Summary
Background
Adenoviral vector-mediated gene therapy might have potential for long-term correction of the monogenic disease hemophilia A.
Objective
In this study, we tested the efficacy of administering a helper-dependent adenoviral vector (HDV) designed for maximal liver-restricted canine factor VIII (cFVIII) expression on three out-bred hemophilia A dogs.
Methods
Three FVIII-deficient animals from the University of North Carolina colony were injected with 1 × 1012 (Dog A), and 3 × 1012 (Dog B and C) vp kg−1 helper-dependent adenoviral vector, and we performed systematic analysis of toxicity, persistence of therapeutic gene expression, and molecular analysis of gene transfer.
Results
We observed acute dose-dependent elevation in liver enzymes and thrombocytopenia after injection, although both were transient and resolved within 2 weeks. The whole blood clotting time (WBCT), plasma FVIII concentration, FVIII activity, and activated partial thromboplastin time in all animals improved significantly after treatment, and two animals receiving a higher dose reached near normal WBCT with low-level FVIII activity until terminal sacrifice at 3 months, and 2 years. Importantly, the treated dogs suffered no bleeding events after injection. Moreover, we observed persistent vector-specific DNA and RNA in liver tissue collected from one high-dose animal at days 18 and 79, and could not detect the formation of inhibitory antibodies.
Conclusion
Although vector-associated toxicity remains an obstacle, a single injection of HDV led to long-term transgene expression and vector persistence in two FVIII-deficient animals with conversion of their severe phenotype to a moderate one.
Keywords: FVII, gene therapy, helper-dependent adenovirus, hemophilia A
Introduction
A deficiency in coagulation factor (F) VIII results in the bleeding disorder, hemophilia A (HA) [1]. Recombinant and plasma-derived FVIII protein therapy can effectively treat spontaneous bleeding in the joints, muscles and internal organs, leading to prolonged life expectancy [2–4]. However, this mode of therapy is costly, requires repeated administration, and is rendered ineffective by the emergence of FVIII-specific antibodies [5,6]. Gene therapy strategies are attractive treatment options because of the potential for sustained production of FVIII.
Adenoviral vectors can accommodate large cDNAs and exhibit efficient transduction of both dividing and non-dividing cells. Initial studies using adenovirus in hemophilic dogs were conducted with the human FVIII cDNA but expression was short term [7]. However, biphasic toxicity of earlier generation vectors (deleted for some, but not all, of the native viral coding DNA sequences) was likely responsible for the short-term expression [8,9]. Recent studies concluded that adenoviral vectors devoid of all viral coding sequences (Helper Dependent Vectors, HDV) alleviate the biphasic toxicity associated with low-level expression of viral genes [10–12]. Moreover, recent works suggest pegylation of virions [13–15], or possibly surgical hepatic isolation [16] could limit the dose requirement and thereby reduce the systemic toxicity associated with HDV administration. Therefore, combining the limited toxicity of HDV with advances in production, purification, and vector design suggests HDV-mediated gene transfer warrants further investigation for the treatment of HA [17].
When HDVs were used to deliver the canine FVIII transgene to a dog model of HA, plasma FVIII levels decreased significantly from peak levels over the course of a few weeks [18]. Although the reason for this is unclear, one possibility is that vector DNA remains episomal in transduced cells, and vector genome loss during cell division could account for the loss of plasma FVIII protein [19].
We investigated the efficacy of HDV-mediated gene therapy in the University of North Carolina canine model of severe HA [20]. We observed long-term phenotypic correction of FVIII-dependent clotting function, despite a significant decrease in plasma FVIII levels over several weeks. To determine whether this was because of vector genome loss, we performed liver biopsy on an HDV-treated dog at two separate time-points correlating with the peak of plasma FVIII and after it had decreased to minimal levels. The data show long-term presence of HDV-specific DNA and RNA correlates with partial WBCT and APTT correction, as well as the absence of spontaneous bleeding episodes.
Materials and methods
Preparation of helper-dependent Ad vector HDV-PEPCK/BDD-cFVIII/WPRE
The vector (HDV-PEPCK/BDD-cFVIII/WPRE, Fig. 1A) was prepared using the reagents and methods described in detail elsewhere [10,17]. Briefly, canine B-domain deleted-FVIII cDNA was cloned into the NotI restriction site of shuttle plasmid pGem7-PEPCK-WPRE. The shuttle plasmid contained the rat phosphoenolpyruvate carboxykinase (PEPCK) promoter with downstream elements in the following order: intronic DNA, multicloning site, woodchuck hepatitis virus post-transcriptional regulatory element (WPRE), and a human growth hormone poly-A [21]. The PEPCK promoter is tissue restricted and expresses largely in hepatocytes [22]. The WPRE element increases mRNA levels in tissues transduced with viral gene transfer vectors [21,23–25]. AscI digestion released the transgene cassette from the shuttle plasmid and was inserted into a unique AscI site on the pΔ28E4 HDV plasmid [26]. Virion DNA was analyzed by Southern blot and the level of helper virus contamination was determined by phosphorimager analysis as described [17]. Physical titer was determined spectrophotometrically and expressed as vector particles per milliliter (vp mL−1). Average titer for vector preparations was 7 × 1012 vp mL−1.
Fig. 1.
