Abstract
There is no standard therapy for multiple myeloma (MM) relapsing after an autotransplant. We compared the outcomes of a 2nd autotransplant (N=137) with those of an allotransplant (N=152) after non-myeloablative or reduced-intensity conditioning (NST/RIC) in 289 subjects reported to the CIBMTR from 1995–2008. NST/RIC recipients were younger (median age 53 vs. 56 years; p < 0.001) and had a shorter time to progression after their first autotransplant. Non-relapse mortality (NRM) at one-year post-transplant was higher in the NST/RIC cohort, 13% (95% CI, 8–19) vs. 2% (95% CI, 1–5, p = < 0.001). Three year progression-free survival (PFS) and overall survival (OS) for NST/RIC cohort were 6% (95% CI, 3–10%) and 20% (95% CI, 14–27%). Similar outcomes for the autotransplant cohort were 12% (95% CI, 7–19%, p = 0.038) and 46% (95% CI, 37–55%, p = 0.001). In multivariate analyses, risk of death was higher in NST/RIC recipients (HR 2.38 [95% CI, 1.79–3.16], p < 0.001), those with KPS < 90 (HR 1.96 [95% CI, 1.47–2.62], p < 0.001) and transplant before 2004 (HR 1.77 [95% CI, 1.34–2.35] p = < 0.001). In conclusion, NST/RIC was associated with higher TRM and lower survival than an autotransplant. Since disease status was not available for most allotransplant recipients, is not possible to determine which type of transplant is superior after autotransplant failure.
Keywords: Multiple Myeloma, allogeneic, salvage transplant
INTRODUCTION
High-dose chemotherapy followed by autologous hematopoietic cell transplantation is widely used to treat persons with multiple myeloma (MM). However, there is no standard therapy for those who relapse [1, 2]. The outcome of those relapsing after autotransplantation and are also refractory to proteasome inhibitors and immunomodulatory agents is particularly poor [3]. Options for relapsed patients include clinical trials, second autotransplants or an allogeneic stem cell hematopoietic cell transplant. Because of the high morbidity and mortality associated with myeloablative allogeneic transplantation, lower intensity conditioning regimens such as non-myeloablative (NST) or reduced-intensity conditioning (RIC) allogeneic transplants [4] are more commonly used.
There are limited data on the outcomes of NST/RIC in persons with myeloma failing an autotransplant. We used the Center for International Blood and Marrow Transplant Research (CIBMTR) database to compare outcomes of a 2nd autotransplant versus NST/RIC allotransplantation in this setting.
PATIENTS AND METHODS
Data source
The CIBMTR is a voluntary working group of more than 450 transplantation centers worldwide that contribute detailed data on consecutive allogeneic and autologous transplants to a statistical center at the Medical College of Wisconsin. Participating centers are required to register all transplants consecutively; compliance is monitored by on-site audits. Patients are followed up longitudinally, with yearly follow-up. Computerized checks for errors, physicians’ review of submitted data, and on-site audits of participating centers ensure data quality. Observational studies conducted by the CIBMTR are performed with a waiver of informed consent and in compliance with Health Insurance Portability and Accountability Act regulations as determined by the institutional review board and the privacy officer of the Medical College of Wisconsin. All CIBMTR centers contribute to the registration data. Research data are collected on subset of registered patients and include detailed disease and pre-transplantation and post-transplantation clinical information.
Patients
The study population comprised of MM patients <65 years who had relapsed/progressed after prior autologous transplant and subsequently received NST/RIC allogeneic transplant or a 2nd autotransplant between 1995 and 2008. The age limit of 65 was used since most transplant centers would not perform full myeloablative allogeneic transplants in patients 65 or older.
Recipients of planned tandem transplants (n = 931) were excluded from the study. The following allogeneic transplant recipients were excluded: those receiving NST/RIC for graft failure (n = 15) or second malignancies (n = 4) as well as patients who received cord blood transplants (n = 2).
Definitions
The intensity of conditioning regimens was categorized as RIC or NST using established consensus criteria [5]. Previously established criteria for categorizing the degree of HLA matching were used for unrelated donor transplants [6].
