Abstract
The function and mode of action of curcumin in modulating the formation of lipid raft domains were investigated by microscopic observation using model membranes. Curcumin induces fusion of lipid raft domains at extremely low concentrations through the alteration of the boundary between the ordered and disordered phases.
In biological systems, heterogeneity of the cell membrane plays important roles as postulated by the lipid raft hypothesis.1 Lipid rafts are functional domains in a cell membrane, which recruit specific lipids and membrane proteins in a cluster to regulate their activity. Many lipid raft-related cellular events such as signal transduction,2 protein sorting3 and membrane transport4 have been reported so far. In addition, lipid rafts have been studied for their roles in the development of membrane-associated diseases including viral and bacterial infections, Alzheimer’s disease, prion disorders, diabetes as well as cardiovascular disease.5 Therefore, it is important to find a chemical approach to control the properties of lipid rafts for biological and medicinal applications.
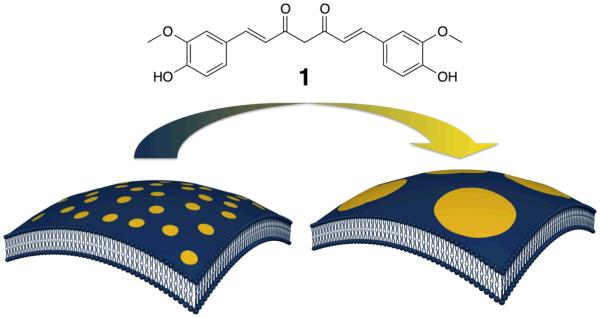
We report herein that curcumin, a major component of traditional spice turmeric, actively modulates the structure of raft domain in a lipid membrane (Fig. 1). Curcumin has attracted much attention because of its broad-spectrum of pharmacological activities including antioxidant, anticancer, antimutagenic, antibiotic, antiviral, antifungal, antiamyloid, antidiabetic and anti-inflammatory properties.6 Recently, it has been found that curcumin can regulate various structurally-unrelated membrane proteins such as transcription factors, receptors and ion channels.7 These reports suggest that curcumin is a membrane-active compound that modulates the activity of membrane proteins by changing the properties of the surrounding lipid bilayer rather than directly binding to proteins.8 In this study, we have investigated the function and mode of action of curcumin in modulating the raft domains by a microscopic observation of lipid raft reconstituted model membranes.
Fig. 1.
Chemical structure of curcumin (1) and a schematic illustration of curcumin-induced growth of lipid do-mains in membrane.
We first evaluated the effect of curcumin on the lipid raft domain in cell-sized giant unilamellar vesicles (GUVs). GUVs were prepared by gentle hydration of thin lipid film formed with an equimolar mixture of 1,2-dioleoyl-sn-glycero-3-phosphocholine (DOPC), sphingomyelin from porcine brain (SM) and cholesterol (Chol). This system forms liquid-ordered (lo) raft domain, which was enriched in SM and Chol, in the surrounding DOPC-rich liquid-disordered (ld) membrane.9 Prior to the addition of curcumin, the GUVs have several dark domains with a diameter <5 μm (Fig. 2, panel a), which are the lo raft domains, in the bright surrounding DOPC-rich ld phase, to which fluorescent Texas Red® 1,2-dihexadecanoyl-sn-glycero-3-phosphoethanolamine, triethylammonium salt (TR-DHPE) is preferentially distributed.10 All domains are circular and moved laterally in the membrane, suggesting that the domain formation is due to the phase separation between liquid (ld and lo) phases.11 Interestingly, upon the addition of curcumin at 0.3 mole % to the total lipids, the raft (dark) domains started merging and completely covered the hemisphere of GUVs after 2 hours (Fig. 2, panels b-d). The fused domains still adopt a circular shape, indicating that the line tension at the domain boundary determines the domain formation in these liquid phases. In a control experiment, the injection of only solvent did not induce the fusion of domains (see ESI†, Fig. S1). Curcumin appears to induce the growth of domain by fusing the existing domains, but not by the Ostwald ripening, which otherwise might cause the growth of large domains with concomitant shrinkage of small raft domains. In addition, we did not observe any significant morphological changes or deformation of vesicles such as rupture, shrinkage, budding and fission over 2 hours, indicating that vesicular structure remains intact during the domain fusion. The cucumin concentration of 0.3 mole % is likely to be the threshold concentration for the domains fusion as the domains were intact below 0.2 mole% (see ESI†, Fig. S2). This also suggests that a small quantity of curcumin can modulate the macroscopic phase property of lipid bilayer.
