Abstract
Background
Eph receptor tyrosine kinases EphB2 and EphB3, and ephrin-B1 ligand play a critical role in regulating small intestinal epithelial cell migration. Although well studied in developing brain, the expression pattern of Ephs/ephrins has not been delineated in the developing small intestine.
Aims
To examine the gene expression of all known members of Ephs/ephrins during development of mouse small intestine.
Methods
We examined the expression of 21 A- and B-Ephs/ephrins in mouse small intestine or the Caco2 cell-line using RT-PCR, qRT-PCR, and immunohistochemical analyses. EphB2 expressing cells from isolated crypts were detected by immunofluorescence and FACS analyses.
Results
With the exception of EphA5, all family members were expressed throughout the intestine at all ages examined. Most were uniformly expressed. In contrast, levels of EphA4, -A8, -B4, and ephrin-B2 mRNA were highest during early fetal development and declined with age. At E15, EphB2 and EphB4 proteins were diffusely expressed in proliferating stratified intestinal epithelial cells. By E18, the proteins had become localized to cell membranes of columnar epithelial cells within intervillus regions, and later were expressed on epithelial cell membranes in adult crypts. EphB2 expressing cells can be specifically isolated from crypt cell fractions.
Conclusions
The current study represents the first analysis of Ephs/ephrins during intestinal development. The elevated expression of EphA4, -A8, -B4, and ephrin-B2 during the fetal period of intestinal morphogenesis suggests an important role in development. Continued intestinal expression of other family members implicates a role in differentiation.
Keywords: Development, Small Intestine, Eph, Ephrin, Receptor, Ligands
Introduction
The Ephs constitute the largest known family of receptor tyrosine kinases, comprising at least 14 distinct receptors that are highly conserved from Xenopus to humans [1-3]. The Ephs interact with an 8 member family of cell surface bound ligands, the ephrins. Because of their diverse history of isolation and characterization, Ephs and ephrins are known by multiple names (Table S1), making analysis of the older literature challenging. Eph receptors are divided into two groups based on the relatedness of their extracellular domains. Receptors of the EphA group preferentially bind glycosylphosphatidylinositol (GPI)-linked ligands of the ephrin-A subclass. The EphB group preferentially interacts with transmembrane ligands, the ephrin-B subclass (Figure S1). Within a subclass, ephrins show significant structural and sequence homologies, and a single ephrin may bind with high affinity to several Eph receptors. It has been suggested that the evolutionary expansion of the Eph/ephrin families may have served to establish subtle functional differences and a combinatorial code of expression patterns that regulate complex tissue architecture [4].
Both Ephs and ephrins mediate signaling after receptor-ligand interaction [5-8]. This bi-directional signaling affects cellular interactions such as cell adhesion, cell migration, and tissue boundary formation [9-11]. Much of Eph/ephrin function is achieved by regulating cell movement. Eph/ephrin interactions are critical for processes such as embryogenesis [12], vasculogenesis [13], and cell motility [14]. Eph receptors exhibit a variety of distribution patterns during early stages of embryonic development [15-17]. For example, the localized expression of the mRNA for different Ephs and ephrins in the developing brain occurs in association with the formation of specific structures, suggesting a regulatory role in their formation [18]. Using knockout mice, specific roles for several EphB receptors have been found in the developing forebrain [19].
The small intestinal epithelium consists of a highly dynamic cell population that undergoes rapid turnover throughout life in conjunction with structural and functional differentiation. Examination of normal adult human small intestine revealed expression of mRNA for all Ephs and ephrins, with the exception of EphA8 [20]. In the mature intestine, four epithelial cell types arise from stem cells in the crypts, three cell types moving upward out of the crypts onto the villi as they differentiate and a fourth, the Paneth cell, moving to the base of the crypt. Although cell-cell interactions have long been thought to be critical in migration and differentiation of these epithelial cells, few mechanisms have been understood until recently. Analysis of EphB2 and EphB3 null mice by Batlle et al. [21] demonstrated that the normal movement of different cell types was disordered: Paneth cells migrating up the villi, while differentiated absorptive cells mingled with proliferating crypt cells. These authors suggested that there are complimentary gradients of EphB2, -B3 and ephrin-B1 and that as intestinal cells differentiate, interaction of EphB2 and -B3 with ephrin-B1 directs cell movements upward or downward [22]. It has been reported that signaling between EphB2 and ephrin-B2 regulates cell proliferation in the small intestine [23,24]. Expression of mouse EphA2 has been identified in E7.5 endoderm and persists through E10.5, suggesting a possible role in early patterning of the gut tube [25]. In contrast to the focus on EphB2, EphB3, ephrin-B1 and -B2, the expression and potential roles of other family members in the developing gastrointestinal tract have been little examined.
We screened the mRNA expression for all the members of the mouse Ephs and ephrins in the developing intestine by RT-PCR; and quantified and performed immunohistochemistry on subsets of Ephs/ephrins. Most members were expressed at all ages examined, except for EphA5. EphA4, -A8, -B4, and ephrin-B2 had their highest levels of expression during fetal development, with EphA8 barely detectable in adult intestine. Since Caco-2 cells (human colon carcinoma cells) provide a relevant model for studying epithelial cell differentiation and polarization in vitro and no comparable mouse intestinal cell line has been well characterized [26,27], we extended our data for EphB-family members to their expression during proliferation and differentiation in these cells. When cultured, these cells proliferate, form tight junctions and exhibit the phenotype of human small intestinal epithelial cells [26]. In addition, studies were undertaken to assess the relationship between EphB2 and -B4 and its cognate ligand ephrin-B1 in crypt and villus cells as well as to isolate EphB2 expressing cells from the intestinal epithelium. The present data suggest that the widespread expression of most of these receptors at all developmental stages examined indicates important roles in differentiation of the intestine (and could be candidates for further analysis for cell-based therapies).
