Abstract
Background
Our previous analysis of papillary thyroid carcinomas (PTC) from the Ukrainian-American cohort exposed to 131I from the Chernobyl accident found RET/PTC rearrangements and other driver mutations in 60% of tumors.
Methods
In this study, we analyzed the remaining, mutation-negative tumors using RNA-Seq and RT-PCR to identify novel chromosomal rearrangements and characterize their relationship with radiation dose.
Results
The ETV6-NTRK3 rearrangement was identified by RNA-Seq in a tumor from a patient who received a high 131I dose. Overall, it was detected in 9/62 (14.5%) of post-Chernobyl and in 3/151 (2%) of sporadic PTCs (p=0.019). The most common fusion type was between exon 4 of ETV6 and exon 14 of NTRK3. The ETV6-NTRK3 prevalence in post-Chernobyl PTCs was associated with increasing 131I dose, albeit at borderline significance (p=0.126). The group of rearrangement-positive PTCs (ETV6-NTRK3, RET/PTC, PAX8-PPARγ) was associated with significantly higher dose response compared to the group of PTCs with point mutations (BRAF, RAS) (p<0.001). In vitro exposure of human thyroid cells to 1 Gy of 131I and γ-radiation resulted in the formation of ETV6-NTRK3 with a rate of 7.9 × 10−6 and 3.0 ×10−6 cells, respectively.
Conclusions
We report here the occurrence of ETV6-NTRK3 rearrangements in thyroid cancer and show that this rearrangement is significantly more common in tumors associated with exposure to 131I and has a borderline significant dose response. Moreover, ETV6-NTRK3 can be directly induced in thyroid cells by ionizing radiation in vitro and therefore may represent a novel mechanism of radiation-induced carcinogenesis.
Keywords: thyroid cancer, radiation, chromosomal rearrangements, NTRK3, Chernobyl
INTRODUCTION
Thyroid cancer is the most common type of endocrine malignancy, and its incidence has been steadily growing in the U.S. and many other countries during the last four decades.1 Exposure to ionizing radiation during childhood is a well-established risk factor for thyroid cancer. The increased risk of thyroid cancer after radiation exposure was first suggested in 1950 in infants who received external beam radiation for enlarged thymus.2 This has been later confirmed in multiple studies of patients exposed to environmental or medical radiation, including X-ray or γ-radiation as wells as radioiodines, mainly iodine-131 (131I).3 In the decades following the 1986 Chernobyl accident, the surrounding geographic area experienced a marked increase in incidence of thyroid cancer among those who were children or young adults at the time of the accident.4 The post-Chernobyl case-control and cohort studies confirmed previous observations that papillary thyroid carcinoma (PTC) is the predominant type of thyroid cancer associated with radiation exposure,4–6 and that the risk for PTC following131I exposure increases with dose, with the magnitude of the increase comparable to that following external radiation.7–10
Activating mutations in the mitogen activated protein kinase (MAPK) signaling cascade are common in thyroid cancer and believed to be essential for tumorigenesis.11 The most common events include point mutations in the BRAF and RAS genes as well as chromosomal rearrangements involving the RET gene, known as RET/PTC. However, the mutational mechanisms leading to MAPK activation in sporadic and radiation-related PTCs appear to be different. Whereas in sporadic tumors, point mutations in BRAF and RAS genes are by far most common (∼60% of all PTCs), in post-Chernobyl or post-radiotherapy PTCs, 50–80% of tumors typically harbor chromosomal rearrangements of the RET gene known as RET/PTC whereas point mutations are rare.12–14 Other chromosomal rearrangements, such as AKAP9-BRAF and those involving the NTRK1 and PPARγ genes, are also more frequently found in radiation-associated PTCs.15,16 However, a significant proportion of radiation-associated tumors harbor none of the known mutations, suggesting that other, unknown genetic events may occur in these tumors.
In our recent study, we performed genotypic analysis of 62 PTCs from a well-characterized cohort of Ukrainian individuals (UkrAm) who received 0.008–8.6 Gy of 131I to the thyroid after the Chernobyl accident.15 The study confirmed the RET/PTC rearrangements as the most common genetic event in these tumors and found different trends with dose in PTCs harboring chromosomal rearrangements and point mutations. However, 40% of these tumors had none of the known genetic events and were further analyzed in this study using the RNA-Seq analysis to discover novel genetic events that might occur in radiation-related thyroid cancer. This analysis revealed the ETV6-NTRK3 chromosomal rearrangement, previously unknown to occur in thyroid cancer, is a common genetic event in radiation-related but not in sporadic thyroid cancer, and showed that ETV6-NTRK3 can be directly induced in human thyroid cells by ionizing radiation in vitro.
MATERIALS AND METHODS
Study cases and samples
The study was approved by the University of Pittsburgh, National Cancer Institute, and Institute of Endocrinology and Metabolism (IEM) (Kyiv, Ukraine) Institutional Review Boards. The cases of radiation-associated PTCs were diagnosed among individuals from the UkrAm study who were younger than 18 years old at the time of the Chernobyl accident.17 Individual radioactivity measurements in thyroid gland were performed within the two months following the accident and individual 131I thyroid doses were estimated based on these measurements, interview data concerning dietary and lifestyle habits, and environmental transfer models.18,19 PTC was diagnosed in 104 individuals (age at surgery: range, 14–32 years; mean, 22.7±5.1 years) between 1998 and 2008 at the Laboratory of Morphology of Endocrine System of the IEM, after four sequential screenings.20 Pathologic diagnoses were reviewed by the International Pathology Panel of the Chernobyl Tissue Bank (CTB). Frozen tissue samples were available for 75 cases of PTCs. For 74 PTCs DNA and/or RNA were extracted at IEM or Imperial College (London, UK) and received through the CTB. Four cases exposed to 131I in utero and 8 cases that lacked either DNA (n=3) or RNA (n=5) were excluded. In addition, a series of 151 consecutive sporadic PTC cases (age at surgery: range, 15–97 years; mean, 45.6±17.7 years) and additional 92 PTC cases (age at surgery: range, 4–77 years; mean, 44.2±16.7 years) previously found to be negative for known genetic alterations,21 were available through the University of Pittsburgh Health Sciences Tissue Bank (HSTB).
