, connective tissue
INTRODUCTION
Recombinant antibodies are valuable biological compounds synthesized as complex full-length molecules or as mini-antibody variants. The mini-versions of antibodies have a number of advantages over their full-length variants which may include reduced immunogenicity, improved pharmacokinetic properties, and better folding characteristics [1, 2]. One form of mini-antibody is the single chain fragment variable (scFv) whose therapeutic utilities have been demonstrated in a number of tests [1]. Although the scFv constructs are mostly applied against cellular targets, they were also effective in blocking the amyloid-derived Aβ peptide whose extracellular aggregation is associated with Alzheimer's disease [3]. As a therapeutic approach to limit the growth of collagen-rich fibrotic deposits, we have suggested blocking the extracellular collagen I-collagen I interaction that drives the formation of collagen fibrils [4]. We have demonstrated that blocking a critical α2 C telopeptide of collagen I (α2Ct) with anti-α2Ct antibodies inhibits the fibril formation process and reduces the formation of fibrotic deposits [4, 5]. Recently, we tested this concept by employing a genetically engineered chimeric mouse-human IgG (chIgG) variant of the original monoclonal IgA-type anti-α2Ct antibody [5]. We have demonstrated that this variant, consisting of mouse-type variable domains and human-type constant domains of the heavy γ and the light κ chains, is properly folded, retains the epitope-binding specificity, and is active in blocking collagen fibril formation [4, 5].
The study described here tests the utility of the scFv variant of the anti-α2Ct antibody to serve as an inhibitor of excessive formation of collagen-rich deposits with a special emphasis put on testing its antifibrotic utility in an orthopaedic-relevant model.
MATERIALS AND METHODS
DNA construct encoding the anti-α2Ct scFv
A DNA construct for the anti-α2Ct scFv was engineered by employing the sequences encoding the variable regions of the heavy α (VH) and the light κ (VL) chains of the original mouse IgA-type anti-α2Ct antibody, as described [4, 5]. The DNA fragments for the VH and the VL were connected via a sequence encoding the (GGGGS)6 linker. The entire DNA construct was synthesized commercially (Blue Heron Biotechnology) and then cloned into the pPIC-9K yeast-expression vector downstream of the sequence that encodes the α-Factor signal peptide which directs the expressed protein of interest to the extracellular space (Invitrogen). The sequence for the His-tag was incorporated at the 3’ end of the construct.
Computer modeling of the scFv structure
The amino acid sequence of the scFv construct has been submitted for homology modeling to the Swiss-Model server (http://swissmodel.expasy.org/) [6]. Subsequently, the quaternary structure of the scFv was displayed with the use of Sybyl software (Tripos). The complementarity determining regions (CDRs) of the variable domains were identified with Rosetta software (http://rosie.graylab.jhu.edu/).
Purification of the scFv expressed in Pichia pastoris
Yeast cells expressing the scFv variant were selected and then cultured according to the manufacturer’s protocol (Invitrogen). In one set of experiments, cell culture media were supplemented with 0.1 M trimethylamine N-oxide (TMAO, Sigma-Aldrich) to explore the utility of this chemical chaperone to facilitate the folding of the scFv molecules [7]. The secreted scFv variant was purified from cell culture media with the use of a nickel column (Invitrogen). Subsequently, purified scFv was analyzed by size exclusion chromatography and polyacrylamide gel electrophoresis. For all subsequent experiments, a stock solution of the scFv variant concentrated to 0.5 mg/ml was prepared by ultrafiltration.
Size exclusion chromatography
The molecular mass of purified scFv was analyzed by size exclusion high pressure liquid chromatography (SEC-HPLC) performed in non-denaturing conditions.
Western blot assays of scFv-procollagen I binding
Human procollagen I was purified from the medium of cultured dermal fibroblasts, as described [8]. Pro-α1 and pro-α2 chains of human procollagen I were separated in a polyacrylamide gel in reducing conditions and then transferred onto a nitrocellulose membrane. Subsequently, the membrane was exposed to the scFv variant added at a concentration of 5 µg/ml. Binding of the His-tagged scFv was visualized by chemiluminescence with the use of the mouse anti-His antibody (Invitrogen) [5]. Following Western blots, procollagen and procollagen-derived bands were visualized with the Ponceau S to determine the identity of bands detected via chemiluminescence. In a similar experiment, a His-tagged anti-p53 scFv was employed as a negative control (Advanced Targeting Systems, Inc.).
Mapping epitopes recognized by the scFv variant
A set of four overlapping biotinylated peptides (Genemed Synthesis, Inc.) spanning the amino acid sequence GGGYDFGYDGDFYRA representing the α2Ct has been employed to define epitopes of the scFv variant, as described [9]. In brief, the original anti-α2Ct IgA-type antibody and its chIgG and scFv variants were immobilized on nitrocellulose membranes by slot blot, and then the membranes were blocked with 1% bovine serum albumin (BSA; Sigma-Aldrich) [4, 5]. Subsequently, the individual membranes were incubated with specific biotinylated peptides followed by washes with PBS containing 0.02% Tween-20. Next, the membranes were incubated with horseradish peroxidase conjugated with streptavidin (Millipore), and then the bands were detected with chemiluminescence. In addition to the overlapping fragments, the full-length biotinylated α2Ct peptide was used as control.
scFv-procollagen I binding assays
Binding interactions were analyzed with the use of the SensiQ Pioneer biosensor (SensiQ Technologies Inc.), as described [5]. In brief, purified procollagen I was immobilized on the sensor’s surface and then free scFv was added to the system at concentrations ranging from 6.25 nM to 800 nM. In this series of experiments, the association and dissociation rates were recorded. Subsequently, data were analyzed with the use of the QDat software (SensiQ Technologies Inc.) [10]. In addition, binding interactions of procollagen I and control human IgG (Bethyl Laboratories Inc.) were also analyzed, as described [5].
