Abstract
BACKGROUND
Chemoradiotherapy has become the standard of care for head and neck squamous cell carcinoma; however, those patients often experience multiple treatment-related symptoms or symptom clusters. Two symptom clusters have been identified for this population. Little is known about the risk factors of these symptom clusters.
METHODS
Subjects comprised 684 patients who were treated with concurrent chemoradiotherapy in a phase III randomized clinical trial. This trial compared standard fractionation radiotherapy to accelerated fractionation radiotherapy. Symptom clusters were evaluated at the end of the first and the second cycle of chemotherapy, and 3 months after the start of radiotherapy. Mixed-effect modeling was used to observe risk factors for symptom clusters.
RESULTS
Race and education were independent predictors for the head and neck cluster, while gender and history of tobacco use were for the gastrointestinal cluster. Primary cancer site was only significant for the head and neck cluster when other factors were not controlled: oropharyngeal cancer patients had more severe symptoms in the head and neck clusters than laryngeal cancer patients. Additionally, patients receiving accelerated fractionation radiotherapy experienced more symptoms of radiomucositis, pain, and nausea at 3 months after the start of radiotherapy than those receiving standard fractionation radiotherapy.
CONCLUSION
Demographic characteristics were more predictive to symptom clusters, while clinical characteristics, such as cancer site and treatment arms, were more significant for individual symptoms. Knowing the risk factors will enhance the capability of clinicians to evaluate patients’ risk of severe symptom clusters and to personalize management strategies.
Keywords: Head and neck neoplasms, Chemoradiation, Symptom clusters, Risk factors
INTRODUCTION
Chemoradiotherapy has become the standard of care for head and neck squamous cell carcinoma because of the radiosensitizing effect of certain chemotherapeutic agents.1 Individually, chemotherapy and radiotherapy are both linked to multiple, adverse, treatment-related symptoms.2 These symptoms become even worse when patients receive chemoradiotherapy because the effect of the modalities is synergistic. Many of these symptoms negatively affect patients’ quality of life and functional status, especially when they present either temporally or biologically as a symptom cluster.3 However, the cascade of symptoms may be prevented or alleviated by treating the first presenting or the most influential symptom in a cluster.
Our previous study4 found two stable symptom clusters for head and neck cancer (HNC) patients who received concurrent chemoradiotherapy in a randomized phase III clinical trial, the Radiation Therapy Oncology Group (RTOG) 0129. One symptom cluster was specific to the head and neck, the other to the gastrointestinal system. The symptom cluster of the former comprises seven symptoms: dry mouth, dysphagia, fatigue, pain, radiodermatitis, radiomucositis, and taste disturbance; the symptom cluster of the latter comprises three symptoms: dehydration, nausea, and vomiting.4 Presently, little is known about the risk factors for symptom clusters due to inconsistent results in other cancer populations and the paucity of HNC studies.5,6 Knowing risk factors for symptom clusters allows clinicians to design personalized plans for symptom prevention and management, which might eventually benefit multiple symptoms in a cluster. The purpose of this paper, therefore, is to examine the demographic and clinical risk factors for the two previously identified symptom clusters in a large cohort of patients treated uniformly with concurrent chemoradiotherapy in a prospective randomized phase III clinical trial.
METHODS AND MATERIALS
Study design
This study analyzed data from the RTOG 0129, a phase III randomized clinical trial that compared standard fractionation radiotherapy (SFRT) with accelerated fractionation radiotherapy (AFRT), both in combination with concurrent cisplatin. Adult patients were eligible for this trial if they had selected stage III–IV7 (T2N2-3M0, T3–4 any N M0) head and neck squamous cell carcinoma without metastasis and major organ disease and had a Zubrod Performance Status of 0–1. Patients were randomized to either SFRT with 70 Gy in 35 fractions within 7 weeks plus three cycles of cisplatin (100 mg/m2 on days 1, 22, and 43) or AFRT with a concomitant boost to a total dose of 72 Gy in 42 fractions within 6 weeks plus two cycles of cisplatin (100 mg/m2 on days 1 and 22). All patients who agreed to participate in the study signed informed consent.
Sample
Seven hundred twenty-one patients who received treatment in the RTOG 0129 were included in this study. They were required to meet additional inclusion criteria. Patients had to have had (a) their symptoms assessed at one of three time points: Time 1, at the end of the first cycle of chemotherapy, which was after the first day of radiotherapy (week 1); Time 2, at the end of second cycle of chemotherapy, which was after the twenty-first day of starting radiotherapy (week 4); and Time 3, 3 months after the start of radiotherapy (week 13); (b) at least 90% of radiation per protocol for each arm; and (c) at least one cycle of chemotherapy.