RNA-Seq and data analysis
Tumor RNA samples were processed to remove ribosomal RNA using the Ribozero Magnetic Gold kit (Illumina), followed by library preparation for RNA sequencing using the IlluminaTruSeq RNA Sample Preparation Kit v2. Briefly, polyadenylated RNA was fragmented, reverse transcribed, indexed, amplified and purified to produce individual barcoded libraries, according to the manufacturer’s instructions. The prepared libraries were assessed using a Bioanalyzer and the High Sensitivity DNA kit (Agilent). Paired-end sequencing was performed on Illumina HiSeq2000 at the High Throughput Genome Center at the Department of Pathology, University of Pittsburgh. Sequence reads obta were analyzed for gene fusion events using the ChimeraScan22,23 and deFuse24 programs. The predicted fusion events from the two programs were integrated and combined with genomic annotation to generate a list of candidate gene fusions. Before the analysis, sequences with low quality (base quality < 13) at both ends of reads were trimmed and trimmed reads with less than 25 bp were removed. The reference human genome (NCBI build 37.1, hg19) and gene annotation database (Ensembl v69 and UCSC hg19) were used for the analysis. To reduce false positive findings, the fusion events detected by both programs were further narrowed down by excluding (i) fusion events between adjacent genes (called as read-through), (ii) fusion events with no reads spanning the predicted breakpoints, and (iii) fusion events predicted to have five or more fusion partners and lacking specificity of target regions.
Detection of ETV6-NTRK3 fusions by RT-PCR
Tumor RNA was reverse transcribed and amplified using the following primers: 5’-CATTCTTCCACCCTGGAAAC-3’ (forward ETV6 exon 4), 5’-AAGCCCATCAACCTCTCTCA-3’ (forward ETV6 exon 5), 5’-TCCTCACCACTGATGACAGC-3’ (common reverse NTRK3). PCR product was analyzed by agarose gel electrophoresis. The presence of the fusion was confirmed by Sanger sequencing.
Cell irradiation and in vitro induction of ETV6-NTRK3 fusions
HTori-3 human thyroid cells25 were grown in RPMI1640 supplemented with 10% FBS. Cells were authenticated using the STRS analysis.26 For γ-radiation, 1×106 HTori-3 cells were exposed to 1Gy from 137Cs source (Gamma Cell 40 irradiator) at a dose-rate of 0.58 Gy per minute. For 131I irradiation, 1×106 HTori-3 cells in a T25 flask were incubated for 24 hours in 2 mL of culture media in the presence of NaI131 to deliver 1 Gy of radiation. Calculation of dose received by a monolayer of cells growing in the T25 flask and exposed to 1 Gy of 131Iwas performed based on Kernel integrations as previously described27 and was found to be 1.02 Gy per hour per 1 mCi. Two replicates of each experiment were performed. Delivery of radiation was monitored by formation of γH2AX nuclear foci using nnti-phosphorylated histone H2AX primary antibody (Upstate Biotechnology). Following γ or 131I irradiation, HTori-3 cells were split into thirty T25 flasks, transferred to T75 flasks for continuous growth, and harvested 9 days after irradiation. RNA was extracted using Trizol (Invitrogen), mRNA was purified using Oligotex mRNA kit (Qiagen). Following the reverse transcription step, multiplex PCR was performed to detect ETV6e4-NTRK3e14 and ETV6e5-NTRK3e14 rearrangements using the sequence specific primers described above. PCR products were resolved in the agarose gel and detected by Southern blot hybridization with 32P-labeled oligonucleotide probes specific for ETV6e4-NTRK3e14 (5’-ACCATGAAGAAGGTCCCGT-3’) and ETV6e5-NTRK3e14 (5’-AGAATAGCAGGTCCCGTGG-3’).
Statistical analysis
Univariate analyses were performed using the two-sample T-test. Mutation prevalence data was analyzed using standard logistic regression models as previously described. (18) Briefly, the following model was used to examine the probability of ETV6-NTRK3rearrangement with 131I dose D (in Gy) controlling for effect of age at surgery a, gender s, oblast (province) of residence O:
For some analyses quartic polynomials or categorical functions of age replaced the α1(a–25) term. Most analyses used log-linear functions of dose, D, but a few involved log-quadratic functions of dose. Twenty five years were subtracted from age (at surgery) to aid convergence of fitted models. All tests were two-sided and based on the likelihood-ratio test, and confidence intervals for the logistic regression analyses were derived from the profile likelihood.28 Likelihood-ratio tests were also used to assess heterogeneity by endpoint, using an extension of methods previously described.29 Linear regression analyses were performed using Stata and log-linear logistic regression analyses using Epicure.30
RESULTS
Identification and prevalence of ETV6-NTRK3 rearrangements in radiation-related and sporadic PTCs
The previous genotyping analysis of 62 post-Chernobyl PTCs from the UkrAm cohort identified driver mutations in 37 tumors (60%), whereas 25 (40%) of the tumors did not harbor any other mutations previously reported to occur in thyroid cancer.15 From this group, two tumors had a sufficient amount of RNA and were selected for whole-transcriptome (RNA-Seq) analysis to search for novel chromosomal rearrangements. One of these tumors was from a patient who was 1 year old at the time of the Chernobyl accident and received an estimated 131I dose of 7.5 Gy to the thyroid, among the highest doses in this series. Another tumor was from a patient who was 10 years old at the time of exposure and received an estimated 131I dose of 0.34 Gy. The analysis of PTC from the first patient revealed an in-frame fusion event between exon 4 of the ETS variant gene 6 (ETV6) and exon 14 of the neurotrophin receptor 3 (NTRK3) gene, which was detected by both programs, ChimeraScan and deFuse. The RNA-seq analysis of PTC sample from the second patient did not yield any promising gene fusions involving potential oncogenes. The presence of the ETV6-NTRK3 rearrangement was validated by RT-PCR and confirmed by Sanger sequencing (Fig. 1).