Study Endpoints and statistical analysis
Primary outcomes were non-relapse mortality (NRM), progression/relapse, progression-free survival (PFS), and overall survival (OS) after the second transplant. NRM was defined as death from any cause within the first 28 days after transplantation or death thereafter in the absence of relapse/progression. Relapse/progression was defined according to the standard EBMT/IBMTR/ABMTR criteria. Probabilities of NRM and myeloma progression/relapse were calculated using cumulative incidence curves to accommodate competing risks [8, 9]. OS interval was defined as the time from second transplant to death from any cause. PFS interval was defined as the time from second transplant to relapse/progression or death from any cause whichever occurs first. Patients alive without evidence of disease relapse/progression were censored at last follow up. Probabilities of PFS and OS were calculated using the Kaplan-Meier product limit estimate. Other outcomes analyzed included acute and chronic graft versus-host disease (GVHD) and cause of death. Acute GVHD was defined and graded based on the pattern and severity of organ involvement using established criteria [7]. Chronic GVHD was defined as the development of any chronic GVHD based on clinical criteria. Both of these events were summarized by the corresponding cumulative incidence estimate, with death without development of GVHD as the competing risk.
Associations between patient-, disease-, and transplant-related factors and survival were assessed using multivariate Cox proportional hazards regression [10]. The variables considered in the multivariate analysis were: age (< 50 vs. ≥ 50), sex, Karnofsky performance score (KPS), Durie-Salmon (DS) stage, immunochemical subtype of MM, conditioning regimen for second transplant, interval from diagnosis to first transplant, interval from first transplant to relapse/progression, time interval from first to second transplant and the year of second transplant. Stepwise variable selection at a 0.05 significance level was used to identify covariates. In the model, the assumption of proportional hazards was tested for each variable using a time-dependent covariate and graphical methods. All variables considered in the multivariate analysis satisfied the proportionality assumption. All computations were made using the statistical package SAS version 9.1 (SAS Institute, Cary, NC).
RESULTS
Subject-, Disease-, and Transplant-Related Variables (Table 1)
Table 1.
Characteristics of patients who underwent second autologous, HLA-identical or unrelated non-myeloablative/reduced intensity conditioning transplantation as therapy for persistent or recurrent disease after autologous transplantation for Multiple Myeloma in North American, reported to CIBMTR between 1995 and 2008.
| Characteristics of patients | Autologous | Allogeneic | P-value |
|---|---|---|---|
| Patients-related | |||
| Number of patients | 137 | 152 | |
| Number of centers | 54 | 65 | |
| Age at 2nd transplant, median (range), years | 56 (28–65) | 53(32–65) | 0.001* |
| 18–29 | 1 (<1) | 0 (0) | 0.039* |
| 30–39 | 4 ( 3) | 9 ( 6) | |
| 40–49 | 32 (23) | 47 (31) | |
| 50–59 | 60 (44) | 72 (47) | |
| 60–65 | 40 (29) | 24 (16) | |
| Gender | |||
| Male | 84 (61) | 90 (59) | 0.720 |
| Karnofsky score pre-transplant | |||
| ≥90% | 68 (50) | 76 (50) | 0.884 |
| <90% | 55 (40) | 63 (41) | |
| Missing | 14 (10) | 13 ( 9) | |
| Disease-related | |||
| Immunochemical subtype of MM | |||
| IgG | 67 (49) | 63 (41) | 0.004* |
| IgA | 20 (15) | 37 (24) | |
| Light chain | 26 (19) | 36 (24) | |
| Othersa | 7 ( 5) | 12 ( 8) | |
| Missing | 17 (12) | 4 ( 3) | |
| Durie-Salmon stage at diagnosis | |||
| Stage I | 8 ( 6) | 13 ( 9) | 0.293 |
| Stage II | 32 (23) | 41 (27) | |
| Stage III | 80 (58) | 88 (58) | |
| Missing | 17 (13) | 10 ( 7) | |
| Percent of plasma cell prior to transplant | 6 (1–95) | 12 (1–95) | --- |
| Evaluable | 85 (62) | 27 (18) | |