Measurements
Demographic and clinical characteristics were collected by case reports. Clinicians used the National Cancer Institute’s Common Toxicity Criteria (CTC) version 2.0 to report treatment-related symptoms. Treatment-related symptoms were rated on a 5-point scale: 1 = minimal severity, 4 = maximum severity, and 5 = death. The CTC v 2.0 included adverse events that were laboratory or diagnostic based (e.g., hyponatremia, leukopenia, or neutropenia). Symptoms in this study included only those adverse events that a patient could report.
The identification of symptom clusters was based on symptoms with more than a 10% average prevalence across the three time points. The clusters were first examined by exploratory factor analysis of 50% of a randomly selected sample; they were then verified by confirmatory factor analysis of the remaining 50% sample that was not used in exploratory factor analysis. Symptom cluster scores were calculated for each patient at each time point, after the identification of symptom clusters. Standardized unit weighting8 was used for cluster score calculation. Raw scores for each symptom within a cluster were added, then ranked, and finally standardized to T scores.9
Data analysis
Mixed-effect modeling was generated to assess risk factors for the two symptom clusters over time. Dependent variables were symptom cluster scores at each time point; independent variables were symptom assessment time point, age, gender (male vs. female), years of education (≤12 years vs. >12 years), race (White vs. non-White), marital status (married vs. unmarried), history of tobacco use (no vs. yes), treatment (SFRT vs. AFRT), dose of radiation, primary cancer site (oral cavity, oropharynx, hypopharynx, or larynx), cancer stage (stage III vs. stage IV), and tube feeding (no vs. yes).
Selecting independent variables (risk factors) for the final mixed-effect model involved three steps. First, the bivariate relationships between risk factors were examined for multicolinearity. The t-test, chi-square test, and correlation coefficients were selected for the relationship between categorical and continuous variables, between categorical variables, or between continuous variables, respectively. Variables that were significantly associated with others were excluded from the next step based on their contribution. Second, univariate mixed-effect models were used to observe the effects of the each remaining risk factors on either cluster. Variables with a level of significance less than 0.1 over two time periods (i.e., from Time 1 to Time 2 and from Time 2 to Time 3) were entered into the next step as the risk factors for the stepwise multivariate mixed-effect analysis. The variables with a significance level less than 0.1 for either time period were used to detect the interaction effect with time on clusters. Third, variables at a significance level greater than 0.05 at each step were excluded from the final stepwise mixed-effect model. All data were analyzed using SAS 9.2.
RESULTS
Of the 721 patients enrolled in the RTOG 0129 trial, 19 were excluded for lack of symptoms. Patients who received less than 90% of the radiation dose per protocol for each arm and those who did not receive any chemotherapy were also excluded from the analysis. As a result, 684 patients were involved in the final data analysis. Demographic and clinical characteristics are shown in Table 1. With the exception of gender and history of tobacco use, all predictors were evenly distributed between the two treatment arms. Patients in the SFRT arm included more men (85%) than women (15%), and more smokers (86%) than nonsmokers (14%). Correspondingly, patients in the AFRT arm included more men (79%) than women (21%), and more smokers (78%) than nonsmokers (22%).
Table 1.
Patient Characteristics (N=684)
| Variables | SFRT (N=349) Mean±SD or N (%) |
AFRT (N=335) Mean±SD or N (%) |
|
|---|---|---|---|
| Age (year) | 56.19 ± 8.35 | 55.19 ± 9.30 | |
| Gender | Male | 298 (85) | 266 (79) |
| Female | 51 (15) | 69 (21) | |
| Racea | White | 280 (80) | 278 (83) |
| Non-White | 68 (20) | 56 (17) | |
| Educationa | ≤12 years | 183 (61) | 196 (65) |
| >12 years | 118 (39) | 104 (35) | |
| Marital statusa | Married | 175 (54) | 186 (58) |
| Unmarried | 151 (46) | 132 (42) | |
| History of tobacco usea | No | 44 (14) | 66 (22) |
| Yes | 264 (86) | 236 (78) | |
| Radiation dose (Gy) | 70.37± 1.13 | 72.15 ± 1.12 | |
| Primary cancer Site | Oral cavity | 22 (6) | 14 (4) |
| Oropharynx | 212 (61) | 204 (61) | |
| Hypopharynx | 29 (8) | 27 (8) | |
| larynx | 86 (25) | 90 (27) | |
| Stage | III | 74 (21) | 75 (22) |
| IV | 275 (79) | 260 (78) | |
| Tube feeding | No | 267 (77) | 264 (79) |
| Yes | 82 (23) | 71 (21) | |
Abbreviations: SD = Standard deviation, SFRT = Standard fractionation radiotherapy, AFRT = Accelerated fractionation radiotherapy. Statistical significant differences are highlighted in bold text.
Married: married or living as married; unmarried: single, separated, divorced, or widowed.
Having missing cases: Race (2); Education (83); Marital status (40); History of tobacco use(74).