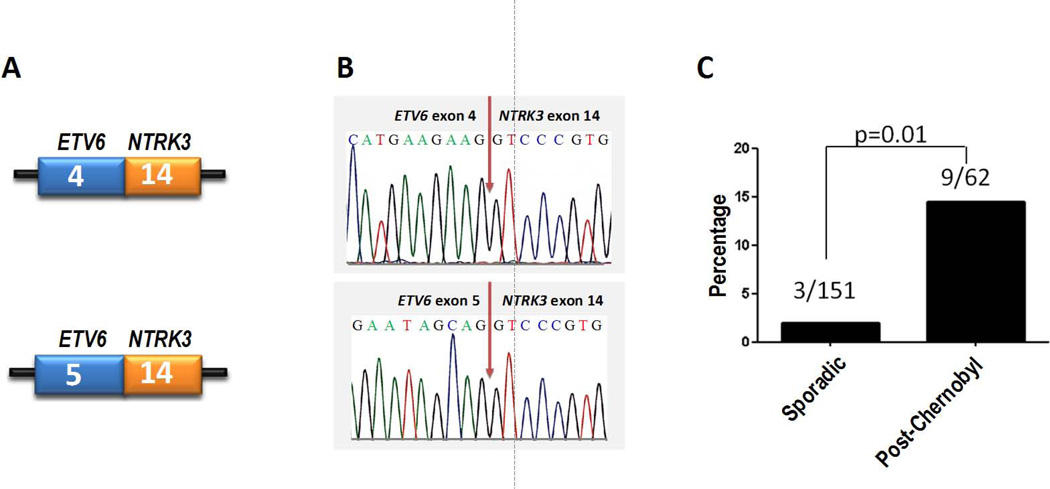
Figure 1.
ETV6-NTRK3 fusions identified in post-Chernobyl and sporadic thyroid tumors. (A) Schematic representation of the fusion point between exons of the two gene in mRNA. (B) Confirmation of ETV6-NTRK3 fusions by Sanger sequencing. (C) Frequency of ETV6-NTRK3 fusions in sporadic and post-Chernobyl PTCs.
Upon screening the rest of post-Chernobyl tumors using RT-PCR, 8 additional cases positive for ETV6-NTRK3 fusion were found, all involving exon 4 of ETV6 and exon 14 of NTRK3 genes. Overall, 9 out of 62 (14.5%) of post-Chernobyl PTCs harbored this rearrangement. One of these tumors also harbored BRAF V600E mutation and another tumor also had the RET/PTC1 rearrangement, whereas the remaining seven ETV6-NTRK3 positive tumors lacked known common driver mutations (BRAF, RAS, RET/PTC, or PAX8-PPARγ).
Screening of 151 consecutive sporadic PTCs from the general U.S. population revealed three positive cases, resulting in a prevalence of 2%. None of the three ETV6-NTRK3 positive sporadic tumors harbored other common driver mutations known to occur in thyroid cancer. The prevalence of ETV6-NTRK3 in post-Chernobyl PTCs was significantly higher than in sporadic PTCs, including both crude prevalence (p=0.01) and prevalence after adjustment for age and gender (p=0.019).
Analysis of additional 92 sporadic PTCs selected based on the lack of other known driver mutations identified 4 tumors positive for ETV6-NTRK3 (4.3%). Of the seven ETV6-NTRK3 rearrangements totally identified in sporadic PTCs, 6 involved the fusion of exon 4 of ETV6 to exon 14 of NTRK3 (ETV6e4-NTRK3e14) and one revealed a larger PCR product, which on Sanger sequencing was found to result from the fusion of exon 5 of ETV6 to exon 14 of NTRK3 (ETV6e5-NTRK3e14) (Fig. 1). None of the 7 patients with sporadic PTCs carrying ETV6-NTRK3 rearrangement was found to have a documented history of radiation exposure. Re-screening of post-Chernobyl tumors for ETV6e5-NTRK3e14 rearrangement revealed no additional positive cases. No ETV6-NTRK3 was found in TPC1 cell line established from PTC.
Exposure-related features of ETV6-NTRK3-positive tumors
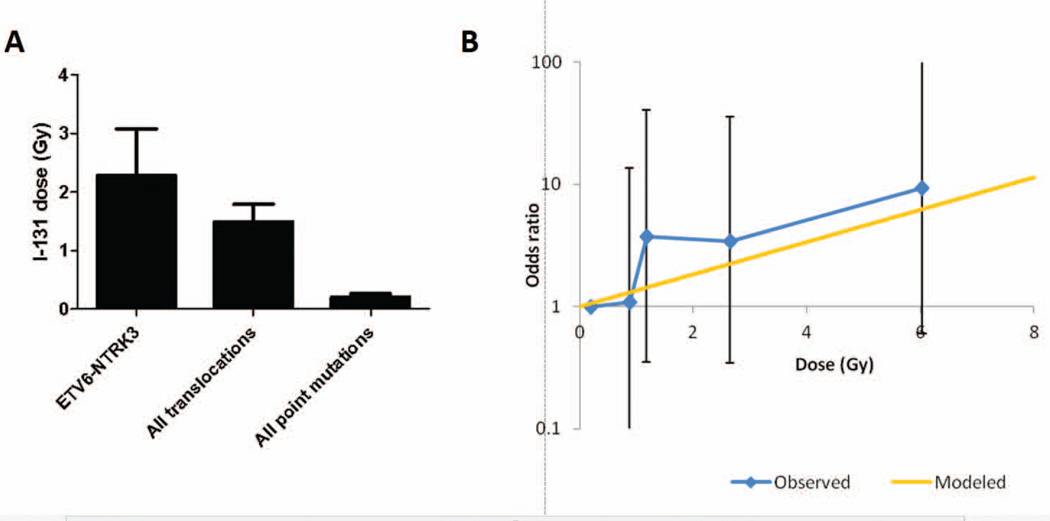
In 62 post-Chernobyl PTCs, an average thyroid dose received from 131I was 1.27 Gy. Among the ETV6-NTRK3-positive tumors (n=9), the average 131I dose was 2.27 Gy (Fig. 2A). The age of these individuals at the time of exposure ranged from 0.5 to 17.2 years (mean, 8.1 years) and the age at surgery ranged from 14.2 to 35.1 years (mean, 23.9 years) with the average time between exposure and surgery of 15.8 years (Table 1). In multivariate analysis (adjusting for age at surgery, gender, and place (oblast) of residence), tumors harboring the ETV6-NTRK3 rearrangement were found to be associated with increasing 131I dose, albeit at borderline significance (p=0.126) (Table 2). The dose response adjusted for these variables is shown in Fig. 2B.