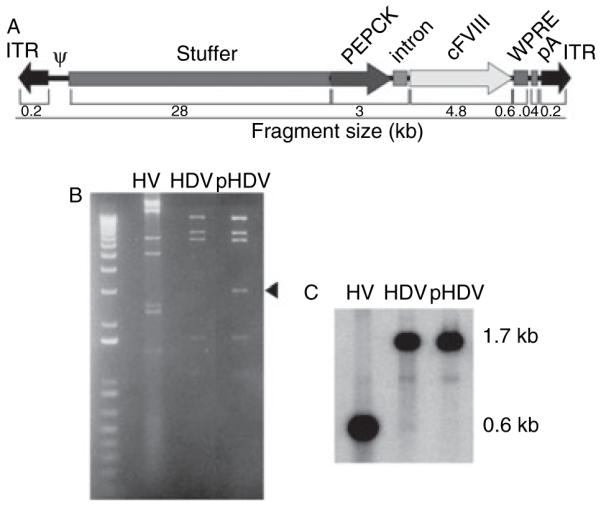
Construction and analysis of helper-dependent adenoviral vector HDV-PEPCK/BDD-cFVIII/WPRE. (A) Schematic of DNA used to construct HDV-PEPCK/BDD-cFVIII/WPRE. ψ is the viral packaging signal, and the inverted terminal repeats are the only native viral DNA in the vector. Stuffer DNA was derived from intronic human sequences [26]. The expression cassette contains the phosphoenolpyruvate carboxykinase (PEPCK) promoter, the ApoA1 intron, canine B-domain-deleted factor VIII cDNA, woodchuck hepatitis virus post-transcriptional regulatory element (WPRE), and a human growth hormone poly-A [21]. (B) ApaLI digest of helper-virus (HV), helper-dependent adenoviral vector (HDV), and PmeI/ApaLI digest of the cloning vector (pHDV), demonstrating no gross rearrangements, occurred during production. Arrowhead indicates plasmid-derived sequence. (C) Southern analysis of the same filter probed for the adenoviral packaging signal. In the HDV lane, comparison of the vector signal at 1.7 kb with the fragment containing the packaging signal at 0.6 kb by phosphoimager analysis reveals < 0.2% HV contamination.
Animal procedures
The animals used for these experiments were mixed breed dogs from the University of North Carolina at Chapel Hill colony. Animals were sedated with ketamine prior to the procedure. Next, HDV was diluted in phosphate buffered saline (PBS), and delivered by forelimb vein catheter over 50 min. The catheter was flushed with PBS following vector administration. During the administration of vector, no transfusion of cryoprecipitate occurred. Dog C underwent liver biopsies under general anesthesia via midline abdominal incision with direct inspection of the liver. Normal canine plasma was given prior to the procedure for hemostasis. Two biopsy samples were taken on day 18, and again on day 79 from two different areas of the liver. Separate DNA preparations are indicated in the figures as 18-1 and 18-2, 79-1 and 79-2, for each independent biopsy site.
All animals were treated in accordance with the standards set in Guide for the Care and Use of Laboratory Animals (National Institute of Health no. 85–23). The Institutional Animal Care and Use Committee approved all experiments.
Toxicity assays
All blood samples for toxicity and hemostatic measurements were obtained at indicated time-points by cephalic vein phlebotomy. Serum was prepared to quantify liver enzymes and other liver function studies including aspartate aminotransferase (AST), and alanine aminotransferase (ALT) (ANTECH Inc., Cary, NC, USA). Complete blood counts were performed on ethylenediaminetetra-acetic acid (EDTA)-anticoagulated blood with a cell counter (Heska Co., Fort Collins, CO, USA) calibrated for dogs.
Whole blood clotting times (WBCT)
This assay is performed by a two-tube procedure at 28 °C. One milliliter of whole blood is distributed equally between two siliconized tubes (Vacutainer™, #6431, Becton-Dickinson, Rutherford, NJ, USA). The first tube is tilted every 30 s. After a clot forms, the second tube is tilted and observed every 30 s. The endpoint measurement is the clotting time of the second tube. The WBCT was assayed prior to infusion of vector (baseline), at 1, 8, and 24 h, and weekly thereafter.
Activated partial thromboplastin time (APTT)
Activated partial thromboplastin time was determined on plasma samples from the indicated time-points by analysis in the ST4 coagulation instrument (Diagnostica Stago, Asnieres, France) or the Multiple Discrete Analyzer (MDA) 180 (Organon Teknika, Durham, NC, USA) that has the capacity to process rapidly a large number of samples. Whether APTTs are determined on the ST4 coagulation instrument, or the Orgenon Teknika MDA 180, the controls and reagents are of the same type. For the APTT test, mixtures consist of equal portions of partial tromboplastin (Automated APTT, Bio-Mérieux, Durham, NC, USA), 0.025 m CaCl2, and citrated test plasma [27,28]. Normal canine reference plasma consists of pools from 5–10 normal dogs from our inbred colony and a reference range is established for each preparation, usually 24–34 s.
Chromogenic assay for FVIII activity
Biologically active canine FVIII was measured in the dog plasma using the COATEST chromogenic bioassay according to the manufacturer’s specifications (Coamatic assay for FVIII, Chromogenix Instrumentation Laboratory from Milan, Italy; distributed by Diapharma, Westchester, OH, USA). Standard curve, generated using pooled normal canine reference plasma, was provided by Dr Nichols (University of North Carolina, Chapel Hill, NC, USA).
ELISA assay for FVIII antigen
The FVIII enzyme-linked immunosorbent assay (ELISA) was performed according to manufacturers protocol. (Affinity Biologicals, Ancaster, ON, Canada). Data represented as percent level of pooled human plasma standard.
DNA and RNA preparation
Liver biopsies were collected and immediately frozen (−80 °C) at days 18 and 79 postinjection for dog C. DNA was extracted as described elsewhere so as to avoid polymerase chain reaction (PCR) interference by hemoglobin [29]. Total genomic DNA was then extracted by phenol–chloroform separation.