Methods
Reagents
Quantitative RT-PCR (qRT-PCR) reagents were from BioRad (Hercules, CA). Primer pairs (Table S2) were purchased from Superarray Bioscience Corporation (Frederick, MD), or designed using Beacon designer software and synthesized by Invitrogen Life Technologies (Carlsbad, CA). The sequence of primer pairs obtained from Superarray Bioscience Corporation are proprietary. RT-PCR reagents were from Invitrogen. Anti-EphB2, -B4 and ephrin-B1 antibodies were obtained from R&D Systems (Minneapolis, MN). Alexa anti-goat antibody was obtained from Molecular Probes; and FITC labeled antibodies were obtained from R & D Systems. All other chemicals and reagents were purchased from Sigma (St. Louis, MO), Gibco-BRL (Grand Island, NY), or Fisher Scientific (Fair Lawn, NJ).
Sample Preparation and RT-PCR Analysis
Mouse Eph receptors (EphA1–EphA8; EphB1–B4, -B6), ephrin ligands (ephrin-A1–A5; ephrin-B1–B3) and GAPDH (as a control) mRNA expression was determined by RT-PCR analysis. Total RNA from mouse fetal (E13.5, 14.5, 15.5, 16.5, 17.0 and 17.5 days), newborn (NB), and adult intestine was isolated according to the manufacturer's instructions (RNeasy kit, Qiagen Inc; Valencia, CA). Newborn mice had a first nursing prior to sacrifice. Adult mice were usually 4 weeks of age. To make certain that recovery of Eph/ephrin was complete, whole intestine was used rather than cellular fractions. Tissues from each developmental stage were pooled to minimize inter-individual variance (n=2-10 animals). RNA samples from at least three different preparations were used for gene-expression analysis. Total RNA from Caco-2 cells was also isolated following manufacturer's (Qiagen) specifications. Samples were digested with DNAse (Ambion Inc., Austin, TX) to eliminate genomic DNA contamination. No-template controls were run with each reaction to verify the absence of genomic DNA.
For the RT-PCR analysis, first-strand cDNA synthesis was performed using 1.0–2.0 μg of total RNA from different stages and regions (proximal = duodenum; middle = jejunum; and distal = ileum) of mouse intestine using either the SuperScript III first-strand synthesis system (InVitrogen) or the iScript cDNA synthesis kit (BioRad) in a final reaction volume of 25 μl according to the manufacturer's instructions. After the completion of the reaction, the cDNA was further diluted 4-5 times with RNase-free water and 1-2 μl diluted cDNA was used for PCR amplification.
For A-family Ephs/ephrins, the amplification reaction was carried out for 30-35 cycles with an initial hot start at 95C for 15 min, followed by denaturation for 30 sec at 95C, annealing for 30 sec at 55C and extension for 30 sec at 72C. For the mRNA expression of EphA4, EphA5, and B-family Ephs/ephrins, the amplification reaction was carried out using similar conditions as for A-family members except there was no initial hot start. The amplification reaction for sucrase-isomaltase was carried out for 30 cycles with denaturation for 30 s at 94C, annealing for 1 min at 51C, and extension for 1 min at 72C, followed by a final extension at 72C for 7 min.
Aliquots of the amplified products were analyzed on 1.2 – 2.0% agarose gel (Tris-borate-EDTA containing ethidium bromide) electrophoresis. For each analysis, a negative control, prepared using all reagents except SuperScript II H− reverse transcriptase and an aliquot of the matching RNA, yielded no detectable products, indicating that all RNAs were free of DNA contamination.
As positive controls, we initially validated primer pairs using RT-PCR analysis on RNA isolated from mouse brain which is known to express all members of the Eph/Ephrin family except EphA8 and ephrin-A2 [20].
Real-time qRT-PCR Analysis
To quantify the mRNA expression for EphA4 and EphB4 receptors, and their cognate ligand ephrin-B2, and to confirm the differential and spatial expression of EphA8 receptor, real-time RT-PCR was performed using SYBR green detection platform and determined using a standard curve method and a calibrator sample [28]. Thermal cycling parameters were used as per instructions (Superarray Bioscience Corporation). Briefly, cDNA was synthesized using the reverse transcription kit from BioRad according to the manufacturer's protocol. Melting curve experiments established that the fluorescent signal for each amplicon was derived from product only and not from primer dimers. Quantification was obtained by comparing the threshold cycles of unknown samples against calibration curves with known copy numbers. The amount of gene-specific mRNA is displayed as the x-fold gene expression of each experimental sample compared with the calibration sample after normalization and as described [28]. All experiments were performed as duplicates or triplicates in 4 or 5 separate experiments. Statistical significance (p<0.05) was determined by ANOVA (GraphPad Software; San Diego, CA). In some cases, amplification of the expected single products was confirmed using 2% agarose gel and ethidium bromide staining. The amount of GAPDH mRNA in each sample was quantified and used as an internal control for comparison among different samples.
Cell Culture
Caco-2 cells were obtained from the American Type Culture Collection (Manassas, VA) and routinely maintained in Dulbecco's modified Eagle's medium containing 10% fetal bovine serum. To further substantiate the specificity of the results for the epithelial compartments, in this series of experiments Caco-2 cells were harvested at pre-confluence (2 d; proliferative stage) and post-confluence (21 d; differentiated stage) and used for gene expression studies.
Intestinal Cell Isolation
The isolation of villus to crypt gradients of intact isolated intestinal epithelial cells from adult mouse small intestine was performed as originally described [29,30]. Since fetal and newborn intestines do not have crypts which are developed at a later stage, intestinal crypt cells were extracted from adult animals only. Small intestine was obtained from C57BL/wt mice and luminal contents gently removed from the intestine. The intestine was then rinsed thoroughly with 0.15M NaCl and 1 mM dithiothretitol as described in the Weiser “rinse” [30]. Subsequently, the intestine was incubated with Weiser Solution A (1.5 mM KCl, 96mM NaCl, 27 mM sodium citrate, 8mM KH2PO4, 5.6 mM Na2HPO4; pH, 7.3) for 15 minutes at 37C. After rinsing the intestine with phosphate buffered saline, it was refilled with Solution B (phosphate buffered saline containing 1.5 mM EDTA and 0.5 mM dithiothreitol) and incubated for 30 minutes at 37C; no proteases or other enzymes were used. This method isolates only epithelial cells and excludes serosal and interstitial cells. After dissociation, the cells were washed repeatedly with phosphate-buffered saline solution. By a series of incubations at 37C and washing of gut loops, sequential fractions of isolated epithelial cells were obtained with a gradient of cells from the villus tip to the lower villus and then the crypts. Harvested crypt cells (at 60 minutes from the start of experiment) were used for gene expression, immunofluorescence or FACS analyses.