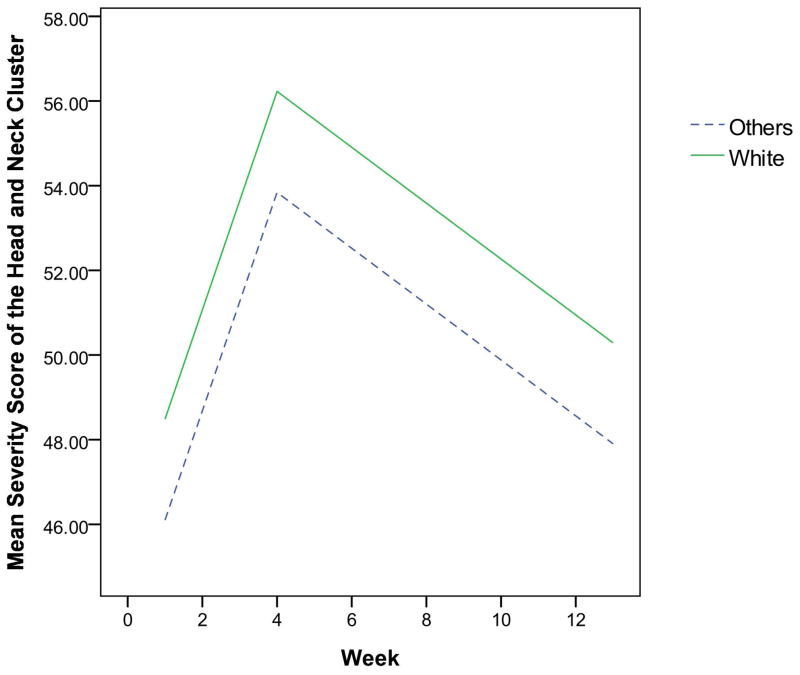
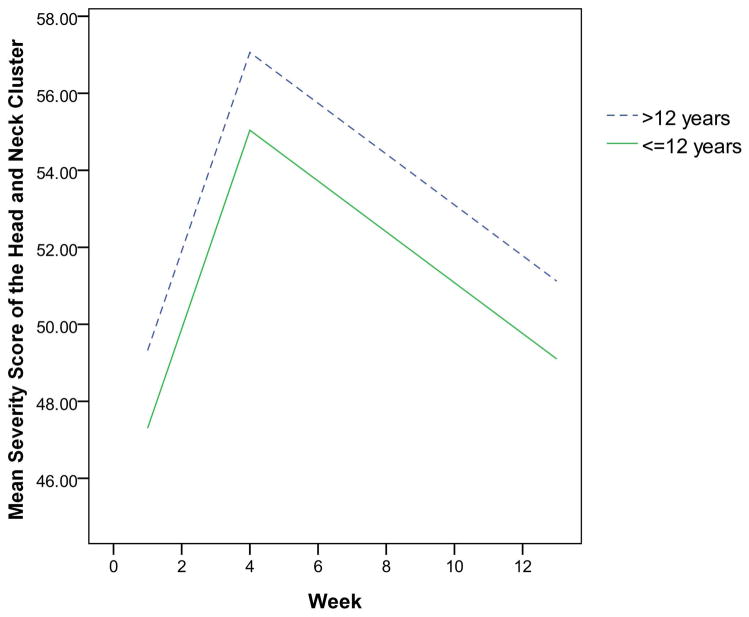
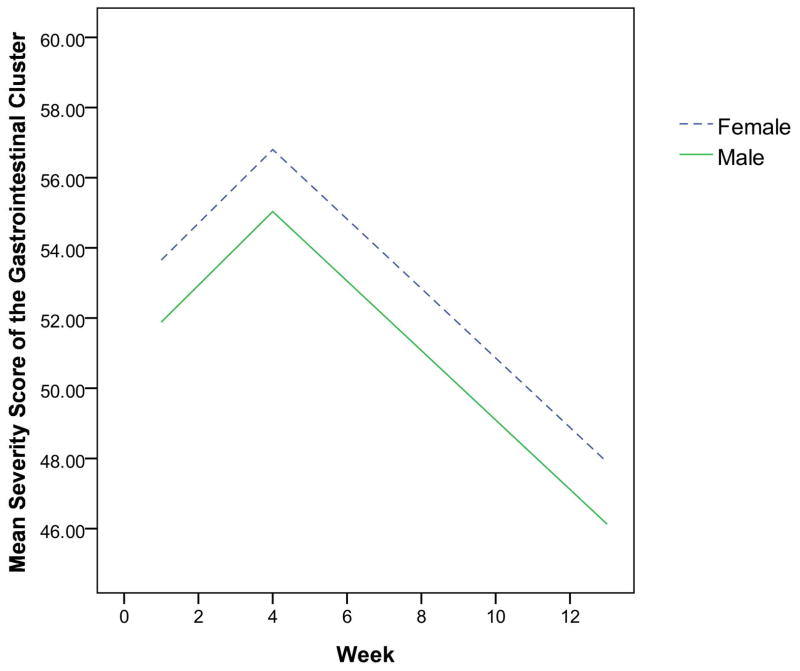
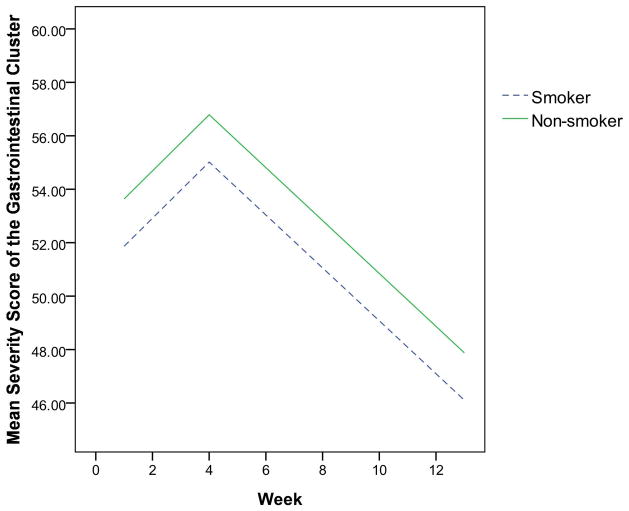
The final multivariate mixed-effect models for the two clusters showed that the demographic characteristics dominated, while clinical characteristics were eliminated. As displayed in Table 2, the effect of race and education were independent risk factors for the head and neck symptom cluster. Patients who were White or had more than 12 years of education were more likely to have severe symptom clusters than those who were non-White or had less than or equal to 12 years of education (Figures 1 and 2). Characteristics that independently predicted the gastrointestinal symptom cluster were gender and history of tobacco use. Patients who were female or never smoked were more likely to experience a more severe gastrointestinal symptom cluster than those who were male or those with a history of tobacco use, as shown in Figures 3 and 4.
Table 2.
Final mixed effect modeling results (N=684)
| Estimates | SE | p | |
|---|---|---|---|
| The HNC specific cluster | |||
| Time | 3.39 | 0.19 | <.0001 |
| Racea(non-White) | 2.20 | 0.66 | 0.0010 |
| Educationa(>12years) | −1.88 | 0.53 | 0.0005 |
| Time × time | −0.27 | 0.02 | <.0001 |
|
| |||
| The gastrointestinal cluster | |||
|
| |||
| Time | 1.56 | 0.21 | <.0001 |
| Gender (female) | −1.87 | 0.69 | 0.0068 |
| History of tobacco usea (yes) | 1.87 | 0.67 | 0.0057 |
| Time × time | −0.17 | 0.02 | <.0001 |
Abbreviation: HNC, head and neck cancer; SE, standard error. Parenthetical groups indicate the reference level.
Having missing cases: Race (2) and Education (83), which led to a total of 84 missing cases for the HNC specific cluster model; History of tobacco use (74).
Fig. 1.