Formation of collagen fibrils in vitro
In vitro fibril formation assays were employed to analyze the effects of the binding of the scFv to the α2Ct on the self-assembly of collagen molecules into fibrils, as described [4, 5]. The anti-α2Ct scFv was added to the separate collagen samples at the following scFv:collagen I molar ratios: 16:1, 4:1, 1:1, 1:4, and 1:16. Specifically, the concentration of collagen I employed in these studies was 120 µg/ml, while the scFv construct has been added at 180 µg/ml, 45 µg/ml, 11 µg/ml, 3 µg/ml, and 0.7 µg/ml, respectively. In addition, a control sample containing the anti-p53 scFv added at a 16:1 ratio was also prepared. The scFv-collagen I mixtures were then pre-incubated for 1 h at 25°C, a temperature at which collagen fibril formation does not occur [8]. After that time, the temperature was raised to 37°C and the samples were incubated for 24h. Subsequently, the morphology of the collagen assemblies was evaluated by dark-field light microscopy and transmission electron microscopy (TEM), as described [11].
Binding of scFv to collagen fibrils
TEM has been employed to test the ability of the anti-α2Ct scFv to bind to the epitopes present on the surface of collagen fibrils. In this assay, done according to the method described by Hagg et al., collagen fibrils formed in the absence of the scFv construct were deposited on the surfaces of the copper grids coated with the Formvar/carbon films (Ted Pella, Inc.) [12]. Subsequently, the grids were washed with phosphate buffered saline (PBS). Next, the samples were treated with 2% BSA for 30 min and then exposed to a biotinylated form of the anti-α2Ct scFv in 0.2% BSA for 2h. Following extensive washing, the samples were exposed to 0.2% BSA containing 15-µm colloidal gold particles conjugated with streptavidin (Ted Pella, Inc.). Finally, the collagen fibrils were stained negatively with the use of 1% phosphotungstic acid. In a parallel experiment, control samples treated with 1 µg/ml of a biotinylated chIgG form of the anti-α2Ct antibody were also prepared [5]. In addition, a control sample in which the biotinylated antibodies were omitted was also prepared. Collagen fibrils were observed with the use of an electron microscope.
A model representing tendon and tendon sheath
The presence of a tendon sheath facilitates the gliding motion of a tendon. This tubular fibrous membrane includes a synovial layer that produces a lubricating substance, thereby reducing the tendon's friction. In pathological situations, collagen-rich fibrotic deposits may be formed around a tendon in response to trauma. By adhering to the tendon and the tendon sheath, these fibrotic structures may limit the movement of the tendon, a consequence associated with joint stiffness. Here, we tested the ability of the anti-α2Ct scFv to limit the formation of the collagen-rich deposits in the area located between cell layers representing the tendon and the tendon sheath. Such a simplified model of the tendon sheath-tendon structure was created by seeding tendon-derived and tendon-sheath-derived fibroblasts into individual wells of a two-well silicon chamber (Ibidi, LLC). In brief, a removable leak-proof chamber was positioned in a well of a 24-well cell culture plate. One well of the chamber was populated with 5×103 fibroblasts isolated from the tendon, while the second chamber was populated with the same number of the tendon sheath-derived fibroblasts. The cells were cultured in individual wells separated by a silicon barrier in Dulbecco's Modified Eagle Medium supplemented with 10% fetal bovine serum and 40 µg/ml of L-ascorbic acid phosphate magnesium salt (Wako Pure Chemical Industries, Ltd.) for 4 days. After that time, the silicon chamber was removed together with a barrier separating the two cell populations and the cell culture was continued in the presence of 30 µg/ml of the anti-α2Ct scFv variant for an additional 4 days. Subsequently, the cell layers were washed with PBS and then fixed with methanol. Collagen fibrils deposited in cell layers were stained with primary polyclonal anti-collagen I antibodies and secondary anti-rabbit-IgG antibodies conjugated to the Alexa Fluor 594 (Invitrogen), as described [5]. Subsequently, the nuclei of cells in the analyzed samples were stained with 4',6-diamidino-2-phenylindole.
Cell viability assays
Employing a colorimetric assay (CCK-8; Sigma-Aldrich), the effects of the presence of the scFv variant on the viability of human fibroblasts isolated from a tendon and a tendon sheath were tested, as described [5]. The utility of the applied test to detect changes in the viability of selected cells was determined by employing tissue necrosis factor α (TNFα). In brief, fibroblasts seeded at 1×104 cells/well were treated with increasing concentrations of TNFα for 24h (Sigma-Aldrich). After that time, the viability of cells in response to TNFα was measured. Next, we measured the potential of the scFv variant to induce acute cytotoxicity by measuring the viability of cells treated for 24h with increasing concentrations of the scFv variant (up to 200 µg/ml). Subsequently, we analyzed the proliferation of fibroblasts treated with 100 µg/ml of the anti-α2Ct scFv for 4 days, as described [5]. Employing a control group consisting of cells cultured in the presence of anti-proliferative actinomycin D validated the test.