Immunohistochemistry
Paraffin-embedded sections (5 μm) were deparaffinized with xylene, and rehydrated with a graded series of ethanols. Antigen retrieval was carried out by heating the sections in a microwave (95C for 20 min) in 0.01M citrate buffer, pH 6.0. Sections were incubated with 2.0% hydrogen peroxide for 20 min to block endogenous peroxidase, then incubated with blocking serum (ABC kit, Vector Laboratories, Burlingame, CA) in TBS (0.1M Tris-HCl, pH 7.5; 0.15M NaCl) for 30 min at 37C. Incubations with primary antibodies against EphB2, -B4 (1:375 dilutions), and ephrin-B1 (1:400 dilutions) were performed for 3h at 37C followed by incubation with secondary biotinylated antibody, then with preformed avidin-biotin horseradish peroxidase macromolecular complex (Vectastain ABC kit) for 30 min at 37C and according to the manufacturer's protocol. Signal detection was performed with 3, 3′-diaminobenzidine tetrahydrochloride (DAB) as the substrate according to the manufacturer's instructions.
Immunofluorescence Staining and FACS Analyses
For immunofluorescence analyses of EphB2 protein in mouse small intestinal tissues or isolated intestinal epithelial crypt cells, incubations with primary antibodies against EphB2 (1:375 dilution) were performed for 3h at 37C followed by incubation with secondary Alexa anti-goat antibody (1:100 dilution; Molecular Probes) in a humidified incubator for 30 min at 37C. Sections were washed thoroughly with TBS and mounted with Mowiol (Calbiochem). For FACS analysis, isolated intestinal epithelial cells were placed on ice and incubated with primary antibodies against EphB2 (1:375 dilution; R & D Systems) for 30 min. After three washes with staining medium (enzyme free cell dissociation buffer containing 3% fetal bovine serum; Gibco-BRL), the cells were incubated with secondary FITC antibody (1:100) for 30 min in a total volume of 1 ml, and the tubes were placed on their sides for proper mixing during incubation. The cells were washed three times with phosphate buffered saline and the FACS-Vantage SE (Beckton Dickinson) was used to perform FACS analysis.
Microscopy
Immunohistochemical images were captured under brightfield illumination using an Olympus BX41 (Olympus America Inc., Center Valley, PA) microscope equipped with an Olympus DP70 camera. Immunofluorescence images of tissue sections or isolated intestinal epithelial cells were captured with an Olympus AX70 microscope.
Results
Expression of Ephs/ephrins in Mouse tissues
We initially validated all of the primer pairs on RNA from mouse brain as positive controls using RT-PCR analysis [18,31]. As expected, the A- and B-family Ephs/ephrin were expressed in the E14.5 or adult brain (Fig. S2A and S2C). Interestingly, EphA8 was not detectable in the adult brain but was expressed at E14.5 (Fig. S2B).
Ephs/ephrins Expression in Staged Developmental Mouse Series
Adult human small intestine exhibits the presence of a broad spectrum of A- and B-class Ephs/ephrins [20,32]. To investigate the expression of these mRNAs in developing mouse small intestine, we performed RT-PCR analysis on E13.5-E17.5, NB, and adult isolates from proximal, mid- and distal regions. Examples of Eph and ephrin expression are shown in Fig. 1. Our data show that with the exception of EphA5 and EphA8, mRNAs for the A- and B-family Ephs and ephrins are expressed constitutively during intestinal development (Fig. 1A and 1C; Table 1) suggesting an important role for their translated proteins. Although EphA5 is expressed in E14.5 brain, it was undetectable in the developing intestine. Distinct differences in EphA8 expression were observed during development, with greater intensity found at E13.5-E17.5 and lower levels in NB and adult (Fig. 1B). Most strikingly at the early stages, EphA8 expression was higher in the mid- and distal regions than in the proximal region.
Fig. 1.
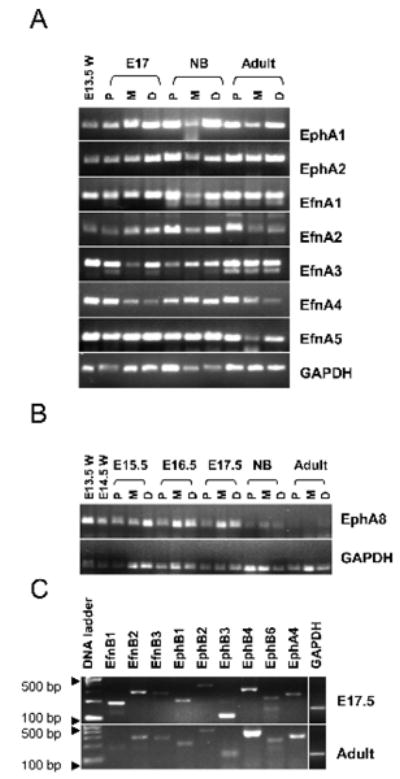
Table 1.
Expression of Eph receptor and ephrin ligand families in known tissues and a summary of our data.