| Albumin prior to transplant | |||
| <3.5 g/dL | 44 (32) | 12 (8) | --- |
| ≥3.5g/dL | 88 (64) | 20 (13) | |
| Missing | 5 ( 4) | 120 (79) | |
| β2-microglobulin level prior to transplant | |||
| <3.5 mg/L | 63 (46) | 19 (13) | --- |
| ≥3.5 mg/L | 22 (16) | 6 ( 4) | |
| Missing | 52 (38) | 127 (84) | |
| Transplant-related | |||
| Conditioning regimen | |||
| Melphalan alone | 116 (85) | 3 ( 2) | <0.001* |
| Melphalan + TBI ± others | 4 ( 3) | 7 ( 5) | |
| Melphalan (no TBI) + others | 11 ( 8) | 58 (38) | |
| TBI (no Melphalan) ± others | 2 ( 1) | 37 (24) | |
| Busulfan + cyclophosphamide ± others | 3 ( 2) | 1 (<1) | |
| Busulfan + fludarabine ± others | 0 ( 0) | 30 (20) | |
| Cyclophosphamide + fludarabine ± others | 0 ( 0) | 16 (11) | |
| Othersb | 1 (<1) | 0 (0) | |
| Disease status prior 2nd transplant | |||
| CR/PR | 54 (39) | 1(<1) | <0.001* |
| MR/NR/SD | 60 (44) | 7 (5) | |
| Relapse/progression | 23 (17) | 25 (16) | |
| Missing | -- | 119 (78) | |
| Graft type | |||
| Bone marrow | 0 ( 0) | 26 (17) | <0.001* |
| Peripheral blood | 137(100) | 126 (83) | |
| Donor type | |||
| Related | -- | 32 | |
| Unrelated | -- | 120 | |
| Reason for 2nd transplant | |||
| Persistent malignancy | 54 (39) | 57 (38) | 0.809 |
| Recurrent malignancy | 83 (61) | 95 (62) | |
| Donor age, years | NA | 37 (19–78) | --- |
| Donor-recipient sex match | |||
| Male-Male | NA | 65 (43) | --- |
| Male-Female | NA | 25 (16) | |
| Female-Male | NA | 33 (22) | |
| Female-Female | NA | 29 (19) | |
| GVHD prophylaxis | |||
| FK506+MTX ± other | NA | 53 (35) | --- |
| FK506 ± other | NA | 41 (27) | |
| MTX+CSA ± other | NA | 9 ( 6) | |
| CSA ± other | NA | 49 (32) | |
| RIC vs. Non-myeloablative | |||
| RIC | NA | 111 (73) | --- |
| Non-myeloablative | NA | 41 (27) | |
| Time from diagnosis to 1st transplant, median(range), months | 7 (4–69) | 8 (<1–119) | 0.114 |
| <12 months | 102 (74) | 110 (72) | 0.790 |
| ≥12 months | 35 (26) | 42 (28) | |
| Time from 1st transplant to relapse/progression, months | 17 (<1–121) | 12 (<1–61) | 0.009* |
| <6 months | 26 (19) | 36 (24) | 0.135 |
| 6–12 months | 22 (16) | 27 (18) | |
| 12–24 months | 39 (28) | 31 (20) | |
| 24–36 months | 24 (18) | 14 ( 9) | |
| >36 months | 26 (19) | 17 (11) | |
| Missing | 0 ( 0) | 27 (18) | |
| Time from 1st to 2nd transplant, median(range), months | 30 (6–122) | 23 (6–78) | 0.014* |
| 12–24 months | 44 (32) | 78 (51) | 0.001* |
| >24 months | 93 (68) | 74 (49) | |
| Year of transplant | |||
| 1995–1996 | 3 ( 2) | 0 ( 0) | <0.001* |
| 1997–1998 | 5 ( 4) | 3 ( 2) | |
| 1999–2000 | 9 ( 7) | 11 ( 7) | |
| 2001–2002 | 16 (12) | 28 (18) | |
| 2003–2004 | 26 (19) | 47 (31) | |
| 2005–2006 | 39 (28) | 54 (36) | |
| 2007–2008 | 39 (28) | 9 ( 6) | |
| Post-transplant | |||
| Maintenance therapy | |||
| No | 68 (50) | 47 (31) | <0.001 |
| Yes | 69 (50) | 17 (11) | |
| Missing | 0 | 88 (58) | |
| DLI given | |||
| Yes | 0 | 19 (13) | <0.001 |
| No | 137 (100) | 133 (88) | |
| Median follow-up of survivors (range), months | 29 (3–97) | 30 (12–98) | |
Abbreviations: TBI = total body irradiation; RIC = reduced intensity; conditioning; FK506 = tacrolimus; CSA = cyclosporine; MTX = methotrexate.
Follow-up completeness index as of 12/31/2009: @ 1year (96%), @ 3 year (92%), and @ 5 year 91%.
- Autologous: non-secretory (n=5), IgD (n=1), and IgM (n=1)
- Allogeneic: non-secretory (n=11), and IgD (n=1)
- Autologous: CY+ARAC+ETOP+NITRO (n=1)
Between 1995 and 2008, 152 subjects received NST/RIC (32 from HLA-identical siblings and 120 from HLA-matched unrelated donors) for relapsed/progressive MM after a prior autotransplant. 137 subjects received a 2nd autotransplant in the same setting. Median follow-up of NST/RIC survivors is 30 months (range, 2–98 months) and 29 months for patients who underwent a 2nd autotransplant (range, 3–97 months). The NST/RIC cohort was younger: median age, 53 years versus 56 years (p = 0.001). Gender distribution and Karnofsky performance score (KPS) were similar.