Figure 2.
Exposure-related characteristics of ETV6-NTRK3-positive tumors. (A) Mean 131I thyroid dose received after the Chernobyl accident by individuals who subsequently developed PTC carrying specific molecular alterations. (B) Observed and modeled dose response for post-Chernobyl PTCs adjusted for age at surgery, gender and place of residence.
Table 1.
131I dose and other exposure-related characteristics by mutation type in post-Chernobyl papillary thyroid cancer
| Genetic Alteration | N (%)* |
131I dose, mean (Gy) |
Age at exposure, mean (yrs) |
Age at surgery, mean (yrs) |
Latency, mean (yrs) |
|---|---|---|---|---|---|
| BRAF | 9 (14.5%) | 0.27 | 10.2 | 27.0 | 16.8 |
| RAS | 6 (9.7%) | 0.21 | 10.9 | 29.5 | 18.6 |
| PAX8-PPARγ | 2 (3%) | 0.62 | 12.2 | 25.8 | 13.5 |
| RET/PTC | 22 (36%) | 1.22 | 6.4 | 22.3 | 15.9 |
| ETV6-NTRK3 | 9 (14.5%) | 2.27 | 8.1 | 23.9 | 15.8 |
| None | 18 (29%) | 1.71 | 7.9 | 24.6 | 16.7 |
| Total | 62 | 1.27 | 8.1 | 24.5 | 16.5 |
Four tumors had two mutations each: BRAF and NRAS, NRAS and PAX8-PPARγ, BRAF and ETV6-NTRK3, RET/PTC1 and ETV6-NTRK3.
Table 2.
Dose response for different groups of mutations in post-Chernobyl papillary thyroid cancer*
| Genetic Alteration | Risk*** Gy−1 (95% CI) | p |
| Assessment of trend | ||
| ETV6-NTRK3 | 0.30 (−0.09, 0.74) | 0.1263** |
| Assessment of heterogeneity (log-linear dose response) | ||
| All translocations | 0.09 (−0.24,0.46) | <0.0001^ |
| All point mutations (BRAF, NRAS, HRAS) |
−3.29 (−6.06, −1.38) | |
| Assessment of heterogeneity (log linear-quadratic dose response for translocations) | ||
| All translocations (linear term) | 0.77 (−0.07,1.69) | <0.0001^ |
| All point mutations (BRAF, NRAS, HRAS) |
−3.20 (−5.94, −1.32) | |
All analyses adjusted for age at surgery, gender, and oblast.
p-value for linear trend.
p-value for heterogeneity in linear terms.
Based on log-linear and log-linear quadratic models.
When the ETV6-NTRK3-positive tumors were grouped with tumors that harbored other types of chromosomal rearrangements, i.e. RET/PTC orPAX8/PPARγ, rearrangement-positive tumors were associated with a significantly higher dose response compared to the tumors with point mutations (BRAF, NRAS, HRAS) adjusting for age, gender, and oblast (p<0.0001) (Table 2). Specifically, the adjusted excess odds ratio (EOR) per Gy for all chromosomal rearrangements was 0.09 (95% CI: −0.24, 0.46) while that for point mutations was −3.29 (95% CI: −6.06, −1.38) (Table 2). There was a similar degree of heterogeneity (p<0.0001) if adjustment was also made for a possible quadratic term in the dose response for rearrangements.
Histopathologic features of ETV6-NTRK3-positive PTCs
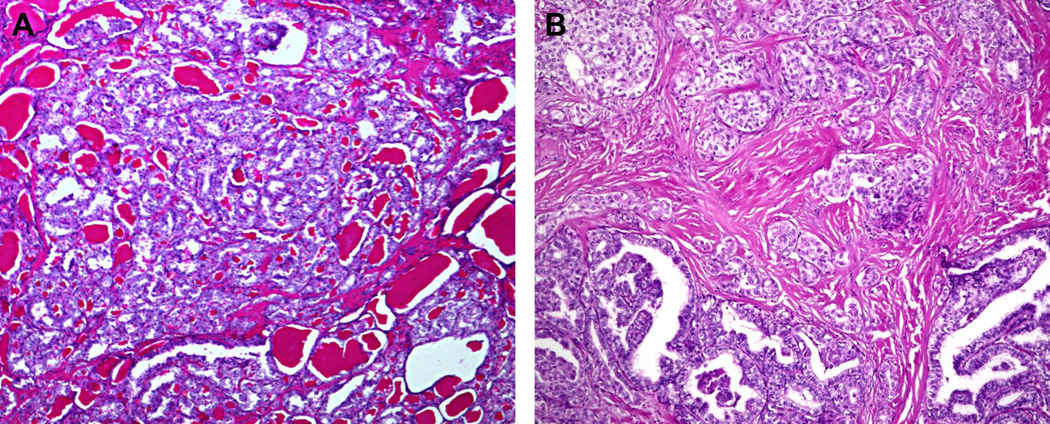
The majority (6/9, 67%) of post-Chernobyl PTCs found to have the ETV6-NTRK3 rearrangement demonstrated a follicular growth pattern and were classified as the follicular variant of PTC (Fig. 3A). The remaining tumors (3/9, 33%) had a significant papillary component and were classified as classic papillary type of PTC (Fig. 3B). All nine tumors showed some component of a solid growth pattern, comprising approximately 10% of the examined tumor in seven cases and 20–30% of the examined tumor in two cases (Fig. 3B). Among sporadic PTCs positive for ETV6-NTRK3rearrangement, four were classic papillary type (57%) and three were follicular variant PTC (43%). All four classic papillary PTCs had a significant component of follicular growth, but no well-defined solid component was observed in any of the sporadic tumors. Among radiation-associated tumors, all were AJCC/UICC stage I at presentation, whereas among sporadic tumors, 5 were stage I and two were stage III based on the presence of minimal extrathyroidal extension in patients over the age of 45.