RESULTS
Anti-α2Ct scFv
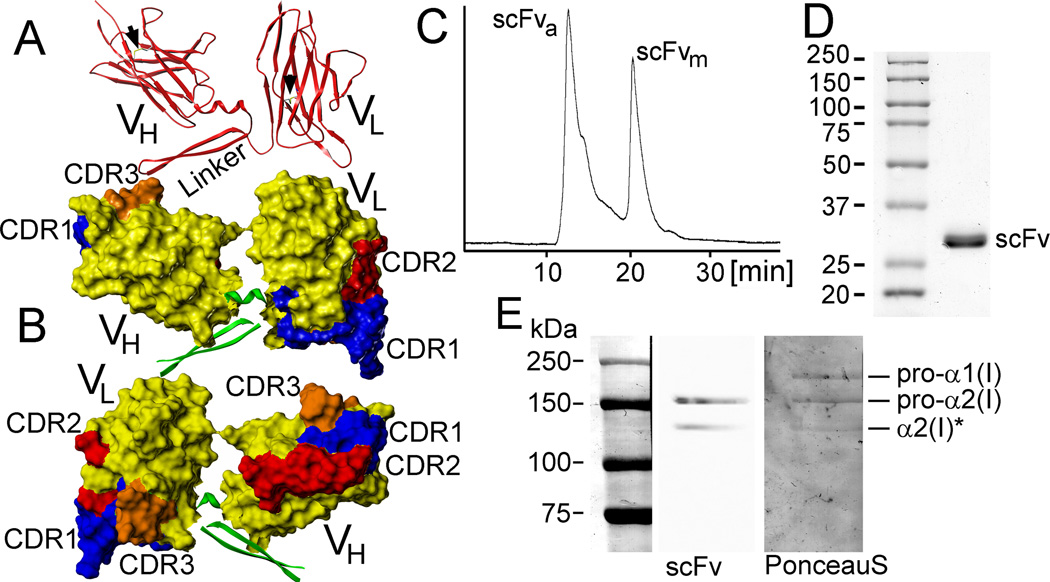
The scFv form of the anti-α2Ct antibody was designed to include the VH and VL regions of the original anti-α2Ct IgA-type antibody [4]. A (GGGGS)6 linker was introduced to bridge the variable domains (Fig. 1A & 1B). Homology modeling indicates that the applied design of the scFv construct allows it to fold into immunoglobulin-like domains. Specifically, analysis of the model indicates that the VH and VL regions of the scFv may fold into characteristic immunoglobulin barrel-shaped folds created by the β-sheet structures, and that the positions of the cysteine residues permit the formation of the disulfide bridges that stabilize them (Fig. 1A). Computer modeling also identifies the CDRs whose predicted locations are indicated in Figure 1B.
Figure 1.
Structural and functional analyses of the anti-α2Ct scFv construct. A, B: Computer models depicting the barrel-shaped immunoglobulin folds and organization of the CDRs. Arrows indicate predicted disulfide bonds formed within the variable regions (A). C: A chromatography profile indicates the presence of the aggregated (scFva) and monomeric (scFvm) forms of the scFv variant expressed in the presence of TMAO. D: Electrophoretic migration of the scFv variant indicating its expected mass of 28 kDa. E: Western blot assay of the binding of the scFv construct to the band corresponding to the intact pro-α2 chain of human procollagen I and its partially processed form (asterisk) indicates the specificity of this antibody variant to recognize the α2Ct epitope. The middle line (scFv) of panel E depicts a chemiluminescence-based image of procollagen I bands detected on the nitrocellulose membrane by the specific binding of the scFv variant. The right lane shows the same membrane stained with Ponceau S in which pro-α1 chains are also apparent.
SEC-HPLC of purified scFv, however, indicates that this protein has a tendency to form soluble aggregates (Fig. 1C). At a concentration of 200 µg/ml, about 60% of the scFv molecules produced in the presence of TMAO existed in the aggregated form, while the remaining 40% were present in a monomeric form. Upon concentrating the scFv samples to 350 µg/ml, all scFv molecules were present in the form of soluble aggregates with a mass of about 300 kDa (not shown). In preparations done in the absence of TMAO, all the scFv molecules existed only in the form of soluble aggregates even at concentrations below 80 µg/ml (not shown). Electrophoretic migration of the purified scFv in denaturing and reducing conditions, however, was consistent with its predicted mass of 28 kDa (Fig. 1D). Since all batches of purified scFv were concentrated to 0.5 mg/ml, the soluble-aggregate form of this protein construct has been applied in all subsequent assays.
Western blot assays of scFv-procollagen I binding
As demonstrated in Fig. 1E,the scFv variant binds exclusively to the pro-α2 chain. The lack of binding of the scFv construct to the pro-α1 chain, which does not include the α2Ct epitope, corroborates its binding specificity. This specificity is further indicated by the lack of binding of the anti-p53 scFv control to procollagen I (not shown).
Epitope mapping
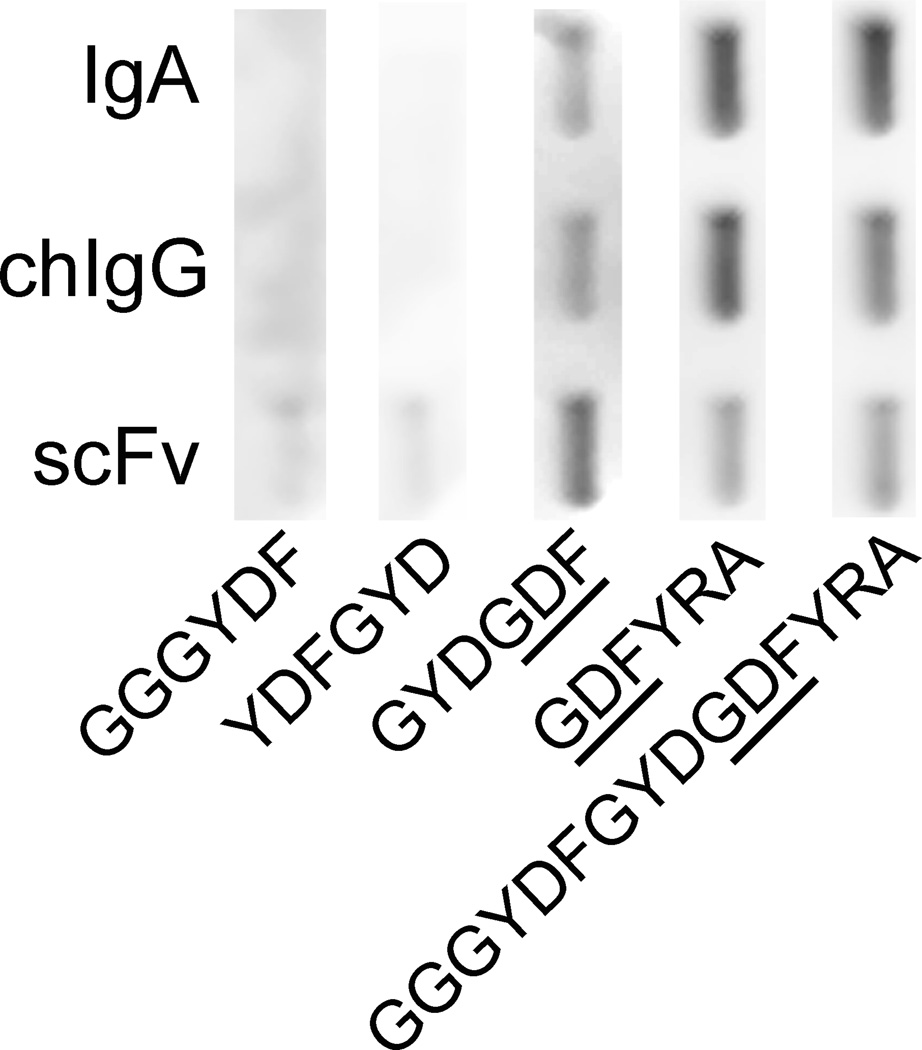
Qualitative binding experiments with the use of the biotinylated peptides representing the overlapping fragments of the α2Ct demonstrated that the region containing the GDF motif was well recognized by all antibody variants (Fig. 2). The binding patterns of IgA, chIgG, and scFv antibody variants to the GYDGDF and the GDFYRA peptides that contain this region were similar to that shown for the binding of the full-length α2Ct represented by the GGGYDFGYDGDFYRA sequence (Fig. 2). In contrast, the binding of the analyzed antibodies to the GGGYDF and YDFGYD peptides located toward the N terminus of the α2Ct demonstrated only relatively weak background-level signals (Fig. 2).
