Abstract
Objective
Despite the high frequency of CD4+ T cells with a regulatory phenotype (CD25+CD127lowFoxP3+) in the joints of patients with rheumatoid arthritis (RA), inflammation persists. One possible explanation is that human Tregs are converted into pro-inflammatory IL-17-producing cells by inflammatory mediators and thereby lose their suppressive function. We investigated whether activated monocytes, which are potent producers of inflammatory cytokines and abundantly present in the rheumatic joint, induce pro-inflammatory cytokine expression in human Tregs and impair their regulatory function.
Methods
The presence and phenotype of CD4+CD45RO+CD25+CD127low T cells (memory Tregs) and CD14+ monocytes in the peripheral blood (PB) and synovial fluid (SF) from patients with RA was investigated by flow cytometry. FACS-sorted memory Tregs from healthy controls were co-cultured with autologous activated monocytes and stimulated with anti-CD3 monoclonal antibody. Intracellular cytokine expression, phenotype and function of cells were determined by flow cytometry, ELISA and proliferation assays.
Results
Patients with RA showed higher frequencies of CD4+CD45RO+CD25+CD127low Tregs and activated CD14+ monocytes in SF relative to PB. In vitro-activated monocytes induced an increase in the percentage of IL-17+, IFNγ+ and TNF-α+, but also IL-10+ Tregs. The observed increase in IL-17+ and IFNγ+ Tregs was driven by monocyte-derived IL-1β, IL-6 and TNF-α and was mediated by both CD14+CD16− and CD14+CD16+ monocyte subsets. Despite enhanced cytokine expression, cells maintained their CD25+FoxP3+CD39+ Treg phenotype and showed enhanced capacity to suppress proliferation and IL-17 production by effector T cells.
Conclusion
Tregs exposed to a pro-inflammatory environment show increased cytokine expression as well as enhanced suppressive activity.
Immune regulation is essential for the maintenance of peripheral tolerance, prevention of autoimmune diseases and the limitation of chronic inflammation. Human CD4+CD25+CD127low regulatory T cells (Tregs), characterised by the expression of the lineage-specific transcription factor FoxP3, are important immune regulators through their ability to suppress activation, proliferation and effector functions of a wide range of immune cells including CD4+ and CD8+ T cells, B cells, NK cells and APCs (reviewed in (1, 2)). The notion that CD4+CD25+CD127lowFoxp3+ T cells are terminally differentiated suppressor cells has been challenged by reports showing that Tregs can display significant plasticity during development and differentiation in the periphery in response to extrinsic cues (reviewed in (3, 4)). The concept of Treg plasticity has raised fundamental questions regarding the significance of CD4+CD25+CD127lowFoxp3+ Tregs present at sites of inflammation as well as the stability and safety of ex vivo-expanded human Tregs for the use in immunotherapy.
A landmark study by Miyara et al. revealed that Tregs from human peripheral blood are heterogeneous, comprising at least three phenotypically and functionally distinct populations. The so called population III (CD45RA−FoxP3low) was shown to be non-suppressive and able to convert into IL-17-producing cells (5). The in vivo existence of IL-17+ Tregs has been demonstrated in human peripheral blood (6, 7) as well as in periodontitis lesions (8) and skin lesions of patients with severe psoriasis (9). Several groups have identified the pro-inflammatory cytokine IL-1β as a critical mediator in the conversion of human Tregs into IL-17-producing cells in vitro (6, 7, 10-13). As yet, data are conflicting as to whether these pro-inflammatory cytokine-producing Tregs are impaired in their regulatory function. Furthermore, since most of these studies have been performed using α-CD3/CD28 beads and recombinant cytokines, data on human Treg conversion in a physiological context are scarce.
IL-17 has been associated with inflammatory diseases such as rheumatoid arthritis (RA), inflammatory bowel disease, multiple sclerosis, asthma, systemic lupus erythematosus, psoriasis and type 1 diabetes (reviewed in (14)). Previous work from our lab has shown that CD14+ cells are present in large numbers in the synovial fluid of patients with RA and that these cells preferentially promote Th17 responses in CD4+ T cells (15). CD14+ monocytes are important contributors to inflammation through the production of pro-inflammatory cytokines such as IL-1β. Based on these findings we sought to determine whether activated monocytes drive the expression of IL-17 in highly purified CD4+CD45RO+CD25+CD127low regulatory T cells (memory Tregs), and whether this affects Treg phenotype and function. We report here that human memory Tregs, in the presence of activated monocytes, display increased expression of both pro- and anti-inflammatory cytokines. These cells maintain their Treg phenotype and exert enhanced suppressive effects on T cell proliferation and cytokine production.
Materials & Methods
Patients and healthy volunteers
Peripheral blood (PB, n=29) and synovial fluid (SF, n=12) was obtained from patients with rheumatoid arthritis (RA) recruited from Guy’s and St Thomas’ Hospital NHS Trust. PB was also collected from adult healthy controls (HC). The mean age of patients and HC was 58±2.8 and 36±2.2 years, respectively. Female to male ratios were 26:3 (patients) and 24:12 (HC). The mean patients’ DAS28 score was 5.2±0.3 (mean±SEM, n=18); 5/29 patients were on TNF inhibitor therapy, 18/29 on DMARD, and 3/29 on steroids or NSAIDs. All participants gave written informed consent. Ethics approval for this study was given by the Bromley Research Ethics Committee (06/Q0705/20). Mononuclear cells were isolated from PB and SF using Ficoll-Hypaque (LSM 1077, PAA, Pasching, Austria) density gradient centrifugation.
Phenotypic analysis
The following monoclonal antibodies (mAb) were used: CD2-PacificBlue (clone: TS18), CD3-APC/Cy7 (clone: HIT3a), CD4-PerCP/Cy5.5 (clone: SK3), CD14-APC/Cy7 (clone: HCD14), CD16-AlexaFluor488 (clone: 3G8), CD39-PE/Cy7 (clone: A1), CD45RO-PacificBlue (clone: UCHL1), CD54-AlexaFluor647 (clone: HCD54), CD86-PacificBlue (clone: IT2.2), CD127-AlexaFluor488 (clone: HCD127) and CD161-AlexaFluor647 (clone: HP-3G10) all from BioLegend (San Diego CA, USA), CD25-PE (clone: 4E3) from Miltenyi Biotec (Bergisch Gladbach, Germany), CD40-PE (clone: LOB7/6) and CD69-PE (clone: FN50) from AbD Serotec (Kidlington, UK) and HLA-DR-PerCP/Cy5.5 (clone: G46-6) from BD (Franklin Lakes NJ, USA). For intracellular cytokine staining (ICCS), cells were stained for CD2 and CD14, followed by fixation with 2% PFA. Cells were then stained intracellularly with IL-10-AlexaFluor488 (clone: JES3-9D7), IL-17A-PE (clone: BL168), TNF-α-APC (clone: MAb11) and IFNγ-PerCP/Cy5.5 (clone: 4S.B3) (all from BioLegend) using 0.5% Saponin. For intranuclear staining, cells were extracellularly stained and fixed as described above followed by permeabilization with 1× FoxP3 perm buffer (BioLegend). Cells were then stained with FoxP3-AlexaFluor647 (clone: 259D) and Ki-67-AlexaFluor488 (clone: Ki-67) from BioLegend in combination with IL-17-PE. Cells were acquired on a BD FACSCantoII and analysed using FlowJo 7.6.1 software (Tree Star Inc., Ashland OR, USA).
