Abstract
Previous studies have suggested that TGF-β functions as a tumor promoter in metastatic, mesenchymal-like breast cancer cells and that TGF-β inhibitors can effectively abrogate tumor progression in several of these models. Here we report a novel observation with the use of genetic and pharmacological approaches, and murine mammary cell injection models in both syngeneic and immune compromised mice. We found that TGF-β receptor II (TβRII) knockdown in the MMTV-PyMT derived Py8119, a mesenchymal-like murine mammary tumor cell line, resulted in increased orthotopic tumor growth potential in a syngeneic background and a similar trend in an immune compromised background. Systemic treatment with a small-molecule TGF-β receptor I kinase inhibitor induced a trend towards increased metastatic colonization of distant organs following intra cardiac inoculation of Py8119 cells, with little effect on the colonization of luminal-like Py230 cells, also derived from MMTV-PyMT tumors. Taken together, our data suggest that the attenuation of TGF-β signaling in mesenchymal-like mammary tumors does not necessarily inhibit their malignant potential, and anti-TGF-β therapeutic intervention requires greater precision in identifying molecular markers in tumors with an indication of functional TGF-β signaling.
Keywords: TGF-β, MMTV-PyMT mammary tumor, syngeneic model, metastasis
1. Introduction
The cytokine transforming growth factor beta (TGF-β) is pivotal in orchestrating multiple events during embryonic development, events that encompass its diverse roles in mammary gland morphogenesis [1; 2]. In normal epithelial cells, TGF-β signaling antagonizes cell proliferation, whereas in mesenchymal cells it is mitogenic. In addition to inducing cell cycle arrest, it also promotes apoptosis as well as cell senescence to maintain tissue homeostasis [3; 4; 5; 6].
TGF-β signaling and its functions in normal epithelial tissues have been extensively reviewed [7; 8; 9; 10]. A substantial body of evidence from clinical studies supports the fact that TGF-β has diverse effects on tumor progression in multiple tissues. Loss of TGF-β signaling through loss/mutation of its intracellular mediators Smad4 and Smad2/3, and its receptors as well as epigenetic silencing of Smad3 is prevalent in various types of cancer including pancreatic, colorectal, gastro-intestinal, renal cancers and acute lymphoblastic leukemia (ALL) [11; 12]. Conversely, over expression of TGF-β is associated with poor prognosis in several advanced metastatic tumors, which include breast tumors and melanomas [13]. In such cases, although tumors may exhibit longer latency in formation, they metastasize very rapidly once they are formed. This is in concordance with studies that have used either soluble TGF-β receptors or antibodies against TGF-β and have demonstrated inhibition of metastases in allograft or xenograft models with advanced metastatic tumors [14; 15].
Thus, for most tumor models, the current paradigm is that during the early stages of tumor development, TGF-β functions as a growth inhibitor [16; 17]. However, it undergoes a role reversal during the advanced stages of tumor progression, where this cytokine is capable of enhancing tumor invasiveness and distant metastases. Unlike several other tumors, particularly gastro-intestinal tumors that undergo loss of one or more components of the TGF-β pathway and therefore possess an impaired signaling activity [18; 19], most breast tumors retain a functional TGF-β pathway. Essentially, these tumor cells are no longer responsive to growth inhibition by TGF-β, they undergo TGF-β mediated epithelial to mesenchymal transition, and thus metastasize more efficiently than the luminal-like counterparts [20; 21; 22]. This makes the TGF-β pathway a potential target for therapy in advanced and metastatic breast cancers. Contradictory to these findings, abrogation of TGF-β signaling in transgenic mouse models has reportedly enhanced primary tumorigenesis and even increased metastases in various genetic backgrounds in different tumor models [23; 24; 25]. Furthermore, data from clinical studies have shown that TβRII expression levels correlate inversely with pathological grades in ductal carcinoma in situ and invasive metastatic breast cancer [26]. These numerous and often contradictory findings have created a conundrum, making the proper identification of TGF-β as a therapeutic target difficult. Some studies in xenograft and allograft mouse models have illustrated the therapeutic efficacy of several TGF-β inhibitors in reducing distant organ metastases in mesenchymal-like tumors [14; 27]. However, data from most existing studies on TGF-β inhibitors have utilized human xenografts in an immune compromised background. TGF-β is an important modulator of the immune system, particularly as a regulator of T-cells and myeloid cells [28; 29]. These aforementioned issues necessitate a closer evaluation of the effects of systemic and genetic abrogation of TGF-β function in a mesenchymal invasive mammary epithelial system in a syngeneic, immune-competent background.
We describe here for the first time, that the abrogation of endogenous TGF-β signaling in Py8119 orthotopic tumors enhanced tumor outgrowth in the immune competent syngeneic C57Bl/6 mice. A similar trend towards an increase in tumor volume was observed in immune compromised mice. Along similar lines, we found that the abrogation of TGF-β signaling in the mesenchymal-like Py8119 cells did not inhibit their metastatic potential, but moderately increased their colonization in secondary organs in the syngeneic mice. Therefore, our results present a novel cell model, which did not show inhibition in tumor growth and metastatic colonization with abrogation in TGF-β signaling in vivo, despite its mesenchymal- and myofibroblast-like phenotype.
2. Materials and Methods
2.1. Cell lines and reagents
Py230 and Py8119 cells were obtained from spontaneously arising tumors in MMTV-PyMT C57Bl/6 female mice by serial trypsinization and limiting dilution [30]. The mouse model used for obtaining these tumors has been described in detail previously [31; 32]. Cells were maintained in F12K nutrient culture media (Mediatech, Manassas, VA) with 5% fetal clone II (Fisher Scientific, Pittsburgh, PA) and supplemented with MITO serum extender (BD biosciences, San Jose, CA). Py230 cells were cultured until confluent and then passaged. Once confluent, they formed well-differentiated colonies. Py8119 cells, on the other hand, grew much more rapidly, without differentiating into domes/colonies.
The TGF-β receptor I kinase inhibitor (TβRI-KI) used in our study, HTS 466284/LY364947 has been reported to be an ATP competitive inhibitor of the TβRI kinase [33; 34]. This compound, [3-(pyridine-2yl)-4-(4-quinonyl)]-1H pyrazole was synthesized according to the procedure described previously [34]. We have also utilized a recombinant ligand trap molecule BGERII that involves the fusion of the extracellular domain of TβRII and the endoglin domain of betaglycan. This fusion molecule acts as a potent antagonist of TGF-β1, TGF-β2, and TGF-β3. This antagonist was initially expressed and purified from bacterial cells and compared for its efficiency to inhibit TGF-β signaling activity against other commercial inhibitors with various assays. It was found to be more effective than either commercially available soluble TβRII or betaglycan ectodomain alone in inhibiting TGF-β isoforms [35]. It was later expressed and purified from mammalian CHO (Chinese Hamster Ovarian) cells and used in this study.
2.2. Western Blot analyses
Whole cell lysates were obtained and fractionated by using 10% SDS-PAGE followed by transferring to a PVDF membrane according to the manufacturer’s protocols, Bio-Rad (Hercules, CA). Following transfer, membranes were blocked in 5% non-fat milk in TBST for 2 hours at room temperature. The membranes were then incubated in the primary antibodies in 5% milk or 3% BSA (as per manufacturer’s instructions) overnight at 4°C. Antibodies purchased were: p-Smad2: Cell Signaling (1:1000), Smad2 (1:3000): Cell Signaling, p-Smad3: Epitomics (1:2000), Smad3: (1:1000) Cell Signaling, TβRI: (1:500), Santa Cruz, TβRII: Santa Cruz, GAPDH: Calbiochem (1:500), p-p38: Cell Signaling (1:1000), p38: Cell Signaling (1:1000), p-Src (Y416): Cell Signaling (1:1000), E-Cadherin: BD Biosciences (1:500), N-Cadherin: BD Biosciences (1:500), Vimentin: V5255 (1:200), Slug: Abcam (1:1000), CK-8: Abcam (1:1000), CK-14 (1:200): Abcam. p-TβRI Abbomax, (1:500). The membranes were washed three times for 10 minutes in TBST and then incubated in secondary antibody diluted in 5% milk in TBST for 1 hour at room temperature. Membranes were developed using the ECL reagents, Amersham (Pittsburgh, PA). Blots were developed using an ECL kit (Amersham Biosciences, Piscataway, NJ) and exposed with Blue Basic Autorad Film (ISC BioExpress).
2.3. Immunocytochemical staining
Cells were plated on coverslips in 24-well plates, Corning Life Sciences (Corning, NY), at required density and fixed using 4% paraformaldehyde solution in 1X PHEM buffer (60 mM PIPES, 25 mM HEPES, 10 mM EGTA, 2 mM MgCl2). They were then permeabilized in 0.1% Triton X-00 in 1X PHEM and blocked in 10% normal goat serum for one hour at room temperature. Primary antibody was diluted as required in blocking buffer and fixed cells were incubated overnight. Incubation with secondary antibody (Alexa 488/ Alexa 568) was performed at room temperature for one hour in the dark, after which samples were washed and mounted with Vectashield mounting medium (Burlingame, CA) and viewed under confocal microscope at 60X objective (Olympus FV-1000). Images were generated using Olympus Viewer2 software.
2.4. Transwell migration assays
Cells were seeded at a density of 40,000 cells /well for Py230 and 20,000 cells/well for Py8119 in serum free media into 8µm pore-size inserts (BD Falcon) in 24-well plates. TGT-β1 (2ng/mL) and TβRI-KI (100 nM) were added to the inserts in the basic media, either alone or in combination. The bottom chamber had media with 5% serum to create a chemo-attractive gradient. The cells in the inserts were allowed to migrate for 18 hours in a cell culture incubator at 37°C. After 18 hours, the cells on the inner surface of the inserts were cleared using cotton swabs. Migrated cells were then fixed, stained using HEMA staining kit, Fisher Scientific (Pittsburgh, PA) according to the manufacturer’s protocol, and counted under a bright field microscope.
2.5. MTT cell proliferation assays
Py230 and Py8119 and MCF10A (normal human mammary epithelial cells) were plated in triplicate at a density of 3,000 cells/well into 96 well plates in F12K media and treated with different concentrations of TGF-β (0, 0.2, 1, 5 ng/ml). After 4 days, MTT solution (50 µl, 2 mg/ml in PBS) was added to each well and allowed to incubate for 2 hours in a cell culture incubator. DMSO (100 µl) was added into each well after removing the media. To allow homogenous dissolution of the precipitate, the plate was gently agitated on a shaker for 10 min. The absorbance was measured at 595 nm with a microplate reader (BioTek Instrument, Winooski, VT).
