Abstract
About 15% of lung cancer cases are of the small cell subtype, but this variant is highly aggressive and often diagnosed at advanced stages. Outcomes after current treatment regimens have been poor, with 5-year survival rates as low as 25% for patients with limited-stage disease. Advances in therapy for small cell lung cancer have included the development of more effective chemotherapeutic agents and radiation techniques. For example, hyperfractionated radiation therapy given early in the course of the disease can reduce local recurrence and extend survival. Other technologic advances in radiation planning and delivery such as intensity-modulated radiation therapy, image-guided adaptive radiation therapy, and four-dimensional computed tomography/positron emission tomography have facilitated the design of treatment volumes that closely conform to the shape of the tumor, which allows higher radiation doses to be given while minimizing radiation-induced toxicity to adjacent structures. Future improvements in outcomes will require clarifying the molecular basis for this disease.
Introduction
More than 1.6 million new cases of lung cancer were diagnosed worldwide in 2008, with an estimated 1,378,400 deaths from the disease.1 Small cell lung cancer (SCLC) accounts for 15%–20% of all lung cancers, and the overwhelming majority (>95%) are associated with tobacco exposure. The incidence of all types of lung cancer, including SCLC, has been declining in the United States with the onset of tobacco smoking cessation programs, although this trend took nearly 20 years to become evident among men.2 Overall survival (OS) rates for patients with lung cancer have also increased by about 5% since the advent of low-dose spiral computed tomography (CT) scanning to detect early lung cancer.3 The prognosis for patients with SCLC continues to be poor but has improved with the advent of smoking cessation campaigns, more effective chemotherapy agents and radiation planning and delivery techniques, and the use of prophylactic cranial irradiation (PCI) for those who experience a complete response to therapy.4
SCLC typically presents in patients aged ≥70 years with a history of heavy tobacco smoking. Disease often presents as bulky symptomatic masses, and mediastinal involvement is common. Extrathoracic spread (i.e., extensive-stage disease) is also quite common, being present in 75%–80% of cases at diagnosis.5 Brain metastases are present in approximately 20% of patients at diagnosis; roughly half of these metastases are symptomatic and the other half are detected by imaging.6 The rate of brain metastasis increases among patients who survive for at least 2 years after diagnosis.7 Given the highly aggressive nature of SCLC, 5-year OS rates are only about 25% for patients with limited-stage SCLC (disease confined to one hemithorax and regional nodes).8,9 Predictors of poor prognosis include poor performance status, older age, and being male.10 The pathologic subtypes of the disease (small cell carcinoma and combined small cell carcinoma) all carry a similarly poor prognosis.11
Disease Staging
Although a tumor–node–metastasis (TNM) classification has been proposed for staging SCLC,12,13 many institutions continue to use a simplified two-stage system developed by the Veterans Administration Lung Cancer group that categorizes disease as either limited-stage or extensive-stage.14 Current guidelines of the U.S. National Comprehensive Cancer Network recommend the use of positron emission tomography (PET) and CT scanning, or fused PET/CT scanning, of the chest, liver, adrenals, bone, and other areas of concern in the diagnosis and staging of SCLC. In one small study comparing the use of CT versus PET/CT for disease staging in 51 patients with SCLC, PET/CT detected all 51 primary lung cancers that had been observed on CT. However, PET/CT scanning led to changes in the assigned disease stage for 8 patients, with 2 of 18 cases originally diagnosed as limited-stage cancer being reclassified as extensive disease and 6 of 33 cases of extensive disease being reclassified as limited-stage disease.15
Several histologic and immunohistochemical markers have been evaluated for diagnosing or monitoring treatment response in SCLC, including transcription thyroid factor-1 (positive in >85% of SCLC cases); cytokeratin 7; deletions in chromosome 3; Leu-7; chromogranin A; synaptophysin; myc amplification; and p53 mutations (present in ~75% of cases).16 Deletions of tumor-suppressor genes are also relatively common and include fragile histidine triad (FHIT) (80%); RAS effector homologue (RASSF1) (>90%); TP53 (>75%); retinoblastoma-1 (RB1) (>90%); and retinoic acid receptor-beta (72%).17,18 However, to date no biomarkers have been validated for use in diagnosing SCLC. Moreover, mutations that are often present in non-small cell lung cancer (such as epidermal growth factor receptor [EGFR] mutations and anaplastic lymphoma kinase [ALK]) are rare in SCLC. Several clinicopathologic features have been linked with worse prognosis, such as poor performance status, significant weight loss, high lactate dehydrogenase levels, large numbers of metastatic sites, and the presence of paraneoplastic syndromes.19
Recognizing and Managing Paraneoplastic Syndrome
Paraneoplastic syndromes are fairly common in SCLC, with the syndrome of inappropriate antidiuretic hormone (SIADH) appearing in up to 15% of cases, Cushing or adrenocorticotropic hormone production syndrome in 2% to 5% of cases, and Lambert-Eaton syndrome in 3%.18,20 SIADH is the most common paraneoplastic disorder associated with SCLC. Indeed, among patients with SIADH, SCLC is an incidental finding in nearly 50%.21 A review of 244 patients with patients with limited-stage SCLC treated in 1981–1998 revealed that 14 (6%) had SIADH at presentation, with symptoms including weakness, altered consciousness, seizures, and low overall sodium levels (110–129 mEq/L).22 Moreover, 10 of these 14 patients who originally presented with SIADH had recurrent SIADH when the SCLC recurred, suggesting that serum sodium levels may be a useful marker in post-treatment surveillance for tumor recurrence. Another group found that both initial hyponatremia (p<0.001) and an inability to normalize sodium levels during chemotherapy (p=0.027) were poor prognostic indicators for OS.23 They also found an increased proportion of metastatic disease at presentation among those who presented with low serum sodium levels. The usefulness of hyponatremia as a prognostic indicator is supported by other studies as well.24,25
Rare cases have been reported of patients presenting with paraneoplastic complications such as gastroparesis and pseudoachalasia.26,27 These symptoms resolved with the treatment of SCLC. Interestingly, antinuclear antibodies such as ANNA-1 (also known as anti-Hu) have been found in some cases of gastrointestinal dysmotility secondary to paraneoplastic disease.28,29 SCLC has also been associated with combined antidiuretic hormone secretion and ectopic adrenocorticotropic hormone production.30 Another paraneoplastic disorder called oncogenic osteomalacia, a rare bone disorder presenting with increased renal phosphate excretion, has been identified in several cases of SCLC.31 Guillain-Barre syndrome, although more often present in association with other cancers, was identified as a paraneoplastic syndrome in a patient with SCLC.32 Finally, Lambert-Eaton myasthenic syndrome, which occurs in only 1%–3% of patients with SCLC, strongly correlates with underlying cancer, as nearly 50% who present with this syndrome are found incidentally to have SCLC.33,34 Hence the presence of Lambert-Eaton syndrome should prompt screening for SCLC.34 Treatment for paraneoplastic syndromes usually includes correction of electrolyte abnormalities and symptom management, but they generally resolve when the cancer is treated.