Figure 3.
Histopathologic features of ETV6-NTRK3-positive tumors. (A) Follicular variant of PTC showing follicular growth pattern and no well-formed papillary structures. (B) Classic papillary type of PTC showing well-formed papillae (bottom) and focal areas of solid growth (top).
In vitro induction of ETV6-NTRK3 rearrangements by ionizing radiation
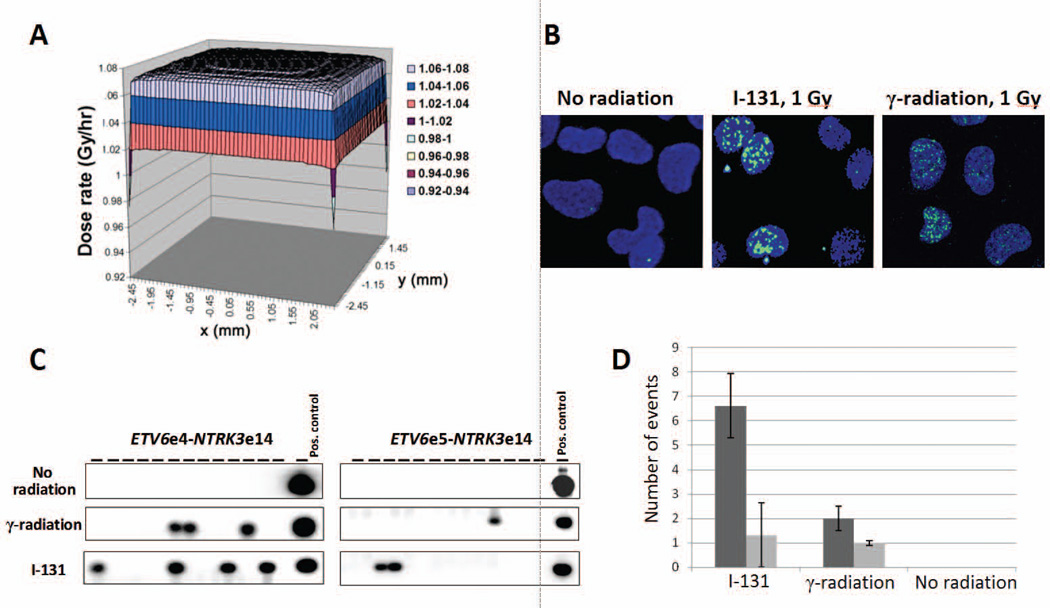
High prevalence of ETV6-NTRK3 rearrangement in radiation-associated PTCs and its association with high thyroid dose suggested that this rearrangement could be induced by ionizing radiation. To test this possibility, we studied the induction of ETV6-NTRK3 rearrangement in HTori-3 human thyroid cells after exposure to 1 Gy of 131I or γ-radiation. The 131I dose distribution in the monolayer of cells within a T25 flask generated by Kernel integration is shown in Fig. 4A. Induction of double-stranded DNA breaks by radiation was monitored by formation of γH2AX nuclear foci (Fig. 4B).Whereas no rearrangements were observed in the unexposed cells, both radiation types resulted in the generation of ETV6-NTRK3 rearrangements (Fig. 4C).The average rate of ETV6-NTRK3 induction was 7.9 ×10−6 cells per 1 Gy of 131I and 3.0 × 10−6 per 1 Gy of γ-radiation. Both ETV6e4-NTRK3e14 and ETV6e5-NTRK3e14 fusions were induced by ionizing radiation, with the predominance of ETV6e4-NTRK3e14 after both 131I and γ-radiation (Fig. 4D).
Figure 4.
In vitro induction of ETV6-NTRK3 rearrangements by radiation exposure. (A) Distribution of the 131I dose in the monolayer of cells within a T25 flask generated by Kernel integration. (B) Induction of DSBs by ionizing radiation as evident by formation of gH2AX nuclear foci. (C) Identification of ETV6-NTRK3 rearrangements in human thyroid cells after exposure to 1 Gy of 131I or γ-radiation. (D) Frequency of specific types of ETV6-NTRK3 rearrangements induced in vitro by 131I and g-radiation.
DISCUSSION
In this study, we report for the first time the occurrence of the ETV6-NTRK3 chromosomal rearrangement in thyroid cancer, which was found to be common in PTCs associated with 131I exposure from the Chernobyl accident. In fact, in this well-characterized series of radiation-related thyroid cancer, ETV6-NTRK3 was the second most common rearrangement type after RET/PTC. Moreover, this study demonstrates that this rearrangement can be directly induced in human thyroid cells in vitro by exposure to both 131I and γ-radiation.