The estimated severity score for the head and neck cluster over time by race
Fig. 2.
The estimated severity score for the head and neck cluster over time by education
Fig. 3.
The estimated severity score for the gastrointestinal cluster over time by gender
Fig. 4.
The estimated severity score for the gastrointestinal cluster over time by history of tobacco use
Clinical characteristics such as cancer site and treatment arm were only significant in univariate analyses, but not in multivariate analyses, which means that the effect of these characteristics disappeared after controlling for other characteristics such as race, gender, and education. The primary cancer site was the only significant clinical predictor for the head and neck symptom cluster over time (F = 4.72; p = .0029). Patients diagnosed with oropharyngeal cancer had more severe symptoms in the head and neck cluster than those diagnosed with laryngeal cancer. Although treatment arm was not a significant risk factor for either cluster over time, this characteristic was significant for individual symptoms of radiomucositis (p = .020), pain (p = .027), and nausea (p = .033) at 3 months after the initiation of radiotherapy; patients in the AFRT group experienced more severe symptoms than those in the SFRT group. Furthermore, the frequency of those symptoms was significantly higher in the AFRT than in the SFRT group.
DISCUSSION
This study, which used data from a large randomized clinical phase III trial, is the first to explore risk factors for symptom clusters in HNC patients. The findings show that demographic characteristics are significant risk factors for the head and neck and the gastrointestinal symptom clusters over time when controlling for other characteristics; clinical characteristics are only significant when other characteristics are not controlled.