Cell isolation
Peripheral blood mononuclear cells (PBMC) were incubated with CD14 MicroBeads (Miltenyi Biotec) for positive selection of CD14+ monocytes by magnetic cell separation. Purity was confirmed by flow cytometry and was consistently >97%. The CD14− fraction was used for memory CD4+ T cell isolation by negative selection (Miltenyi Biotec) according to the manufacturer’s instructions and purity of cells was always >96%. Memory CD4+ T cells were stained with CD25-PE and CD127-FITC (clone: A019D5, BioLegend) and sorted into CD25+CD127low Tregs and CD25low/−CD127+ effector T cells (Teff) using a BD FACS Aria™ II cell sorter (purities >97%, Supplementary Figure 1). Where indicated, isolated CD14+ monocytes were stained with CD16-AlexaFluor488 after incubation with FcR blocking reagent (Miltenyi Biotec) and sorted into CD16+ and CD16− cells (Supplementary Figure 2).
Co-culture experiments
Cells were cultured in culture medium (RPMI 1640 (Gibco®, Camarillo CA, USA), 20mM L-glutamine (Gibco®), 1% Penicillin-Streptomycin (Gibco®) and 10% FCS (Sigma, St. Louis MO, USA)) at 37°C and 5% CO2. Monocytes (10×106/ml) were pre-incubated with medium, 100ng/ml LPS (Sigma), or a cytokine cocktail consisting of hrIL-1β, IL-6, IL-10, IL-17, TNF-α, OPN (all at 10ng/ml) and 10U/ml IFNγ (all from R&D, Minneapolis MN, USA) for 30 min. Following incubation, cells were washed twice with 5-10ml of medium and recounted.
Monocytes (1×105) were co-cultured at a 1:1 ratio with sorted CD4+CD45RO+CD25+CD127low Tregs or CD4+CD45RO+CD25low/−CD127+ Teff and 100ng/ml soluble anti-CD3 mAb (Okt-3, Janssen-Cilag, Buckinghamshire, UK) in a total volume of 250μl. At day 3, cells were stimulated with 50ng/ml PMA (Sigma) and 750ng/ml ionomycin (Sigma) for 6hrs, with GolgiStop™ (BD) present for the last 3hrs. Where supernatants of activated monocytes were transferred, these were collected from autologous or allogeneic LPS-pre-activated monocytes after 40hrs and added 1:1 (v/v) to co-cultures. For blocking experiments, neutralising antibodies against IL-1β (1μg/ml, clone: 8516, mIgG1), IL-6 (10μg/ml, clone: 1936, mIgG2b) and TNF-α (1μg/ml, clone: 1825, mIgG1) (all from R&D) were added at the start of culture.
Suppression assay
Freshly sorted Teff (2-5×106/ml) were labelled with 2μM carboxyfluorescein succinimidyl ester (CFSE, Molecular Probes™, Eugene OR, USA) according to manufacturer’s instructions. Cells were washed, recounted and plated in a 24-well plate (1×106/ml). At the same time, unlabelled, sorted Tregs at various concentrations (1×104-5×104) were cultured with anti-CD3 mAb and 5×104 monocytes pre-treated with either LPS or medium in a total volume of 100μl. The next day, CFSE-labelled Teff were taken off the plate, washed, recounted and 5×104 cells added to the Treg-monocyte co-cultures. To determine proliferation, fluorescence was assessed 2 days later by flow cytometry and percentage of suppression was calculated using the formula: {100 − [(% dividing cells (condition w/ Treg) / % dividing cells (condition w/o Treg)] * 100)}.
Cytokine analysis
Supernatants of co-cultures were collected on day 3, after re-stimulation with PMA and ionomycin, centrifuged to remove cell debris and stored at −80°C until analysed. IL-1β (R&D Systems), IL-6, IL-17, IFNγ and TNF-α (BioLegend) levels were determined using enzyme-linked immunosorbent assay (ELISA) according to manufacturers’ protocols. Supernatants from monocytes stimulated with either medium or LPS for 40hrs, were analysed using the Human Cytokine 25-plex kit (Invitrogen Life Technologies, Camarillo CA, USA) on the Luminex® platform (Austin TX, USA). For detection of cytokines in SF of patients with RA, FlowCytomix™ Th1/Th2 assay (eBioscience, Hatfield, UK), IL-17 and OPN ELISA (R&D Systems) were used.
Statistical analysis
Significance testing was performed using Prism 5 software (GraphPad, San Diego CA, USA). Data were tested for normality using D’Agostino and Pearson omnibus normality test, followed by the appropriate parametric or non-parametric test, as indicated in the figure legends.
Results
CD4+CD45RO+ T cells with a regulatory phenotype and activated CD14+ monocytes are abundantly present at the site of inflammation in patients with RA
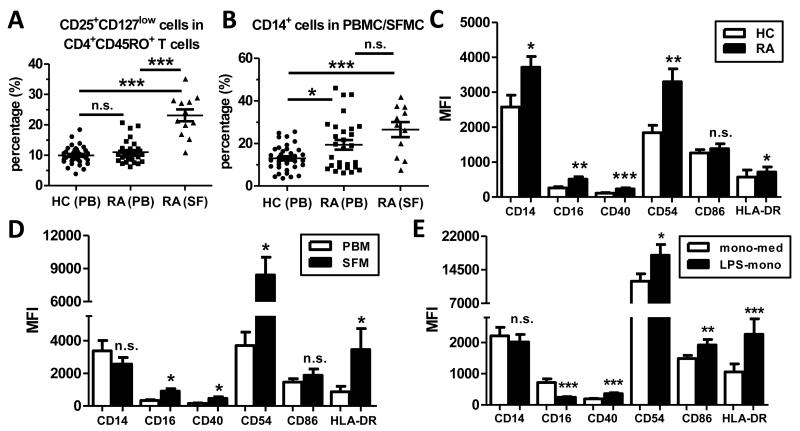
Various reports, including our previous work, have demonstrated increased frequencies of CD4+CD25+ Tregs in the synovial fluid (SF) of patients with rheumatoid arthritis (RA) compared to peripheral blood (PB) (16-23). However, these studies did not account for the fact that virtually all T cells in SF have a memory CD45RO+ phenotype, whilst their blood counterparts comprise both CD45RO+ and CD45RO− T cells. We therefore determined the percentage of memory CD4+ T cells with a regulatory phenotype (CD25+CD127low) in PB and SF from patients with RA, and in PB from healthy controls (HC) (gating strategy shown in Supplementary Figure 3). Our data show that there was no significant difference in the percentage of CD25+CD127low cells within CD3+CD4+CD45RO+ T cells in the peripheral blood of patients with RA (n=29) and HC (n=36) (10±0.5% vs. 11±0.7%, Figure 1A), even after correcting for age differences between the groups (data not shown). However, the percentage of CD25+CD127low cells within CD3+CD4+CD45RO+ T cells was significantly elevated in SF (n=12) (23±2.0%, p<0.0001) compared to PB (n=29) from patients with RA (Figure 1A). A similar increase was observed when analysing paired PB and SF samples only (n=11, p=0.0005). We also determined the presence of CD14+ monocytes in HC and patients with RA, and found a significant increase in the percentage of CD14+ monocytes in PB of patients compared to HC (19±2.3% vs. 13±1.0%, p<0.05), and an even higher percentage at the site of inflammation (26±3.5%) (Figure 1B).
Figure 1. Memory CD4+ T cells with a Treg phenotype and activated CD14+ monocytes are increased at the site of inflammation in patients with RA.
(A, B) Mononuclear cells from PB of healthy controls (HC, n=36) and from PB (n=28-29) and SF (n=11-12) from patients with RA were analysed for the percentage of CD25+CD127low cells within CD4+CD45RO+ T cells (A) and for the percentage of CD14+ cells in PBMC/SFMC. Lines indicate mean ± SEM; data were analysed by ANOVA: * p<0.05, *** p<0.001. (C-E) Surface expression of the indicated markers was determined in PB CD14+ monocytes (PBM) from patients with RA (n=17) and HC (n=16) (C), in paired PBM and SF-derived CD14+ monocytes (SFM) from patients with RA (n=7) (D) and in PBM from HC (n=18) that were treated with medium (mono-med) or LPS (LPS-mono) for 30 min and cultured for 16 hours (E). Results are shown as mean ± SEM and analysis was performed using Wilcoxon matched-pairs signed rank test: * p<0.05, ** p<0.01, *** p<0.001.