| Mouse Eph Receptors and Ligands (Accession numbers) | Tissue Expression (preferential) | References | Our data (developing mouse small intestine) |
|---|---|---|---|
| Eph Receptors | |||
| EphA1 (NM_023580) | widely expressed* | [20,43] | constitutively expressed• |
| EphA2 (NM_010139) | skin, ovary; widely expressed | [20,43,44] | constitutively expressed |
| EphA3 (NM_010140) | (uterus, bladder, prostate) | [20] | constitutively expressed |
| EphA4 (NM_007936) | widely expressed | [20] | developmentally regulatedΔ |
| EphA5 (NM_007937) | assay design failed repeatedly | [20] | undetectable |
| EphA6 (NM_007938) | (brain, testis) | [20] | constitutively expressed |
| EphA7 (NM_010141) | widely expressed; (kidney) | [20] | very low expression |
| EphA8 (NM_007939) | (brain, testis), colon tumor | [20,45] | developmentally regulated |
| EphB1 (NM_173447) | (colon, brain, testis) | [21] | constitutively expressed |
| EphB2 (NM_010142) | (intestine) | [21] | constitutively expressed |
| EphB3 (NM_010143) | widely expressed | [20,21] | constitutively expressed |
| EphB4 (NM_010144) | widely expressed | [20,43] | developmentally regulated |
| EphB6 (NM_007680) | (Brain, thymus) | [20] | constitutively expressed |
| Ephrins | |||
| Ephrin-A1 (NM_010107) | widely expressed | [20] | constitutively expressed |
| Ephrin-A2 (NM_007909) | (colon), lung tumor, brain, intestine | [20,43] | constitutively expressed |
| Ephrin-A3 (NM_010108) | (brain) | [20] | constitutively expressed |
| Ephrin-A4 (NM_007910) | (colon) | [20] | constitutively expressed |
| Ephrin-A5 (NM_207654, transcript variant1; NM_010109, transcript variant 2) | widely expressed | [20] | constitutively expressed |
| Ephrin-B1 (NM_010110) | widely expressed | [20,21] | constitutively expressed |
| Ephrin-B2 (NM_010111) | widely expressed | [20] | developmentally regulated |
| Ephrin-B3 (NM_007911) | (brain), colon, lung, kidney | [20,37] | constitutively expressed |
Widely expressed tissues include normal adult brain, lung, liver, spleen, colon, small intestine, kidney, bladder, prostate, testis, uterus, thymus, and bone marrow [20]. Bone marrow showed the lowest Eph receptor and ephrin expression.
Expression in developmental stages and regions of mouse small intestine assessed by RT-PCR analysis.
Confirmed by quantitative real-time RT-PCR analysis (n=3 or more individual experiments).
Real-time qRT-PCR Analysis for Ephs/ephrins in Developing Mouse Intestine
Since no previously published data are available for EphA8 expression in normal small intestine, and based on our observation that EphA8 was expressed at a minimal level in NB as well as in adult small intestine by RT-PCR analysis (Fig. 1B), we performed qRT-PCR analysis. Our data show that EphA8 mRNAs exhibit similar patterns as seen for RT-PCR analysis (Fig. 2A). The relative abundance of EphA8 mRNA showed dramatic differences in the different regions of fetal intestine. It was expressed at higher levels distally than proximally; levels were, however, consistently higher in early stages compared to adult. Taken together, our data indicate that EphA8 mRNA expression is temporally and spatially regulated.
Fig. 2.
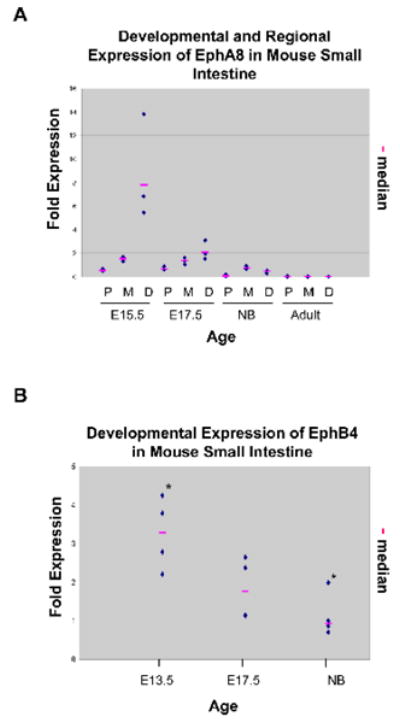
Because EphB4 transcripts have been shown to be enriched in mouse gastric epithelial progenitor cells [33]; and EphA4 is the only receptor which binds to members of ephrin-A ligands as well as ephrin-B2 and -B3 (Fig. S1), [34] quantitative analysis was focused on EphA4, -B4, and their cognate ligand, ephrin-B2. As shown in Fig. 2B, EphB4 expression is highest in early fetal development and significantly declines with age. EphA4 and ephrin-B2 showed a similar expression pattern (data not shown). We were able to ascertain developmental and/or spatial differences in expression levels by the shift in the threshold cycle for EphA8, -A4, -B4 and ephrin-B2; there was no difference for ephrin-B1 (data not shown) during development. Our data for EphA4, -B4, and ephrin-B2 expression indicate that their mRNA levels are highest in the developing intestine at E13.5 (when epithelial cells are rapidly proliferating and form a stratified epithelium) but decline after villus morphogenesis.
Expression of Ephs/ephrins in Caco-2 and Intestinal Crypt Cells
To obtain a better understanding of the role of Ephs/ephrins in intestinal cells we examined the expression of EphA4 and B-family Ephs/ephrins in Caco-2 cells and in isolated crypt cells using RT-PCR analysis (Table 2). Since crypt cells develop post-natally our crypt cells data are for the adult only. EphA4 and EphB2 receptors are expressed in pre- and post-confluent Caco-2 cells and in crypt cells. EphB3 was expressed only in pre-confluent Caco-2 cells as well as in crypt cells, suggesting that EphB3 expression is required only in proliferating cells. EphB1 and -B6 showed very low expression levels in Caco-2 and crypt cells consistent with previously published microarray data [35]. Ephrin-B1, -B2, and -B3 mRNAs were also undetectable or very low in pre-confluent (2 d) Caco-2 cells. However, after the cells reached confluence and differentiated (21 d), ephrin-B1 and -B3 were expressed. Isolated crypt cells showed expression of ephrin-B1 and -B2 but not -B3. We used sucrase-isomaltase mRNA as a positive control to validate the isolation of crypt and villus cell fractions from the enterocytes. As expected, sucrase-isomaltase mRNA was expressed only in the villus fractions but was undetectable in the isolated crypt cells (Fig. S3) consistent with previously published observations [29,36]. Overall, these data suggest differential roles for these receptors and ligands in regulating intestinal cell proliferation and differentiation.
Table 2.
Distribution of EphA4 and B-family Eph/ephrin transcripts in epithelial cells using RT-PCR analysis. This is a representative of n=3 individual experiments.
| Ephs/ephrins | Caco-2 BBe (culture) | Adult crypt cells | |
|---|---|---|---|
| 2 d | 21 d | ||
| EphA4 | + | + | + |
| EphB1 | VL* | - | VL |
| EphB2 | + | + | + |
| EphB3 | + | - | + |
| EphB4 | + | +/- | + |
| EphB6 | VL | VL | VL |
| Ephrin-B1 | - | + | + |
| Ephrin-B2 | VL | - | + |
| Ephrin-B3 | - | + | VL |
VL, very low expression.