Risk Factors for the Head and Neck Symptom Cluster
Race and education are independent risk factors for the head and neck symptom clusters; however, when the other characteristics were not controlled, the primary cancer site showed a significant predictive effect to this cluster. Patients diagnosed with oropharyngeal cancer had more severe head and neck symptom clusters than those diagnosed with laryngeal cancer. This finding is consistent with other reports of individual symptoms by site where the symptoms in the head and neck cluster (i.e., radiomucositis and dysphagia) are most frequently associated with treatment of the oropharynx.10,11 Paired comparisons between these two and other cancer sites were not significant
We hypothesized that patients who received AFRT would have more severe head and neck symptom clusters, but the data supported this hypothesis only in part. Treatment arm was not a significant risk factor for the head and neck cluster in either multivariate or univariate models over time; however, it is a significant risk factor for two symptoms within this cluster, radiomucositis and pain, at 3 months after initiation of radiotherapy. These findings are consistent with previous studies that compared AFRT with SFRT,12–16 in which significantly higher incidences of acute mucositis were documented for AFRT than for SFRT. In sum, treatment arm (SFRT vs. AFRT) is a significant predictor of individual symptoms like mucositis and pain, but it may not be a risk factor for the head and neck symptom cluster as a whole.
Race
This study showed that patients who were White were more likely to have severe head and neck symptom clusters than those who were non-White. This higher severity was significant even though Whites had more symptom-protective factors than non-Whites such as less tube feeding and lower radiation doses. As in this study, Whites had more intense upper gastrointestinal symptom clusters than non-Whites in patients with breast cancer.6 This trend has also been observed in studies of individual symptoms.17,18 In contrast, some studies have shown that non-Whites report higher symptom severity scores than Whites.19,20 These conflicting findings in literature make it unclear whether the Whites truly experienced more severe symptoms than non-Whites or whether other dynamics might have been at play. For instance, physicians may underestimate symptom severity in minority patients more than in nonminority patients.21 In addition, social and cultural factors like language and cultural differences can make communication between physicians and minority patients difficult.22,23 Given the inconsistent findings in the literature, more research is needed to examine race differences in symptom report and evaluation.
Education
Patients receiving more than 12 years of education were more likely to have severe head and neck symptom clusters. Well-educated patients, who may be better informed about treatment-related symptoms, might find it easier to discuss symptoms with their physician. In a study of women’s awareness of ovarian cancer risks and symptoms, researchers found that women with a college degree or higher were significantly more likely to identify symptoms correctly than those with a high school education or less.24 Other studies also showed that having higher education and more knowledge of a disease encourages behavior to seek health care assistance.25 Similarly, the more highly educated patients in this study may seek more health care assistance and therefore have the higher potential of reporting more symptoms in these clusters. However, our finding conflicts with some previous studies, in which educated persons were less likely to report problematic symptoms.18,26 As the effect of education on symptom report is inconclusive, future research is warranted.
Risk Factors for the Gastrointestinal Symptom Cluster
No significant clinical risk factor for the gastrointestinal cluster was found over time after multivariate and univariate analyses. However, the treatment arm is significant for the symptom of nausea at 3 months after initiation of radiotherapy. Patients in the AFRT group experienced more severe and more frequent nausea than those in the SFRT group after treatment, which is independent of gender and history of tobacco use. Patients in both arms received chemotherapy and radiotherapy but with different chemotherapy cycles and radiation doses. The AFRT group had a higher radiation dose for a shorter time and two cycles of cisplatin; the SFRT group had a lower radiation dose for a longer time but with three cycles of cisplatin. It seems that higher doses of radiotherapy in a shorter time would pose a higher emetic risk than more cycles of chemotherapy. However, the underlying biological mechanisms of this phenomenon are still unclear. Further studies of the effects of different chemotherapy cycles plus different radiotherapy fractionations and doses on nausea are recommended.
Gender
Women in this study tended to report more severe gastrointestinal symptom clusters. Evidence from literature has documented that female gender is a risk factor for nausea and vomiting.27 For instance, women report more chemotherapy-related nausea and vomiting than men.28 A similar association has also been recognized in patients who have received only radiation.29 This association has been further demonstrated in patients who have been treated with concurrent chemoradiation therapy.30 Several large clinical trials have found that antiemetic protection for women is significantly inferior to that for men,31,32 which may explain the connection. Although the precise mechanism underlying the increased risk for female patients remains to be established, the fact that women have a higher risk of nausea and vomiting than men is well-recognized in cancer treatment populations.
History of tobacco use
Patients with a history of tobacco use were less likely to experience a gastrointestinal cluster of dehydration, nausea, and vomiting than patients without a history of tobacco use. This effect is independent of other characteristics such as primary cancer site, treatment arm, and education. Investigations on postoperative nausea and vomiting have shown a similar finding, in which smokers experienced less incidence of the symptoms than non-smokers.33,34 This result was not expected because tobacco and nicotine use have shown an emetogenic effect.35,36 However, given the fact that many studies have found less symptoms of nausea and vomiting for smokers,33,34 some researchers hypothesize that chronic exposure to nicotine or tobacco would desensitize central nicotine receptors, and, subsequently, increase tolerance to the emetogenic effects of anesthesia and surgery.37 Unfortunately, randomized clinical trials that used either nicotine patch or transcutaneous nicotine to reduce postoperative nausea and vomiting have shown inconsistent results.34,37 The underlying biological mechanisms for this phenomenon warrant future examination, which may lead to new antiemetogenic drugs.