We assessed the phenotype of peripheral blood monocytes (PBM) from patients with RA and healthy controls by flow cytometry. PBM from patients with RA had a significantly higher expression of CD14, CD16, CD40, CD54 and HLA-DR compared to HC (Figure 1C), indicating an activated phenotype. CD14+ monocytes were found to be further activated in SF from patients with RA, as shown by a significantly increased expression of CD16, CD40, CD54 and HLA-DR by SF-derived monocytes (SFM) relative to paired PBM (n=7, Figure 1D). In order to investigate the effect of monocytes with an activated phenotype on human Tregs, we established an in vitro-system using 100ng/ml LPS to activate monocytes from HC. These LPS-treated monocytes (LPS-mono) (n=18) showed a significant upregulation of the activation markers CD40, CD54, CD86 and HLA-DR, but a down-regulation of CD16 compared to medium-treated monocytes (mono-med) (Figure 1E), indicating an in vitro-activated phenotype that is similar but not identical to in vivo-activated SF monocytes.
In vitro-activated monocytes induce cytokine expression in CD4+CD45RO+CD25+CD127low Tregs
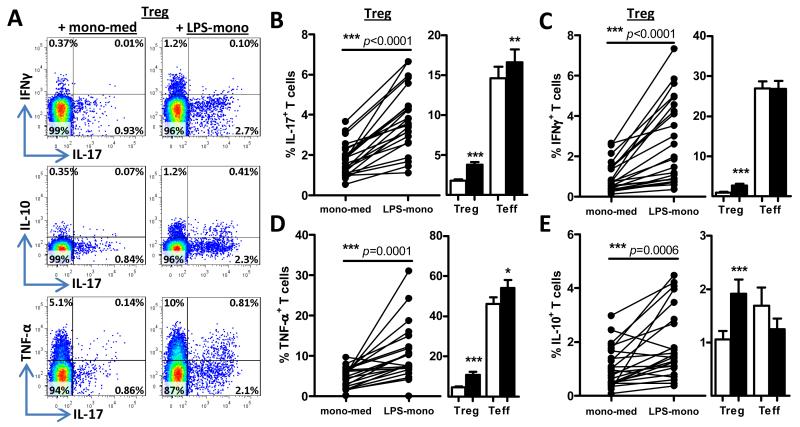
We next assessed the effects of activated monocytes on Treg phenotype and function. CD4+CD45RO+CD25+CD127low T cells (memory Tregs) from healthy controls were sorted to high purity (Supplementary Figure 1) and co-cultured with autologous CD14+ monocytes that had been pre-treated with either 100ng/ml LPS or medium for 30 min followed by extensive washing. Soluble anti-CD3 mAb was added to the co-cultures to activate Tregs. In the presence of LPS-mono, a significant increase in the percentage of memory Tregs expressing IL-17, IFNγ or TNF-α was observed relative to mono-med (Figure 2A-D). Notably, the presence of LPS-mono also significantly increased the percentage of IL-10+ Tregs (Figure 2A, E). In order to determine the relative magnitude of the percentage of cytokine-expressing Tregs, we performed a similar analysis within effector memory T cells, by setting up parallel co-cultures of CD4+CD45RO+CD25low/−CD127+ T cells (Teff) with medium-treated or LPS-activated monocytes. This analysis showed that although pro-inflammatory cytokine-expressing Tregs were increased in the presence of activated monocytes, the percentage was still relatively low compared to pro-inflammatory cytokine-expressing Teff (e.g. 3.8±0.4% vs. 17±1.6% IL-17+ cells) (Figure 2B-D). The percentage of pro-inflammatory cytokine-expressing Tregs was also low compared to unsorted memory CD4+ T cells (data not shown). In contrast, the percentage of IL-10+ cells was highest in Tregs following incubation with activated monocytes (Figure 2E). Of note, although pro-inflammatory cytokine-expressing regulatory T cells are not “Tregs” per definition, for the purpose of clarity, we will continue to refer to these cytokine-expressing CD4+CD45RO+CD25+CD127low T cells as Tregs in this paper.
Figure 2. In vitro-activated monocytes induce cytokine expression in CD4+CD45RO+CD25+CD127low Tregs.
(A-E) Sorted CD4+CD45RO+CD25+CD127low (Tregs) or CD4+CD45RO+CD25−/lowCD127+ T cells (Teff) were co-cultured with autologous CD14+ monocytes, which were either pre-treated with medium or LPS, in the presence of anti-CD3 mAb. After 3 days, cells were re-stimulated with PMA and ionomycin for 6 hours, with GolgiStop present for the last 3 hours and intracellularly stained for IL-17 (B), IFNγ (C), TNF-α (D) and IL-10 (E). Dot plots of one representative experiment from co-cultures of Tregs with monocytes (A), and the individual (Treg) and cumulative data from 18-23 experiments (B-E) of either Treg or Teff co-cultures with monocytes are shown. Results are plotted as % of CD2+CD14− cells expressing the indicated cytokine (mean ± SEM); data were analysed using Wilcoxon matched-pairs signed rank test: * p<0.05, ** p<0.01, *** p<0.001.
Cytokine-activated monocytes as well as CD14+CD16− and CD14+CD16+ subsets can induce pro-inflammatory cytokine expression in memory Tregs
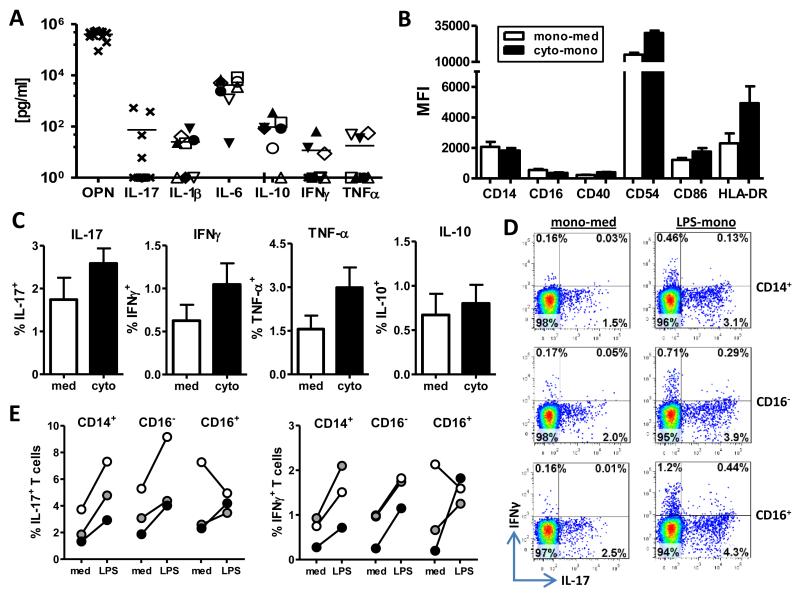
The composition of RA synovial fluid varies between patients and contains varying levels of pro- and anti-inflammatory cytokines (Figure 3A) (24). To mimic the environment monocytes may be exposed to in the inflamed joint, we incubated monocytes with either medium (mono-med) or a cocktail of cytokines commonly associated with SF (IL-1β, IL-6, IL-17, IFNγ, IL-10, TNF-α and OPN) (cyto-mono) for 30 min, followed by extensive washing. Monocytes were cultured overnight and their phenotype assessed. A consistent increase in the expression of the monocyte activation markers CD40, CD54, CD86 and HLA-DR, but a concomitant decrease in CD16 was found on cytokine-activated monocytes compared to mono-med (Figure 3B, n=5), indicating that these cytokine-activated monocytes are similar, but not identical to in vivo-activated SF monocytes (Figure 1D). Co-culture experiments revealed that cytokine-activated monocytes also increased the percentage of memory Tregs expressing IL-17, IFNγ or TNF-α, and to a lesser extent IL-10 (Figure 3C).