Figure 2.
Mapping of epitopes recognized by the scFv construct. The binding of biotinylated overlapping peptides spanning the α2Ct sequence to the anti-α2Ct antibody variants immobilized on nitrocellulose membranes was visualized by chemiluminescence. The sequences of employed biotinylated peptides are indicated. The underlined GDF sequence represents the critical region recognized by all antibody variants.
Biosensor assays of scFv-procollagen I binding
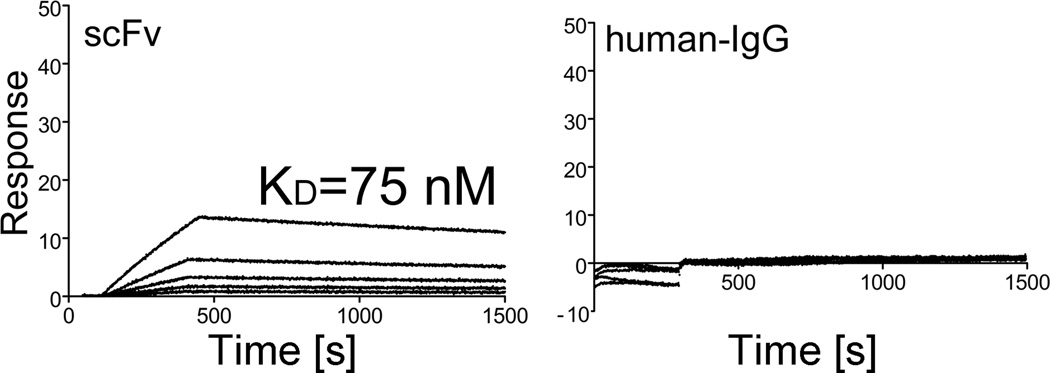
Employing a biosensor, we analyzed the binding interactions between the anti-α2Ct scFv variant and its epitope present in native procollagen I. These analyses have determined that the KD value for the scFv-procollagen I interaction is 75 nM. In the same experimental conditions, no binding interaction was observed between procollagen I and control (Fig. 3)
Figure 3.
Kinetics of the binding of the anti-α2Ct scFv construct and control human IgG to procollagen I. In each panel, the curves represent association and dissociation events during analyzed binding interactions between procollagen I and a free interactant present at concentrations ranging from 6.25 nM to 800 nM.
Inhibition of collagen fibril formation in vitro
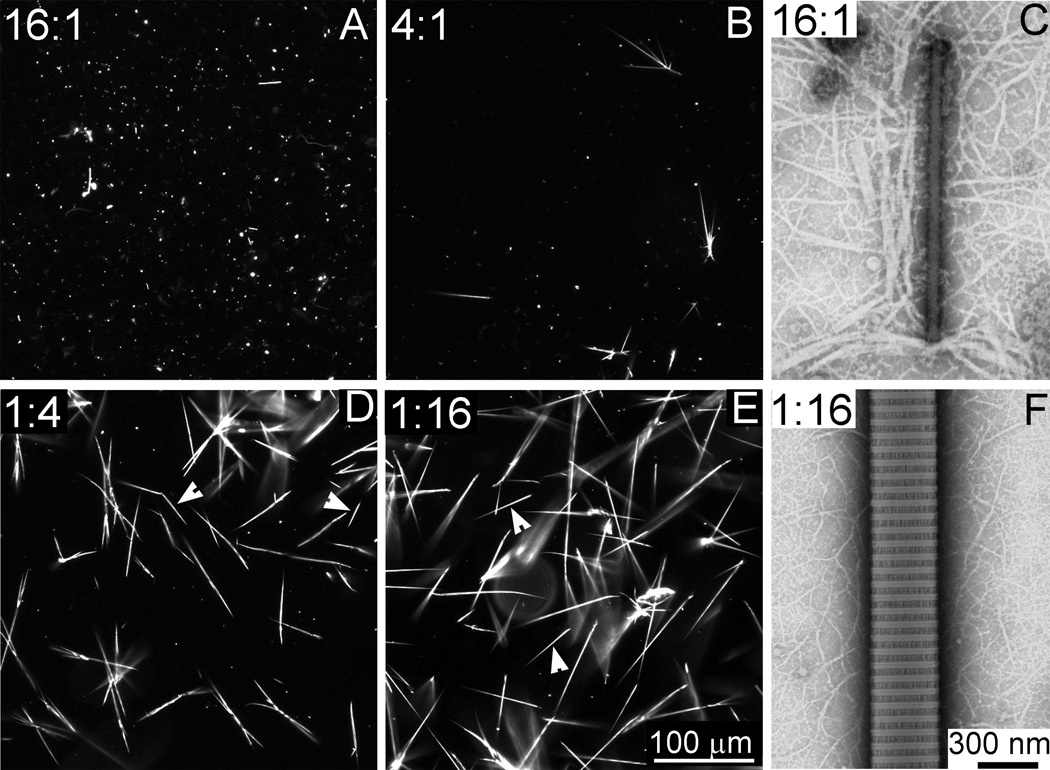
The potential of the anti-α2Ct scFv to inhibit collagen fibril formation was analyzed by blocking critical collagen I-collagen I interaction mediated by the site-specific binding of the α2Ct [4, 9]. As depicted in Fig. 4, adding the scFv variant at the scFv:collagen molar ratio of 16:1 completely inhibited the formation of collagen fibrils. In these experimental conditions, only atypical punctate aggregates were observed with the use of dark-field light microscopy. When viewed using TEM, these aggregates appeared as filaments of varying sizes. Decreasing the concentration of the scFv variant to a scFv:collagen ratio of 4:1 resulted in the formation of a mixed population of collagen assemblies. This mixture consisted of atypical aggregates and fibrils whose morphology was consistent with that of native collagen fibrils. As indicated in Fig. 4, further decrease in the concentration of the scFv variant eliminated its ability to inhibit the formation of collagen fibrils. In these sub-inhibitory concentrations of the scFv construct, the morphology of formed collagen assemblies was consistent with that of control fibrils formed in the absence of the scFv inhibitor (Fig. 5). Specifically, the fibrils observed via dark-field light microscopy