Early Thoracic Radiotherapy
Surgery currently has little role in the treatment of SCLC unless it is diagnosed quite early. The superiority of radiation therapy over surgery was established several decades ago by a study by the British Medical Research Council (median OS time 9.9 months for those treated with radiation vs. 6.5 months for those treated with surgery),35,36 and radiation therapy has been the standard treatment for limited-stage SCLC since that time. The next series of studies investigated more effective chemotherapy strategies for limited-stage SCLC. Although several large studies have demonstrated substantial improvements with the use of etoposide-containing regimens and cisplatin,37,38 the optimal combination of radiation and chemotherapy has yet to be defined. Studies now ongoing are analyzing various combination regimens for their ability to control tumors while minimizing toxicity.
The combination of radiation and chemotherapy for limited-stage disease is well supported by two meta-analyses.39,40 One meta-analysis, reported by Pignon et al.,39 reviewed 2140 patients from 13 trials and found an improvement in 3-year OS for those treated with chemoradiation (14.3%) versus with chemotherapy alone (8.9%). In the other meta-analysis, Warde and Payne40 combined data from 11 randomized trials with or without chemoradiation and found that receipt of chemoradiation led to better 2-year intrathoracic tumor control rates than did chemotherapy alone (34.1% vs. 16.5%). Two-year OS rates were also 5.4% higher (p<0.05) in the chemoradiation group. Further fine-tuning of the treatment approach with altered fractionation and perhaps targeted agents could further improve survival rates.
In terms of the optimal timing of thoracic radiation and chemotherapy, several large studies have shown that concurrent chemotherapy, or radiation begun early in the course of the chemotherapy, produces better disease control than chemotherapy followed sequentially by radiation.41–43 Murray et al.42 studied patients treated with cyclophosphamide, doxorubicin, and vincristine, alternating with etoposide and cisplatin, and beginning thoracic radiation either on week 3 or on week 15. Patients who achieved a complete response to this therapy were also given PCI. Patients who received radiation early during chemotherapy had significantly better progression-free survival (PFS) (p=0.036), OS (p=0.008) and freedom from brain metastasis (p=0.006) (Fig. 1). JCOG 9104, a phase III trial by the Japanese Clinical Oncology Group,41 compared concurrent chemoradiation with sequential chemotherapy followed by radiation for limited-stage SCLC. All patients received cisplatin and etoposide. Patients who received concurrent chemoradiation seemed to have better 2-year, 3-year, and 5-year OS rates than did patients receiving sequential treatment (2-year 54.4% vs. 35.1%; 3-year 29.8% vs. 20.2%; and 5-year 23.7% vs. 18.3%), but these apparent differences were not statistically significant (p=0.097). Further support for beginning thoracic radiation therapy early for limited-stage SCLC was demonstrated by another meta-analysis of trials in 1985–2002,44 which showed a small but significant improvement in 2-year OS from early radiation treatment that was more evident among those receiving hyperfractionated regimens and platinum-based chemotherapy. A more recent meta-analysis45 of 7 randomized trials evaluating when to start radiation treatment relative to platinum-based chemotherapy showed that radiation treatment begun within 30 days of the start of chemotherapy produced better 2- and 5-year survival rates than radiation that was begun more than 30 days after chemotherapy (hazard ratio 0.65, 95% confidence interval 0.45–0.93, p=0.02). The inferiority of sequential chemotherapy and radiation compared with concurrent chemoradiation probably reflects the development of chemoresistant clones, which often become resistant to radiation and lead to tumor repopulation.
Figure 1.
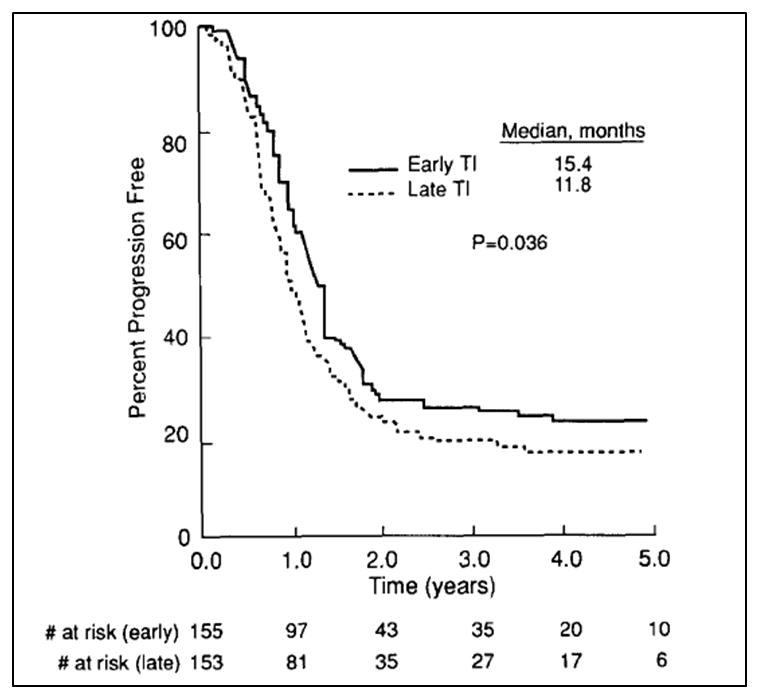
Progression-free survival rates for early versus late thoracic irradiation with concurrent chemotherapy in the treatment of limited-stage small cell lung cancer, as studied by the National Cancer Institute of Canada Clinical Trials Group (Murray et al. J Clin Oncol 1993). TI, thoracic irradiation.
Hyperfractionated and Accelerated Thoracic Radiation Therapy
Concurrent chemoradiation therapy has become the standard of care for SCLC, at least for patients who can tolerate the therapy.19 The next advancements in treatment came from considering the potential benefits of hyperfractionated radiation on tumor control. The fact that local tumor control rates remain less than optimal at 30%–50% suggested that increasing the radiation dose, either by escalation, hyperfractionation, or a combination of the two, might improve outcomes. Several studies have been done to evaluate the effectiveness of higher-dose radiation for limited-stage SCLC.46–48 One such study by the Radiation Therapy Oncology Group (RTOG 9712) sought to establish the maximum tolerated dose of thoracic radiation therapy with concurrent cisplatin and etoposide for limited-stage disease.48 Radiation was initially given in once-daily fractions of 1.8 Gy/fraction and subsequently increased to twice-daily fractions such that the maximum doses were 50.4 Gy, 54.0 Gy, 57.6 Gy, 61.2 Gy, and 64.8 Gy. The maximum tolerated dose in that study was 61.2 Gy. At 18 months, the median OS rate for those receiving 61.2 Gy was 82% compared with 25% for those given 50.4 Gy.