Figure 3. Cytokine-activated monocytes as well as CD14+CD16− and CD14+CD16+ subsets can induce pro-inflammatory cytokine expression in memory Tregs.
(A) The presence of cytokines in RA SF (n=8-13) was determined by ELISA or a FlowCytomix Th1/Th2 assay for the indicated cytokines. For TNF-α, IL-1β, IL-6, IFNγ and IL-10 identical symbols reflect the same sample. (B) Expression of the indicated markers was determined on cytokine-activated monocytes (cyto-mono) and compared to medium-treated monocytes (mono-med) from HC (n=5). (C) Memory Tregs were co-cultured with medium-treated (med) or cytokine-activated monocytes (cyto) from HC in the presence of anti-CD3 mAb for 3 days and the percentage of cytokine-expressing T cells was assessed by ICCS (n=5). (D, E) Memory Tregs were co-cultured with MACS-isolated total CD14+ monocytes, or with sorted CD14+CD16− or CD14+CD16+ monocytes in the presence (LPS) or absence (med) of LPS, with anti-CD3 mAb added. The percentage of IL-17+ or IFNγ+ T cells was analysed at day 3 by ICCS after re-stimulation with PMA and ionomycin. Dot plots of one representative experiment (D) and the individual data of 3 experiments (E) are shown.
It was shown recently that CD14+CD16+ monocytes are increased in PB of patients with RA and that this subpopulation of monocytes is most potent in the induction of IL-17 production by CD4+ T cells (25). In agreement with that study, we observed a significant increase in CD14+CD16+ monocytes in PB of patients with RA (n=22) compared to HC (n=26) (10±1.2% vs. 7±0.6% of CD14+ monocytes, p=0.016, data not shown). Since MACS-isolated CD14+ monocytes contain both “pro-inflammatory” CD14+CD16+ and “classical” CD14+CD16− populations, we sorted CD14+ monocytes into CD16− and CD16+ cells (Supplementary Figure 2) and co-cultured them with memory Tregs with or without LPS. Both CD16+-depleted (CD16−) and CD14+CD16+ (CD16+) monocytes showed a similar capacity as total CD14+ monocytes to induce pro-inflammatory IL-17 and IFNγ expression in memory Tregs (Figure 3D, E). Together, these findings indicate that TLR4-stimulated as well as cytokine-activated monocytes can induce pro-inflammatory cytokine expression in CD4+CD45RO+CD25+CD127low Tregs, and that this is not a unique feature of a particular CD14+ monocyte population.
The increased expression of IL-17 and IFNγ in memory Tregs is driven by monocyte-derived IL-1β, IL-6 and TNF-α
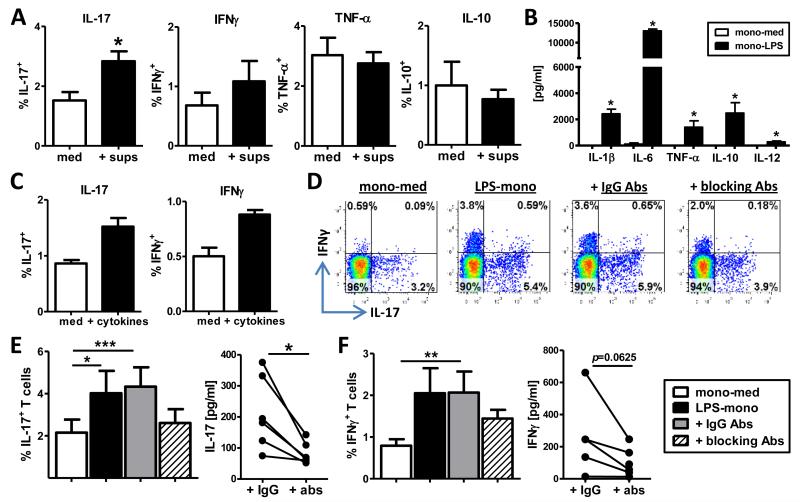
To determine the mechanism via which activated monocytes drive the observed increase in cytokine-expressing Tregs, we first investigated whether soluble factors were involved. Monocytes were pre-activated with LPS, extensively washed and cultured for 40hrs. Supernatants were collected and transferred to monocyte-Treg co-cultures and intracellular cytokine expression was determined at day 3. The addition of supernatants from activated monocytes led to a significant increase in IL-17+ Tregs, and a trend towards increased IFNγ+ Tregs (p=0.09, n=6), whilst IL-10 and TNF-α expression were unaffected (Figure 4A). To elucidate which soluble factors secreted by LPS-mono could drive the increased expression of pro-inflammatory IL-17 and IFNγ in memory Tregs, supernatants from mono-med and LPS-mono were analysed by a 25-plex cytokine array. LPS-mono produced significantly increased amounts of pro-inflammatory (IL-1β, IL-6, TNF-α, IL-12p40/p70) and anti-inflammatory (IL-10) cytokines (Figure 4B), which are also present in SF of patients with RA (Figure 3A and data not shown). IL-1β, IL-6 and TNF-α are known inflammatory mediators in the pathogenesis of RA and can drive the induction of IL-17-producing T cells (26). To test the role of these cytokines, we performed reconstitution and blocking experiments. Addition of hrIL-1β, IL-6 and TNF-α to co-cultures of memory Tregs with monocytes led to an increase in the percentage of IL-17+ and IFNγ+ Tregs compared to medium control (Figure 4C). Conversely, neutralisation of IL-1β, IL-6 and TNF-α during co-culture of memory Tregs with LPS-mono consistently prevented the increase in IL-17 expression and to some extent IFNγ (Figure 4D-F), but did not affect IL-10 or TNF-α expression (data not shown). The addition of neutralising Abs also reduced IL-17 and to a lesser extent IFNγ secretion (Figure 4E, F).
Figure 4. Increased IL-17 and IFNγ expression in memory Tregs is mediated by monocyte-derived IL-1β, IL-6 and TNF-α.
(A) CD4+CD45RO+CD25+CD127low Tregs were co-cultured with mono-med alone or with the addition of supernatants from LPS-pre-treated monocytes (sups) in the presence of anti-CD3 mAb. The percentage of cytokine-expressing T cells was determined as described in Figure 2 (mean ± SEM, n=6). (B) Monocytes from HC were pre-treated with medium or LPS (100ng/ml) and cultured for 40 hours. Supernatants were quantified by 25-plex cytokine array (mean ± SEM, n=6). (C) Sorted memory Tregs were co-cultured with mono-med for 3 days with or without hrIL-1β, IL-6 and TNF-α, and IL-17 and IFNγ expression assessed (n=3). (D-F) Memory Tregs were co-cultured with mono-med, LPS-mono or LPS-mono and neutralising antibodies against IL-1β, IL-6 and TNF-α (blocking Abs) or the appropriate IgG isotype controls (IgG Abs). At day 3, cells were analysed for IL-17 (E) and IFNγ (F) expression by ICCS and secretion by ELISA. One representative experiment (D) and the cumulative data (mean ± SEM, n=6) (E, F) are shown. Data were analysed by Wilcoxon matched-pairs signed rank test or Friedman test with Dunn’s Multiple Comparison Test: * p<0.05, ** p<0.01, *** p<0.001.