Distribution Pattern of B-Family Eph/ephrin Proteins in Developing Small Intestine
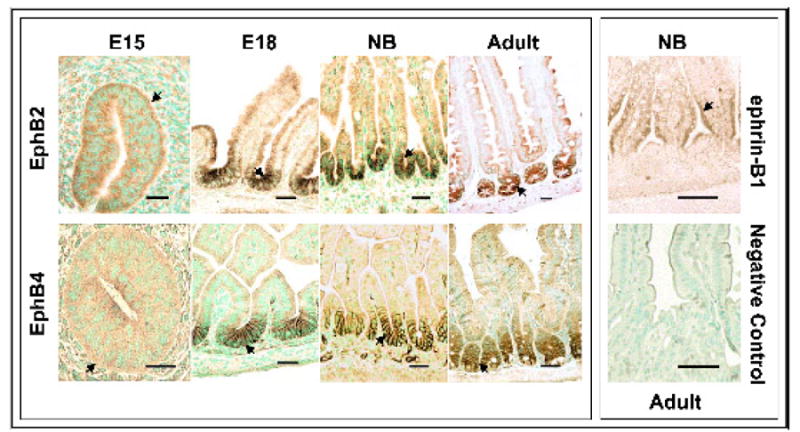
The spatial distribution of EphB2 and EphB4 proteins was investigated in mouse small intestine at days E15, E18, NB, and adult stages of development using immunohistochemical analysis (Fig. 3). Comparison of the expression pattern of EphB2 and EphB4 in embryonic intestine with that of the adult revealed marked differences. There was low expression, diffuse and cytoplasmic, of both EphB2 and EphB4 receptors in stratified epithelium at E15. In the early stages of villus formation, the proteins were expressed on the cell membranes of all epithelial cells in the single layer epithelium. At E18 and in newborns, expression was restricted to the membranes of cells in the intervillus regions. Thus, villus formation appears to occur prior to restriction of the receptors to the intervillus regions (Fig. 3). This pattern suggests that morphogenesis of villi from a multilayer stratified epithelium likely does not result from interactions between these Eph proteins. In the adult, the immunoreactivity for these receptors was prominent in cells toward the base of crypts, marking the proliferating cell zone. In contrast, ephrin-B1 expression was detected in cells on the lower halves of the villi in newborn animals, but not in the intervillus region. No signals were detected in the goblet cells or in the lamina propria. As expected, the negative control did not show any immunoreactivity to the antibodies. These data indicate that both EphB2 and EphB4 are expressed in the proliferative zone whereas ephrin-B1 is expressed in differentiating cells during intestinal development.
Fig. 3.
Expression of EphB2 in isolated intestinal crypt cells by immunofluorescence and FACS analysis
Since our data indicate that EphB2 is expressed throughout the developmental stages analyzed, and could be used to characterize stem cells, we reconfirmed its expression in the adult mouse intestine. Immunofluorescent staining with EphB2 primary and a secondary Alexa anti-goat antibody revealed EphB2 expression in the lower crypt in adult mouse small intestine (data not shown) suggesting that a similar approach could be used to stain and isolate EphB2 expressing cells from the intestinal epithelium.
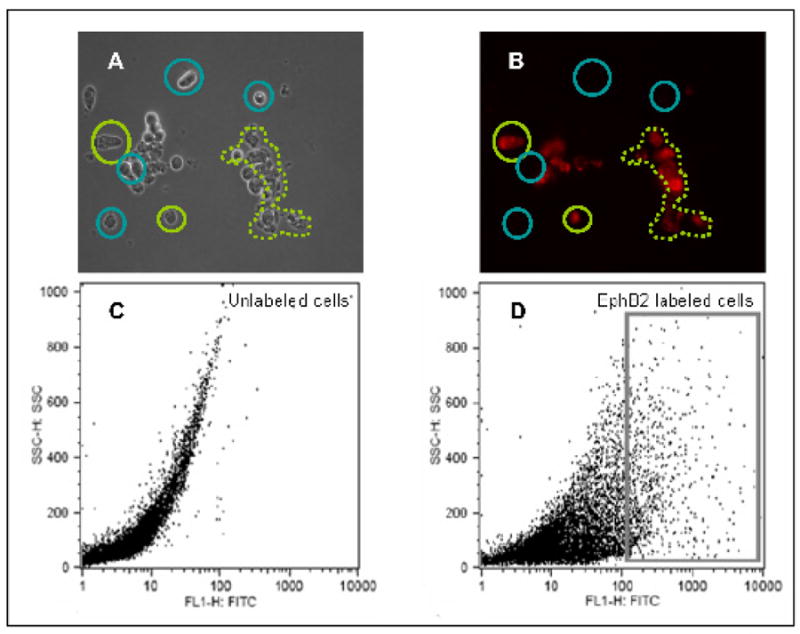
Once crypt cells were isolated using the Weiser technique [30], as shown in the dark field image, they were fluorescently labeled with EphB2 antibody in preparation for FACS analysis. A substantial number of cells expressed EphB2 on their surface. The cells in the dark field image (Fig. 4A) indicated by the green circle or dotted area is labeled positively for EphB2 (Fig. 4B) as detected by immunofluorescence analysis, whereas the cells indicated by the blue circle were not labeled presumably because they did not express this receptor. Furthermore, using FACS analysis, it is possible that a population of cells representing intestinal stem cells could be isolated from the crypts as observed by EphB2 surface labeled cells shown in the box (Fig. 4D) compared to the unlabeled control cells (Fig. 4C).
Fig. 4.
Discussion
The adult small intestinal epithelium consists of two compartments of cells, the proliferating cells in the crypts and non-proliferating, differentiated cells on the villi; and paneth cells at crypt bases. Cells in both compartments are constantly moving, as the epithelial, enteroendocrine and goblet cells progress from crypt base to villus tip. The interaction of EphB2 and EphB3 with ephrin-B1, normally expressed in reciprocal gradients along the crypt/villus axis, maintains such orderly progression [21]. As the expression of other members of the Eph/ephrin family had not been delineated, we examined all the known family members in adult intestine and during the fetal morphogenetic process when a stratified epithelium is transformed into a single layer lining nascent villi. We hypothesized that other critical Ephs or ephrins might be identified by their expression pattern. Our data indicate that while most family members are constitutively expressed, EphA4, EphA8, EphB4, and ephrin-B2 expression levels are maximal in fetal life, suggesting that they may have an important role in intestinal morphogenesis.