were characterized by an elongated shape and tapered ends. Depending on the growth phase, fibrils were flanked by pointed and blunt tips or by pointed tips present on both ends of a fibril (Fig. 4 and Fig. 5), a characteristic of fibrils formed de novo (Fig. 4 and Fig. 5) [13]. The same fibrils seen via TEM had a specific D-periodic banding pattern, thereby indicating a proper packing of individual collagen molecules that form them. The thin fibrils seen in the background of the thick banded fibrils represent intermediates formed during the growth of the thick fibrils and are regularly observed in the employed fibril-formation system (Fig. 4) [11].
Figure 4.
Morphology of the collagen assemblies formed in the presence of the anti-α2Ct scFv construct added at indicated scFv:collagen I molar ratios. As indicated by the punctate staining seen in panels A and B, at the highest scFv:collagen I molar ratios, the formation of properly folded spindle-shaped collagen fibrils is inhibited. In contrast, at the relatively low concentrations of inhibitory scFv, abundant spindle-shaped collagen fibrils are formed (D, E). Panels C and F depict the ultrastructure of assemblies formed at high (C) and low (F) scFv: collagen I molar ratios. Arrows indicate fibrils with pointed and blunt ends.
Figure 5.
Morphology of collagen fibrils formed in the absence of the scFv construct. A: Low magnification-image depicting the morphologies of the population of fibrils present in the analyzed sample. B: panels depicting magnified views of selected fibrils flanked with pointed (narrow arrows) ends and those flanked with both pointed and blunt ends (wide arrows). Bars=100 µm.
The specificity of the anti-α2Ct scFv-mediated inhibition of collagen fibril formation was confirmed by employing control anti-p53 scFv, whose presence did not prevent collagen molecules to self-assemble into fibrils (not shown).
Interaction of the anti-α2Ct scFv with collagen fibrils
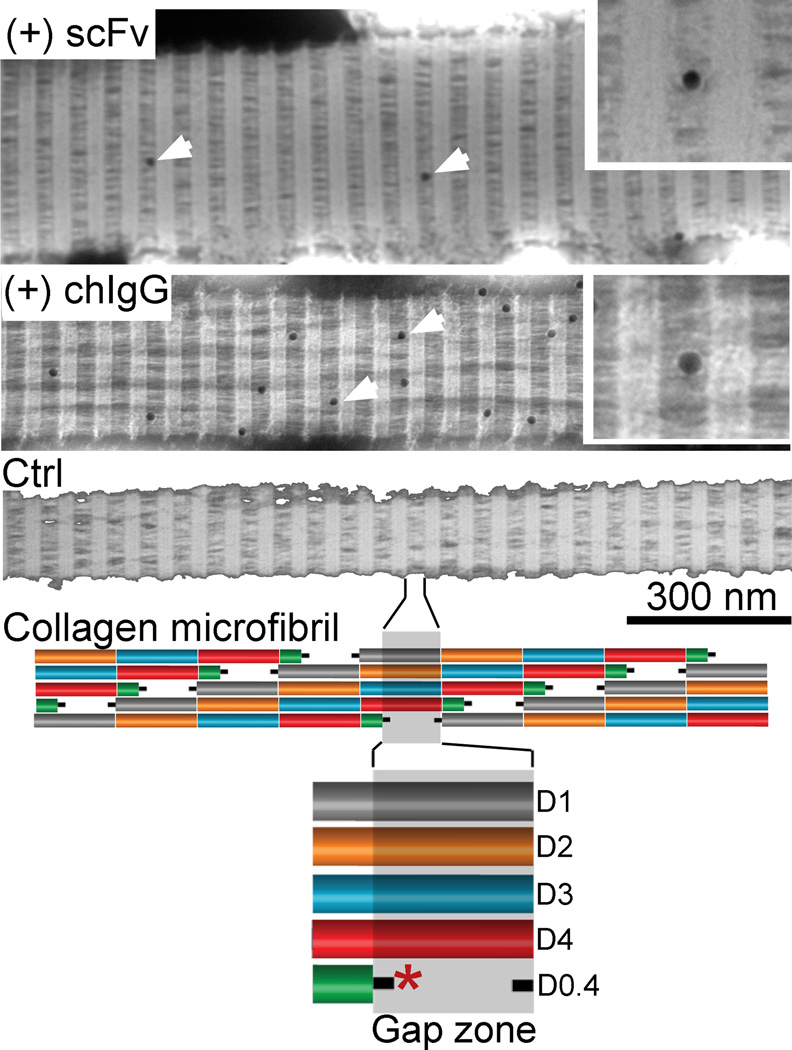
We have also studied the ability of the anti-α2Ct scFv to bind to fibrils pre-formed in the absence of this inhibitor. As demonstrated in Fig. 6, the scFv interacts with collagen fibrils, primarily at the boundaries of a gap region in which the scFv's epitope, i.e. the α2Ct, is present [14]. A similar pattern of binding was observed for the chIgG variant of the anti-α2Ct antibody (Fig. 6). In the absence of biotinylated scFv or the chIgG variants, no binding of the colloidal gold particles was observed (Fig. 6).