CD4+CD45RO+CD25+CD127low Tregs maintain a regulatory phenotype after co-culture with in vitro-activated monocytes
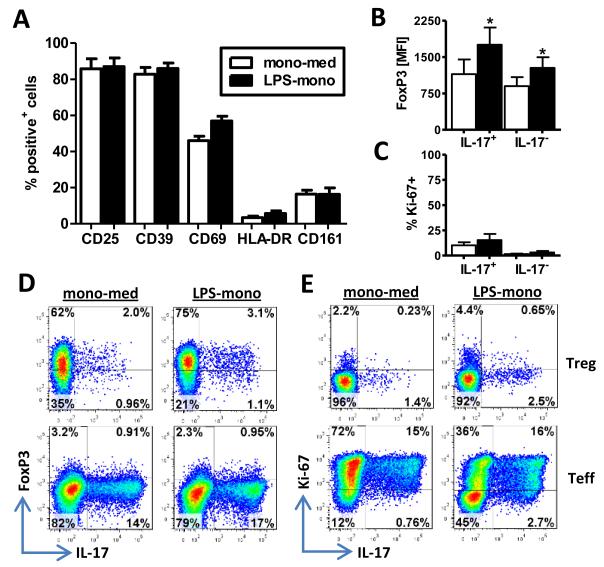
We determined whether CD4+CD45RO+CD25+CD127low Tregs still displayed a regulatory phenotype following incubation with activated monocytes. After co-culture with LPS-mono, Tregs remained positive for CD25 and CD39 (Figure 5A). A slight increase was seen in the percentage of cells positive for CD69 and HLA-DR, indicating an activated status of the cells. Despite the increase in IL-17-expressing cells, we did not observe an increase in the Th17 marker CD161 (27) (Figure 5A). IL-17+ Tregs showed sustained FoxP3 expression following co-culture with activated monocytes (Figure 5B, D). In fact, FoxP3 expression in LPS-mono-activated Tregs was significantly higher in both IL-17+ and IL-17− cells compared to their respective mono-med-cultured counterparts (Figure 5B, D). In contrast, virtually all IL-17+ Teff, which were induced under the same conditions, were FoxP3-negative (Figure 5D). IL-17+ Tregs from co-cultures with both mono-med and LPS-mono contained a low percentage of Ki-67+ cells (10±3.1% and 15±6.1%, respectively) (Figure 5C, E) whilst IL-17+ Teff were pre-dominantly Ki-67+ (91±2.7% and 75±5.7%) (Figure 5E, data not shown). Together, these data demonstrate that although CD4+CD45RO+CD25+CD127low Tregs show increased pro-inflammatory cytokine expression following co-culture with activated monocytes, their Treg phenotype is maintained.
Figure 5. CD4+CD45RO+CD25+CD127low T cells maintain a Treg phenotype after co-culture with activated monocytes.
(A) CD4+CD45RO+CD25+CD127low Tregs were co-cultured with mono-med or LPS-mono in the presence of anti-CD3 mAb. At day 3, cells were stained for CD69, CD25, CD39, HLA-DR and CD161. Data (n=3, mean ± SEM) are shown as the % of CD2+CD14− cells expressing the indicated marker. (B-E) Memory Tregs or Teff of a three day culture with monocytes were intranuclearly stained for FoxP3, Ki-67 and IL-17 after a PMA/ionomycin re-stimulation. Data depict the average data (mean ± SEM, n=6) of FoxP3 expression (B) and the percentage of Ki-67-positive cells within IL-17+ and IL-17− CD2+CD14− cells (C) with representative dot plots showing FoxP3 vs. IL-17 (D) and Ki-67 vs. IL-17 (E) for Tregs and Teff. Data were analysed using Wilcoxon matched-pairs signed rank test: * p<0.05.
Activated memory Tregs show an enhanced capacity to suppress cytokine secretion and T cell proliferation
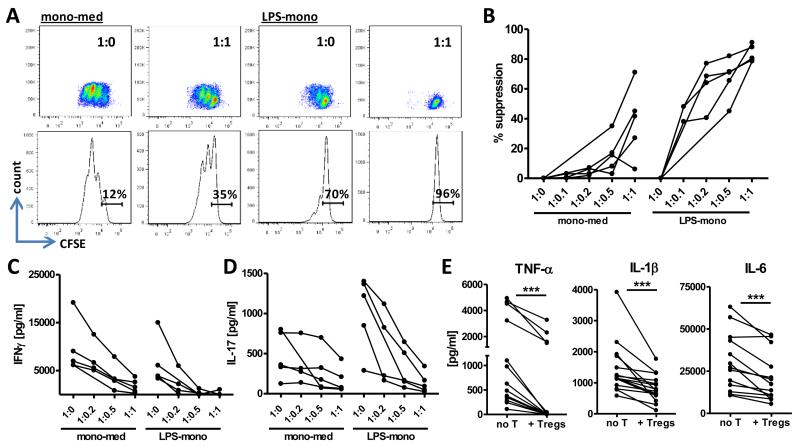
Finally, we determined whether enhanced pro-inflammatory cytokine expression in CD4+CD45RO+CD25+CD127low Tregs impaired their ability to suppress T cell proliferation as well as monocyte- and T cell-derived cytokine production. We co-cultured memory Tregs with either mono-med or LPS-mono in the presence of anti-CD3 mAb overnight at various cell ratios to allow interaction between cells. The next day, CFSE-labelled, autologous Teff were added to the cultures and proliferation was assessed by flow cytometry two days later. Teff proliferated strongly in the presence of mono-med, which was suppressed by the presence of Tregs (Figure 6A, B). Although Teff proliferated less profoundly in the presence of LPS-activated monocytes, their proliferation was still strongly suppressed by the presence of Tregs. In fact, when we calculated the percentage of suppression of proliferation, we found an increased suppressive capacity when Tregs were pre-cultured with LPS-mono at all Teff:Treg ratios (Figure 6B). We also assessed the suppressive effects of Tregs on the secretion of IL-17 and IFNγ by Teff, and found that following interaction with LPS-mono, memory Tregs were more efficient in suppressing cytokine secretion, which was particularly evident for IL-17 (Figure 6C, D); LPS-mono-activated Tregs suppressed IL-17 secretion already at a 1:0.2 ratio (Teff:Treg) whilst mono-med-cultured Tregs in most cases only suppressed at a 1:1 ratio (Figure 6D). Finally, the addition of memory Tregs to cultures of LPS-activated monocytes significantly suppressed the secretion of TNF-α, IL-1β and IL-6 by monocytes (Figure 6E), but did not affect IL-10 secretion (data not shown). Overall, these data indicate that monocyte-activated memory Tregs, despite an increased expression of pro-inflammatory cytokines, maintain their Treg phenotype and function, and in fact show an enhanced capacity to suppress T cell proliferation and IL-17 production.
Figure 6. Activated memory Tregs show an enhanced capacity to suppress T cell proliferation and IL-17 production.
(A-D) Sorted CD4+CD45RO+CD25+CD127low Tregs (1-5×104) were co-cultured overnight with 5×104 mono-med or LPS-mono in the presence of anti-CD3 mAb at the indicated cell ratios. The next day, CFSE-labelled memory Teff (5×104) were added to the co-cultures and proliferation was assessed on day 3. Dot plots and histograms from one representative experiment (A) and the percentage suppression of proliferation for the individual experiments (n=5) (B) are shown. Supernatants from the co-cultures in (A, B) were analysed for IFNγ (C) and IL-17 secretion (D) by ELISA. (E) Supernatants from cell cultures of monocytes with or without Tregs (no T) were collected at day 3 and analysed for TNF-α (n=14), IL-1β (n=15) and IL-6 (n=13) secretion. Analysis was performed using Wilcoxon matched-pairs signed rank test: *** p<0.001.