Fig. 2.

Fluorescence microscopic observation of curcumin-induced growth of domains in a giant vesicle consisting of DOPC / SM / Chol. Images were acquired at (a) 0, (b) 30, (c) 60 and (d) 120 minutes after the addition of curcumin. Dark and bright regions are corresponding to the liquid-ordered (lo) raft domains and liquid-disordered (ld) DOPC-rich membrane, respectively. [curcumin] / [lipid] = 3 ×10−3 at 25 °C. 1 mole % of TR-DHPE was added to the ternary mixture of lipids.
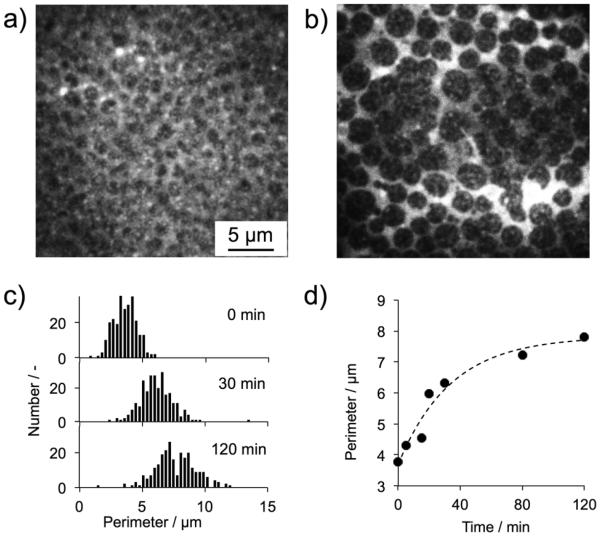
The domain growth was further characterized by total internal reflection fluorescence microscopy (TIRF). A planar lipid bilayer with DOPC/SM/Chol (1:1:1) containing 1 mole % of TR-DHPE, gave dark raft domains and bright DOPC-rich phase (Fig. 3, panel a). The entire field of substrate was uniformly covered by the lipid bilayer with domain structures. Upon the addition of a small quantity of curcumin (8 × 10−4 mole % to total lipids), the size of each domain gradually increased over 2 hours (Fig. 3, panel b). The control experiment confirmed that, in the absence of curcumin, the domains did not grow spontaneously within the observation time (see ESI†, Fig. S3). Prior to the addition of curcumin, the perimeter of the domain displayed a mean value of 3.8 μm (S.D.: 0.90 μm, Fig. 3, panel d). It should be noted that the spatial resolution of TIRF used in this study is around 300 nm, providing quantitative analysis of data. After the addition of curcumin, the domain perimeter monotonically increased with time, and the mean perimeter appears to eventually level off around 8 μm after 2 hours. A single Gaussian distribution of the domain perimeter without new additional populations was observed over all the observation times (Fig. 3, panel c). In corroboration with the results of GUVs, this result suggests that the domain growth is likely due to a random fusion of existing domains neighbouring to each other rather than fusion of domains with specific sizes or properties.
Fig. 3.
Characterization of curcumin-induced domain growth in a planar lipid bilayer consisting of DOPC / SM / Chol. TIRF images of the lipid bilayer before (a) and 2 hours after (b) the addition of curcumin. (c) Time-dependence of the distribution of domain perimeter and (d) their mean values. 250 domains were subjected to the perimeter analysis at each observation time. [curcumin] / [lipid] = 8 ×10−6 at 25 °C.