Similar to the observations of Hafner et al. [20] in the adult human intestine, most of the Ephs and ephrins examined were expressed ubiquitously in the mouse small intestine. In fact, Hafner et al. [20], in a screen of 13 organs, found that almost all of the Ephs and ephrins were expressed at some level in every organ, although levels varied widely. Relative to other organs, expression of EphB2 was highest in colon and small intestine, consistent with its critical role in the regulation of directional intestinal epithelial cell migration along the crypt-villus axis [21]. These investigators did not assay for EphA5, which in our study was undetectable in the intestine but was successfully amplified from fetal brain consistent with previously published data in which EphA5 is reported to be almost exclusively expressed in the nervous system; reviewed in [37].
Interestingly, our study detected EphA8 in normal fetal intestine but not in adult intestine. Hafner et al. [20] detected expression of EphA8 only in human colon tumor cells, in keeping with the concept that cancer cells recapitulate gene expression of early developmental stages. These data imply that examination of developing human intestine would reveal developmentally regulated EphA8 expression.
EphA2 has been identified as a marker of the posterior endoderm at E7.5 as the primitive gut tube is forming [25]. Our data demonstrated that EphA2 is expressed at all developmental and adult stages. Aasheim et al. [38] reported ephrin-A2 in fetal human intestine and strong expression in adult intestine; we found a similar pattern during mouse development (Fig. 1A). Mice lacking ephrin-B2 display severe anorectal malformations, including absence of the rectum [39]. In the present study ephrin-B2 was expressed at comparable levels in E17.5 and adult intestine. Taken together, expression patterns suggest an essential role for these Eph receptors and ephrins, not only in development, but also in maintenance of the organization of adult intestine.
Key roles for EphB2, -B3, and ephrin-B1 in localizing crypt and villus cells, especially Paneth cells, have been demonstrated in EphB2 and EphB3 null mice [21] and conditional ephrin-B1 knockout mice [22]. Analysis of isolated mouse crypt cells in the present study confirmed the expression of EphB2 and EphB3 in these epithelial cells and additionally demonstrated expression of EphA4 and EphB4, suggesting that these receptors may also be important in crypt cell regulation. Previous studies by Saaf et al. [40] in a microarray analysis of Caco-2 cell differentiation, found that EphB2 expression decreased with differentiation, while ephrin-B1 increased. Consistent with this, we found that ephrin-B1 and ephrin-B3 were undetectable in preconfluent cells, but expressed in post-confluent cells, while EphB3 and EphB4 levels decreased over the same period. EphA4 and EphB2 were expressed at both stages, suggesting a complex interaction involving multiple A and B receptor and ligand family members in the regulation of Caco2 cell differentiation. Although Batlle et al. [21] reported a lack of protein staining for ephrin-B2 in small intestine, the mRNA was successfully detected by RT-PCR (Fig. 1C); this maybe a reflection of the high sensitivity of this method, or post transcriptional regulation of the protein. In a microarray analysis of small intestinal progenitor cells isolated by laser capture, Stappenbeck et al. [41] identified EphB3, EphB4, and EphA5 as upregulated genes. Thus, one or a combination of these proteins may have a role in the small intestinal stem cell.
Since EphB2 and EphB3 apparently organize adult crypt/villus architecture by a gradient of expression interacting with a reciprocal gradient of ephrin-B1 controlled by Wnt signaling targets, β-catenin and TCF [21,22], we hypothesized that the initial formation of the crypt/villus structures might be due to an early complex expression of the receptors and ligands leading to sorting of the cells into two compartments. Indeed our data do demonstrate complex patterns of gene expression. While expression of both EphB2 and EphB3 mRNA was high prior to villus formation, the proteins were diffusely expressed in all of the stratified epithelial cells; then as the villi formed, EphB2 and -B3 proteins became restricted to the membranes of cells in the intervillus spaces consistent with the previously published observation for TCF-4 expression in the intervillus region [42]. The temporal progression indicates that intervillus cell-specific expression of these proteins may be a result, rather than a cause of morphogenesis. These data also suggest that cell-specific expression of EphB2 and EphB4 proteins is not part of such a developmental mechanism, but this does not rule out such a role for other family members. However, the importance of Wnt/Eph/ephrin interactions for maintenance of cell proliferation during development requires additional study.
Eph and ephrin expression analysis in the developing intestine demonstrates that almost all family members are expressed throughout the developmental period examined, suggesting roles in continuing tissue maintenance. EphA4, -A8, -B4, and ephrin-B2 displayed peaks during fetal intestinal morphogenesis, suggesting an important role in that process and indicating that further investigation of their roles would be worthwhile. The unique pattern of EphA8 is particularly of interest since it is strongly expressed in the developing intestine, but alone among all the family members, becomes undetectable in adulthood. It would also be of interest to determine if human EphA8 is expressed in developing human intestine, as it is expressed in colon tumors but not in normal adult intestine. Further investigation of EphB3 and EphB4 as possible stem cell markers is also warranted. These studies confirm previously published results and identify new members of the Eph/ephrin family that may also play an important role during development of the intestine. They provide a guide for detailed evaluation of these functions in the future.
Supplementary Material
Acknowledgments
We would like to thank Dr. Stephen D Krasinski (Division of Gastroenterology and Nutrition, Children's Hospital Boston, Boston, MA) for statistical analysis of EphB4 expression data; NanaYaa Baffour-Awuah for technical help in qRT-PCR experiments; Sacha de Stoppelaar for maintaining Caco-2 cell-lines; and Dr. Daniel J Liebl (University of Miami, FL) for providing primer sequence for EphB3/B4. We thank Ms. Suzanne White (Histology Core, Harvard Digestive Disease Center, Boston, MA) for preparation of paraffin-embedded tissue sections.