The fusion between the ETV6 gene on chromosome 12 and the NTRK3 gene on chromosome 15 was first described in congenital fibrosarcoma in 1998.31 Since then, ETV6-NTRK3 rearrangements have been found in several other tumor types including acute myeloid leukemia (AML),32,33 chronic eosinophilic leukemia (CEL),34 congenital mesoblastic nephroma, 35 secretory breast carcinoma,36 and mammary analogue secretory carcinoma of the salivary gland.37 ETV6, also known as TEL, is a transcription factor from the ETS transcription factor family, which is involved in various oncogenic gene fusions resulting from chromosomal translocations, mostly reported in subtypes of AML. NTRK3 is a transmembrane receptor tyrosine kinase, for which ligand is neurotrophin-3, which is primarily involved in neuronal cellular processes.38 The rearrangement results in fusion of the SAM domain of ETV6, which is required for dimerization, with the tyrosine kinase domain of NTRK3, such that the transcribed product is a constitutively active tyrosine kinase.38
Most of ETV6-NTRK3 fusions that occur in various tumor types involve the fusion point initially identified in congenital fibrosarcoma, i.e. between exon 5 of ETV6 and exon 13 of NTRK3 genes31 A shorter variant, in which exon 4 of ETV6 is fused to NTRK3, has been found in cases of AML and CEL.32,34 Here, we report the occurrence of ETV6-NTRK3 rearrangements in radiation-related and sporadic PTCs, with fusion points that differ from those previously identified in other tumor types as they lack exon 13 of NTRK3.
Our results provide several lines of evidence that link ETV6-NTRK3 rearrangements in papillary thyroid cancer with radiation exposure. First, this rearrangement is found with a significantly higher prevalence in PTC’s of patients exposed to 131I from the Chernobyl accident than in PTCs arising in the general U.S. population. Second, among post-Chernobyl PTCs we observed a borderline statistically significant association between 131I dose and prevalence of ETV6-NTRK3 rearrangement. Finally, our in vitro experiments demonstrated the induction of both types of ETV6-NTRK3 fusions in human thyroid cells by radiation. Both 131I and γ-radiation were efficient at inducing this rearrangement in cultured human cells. This suggests that, in addition to patients exposed to 131I after the Chernobyl accident, thyroid cancers developing after external beam radiation therapy may also harbor these rearrangements.
Among post-Chernobyl tumors in this study, ETV6-NTRK3-positive PTCs arose in individuals exposed to an average 131I dose of 2.3 Gy, higher than the average dose received by patients who developed PTCs driven by other oncogenes. The average age of patients with PTCs positive for ETV6-NTRK3 was about 8 years old at the time of the accident and 24 years old at the time of surgery, resulting in the average time between exposure and thyroid surgery of 16 years. The UkrAm study includes individuals followed since 1998, and therefore it remains unknown whether ETV6-NTRK3 may also be common in tumors that developed less than 12 years after the accident. Of note, a sharp increase in thyroid cancer incidence in the area surrounding the Chernobyl nuclear power plant was observed as early as 4–6 years following the accident.39
Phenotypically, both radiation-associated and sporadic tumors harboring ETV6-NTRK3rearrangement were either follicular variant of PTC or classic papillary cancer. Interestingly, the presence of solid growth pattern was a microscopic feature found only in radiation-associated PTCs carrying this rearrangement. We and others have previously demonstrated a common presence of solid growth pattern in post-Chernobyl tumors as compared to sporadic PTCs, and the association of this growth pattern with RET/PTC3 rearrangement.40,41 The results of this study suggest that ETV6-NTRK3 may represent another type of chromosomal rearrangement associated with the solid growth pattern of PTC in patients exposed to radiation.
Findings in this study provide additional evidence for association between specific types of mutations and etiologic factors implicated in the development of thyroid cancer. Previous studies have shown that thyroid cancers developing after exposure to ionizing radiation have a high prevalence of chromosomal rearrangements such as RET/PTC and AKAP9-BRAF, and low prevalence of point mutations.12–14 The results of this study extend evidence supporting such as association, adding ETV6-NTRK3 to the list of fusions that preferentially occur in patients exposed to radiation and that can be induced by radiation in vitro.
The association between ETV6-NTRK3 and radiation exposure of thyroid gland found in this study raises a possibility that these fusion observed in other cancer types may also be related to radiation exposure. This is particularly plausible for AML, which has a strong dose-dependent relationship with environmental or medical irradiation.42
In summary, we report here the occurrence of ETV6-NTRK3 rearrangement in papillary thyroid cancer and show that this is common event in thyroid tumors associated with 131I radiation exposure. Moreover, we demonstrate that this rearrangement can be directly induced by 131I or γ-radiation in vitro and therefore may represent a novel molecular mechanism contributing to the development of radiation-induced thyroid cancer.
Acknowledgements
We want to that to the staff of the University of Pittsburgh Health Sciences Tissue Bank (HSTB) for providing samples of sporadic thyroid tumors used in this study. We are grateful to Dr. Geraldine Thomas (Imperial College, London, UK) for her valuable assistance and support of the study. The authors gratefully acknowledge the confirmation of diagnoses provided by the International Pathology Panel of the Chernobyl Tissue Bank. We are also grateful to our dosimetry colleagues, including Drs. Likhtarev, IA, Kovgan, LN, Bouville, A and Drozdovitch, V., for their valuable contributions to I-131 thyroid dose estimates.
Financial support: This work was supported by the NIH grant R01 CA88041 and in part by the Intramural Research Program of the NIH, National Cancer Institute.
Footnotes
Conflict of interest, financial disclosures: The authors declare no actual, potential, or perceived conflict of interest nor any financial disclosures.