Directions for Personalized Symptom Management
Different predictors for different symptom clusters during and post concurrent chemoradiotherapy may help clinicians anticipate symptom profiles and tailor specific symptom management plans. For instance, clinicians may expect head and neck symptom clusters to be more severe for White and well-educated patients and gastrointestinal symptom clusters to be more severe for women and nonsmokers. Consequently, prevention or treatments could be recommended or prescribed for patients with higher risks of certain clusters. Personalized symptom management strategies may alter the course of symptom burden, thereby improving adherence to treatment regimens, short-and long-term quality of life, and possibly survival. Future research on genomic information in high-risk patients who suffer from these symptom clusters will expand the body of knowledge needed to personalized preventive measures and management.
Limitations
Notably, no information was provided on patients’ symptom management strategies. These strategies may have an influence on symptom cluster changes over time and, consequently, may change the predictive effects of the risk factors for these clusters. Additionally, symptoms in this study were clinician-not patient-reported, which might be a limitation when considering the literature on patient-reported symptom clusters. However, evidence shows the value of both symptom reporting approaches: symptoms reported by clinicians are more predictive of unfavorable clinical events, while patient-reported symptoms are associated more with their quality of life.16,38 A collaborative reporting approach has been proposed to value both clinician- and patient-reported symptoms.16,38 Finally, our data analysis might be limited by a relatively small sample of non-White patients, which may not be representative of the larger population of HNC patients.
CONCLUSION
This study investigated the effect of demographic and clinical characteristics as risk factors for two previous identified symptom clusters in HNC patients who received concurrent chemoradiotherapy. Demographic characteristics were more predictive of symptom clusters than clinical characteristics. The risk factors for the two clusters differ: race and education are predictive of head and neck symptom clusters, while gender and history of tobacco are associated with gastrointestinal symptom clusters. Clinical characteristics such as treatment arm are more predictive of certain individual symptoms like radiomucositis, pain, and nausea than of the symptom clusters. The findings are generalizable, because this study used data from the RTOG 0129, a large sample randomized clinical trial that collected data from multiple sites across Canada and the United States. Future research on genomic information in high-risk patients who suffer from these symptom clusters will expand the body of knowledge needed to personalize prevention and management.
Acknowledgments
This project was supported by RTOG grant U10 CA21661, CCOP grant U10 CA37422, Stat grant U10 CA32115 from the National Cancer Institute (NCI), and American Nursing Foundation (ANF). This manuscript’s contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Cancer Institute. The authors thank the contributions from Dr.’s Sarah H. Kagan and Andrea M. Barsevick for consultation on the study design.
Footnotes
Conflict of interest: None.
References
- 1.Cooper JS, Porter K, Mallin K, et al. National Cancer Database report on cancer of the head and neck: 10-year update. Head Neck. 2009 Jun;31(6):748–758. doi: 10.1002/hed.21022. [DOI] [PubMed] [Google Scholar]
- 2.Beck SL. Mucositis. In: Yarbro CH, Frogge MH, Goodman M, editors. Cancer symptom management. 3. Sudbury: Jones and Bartlett; 2004. p. 765. [Google Scholar]
- 3.Given B, Given C, Azzouz F, Stommel M. Physical functioning of elderly cancer patients prior to diagnosis and following initial treatment. Nurs Res. 2001 Jul-Aug;50(4):222–232. doi: 10.1097/00006199-200107000-00006. [DOI] [PubMed] [Google Scholar]
- 4.Xiao C, Hanlon A, Zhang Q, et al. Symptom clusters in patients with head and neck cancer receiving concurrent chemoradiotherapy. Oral oncology. 2012 Nov 2; doi: 10.1016/j.oraloncology.2012.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang SY, Tsai CM, Chen BC, Lin CH, Lin CC. Symptom clusters and relationships to symptom interference with daily life in Taiwanese lung cancer patients. J Pain Symptom Manage. 2008 Mar;35(3):258–266. doi: 10.1016/j.jpainsymman.2007.03.017. [DOI] [PubMed] [Google Scholar]