The domains in the planar lipid bilayer were fused by significantly small amount of curcumin, while domains in GUV membrane were intact at the same concentration (see ESI†, Fig. S4). This effect is likely related to the difference in lateral tension of the lipid membranes, which will be discussed later. In addition, when the membrane lacks cholesterol, the vesicles formed SM-rich gel (so) phase domains in the DOPC-rich ld phase. Curcumin did not induce domain fusion in this so-ld membrane, indicating that curcumin is not able to modulate gel domains (see ESI†, Fig. S5).
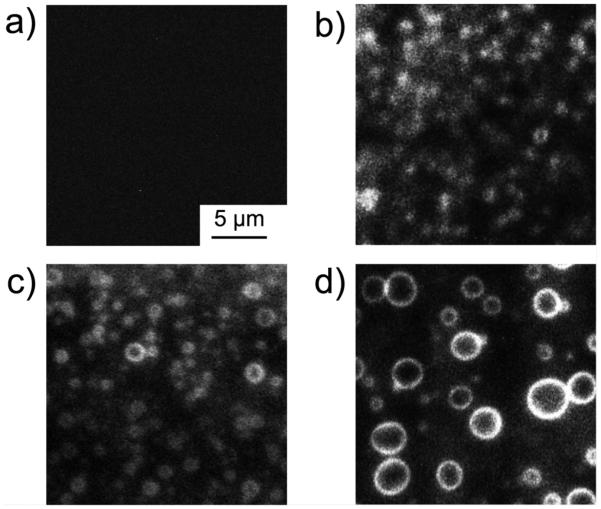
To identify the binding sites of curcumin in raft-forming membrane, we further visualized curcumin by TIRF according to the fluorescence property of curcumin.12 Upon the addition of curcumin, the domain perimeters became fluorescent, giving many bright circles, and their size increased over time as the domains grow (Fig. 4). This indicates that curcumin is localized at the domain boundary between lo raft and surrounding ld phase during the domain fusion process. We further evaluated the domain selective action of curcumin in Langmuir monolayer experiment; small quantity of curcumin (0.8 × 10−4 mole % to total lipids) reduced the surface pressure of raft-forming DOPC/SM/Chol monolayer, (see ESI†, Fig. S6), whereas no effect of curcumin was observed on the surface pressure of monolayers mimicking the lipid composition of each domain (DOPC or SM/Chol). In addition, we observed that curcumin induced the marked shifting of the π-A isotherm of DOPC/SM/Chol monolayer to lower molecular area, whereas there is no change in the isotherms of DOPC and SM/Chol monolayers (see ESI†, Fig. S7). These results support the notion that curcumin reduces the intrinsic molecular area of lipids in membrane by binding to the raft domain boundaries.
Fig. 4.
Visualization of curcumin localization at the domain boundaries. TIRF images of the planar lipid bilayer consisting of DOPC / SM / Chol were acquired (a) before the addition and at (b) 30, (c) 45 and (d) 60 minutes after the addition of curcumin. Curcumin was excited at 405 nm by a diode laser. [curcumin] / [lipid] = 8 ×10−6 at 25 °C.
From these results, we here propose that the domain fusion is attributed to the synergism of two plausible mechanisms: (i) increased line tension at the phase boundary due to the local membrane thinning and (ii) subsequent global increase in lateral tension of the membrane (Fig. 5). Raft domains are thicker than the surrounding ld domain, which causes local deformation of the lipid bilayer resulting in the high line tension at the phase boundary.13 The bilayer structure at the boundary is highly deformed and disordered. This effect creates packing defects in the membrane and provides binding sites for curcumin. It has been previously reported that curcumin causes thinning of DOPC bilayers at a low curcumin concentration ([curcumin] / [DOPC] < 1:50).14 This suggests that the binding of curcumin to the phase boundary might induce thinning of the DOPC-rich domain around the boundary, increasing the difference in the membrane thickness. This would increase the line tension, triggering domain fusion to reduce the total length of the domain boundary and minimize the interface energy in whole system. Ganglioside GM1, which is localized in the lo phase, is also known to modulate the raft domain.15 However, in contrast to curcumin, ganglioside GM1 requires relatively large quantity (2 mole %) for the raft modulation. Thus, significantly small quantity of curcumin for the raft fusion is originated in the boundary-specific binding of curcumin. Interestingly, dimethoxycurcumin, which lacks hydrophilic hydroxyl groups of curcumin, induced the rupture of GUVs in 7 minutes without the fusion of domains (see ESI†, Fig. S8), indicating that the hydroxyl groups are necessary for modulating the domain structure. This is probably due to the interaction between the hydroxyl groups of curcumin and lipid head groups,16 which might enhance the specific binding to the lipid packing defects at the domain boundaries.