2.6. Luciferase reporter assays
The firefly luciferase reporter (pSBE4-Luc) plasmid containing a Smad responsive promoter was used for the transcriptional reporter assays [36]. Cells were plated into 24 well plates at a density of 0.1 × 106 per well and grown to approximately 70% confluence. They were then transfected with 0.4 µg of pSBE4-Luc and 0.1 µg of a β-galactosidase expressing plasmids using Fugene HD transfection reagent, Promega (Madison, WI) as per the manufacturer’s instructions. After 6 hours the media was changed and cells were treated with TGF-β1 (2ng/mL) or TβRI-KI (100 nM), either individually, or in combination. After 24 hours, cell lysates were obtained using lysis buffer [100 mM K2HPO4 (pH 7.8) and 1 mM DTT]. Luciferase activity was measured and normalized with β-galactosidase readings as described previously [37].
2.7. Cell cycle analysis
Py230 cells were plated into 60 mm culture dishes and grown to approximately 70% confluence. Cells were serum starved for 24 hours to synchronize the cell cycles and then treated with TGF-β1 (2ng/mL) for 24 hours at 37°C. Treated and untreated cells were then harvested by trypsinization, and suspended in 1X PBS. Cells were fixed using cold 70% ethanol on ice for 30 minutes, RNase treated, and stained with propidium iodide. Cell cycle data was acquired with a BD Bioscience LSRII flow cytometer (San Jose, CA) and analyzed with the FlowJo software (Ashland, OR) at the flow cytometry core at UT Health Science Center, San Antonio. Single cell discrimination was performed using PI-Area and PI-Width. The Dean-Jett-Fox mathematical model was used for calculating percentage of cells in each cell cycle stage.
2.8. Lentiviral shRNA knockdown studies
A PLKO.1 vector expressing a small hairpin RNA against TβRII was purchased from Thermo Scientific (Pittsburgh, PA), and 293T packaging cells were transfected to generate TβRII lentiviral sh-RNA, which was used to infect Py8119 cells as per the manufacturer’s instructions. Knockdown of TGF-β receptor II was confirmed by real-time PCR and Western blot analyses. PLKO.1 backbone lentiviral plasmid was used as a control.
2.9. Real-time PCR analysis
RNA was isolated using Tri reagent (Sigma T9424), cDNA was generated using random primers and M-MLV reverse transcriptase from Invitrogen (Grand Island, NY) and real-time PCR was performed using SYBR reagent (Invitrogen) to detect Ct values for TβRII, which were then normalized to β-actin levels.
Primers for TβRII: F- 5’-TTC GCC GAG GTC TAC AAG-3’, R- 5’-CAG CCA CGG TCT CAA ACT-3’ Primers for β-actin: F- 5’-TCG TCA TCC ATG GCG AAC TGGT-3’, R- 5’-CTG TCG AGT CGC GTC CACC-3’. Primer pair specificity was determined by generation of a single peak for dissociation curve, through melting curve analysis at the end of RT-PCR cycling program.
2.10. Animal Studies
Ethics Statement
All animal experiments were conducted following appropriate guidelines. They were approved by the ethics committee/institutional review board, ‘Institutional Animal Care and Use Committee’ (IACUC approval ID 99142×3411A) and monitored by the Department of Laboratory Animal Resources (DLAR) at the University of Texas Health Science Center at San Antonio (UTHSCSA).
Four-week old female C57Bl/6 mice were obtained from Jackson laboratories Inc. (Bar Harbor, Maine). Female Nu/Nu mice at similar ages were purchased from Harlan Sprague Dawley Inc. (Indianapolis, IN). All mice were housed in specific pathogen free conditions in the animal housing facility with the experiments performed in accordance with the guidelines for animal care at the UTHSCSA. For the orthotopic tumor studies in C57Bl/6 mice, Py8119 cells tagged with Luciferase-GFP (luciferase-GFP plasmid, kindly provided by Dr. Brian Rabinovich, MD Anderson Cancer Center, Houston) were used for injections into both inguinal mammary gland areas. Four-week old mice were injected with 0.1 × 106 cells in 1X PBS mixed with Matrigel (1:1). Animals were treated with placebo (1X PBS) or TβRI-KI (1mg/kg body weight) every alternate day, through the intra-peritoneal route. Animals that received cells with an endogenous knockdown of TβRII were treated only with the placebo. Tumor volumes were measured twice weekly with a caliper and tumor volumes were calculated using the formula V = (L×W2)×0.5, where L is length and W is width of a tumor.
For orthotopic studies in Nu/Nu mice, similar procedures as above were performed for orthotopic transplants in Nu/Nu mice. Animals receiving control (PLKO.1 vector infected) cells received either placebo, TβRI-KI (1mg/kg body weight) or BGERII (100 µg/animal) through intraperitoneal injections. Animals injected with TβRII knockdown cells received only placebo injections.
2.11. Experimental metastasis models
Animals were anaesthetized with 1–3% isoflurane inhalation. Py8119 cells were suspended in sterile 1X PBS and 1×105 cells were injected into the left ventricle of five-week old C57Bl/6 mice. The mice were treated intraperitoneally with placebo or TβRI-KI as mentioned above, 24 hours after cell inoculation.
2.12. In vivo bioluminescence imaging
Mice were administered D-luciferin substrate at 150 mg/kg via intra-peritoneal injections. Bioluminescence imaging was performed using the Xenogen IVIS spectrum imaging system as described previously [38] at the imaging core facility at UTHSCSA.
2.13. Histology
Brain tissues were fixed in 10% neutral buffered formalin Fisher Scientific (Pittsburgh, PA) and embedded in paraffin. Paraffin-embedded sections were stained with hematoxylin and eosin. To analyze tibial metastases in the intracardially injected mice, bone tissues were fixed similarly for 48 hours at room temperature, decalcified in 10% EDTA and embedded in paraffin. Paraffin-embedded sections were stained with hematoxylin and eosin as previously described. TUNEL assays were also performed to detect apoptosis in the tumor samples as described previously [39].
2.14. Statistical analyses
All statistical analyses were performed using Prism version 5.0 software
3. Results
3.1. Py230 and Py8119 cells exhibit a differential profile of epithelial/mesenchymal cell markers
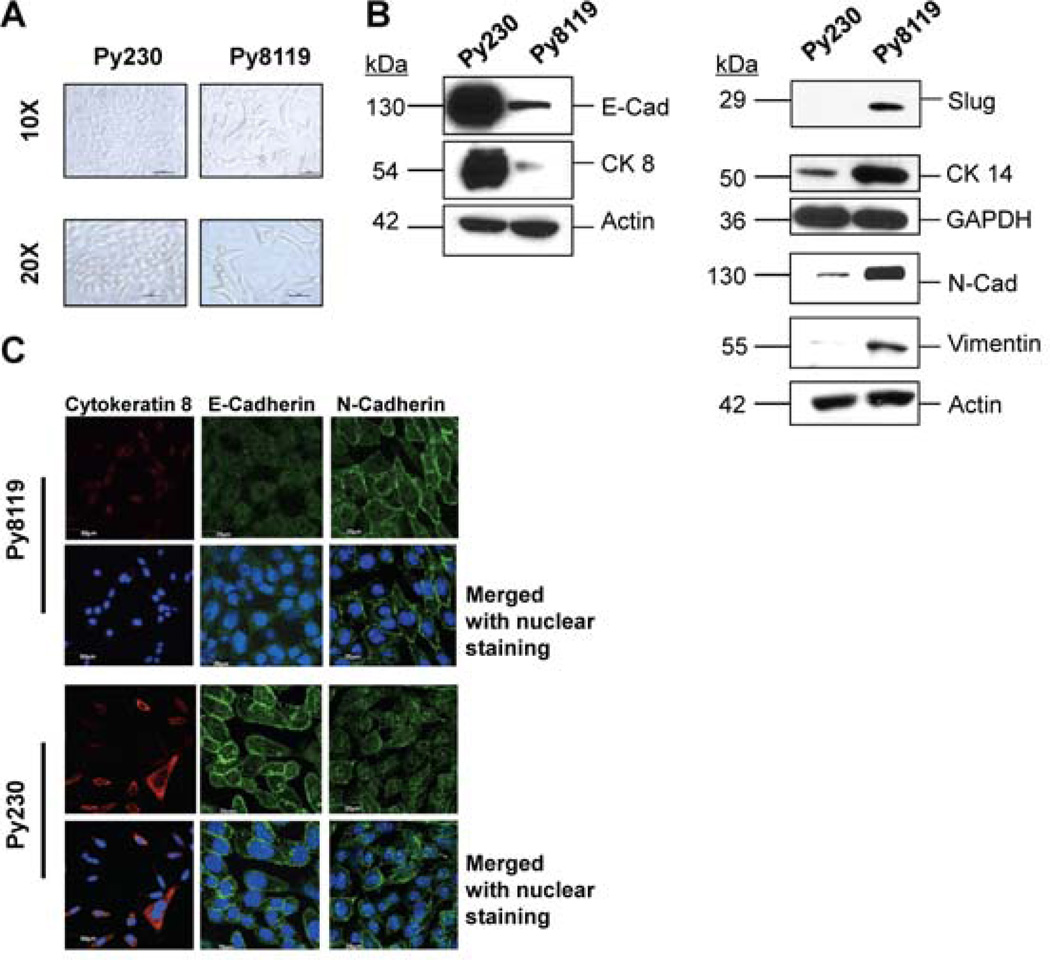
To determine and compare the role of TGF-β signaling in the regulation of growth and malignant phenotypes in transformed epithelial cells with or without a mesenchymal phenotype, we used the Py230 and Py8119 cell lines, which were isolated from mammary tumors in MMTV-PyMT (mouse mammary tumor virus promoter driven polyoma middle T-antigen) transgenic C57Bl/6 mice. Both cell lines were isolated by serial trypsinization and limiting dilution of tumor cells and maintained in culture medium with low serum [30]. These cells showed remarkably distinct morphologies, Py230 cells were more cuboidal, and grew in well-differentiated domes or colonies. The Py8119 cells were spindle-shaped and did not form colonies (Figure 1A). Py8119 cells expressed relatively high levels of mesenchymal markers, like N-cadherin, vimentin and Slug. They also expressed higher levels of the basal epithelial cytokeratin 14 as compared to the Py230 cells. The Py230, on the other hand, expressed higher levels of luminal-epithelial markers, like E-Cadherin and cytokeratin 8 (Figure 1B). These observations were further validated with immunofluorescence studies, which demonstrated high levels of expression for CK8 and E-Cadherin and moderate expression for N-Cadherin in Py230 cells. Conversely, Py8119 cells showed relatively high levels of expression for N-Cadherin and relatively low levels of the luminal epithelial markers (Figure 1C). These data suggest that in addition to their morphological differences, the two cell lines had explicitly differential expression profiles of epithelial and mesenchymal markers [30].