Grant Sponsor: NIH Research Grant (MERIT) and Harvard Digestive Disease Center.
Grant number: R37 DK32658 and P30 DK 34854.
References
- 1.Lhotak V, Greer P, Letwin K, Pawson T. Characterization of elk, a brain-specific receptor tyrosine kinase. Mol Cell Biol. 1991;11:2496–2502. doi: 10.1128/mcb.11.5.2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jones TL, Karavanova I, Maeno M, Ong RC, Kung HF, Daar IO. Expression of an amphibian homolog of the eph family of receptor tyrosine kinases is developmentally regulated. Oncogene. 1995;10:1111–1117. [PubMed] [Google Scholar]
- 3.Scales JB, Winning RS, Renaud CS, Shea LJ, Sargent TD. Novel members of the eph receptor tyrosine kinase subfamily expressed during xenopus development. Oncogene. 1995;11:1745–1752. [PubMed] [Google Scholar]
- 4.Murai KK, Pasquale EB. ‘Eph’ective signaling: Forward, reverse and crosstalk. J Cell Sci. 2003;116:2823–2832. doi: 10.1242/jcs.00625. [DOI] [PubMed] [Google Scholar]
- 5.Holland SJ, Gale NW, Mbamalu G, Yancopoulos GD, Henkemeyer M, Pawson T. Bidirectional signalling through the eph-family receptor nuk and its transmembrane ligands. Nature. 1996;383:722–725. doi: 10.1038/383722a0. [DOI] [PubMed] [Google Scholar]
- 6.Davy A, Robbins SM. Ephrin-A5 modulates cell adhesion and morphology in an integrin-dependent manner. EMBO J. 2000;19:5396–5405. doi: 10.1093/emboj/19.20.5396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davy A, Gale NW, Murray EW, et al. Compartmentalized signaling by GPI-anchored ephrin-A5 requires the fyn tyrosine kinase to regulate cellular adhesion. Genes Dev. 1999;13:3125–3135. doi: 10.1101/gad.13.23.3125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schmucker D, Zipursky SL. Signaling downstream of eph receptors and ephrin ligands. Cell. 2001;105:701–704. doi: 10.1016/s0092-8674(01)00391-9. [DOI] [PubMed] [Google Scholar]
- 9.Pandey A, Shao H, Marks RM, Polverini PJ, Dixit VM. Role of B61, the ligand for the eck receptor tyrosine kinase, in TNF-alpha-induced angiogenesis. Science. 1995;268:567–569. doi: 10.1126/science.7536959. [DOI] [PubMed] [Google Scholar]
- 10.Boyd AW, Lackmann M. Signals from eph and ephrin proteins: A developmental tool kit. Sci STKE. 2001;2001:RE20. doi: 10.1126/stke.2001.112.re20. [DOI] [PubMed] [Google Scholar]
- 11.Kullander K, Klein R. Mechanisms and functions of eph and ephrin signalling. Nat Rev Mol Cell Biol. 2002;3:475–486. doi: 10.1038/nrm856. [DOI] [PubMed] [Google Scholar]
- 12.Frisen J, Holmberg J, Barbacid M. Ephrins and their eph receptors. Multitalented directors of embryonic development. EMBO J. 1999;18:5159–5165. doi: 10.1093/emboj/18.19.5159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oike Y, Ito Y, Hamada K, et al. Regulation of vasculogenesis and angiogenesis by ephB/ephrin-B2 signaling between endothelial cells and surrounding mesenchymal cells. Blood. 2002;100:1326–1333. [PubMed] [Google Scholar]
- 14.Huynh-Do U, Vindis C, Liu H, et al. Ephrin-B1 transduces signals to activate integrin-mediated migration, attachment and angiogenesis. J Cell Sci. 2002;115:3073–3081. doi: 10.1242/jcs.115.15.3073. [DOI] [PubMed] [Google Scholar]
- 15.Becker N, Seitanidou T, Murphy P, et al. Several receptor tyrosine kinase genes of the eph family are segmentally expressed in the developing hindbrain. Mech Dev. 1994;47:3–17. doi: 10.1016/0925-4773(94)90091-4. [DOI] [PubMed] [Google Scholar]
- 16.Ganju P, Shigemoto K, Brennan J, Entwistle A, Reith AD. The eck receptor tyrosine kinase is implicated in pattern formation during gastrulation, hindbrain segmentation and limb development. Oncogene. 1994;9:1613–1624. [PubMed] [Google Scholar]
- 17.Patel K, Nittenberg R, D'Souza D, et al. Expression and regulation of Cek-8, a cell to cell signalling receptor in developing chick limb buds. Development. 1996;122:1147–1155. doi: 10.1242/dev.122.4.1147. [DOI] [PubMed] [Google Scholar]
- 18.Liebl DJ, Morris CJ, Henkemeyer M, Parada LF. mRNA expression of ephrins and eph receptor tyrosine kinases in the neonatal and adult mouse central nervous system. J Neurosci Res. 2003;71:7–22. doi: 10.1002/jnr.10457. [DOI] [PubMed] [Google Scholar]
- 19.Mendes SW, Henkemeyer M, Liebl DJ. Multiple eph receptors and B-class ephrins regulate midline crossing of corpus callosum fibers in the developing mouse forebrain. J Neurosci. 2006;26:882–892. doi: 10.1523/JNEUROSCI.3162-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hafner C, Schmitz G, Meyer S, et al. Differential gene expression of eph receptors and ephrins in benign human tissues and cancers. Clin Chem. 2004;50:490–499. doi: 10.1373/clinchem.2003.026849. [DOI] [PubMed] [Google Scholar]
- 21.Batlle E, Henderson JT, Beghtel H, et al. Beta-catenin and TCF mediate cell positioning in the intestinal epithelium by controlling the expression of ephB/ephrinB. Cell. 2002;111:251–263. doi: 10.1016/s0092-8674(02)01015-2. [DOI] [PubMed] [Google Scholar]
- 22.Cortina C, Palomo-Ponce S, Iglesias M, et al. EphB-ephrin-B interactions suppress colorectal cancer progression by compartmentalizing tumor cells. Nat Genet. 2007;39:1376–1383. doi: 10.1038/ng.2007.11. [DOI] [PubMed] [Google Scholar]
- 23.Holmberg J, Genander M, Halford MM, et al. EphB receptors coordinate migration and proliferation in the intestinal stem cell niche. Cell. 2006;125:1151–1163. doi: 10.1016/j.cell.2006.04.030. [DOI] [PubMed] [Google Scholar]