Fig. 5.
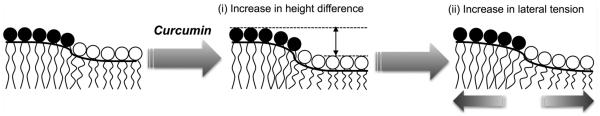
Proposed mechanism of curcumin-induced raft domain fusion. The fusion was driven by a synergetic effect of local membrane thinning of ld domain (represented by white lipid headgroup) and a global increase in lateral tension of membrane.
The domain fusion by curcumin increases the global lateral tension of the membrane; the decrease in the surface pressure of lipid monolayer (see ESI† Fig. S6) results from an increase in the surface tension of the monolayer because the surface tension of water / air interface without lipids is constant. The reduced surface pressure is likely due to the global reduction of average molecular area of lipids in the membrane, associated with the decrease in the number of deformed lipids at the boundary as the raft domains are fused. The increase in the lateral tension of the membrane further increases the line tension at the domain boundary, enhancing the domain fusion.17 As shown above, curcumin induced domain fusion in the planar lipid bilayer at the lower curcumin/lipid ratio than in the GUVs. The planar lipid bilayer may yield a high lateral tension due to the strong interaction of lipid membrane with a glass substrate as compared to GUVs where the lipid bilayer is closed. Therefore, the higher lateral tension could induce the domain fusion more effectively in the planar lipid bilayer than GUVs. Accordingly, the domain fusion by curcumin could be driven by synergetic effect of the membrane thinning at the domain boundaries and sub-sequent global lateral tension.
In conclusion, we found that curcumin modulates the lipid raft domains in the membrane effectively at significantly low concentrations by the specific attacking to the boundary of raft and non-raft domains. Boundary specific action of curcumin may explain the mystery that very low concentration of curcumin is needed to expresses a variety of pharmacological activities in the body.6 Although it is beyond the scope of this study, it will be of interest if other molecules with resembled structures or natural products in traditional medicines would exhibit their biological activities by acting on membranes. In addition, boundary-targeting molecule is expected to open a novel chemical approach to control raft-related cellular events as well as the molecular therapeutics.
Supplementary Material
Acknowledgments
This work was supported in part by a Grant-in-aid for Young Scientists A (K. Y., No. 24681028) and Challenging Exploratory Research (K. Y., No. 25650053) from the Japan Society for the Promotion of Science (JSPS), NSF CAREER Award (K. K., DMR-0845592), Support from Department of Biologic and Materials Sciences, University of Michigan School of Dentistry (K. K.), and NIH grants (A. R., GM084018 and GM095640).