Control DNA was prepared from normal canine whole blood using the Puregene DNA purification system (Gentra Systems, Minneapolis, MN, USA). Total RNA was isolated from biopsied livers using Trizol™ (Invitrogen, Carlsbad, CA, USA) according to manufacturer’s instructions. All nucleotide concentrations were determined with Pico Green reagent (DNA), or Ribo Green (RNA) (Molecular Probes, Eugene, OR, USA) using a Fluostar fluorescent plate reader (BMG Lab technologies GmbH, Durham, NC, USA) according to manufacturer’s protocol.
Quantitative and semi-quantitative PCR
Forty nanograms of template DNA was added to each PCR reaction using AmpliTaq™ gold thermostable polymerase. An initial activation cycle of 95 °C for 16 min was followed by 33 cycles at 95 °C, 52 °C, and 72 °C each at 30 s and a final extension cycle of 72 °C for 7 min.
Real-time PCR was conducted using a Roche Lightcycler and Roche LC FS DNA Master SYBR Green I reaction chemistry (Roche, Indianapolis, IN, USA) according to manufacturer instructions. Five nanograms of each sample was amplified for 45 cycles at 58 °C annealing temperature; otherwise, all PCR and melting curve profiles were performed using default instrument parameters. WPRE-specific primers used for both semi-quantitative PCR were: forward primer 5′-TCTCTTTATGAGGAGTTGTGGCCC-3′ reverse 5′-ACTGACAATTCCGTGGTGTTGTCG-3′.
RT-PCR
qPCR was performed in a Roche Lightcycler 1.0 using Roche Lightcycler Faststart DNA Master SYBR Green I chemistry using the following WPRE-specific primers: sense, 5′-TCTCTTTATGAGGAGTTGTGGCCC-3′ and antisense, 5′-ACTGACAATTCCGTGGTGTTGTCG-3′. Semi-quantitative PCR was performed using equal quantities of cDNA for each sample in an MJ thermocycler by standard PCR chemistry, using WPRE-specific primers with annealing at 62 °C, products were removed before the plateau phase of PCR.
Southern analysis
Eight micrograms of genomic DNA was digested with XmaI, electrophoresed through a 0.8% agarose gel and transferred to a nylon membrane. The probe specific for the WPRE sequence was prepared by excision from the shuttle plasmid followed by phenol–chloroform extraction and ethanol precipitation. The purified probe was labeled with [α32P] dCTP by random priming. Hybridization occurred at 65 °C as did successive washes with first 2.0X SSC/0.1% SDS and then with 0.5 × SSC/0.1% SDS. Relative abundance of hybridized fragments was detected by Phosphoimager analysis (Molecular Dynamics, Sunnyvale, CA, USA).
Results
Analysis of helper-dependent adenoviral vectors
The HDV used in this study, HDV-PEPCK/BDD-cFVIII/WPRE, carries a liver-restricted canine FVIII expression cassette and was produced as previously described [17]. Figure 1A depicts an illustration of the vector genome. To confirm vector integrity, the HDV and the helper-virus (HV) were digested with ApaLI, and the pΔ28E4-PEPCK/BDD-cFVIII/WPRE plasmid (pHDV) from which the vector was derived was digested with PmeI and ApaLI, and electrophoresed through a 1% agarose gel. No rearrangements were evident as the HDV and plasmid were similar save for the presence of a 2.5 kb ApaLI–PmeI fragment bearing the bacterial plasmid sequences in the pHDV lane (Fig. 1B arrowhead). This gel was transferred to a nylon membrane, and Southern blot was performed using a probe specific to the packaging signal (Fig. 1C). The degree of helper virus contamination was calculated as the ratio of the signal from the helper virus band divided by the signal from the HV band plus helper-dependent vector band [HV/(HV + HDAd)] in the helper-dependent vector lane. The Southern blot was quantified by phosphoimager analysis, and HV contamination was approximately < 0.2% (Fig. 1C).
Transient acute toxicity associated with HDV administration
Three dogs replicating the severe HA phenotype were injected via the forelimb vein with HDV-PEPCK/BDD-cFVIII/WPRE (Fig. 1A). Dog A received 1 × 1012 vector particles per kilogram (vp kg−1) and dogs B and C received 3 × 1012 vp kg−1. Laboratory measurements of acute toxicity were assessed throughout, and in the days following vector infusion. The animal receiving 1 × 1012 vp kg−1 (dog A) showed marginal changes in hepatic transaminases (Fig. 2A,B) and transiently decreased platelet counts, but did not deviate from the normal range (Fig. 2C), suggesting the lower 1 × 1012 vp kg−1 dose results in transient hematological toxicity but not liver-associated toxicity. Two animals receiving 3 × 1012 vp kg−1 (dogs B and C) showed an acute transient elevation of hepatic transaminases that returned to baseline by 72 h after injection (Fig. 2A,B). Platelet levels dropped from 400 000 to 100 000 mm−3 in dog B, and from 200 000 to 59 000 mm−3 in dog C by day 2, and returned to the normal range by day 7 in dog B, and by day 16 in dog C (Fig. 2C). The observed change in liver transaminases and drop in platelet levels are, in general, consistent with previous studies [8,10–12,30]. As the change in liver transaminases and platelet levels were not consistent with severe liver damage or bleeding, neither dog required infusion of normal canine plasma, although this was not the case in a similar study [31], and as each parameter resolved after a few days, we conclude that both doses were not associated with life-threatening toxicity in this experiment. Importantly, clinical parameters of toxicity were monitored in dog B for 2 years after vector administration with no significant changes, indicating the absence of chronic toxicity.
Fig. 2.