REFERENCES
- 1.Aschebrook-Kilfoy B, Schechter RB, Shih YC, et al. The clinical and economic burden of a sustained increase in thyroid cancer incidence. Cancer Epidemiol Biomarkers Prev. 2013;22:1252–1259. doi: 10.1158/1055-9965.EPI-13-0242. [DOI] [PubMed] [Google Scholar]
- 2.Duffy BJ, Jr, Fitzgerald PJ. Cancer of the thyroid in children: a report of 28 cases. J Clin Endocrinol Metab. 1950;10:1296–1308. doi: 10.1210/jcem-10-10-1296. [DOI] [PubMed] [Google Scholar]
- 3.Ron E, Lubin JH, Shore RE, et al. Thyroid cancer after exposure to external radiation: a pooled analysis of seven studies. Radiat Res. 1995;141:259–277. [PubMed] [Google Scholar]
- 4.Stsjazhko VA, Tsyb AF, Tronko ND, Souchkevitch G, Baverstock KF. Childhood thyroid cancer since accident at Chernobyl. BMJ. 1995;310:801. doi: 10.1136/bmj.310.6982.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.LiVolsi VA, Abrosimov AA, Bogdanova T, et al. The Chernobyl thyroid cancer experience: pathology. Clin Oncol (R Coll Radiol) 2011;23:261–267. doi: 10.1016/j.clon.2011.01.160. [DOI] [PubMed] [Google Scholar]
- 6.Pacini F, Vorontsova T, Demidchik EP, et al. Post-Chernobyl thyroid carcinoma in Belarus children and adolescents: comparison with naturally occurring thyroid carcinoma in Italy and France. J Clin Endocrinol Metab. 1997;82:3563–3569. doi: 10.1210/jcem.82.11.4367. [DOI] [PubMed] [Google Scholar]
- 7.Brenner AV, Tronko MD, Hatch M, et al. I-131 dose response for incident thyroid cancers in Ukraine related to the Chornobyl accident. Environ Health Perspect. 2011;119:933–939. doi: 10.1289/ehp.1002674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cardis E, Kesminiene A, Ivanov V, et al. Risk of thyroid cancer after exposure to 131I in childhood. J Natl Cancer Inst. 2005;97:724–732. doi: 10.1093/jnci/dji129. [DOI] [PubMed] [Google Scholar]
- 9.Davis S, Stepanenko V, Rivkind N, et al. Risk of thyroid cancer in the Bryansk Oblast of the Russian Federation after the Chernobyl Power Station accident. Radiat Res. 2004;162:241–248. doi: 10.1667/rr3233. [DOI] [PubMed] [Google Scholar]
- 10.Tronko MD, Howe GR, Bogdanova TI, et al. A cohort study of thyroid cancer and other thyroid diseases after the chornobyl accident: thyroid cancer in Ukraine detected during first screening. J Natl Cancer Inst. 2006;98:897–903. doi: 10.1093/jnci/djj244. [DOI] [PubMed] [Google Scholar]
- 11.Nikiforov YE, Nikiforova MN. Molecular genetics and diagnosis of thyroid cancer. Nat Rev Endocrinol. 2011;7:569–580. doi: 10.1038/nrendo.2011.142. [DOI] [PubMed] [Google Scholar]
- 12.Bounacer A, Wicker R, Caillou B, et al. High prevalence of activating ret proto-oncogene rearrangements, in thyroid tumors from patients who had received external radiation. Oncogene. 1997;15:1263–1273. doi: 10.1038/sj.onc.1200206. [DOI] [PubMed] [Google Scholar]
- 13.Nikiforov YE, Rowland JM, Bove KE, Monforte-Munoz H, Fagin JA. Distinct pattern of ret oncogene rearrangements in morphological variants of radiation-induced and sporadic thyroid papillary carcinomas in children. Cancer Res. 1997;57:1690–1694. [PubMed] [Google Scholar]
- 14.Rabes HM, Demidchik EP, Sidorow JD, et al. Pattern of radiation-induced RET and NTRK1 rearrangements in 191 post-chernobyl papillary thyroid carcinomas: biological, phenotypic, and clinical implications. Clin Cancer Res. 2000;6:1093–1103. [PubMed] [Google Scholar]
- 15.Leeman-Neill RJ, Brenner AV, Little MP, et al. RET/PTC and PAX8/PPARgamma chromosomal rearrangements in post-Chernobyl thyroid cancer and their association with iodine-131 radiation dose and other characteristics. Cancer. 2013;119:1792–1799. doi: 10.1002/cncr.27893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ciampi R, Knauf JA, Kerler R, et al. Oncogenic AKAP9-BRAF fusion is a novel mechanism of MAPK pathway activation in thyroid cancer. J Clin Invest. 2005;115:94–101. doi: 10.1172/JCI23237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stezhko VA, Buglova EE, Danilova LI, et al. A cohort study of thyroid cancer and other thyroid diseases after the Chornobyl accident: objectives, design and methods. Radiat Res. 2004;161:481–492. doi: 10.1667/3148. [DOI] [PubMed] [Google Scholar]
- 18.Likhtarev I, Bouville A, Kovgan L, Luckyanov N, Voilleque P, Chepurny M. Questionnaire- and measurement-based individual thyroid doses in Ukraine resulting from the Chornobyl nuclear reactor accident. Radiat Res. 2006;166:271–286. doi: 10.1667/RR3545.1. [DOI] [PubMed] [Google Scholar]
- 19.Likhtarev I, Minenko V, Khrouch V, Bouville A. Uncertainties in thyroid dose reconstruction after Chernobyl. Radiat Prot Dosimetry. 2003;105:601–608. doi: 10.1093/oxfordjournals.rpd.a006310. [DOI] [PubMed] [Google Scholar]