Figure 1. Py230 and Py8119 exhibit differential expression profile for cell adhesion markers and cytoskeletal proteins.
A. Bright field images, at 10X and 20X objectives, depicting differences in morphology of murine mammary tumor cells Py230 and Py8119. Py230 cells showed a cuboidal (cobblestone like) morphology, while Py8119 cells showed a mesenchymal-like morphology. B. Western blots were performed on cell lysates from both the cell lines to detect the constitutive expression of epithelial markers. Figure shows that Py230 cells expressed higher levels of epithelial cell markers E-Cadherin, and cytokeratin 8. Py8119 cells expressed relatively higher levels of mesenchymal markers like N-Cadherin, vimentin, Slug and the basal epithelial cytokeratin 14. C. Immunofluorescence images (60X) showed high levels of E-Cadherin and cytokeratin 8 in Py230 cells, as compared to Py8119 cells. Py230 cells expressed low levels of N-Cadherin. Approximately, 10,000 cells were plated on top of round glass coverslips in 24-well plate, and fixed in 4% paraformaldehyde. The cells were then permeabilized in Triton X100 and blocked in normal goat serum, following primary and secondary antibody incubations. Coverslips were mounted using Vectashield mounting medium, and visualized under oil immersion lens, using 60X objective. Experiments were performed in triplicates and representative images from independent experiments have been presented.
3.2. TGF-β signaling pathway is functional in both Py230 and Py8119 cell lines, but inhibits growth only in Py230 cells
Complete loss of TGF-β signaling in terms of losing the receptors or Smad is rare in breast cancers. Instead, it is more common for breast cancer cells to retain functional signaling, while developing resistance to TGF-β’s growth suppression and pro-apoptotic functions. Indeed, we found that TFG β receptor-I (TβRI), and receptor-II (TβRII) proteins were expressed at comparable levels in both the cell lines (supplemental data, Fig. S1). To characterize TGF-β signaling activity in Py230 and Py8119 cells, we investigated the effects of TGF-β signaling on gene transcription, Smad phosphorylation, cell growth and migration.
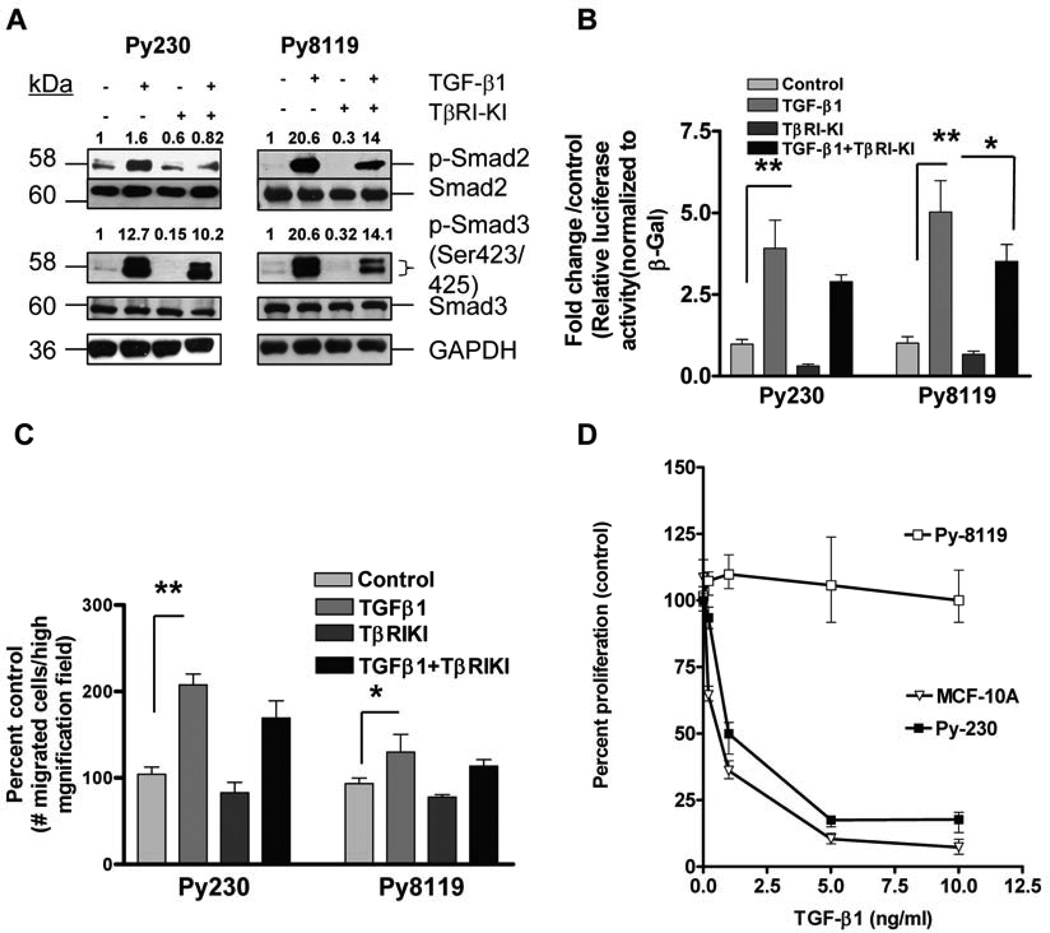
Western blot analyses showed enhanced levels of phosphorylated Smad2/Smad3 in both the cell lines when they were treated with TGF-β, which again indicated signaling activity of TGF-β in both the cell lines (Fig. 2A). Furthermore, both cells expressed detectable levels of constitutive p-Smad2 and p-Smad3, which were attenuated by the treatment with TβRI-KI suggesting the presence of the autocrine TGF-β signaling activity in both cells. Pretreatment of both cell lines with TβRI-KI also reduced the level of p-Smad2/3 activation by exogenous TGF-β. Similar results were also observed in the untransformed human mammary epithelial MCF 10A cells (Fig. S2).
Figure 2. TGF-β signaling is functional in luminal-epithelial Py230 and mesenchymal-like Py8119 cells.
A. Western blot analyses showed a clear activation of receptor-regulated Smads (R-Smads) with TGF-β treatment, indicating that TGF-β signaling is functional in both the cell lines. TβRI-KI reduced both the constitutive expression and TGF-β induced expression of p-Smad2/3 in both the cell lines. Cells were plated in 60-mm culture dishes, to approximately 70% confluence, and were treated with TβRI-KI (100 nM) overnight, then treated with TGF-β1 (2ng/ml) for 60 minutes, following which cell lysates were obtained. Western blot analyses were performed for p-Smad2/3 expression and total Smad levels were detected for controls. GAPDH was detected for loading control. Densitometry analyses were performed (Image J software) and densities were expressed relative to control (untreated) for each cell line. Representative images from two independent experiments have been presented. B. Luciferase reporter assays were performed using both the cell lines. Approximately, 1 × 105 cells for each cell line were plated in 24-well plate, and co-transfected with plasmids expressing luciferase reporter with Smad binding element promoter, and plasmids expressing beta galactosidase as controls for transfection efficiency. After 6 hours the cells were treated with TβRI-KI, TGT-β1, either alone or in combination for another 24 hours. The cell lysates were obtained, luciferase assays were performed, and relative luciferase activity with respect to beta galactosidase was determined. Fold changes in relative activity were plotted with respect to untreated controls. Double asterisks indicate statistical significance, P<0.001, one-way ANOVA, Tukey’s post-hoc test, N=3. C. Transwell migration assays were performed in 24 well plates using transwell inserts. Cells were plated into inserts and chemical gradient was set up, using media with 5% serum in the lower chambers. Results showed increase in migration potential with TGF-β treatment. This response was more robust for the Py230 cells as compared to the Py8119 cells. TβRI-KI treatment reduced the basal level of migration, although not statistically significant. Similarly, TGF-β mediated increase in migration potential was also inhibited by kinase inhibitor treatment, which was statistically non-significant. The number of cells that migrated per high magnification field per well was normalized as percent of the respective controls. Five high magnification fields were chosen per replicate. The data are presented as mean + SEM of three replicates per group. Asterisk indicate significant difference at P< 0.05 with one-way ANOVA. D. MTT viability assays (96 hours) showed a clear dose-dependent growth inhibition in response to increasing concentrations of TGT-β1 in the Py230 cells, whereas the Py8119 cells remained non-responsive to this growth suppression. Human mammary epithelial cell line MCF10A was used as control to determine the effect of TGF-β mediated inhibition of proliferation. As expected, TGF-β inhibited the proliferation of MCF10A in a dose dependent manner. Briefly, about 3 × 103 cells per cell line were plated in 96 well plate, in triplicates and treated with increasing concentrations of TGF-β. The cells were then treated with MTT reagent after 96 hours, and readings were recorded at 595 nm. Percent proliferation with repect to control (untreated) was calculated to plot the growth curves.
Luciferase assays using the reporter plasmid (containing the 8 base pair Smad binding element (SBE-4) [36] demonstrated significantly higher levels of luciferase activity in both Py230 and Py8119 cell lines upon treatment with TGF-β (Figure 2B) as compared to the untreated controls. Treatment with small molecule TβRI-KI (TGF-β receptor I- kinase inhibitor) reduced the basal levels of luciferase activity in Py230 cells (although not statistically significantly) demonstrating likely, the autocrine function of the signaling pathway in these cells. Similar trend was noticed in Py8119 cells. Treatment with TβRI-KI in the presence of TGF-β1 reduced the SBE luciferase activity, which was not statistically significant for Py230 cells. For Py8119 cells however, it showed significant reduction at P<0.05.