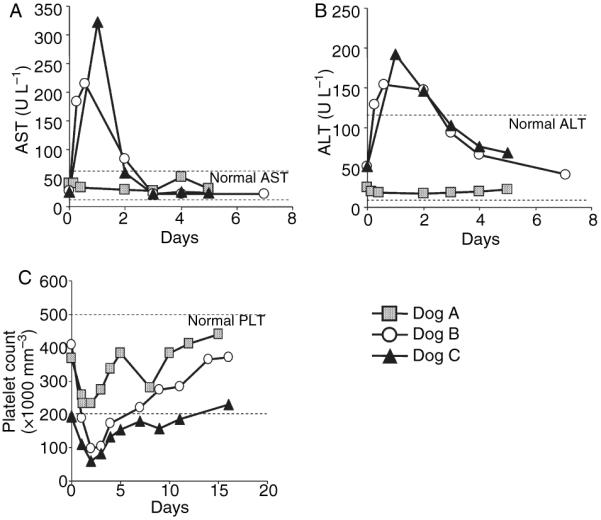
Toxicity profiles in two hemophilic dogs treated with HDV-PEPCK/BDDcFVIII/WPRE. Plasma was collected from the dogs at the indicated times prior to and after vector administration. (A) aspartate aminotransferase aspartate aminotransferase and (B) alanine aminotransferase analyses of three dogs receiving either 1 × 1012 vp kg−1 (dog A) or 3 × 1012 vp kg−1 (dogs B and C) HDV on day zero. (C) Platelet levels were measured for each dog throughout vector administration.
Phenotypic correction of bleeding disorder in FVIII-deficient dogs
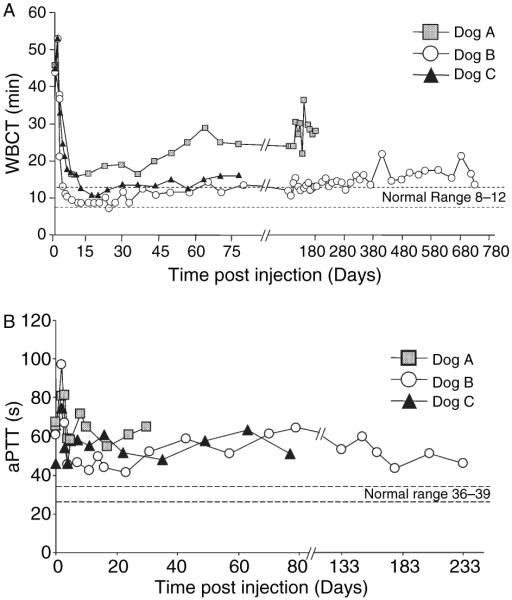
To determine whether the HDV- PEPCK/BDDcFVIII/WPRE vector would result in phenotypic correction of FVIII deficiency, WBCT was followed in the three animals after vector administration (Fig. 3A). In dog A, WBCT decreased from a baseline of 45–15 min, 2 weeks after vector delivery (Fig. 3A, boxes). The animal was followed until terminal sacrifice at 24 weeks at which time the WBCT was 28 min. The WBCT of dog B decreased from 40 to 8 min after 2 weeks postinjection (Fig. 3A, circles). This animal was followed for 2 years until terminal sacrifice, and maintained WBCTs in the 14–16 min range. Dog C received the same dose of vector as dog B and the WBCT of dog C decreased from 45.5 min to a minimum of 10.5 min on day 16, and maintained WBCT in the 10–16 min range until sacrifice at 12 weeks (Fig. 3A, triangles).
Fig. 3.
Whole blood clotting time (WBCT) and activated partial thromboplastin time (APTT) after injection of HDV-PEPCK/BDD-cFVIII/WPRE. Blood samples were measured periodically for WBCT and APTT as described in Materials and methods. (A) WBCT was monitored for the indicated time. Dog A received 1 × 1012 vp kg−1, and dogs B and C received 3 × 1012 vp kg−1. WBCT of dog B was measured for 2 years, and dog C for 79 days until terminal sacrifice. Normal WBCT = 8–12 min.
Likewise, APTT measurements for each dog decreased from the preinjection levels of 61, 64, and 66 s, for dogs A, B, and C, respectively (Fig. 3B). Although APTT for dog A were followed for only 20 days (Fig. 3B, boxes), dogs B and C were measured for 77 and 233 days, and the lowest APTT values were observed on days 11 and 4 for dogs B and C, respectively, when it dropped to nearly 45 s (Fig. 3B, circles and triangles). It is important to note that a significantly reduced APTT was observed on day 233 for dog B (53 s). However, the normal APTT for wild-type dogs is between 36 and 39 s [32], and thus we did not observe complete correction of this parameter in any of the dogs. Regardless, this is the longest observed correction of WBCT after a single HDV gene therapy treatment. Moreover, as untreated HA dogs would be expected to have ~5 bleeding events per year requiring plasmaphoresis, and as both dogs receiving the higher dose of HDV-PEPCK/BDD-cFVIII/WPRE did not manifest any bleeding episodes, significant clinical complications of the HA were avoided. Taken together, these data indicate that the higher dose animals converted from severe-to-moderate hemophilia after HDV infusion.