The propensity of SCLC for tumor repopulation and resistance has prompted intense study of hyperfractionated accelerated chemoradiation. Intergroup (INT) trial 0096 (also known as RTOG 8815) demonstrated significant benefit from hyperfractionated therapy, specifically comparing the standard dose of 45 Gy given in once-daily fractionation over 5 weeks to a dose of 45 Gy given twice daily over 3 weeks.49 Patients given the hyperfractionated regimen had significantly better 5-year OS rates (26% vs. 16%, p=0.04) (Fig. 2); however, the twice-daily regimen was associated with high rates of toxicity (grade 3 esophagitis). Notably, at about 60 Gy, the biologically effective dose (BED) of the twice-daily regimen was considerably higher than the BED of the once-daily dose to 45 Gy used in that trial, a dose that is now considered below the current standard. A meta-analysis of individual patient data published in 2012 also suggested that accelerated or hyperfractionated radiotherapy may have been beneficial in terms of OS, but this potential benefit again came at the cost of higher rates of acute esophagitis (odds ratio 2.41, p<0.001).50 However, that meta-analysis included patients treated since 1970, and thus the older, less conformal radiation techniques in use at that time may have accounted for the high rates of toxicity. Another more recent study, RTOG 0239, was a phase II trial of accelerated high-dose thoracic radiation therapy to 61.2 Gy given over 5 weeks (16 once-daily fractions of 1.8 Gy followed by 18 twice-daily fractions of 1.8 Gy) given with concurrent cisplatin and etoposide.51 The 2-year local control rate of 80% was much better than the 64% in INT 0096, supporting the use of accelerated hyperfractionation to achieve a high BED (Fig. 3); however, rates of severe acute esophagitis (18%) and myelosuppression (90%) underscore the need to remain cognizant of the toxicity of this regimen. Nevertheless, at 2.8% the treatment-related death rate was similar to that of other chemoradiation regimens. This treatment regimen was initially included in the ongoing phase III trial RTOG 0538, which compares outcomes among patients randomly assigned to one of three groups: 45 Gy in 30 fractions twice daily; 70 Gy in 35 fractions once daily; and 61.2 Gy in 34 fractions given first once daily and later twice daily (the RTOG 0239 regimen) (Fig. 4). However, the RTOG 0239 treatment arm was closed recently when higher treatment-related toxicity was noted on an interim analysis. A similar European collaborative phase III trial (CONVERT) is also ongoing. At this time, the current standard of care remains 45 Gy, given in 1.5-Gy fractions twice daily.
Figure 2.
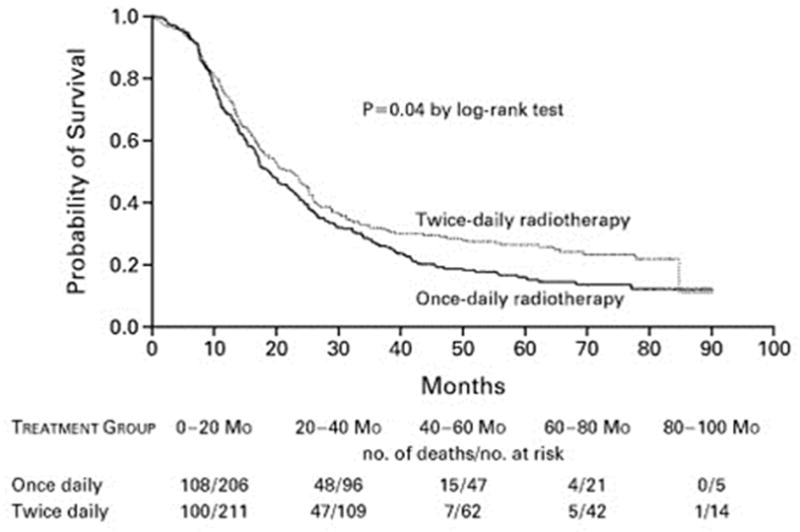
Kaplan-Meier estimates of overall survival for patients receiving twice-daily versus once-daily thoracic radiotherapy for limited-stage small cell lung cancer in the Intergroup trial 0096 (Turrisi et al., NEJM 1999).
Figure 3.
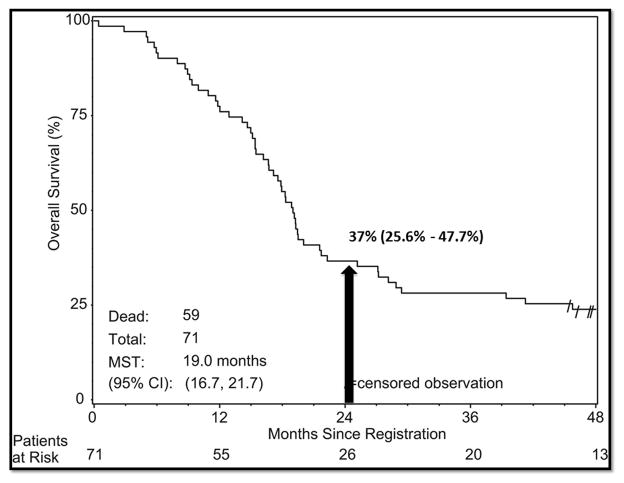
Kaplan-Meier estimates of overall survival for high-dose thoracic radiation given twice daily with concurrent cisplatin-etoposide in the Radiation Therapy Oncology Group (RTOG) trial 0239 (Komaki et al., IJROBP 2012). MST, median survival time; CI, confidence interval.
Figure 4.
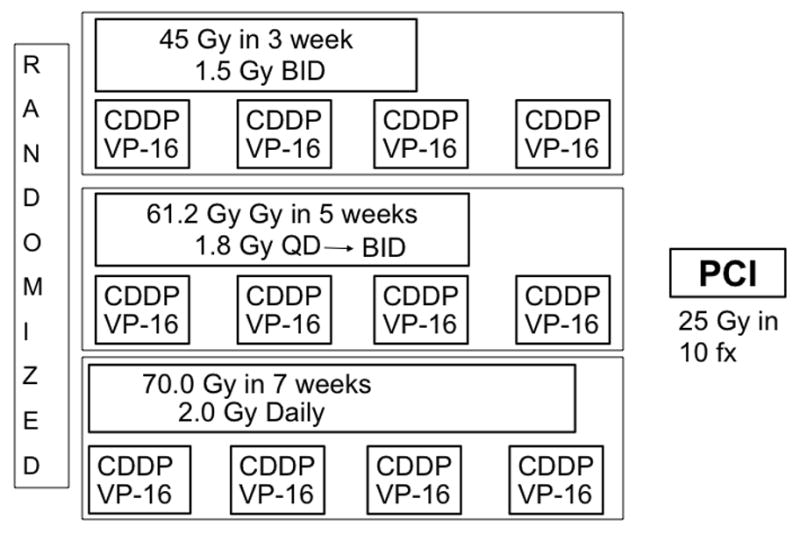
Treatment schema for Cancer and Leukemia Group B (CALGB) 30610/RTOG 0538, an ongoing phase III trial comparing thoracic radiotherapy regimens for limited-stage small cell lung cancer. CDDP, cisplatin; VP-16, etoposide; PCI, prophylactic cranial irradiation; QD, daily; BID, twice daily.