Epithelial to mesenchymal transition is one of the hallmarks of cancer cells during the acquisition of metastatic behavior and is characterized by the increase in cell motility [40]. TGF-β is known to switch on the mesenchymal-like phenotype, particularly in tumor cells that have evaded its growth inhibition effect [41]. In order to determine the motility-inducing effects of TGF-β on these cells, we performed Boyden chamber transwell migration assays and found significant enhancement in the number of migrating cells per field with TGF-β treatment, particularly in Py230 cells (Figure 2C). The mesenchymal-like Py8119 cells have a constitutively higher migration potential as compared to Py230 cells when untreated, and seeded at the same cell density. However, at such high cell densities the effect of TGF-β was not observed (data not shown). At lower seeding density as presented in Fig. 2C, TGF-β treatment induced migration in Py8119 cells, although less robustly as compared to the Py230 cells. This was statistically significant in both cell lines. Interestingly, treatment with TβRI-KI only moderately inhibit the TGF-β mediated increase in migration potential for both cell lines. These experiments were performed with transient treatments that lasted for 18 hours. Duration of treatment was chosen to ensure that there was little cell division of migrating cells within this time period, which would contribute to false positive quantification of migration. On the other hand, the short duration of treatment might be too short for TβRI-KI to show a significant blockade of TGF-β-induced migration. Unlike the clear inhibition of p-Smad2/p-Smad3 expression in both cell lines, only slight reduction in migration was observed in cells treated with TβRI-KI alone, again indicating limited effect of TβRI-KI on the migration with the short duration of treatment.
Because in normal epithelial cells TGF-β can inhibit cell proliferation by blocking cell-cycle progression [42], we next determined the effect of TGF-β on the growth of Py230 and Py8119 cells by performing MTT viability tests on the cells with increasing doses of the cytokine. After 96 hours of treatment, there was a clear dose-dependent growth inhibition evident in the Py230 cells; however, the Py8119 cells showed very little response (Figure 2D), suggesting that Py8119 cells have circumvented the TGF-β mediated growth inhibition. Additionally, MCF10A cells were plated as normal epithelial cell controls to determine the effect of TGF-β on proliferation. As expected TGF- β induced a clear dose dependent growth inhibition in these cells.
3.3. Blockade of endogenous TGF-β signaling aggravates tumor formation at orthotopic sites in immune competent mice
TGF-β inhibitors are known to be effective in containing mesenchymal mammary tumor allografts in a syngeneic background. It has been demonstrated that systemic administration of Fc:TβRII can significantly reduce the tumor sizes at the orthotopic site as well as limit local invasion to lymph nodes when using 4T1 and EMT6 cell lines injected into BALB/C mice [14]. Because Py8119 cells are mesenchymal like and are not growth inhibited by TGF-β in vitro, we wanted to examine the effects of attenuating TGF-β signaling on tumorigenesis in vivo. We began with transducing the enhanced GFP-luciferase tagged Py8119 cells with a lentiviral vector expressing an shRNA against TβRII or empty vector (PLKO.1).
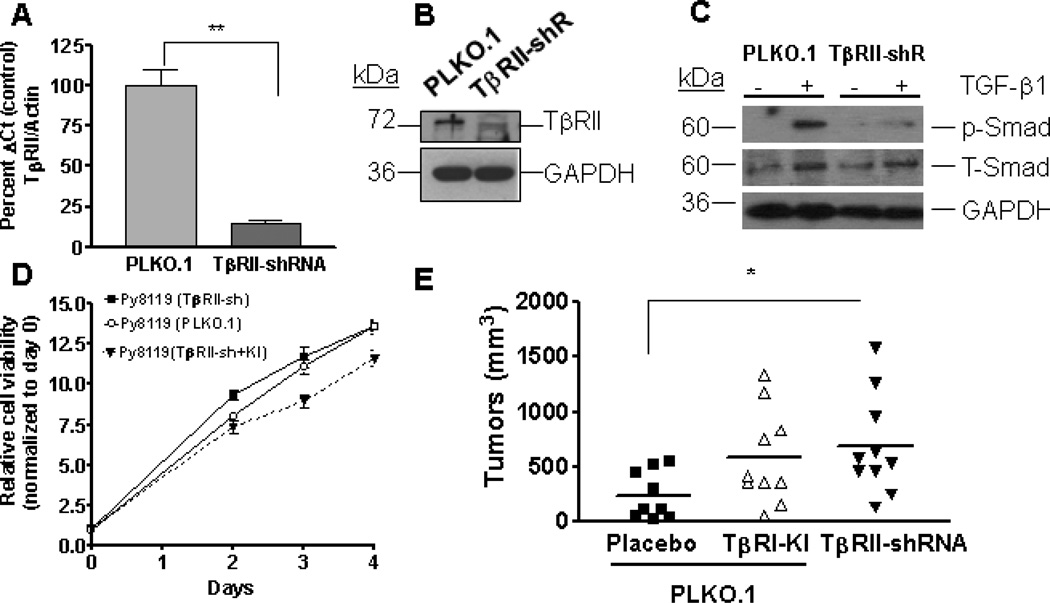
The endogenous TβRII knockdown in these cells was confirmed through real-time PCR analyses for TβRII gene expression, and showed reduced mRNA levels with the knockdown (Figure 3A). Western blot analysis for TβRII protein levels (Figure 3B) and the phosphorylated Smad2 (p-Smad2) (Figure 3C) in the cell line with knockdown showed reduced levels of receptor II (RII) expression and reduced phosphorylated Smad2 on TGF-β stimulation, respectively. However, disruption of endogenous TGF-β receptor did not have significant effect on the growth of these cells, as seen in the later time points (72h and 96h) in MTT viability assays (Figure 3D). Addition of TβRI-KI to Py8119 TβRII-sh cells, moderately inhibited the cell proliferation as compared to untreated controls, although not statistically significant.
Figure 3. Abrogating TGF-β signaling enhances orthotopic mammary tumor growth in Py8119 cell model in C57Bl/6 mice.
A. Real time-PCR analysis showed significant reduction in TGF-β receptor II mRNA levels after knock down as compared to control (PLKO.1) transduced cells (Student’s T-test, P<0.05, N=3). RNA was extracted using tri reagent and reverse transcription was performed using MMLV-RT reverse transcriptase and random primers. Real-time PCR was performed using primers specific for TβRII and Actin (for endogenous controls). Ct values from TβRII were normalized with respect to Actin. Results expressed as mean+SEM of three replicates. B. Western blot analyses showed reduced TβRII protein levels after knock down of TGF-β receptor II gene expression and was performed twice. C. Py8119 cells with TβRII knockdown showed reduced levels of phosphorylated Smad2 with TGF-β treatment. Briefly, Py8119 cells infected with either PLKO.1 or TβRII sh-RNA lentivirus were treated with TGF-β (2ng/ml) for 60 minutes and lysates were obtained for western blot analyses for p-Smad2. Untreated cell lysates for each cell line were used as controls. Densitometry quantitations were performed using ImageJ and expressed as ratio of p-Smad2/ T-Smad2 for treated samples from both cell lines. D. MTT viability assays showed moderate increase in growth of Py8119 cells after knocking down the TβRII gene expression at 48 hours, but the rates of proliferation were comparable for both cell lines at the later time points. TβRI-KI (100 nM) treatment in Py8119 cells infected with TβRII-shRNA inhibited proliferation, although not statistically significantly. Approximately, 3×103 cells/well for each cell line were plated in 96-well plates and allowed to proliferate. Cells were treated with MTT reagent at day 0–4, and absorbance was recorded at 595 nm. Data presented as relative cell viability with respect to viability at day 0 for each cell line. E. Py8119 cells tagged with luciferase-GFP were infected with either control (PLKO.1) or TβRII (TGF-β receptor II) sh-RNA lentivirus and were injected into mammary fat pads on each side of C57Bl/6 mice (contralateral injections) and treated with placebo or TβRI-KI. Shown here is the mean of tumor volumes as measured before termination of experiment. Data suggest that abrogating TGF-β signaling both systemically and at the endogenous level increased tumor formation at the orthotopic site. (The asterisk indicates significant difference at P<0.05 with one-way ANOVA and Tukey’s post-hoc test)
The control and TβRII shRNA infected Py8119 cells were injected into the mammary fat pad of female C57Bl/6 mice and treated with placebo or TβRI-KI. The small molecule inhibitor used in our studies, HTS 466284/LY364947 is a pyrazole-based TβRI-KI that inhibits the kinase of the type-I receptor. Previous studies have shown that systemic administration of this inhibitor can effectively inhibit tumor metastases [43].
Knockdown of TβRII significantly increased tumor growth, in comparison to the control group (Figure 3E). This indicates that the endogenous inhibition of TGF-β signaling in these tumors enhanced their outgrowth. Mice with Py8119 allografts that received systemic treatment with TβRI-KI showed an increase in the mammary tumor volumes as compared to the placebo group, even though the increase did not reach statistical significance at P<0.05. This presents an interesting and novel contrast with the previously studied models mentioned in the Introduction.