There was a higher proportion of pts with IgG MM in the autotransplantation cohort (p = 0.004). Stage at diagnosis was similar with 58% of patients in Durie-Salmon stage III in both cohorts.
As expected, conditioning regimens differed between cohorts. Most (85%) recipients of 2nd autotransplant received high-dose melphalan alone. Melphalan containing regimens were used in only 43% of the NST/RIC group. Only 4% of the autotransplant cohort received total body irradiation as part of their conditioning, in contrast to 29% of the NST/RIC.
The amount of missing myeloma related data—beta-2 microglobulin, albumin, and response status prior to 2nd transplant—between the two groups was strikingly different, with 25% of the NST/RIC patients having these data available. There was no meaningful way to compare the disease state prior to transplant in the 2 cohorts. Among autotransplant recipients 54 (39%) were in complete or partial remission where as 78% of the NST/RIC cohort had missing disease status data.
Median interval from diagnosis to first transplant was similar in both cohorts. In contrast, interval from 1st transplant to relapse/progression was significantly shorter in the NST/RIC cohort: 12 months (range, < 1–61 months) vs. 17 months (range, < 1–121 months; p = 0.009) in the autotransplant cohort. Interval from 1st to 2nd transplants was also shorter for the NST/RIC cohort, 23 months (range 6–78 months) versus 30 months (range, 6–122 months; p = 0.014). Between 1995 and 2000, comparable numbers of patients were salvaged with autotransplants and NST/RIC, but between 2001 and 2006, NST/RIC appeared to be favored, while from 2007–2008, the trend appeared to reverse itself, favoring autotransplantation. One-half of subjects receiving an autotransplant received maintenance therapy, but only 11% of the NST/RIC group was reported to receive maintenance; comparisons are again confounded by missing maintenance data for 58% of patients in the NST/RIC group.
Patient outcomes
Table 2 demonstrates unadjusted outcomes. Platelet engraftment at 28 and 60 days was inferior in the NST/RIC group. NRM (Figure 1) was 13% (95% CI, 8–19) at one year for subjects receiving NST/RIC versus 2% (95% CI, 1–5%) for AHCT recipients (p = < 0.001). Three-year probabilities of NRM were 14% (95% CI, 9–20) versus 4% (95% CI, 2–8; p ≤ 0.001). Relapse rates differed at 12 months, favoring the autotransplant group, but this difference did not persist long term (Figure 2). There was a trend toward better 5-year progression rates in the NST/RIC group. PFS results also favored the autotransplant group, with the most striking differences at 12 and 36 months (Figure 3). Finally, OS was far superior at all time points in the autotransplant group (Figure 4).
Table 2.
Unadjusted univariate analysis
| Outcomes | Autologous | NST/RIC | P-value | ||
|---|---|---|---|---|---|
| N | Prob (95% CI) | N | Prob (95% CI) | ||
| Engraftment | |||||
| ANC ≥ 500/mm3 | |||||
| @ 28 days | 137 | 96 (92–98) | 152 | 96 (93–98) | 0.999 |
| Platelets ≥ 20 × 109/L | |||||
| @ 28 days | 137 | 90 (84–94) | 152 | 77 (70–83) | 0.007* |
| @ 60 days | 137 | 93 (89–97) | 152 | 84 (78–89) | 0.009* |
| Acute graft-versus-host disease | |||||
| @ 30 days | NA | 152 | 21 (15–28) | -- | |
| @ 60 days | NA | 35 (28–43) | -- | ||
| Chronic graft-versus-host disease | |||||
| @ 12 months | NA | 152 | 42 (34–50) | -- | |
| @ 36 months | NA | 44 (36–52) | -- | ||
| Relapse/Progression | |||||
| @ 12 months | 137 | 53 (44–61) | 152 | 72 (64–79) | <0.001* |
| @ 36 months | 137 | 84 (76–90) | 80 (73–86) | 0.655 | |
| @ 60 months | 137 | 91 (85–96) | 83 (77–89) | 0.046* | |
| Non-relapse mortality | |||||
| @ 12 months | 137 | 2 (1–5) | 152 | 13 ( 8–19) | <0.001* |
| @ 36 months | 4 (2–8) | 14 ( 9–20) | <0.001* | ||
| @ 60 months | 4 (2–8) | 15 (10–21) | <0.001* | ||
| Progression free survival | |||||
| @ 12 months | 137 | 45 (37–54) | 152 | 15 (10–21) | <0.001* |
| @ 36 months | 12 (7–19) | 6 ( 3–10) | 0.038* | ||
| @ 60 months | 4 (1–11) | 2 (<1–5) | 0.235 | ||
| Overall survival | |||||
| @ 100 days | 137 | 97 (91–98) | 152 | 77 (69–83) | <0.001* |
| @ 12 months | 82 (75–88) | 51 (42–58) | <0.001* | ||
| @ 36 months | 46 (37–55) | 20 (14–27) | <0.001* | ||
| @ 60 months | 29 (20–40) | 9 ( 5–15) | <0.001* | ||
significant difference
NST/RIC = non-myeloablative stem cell transplant/reduced intensity conditioning
Figure 1. Non-relapsed mortality (NRM) after second hematological cell transplant by type of second transplant.