Figure 6.
Binding of the anti-α2Ct scFv and the chIgG variants to the collagen fibrils. In the demonstrated fibrils arrows point to the gold particles representing positions of the fibril-bound scFv or chIgG constructs. Included inserts represent magnified views of the gap regions with gold particles representing fibril-bound antibody variants. Control (Ctrl) fibrils not exposed to the anti-α2Ct constructs show no binding of the gold particles.
Lower portion of this figure depicts a telopeptide-region with a possible binding site (asterisk) of the anti-α2Ct constructs presented in the context of the specific arrangement of collagen molecules that form a collagen microfibril. Specific regions corresponding to particular D-periods of the collagen molecule are also indicated.
Formation of collagen deposits in cell layers
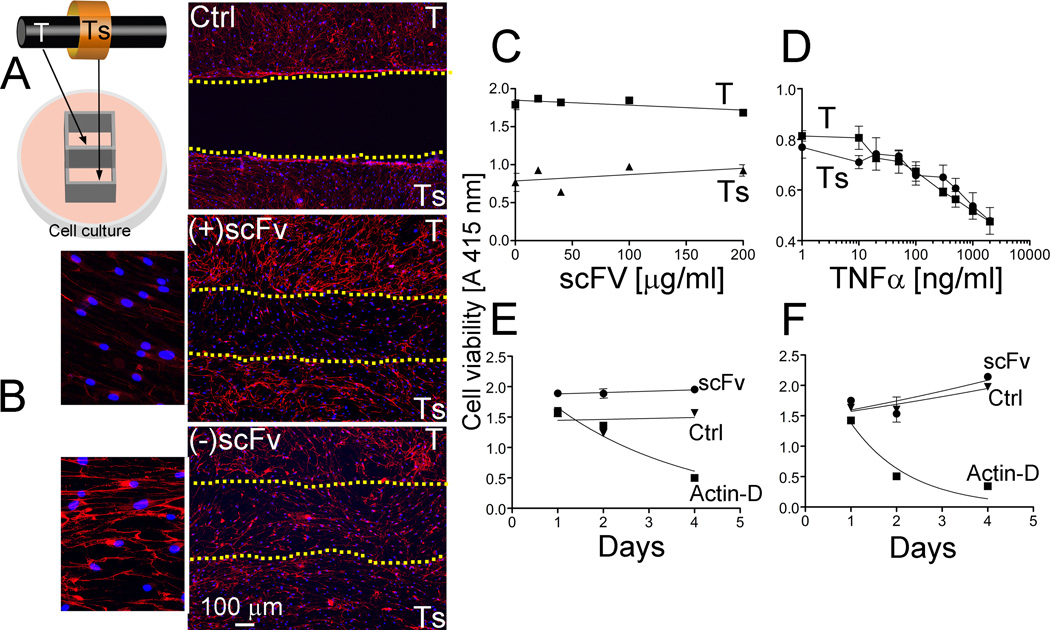
The cellular aspect of the complex structure of the tendon and tendon sheath was represented by co-cultures of cells isolated from these tissues (Fig. 7A). When cultured in the absence of the inhibitory anti-α2Ct scFv variant, both cell types were able to form collagen-rich deposits readily visible in a fluorescence microscope (Fig. 7B). In contrast, in cell culture conditions in which this inhibitory scFv variant was present, the formation of collagen-rich deposits was blocked in the gap region existing between the two cell types (Fig. 7B). The presence of the scFv, however, did not alter the ability of cells to proliferate and migrate into the border region (Fig. 7B).
Figure 7.
Effects of the presence of the anti-α2Ct scFv variant on the deposition of collagen fibrils and viability of cells isolated from tendon (T) and tendon sheath (Ts). A: An experimental setting of co-cultures of employed cells is indicated. B: In the presence of the scFv (+scFv), no collagen deposits have been formed in the region separating layers of cells derived from tendon and tendon sheath. In contrast, in the absence of the scFv variant (−scFv), collagen-rich deposits have been formed in the border region. Control (Ctrl) depicts a border region in a sample in which a dividing barrier has not been removed. Dotted lines indicate the borders of cell layers formed by T and Ts cells and define a region separating them. C: The viability of cells has been analyzed as the function of increasing concentrations of the scFv construct. D: The utility of the employed test was validated by the observation of viability reduction in response to the increasing concentrations of TNFα. E, F: The effects of the anti-α2Ct scFv on the proliferation of tendon-derived (E) and tendon sheath-derived (F) cells has been analyzed in comparison to untreated control (Ctrl) and to control treated with actinomycin D (Actin-D).