FVIII activity and FVIII plasma concentration analysis
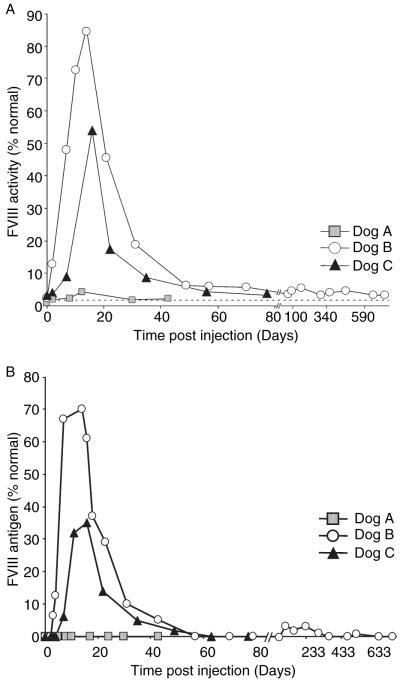
Plasma levels of the canine FVIII transgene product were determined by both FVIII ELISA and chromogenic bioassay (COATEST) (Fig. 4). Prior to treatment, all dogs had undetectable plasma FVIII as measured by either COATEST or antigen ELISA. We measured FVIII activity at peak levels of 4% on day 7 that quickly fell below detectable levels by day 12 in dog A (Fig. 4A, boxes), which corresponded to a plasma FVIII concentration peak level of 1.3% normal on day 8 (Fig. 4B, boxes), and then declined to < 1%, 43 days after vector administration. In summary, dog A received the lowest dose tested (1 × 1012 vp kg−1) and achieved detectable, albeit transient FVIII concentrations which correlated with significant decrease in WBCT but not APTT, transient thrombocytopenia, and modest acute liver toxicity. These data highlight the importance of overcoming the dose dependent effect of systemic adenoviral injection in order to achieve sufficient FVIII protein expression for long-term therapy.
Fig. 4.
Plasma factor VIII (FVIII) activity in canine hemophilia A dogs treated with HDV-PEPCK/BDDcFVIII/WPRE. Each dog was administered HDV on experimental day 0. Dog A received 1 × 1012 vp kg−1 body weight and dogs B and C each received 3 × 1012 vp kg−1 body weight. Periodic blood samples were collected and percent normal FVIII activity was measured by COATEST assay (A) and FVIII antigen level was measured by enzyme-linked immunosorbent assay (B). Dotted line represents lower limit of detection.
In dog B, we observed peak activity of 84% normal by COATEST at 14 days that decreased and stabilized around 3–6% normal activity until terminal sacrifice on day 724 (Fig. 4A, circles). Dog B also peaked in detectable antigen level on day 14, reaching 70% of the normal control, and nominal levels of over 1% were detected at days 119, 162, 233, and 519 (Fig. 4B circles). In dog C, we observed peak activity of 54% on day 16 that decreased and remained detectable for 79 days until terminal sacrifice. We noted variable percentages of normal FVIII levels in the two dogs receiving the same dose (Compare Fig. 4B circles and triangles). We speculate that the discrepancy between dogs B and C may be because of inter-animal variability, or possibly a reflection of the relative differences in infectivity in the vector preparations used for each dog. Nevertheless, both levels were sufficient to substantially reduce the WBCT defect long term (Fig. 3, triangles, 79 days until terminal sacrifice, and circles, 2 years and terminal sacrifice). These WBCTs are consistent with the relative effects of low levels of FVIII (Supplemental Fig. 1A). Taken together, these data are consistent with the correlation of WBCT and FVIII activity in the canine model, where minimal amounts of FVIII activity are sufficient to partially correct WBCT.
Long-term persistence of vector genome and message after HDV administration
We sought to determine if vector genome loss was a major factor in the precipitous decline of plasma FVIII activity from weeks 2–12. Liver biopsy tissue from dog C was collected at day 18 postvector administration and at day 79. These time-points correspond to the peak of plasma FVIII activity (54% normal at day 16, Fig. 4, triangles) and to the time when activity had fallen to nearly 3% normal (day 77).
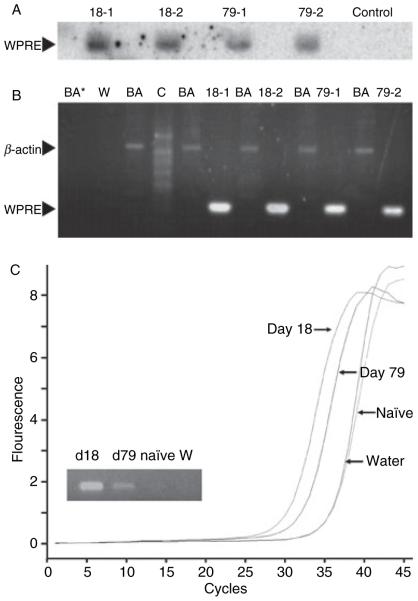
To determine whether the decrease of plasma FVIII activity over time could be explained by loss of vector genome, total DNA was isolated from the days 18 and 79 liver samples. Genomic Southern analyses were performed using a vector-specific probe to the WPRE sequence. A canine β-actin probe was used to control for DNA input. DNA isolated from the blood of an unaffected dog was used as control. We observed an approximate 2-fold decrease of vector-specific DNA at the later time-point (Fig. 5A, compare lanes 18-1 and 18-2, to lanes 79-1 and 79-2). This result was confirmed by semi-quantitative PCR, which showed a similar 2-fold decrease at the later time-point (Fig. 5B), as well as by real-time PCR (data not shown). The lack of a band in the control lane (Fig. 5B lane C) of the appropriate size indicates that there was no cross-reactivity with normal canine DNA and the WPRE-specific probe, and thus the probe is specific to the HDV intervention.
Fig. 5.