- 6.Kim HJ, Barsevick AM, Tulman L. Predictors of the intensity of symptoms in a cluster in patients with breast cancer. J Nurs Scholarsh. 2009;41(2):158–165. doi: 10.1111/j.1547-5069.2009.01267.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fleming ID, Cooper JS, Henson DE, et al. AJCC Cancer Staging Manual. 5. Philadelphia: Lippincott Williams & Wilkins; 1997. [Google Scholar]
- 8.Wainer H. Estimating coefficients in linear models: It don’t make no nevermind. Psychological Bulletin. 1976;83:213–217. [Google Scholar]
- 9.Thorndike RL. Applied psychometrics. Boston: Houghton Mifflin; 1982. [Google Scholar]
- 10.Elting LS, Cooksley CD, Chambers MS, Garden AS. Risk, outcomes, and costs of radiation-induced oral mucositis among patients with head-and-neck malignancies. Int J Radiat Oncol Biol Phys. 2007 Jul 15;68(4):1110–1120. doi: 10.1016/j.ijrobp.2007.01.053. [DOI] [PubMed] [Google Scholar]
- 11.Vera-Llonch M, Oster G, Hagiwara M, Sonis S. Oral mucositis in patients undergoing radiation treatment for head and neck carcinoma. Cancer. 2006 Jan 15;106(2):329–336. doi: 10.1002/cncr.21622. [DOI] [PubMed] [Google Scholar]
- 12.Corvo R. Evidence-based radiation oncology in head and neck squamous cell carcinoma. Radiother Oncol. 2007 Oct;85(1):156–170. doi: 10.1016/j.radonc.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 13.Fu KK, Pajak TF, Trotti A, et al. A Radiation Therapy Oncology Group (RTOG) phase III randomized study to compare hyperfractionation and two variants of accelerated fractionation to standard fractionation radiotherapy for head and neck squamous cell carcinomas: first report of RTOG 9003. International journal of radiation oncology, biology, physics. 2000 Aug 1;48(1):7–16. doi: 10.1016/s0360-3016(00)00663-5. [DOI] [PubMed] [Google Scholar]
- 14.Mortensen HR, Overgaard J, Specht L, et al. Prevalence and peak incidence of acute and late normal tissue morbidity in the DAHANCA 6&7 randomised trial with accelerated radiotherapy for head and neck cancer. Radiother Oncol. 2012 Apr;103(1):69–75. doi: 10.1016/j.radonc.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 15.Overgaard J, Mohanti BK, Begum N, et al. Five versus six fractions of radiotherapy per week for squamous-cell carcinoma of the head and neck (IAEA-ACC study): a randomised, multicentre trial. The lancet oncology. 2010 Jun;11(6):553–560. doi: 10.1016/S1470-2045(10)70072-3. [DOI] [PubMed] [Google Scholar]
- 16.Chitapanarux I, Tharavichitkul E, Kamnerdsupaphon P, Pukanhapan N, Vongtama R. Randomized phase III trial of concurrent chemoradiotherapy vs accelerated hyperfractionation radiotherapy in locally advanced head and neck cancer. Journal of radiation research. 2013 Jun 5; doi: 10.1093/jrr/rrt054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yoon J, Malin JL, Tao ML, et al. Symptoms after breast cancer treatment: are they influenced by patient characteristics? Breast Cancer Res Treat. 2008 Mar;108(2):153–165. doi: 10.1007/s10549-007-9599-3. [DOI] [PubMed] [Google Scholar]
- 18.Stanton AL, Bernaards CA, Ganz PA. The BCPT symptom scales: a measure of physical symptoms for women diagnosed with or at risk for breast cancer. J Natl Cancer Inst. 2005 Mar 16;97(6):448–456. doi: 10.1093/jnci/dji069. [DOI] [PubMed] [Google Scholar]
- 19.Green CR, Hart-Johnson T, Loeffler DR. Cancer-related chronic pain: examining quality of life in diverse cancer survivors. Cancer. 2011 May 1;117(9):1994–2003. doi: 10.1002/cncr.25761. [DOI] [PubMed] [Google Scholar]
- 20.Eversley R, Estrin D, Dibble S, Wardlaw L, Pedrosa M, Favila-Penney W. Post-treatment symptoms among ethnic minority breast cancer survivors. Oncol Nurs Forum. 2005 Mar;32(2):250–256. doi: 10.1188/05.ONF.250-256. [DOI] [PubMed] [Google Scholar]
- 21.Cleeland CS, Gonin R, Baez L, Loehrer P, Pandya KJ. Pain and treatment of pain in minority patients with cancer. The Eastern Cooperative Oncology Group Minority Outpatient Pain Study. Ann Intern Med. 1997 Nov 1;127(9):813–816. doi: 10.7326/0003-4819-127-9-199711010-00006. [DOI] [PubMed] [Google Scholar]
- 22.Berkman ND, Sheridan SL, Donahue KE, Halpern DJ, Crotty K. Low health literacy and health outcomes: an updated systematic review. Annals of internal medicine. 2011 Jul 19;155(2):97–107. doi: 10.7326/0003-4819-155-2-201107190-00005. [DOI] [PubMed] [Google Scholar]