Effects of the scFv on the viability of cells
As seen in Fig. 7C, increasing the concentration of the scFv variant to 200 µg/ml, a 6.5-fold increase over that employed for the cell-based inhibitory assays (Fig. 7B), did not have any significant acute toxic effects on the viability of the analyzed cells. The ability of this test to detect changes in the viability of cells treated with increasing concentrations of TNFα, validates its utility (Fig. 7D). The effects of the scFv on the viability of selected cells was also analyzed by monitoring their proliferation. As shown in Fig. 7E and Fig. 7F, the presence of the analyzed scFv variant added to the cell culture media at a concentration of 100 µg/ml did not have any effect on proliferation. The proliferation characteristics of cells cultured in the presence of the ant-α2Ct scFv, illustrated by the fitted curves, were similar to those observed for cells cultured in the absence of this inhibitor. The lack of proliferation in the presence of actinomycin D validates the utility of the applied assay (Fig. 7E and 4F). Although the above proliferative behavior was similar in the tested tendon-derived fibroblasts (Fig. 7E) and cells isolated from the tendon sheaths (Fig. 7F), the specific dynamics of proliferation differed in these cell types.
DISCUSSION
In our earlier reports, we provided experimental evidence for the notion that the process of collagen fibril formation represents a valid target to limit excessive production of collagen-rich deposits seen in localized fibrosis [4, 5]. In the present study, we have characterized the scFv variant of the original anti-α2Ct antibody engineered as a potential inhibitor of collagen fibril formation. The scFv variant described here includes domains derived from the original variable regions of the mouse monoclonal anti-α2Ct antibody of the IgA type [4, 5]. The ability of the scFv construct in which the VH and the VL domains are connected via a flexible linker to fold correctly is indicated by an energy-minimized computer model of this molecule. In this theoretical model, the structure of the scFv variant includes an immunoglobulin fold, a landmark structure of antibodies [15]. Moreover, the typical position of the CDR loops and a confirmation that permits formation of stabilizing disulfide bonds further indicate the potential of the scFv construct to form a correct structure.
Chromatographic assays of the scFv variant done in native conditions have revealed that this construct is prone to formation of soluble aggregates. This property is a common unwanted characteristic of engineered recombinant antibody fragments that may limit their biological activity and increase the risk of toxicity [16]. It has been established that mechanisms of the aggregation of the antibody fragments such as scFv variants are complex and include atypical folding and unfolding kinetics. It has been demonstrated that aggregation may be triggered by external environmental factors such as high concentration, extreme pH, changes in ionic strength, agitation, and temperature. A number of strategies to minimize the aggregation of antibody constructs have been described [17]. Some of these include designing stabilizing disulfide bonds that link the VH and the VL domains and introducing hydrophilic amino acid sequences that increase the solubility of engineered antibody variants [17]. Another strategy employs osmolytes, small organic molecules that increase the solubility of proteins. Employing TMAO, a naturally occurring osmolyte, we were able to limit the aggregation of the scFv variant present at a relatively low concentration. This method, however, did not prevent aggregation of the scFv monomers during the concentration of these molecules above 350 µg/ml.
Despite the aggregation, the anti-α2Ct scFv variant recognized the α2Ct-specific epitope with a high degree of specificity comparable to that of the IgA and chIgG counterparts. Its binding affinity, however, was lower than the affinities determined for the original IgA-type anti-α2Ct monoclonal antibody and for the recombinant chIgG variant [5]. Specifically, the KD values reported for the IgA and chIgG variants are 0.2 nM and 3.2 nM, respectively, while the corresponding value reported here for the scFv-procollagen I interaction is 75 nM [5]. The relatively low affinity of the scFv variants is frequently observed and is attributed to structural changes that may alter antigen-binding properties [18, 19].
The effects of the scFv variant’s binding to collagen I molecules on their ability to self-assemble into fibrils were analyzed in vitro. At the highest scFv:collagen I ratio employed in these studies, no collagen fibrils were formed. Instead, microscopic assays revealed the presence of atypical filamentous aggregates in which the characteristic banding pattern seen in correctly-folded collagen fibrils was not apparent. As the banding pattern of collagen fibrils is a consequence of the D-staggered arrangement of the individual collagen molecules, we propose that the presence of inhibitory scFv prevents the correct collagen-collagen interaction. Instead, the formation of atypical collagen assemblies seen in Fig. 4 could be a result of the clustering of collagen molecules via binding to the α2Ct-binding regions present on the surface of multifaceted anti-α2Ct scFv aggregates. The inhibitory effect of the scFv variant has been concentration-dependent; decreasing the relative amount of scFv in collagen I samples has resulted in the formation of an increasing number of collagen fibrils characterized by native-like morphology. Specifically, collagen fibrils seen in a dark-field microscope had a spindle-like shape and were flanked by tapered ends. In some fibrils pointed and blunt ends were observed. As demonstrated by Kadler et al., the specific shapes of the ends of a fibril reflect the mechanism of growth of the fibril. In particular, the initial growth of the fibril progresses from the pointed end while the blunt end serves as a nucleation site for the growth of the fibril in the opposite direction [13]. The specificity of the inhibitory effects imposed by the anti-α2Ct scFv has been confirmed by the lack of inhibitory activity of the anti-p53 scFv variant with no specificity for collagen I binding.
We have also demonstrated the ability of the anti-α2Ct scFv variant to bind to the α2Ct epitopes present in the gap region of the collagen fibril preformed in the absence of this construct. When compared to the anti-α2Ct scFv, a relatively larger number of bound chIgG molecules was observed at the boundaries of the gap regions, a result that may reflect the higher affinity of this construct for binding to the α2Ct [5].
The potential of the scFv variant to limit accumulation of collagen fibrils produced by cells was demonstrated in a model formed by co-cultures of cells isolated from the tendon and the tendon sheath. Microscopic assays of the areas between layers formed by the employed cells cultured in the presence of the anti-α2Ct scFv have demonstrated a reduced amount of the collagen fibrils formed by cell migrating to these areas, thereby indicating the inhibitory activity of the scFv in the cell culture condition. Although the microscopy technique we employed was sufficient to determine the reduced number of the collagen fibrils, its technical limitations do not permit visualization of small collagen filaments that could have been formed in cultures of analyzed cells.