Persistence of vector-specific DNA in liver after administration of HDV-PEPCK/BDD-cFVIII/WPRE. (A) Vector-specific Southern blot. Genomic DNA from days 18 and 79 liver biopsy tissue was probed with vector-specific WPRE element. Control is normal canine genomic DNA. (B) Semi-quantitative polymerase chain reaction (PCR) with WPRE and beta-actin-specific primers. BA is β actin control, C is DNA derived from a normal dog, W is WPRE primer run in water, and BA* is β actin-specific primers run in water. (C) RT-PCR and qualitative RT-PCR from days 18 and 79 liver biopsy tissue were performed with vector-specific WPRE primers. Naïve indicates an untreated FVIII-deficient animal, W is water control.
Given the persistence of vector genome, we hypothesized that vector-specific message was also persistent in the biopsy tissue. We measured total RNA by RT PCR using a probe against the WPRE element because it was unique to the vector RNA. On qualitative analysis, we observed WPRE-specific RNA in liver tissue isolated from dog C collected on days 18, and 79, but not liver from a control uninjected FVIII-deficient dog from the same colony (Fig. 5C, inset). Similarly, when the cDNA samples were analyzed by real-time PCR, we observed a 1.89 cycle difference in crossing points comparing day 18 (30.55 cycles) to day 79 (32.44 cycles). As the crossing point analysis is dependent upon the concentration of input cDNA (determined by fluorometry and normalized for each sample), we calculate a 3.8-fold decrease in RNA from days 18–79. This is on par with the decrease observed in vector-specific DNA. Taken together, this data suggest that long-term vector persistence, expression, and therapeutic effect are achievable, at least in this canine HA model, after a single injection of HDV-PEPCK/BDD-cFVIII/WPRE.
Discussion
A similar recent study tested the feasibility of systemic injection of HDV-expressing cFVIII into a FVIII-deficient canine model [18]. Of note, the animal colony, vector construct (including promoter), dose of vector (1.25–2 vs. 3 × 1012 vp kg−1), resulting FVIII expression maximum (10–20% vs. 84%), and duration of analysis (1% at day 168 vs. 3.5% at day 724) differed from the current study. In addition, one animal in the Brown et al. study received 2 × 1012 vp kg−1, and reached 20% FVIII activity, but soon developed immunologic clearance of antigen. Neither the 1 × 1012 vp kg−1 nor the two 3 × 1012 vp kg−1 animals in the present study developed inhibitory antibodies (data not shown). We speculate that these observed differences are accounted for by variability between the animal colonies, as well as differences in vector construction, amplification and purification, and the experimental limitations inherent in small numbers used in large animal experiments. Hence, each experimental condition substantially contributes to the overall preclinical experience of HDV and canine HA.
Another study observed loss of therapeutic antigen over time after a HDV expressing cFIX was administered to hemophilia B dogs [33]. As HDV genomes exist as episomal DNA, it was postulated that loss of vector genomes over time might explain the loss of transgene production. To directly test this, we performed survival liver biopsies on a HDV-treated dog at times correlating with high and low levels of plasma FVIII. We determined that vector genome loss could not explain the magnitude of decrease in FVIII. We also observed persistence of vector-specific mRNA. In addition, dog B doubled in size from the time of injection (9.4 kg) until terminal sacrifice (21 kg), yet the single injection of HDV was responsible for a 2-year conversion from severe-to-moderate hemophilia. Together, these data strongly suggest that neither animal growth, tissue turn-over, nor promoter shut-off is responsible for the decrease in FVIII antigen.
Despite these data, the putative FVIII clearance mechanism remains unknown. Indeed, our attempts to identify cFVIII-specific antibodies which might cause the observed decline in circulating FVIII were unsuccessful by both Western analyses, and ELISA (data not shown), although this was primarily limited by the lack of sufficient canine-specific reagents.
These studies underscore the ‘threshold effect’ evident after systemic adenovirus injection, where linear dose escalation does not correlate with protein expression [34]. Moreover, it is likely that the therapeutic threshold is different across species. In fact, mice and non-human primates are apparently more tolerant to high doses of vector compared with humans, and more than one human trial was abruptly halted because of vector-associated toxicity to 50–100-fold less virus than the dose tested in the present study [35]. Although the dose tested was not clinically relevant per se, the observation that vector genome and expression can persist for at least 2 years is itself important, and unprecedented with other adenoviral vectors in this model. Moreover, it suggests that if innate toxicity to adenovirus is overcome, relatively long-term clinical effect can be achieved without risk of genotoxicity.
Methods that restrict viral exposure to specific organs [16] and PEG-ylation strategies that shield the virus from the immune system [13–15] are a few examples of current efforts to overcome the threshold effect, and hence, dose-related acute toxicity.
Clearly, further studies are needed to elucidate and ultimately avoid all of the potential toxic and immunologic responses to HDV-mediated gene therapy for HA. Encouragingly, the production of cFVIII from this HDV construct was sufficient to overcome a putative antigen clearance mechanism, and confer long-term reduction in WBCT. Thus, if the dose threshold were to be overcome, then HDV gene transfer for FVIII deficiency should result in long-term therapeutic benefit.
Supplementary Material
Acknowledgements
This manuscript is in memory of Dr W. Michael McCormack. We thank Dr David Lillicrap for providing the canine FVIII cDNA, and Wyeth Research for providing the FVIII infusion fall-off study. We thank Christian Clarke for excellent technical assistance, and Monique Land for administrative support. The authors thank the Texas Gulf Coast Digestive Disease Center NIH DK58338, the Baylor MRDDRC NIH HD024064.