Prophylactic Cranial Irradiation
Brain metastases are common in SCLC, appearing in more than 50% of patients within 2 years of diagnosis and in up to 60% within 5 years.52,53 Chemotherapy is largely ineffective in preventing or treating brain metastases owing to the presence of the blood-brain barrier. However, PCI has shown some ability to prevent or control brain metastases in some patients with SCLC, predominantly those with limited-stage disease that responds completely to chemoradiation.
In 1993, Rosen et al.54 suggested that receipt of PCI improved survival in patients who showed a complete response to treatment. These findings led to PCI being offered routinely to patients with complete response to treatment of limited-stage disease. A large meta-analysis of data from 7 trials showed that patients who received PCI had a lower incidence of brain metastasis at 3 years after treatment (33% vs. 59%) and a higher 3-year OS rate (20.7% vs. 15.3%).55 A large randomized phase III study with 720 patients was undertaken to define the optimal dose for PCI; in that study, patients were to receive 25 Gy in 2.5-Gy once-daily fractions, 36 Gy in 2-Gy once-daily fractions, or 36 Gy in 1.5-Gy twice-daily fractions.56 Receipt of 36 Gy did not substantially reduce the 2-year rate of brain metastasis relative to the 25-Gy dose (23% vs. 29%, p=0.18), and the higher dose was associated with more severe toxicity and worse 2-year OS rate (37% vs. 43%, p=0.05). A follow-up analysis of quality of life revealed communication deficits, leg weakness, intellectual deficits, and memory problems to be more common in the higher-dose group (p<0.005).57 The findings from this study support the use of 25 Gy in 10 fractions, which continues to be the standard dose used at this time. Studies have also supported the use of PCI for patients with extensive SCLC that responded to chemotherapy, demonstrating overall improvements in survival and disease-free progression.58
The potential benefit of PCI for patients who achieve only a partial or incomplete response to chemoradiation has not been well studied. One recent attempt to address this issue in reviewing patients with limited-stage SCLC treated in 1981–2007 found some benefit from PCI in reducing the rate of brain metastasis (6.1% vs. 27.6%, p=0.05) and delaying its onset (time to symptom onset 20.7 vs. 10.6 months, p<0.0001) in patients with an incomplete response to therapy.59 However, no benefit was found in OS (p=0.32).59 The authors of this report suggested that some clinical predictors may be useful for identifying a subgroup of incomplete responders who may benefit from PCI. This issue will require further study to identify and validate clinical or biological markers for this purpose. Current guidelines support the use of PCI for patients with limited-stage SCLC who experience a complete response to therapy,9 with some additional evidence supporting the use of PCI for patients who show any response.60
Future Directions
Use of modern techniques for radiation planning and delivery such as intensity-modulated radiation therapy can greatly improve outcomes in limited-stage SCLC because such techniques provide highly conformal radiation doses, offering the possibility of dose escalation to tumors while minimizing treatment-related toxicity resulting from inadvertent irradiation of surrounding normal tissues. Indeed, a group at MD Anderson Cancer Center recently reported that intensity-modulated radiation therapy produced equivalent oncologic outcomes and reduced the need for feeding tube placement relative to 3-dimensional conformal techniques for patients undergoing radiation therapy for limited-stage SCLC.61 The incorporation of image-guided adaptive radiation therapy further improves the accuracy of delivery by allowing treatment volumes to be modified based on tumor response during treatment.62 Use of innovative radiation delivery techniques such as these, and possibly proton therapy as well,63,64 in combination with modifying fractionation schedules (daily fractions vs. hyperfractionated treatment) to deliver the maximum BED while minimizing toxicity should help to establish the optimal treatment for SCLC that will translate into better local control.
Tailoring the radiation treatment to better address the biological characteristics of SCLC, specifically accelerated proliferation, is an important area of research. Means of maximizing the BED while respecting dose limitations to nearby critical normal organs are actively being sought. In one retrospective review, patients who had received doses exceeding a BED of 57 Gy had better local control (p=0.024), PFS (p=0.006), and OS (p=0.005) than those who received a BED <57 Gy.65 As described previously, several studies have found that shortening the duration of radiation and effectively maintaining or increasing the BED may improve outcomes. Modern trials are using this concept to evaluate various radiation doses and fraction sizes to achieve the optimal response. The INT 0096 trial initially established that accelerated fractionation was beneficial over the original once-daily fractionation in terms of 5-year OS rates.49 More recently, RTOG 0239 incorporated updated radiation techniques to deliver doses in once-daily fractionation followed by a hyperfractionated boost, both with concurrent chemotherapy.51,66 Although the higher dose led to better tumor response rates, it was also associated with myelosuppression.
The advent of PET/CT has greatly improved the ability to identify the extent of tumor and nodal involvement in lung cancer.67,68 The use of PET to evaluate tumor response before and after initial cycles of chemotherapy has raised the question of whether radiation volumes should include pre- or post-induction chemotherapy fields. This question is being addressed in a prospective randomized trial in which all patients receive two cycles of induction chemotherapy followed by either concurrent chemoradiation using pre-chemotherapy volumes or concurrent chemoradiation using post-chemotherapy volumes.69 Interim results indicated no difference in local recurrence rates between the post-chemotherapy (31.6%) and pre-chemotherapy (28.6%) groups (p=0.81).
Progress in developing more effective chemotherapy agents has been slow. Newer agents for use with platinum-based chemotherapy have produced only minimal improvements thus far. For example, maintenance therapy with vandetanib, a vascular endothelial growth factor receptor tyrosine kinase inhibitor, did not improve OS or PFS compared with a placebo.70 A phase I study evaluating topotecan, a topoisomerase I inhibitor previously used as second-line therapy for SCLC, with paclitaxel in an attempt to enhance the cytotoxicity of induction therapy before chemoradiation showed some promise.71 Research to clarify the molecular architecture of SCLC and its resistance to treatment may lead to the discovery of additional, more effectively targeted agents. One example of this approach led to the identification of poly (ADP-ribose) polymerase-1 (PARP1), a DNA repair protein, as a potential therapeutic target. Proteomic profiling revealed high PARP1 levels in SCLC tumors and cell lines, and PARP1 inhibition, both as single-agent therapy and in combination with chemotherapy, has had some antitumor activity in preclinical models of SCLC.72 Based on these results, several PARP inhibitors are being tested in clinical trials for SCLC, including one study (Eastern Cooperative Oncology Group [ECOG] E2511) in which addition of the PARP1 inhibitor ABT-888 to chemoradiation is being investigated for limited-stage SCLC.