3.4. Abrogation of TGF-β signaling does not inhibit tumor outgrowth in immune-compromised mice
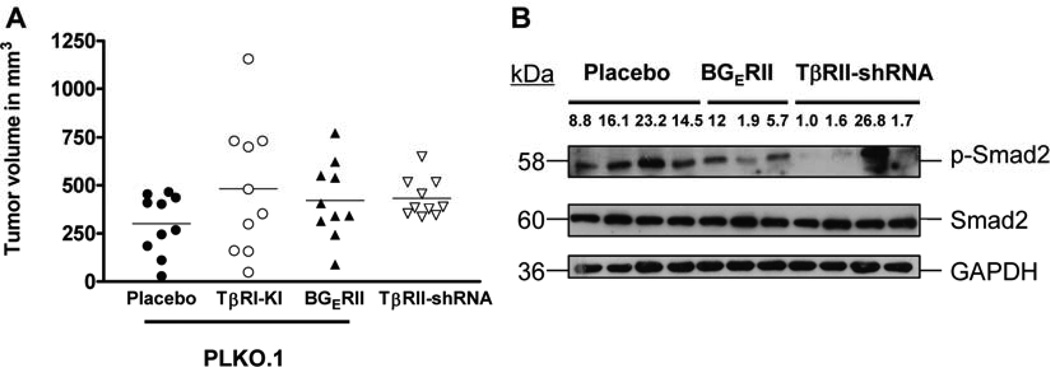
Efficacy of TGF-β inhibitors has been largely demonstrated in pre-clinical models of triple negative breast cancer. Typically, these models include human breast cancer cell xenografts in immune compromised mice. TGF-β is a potent immune suppressor and as such the abrogation of TGF-β signaling may lead to inflammatory responses at the primary tumor site. In order to determine whether the increase in tumorigenesis in the C57Bl/6 model was specifically due to the attenuation of TGF-β signaling in the epithelial tissue, and not due to host immune response via T lymphocytes, we transplanted Py8119 cells in immune compromised Nu/Nu mice. Results from the systemic administration of the TβRI-KI or the ligand trap BGERII as well as the knockdown of TβRII did not inhibit the tumor outgrowth at the orthotopic sites. As in the immune-competent mice, we found a trend towards increased tumor growth (although not statistically significant) with the attenuation of TGF-β signaling (Figure 4A) in the nude mice. Based on the above results, we are tempted to speculate that the abrogation of TGF-β signaling in tumor cells led to immune cell-mediated responses (via modulation of tumor microenvironment) in the Bl/6 background and enhanced the tumor outgrowth. However, future experiments are required for the further validation of this speculation. Western blot analyses from tumor lysates showed reduced levels of p-Smad2 in the tumors with endogenous knockdown of TβRII and tumors receiving systemic BGERII as compared to the controls except one tumor in the TβRII-shRNA group (Figure 4B). This suggests that the TGF-β signaling remains abrogated in the majority tumors during the course of the in vivo experiment.
Figure 4. Abrogation of TGF-β signaling either by using systemic inhibitors or by knocking down the endogenous TβRII gene induces a trend of increase in tumor size in immune-compromised mice.
A. Py8119 cells were similarly infected with either PLKO.1 or sh-RNA lentivirus against TβRII, and injected contra-laterally into mammary fat pads of Nu/Nu mice. 0.1×106 cells were injected into each fat pad, along with matrigel (1:1) and tumor volumes were calculated as V = (L×W2)×0.5. Results show mean of tumor volumes from each group. The abrogation of TGFβ signaling in immune-compromised mice induced tumors with larger volumes as seen earlier in C57Bl/6 mice, although not statistically significant. B. Lysates obtained from tumor samples showed reduced levels of p-Smad2 when TGF-β receptor II was knocked down. This suggests that the tumors retained the effect of TβRII knockdown and had down regulated Smad signaling during their course of growth in vivo. The numbers represent the density of each band relative to the band showing the lowest density.
3.5. Abrogation of TGF-β signaling reduces apoptosis and increases proliferation in Py8119 tumors
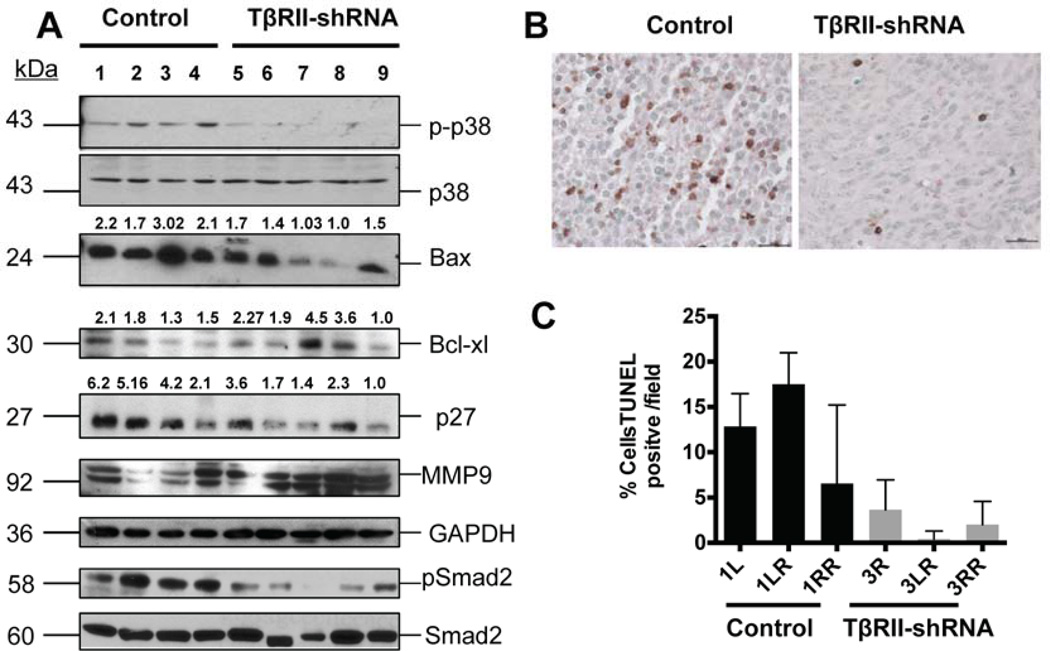
Western blot analyses performed on whole tissue lysates from tumor samples (from C57Bl/6 mice) demonstrated reduced levels of p-p38 MAPK in tumors formed by TβRII knockdown cells (Figure 5A). p38 MAPK is a stress activated kinase that has been shown to be involved in mediating pro-apoptotic responses in epithelial and endothelial cells, either directly or in cross-talk with other pathways [44; 45; 46] , and has been shown to mediate TGF-β induced apoptosis, operating in a Smad-independent manner [46]. A similar trend in the reduction of p-p38 MAPK was observed in tumors generated in nude mice treated with the fusion ligand trap BGERII (data not shown). Concomitantly, there was an overall decrease in the level of Bax expression, a key pro-apoptosis protein, and a moderate increase in the expression of anti-apoptotic protein Bcl-xl (Figure 5A). These observations indicate a potential decrease in cell death in the tumors formed by TβRII knockdown cells. Indeed, apoptosis TUNEL assay results from representative tumor sections also indicated a trend of reduced apoptosis in the tumors with abrogated TGF-β signaling (Figure 5 C , D).
Figure 5. Py8119 tumors with abrogated TGF-β receptor II (TβRII) gene expression show reduced phosphorylation of stress-activated kinase, reduced apoptosis and an increase in MMP-9 expression.
A. Western blot analyses showing reduced levels of activated p38-MAPK as detected in tumors with TβRII knockdown. These tumors expressed lower levels of pro-apoptotic protein Bax and increased levels of anti-apoptotic protein Bcl-xl. The numbers represent the density of each band relative to the band showing the lowest density. Abrogation of TGF-β signaling in these tumors was associated with increase in extracellular remodeling protein MMP-9 (matrix metalloprotease-9. They also showed reduced levels of p27Kip1 cell-cycle inhibitor protein. Efficiency of TβRII knockdown was determined by probing for p-Smad2 levels, total Smad levels were determined for endogenous controls. Representative blots have been presented from repeated analyses from tumors in both immune compromised and immune competent mice.
B. Representative images (40x) from TUNEL assay on paraffin embedded tumor sections showing nuclei stained positive for apoptosis. C. Quantitative estimation for percent nuclei stained TUNEL positive in each field. Bars represent average of five fields from individual tumors (error bars represent S.E.M). PLKO.1 (empty vector) transduced tumors showed higher percent apoptosis when compared to the tumors with abrogated TβRII gene expression.
Furthermore, in comparison with the control tumors, a reduction in levels of cell cycle inhibitor p27Kip1 was also observed in tumors formed by TβRII knockdown Py8119 cells (Figure 5A), suggesting a potential increase in proliferation in these tumors (Figure 5A). Another interesting observation was that, in comparison to the control tumors, the tumors with TβRII knockdown showed an increased expression of matrix metalloproteinase 9 (MMP-9), which might have contributed to their faster growth (Figure 5B) as MMP-9 has been shown to contribute to tumor growth [47; 48].
Taken together, these results suggest that TGF-β signaling in the context of tumor micro-environment appears to inhibit Py8119 cell growth and MMP-9 expression in vivo resulting in the inhibition of allograft tumors.
3.6. TGF-β inhibitors do not inhibit metastatic colonization of Py8119 cells
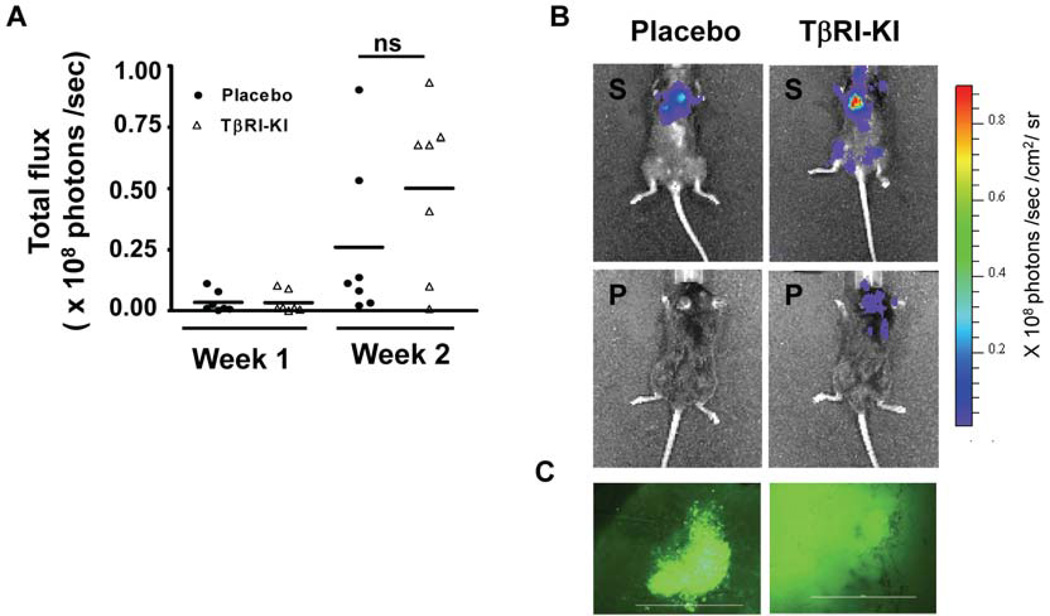
TGF-β inhibitors have been found to be highly effective in reducing metastatic colonization to distant organs in several studies performed with experimental models (via intracardiac or tail vein injections of tumor cells directly into the circulation) and also spontaneously occurring metastasis from orthotopic sites [12; 14; 39; 49]. Because the blockade of TGF-β signaling stimulated orthotopic growth of injected Py8119 cells, we next examined how TGF-β inhibitors affect the metastatic colonization of Py8119 cells. We found that in an experimental model of intracardiac injection of luciferase-GFP tagged Py8119 cells, systemic inhibition of TGF-β signaling with the TβRI-KI did not inhibit metastases to various organs as detected with whole body bioluminescence imaging (Figure 6A, B). However, there was a trend towards an increase in metastatic colonization to distant organs such as brain and tibia (Figure S3). The overall metastases were determined based on the total flux readings obtained from the animals at the end of Week 1 and Week 2 following the initial injections. The Py8119 cell line forms very aggressive tumors, and the tumor extravasation and colonization occurred very rapidly. Fluorescent imaging of sections from metastatic brains revealed severe metastases in the kinase inhibitor treated group, as compared to placebo treated group (Figure 6C). H&E staining of tissue sections from tibia and brain confirmed the presence of metastatic lesions to these organs (Figure S3). However, in a similar experimental model with Py230 cells, the systemic treatment with TβRI-KI did not aggravate the extent of metastases (Figure S4). Thus, data from both the metastatic colonization model and the orthotopic tumor models strongly suggest that disruption of TGF-β signaling does not inhibit metastases and tumor outgrowth in the MMTV-PyMT mesenchymal-like mammary tumor cells, unlike many previous reports with similar approaches. On the contrary, endogenous disruption of TGF-β signaling enhanced the orthotopic tumor outgrowth in this syngeneic mouse model. There was also an overall trend of an increase in both orthotopic tumor volumes and the extent of metastatic colonization of Py8119 cells with the systemic administration of TGF-β signaling inhibitors.