References
- 1.Kaufman RJ. Advances toward gene therapy for hemophilia at the millennium. Hum Gene Ther. 1999;10:2091–107. doi: 10.1089/10430349950017095. [DOI] [PubMed] [Google Scholar]
- 2.Fijnvandraat K, Berntorp E, ten Cate JW, Johnsson H, Peters M, Savidge G, Tengborn L, Spira J, Stahl C. Recombinant, B-domain deleted factor VIII (r-VIII SQ): pharmacokinetics and initial safety aspects in hemophilia A patients. Thromb Haemost. 1997;77:298–302. [PubMed] [Google Scholar]
- 3.Eaton DL, Hass PE, Riddle L, Mather J, Wiebe M, Gregory T, Vehar GA. Characterization of recombinant human factor VIII. J Biol Chem. 1987;262:3285–90. [PubMed] [Google Scholar]
- 4.Gill JC. Therapy of factor VIII deficiency. Semin Thromb Hemost. 1993;19:1–12. doi: 10.1055/s-2007-994001. [DOI] [PubMed] [Google Scholar]
- 5.Fijnvandraat K, Bril WS, Voorberg J. Immunobiology of inhibitor development in hemophilia A. Semin Thromb Hemost. 2003;29:61–8. doi: 10.1055/s-2003-37940. [DOI] [PubMed] [Google Scholar]
- 6.Oldenburg J, El-Maarri O, Schwaab R. Inhibitor development in correlation to factor VIII genotypes. Haemophilia. 2002;8:23–9. doi: 10.1046/j.1351-8216.2001.00134.x. [DOI] [PubMed] [Google Scholar]
- 7.Connelly S, Mount J, Mauser A, Gardner JM, Kaleko M, McClelland A, Lothrop CD., Jr Complete short-term correction of canine hemophilia A by in vivo gene therapy. Blood. 1996;88:3846–53. [PubMed] [Google Scholar]
- 8.Morral N, O’Neal W, Zhou H, Langston C, Beaudet A. Immune responses to reporter proteins and high viral dose limit duration of expression with adenoviral vectors: comparison of E2a wild type and E2a deleted vectors. Hum Gene Ther. 1997;8:1275–86. doi: 10.1089/hum.1997.8.10-1275. [DOI] [PubMed] [Google Scholar]
- 9.Gallo-Penn AM, Shirley PS, Andrews JL, Tinlin S, Webster S, Cameron C, Hough C, Notley C, Lillicrap D, Kaleko M, Connelly S. Systemic delivery of an adenoviral vector encoding canine factor VIII results in short-term phenotypic correction, inhibitor development, and biphasic liver toxicity in hemophilia A dogs. Blood. 2001;97:107–13. doi: 10.1182/blood.v97.1.107. [DOI] [PubMed] [Google Scholar]
- 10.Brunetti-Pierri N, Palmer DJ, Beaudet AL, Carey KD, Finegold M, Ng P. Acute toxicity after high-dose systemic injection of helper-dependent adenoviral vectors into nonhuman primates. Hum Gene Ther. 2004;15:35–46. doi: 10.1089/10430340460732445. [DOI] [PubMed] [Google Scholar]
- 11.Morsy MA, Gu M, Motzel S, Zhao J, Lin J, Su Q, Allen H, Franlin L, Parks RJ, Graham FL, Kochanek S, Bett AJ, Caskey CT. An adenoviral vector deleted for all viral coding sequences results in enhanced safety and extended expression of a leptin transgene. Proc Natl Acad Sci USA. 1998;95:7866–71. doi: 10.1073/pnas.95.14.7866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morral N, Parks RJ, Zhou H, Langston C, Schiedner G, Quinones J, Graham FL, Kochanek S, Beaudet AL. High doses of a helper-dependent adenoviral vector yield supraphysiological levels of alpha1-antitrypsin with negligible toxicity. Hum Gene Ther. 1998;9:2709–16. doi: 10.1089/hum.1998.9.18-2709. [DOI] [PubMed] [Google Scholar]
- 13.Croyle MA, Le HT, Linse KD, Cerullo V, Toietta G, Beaudet A, Pastore L. PEGylated helper-dependent adenoviral vectors: highly efficient vectors with an enhanced safety profile. Gene Ther. 2005;12:579–87. doi: 10.1038/sj.gt.3302441. [DOI] [PubMed] [Google Scholar]
- 14.Mok H, Palmer DJ, Ng P, Barry MA. Evaluation of polyethylene glycol modification of first-generation and helper-dependent adenoviral vectors to reduce innate immune responses. Mol Ther. 2005;11:66–79. doi: 10.1016/j.ymthe.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 15.O’Riordan CR, Lachapelle A, Delgado C, Parkes V, Wadsworth SC, Smith AE, Francis GE. PEGylation of adenovirus with retention of infectivity and protection from neutralizing antibody in vitro and in vivo. Hum Gene Ther. 1999;10:1349–58. doi: 10.1089/10430349950018021. [DOI] [PubMed] [Google Scholar]
- 16.Brunetti-Pierri N, Palmer DJ, Mane V, Finegold M, Beaudet AL, Ng P. Increased hepatic transduction with reduced systemic dissemination and proinflammatory cytokines following hydrodynamic injection of helper-dependent adenoviral vectors. Mol Ther. 2005;12:99–106. doi: 10.1016/j.ymthe.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 17.Palmer D, Ng P. Improved system for helper-dependent adenoviral vector production. Mol Ther. 2003;8:846–52. doi: 10.1016/j.ymthe.2003.08.014. [DOI] [PubMed] [Google Scholar]
- 18.Brown BD, Shi CX, Powell S, Hurlbut D, Graham FL, Lillicrap D. Helper-dependent adenoviral vectors mediate therapeutic factor VIII expression for several months with minimal accompanying toxicity in a canine model of severe hemophilia A. Blood. 2004;103:804–10. doi: 10.1182/blood-2003-05-1426. [DOI] [PubMed] [Google Scholar]