Discussion
Here we show that human CD4+CD45RO+CD25+CD127low Tregs can be induced to express pro-inflammatory (IL-17, IFNγ, TNF-α) but also anti-inflammatory (IL-10) cytokines upon interaction with activated monocytes. Importantly, despite the observed increase in pro-inflammatory cytokine expression, Tregs maintain their regulatory phenotype and appear enhanced, rather than impaired, in their ability to suppress T cell proliferation and cytokine production, and IL-17 in particular. Our data suggest that cytokine-expressing Tregs at sites of inflammation may still exert potent immune suppression.
We found that the increase in IL-17+ and to a lesser extent IFNγ+ Tregs was driven by monocyte-derived IL-6, TNF-α and IL-1β. TNF-α was the most abundantly induced cytokine in Tregs following interaction with activated monocytes, and TNF-α+ Tregs have also been observed in other in vitro studies (6, 7, 11). However, the mechanism for induction of TNF-α expression in Tregs appeared different as the addition of supernatants from activated monocytes did not increase TNF-α expression. The induction of IL-10 expression in T cells was relatively small overall, but the percentage of IL-10+ cells was highest in LPS-mono-activated Tregs (1.9±0.3%). It should be noted that the cytokine expression we report represents the cytokine profile following PMA and ionomycin restimulation. Although this is a very commonly used system for the detection of cytokine-expressing cells, the levels reported may not reflect the actual levels of cytokine-secreting cells.
Various other reports indicate that human CD4+CD25+FoxP3+ regulatory T cells comprise a heterogeneous cell population that can display plasticity during development, differentiation and when exposed to a pro-inflammatory environment (reviewed in (3, 4)). It was shown that human Tregs can be induced in vitro to express the pro-inflammatory cytokine IL-17 following stimulation with α-CD3/CD28 mAb-coated beads in the presence of hrIL-1β and IL-2 (7, 10-13). IL-17+ Tregs have also been found in vivo at sites of inflammation including periodontitis lesions (8), psoriatic skin (9), human tonsils (7), and the lamina propria of patients with Crohn’s disease (28). Furthermore, Tregs from patients with psoriasis showed an enhanced propensity to differentiate into IL-17-producing cells, which was accompanied by decreased FoxP3 and increased Rorc2 expression (9). These findings overall suggest that Tregs from inflammatory sites may convert into IL-17-producing cells.
The key question is whether human pro-inflammatory cytokine-expressing Tregs maintain their suppressive capacity. Two groups reported that the induction of IL-17 expression in naïve Tregs under inflammatory conditions was accompanied by impaired suppression (11, 12). Single-cell cloning experiments further suggested that Tregs can transiently lose their suppressive function when actively secreting IL-17, but FoxP3 expression was not affected (13). In contrast, IL-17+ Treg clones (6, 7, 13) as well as IL-17+ Tregs from the inflamed intestinal mucosa of patients with Crohn’s disease, were shown to be suppressive (28). In diabetic patients, IFNγ+ Tregs were reported to be increased, but these cells expressed high levels of FoxP3, and possessed suppressive activity (29). Furthermore, IFNγ production by Tregs was recently suggested to be essential for the prevention of Graft-versus-Host disease (30). Although care needs to be taken when extrapolating in vitro data into the in vivo situation as exemplified by a preclinical model for xenogeneic Graft-versus-Host-Disease (31), our data suggest that Tregs exposed to an inflammatory environment may have enhanced suppressive effects particularly on T cell proliferation and IL-17 production, despite the fact that Tregs themselves become more IL-17+. Th17 cells are thought to be more resistant to Treg-mediated suppression (32-35), and our findings that an increase in IL-17+ Tregs corresponds with enhanced suppression of Th17 cells are in line with elegant studies in mice demonstrating that Tregs adapt to their cytokine milieu through the upregulation of specific transcription factors, thus ensuring appropriate T helper-specific control of inflammation (36-38). Together, these data suggest that the induction of pro-inflammatory cytokine expression in Tregs is not indicative per se of a conversion towards a less suppressive and/or more pathogenic function. Instead, this should be seen in context of the other cytokines Tregs produce (e.g. IL-10), their level of cytokine expression relative to Teff, and their regulatory phenotype and function.
Our findings may have physiological relevance since we show that memory Tregs and activated monocytes are present in abundance in the inflamed rheumatic joint. The well-documented presence of T cells with a regulatory phenotype and function in SF from patients with RA (16-23) thus leaves the paradox as to why inflammation persists despite the presence of these potentially suppressive cells. It has been shown that addition of pro-inflammatory mediators such as IL-7 or TNF-α in vitro can break Treg function (39). TNF-α was also found to downregulate FoxP3 expression and function in humans Tregs (40). Furthermore, DC-derived IL-6 was shown to abrogate Treg-mediated suppression in mice (41), to increase IL-17 production (42), and to induce a loss in FoxP3 expression, which was exacerbated in the presence of IL-1β (43). Inhibition of IL-6 using tocilizumab, a humanised anti-IL-6R antibody, was shown to correct for the imbalance of Th17 cells to Tregs in RA (44) and enhanced the suppressive capacity of SF-derived Tregs (45). A recent study showed that the ability to control monocyte-derived IL-6 production was critical for the ability of Tregs to suppress Th17 responses in patients with RA (46). It should be noted however, that the aforementioned studies do not always allow a distinction between impaired Treg function vs. increased resistance of Teff to suppression. Our data also show a distinct effect of IL-1β, IL-6 and TNF-α on Tregs, since we found that increased IL-17 and IFNγ expression in Tregs was driven by these cytokines. Notwithstanding the above mentioned effects of TNF-α, IL-6 and IL-1β on Treg phenotype and function in vitro, it is evident that SF-derived Tregs are fully suppressive ex vivo (17-23, 47) and in fact, may even be more suppressive than their PB counterparts (17, 47). Recent data also revealed a fully demethylated FoxP3 promoter region in SF-derived Tregs, indicating that these cells may indeed be “true” Tregs, despite low level IL-17 and IFNγ expression upon stimulation (45). Thus, Tregs at sites of inflammation may not be intrinsically defective. Instead, possible defects in immunoregulation may reside within the activated Teff population that becomes resistant to Treg-mediated suppression (17, 48-50).
In conclusion, our data - together with the existing literature - provide evidence that Tregs that have been exposed to an inflammatory environment express pro-inflammatory cytokines but may maintain their suppressive capacity, and in fact may be strengthened rather than weakened in their function.
Supplementary Material
Acknowledgements
The authors are grateful to all donors and patients who consented to participate in this study. This work was supported by Arthritis Research UK, the IMI JU funded project BeTheCure, contract no 115142-2, by the National Institute for Health Research (NIHR) Biomedical Research Centre based at Guy’s and St Thomas’ NHS Foundation Trust and King’s College London. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health. We would like to thank Susanne Heck, Helen Graves and Pj Chana for their help in cell sorting at the BRC Flow Core Facility. The authors would like to acknowledge Ms Cristina Blanco-Gil for help in patient recruitment and data collection.
This work was financially supported by Arthritis Research UK, the IMI JU funded project BTCure 115142-2, and the Department of Health via the National Institute for Health Research (NIHR) Biomedical Research Centre (BRC) award to Guy’s & St Thomas’ NHS Foundation Trust in partnership with King’s College London and King’s College Hospital NHS Foundation Trust.
Footnotes
BWK has received consultancies, speaking fees or honoraria (<$10,000) from Abbott, Pfizer, UCB, Roche, BMS. The other authors report no conflicts of interests related to this work.