Footnotes
Electronic Supplementary Information (ESI) available: Experimental, additional microscopic images and Langmuir monolayer experiments. See DOI: 10.1039/c000000x/
References
- 1.Simons K, Ikonen E. Nature. 1997;387:569. doi: 10.1038/42408. [DOI] [PubMed] [Google Scholar]
- 2.Simons K, Toomre D. Nat. Rev. Mol. Cell Biol. 2000;1:31. doi: 10.1038/35036052. [DOI] [PubMed] [Google Scholar]
- 3.Surma MA, Klose C, Simons K. Biochim. Biophys. Acta. 2012;1821:1059–1067. doi: 10.1016/j.bbalip.2011.12.008. [DOI] [PubMed] [Google Scholar]
- 4.Ikonen E. Curr. Opin. Cell Biol. 2001;13:470. doi: 10.1016/s0955-0674(00)00238-6. [DOI] [PubMed] [Google Scholar]
- 5.(a) Michel V, Bakovic M. Biol. Cell. 2007;99:129. doi: 10.1042/BC20060051. [DOI] [PubMed] [Google Scholar]; (b) Simons K, Ehehalt RJ. Clin. Invest. 2002;1102:597. doi: 10.1172/JCI16390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.(a) Joe B, Vijaykumar M, Lokesh BR. Crit. Rev. Food Sci. Nutr. 2004;44:97. doi: 10.1080/10408690490424702. [DOI] [PubMed] [Google Scholar]; (b) Ono K, Hasegawa K, Naiki H, Yamada M. J. Neurosci. Res. 2004;75:742. doi: 10.1002/jnr.20025. [DOI] [PubMed] [Google Scholar]; (c) De Clercq E. Med. Res. Rev. 2000;20:323. doi: 10.1002/1098-1128(200009)20:5<323::aid-med1>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]; (d) Goel A, Kunnumakkara AB, Aggarwal BB. Biochem. Pharmacol. 2008;75:787. doi: 10.1016/j.bcp.2007.08.016. [DOI] [PubMed] [Google Scholar]; (e) Strimpakos AS, Sharma RA. Redox Signal. 2008;10:511. doi: 10.1089/ars.2007.1769. [DOI] [PubMed] [Google Scholar]; (f) Jagetia GC, Aggarwal BB. J. Clin. Immunol. 2007;27:195. doi: 10.1007/s10875-006-9066-7. [DOI] [PubMed] [Google Scholar]
- 7.(a) Aggarwal BB, Kumar A, Aggarwal MS, Shishodia S. In: Cancer Chemoprevention. Bagchi D, Preuss HG, editors. CRC Press; Boca Raton, FL, USA: 2005. p. 349. [Google Scholar]; (b) Berger AL, Randak CO, Ostedgaard LS, Karp PH, Vermeer DW, Welsh MJ. J. Biol. Chem. 2005;280:5221. doi: 10.1074/jbc.M412972200. [DOI] [PubMed] [Google Scholar]
- 8.Ingolfsson HI, Koeppe RE, Anderson OS. Biochemistry. 2007;46:10384. doi: 10.1021/bi701013n. [DOI] [PubMed] [Google Scholar]
- 9.Dietrich C, Bagatolli LA, Volovyk ZN, Thompson NL, Levi M, Jacobson K, Gratton E. Biophys. J. 2001;80:1417. doi: 10.1016/S0006-3495(01)76114-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baumgart T, Hunt G, Farkas E, Webb W, Feigenson G. Biochim. Biophys. Acta. 2007;1768:2182. doi: 10.1016/j.bbamem.2007.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Veatch SL, Keller SL. Biophys. J. 2003;85:3074. doi: 10.1016/S0006-3495(03)74726-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Karewicz A, Bielska D, Gzyl-Malcher B, Kepczynski M, Lach R, Nowakowska M. Colloids Surf B Biointerfaces. 2011;88:231. doi: 10.1016/j.colsurfb.2011.06.037. [DOI] [PubMed] [Google Scholar]
- 13.Kuzmin PI, Akimov SA, Chizmadzhev YA, Zimmerberg J, Cohen FS. Biophys. J. 2005;88:1120. doi: 10.1529/biophysj.104.048223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hung WC, Chen FY, Lee CC, Sun Y, Lee MT, Huang HW. Biophys. J. 2008;94:4331. doi: 10.1529/biophysj.107.126888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Akimov SA, Hlaponin EA, Bashkirov PV, Boldyrev IA, Mi-khalyov II, Telford WG, Molotkovskaya IM. Biochemistry (Moscow) Suppl. Ser. A: Membr. Cell Biol. 2009;3:216. [Google Scholar]
- 16.Barry J, Fritz M, Brender JR, Smith PE, Lee DK, Ramamoorthy A. J. Am. Chem. Soc. 2009;131:4490. doi: 10.1021/ja809217u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Akimov SA, Kuzmin PI, Zimmerberg J, Cohen FS. Phys. Rev. E. 2007;75:011919. doi: 10.1103/PhysRevE.75.011919. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.