- 20.Bogdanova TI, Zurnadzhy LY, Greenebaum E, et al. A cohort study of thyroid cancer and other thyroid diseases after the Chornobyl accident: pathology analysis of thyroid cancer cases in Ukraine detected during the first screening (1998–2000) Cancer. 2006;107:2559–2566. doi: 10.1002/cncr.22321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nikiforov YE, Ohori NP, Hodak SP, et al. Impact of mutational testing on the diagnosis and management of patients with cytologically indeterminate thyroid nodules: a prospective analysis of 1056 FNA samples. J Clin Endocrinol Metab. 2011;96:3390–3397. doi: 10.1210/jc.2011-1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maher CA, Kumar-Sinha C, Cao X, et al. Transcriptome sequencing to detect gene fusions in cancer. Nature. 2009;458:97–101. doi: 10.1038/nature07638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maher CA, Palanisamy N, Brenner JC, et al. Chimeric transcript discovery by paired-end transcriptome sequencing. Proc Natl Acad Sci U S A. 2009;106:12353–12358. doi: 10.1073/pnas.0904720106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McPherson A, Hormozdiari F, Zayed A, et al. deFuse: an algorithm for gene fusion discovery in tumor RNA-Seq data. PLoS Comput Biol. 2011;7:e1001138. doi: 10.1371/journal.pcbi.1001138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lemoine NR, Mayall ES, Jones T, et al. Characterisation of human thyroid epithelial cells immortalised in vitro by simian virus 40 DNA transfection. Br J Cancer. 1989;60:897–903. doi: 10.1038/bjc.1989.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schweppe RE, Klopper JP, Korch C, et al. Deoxyribonucleic acid profiling analysis of 40 human thyroid cancer cell lines reveals cross-contamination resulting in cell line redundancy and misidentification. J Clin Endocrinol Metab. 2008;93:4331–4341. doi: 10.1210/jc.2008-1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.White JS, Yue N, Hu J, Bakkenist CJ. The ATM kinase signaling induced by the low-energy beta-particles emitted by (33)P is essential for the suppression of chromosome aberrations and is greater than that induced by the energetic beta-particles emitted by (32)P. Mutat Res. 2011;708:28–36. doi: 10.1016/j.mrfmmm.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McCullagh P, Nelder JA. Generalized linear models. Monographs on statistics and applied probability: 37 Boca Raton, FL:Chapman and Hall/CRC. 1989:1–526. [Google Scholar]
- 29.Pierce DA, Preston DL. Joint analysis of site-specific cancer risks for the atomic bomb survivors. Radiat Res. 1993;134:134–142. [PubMed] [Google Scholar]
- 30.Preston DL, Lubin JH, Pierce DA, McConney ME. Epicure release 2.10. Seattle: Hirosoft International. 1998 [Google Scholar]
- 31.Knezevich SR, McFadden DE, Tao W, Lim JF, Sorensen PH. A novel ETV6-NTRK3 gene fusion in congenital fibrosarcoma. Nat Genet. 1998;18:184–187. doi: 10.1038/ng0298-184. [DOI] [PubMed] [Google Scholar]
- 32.Eguchi M, Eguchi-Ishimae M, Tojo A, et al. Fusion of ETV6 to neurotrophin-3 receptor TRKC in acute myeloid leukemia with t(12;15)(p13;q25) Blood. 1999;93:1355–1363. [PubMed] [Google Scholar]
- 33.Setoyama M, Tojo A, Nagamura F, et al. A unique translocation of the TEL gene in a case of acute myelogenous leukemia with inv(12)(p13q15) Blood. 1998;92:1454–1455. [PubMed] [Google Scholar]
- 34.Forghieri F, Morselli M, Potenza L, et al. Chronic eosinophilic leukaemia with ETV6-NTRK3 fusion transcript in an elderly patient affected with pancreatic carcinoma. Eur J Haematol. 2011;86:352–355. doi: 10.1111/j.1600-0609.2011.01576.x. [DOI] [PubMed] [Google Scholar]
- 35.Rubin BP, Chen CJ, Morgan TW, et al. Congenital mesoblastic nephroma t(12;15) is associated with ETV6-NTRK3 gene fusion: cytogenetic and molecular relationship to congenital (infantile) fibrosarcoma. Am J Pathol. 1998;153:1451–1458. doi: 10.1016/S0002-9440(10)65732-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tognon C, Knezevich SR, Huntsman D, et al. Expression of the ETV6-NTRK3 gene fusion as a primary event in human secretory breast carcinoma. Cancer Cell. 2002;2:367–376. doi: 10.1016/s1535-6108(02)00180-0. [DOI] [PubMed] [Google Scholar]
- 37.Skalova A, Vanecek T, Sima R, et al. Mammary analogue secretory carcinoma of salivary glands, containing the ETV6-NTRK3 fusion gene: a hitherto undescribed salivary gland tumor entity. Am J Surg Pathol. 2010;34:599–608. doi: 10.1097/PAS.0b013e3181d9efcc. [DOI] [PubMed] [Google Scholar]
- 38.Lannon CL, Sorensen PH. ETV6-NTRK3: a chimeric protein tyrosine kinase with transformation activity in multiple cell lineages. Semin Cancer Biol. 2005;15:215–223. doi: 10.1016/j.semcancer.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 39.Likhtarev IA, Sobolev BG, Kairo IA, et al. Thyroid cancer in the Ukraine. Nature. 1995;375:365. doi: 10.1038/375365a0. [DOI] [PubMed] [Google Scholar]
- 40.Furmanchuk AW, Averkin JI, Egloff B, et al. Pathomorphological findings in thyroid cancers of children from the Republic of Belarus: a study of 86 cases occurring between 1986 ('post-Chernobyl') and 1991. Histopathology. 1992;21:401–408. doi: 10.1111/j.1365-2559.1992.tb00423.x. [DOI] [PubMed] [Google Scholar]
- 41.Nikiforov Y, Gnepp DR. Pediatric thyroid cancer after the Chernobyl disaster. Pathomorphologic study of 84 cases (1991–1992) from the Republic of Belarus. Cancer. 1994;74:748–766. doi: 10.1002/1097-0142(19940715)74:2<748::aid-cncr2820740231>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 42.Hsu WL, Preston DL, Soda M, et al. The incidence of leukemia, lymphoma and multiple myeloma among atomic bomb survivors: 1950–2001. Radiat Res. 2013;179:361–382. doi: 10.1667/RR2892.1. [DOI] [PMC free article] [PubMed] [Google Scholar]