Figure 6. Systemic inhibition of TGF-β signaling supports a trend of increased metastatic colonization in an experimental model of intra-cardiac inoculation using Py8119 cells in C57Bl/6 mice.
A. Py8119 cells tagged with luciferase-GFP were injected through intracardiac route and animals were treated either with placebo or TβRI-KI every alternate day. Mean value of total flux calculated for individual animals showed a trend of increase in metastasis with TβRI-KI administration (N=10, per group). B. Representative IVIS images, taken after two weeks from the day of inoculation. Left panel shows image of an animal treated with placebo (top left: supine position bottom left: prone position) with signals from the thorax region, indicating lung metastasis. Right panel shows TβRI-KI treated animal (top right: supine position) with moderate to severe metastases in lungs, jaws, and tibia regions. TβRI-KI treated animals also show much higher extent of brain metastases (bottom right: prone position). Measure of total radiance expressed as photons/ sec/ cm2/ sr. C. Fluorescent images (10 X) from whole brain imaging immediately after termination, left panel shows region of metastatic colony in placebo treated group. Right panel shows a region of brain from TβRI-KI treated group with extensive metastasis.
4. Discussion
TGF-β inhibitors have long been tested in pre-clinical models and some of them are currently undergoing clinical trials for multiple therapeutic purposes, including oncological therapy [50; 51]. Although the tumor-suppressive arm of TGF-β commanded more attention during the early years of research in the field, its bimodal effects in tumor development are now widely accepted [52; 53; 54]. Currently, the switch from a tumor suppressor to a tumor promoter is not well defined within the context of all breast tumors. Many of the pre-clinical studies with systemic TGF-β inhibitors involved the use of mesenchymal-like human tumor cell models for which TGF-β is believed to be a growth promoter. The use of human xenografts precludes the effects of abrogating TGF-β signaling on the immune components. Other studies have emphasized TGF-β’s effects exclusively on the epithelial compartment during tumor progression. One such study focused on the effect of conditional knockout of TGF-β receptor II (TβRII) in the mammary glands of PyMT transgenic mice. The conditional loss of TβRII in the mammary epithelium shortened the latency period prior to tumor onset and increased the invasive nature of the tumors, with increased pulmonary metastases [24]. This study was unique compared to the earlier studies in pointing out that abrogation of TGF-β signaling specifically in the mammary epithelium aggravates distant metastases. However, this model among others [23; 25] did not address whether TGF-β signaling can suppress the growth and metastasis during later stages of carcinogenesis.
In this study, we utilized a pair of murine mammary cell lines with distinct epithelial or mesenchymal features derived from an MMTV-PyMT transgene- induced mammary tumors in C57Bl/6 mice for the investigation of the effect of TGF-β signaling on their malignant properties via syngeneic injection. We found that while both cell lines have a functional TGF-β/Smad signaling pathway, only the epithelial-like cells were responsive to TGF-β in growth inhibition assays in vitro. Although we do not know the cause of resistance to TGF-β-induced growth inhibition in Py8119 cell, we did find higher levels of activated p-ERK1/2, which are further increased in the presence of exogenous TGF-β1, in Py8119 cells than those in Py230 cell (Fig. S5) suggesting that the high level of activated ERK pathway may contribute to the resistance to TGF-β-mediated growth inhibition in Py8119 cell. On the other hand, abrogation of TGF-β signaling, either with TβRII knockdown or systemic administration of a TGF-β inhibitor, did not inhibit the growth and metastatic colonization potential of the mesenchymal-like cells, instead it enhanced tumor outgrowth in the syngeneic mice and led to an overall trend in increase of tumor volumes in immune-compromised mice as well as distant organ colonization in immune-competent mice. Systemic treatment with the TGF-β inhibitor showed no effect on the metastatic colonization potential of the luminal epithelial-like cells in immune competent mice. Our novel finding suggests that TGF-β signaling does not always play a tumor-promoting role in mammary tumors, even in those with mesenchymal-like features.
Several different approaches have been adopted to target different components of the TGF-β signaling pathway. The small molecule inhibitor used in our studies, HTS 466284/LY364947 has previously been reported to inhibit breast tumor metastases via systemic administration [43]. We have also used a soluble ligand trap molecule called the BGERII, as mentioned earlier [35]. Together with the systemic administration of these antagonists and the endogenous knockdown of TGF-β receptor-II, we demonstrate in immune-competent orthotopic transplants, in experimental metastatic colonization model and in immune-compromised orthotopic tumor cell injections that TGF-β attenuation does not inhibit the disease phenotype within the context of the mesenchymal-like cell line, Py-8119.
TGF-β’s role has been implicated in MMTV-PyMT transgenic mouse models with regard to the development of resistance to radiation therapy and rchemoresistance [55]. Interestingly, TGF-β receptor II was found to be essential to mediate the radiation resistance in these cells. However, in the absence of radiation, PyMT tumor cells with loss of TβRII formed larger metastatic nodules as compared to the control cells in the tail vein inoculation studies. This observation is consistent with our study and a previous study using TβRII knockout in the MMTV-PyMT model [24].
Interestingly, the abrogation of TGF-β signaling by TβRI-KI or by TβRII knockdown in Py8119 cells does not affect cell proliferation. However, within the context of tumors, there seems to be a pro-apoptotic role of TGF-β signaling in Py8119 tumors.
Within the same context, MMP-9 was inhibited by TGF-β signaling in vivo. MMP-9 belongs to a family of gelatinase matrix metalloproteinases, known to induce angiogenesis in solid tumors [47; 48]. TGF-β signaling regulates MMP synthesis in a highly context dependent manner. Although there is evidence of TGF-β stimulating MMP-9, abrogation of TGF-β1 with pan neutralizing monoclonal antibody in pancreatic cancer stimulates MMP-9 secretion [56]. In endothelial cells TGF-β is known to inhibit TNF-α mediated MMP-9 secretion [57]. These are among the numerous examples of TGF-β’s function as an inhibitor of MMPs [58; 59]. In our study, abrogation of TGF-β signaling also resulted in increased MMP-9 expression, which likely supported tumor cell proliferation in vivo. Interestingly, we did not observe any significant difference in either the p-p38 MAPK levels or the MMP-9 levels in the Py8119 cell line upon TβRII knockdown (data not shown) in vitro. This could be attributed to the difference in 2D culture conditions as opposed to the 3D tumor microenvironment, in vivo. Three-dimensional mechanical stimuli and stress factors are now increasingly believed to modulate the cell signaling pathways involved in cancer pathogenesis [60; 61]. Besides, tumors in vivo represent a heterogeneous population of various cell types. This cautions the extrapolation of in vitro observations to in vivo outcome. A possible involvement of several other paracrine pro-survival factors in the tumor microenvironment cannot be ruled out at this same time. However, the precise mechanism of increased tumorigenesis due to MMP-9 requires further investigation.
Loss of TβRII in epithelial cells is often accompanied by accumulation of additional genetic aberrations, which frequently leads to the development of frank and invasive tumors [19]. Another recent report from genetically engineered mouse models of advanced pancreatic ductal adenocarcinoma, revealed that TGF-β and αvβ6 integrin signaling function in a common tumor suppressor pathway in these models. Pharmacologic intervention of this axis aggravated pancreatic cancer in the above mentioned mouse models, unlike the previous reports of beneficial effects of TGF-β abrogation in such advanced stage carcinomas [62]. This study, similar to ours, suggested that it was difficult to select a safe therapeutic window for administration of TGF-β inhibitors in preclinical models of mesenchymal, aggressive solid tumors. Although naturally occurring mammary tumors seldom lose TβRII or other key components of the signaling, extraneous inhibition of this signaling pathway has always remained a cause for concern in therapy for the same reasons. Our study points out that the administration of TGF-β inhibitors in mesenchymal-like breast tumors may not have universal effects. As such, additional studies are needed for a better understanding of the molecular markers, the state of cell differentiation as well as the pro-oncogenic role of TGF-β signaling in these systems.
5. Conclusions
Taken together, our data suggest that the attenuation of TGF-β signaling in mesenchymal-like metastatic mammary tumors does not necessarily contribute to tumor abrogation or inhibition of metastases. Therefore, administration of anti-TGF-β therapeutic intervention requires greater precision in identifying molecular markers in tumors with an indication of pro-oncogenic activity of TGF-β pathway.