References
- 1.Sakaguchi S, Miyara M, Costantino CM, Hafler DA. FOXP3+ regulatory T cells in the human immune system. Nat Rev Immunol. 2010;10(7):490–500. doi: 10.1038/nri2785. [DOI] [PubMed] [Google Scholar]
- 2.Shevach EM. Mechanisms of Foxp3+ T regulatory cell-mediated suppression. Immunity. 2009;30(5):636–45. doi: 10.1016/j.immuni.2009.04.010. [DOI] [PubMed] [Google Scholar]
- 3.Hori S. Developmental plasticity of Foxp3+ regulatory T cells. Curr Opin Immunol. 2010;22(5):575–82. doi: 10.1016/j.coi.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 4.Zhou X, Bailey-Bucktrout S, Jeker LT, Bluestone JA. Plasticity of CD4(+) FoxP3(+) T cells. Curr Opin Immunol. 2009;21(3):281–5. doi: 10.1016/j.coi.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miyara M, Yoshioka Y, Kitoh A, Shima T, Wing K, Niwa A, et al. Functional delineation and differentiation dynamics of human CD4+ T cells expressing the FoxP3 transcription factor. Immunity. 2009;30(6):899–911. doi: 10.1016/j.immuni.2009.03.019. [DOI] [PubMed] [Google Scholar]
- 6.Ayyoub M, Deknuydt F, Raimbaud I, Dousset C, Leveque L, Bioley G, et al. Human memory FOXP3+ Tregs secrete IL-17 ex vivo and constitutively express the T(H)17 lineage-specific transcription factor RORgamma t. Proc Natl Acad Sci U S A. 2009;106(21):8635–40. doi: 10.1073/pnas.0900621106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Voo KS, Wang YH, Santori FR, Boggiano C, Arima K, Bover L, et al. Identification of IL-17-producing FOXP3+ regulatory T cells in humans. Proc Natl Acad Sci U S A. 2009;106(12):4793–8. doi: 10.1073/pnas.0900408106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Okui T, Aoki Y, Ito H, Honda T, Yamazaki K. The presence of IL-17+/FOXP3+ double-positive cells in periodontitis. J Dent Res. 2012;91(6):574–9. doi: 10.1177/0022034512446341. [DOI] [PubMed] [Google Scholar]
- 9.Bovenschen HJ, van de Kerkhof PC, van Erp PE, Woestenenk R, Joosten I, Koenen HJ. Foxp3+ Regulatory T Cells of Psoriasis Patients Easily Differentiate into IL-17A-Producing Cells and Are Found in Lesional Skin. J Invest Dermatol. 2011;131(9):1853–60. doi: 10.1038/jid.2011.139. [DOI] [PubMed] [Google Scholar]
- 10.Koenen HJ, Smeets RL, Vink PM, van Rijssen E, Boots AM, Joosten I. Human CD25highFoxp3pos regulatory T cells differentiate into IL-17-producing cells. Blood. 2008;112(6):2340–52. doi: 10.1182/blood-2008-01-133967. [DOI] [PubMed] [Google Scholar]
- 11.Deknuydt F, Bioley G, Valmori D, Ayyoub M. IL-1beta and IL-2 convert human Treg into T(H)17 cells. Clin Immunol. 2009;131(2):298–307. doi: 10.1016/j.clim.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 12.Valmori D, Raffin C, Raimbaud I, Ayyoub M. Human RORgammat+ TH17 cells preferentially differentiate from naive FOXP3+Treg in the presence of lineage-specific polarizing factors. Proc Natl Acad Sci U S A. 2010;107(45):19402–7. doi: 10.1073/pnas.1008247107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Beriou G, Costantino CM, Ashley CW, Yang L, Kuchroo VK, Baecher-Allan C, et al. IL-17-producing human peripheral regulatory T cells retain suppressive function. Blood. 2009;113(18):4240–9. doi: 10.1182/blood-2008-10-183251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wilke CM, Bishop K, Fox D, Zou W. Deciphering the role of Th17 cells in human disease. Trends Immunol. 2011;32(12):603–11. doi: 10.1016/j.it.2011.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Evans HG, Gullick NJ, Kelly S, Pitzalis C, Lord GM, Kirkham BW, et al. In vivo activated monocytes from the site of inflammation in humans specifically promote Th17 responses. Proc Natl Acad Sci U S A. 2009;106(15):6232–7. doi: 10.1073/pnas.0808144106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Michels-van Amelsfort JM, Walter GJ, Taams LS. CD4(+)CD25(+) regulatory T cells in systemic sclerosis and other rheumatic diseases. Expert Rev Clin Immunol. 2011;7(4):499–514. doi: 10.1586/eci.11.28. [DOI] [PubMed] [Google Scholar]
- 17.van Amelsfort JM, Jacobs KM, Bijlsma JW, Lafeber FP, Taams LS. CD4(+)CD25(+) regulatory T cells in rheumatoid arthritis: differences in the presence, phenotype, and function between peripheral blood and synovial fluid. Arthritis Rheum. 2004;50(9):2775–85. doi: 10.1002/art.20499. [DOI] [PubMed] [Google Scholar]
- 18.Cao D, Malmstrom V, Baecher-Allan C, Hafler D, Klareskog L, Trollmo C. Isolation and functional characterization of regulatory CD25brightCD4+ T cells from the target organ of patients with rheumatoid arthritis. Eur J Immunol. 2003;33(1):215–23. doi: 10.1002/immu.200390024. [DOI] [PubMed] [Google Scholar]
- 19.Cao D, van Vollenhoven R, Klareskog L, Trollmo C, Malmstrom V. CD25brightCD4+ regulatory T cells are enriched in inflamed joints of patients with chronic rheumatic disease. Arthritis Res Ther. 2004;6(4):R335–46. doi: 10.1186/ar1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu MF, Wang CR, Fung LL, Lin LH, Tsai CN. The presence of cytokine-suppressive CD4+CD25+ T cells in the peripheral blood and synovial fluid of patients with rheumatoid arthritis. Scand J Immunol. 2005;62(3):312–7. doi: 10.1111/j.1365-3083.2005.01656.x. [DOI] [PubMed] [Google Scholar]
- 21.Mottonen M, Heikkinen J, Mustonen L, Isomaki P, Luukkainen R, Lassila O. CD4+ CD25+ T cells with the phenotypic and functional characteristics of regulatory T cells are enriched in the synovial fluid of patients with rheumatoid arthritis. Clin Exp Immunol. 2005;140(2):360–7. doi: 10.1111/j.1365-2249.2005.02754.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Benito-Miguel M, Garcia-Carmona Y, Balsa A, Perez de Ayala C, Cobo-Ibanez T, Martin-Mola E, et al. A dual action of rheumatoid arthritis synovial fibroblast IL-15 expression on the equilibrium between CD4+CD25+ regulatory T cells and CD4+CD25-responder T cells. J Immunol. 2009;183(12):8268–79. doi: 10.4049/jimmunol.0900007. [DOI] [PubMed] [Google Scholar]
- 23.Raghavan S, Cao D, Widhe M, Roth K, Herrath J, Engstrom M, et al. FOXP3 expression in blood, synovial fluid and synovial tissue during inflammatory arthritis and intra-articular corticosteroid treatment. Ann Rheum Dis. 2009;68(12):1908–15. doi: 10.1136/ard.2008.100768. [DOI] [PubMed] [Google Scholar]
- 24.McInnes IB, Schett G. Cytokines in the pathogenesis of rheumatoid arthritis. Nat Rev Immunol. 2007;7(6):429–42. doi: 10.1038/nri2094. [DOI] [PubMed] [Google Scholar]
- 25.Rossol M, Kraus S, Pierer M, Baerwald C, Wagner U. The CD14(bright) CD16+ monocyte subset is expanded in rheumatoid arthritis and promotes Th17 expansion. Arthritis Rheum. 2011;64(3):671–7. doi: 10.1002/art.33418. [DOI] [PubMed] [Google Scholar]
- 26.Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 Cells. Annu Rev Immunol. 2009;27:485–517. doi: 10.1146/annurev.immunol.021908.132710. [DOI] [PubMed] [Google Scholar]