Additional studies are underway to identify agents to treat relapses. Single-agent topotecan is currently the only drug used for second-line therapy for those who do not respond to or experience relapse after initial treatment.73 Several studies have investigated second-line treatment with agents such as imatinib, bevacizumab, and thalidomide, all with minimal success.74–76 In addition to the PARP-inhibitor studies mentioned above, other research in targeted therapy focuses on apoptotic pathways of tumor cells. For example, inhibiting Bcl-2, a mediator of tumor cell apoptosis, may increase SCLC sensitivity to chemotherapy and thus be a fruitful target for further development.77,78 Other potential therapeutic targets that have emerged from more recent profiling efforts include aurora kinase (especially in myc-amplified tumors); SOX2 amplifications; RLF-MYCL1 fusions; and EZH2.72,79,80
Conclusions
Long-term survival rates for SCLC continue to be poor owing to the highly aggressive nature of this disease. Concurrent chemoradiation remains the standard first-line therapy for limited-stage SCLC, with PCI offered to those whose disease responds to treatment. The use of cisplatin and etoposide are fundamental to the improvements seen in OS. The addition of early, accelerated radiation therapy has also led to improved local control and OS. Further advances in technology will no doubt allow further innovations in radiation delivery techniques such that the maximum treatment effects are obtained with minimal damage to surrounding organs. Research and development of molecularly targeted agents will likely lead to a new generation of treatments for SCLC, as is already occurring in the treatment of other types of cancer.
Acknowledgments
Funding sources: None
References
- 1.American Cancer Society. Global Cancer Facts & Figures. 2. Atlanta: American Cancer Society; 2011. [Google Scholar]
- 2.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 3.Aberle DR, Adams AM, Berg CD, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365:395–409. doi: 10.1056/NEJMoa1102873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cox JD, Ang KK. Radiation Oncology: Rationale, Techniques, Results. 9. Mosby; St Louis: 2010. [Google Scholar]
- 5.Dowell JE. Small cell lung cancer: are we making progress? Am J Med Sci. 2010;339:68–76. doi: 10.1097/MAJ.0b013e3181bccef5. [DOI] [PubMed] [Google Scholar]
- 6.Seute T, Leffers P, ten Velde GP, Twijnstra A. Detection of brain metastases from small cell lung cancer: consequences of changing imaging techniques (CT versus MRI) Cancer. 2008;112:1827–34. doi: 10.1002/cncr.23361. [DOI] [PubMed] [Google Scholar]
- 7.Komaki R, Byhardt RW, Anderson T, et al. What is the lowest effective biologic dose for prophylactic cranial irradiation? Am J Clin Oncol. 1985;8:523–7. doi: 10.1097/00000421-198512000-00013. [DOI] [PubMed] [Google Scholar]
- 8.Sorensen M, Pijls-Johannesma M, Felip E. Small-cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2010;21(Suppl 5):v120–5. doi: 10.1093/annonc/mdq172. [DOI] [PubMed] [Google Scholar]
- 9.Simon GR, Turrisi A. Management of small cell lung cancer: ACCP evidence-based clinical practice guidelines (2nd edition) Chest. 2007;132:324S–39S. doi: 10.1378/chest.07-1385. [DOI] [PubMed] [Google Scholar]
- 10.Foster NR, Mandrekar SJ, Schild SE, et al. Prognostic factors differ by tumor stage for small cell lung cancer: a pooled analysis of North Central Cancer Treatment Group trials. Cancer. 2009;115:2721–31. doi: 10.1002/cncr.24314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beasley MB, Brambilla E, Travis WD. The 2004 World Health Organization classification of lung tumors. Semin Roentgenol. 2005;40:90–7. doi: 10.1053/j.ro.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 12.Detterbeck FC, Boffa DJ, Tanoue LT. The new lung cancer staging system. Chest. 2009;136:260–71. doi: 10.1378/chest.08-0978. [DOI] [PubMed] [Google Scholar]
- 13.Mirsadraee S, Oswal D, Alizadeh Y, Caulo A, van Beek E., Jr The 7th lung cancer TNM classification and staging system: Review of the changes and implications. World J Radiol. 2012;4:128–34. doi: 10.4329/wjr.v4.i4.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Micke P, Faldum A, Metz T, et al. Staging small cell lung cancer: Veterans Administration Lung Study Group versus International Association for the Study of Lung Cancer--what limits limited disease? Lung Cancer. 2002;37:271–6. doi: 10.1016/s0169-5002(02)00072-7. [DOI] [PubMed] [Google Scholar]
- 15.Lee YJ, Cho A, Cho BC, et al. High tumor metabolic activity as measured by fluorodeoxyglucose positron emission tomography is associated with poor prognosis in limited and extensive stage small-cell lung cancer. Clin Cancer Res. 2009;15:2426–32. doi: 10.1158/1078-0432.CCR-08-2258. [DOI] [PubMed] [Google Scholar]
- 16.Poola I, Graziano SL. Expression of neuron-specific enolase, chromogranin A, synaptophysin and Leu-7 in lung cancer cell lines. J Exp Clin Cancer Res. 1998;17:165–73. [PubMed] [Google Scholar]
- 17.Prins J, De Vries EG, Mulder NH. The myc family of oncogenes and their presence and importance in small-cell lung carcinoma and other tumour types. Anticancer Res. 1993;13:1373–85. [PubMed] [Google Scholar]
- 18.van Meerbeeck JP, Fennell DA, De Ruysscher DK. Small-cell lung cancer. Lancet. 2011;378:1741–55. doi: 10.1016/S0140-6736(11)60165-7. [DOI] [PubMed] [Google Scholar]
- 19.Schild SE. Thoracic Malignancies. 2. Demos Medical Publishing; New York: 2012. [Google Scholar]
- 20.Planchard D, Le Pechoux C. Small cell lung cancer: new clinical recommendations and current status of biomarker assessment. Eur J Cancer. 2011;47 (Suppl 3):S272–83. doi: 10.1016/S0959-8049(11)70173-3. [DOI] [PubMed] [Google Scholar]