Supplementary Material
Acknowledgements
This work was supported in part by funding from NIH Grants R01CA75253 and R01CA79683 to LZS, NCI K22118182 to LGE, and the Cancer Therapy and Research Center at University of Texas Health Science Center at San Antonio through the NCI Cancer Center Support Grant 2P30CA054174-17. We thank Dr. Brian Rabinovich at MD Anderson Cancer Center for the pLV411G effLuc-flag- IRES-hrGFP vector. Images were generated in the Core Optical Imaging Facility, supported by NIH-NCI P30 CA54174 (CTRC at UTHSCSA) and NIH-NIA P01AG19316. Histology studies were conducted at the CTRC core Histology facility at UTHSCSA, and cell cycle analysis was performed at the CTRC core facility for flowcytometry supported by NIH-NCI P30 CA54174.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement
None
References
- 1.Serra R, Crowley MR. TGF-beta in mammary gland development and breast cancer. Breast Dis. 2003;18:61–73. doi: 10.3233/bd-2003-18107. [DOI] [PubMed] [Google Scholar]
- 2.Pollard JW. Tumour-stromal interactions. Transforming growth factor-beta isoforms and hepatocyte growth factor/scatter factor in mammary gland ductal morphogenesis. Breast cancer research : BCR. 2001;3:230–237. doi: 10.1186/bcr301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hannon GJ, Beach D. p15INK4B is a potential effector of TGF-beta-induced cell cycle arrest. Nature. 1994;371:257–261. doi: 10.1038/371257a0. [DOI] [PubMed] [Google Scholar]
- 4.Datto MB, Yu Y, Wang XF. Functional analysis of the transforming growth factor beta responsive elements in the WAF1/Cip1/p21 promoter. The Journal of biological chemistry. 1995;270:28623–28628. doi: 10.1074/jbc.270.48.28623. [DOI] [PubMed] [Google Scholar]
- 5.Hagimoto N, Kuwano K, Inoshima I, Yoshimi M, Nakamura N, Fujita M, Maeyama T, Hara N. TGF-beta 1 as an enhancer of Fas-mediated apoptosis of lung epithelial cells. Journal of immunology. 2002;168:6470–6478. doi: 10.4049/jimmunol.168.12.6470. [DOI] [PubMed] [Google Scholar]
- 6.Senturk S, Mumcuoglu M, Gursoy-Yuzugullu O, Cingoz B, Akcali KC, Ozturk M. Transforming growth factor-beta induces senescence in hepatocellular carcinoma cells and inhibits tumor growth. Hepatology. 2010;52:966–974. doi: 10.1002/hep.23769. [DOI] [PubMed] [Google Scholar]
- 7.Shi Y, Massague J. Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell. 2003;113:685–700. doi: 10.1016/s0092-8674(03)00432-x. [DOI] [PubMed] [Google Scholar]
- 8.Massague J. TGF-beta signal transduction. Annu Rev Biochem. 1998;67:753–791. doi: 10.1146/annurev.biochem.67.1.753. [DOI] [PubMed] [Google Scholar]
- 9.Massague J. TGF-beta signaling in development and disease. FEBS Lett. 2012;586:1833. doi: 10.1016/j.febslet.2012.05.030. [DOI] [PubMed] [Google Scholar]
- 10.Schmierer B, Hill CS. TGFbeta-SMAD signal transduction: molecular specificity and functional flexibility. Nature reviews. Molecular cell biology. 2007;8:970–982. doi: 10.1038/nrm2297. [DOI] [PubMed] [Google Scholar]
- 11.Fleming NI, Jorissen RN, Mouradov D, Christie M, Sakthianandeswaren A, Palmieri M, Day F, Li S, Tsui C, Lipton L, Desai J, Jones IT, McLaughlin S, Ward RL, Hawkins NJ, Ruszkiewicz AR, Moore J, Zhu HJ, Mariadason JM, Burgess AW, Busam D, Zhao Q, Strausberg RL, Gibbs P, Sieber OM. SMAD2, SMAD3 and SMAD4 mutations in colorectal cancer. Cancer research. 2013;73:725–735. doi: 10.1158/0008-5472.CAN-12-2706. [DOI] [PubMed] [Google Scholar]
- 12.Parsons R, Myeroff LL, Liu B, Willson JK, Markowitz SD, Kinzler KW, Vogelstein B. Microsatellite instability and mutations of the transforming growth factor beta type II receptor gene in colorectal cancer. Cancer research. 1995;55:5548–5550. [PubMed] [Google Scholar]
- 13.Reiss M, Barcellos-Hoff MH. Transforming growth factor-beta in breast cancer: a working hypothesis. Breast cancer research and treatment. 1997;45:81–95. doi: 10.1023/a:1005865812918. [DOI] [PubMed] [Google Scholar]
- 14.Muraoka RS, Dumont N, Ritter CA, Dugger TC, Brantley DM, Chen J, Easterly E, Roebuck LR, Ryan S, Gotwals PJ, Koteliansky V, Arteaga CL. Blockade of TGF-beta inhibits mammary tumor cell viability migration, and metastases. J Clin Invest. 2002;109:1551–1559. doi: 10.1172/JCI15234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arteaga CL, Hurd SD, Winnier AR, Johnson MD, Fendly BM, Forbes JT. Anti-transforming growth factor (TGF)-beta antibodies inhibit breast cancer cell tumorigenicity and increase mouse spleen natural killer cell activity. Implications for a possible role of tumor cell/host TGF-beta interactions in human breast cancer progression. J Clin Invest. 1993;92:2569–2576. doi: 10.1172/JCI116871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Akhurst RJ. TGF beta signaling in health and disease. Nature genetics. 2004;36:790–792. doi: 10.1038/ng0804-790. [DOI] [PubMed] [Google Scholar]
- 17.Derynck R, Akhurst RJ, Balmain A. TGF-beta signaling in tumor suppression and cancer progression. Nat Genet. 2001;29:117–129. doi: 10.1038/ng1001-117. [DOI] [PubMed] [Google Scholar]
- 18.Massague J, Blain SW, Lo RS. TGFbeta signaling in growth, control cancer and heritable disorders. Cell. 2000;103:295–309. doi: 10.1016/s0092-8674(00)00121-5. [DOI] [PubMed] [Google Scholar]
- 19.Markowitz S, Wang J, Myeroff L, Parsons R, Sun L, Lutterbaugh J, Fan RS, Zborowska E, Kinzler KW, Vogelstein B, et al. Inactivation of the type II TGF-beta receptor in colon cancer cells with microsatellite instability. Science. 1995;268:1336–1338. doi: 10.1126/science.7761852. [DOI] [PubMed] [Google Scholar]
- 20.Labelle M, Begum S, Hynes RO. Direct signaling between platelets and cancer cells induces an epithelial-mesenchymal-like transition and promotes metastasis. Cancer cell. 2011;20:576–590. doi: 10.1016/j.ccr.2011.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McEarchern JA, Kobie JJ, Mack V, Wu RS, Meade-Tollin L, Arteaga CL, Dumont N, Besselsen D, Seftor E, Hendrix MJ, Katsanis E, Akporiaye ET. Invasion and metastasis of a mammary tumor involves TGF-beta signaling. International journal of cancer. Journal international du cancer. 2001;91:76–82. doi: 10.1002/1097-0215(20010101)91:1<76::aid-ijc1012>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 22.Fu H, Hu Z, Wen J, Wang K, Liu Y. TGF-beta promotes invasion and metastasis of gastric cancer cells by increasing fascin1 expression via ERK and JNK signal pathways. Acta biochimica et biophysica Sinica. 2009;41:648–656. doi: 10.1093/abbs/gmp053. [DOI] [PubMed] [Google Scholar]
- 23.Bottinger EP, Jakubczak JL, Haines DC, Bagnall K, Wakefield LM. Transgenic mice overexpressing a dominant-negative mutant type II transforming growth factor beta receptor show enhanced tumorigenesis in the mammary gland and lung in response to the carcinogen 7,12-dimethylbenz-[a]-anthracene. Cancer research. 1997;57:5564–5570. [PubMed] [Google Scholar]
- 24.Forrester E, Chytil A, Bierie B, Aakre M, Gorska AE, Sharif-Afshar AR, Muller WJ, Moses HL. Effect of conditional knockout of the type II TGF-beta receptor gene in mammary epithelia on mammary gland development and polyomavirus middle T antigen induced tumor formation and metastasis. Cancer research. 2005;65:2296–2302. doi: 10.1158/0008-5472.CAN-04-3272. [DOI] [PubMed] [Google Scholar]
- 25.Pierce DF, Jr., Gorska AE, Chytil A, Meise KS, Page DL, Coffey RJ, Jr., Moses HL. Mammary tumor suppression by transforming growth factor beta 1 transgene expression. Proceedings of the National Academy of Sciences of the United States of America. 1995;92:4254–4258. doi: 10.1073/pnas.92.10.4254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gobbi H, Dupont WD, Simpson JF, Plummer WD, Jr., Schuyler PA, Olson SJ, Arteaga CL, Page DL. Transforming growth factor-beta and breast cancer risk in women with mammary epithelial hyperplasia. J Natl Cancer Inst. 1999;91:2096–2101. doi: 10.1093/jnci/91.24.2096. [DOI] [PubMed] [Google Scholar]
- 27.Yin JJ, Selander K, Chirgwin JM, Dallas M, Grubbs BG, Wieser R, Massague J, Mundy GR, Guise TA. TGF-beta signaling blockade inhibits PTHrP secretion by breast cancer cells and bone metastases development. The Journal of clinical investigation. 1999;103:197–206. doi: 10.1172/JCI3523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mehal WZ, Sheikh SZ, Gorelik L, Flavell RA. TGF-beta signaling regulates CD8+ T cell responses to high- and low-affinity TCR interactions. Int Immunol. 2005;17:531–538. doi: 10.1093/intimm/dxh233. [DOI] [PubMed] [Google Scholar]