- 23.Murthy VH, Krumholz HM, Gross CP. Participation in cancer clinical trials: race-, sex-, and age-based disparities. JAMA : the journal of the American Medical Association. 2004 Jun 9;291(22):2720–2726. doi: 10.1001/jama.291.22.2720. [DOI] [PubMed] [Google Scholar]
- 24.Lockwood-Rayermann S, Donovan HS, Rambo D, Kuo CW. Women’s awareness of ovarian cancer risks and symptoms. Am J Nurs. 2009 Sep;109(9):36–45. doi: 10.1097/01.NAJ.0000360309.08701.73. quiz 46. [DOI] [PubMed] [Google Scholar]
- 25.David MM, Ko L, Prudent N, Green EH, Posner MA, Freund KM. Mammography use. J Natl Med Assoc. 2005 Feb;97(2):253–261. [PMC free article] [PubMed] [Google Scholar]
- 26.Movsas B, Scott C, Watkins-Bruner D. Pretreatment factors significantly influence quality of life in cancer patients: a Radiation Therapy Oncology Group (RTOG) analysis. International journal of radiation oncology, biology, physics. 2006 Jul 1;65(3):830–835. doi: 10.1016/j.ijrobp.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 27.Cheung WY, Le LW, Gagliese L, Zimmermann C. Age and gender differences in symptom intensity and symptom clusters among patients with metastatic cancer. Support Care Cancer. 2011 Mar;19(3):417–423. doi: 10.1007/s00520-010-0865-2. [DOI] [PubMed] [Google Scholar]
- 28.Liaw CC, Chang HK, Liau CT, Huang JS, Lin YC, Chen JS. Reduced maintenance of complete protection from emesis for women during chemotherapy cycles. Am J Clin Oncol. 2003 Feb;26(1):12–15. doi: 10.1097/00000421-200302000-00003. [DOI] [PubMed] [Google Scholar]
- 29.Feyer P, Maranzano E, Molassiotis A, Roila F, Clark-Snow RA, Jordan K. Radiotherapy-induced nausea and vomiting (RINV): MASCC/ESMO guideline for antiemetics in radiotherapy: update 2009. Support Care Cancer. 2011 Mar;19( Suppl 1):S5–14. doi: 10.1007/s00520-010-0950-6. [DOI] [PubMed] [Google Scholar]
- 30.Fraunholz I, Grau K, Weiss C, Rodel C. Patient- and treatment-related risk factors for nausea and emesis during concurrent chemoradiotherapy. Strahlenther Onkol. 2011 Jan;187(1):1–6. doi: 10.1007/s00066-010-2196-0. [DOI] [PubMed] [Google Scholar]
- 31.Hesketh PJ, Grunberg SM, Herrstedt J, et al. Combined data from two phase III trials of the NK1 antagonist aprepitant plus a 5HT 3 antagonist and a corticosteroid for prevention of chemotherapy-induced nausea and vomiting: effect of gender on treatment response. Support Care Cancer. 2006 Apr;14(4):354–360. doi: 10.1007/s00520-005-0914-4. [DOI] [PubMed] [Google Scholar]
- 32.Myles PS, Hunt JO, Moloney JT. Postoperative ‘minor’ complications. Comparison between men and women. Anaesthesia. 1997 Apr;52(4):300–306. doi: 10.1111/j.1365-2044.1997.89-az0091.x. [DOI] [PubMed] [Google Scholar]
- 33.Brattwall M, Warren Stomberg M, Rawal N, Segerdahl M, Houltz E, Jakobsson J. Postoperative impact of regular tobacco use, smoking or snuffing, a prospective multi-center study. Acta Anaesthesiol Scand. 2010 Mar;54(3):321–327. doi: 10.1111/j.1399-6576.2009.02140.x. [DOI] [PubMed] [Google Scholar]
- 34.Ionescu D, Badescu C, Acalovschi I. Nicotine patch for the prevention of postoperative nausea and vomiting: a prospective randomised trial. Clinical drug investigation. 2007;27(8):559–564. doi: 10.2165/00044011-200727080-00004. [DOI] [PubMed] [Google Scholar]
- 35.Greenland S, Satterfield MH, Lanes SF. A meta-analysis to assess the incidence of adverse effects associated with the transdermal nicotine patch. Drug Saf. 1998 Apr;18(4):297–308. doi: 10.2165/00002018-199818040-00005. [DOI] [PubMed] [Google Scholar]
- 36.Zhan M, Flaws JA, Gallicchio L, Tkaczuk K, Lewis LM, Royak-Schaler R. Profiles of tamoxifen-related side effects by race and smoking status in women with breast cancer. Cancer Detect Prev. 2007;31(5):384–390. doi: 10.1016/j.cdp.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 37.Czarnetzki C, Schiffer E, Lysakowski C, Haller G, Bertrand D, Tramer MR. Transcutaneous nicotine does not prevent postoperative nausea and vomiting: a randomized controlled trial. British journal of clinical pharmacology. 2011 Mar;71(3):383–390. doi: 10.1111/j.1365-2125.2010.03844.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Basch E, Bennett A, Pietanza MC. Use of patient-reported outcomes to improve the predictive accuracy of clinician-reported adverse events. J Natl Cancer Inst. 2011 Dec 21;103(24):1808–1810. doi: 10.1093/jnci/djr493. [DOI] [PubMed] [Google Scholar]