- 21.Payne M, Bradbury P, Lang B, et al. Prospective study into the incidence of Lambert Eaton myasthenic syndrome in small cell lung cancer. J Thorac Oncol. 2010;5:34–8. doi: 10.1097/JTO.0b013e3181c3f4f1. [DOI] [PubMed] [Google Scholar]
- 22.Tai P, Yu E, Jones K, Sadikov E, Mahmood S, Tonita J. Syndrome of inappropriate antidiuretic hormone secretion (SIADH) in patients with limited stage small cell lung cancer. Lung Cancer. 2006;53:211–5. doi: 10.1016/j.lungcan.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 23.Hansen O, Sorensen P, Hansen KH. The occurrence of hyponatremia in SCLC and the influence on prognosis: a retrospective study of 453 patients treated in a single institution in a 10-year period. Lung Cancer. 2010;68:111–4. doi: 10.1016/j.lungcan.2009.05.015. [DOI] [PubMed] [Google Scholar]
- 24.Dearing MP, Steinberg SM, Phelps R, et al. Outcome of patients with small-cell lung cancer: effect of changes in staging procedures and imaging technology on prognostic factors over 14 years. J Clin Oncol. 1990;8:1042–9. doi: 10.1200/JCO.1990.8.6.1042. [DOI] [PubMed] [Google Scholar]
- 25.Rawson NS, Peto J. An overview of prognostic factors in small cell lung cancer. A report from the Subcommittee for the Management of Lung Cancer of the United Kingdom Coordinating Committee on Cancer Research. Br J Cancer. 1990;61:597–604. doi: 10.1038/bjc.1990.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hejazi RA, Zhang D, McCallum RW. Gastroparesis, pseudoachalasia and impaired intestinal motility as paraneoplastic manifestations of small cell lung cancer. Am J Med Sci. 2009;338:69–71. doi: 10.1097/MAJ.0b013e31819b93e5. [DOI] [PubMed] [Google Scholar]
- 27.Lee HR, Lennon VA, Camilleri M, Prather CM. Paraneoplastic gastrointestinal motor dysfunction: clinical and laboratory characteristics. Am J Gastroenterol. 2001;96:373–9. doi: 10.1111/j.1572-0241.2001.03454.x. [DOI] [PubMed] [Google Scholar]
- 28.Lucchinetti CF, Kimmel DW, Lennon VA. Paraneoplastic and oncologic profiles of patients seropositive for type 1 antineuronal nuclear autoantibodies. Neurology. 1998;50:652–7. doi: 10.1212/wnl.50.3.652. [DOI] [PubMed] [Google Scholar]
- 29.Dalmau J, Furneaux HM, Cordon-Cardo C, Posner JB. The expression of the Hu (paraneoplastic encephalomyelitis/sensory neuronopathy) antigen in human normal and tumor tissues. Am J Pathol. 1992;141:881–6. [PMC free article] [PubMed] [Google Scholar]
- 30.Mussig K, Horger M, Haring HU, Wehrmann M. Syndrome of inappropriate antidiuretic hormone secretion and ectopic ACTH production in small cell lung carcinoma. Lung Cancer. 2007;57:120–2. doi: 10.1016/j.lungcan.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 31.Tantisattamo E, Ng RC. Dual paraneoplastic syndromes: small cell lung carcinoma-related oncogenic osteomalacia, and syndrome of inappropriate antidiuretic hormone secretion: report of a case and review of the literature. Hawaii Med J. 2011;70:139–43. [PMC free article] [PubMed] [Google Scholar]
- 32.Naveed S, Okoli K, Hollingsworth J, Kasmani R. Guillain-Barre syndrome as a paraneoplastic manifestation of small-cell carcinoma of lung. South Med J. 2010;103:156–8. doi: 10.1097/smj.0b013e3181bfd2c0. [DOI] [PubMed] [Google Scholar]
- 33.Maddison P, Lang B. Paraneoplastic neurological autoimmunity and survival in small-cell lung cancer. J Neuroimmunol. 2008;201–202:159–62. doi: 10.1016/j.jneuroim.2008.05.024. [DOI] [PubMed] [Google Scholar]
- 34.Titulaer MJ, Wirtz PW, Willems LN, van Kralingen KW, Smitt PA, Verschuuren JJ. Screening for small-cell lung cancer: a follow-up study of patients with Lambert-Eaton myasthenic syndrome. J Clin Oncol. 2008;26:4276–81. doi: 10.1200/JCO.2008.17.5133. [DOI] [PubMed] [Google Scholar]
- 35.Fox W, Scadding JG. Medical Research Council comparative trial of surgery and radiotherapy for primary treatment of small-celled or oat-celled carcinoma of bronchus. Ten-year follow-up. Lancet. 1973;2:63–5. doi: 10.1016/s0140-6736(73)93260-1. [DOI] [PubMed] [Google Scholar]
- 36.Miller AB, Fox W, Tall R. Five-year follow-up of the Medical Research Council comparative trial of surgery and radiotherapy for the primary treatment of small-celled or oat-celled carcinoma of the bronchus. Lancet. 1969;2:501–5. doi: 10.1016/s0140-6736(69)90212-8. [DOI] [PubMed] [Google Scholar]
- 37.Hirsch FR, Hansen HH, Hansen M, et al. The superiority of combination chemotherapy including etoposide based on in vivo cell cycle analysis in the treatment of extensive small-cell lung cancer: a randomized trial of 288 consecutive patients. J Clin Oncol. 1987;5:585–91. doi: 10.1200/JCO.1987.5.4.585. [DOI] [PubMed] [Google Scholar]
- 38.Sundstrom S, Bremnes RM, Kaasa S, et al. Cisplatin and etoposide regimen is superior to cyclophosphamide, epirubicin, and vincristine regimen in small-cell lung cancer: results from a randomized phase III trial with 5 years’ follow-up. J Clin Oncol. 2002;20:4665–72. doi: 10.1200/JCO.2002.12.111. [DOI] [PubMed] [Google Scholar]
- 39.Pignon JP, Arriagada R. Role of thoracic radiotherapy in limited-stage small-cell lung cancer: quantitative review based on the literature versus meta-analysis based on individual data. J Clin Oncol. 1992;10:1819–20. doi: 10.1200/JCO.1992.10.11.1819. [DOI] [PubMed] [Google Scholar]
- 40.Warde P, Payne D. Does thoracic irradiation improve survival and local control in limited-stage small-cell carcinoma of the lung? A meta-analysis. J Clin Oncol. 1992;10:890–5. doi: 10.1200/JCO.1992.10.6.890. [DOI] [PubMed] [Google Scholar]
- 41.Takada M, Fukuoka M, Kawahara M, et al. Phase III study of concurrent versus sequential thoracic radiotherapy in combination with cisplatin and etoposide for limited-stage small-cell lung cancer: results of the Japan Clinical Oncology Group Study 9104. J Clin Oncol. 2002;20:3054–60. doi: 10.1200/JCO.2002.12.071. [DOI] [PubMed] [Google Scholar]