- 24.Takano-Maruyama M, Hase K, Fukamachi H, Kato Y, Koseki H, Ohno H. Foxl1-deficient mice exhibit aberrant epithelial cell positioning resulting from dysregulated ephB/ephrinB expression in the small intestine. Am J Physiol Gastrointest Liver Physiol. 2006;291:G163–170. doi: 10.1152/ajpgi.00019.2006. [DOI] [PubMed] [Google Scholar]
- 25.Moore-Scott BA, Opoka R, Lin SC, Kordich JJ, Wells JM. Identification of molecular markers that are expressed in discrete anterior-posterior domains of the endoderm from the gastrula stage to mid-gestation. Dev Dyn. 2007;236:1997–2003. doi: 10.1002/dvdy.21204. [DOI] [PubMed] [Google Scholar]
- 26.Hauri HP, Sterchi EE, Bienz D, Fransen JA, Marxer A. Expression and intracellular transport of microvillus membrane hydrolases in human intestinal epithelial cells. J Cell Biol. 1985;101:838–851. doi: 10.1083/jcb.101.3.838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pinto M, Robine-Léon S, Appay MD, et al. Enterocyte-like differentiation and polarization of the human colon carcinoma cell line Caco-2 in culture. Biol Cell. 1983;47:323–330. [Google Scholar]
- 28.Davies PS, Enns CA. Expression of the hereditary hemochromatosis protein hfe increases ferritin levels by inhibiting iron export in HT29 cells. J Biol Chem. 2004;279:25085–25092. doi: 10.1074/jbc.M400537200. [DOI] [PubMed] [Google Scholar]
- 29.Islam S, Montgomery RK, Fialkovich JJ, Grand RJ. Developmental and regional expression and localization of mRNAs encoding proteins involved in RNA translocation. J Histochem Cytochem. 2005;53:1501–1509. doi: 10.1369/jhc.5A6655.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weiser MM. Intestinal epithelial cell surface membrane glycoprotein synthesis. I. An indicator of cellular differentiation. J Biol Chem. 1973;248:2536–2541. [PubMed] [Google Scholar]
- 31.Pickles JO. Expression of ephs and ephrins in developing mouse inner ear. Hear Res. 2003;178:44–51. doi: 10.1016/s0378-5955(03)00029-7. [DOI] [PubMed] [Google Scholar]
- 32.Hafner C, Meyer S, Langmann T, et al. Ephrin-B2 is differentially expressed in the intestinal epithelium in crohn's disease and contributes to accelerated epithelial wound healing in vitro. World J Gastroenterol. 2005;11:4024–4031. doi: 10.3748/wjg.v11.i26.4024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mills JC, Andersson N, Hong CV, Stappenbeck TS, Gordon JI. Molecular characterization of mouse gastric epithelial progenitor cells. Proc Natl Acad Sci U S A. 2002;99:14819–14824. doi: 10.1073/pnas.192574799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wilkinson DG. Multiple roles of eph receptors and ephrins in neural development. Nat Rev Neurosci. 2001;2:155–164. doi: 10.1038/35058515. [DOI] [PubMed] [Google Scholar]
- 35.Mariadason JM, Nicholas C, L'Italien KE, et al. Gene expression profiling of intestinal epithelial cell maturation along the crypt-villus axis. Gastroenterology. 2005;128:1081–1088. doi: 10.1053/j.gastro.2005.01.054. [DOI] [PubMed] [Google Scholar]
- 36.Barth JA, Li W, Krasinski SD, Montgomery RK, Verhave M, Grand RJ. Asymmetrical localization of mRNAs in enterocytes of human jejunum. J Histochem Cytochem. 1998;46:335–343. doi: 10.1177/002215549804600307. [DOI] [PubMed] [Google Scholar]
- 37.Surawska H, Ma PC, Salgia R. The role of ephrins and eph receptors in cancer. Cytokine Growth Factor Rev. 2004;15:419–433. doi: 10.1016/j.cytogfr.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 38.Aasheim HC, Pedeutour F, Grosgeorge J, Logtenberg T. Cloning, chromosomal mapping, and tissue expression of the gene encoding the human eph-family kinase ligand ephrin-A2. Biochem Biophys Res Commun. 1998;252:378–382. doi: 10.1006/bbrc.1998.9618. [DOI] [PubMed] [Google Scholar]
- 39.Dravis C, Yokoyama N, Chumley MJ, et al. Bidirectional signaling mediated by ephrin-B2 and ephB2 controls urorectal development. Dev Biol. 2004;271:272–290. doi: 10.1016/j.ydbio.2004.03.027. [DOI] [PubMed] [Google Scholar]
- 40.Saaf AM, Halbleib JM, Chen X, et al. Parallels between global transcriptional programs of polarizing Caco-2 intestinal epithelial cells in vitro and gene expression programs in normal colon and colon cancer. Mol Biol Cell. 2007;18:4245–4260. doi: 10.1091/mbc.E07-04-0309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stappenbeck TS, Mills JC, Gordon JI. Molecular features of adult mouse small intestinal epithelial progenitors. Proc Natl Acad Sci U S A. 2003;100:1004–1009. doi: 10.1073/pnas.242735899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kim BM, Mao J, Taketo MM, Shivdasani RA. Phases of canonical wnt signaling during the development of mouse intestinal epithelium. Gastroenterology. 2007;133:529–538. doi: 10.1053/j.gastro.2007.04.072. [DOI] [PubMed] [Google Scholar]
- 43.Unified nomenclature for Eph family receptors and their ligands, the ephrins. Eph Nomenclature Committee. Cell. 1997;90:403–404. doi: 10.1016/s0092-8674(00)80500-0. [DOI] [PubMed] [Google Scholar]
- 44.Himanen JP, Chumley MJ, Lackmann ML, et al. Repelling class discrimination: ephrin-A5 binds to and activates EphB2 receptor signaling. Nat Neurosci. 2004;7:501–509. doi: 10.1038/nn1237. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