- 19.Ehrhardt A, Xu H, Kay MA. Episomal persistence of recombinant adenoviral vector genomes during the cell cycle in vivo. J Virol. 2003;77:7689–95. doi: 10.1128/JVI.77.13.7689-7695.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lozier JN, Dutra A, Pak E, Zhou N, Zheng Z, Nichols TC, Bellinger DA, Read M, Morgan RA. The Chapel Hill hemophilia A dog colony exhibits a factor VIII gene inversion. Proc Natl Acad Sci USA. 2002;99:12991–6. doi: 10.1073/pnas.192219599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mian A, McCormack WM, Jr, Mane V, Kleppe S, Ng P, Finegold M, O’Brien WE, Rodgers JR, Beaudet AL, Lee B. Long-term correction of ornithine transcarbamylase deficiency by WPRE-mediated over-expression using a helper-dependent adenovirus. Mol Ther. 2004;10:492–9. doi: 10.1016/j.ymthe.2004.05.036. [DOI] [PubMed] [Google Scholar]
- 22.Cook RF, Cook SJ, Savon S, McGrane M, Hartitz M, Hanson RW, Hodgson CP. Liver-specific expression of a phosphoenolpyruvate carboxykinase-neo gene in genetically modified chickens. Poult Sci. 1993;72:554–67. doi: 10.3382/ps.0720554. [DOI] [PubMed] [Google Scholar]
- 23.Loeb JE, Cordier WS, Harris ME, Weitzman MD, Hope TJ. Enhanced expression of transgenes from adeno-associated virus vectors with the woodchuck hepatitis virus posttranscriptional regulatory element: implications for gene therapy. Hum Gene Ther. 1999;10:2295–305. doi: 10.1089/10430349950016942. [DOI] [PubMed] [Google Scholar]
- 24.Schambach A, Wodrich H, Hildinger M, Bohne J, Krausslich HG, Baum C. Context dependence of different modules for posttranscriptional enhancement of gene expression from retroviral vectors. Mol Ther. 2000;2:435–45. doi: 10.1006/mthe.2000.0191. [DOI] [PubMed] [Google Scholar]
- 25.Glover CP, Bienemann AS, Heywood DJ, Cosgrave AS, Uney JB. Adenoviral-mediated, high-level, cell-specific transgene expression: a SYN1-WPRE cassette mediates increased transgene expression with no loss of neuron specificity. Mol Ther. 2002;5:509–16. doi: 10.1006/mthe.2002.0588. [DOI] [PubMed] [Google Scholar]
- 26.Toietta G, Pastore L, Cerullo V, Finegold M, Beaudet AL, Lee B. Generation of helper-dependent adenoviral vectors by homologous recombination. Mol Ther. 2002;5:204–10. doi: 10.1006/mthe.2002.0532. [DOI] [PubMed] [Google Scholar]
- 27.Barrow EM, Bullock WR, Graham JB. A study of the carrier state for plasma thromboplastin component (PTC, Christmas factor) deficiency, utilizing a new assay procedure. J Lab Clin Med. 1960;55:936–45. [PubMed] [Google Scholar]
- 28.Langdell RD, Wagner RH, Brinkhous KM. Effect of antihemophilic factor on one-stage clotting tests; a presumptive test for hemophilia and a simple one-stage antihemophilic factor assay procedure. J Lab Clin Med. 1953;41:637–47. [PubMed] [Google Scholar]
- 29.Al-Soud WA, Radstrom P. Purification and characterization of PCR-inhibitory components in blood cells. J Clin Microbiol. 2001;39:485–93. doi: 10.1128/JCM.39.2.485-493.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lu H, Sullivan D, Gerber MA, Dash S. Adenovirus induced acute hepatitis in non-human primates after liver-directed gene therapy. Chin Med J (Engl) 2002;115:726–31. [PubMed] [Google Scholar]
- 31.Brunetti-Pierri N, Nichols TC, McCorquodale S, Merricks E, Palmer DJ, Beaudet AL, Ng P. Sustained phenotypic correction of canine hemophilia B after systemic administration of helper-dependent adenoviral vector. Hum Gene Ther. 2005;16:811–20. doi: 10.1089/hum.2005.16.811. [DOI] [PubMed] [Google Scholar]
- 32.Brinkhous K, Sandberg H, Widlund L, Read M, Nichols T, Sigman J, Oswaldsson U, Schaub RG, Mikaelsson M. Preclinical pharmacology of albumin-free B-domain deleted recombinant factor VIII. Semin Thromb Hemost. 2002;28:269–72. doi: 10.1055/s-2002-32661. [DOI] [PubMed] [Google Scholar]
- 33.Ehrhardt A, Xu H, Dillow AM, Bellinger DA, Nichols TC, Kay MA. A gene-deleted adenoviral vector results in phenotypic correction of canine hemophilia B without liver toxicity or thrombocytopenia. Blood. 2003;102:2403–11. doi: 10.1182/blood-2003-01-0314. [DOI] [PubMed] [Google Scholar]
- 34.Morral N, O’Neal WK, Rice K, Leland MM, Piedra PA, Aguilar-Cordova E, Carey KD, Beaudet AL, Langston C. Lethal toxicity, severe endothelial injury, and a threshold effect with high doses of an adenoviral vector in baboons. Hum Gene Ther. 2002;13:143–54. doi: 10.1089/10430340152712692. [DOI] [PubMed] [Google Scholar]
- 35.Chuah MK, Collen D, Van den Driessche T. Clinical gene transfer studies for hemophilia A. Semin Thromb Hemost. 2004;30:249–56. doi: 10.1055/s-2004-825638. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.