- 42.Murray N, Coy P, Pater JL, et al. Importance of timing for thoracic irradiation in the combined modality treatment of limited-stage small-cell lung cancer. The National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 1993;11:336–44. doi: 10.1200/JCO.1993.11.2.336. [DOI] [PubMed] [Google Scholar]
- 43.De Ruysscher D, Pijls-Johannesma M, Bentzen SM, et al. Time between the first day of chemotherapy and the last day of chest radiation is the most important predictor of survival in limited-disease small-cell lung cancer. J Clin Oncol. 2006;24:1057–63. doi: 10.1200/JCO.2005.02.9793. [DOI] [PubMed] [Google Scholar]
- 44.Fried DB, Morris DE, Poole C, et al. Systematic review evaluating the timing of thoracic radiation therapy in combined modality therapy for limited-stage small-cell lung cancer. J Clin Oncol. 2004;22:4837–45. doi: 10.1200/JCO.2004.01.178. [DOI] [PubMed] [Google Scholar]
- 45.Pijls-Johannesma M, De Ruysscher D, Vansteenkiste J, Kester A, Rutten I, Lambin P. Timing of chest radiotherapy in patients with limited stage small cell lung cancer: a systematic review and meta-analysis of randomised controlled trials. Cancer Treat Rev. 2007;33:461–73. doi: 10.1016/j.ctrv.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 46.Bogart JA, Herndon JE, 2nd, Lyss AP, et al. 70 Gy thoracic radiotherapy is feasible concurrent with chemotherapy for limited-stage small-cell lung cancer: analysis of Cancer and Leukemia Group B study 39808. Int J Radiat Oncol Biol Phys. 2004;59:460–8. doi: 10.1016/j.ijrobp.2003.10.021. [DOI] [PubMed] [Google Scholar]
- 47.Choi NC, Herndon JE, 2nd, Rosenman J, et al. Phase I study to determine the maximum-tolerated dose of radiation in standard daily and hyperfractionated-accelerated twice-daily radiation schedules with concurrent chemotherapy for limited-stage small-cell lung cancer. J Clin Oncol. 1998;16:3528–36. doi: 10.1200/JCO.1998.16.11.3528. [DOI] [PubMed] [Google Scholar]
- 48.Komaki R, Swann RS, Ettinger DS, et al. Phase I study of thoracic radiation dose escalation with concurrent chemotherapy for patients with limited small-cell lung cancer: Report of Radiation Therapy Oncology Group (RTOG) protocol 97-12. Int J Radiat Oncol Biol Phys. 2005;62:342–50. doi: 10.1016/j.ijrobp.2004.11.030. [DOI] [PubMed] [Google Scholar]
- 49.Turrisi AT, 3rd, Kim K, Blum R, et al. Twice-daily compared with once-daily thoracic radiotherapy in limited small-cell lung cancer treated concurrently with cisplatin and etoposide. N Engl J Med. 1999;340:265–71. doi: 10.1056/NEJM199901283400403. [DOI] [PubMed] [Google Scholar]
- 50.Mauguen A, Le Pechoux C, Saunders MI, et al. Hyperfractionated or accelerated radiotherapy in lung cancer: an individual patient data meta-analysis. J Clin Oncol. 2012;30:2788–97. doi: 10.1200/JCO.2012.41.6677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Komaki R, Paulus R, Ettinger DS, et al. Phase II study of accelerated high-dose radiotherapy with concurrent chemotherapy for patients with limited small-cell lung cancer: Radiation Therapy Oncology Group protocol 0239. Int J Radiat Oncol Biol Phys. 2012;83(4):e531–6. doi: 10.1016/j.ijrobp.2012.01.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Arriagada R, Le Chevalier T, Borie F, et al. Prophylactic cranial irradiation for patients with small-cell lung cancer in complete remission. J Natl Cancer Inst. 1995;87:183–90. doi: 10.1093/jnci/87.3.183. [DOI] [PubMed] [Google Scholar]
- 53.Arriagada R, Le Chevalier T, Riviere A, et al. Patterns of failure after prophylactic cranial irradiation in small-cell lung cancer: analysis of 505 randomized patients. Ann Oncol. 2002;13:748–54. doi: 10.1093/annonc/mdf123. [DOI] [PubMed] [Google Scholar]
- 54.Rosen ST, Makuch RW, Lichter AS, et al. Role of prophylactic cranial irradiation in prevention of central nervous system metastases in small cell lung cancer. Potential benefit restricted to patients with complete response. Am J Med. 1983;74:615–24. doi: 10.1016/0002-9343(83)91019-7. [DOI] [PubMed] [Google Scholar]
- 55.Auperin A, Arriagada R, Pignon JP, et al. Prophylactic cranial irradiation for patients with small-cell lung cancer in complete remission. Prophylactic Cranial Irradiation Overview Collaborative Group. N Engl J Med. 1999;341:476–84. doi: 10.1056/NEJM199908123410703. [DOI] [PubMed] [Google Scholar]
- 56.Le Pechoux C, Dunant A, Senan S, et al. Standard-dose versus higher-dose prophylactic cranial irradiation (PCI) in patients with limited-stage small-cell lung cancer in complete remission after chemotherapy and thoracic radiotherapy (PCI 99-01, EORTC 22003-08004, RTOG 0212, and IFCT 99-01): a randomised clinical trial. Lancet Oncol. 2009;10:467–74. doi: 10.1016/S1470-2045(09)70101-9. [DOI] [PubMed] [Google Scholar]
- 57.Le Pechoux C, Laplanche A, Faivre-Finn C, et al. Clinical neurological outcome and quality of life among patients with limited small-cell cancer treated with two different doses of prophylactic cranial irradiation in the intergroup phase III trial (PCI99-01, EORTC 22003-08004, RTOG 0212 and IFCT 99-01) Ann Oncol. 2011;22:1154–63. doi: 10.1093/annonc/mdq576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Slotman BJ, Mauer ME, Bottomley A, et al. Prophylactic cranial irradiation in extensive disease small-cell lung cancer: short-term health-related quality of life and patient reported symptoms: results of an international Phase III randomized controlled trial by the EORTC Radiation Oncology and Lung Cancer Groups. J Clin Oncol. 2009;27:78–84. doi: 10.1200/JCO.2008.17.0746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tai P, Assouline A, Joseph K, Stitt L, Yu E. Prophylactic cranial irradiation for patients with limited-stage small-cell lung cancer with response to chemoradiation. Clin Lung Cancer. 2013;14(1):40–4. doi: 10.1016/j.cllc.2012.04.005. [DOI] [PubMed] [Google Scholar]