- 27.Cosmi L, De Palma R, Santarlasci V, Maggi L, Capone M, Frosali F, et al. Human interleukin 17-producing cells originate from a CD161+CD4+ T cell precursor. J Exp Med. 2008;205(8):1903–16. doi: 10.1084/jem.20080397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hovhannisyan Z, Treatman J, Littman DR, Mayer L. Characterization of interleukin-17-producing regulatory T cells in inflamed intestinal mucosa from patients with inflammatory bowel diseases. Gastroenterology. 2011;140(3):957–65. doi: 10.1053/j.gastro.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McClymont SA, Putnam AL, Lee MR, Esensten JH, Liu W, Hulme MA, et al. Plasticity of human regulatory T cells in healthy subjects and patients with type 1 diabetes. J Immunol. 2011;186(7):3918–26. doi: 10.4049/jimmunol.1003099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Koenecke C, Lee CW, Thamm K, Fohse L, Schafferus M, Mittrucker HW, et al. IFN-gamma Production by Allogeneic Foxp3+ Regulatory T Cells Is Essential for Preventing Experimental Graft-versus-Host Disease. J Immunol. 2012;189(6):2890–6. doi: 10.4049/jimmunol.1200413. [DOI] [PubMed] [Google Scholar]
- 31.Vercoulen Y, Guichelaar T, Meerding J, Emmelot M, Pingen M, Storm G, et al. Application of cultured human regulatory T cells requires preclinical in vivo evaluation. J Allergy Clin Immunol. 2012;129(3):852–5 e3. doi: 10.1016/j.jaci.2011.10.037. [DOI] [PubMed] [Google Scholar]
- 32.Evans HG, Suddason T, Jackson I, Taams LS, Lord GM. Optimal induction of T helper 17 cells in humans requires T cell receptor ligation in the context of Toll-like receptor-activated monocytes. Proc Natl Acad Sci U S A. 2007;104(43):17034–9. doi: 10.1073/pnas.0708426104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lohr J, Knoechel B, Wang JJ, Villarino AV, Abbas AK. Role of IL-17 and regulatory T lymphocytes in a systemic autoimmune disease. J Exp Med. 2006;203(13):2785–91. doi: 10.1084/jem.20061341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Annunziato F, Cosmi L, Santarlasci V, Maggi L, Liotta F, Mazzinghi B, et al. Phenotypic and functional features of human Th17 cells. J Exp Med. 2007;204(8):1849–61. doi: 10.1084/jem.20070663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.O’Connor RA, Taams LS, Anderton SM. Translational mini-review series on Th17 cells: CD4 T helper cells: functional plasticity and differential sensitivity to regulatory T cell-mediated regulation. Clin Exp Immunol. 2010;159(2):137–47. doi: 10.1111/j.1365-2249.2009.04040.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chaudhry A, Rudra D, Treuting P, Samstein RM, Liang Y, Kas A, et al. CD4+ regulatory T cells control TH17 responses in a Stat3-dependent manner. Science. 2009;326(5955):986–91. doi: 10.1126/science.1172702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Koch MA, Tucker-Heard G, Perdue NR, Killebrew JR, Urdahl KB, Campbell DJ. The transcription factor T-bet controls regulatory T cell homeostasis and function during type 1 inflammation. Nat Immunol. 2009;10(6):595–602. doi: 10.1038/ni.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zheng Y, Chaudhry A, Kas A, deRoos P, Kim JM, Chu TT, et al. Regulatory T-cell suppressor program co-opts transcription factor IRF4 to control T(H)2 responses. Nature. 2009;458(7236):351–6. doi: 10.1038/nature07674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.van Amelsfort JM, van Roon JA, Noordegraaf M, Jacobs KM, Bijlsma JW, Lafeber FP, et al. Proinflammatory mediator-induced reversal of CD4+,CD25+ regulatory T cell-mediated suppression in rheumatoid arthritis. Arthritis Rheum. 2007;56(3):732–42. doi: 10.1002/art.22414. [DOI] [PubMed] [Google Scholar]
- 40.Valencia X, Stephens G, Goldbach-Mansky R, Wilson M, Shevach EM, Lipsky PE. TNF downmodulates the function of human CD4+CD25hi T-regulatory cells. Blood. 2006;108(1):253–61. doi: 10.1182/blood-2005-11-4567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pasare C, Medzhitov R. Toll pathway-dependent blockade of CD4+CD25+ T cell-mediated suppression by dendritic cells. Science. 2003;299(5609):1033–6. doi: 10.1126/science.1078231. [DOI] [PubMed] [Google Scholar]
- 42.Xu L, Kitani A, Fuss I, Strober W. Cutting edge: regulatory T cells induce CD4+CD25-Foxp3-T cells or are self-induced to become Th17 cells in the absence of exogenous TGF-beta. J Immunol. 2007;178(11):6725–9. doi: 10.4049/jimmunol.178.11.6725. [DOI] [PubMed] [Google Scholar]
- 43.Yang XO, Nurieva R, Martinez GJ, Kang HS, Chung Y, Pappu BP, et al. Molecular antagonism and plasticity of regulatory and inflammatory T cell programs. Immunity. 2008;29(1):44–56. doi: 10.1016/j.immuni.2008.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Samson M, Audia S, Janikashvili N, Ciudad M, Trad M, Fraszczak J, et al. Inhibition of IL-6 function corrects Th17/Treg imbalance in rheumatoid arthritis patients. Arthritis Rheum. 2012;64(8):2499–503. doi: 10.1002/art.34477. [DOI] [PubMed] [Google Scholar]
- 45.Herrath J, Muller M, Amoudruz P, Janson P, Michaelsson J, Larsson PT, et al. The inflammatory milieu in the rheumatic joint reduces regulatory T-cell function. Eur J Immunol. 2011;41(8):2279–90. doi: 10.1002/eji.201041004. [DOI] [PubMed] [Google Scholar]
- 46.McGovern JL, Nguyen DX, Notley CA, Mauri C, Isenberg DA, Ehrenstein MR. Th17 cells are restrained by regulatory T cells from patients responding to anti-TNF antibody therapy via inhibition of IL-6. Arthritis Rheum. 2012;64(10):3129–38. doi: 10.1002/art.34565. [DOI] [PubMed] [Google Scholar]
- 47.de Kleer IM, Wedderburn LR, Taams LS, Patel A, Varsani H, Klein M, et al. CD4+CD25bright regulatory T cells actively regulate inflammation in the joints of patients with the remitting form of juvenile idiopathic arthritis. J Immunol. 2004;172(10):6435–43. doi: 10.4049/jimmunol.172.10.6435. [DOI] [PubMed] [Google Scholar]
- 48.Haufe S, Haug M, Schepp C, Kuemmerle-Deschner J, Hansmann S, Rieber N, et al. Impaired suppression of synovial fluid CD4+CD25-T cells from patients with juvenile idiopathic arthritis by CD4+CD25+ Treg cells. Arhritis Rheum. 2011;63(10):3153–62. doi: 10.1002/art.30503. [DOI] [PubMed] [Google Scholar]
- 49.Schneider A, Rieck M, Sanda S, Pihoker C, Greenbaum C, Buckner JH. The effector T cells of diabetic subjects are resistant to regulation via CD4+ FOXP3+ regulatory T cells. J Immunol. 2008;181(10):7350–5. doi: 10.4049/jimmunol.181.10.7350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wehrens EJ, Mijnheer G, Duurland CL, Klein M, Meerding J, van Loosdregt J, et al. Functional human regulatory T cells fail to control autoimmune inflammation due to PKB/c-akt hyperactivation in effector cells. Blood. 2011;118(13):3538–48. doi: 10.1182/blood-2010-12-328187. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.