We have also studied the impact of the scFv construct on the biological activities of analyzed cells. Our assays have demonstrated that the presence of the anti-α2Ct scFv variant does not negatively impact the viability and proliferation characteristics of employed cells. This observation is in agreement with earlier studies indicating the lack of cytotoxic effects of the anti-α2Ct chIgG variant [5].
The study presented here demonstrates the potential of the anti-α2Ct scFv construct to limit excessive collagen fibril formation and identifies potential limitations associated with its tendency to aggregate. Further studies will explore the possibility of reducing these limitations by applying modified gene engineering and cell culture approaches.
ACKNOWLEDGEMENTS
This work was supported by grants from NIH to A.F. (AR061118 and AR048544).
Footnotes
DECLARATION OF INTEREST
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
REFERENCES
- 1.Ahmad ZA, Yeap SK, Ali AM, Ho WY, Alitheen NB, Hamid M. scFv antibody: principles and clinical application. Clinical & developmental immunology. 2012;2012:980250. doi: 10.1155/2012/980250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huston JS. Engineering antibodies for the 21st century. Protein Eng Des Sel. 2012;25:483–484. doi: 10.1093/protein/gzs069. [DOI] [PubMed] [Google Scholar]
- 3.Robert R, Wark KL. Engineered antibody approaches for Alzheimer's disease immunotherapy. Arch Biochem Biophys. 2012;526:132–138. doi: 10.1016/j.abb.2012.02.022. [DOI] [PubMed] [Google Scholar]
- 4.Chung HJ, Steplewski A, Chung KY, Uitto J, Fertala A. Collagen fibril formation. A new target to limit fibrosis. J Biol Chem. 2008;283:25879–25886. doi: 10.1074/jbc.M804272200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fertala J, Steplewski A, Kostas J, Beredjiklian P, Williams G, Arnold W, Abboud J, Bhardwaj A, Hou C, Fertala A. Engineering and characterization of the chimeric antibody that targets the C-terminal telopeptide of the alpha2 chain of human collagen I: a next step in the quest to reduce localized fibrosis. Connect Tissue Res. 2013;54:187–196. doi: 10.3109/03008207.2013.778839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Benkert P, Biasini M, Schwede T. Toward the estimation of the absolute quality of individual protein structure models. Bioinformatics. 2011;27:343–350. doi: 10.1093/bioinformatics/btq662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kumar R. Role of naturally occurring osmolytes in protein folding and stability. Arch Biochem Biophys. 2009;491:1–6. doi: 10.1016/j.abb.2009.09.007. [DOI] [PubMed] [Google Scholar]
- 8.Kadler KE, Hojima Y, Prockop DJ. Assembly of collagen fibrils de novo by cleavage of the type I pC-collagen with procollagen C-proteinase. Assay of critical concentration demonstrates that collagen self-assembly is a classical example of an entropy-driven process. J Biol Chem. 1987;262:15696–15701. [PubMed] [Google Scholar]
- 9.Prockop DJ, Fertala A. Inhibition of the self-assembly of collagen I into fibrils with synthetic peptides. Demonstration that assembly is driven by specific binding sites on the monomers. J Biol Chem. 1998;273:15598–15604. doi: 10.1074/jbc.273.25.15598. [DOI] [PubMed] [Google Scholar]
- 10.Myszka DG, Morton TA. CLAMP: a biosensor kinetic data analysis program. Trends Biochem Sci. 1998;23:149–150. doi: 10.1016/s0968-0004(98)01183-9. [DOI] [PubMed] [Google Scholar]
- 11.Fertala A, Holmes DF, Kadler KE, Sieron AL, Prockop DJ. Assembly in vitro of thin and thick fibrils of collagen II from recombinant procollagen II. The monomers in the tips of thick fibrils have the opposite orientation from monomers in the growing tips of collagen I fibrils. J Biol Chem. 1996;271:14864–14869. doi: 10.1074/jbc.271.25.14864. [DOI] [PubMed] [Google Scholar]
- 12.Hagg R, Bruckner P, Hedbom E. Cartilage fibrils of mammals are biochemically heterogeneous: differential distribution of decorin and collagen IX. J Cell Biol. 1998;142:285–294. doi: 10.1083/jcb.142.1.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kadler KE, Hojima Y, Prockop DJ. Collagen fibrils in vitro grow from pointed tips in the C- to N- terminal direction. Biochem J. 1990;268:339–343. doi: 10.1042/bj2680339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Malone JP, Veis A. Heterotrimeric type I collagen C-telopeptide conformation as docked to its helix receptor. Biochemistry. 2004;43:15358–15366. doi: 10.1021/bi048304b. [DOI] [PubMed] [Google Scholar]
- 15.Bork P, Holm L, Sander C. The immunoglobulin fold. Structural classification, sequence patterns and common core. J Mol Biol. 1994;242:309–320. doi: 10.1006/jmbi.1994.1582. [DOI] [PubMed] [Google Scholar]
- 16.Sun W, Xie J, Lin H, Mi S, Li Z, Hua F, Hu Z. A combined strategy improves the solubility of aggregation-prone single-chain variable fragment antibodies. Protein expression and purification. 2012;83:21–29. doi: 10.1016/j.pep.2012.02.006. [DOI] [PubMed] [Google Scholar]
- 17.Gil D, Schrum AG. Strategies to stabilize compact folding and minimize aggregation of antibody-based fragments. Advances in bioscience and biotechnology. 2013;4:73–84. doi: 10.4236/abb.2013.44A011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ward ES, Gussow D, Griffiths AD, Jones PT, Winter G. Binding activities of a repertoire of single immunoglobulin variable domains secreted from Escherichia coli. Nature. 1989;341:544–546. doi: 10.1038/341544a0. [DOI] [PubMed] [Google Scholar]
- 19.Borrebaeck CA, Ohlin M. Antibody evolution beyond Nature. Nat Biotechnol. 2002;20:1189–1190. doi: 10.1038/nbt1202-1189. [DOI] [PubMed] [Google Scholar]