- 60.Schild SE, Foster NR, Meyers JP, et al. Prophylactic cranial irradiation in small-cell lung cancer: findings from a North Central Cancer Treatment Group Pooled Analysis. Ann Oncol. 2012;23(11):2919–24. doi: 10.1093/annonc/mds123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shirvani SM, Juloori A, Allen PK, et al. Comparison of 2 common radiation therapy techniques for definitive treatment of small cell lung cancer. Int J Radiat Oncol Biol Phys. 2013;87:139–47. doi: 10.1016/j.ijrobp.2013.05.040. [DOI] [PubMed] [Google Scholar]
- 62.Koay EJ, Lege D, Mohan R, et al. Adaptive/nonadaptive proton radiation planning and outcomes in a phase II trial for locally advanced non-small cell lung cancer. Int J Radiat Oncol Biol Phys. 2012;84:1093–100. doi: 10.1016/j.ijrobp.2012.02.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sejpal S, Komaki R, Tsao A, et al. Early findings on toxicity of proton beam therapy with concurrent chemotherapy for nonsmall cell lung cancer. Cancer. 2011;117:3004–13. doi: 10.1002/cncr.25848. [DOI] [PubMed] [Google Scholar]
- 64.Colaco RJ, Huh S, Nichols RC, et al. Dosimetric rationale and early experience at UFPTI of thoracic proton therapy and chemotherapy in limited-stage small cell lung cancer. Acta Oncol. 2013;52:506–13. doi: 10.3109/0284186X.2013.769063. [DOI] [PubMed] [Google Scholar]
- 65.Xia B, Chen GY, Cai XW, et al. The effect of bioequivalent radiation dose on survival of patients with limited-stage small-cell lung cancer. Radiat Oncol. 2011;6:50. doi: 10.1186/1748-717X-6-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Komaki R, Moughan JD, Ettinger D, et al. Toxicities in a phase II study of accelerated high dose thoracic radiation therapy (TRT) with concurrent chemotherapy for limited small cell lung cancer (LSCLC) (RTOG 0239) ASCO Abstract. 2007 Jun;25(18_supp):7717. [Google Scholar]
- 67.Ashamalla H, Rafla S, Parikh K, et al. The contribution of integrated PET/CT to the evolving definition of treatment volumes in radiation treatment planning in lung cancer. Int J Radiat Oncol Biol Phys. 2005;63:1016–23. doi: 10.1016/j.ijrobp.2005.04.021. [DOI] [PubMed] [Google Scholar]
- 68.Fischer BM, Mortensen J, Langer SW, et al. A prospective study of PET/CT in initial staging of small-cell lung cancer: comparison with CT, bone scintigraphy and bone marrow analysis. Ann Oncol. 2007;18:338–45. doi: 10.1093/annonc/mdl374. [DOI] [PubMed] [Google Scholar]
- 69.Hu X, Bao Y, Zhang L, et al. Omitting elective nodal irradiation and irradiating postinduction versus preinduction chemotherapy tumor extent for limited-stage small cell lung cancer: interim analysis of a prospective randomized noninferiority trial. Cancer. 2012;118:278–87. doi: 10.1002/cncr.26119. [DOI] [PubMed] [Google Scholar]
- 70.Arnold AM, Seymour L, Smylie M, et al. Phase II study of vandetanib or placebo in small-cell lung cancer patients after complete or partial response to induction chemotherapy with or without radiation therapy: National Cancer Institute of Canada Clinical Trials Group Study BR. 20. J Clin Oncol. 2007;25:4278–84. doi: 10.1200/JCO.2007.12.3083. [DOI] [PubMed] [Google Scholar]
- 71.Lu C, Komaki R, Lee JS, et al. A phase I study of topotecan/paclitaxel alternating with etoposide/cisplatin and thoracic irradiation in patients with limited small cell lung cancer. Clin Cancer Res. 2003;9:2085–91. [PubMed] [Google Scholar]
- 72.Byers LA, Wang J, Nilsson MB, et al. Proteomic profiling identifies dysregulated pathways in small cell lung cancer and novel therapeutic targets including PARP1. Cancer Discov. 2012;2:798–811. doi: 10.1158/2159-8290.CD-12-0112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.von Pawel J, Schiller JH, Shepherd FA, et al. Topotecan versus cyclophosphamide, doxorubicin, and vincristine for the treatment of recurrent small-cell lung cancer. J Clin Oncol. 1999;17:658–67. doi: 10.1200/JCO.1999.17.2.658. [DOI] [PubMed] [Google Scholar]
- 74.Dy GK, Miller AA, Mandrekar SJ, et al. A phase II trial of imatinib (ST1571) in patients with c-kit expressing relapsed small-cell lung cancer: a CALGB and NCCTG study. Ann Oncol. 2005;16:1811–6. doi: 10.1093/annonc/mdi365. [DOI] [PubMed] [Google Scholar]
- 75.Horn L, Dahlberg SE, Sandler AB, et al. Phase II study of cisplatin plus etoposide and bevacizumab for previously untreated, extensive-stage small-cell lung cancer: Eastern Cooperative Oncology Group Study E3501. J Clin Oncol. 2009;27:6006–11. doi: 10.1200/JCO.2009.23.7545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pujol JL, Breton JL, Gervais R, et al. Phase III double-blind, placebo-controlled study of thalidomide in extensive-disease small-cell lung cancer after response to chemotherapy: an intergroup study FNCLCC cleo04 IFCT 00-01. J Clin Oncol. 2007;25:3945–51. doi: 10.1200/JCO.2007.11.8109. [DOI] [PubMed] [Google Scholar]
- 77.Rudin CM, Hann CL, Garon EB, et al. Phase II study of single-agent navitoclax (ABT-263) and biomarker correlates in patients with relapsed small cell lung cancer. Clin Cancer Res. 2012;18:3163–9. doi: 10.1158/1078-0432.CCR-11-3090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chiappori AA, Schreeder MT, Moezi MM, et al. A phase I trial of pan-Bcl-2 antagonist obatoclax administered as a 3-h or a 24-h infusion in combination with carboplatin and etoposide in patients with extensive-stage small cell lung cancer. Br J Cancer. 2012;106:839–45. doi: 10.1038/bjc.2012.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sos ML, Dietlein F, Peifer M, et al. A framework for identification of actionable cancer genome dependencies in small cell lung cancer. Proc Natl Acad Sci USA. 2012;109:17034–9. doi: 10.1073/pnas.1207310109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rudin CM, Durinck S, Stawiski EW, et al. Comprehensive genomic analysis identifies SOX2 as a frequently amplified gene in small-cell lung cancer. Nat Genet. 2012;44:1111–6. doi: 10.1038/ng.2405. [DOI] [PMC free article] [PubMed] [Google Scholar]