- 29.Yang L, Huang J, Ren X, Gorska AE, Chytil A, Aakre M, Carbone DP, Matrisian LM, Richmond A, Lin PC, Moses HL. Abrogation of TGF beta signaling in mammary carcinomas recruits Gr-1+CD11b+ myeloid cells that promote metastasis. Cancer Cell. 2008;13:23–35. doi: 10.1016/j.ccr.2007.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gibby K, You WK, Kadoya K, Helgadottir H, Young LJ, Ellies LG, Chang Y, Cardiff RD, Stallcup WB. Early vascular deficits are correlated with delayed mammary tumorigenesis in the MMTV-PyMT transgenic mouse following genetic ablation of the NG2 proteoglycan. Breast cancer research : BCR. 2012;14:R67. doi: 10.1186/bcr3174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guy CT, Cardiff RD, Muller WJ. Induction of mammary tumors by expression of polyomavirus middle T oncogene: a transgenic mouse model for metastatic disease. Molecular and cellular biology. 1992;12:954–961. doi: 10.1128/mcb.12.3.954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Davie SA, Maglione JE, Manner CK, Young D, Cardiff RD, MacLeod CL, Ellies LG. Effects of FVB/NJ and C57Bl/6J strain backgrounds on mammary tumor phenotype in inducible nitric oxide synthase deficient mice. Transgenic research. 2007;16:193–201. doi: 10.1007/s11248-006-9056-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Singh J, Chuaqui CE, Boriack-Sjodin PA, Lee WC, Pontz T, Corbley MJ, Cheung HK, Arduini RM, Mead JN, Newman MN, Papadatos JL, Bowes S, Josiah S, Ling LE. Successful shape-based virtual screening: the discovery of a potent inhibitor of the type I TGFbeta receptor kinase (TbetaRI) Bioorg Med Chem Lett. 2003;13:4355–4359. doi: 10.1016/j.bmcl.2003.09.028. [DOI] [PubMed] [Google Scholar]
- 34.Sawyer JS, Anderson BD, Beight DW, Campbell RM, Jones ML, Herron DK, Lampe JW, McCowan JR, McMillen WT, Mort N, Parsons S, Smith EC, Vieth M, Weir LC, Yan L, Zhang F, Yingling JM. Synthesis and activity of new aryl- and heteroaryl-substituted pyrazole inhibitors of the transforming growth factor-beta type I receptor kinase domain. J Med Chem. 2003;46:3953–3956. doi: 10.1021/jm0205705. [DOI] [PubMed] [Google Scholar]
- 35.Verona EV, Tang Y, Millstead TK, Hinck AP, Agyin JK, Sun LZ. Expression, purification and characterization of BG(E)RII: a novel pan-TGFbeta inhibitor. Protein engineering, design & selection : PEDS. 2008;21:463–473. doi: 10.1093/protein/gzn023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zawel L, Dai JL, Buckhaults P, Zhou S, Kinzler KW, Vogelstein B, Kern SE. Human Smad3 and Smad4 are sequence-specific transcription activators. Molecular cell. 1998;1:611–617. doi: 10.1016/s1097-2765(00)80061-1. [DOI] [PubMed] [Google Scholar]
- 37.Schmidt JV, Carver LA, Bradfield CA. Molecular characterization of the murine Ahr gene. Organization, promoter analysis, and chromosomal assignment. J Biol Chem. 1993;268:22203–22209. [PubMed] [Google Scholar]
- 38.Jenkins DE, Oei Y, Hornig YS, Yu SF, Dusich J, Purchio T, Contag PR. Bioluminescent imaging (BLI) to improve and refine traditional murine models of tumor growth and metastasis. Clinical & experimental metastasis. 2003;20:733–744. doi: 10.1023/b:clin.0000006815.49932.98. [DOI] [PubMed] [Google Scholar]
- 39.Bandyopadhyay A, Wang L, Lopez-Casillas F, Mendoza V, Yeh IT, Sun L. Systemic administration of a soluble betaglycan suppresses tumor growth, angiogenesis, and matrix metalloproteinase-9 expression in a human xenograft model of prostate cancer. The Prostate. 2005;63:81–90. doi: 10.1002/pros.20166. [DOI] [PubMed] [Google Scholar]
- 40.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 41.Xu J, Lamouille S, Derynck R. TGF-beta-induced epithelial to mesenchymal transition. Cell research. 2009;19:156–172. doi: 10.1038/cr.2009.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Datto MB, Li Y, Panus JF, Howe DJ, Xiong Y, Wang XF. Transforming growth factor beta induces the cyclin-dependent kinase inhibitor p21 through a p53-independent mechanism. Proceedings of the National Academy of Sciences of the United States of America. 1995;92:5545–5549. doi: 10.1073/pnas.92.12.5545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bandyopadhyay A, Wang L, Agyin J, Tang Y, Lin S, Yeh IT, De K, Sun LZ. Doxorubicin in combination with a small TGFbeta inhibitor: a potential novel therapy for metastatic breast cancer in mouse models. PloS one. 2010;5:e10365. doi: 10.1371/journal.pone.0010365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nakagami H, Morishita R, Yamamoto K, Yoshimura SI, Taniyama Y, Aoki M, Matsubara H, Kim S, Kaneda Y, Ogihara T. Phosphorylation of p38 mitogen-activated protein kinase downstream of bax-caspase-3 pathway leads to cell death induced by high D-glucose in human endothelial cells. Diabetes. 2001;50:1472–1481. doi: 10.2337/diabetes.50.6.1472. [DOI] [PubMed] [Google Scholar]
- 45.Sheng G, Guo J, Warner BW. Epidermal growth factor receptor signaling modulates apoptosis via p38alpha MAPK-dependent activation of Bax in intestinal epithelial cells. American journal of physiology. Gastrointestinal and liver physiology. 2007;293:G599–606. doi: 10.1152/ajpgi.00182.2007. [DOI] [PubMed] [Google Scholar]
- 46.Yu L, Hebert MC, Zhang YE. TGF-beta receptor-activated p38 MAP kinase mediates Smad-independent TGF-beta responses. Embo J. 2002;21:3749–3759. doi: 10.1093/emboj/cdf366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.London CA, Sekhon HS, Arora V, Stein DA, Iversen PL, Devi GR, A novel antisense inhibitor of MMP-9 attenuates angiogenesis. human prostate cancer cell invasion and tumorigenicity. Cancer Gene Ther. 2003;10:823–832. doi: 10.1038/sj.cgt.7700642. [DOI] [PubMed] [Google Scholar]
- 48.Mira E, Lacalle RA, Buesa JM, de Buitrago GG, Jimenez-Baranda S, Gomez-Mouton C, Martinez AC, Manes S. Secreted MMP9 promotes angiogenesis more efficiently than constitutive active MMP9 bound to the tumor cell surface. J Cell Sci. 2004;117:1847–1857. doi: 10.1242/jcs.01035. [DOI] [PubMed] [Google Scholar]
- 49.Fang Y, Chen Y, Yu L, Zheng C, Qi Y, Li Z, Yang Z, Zhang Y, Shi T, Luo J, Liu M. Inhibition of breast cancer metastases by a novel inhibitor of TGFbeta receptor 1. Journal of the National Cancer Institute. 2013;105:47–58. doi: 10.1093/jnci/djs485. [DOI] [PubMed] [Google Scholar]
- 50.Akhurst RJ, Hata A. Targeting the TGFbeta signalling pathway in disease. Nature reviews. Drug discovery. 2012;11:790–811. doi: 10.1038/nrd3810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Akhurst RJ. Large- and small-molecule inhibitors of transforming growth factor-beta signaling. Current opinion in investigational drugs. 2006;7:513–521. [PubMed] [Google Scholar]
- 52.Sun L. Tumor-suppressive and promoting function of transforming growth factor beta. Frontiers in bioscience : a journal and virtual library. 2004;9:1925–1935. doi: 10.2741/1382. [DOI] [PubMed] [Google Scholar]
- 53.Dumont N, Arteaga CL. Transforming growth factor-beta and breast cancer: Tumor promoting effects of transforming growth factor-beta. Breast cancer research : BCR. 2000;2:125–132. doi: 10.1186/bcr44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wakefield LM, Yang YA, Dukhanina O. Transforming growth factor-beta and breast cancer: Lessons learned from genetically altered mouse models. Breast cancer research : BCR. 2000;2:100–106. doi: 10.1186/bcr41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Biswas S, Guix M, Rinehart C, Dugger TC, Chytil A, Moses HL, Freeman ML, Arteaga CL. Inhibition of TGF-beta with neutralizing antibodies prevents radiation-induced acceleration of metastatic cancer progression. The Journal of clinical investigation. 2007;117:1305–1313. doi: 10.1172/JCI30740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shek FW, Benyon RC, Walker FM, McCrudden PR, Pender SL, Williams EJ, Johnson PA, Johnson CD, Bateman AC, Fine DR, Iredale JP. Expression of transforming growth factor-beta 1 by pancreatic stellate cells and its implications for matrix secretion and turnover in chronic pancreatitis. The American journal of pathology. 2002;160:1787–1798. doi: 10.1016/s0002-9440(10)61125-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vaday GG, Schor H, Rahat MA, Lahat N, Lider O. Transforming growth factor-beta suppresses tumor necrosis factor alpha-induced matrix metalloproteinase-9 expression in monocytes. Journal of leukocyte biology. 2001;69:613–621. [PubMed] [Google Scholar]
- 58.Ogawa K, Chen F, Kuang C, Chen Y. Suppression of matrix metalloproteinase-9 transcription by transforming growth factor-beta is mediated by a nuclear factor-kappaB site. The Biochemical journal. 2004;381:413–422. doi: 10.1042/BJ20040058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hall MC, Young DA, Waters JG, Rowan AD, Chantry A, Edwards DR, Clark IM. The comparative role of activator protein 1 and Smad factors in the regulation of Timp-1 and MMP-1 gene expression by transforming growth factor-beta 1. The Journal of biological chemistry. 2003;278:10304–10313. doi: 10.1074/jbc.M212334200. [DOI] [PubMed] [Google Scholar]
- 60.Huang S, Ingber DE. Cell tension, matrix mechanics, and cancer development. Cancer cell. 2005;8:175–176. doi: 10.1016/j.ccr.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 61.Suresh S. Biomechanics and biophysics of cancer cells. Acta biomaterialia. 2007;3:413–438. doi: 10.1016/j.actbio.2007.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hezel AF, Deshpande V, Zimmerman SM, Contino G, Alagesan B, O’Dell MR, Rivera LB, Harper J, Lonning S, Brekken RA, Bardeesy N. TGF-beta and alphavbeta6 integrin act in a common pathway to suppress pancreatic cancer progression. Cancer Res. 2012;72:4840–4845. doi: 10.1158/0008-5472.CAN-12-0634. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.