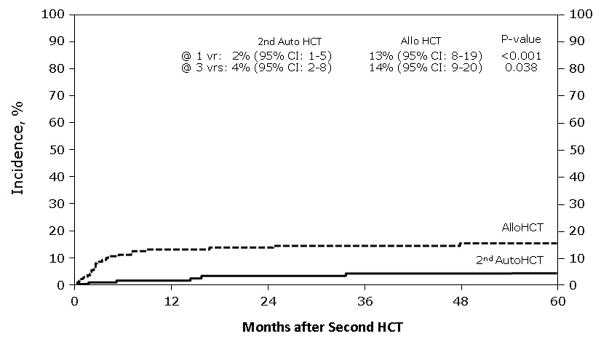
This figure describes the cumulative incidence rate of NRM after second hematological cell transplant by type of second transplant, which are second salvage autologous transplant and allogeneic transplant
Figure 2. Relapse rate (REL) after second hematological cell transplant by type of second transplant.
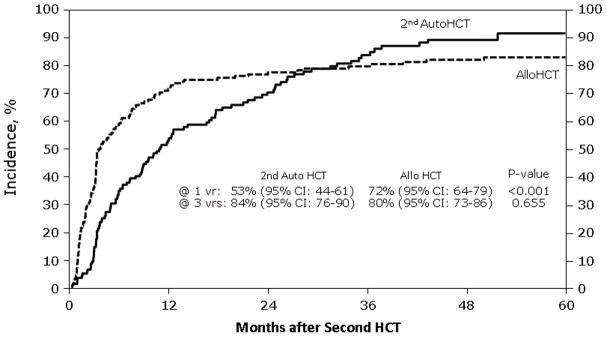
This figure describes the cumulative incidence rate of TRM after second hematological cell transplant by type of second transplant, which are second salvage autologous transplant and allogeneic transplant
Figure 3. Progression-free survival (PFS) after second hematological cell transplant by type of second transplant.
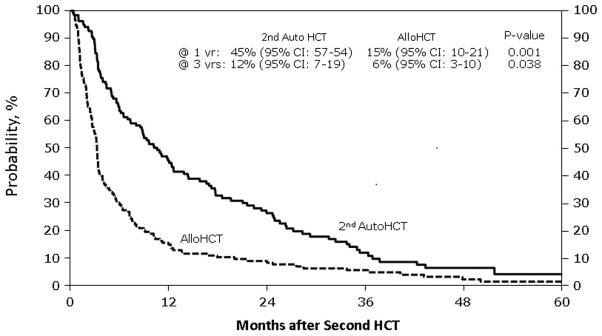
This figure describes the probability estimates of PFS after second hematological cell transplant by type of second transplant, which are second salvage autologous transplant and allogeneic transplant
Figure 4. Overall survival (OS) after second hematological cell transplant by type of second transplant.
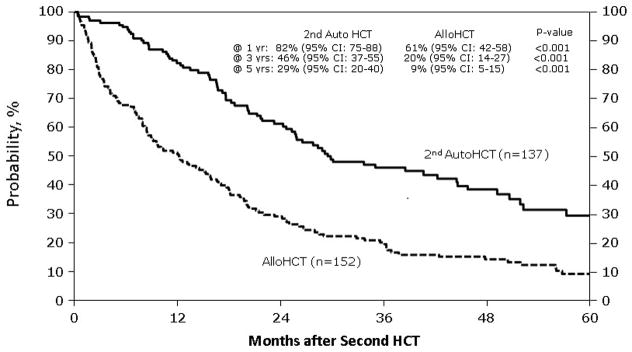
This figure describes the probability estimates of OS after second hematological cell transplant by type of second transplant, which are second salvage autologous transplant and allogeneic transplant
The multivariate analyses were limited by the quality of data requested in the forms (insufficient cytogenetic and FISH data) and the quality of data provided for the patients in the NST/RIC group. Factors that affected overall mortality included NST/RIC (HR 2.38, 95%, CI, 1.79–3.16, p = <0.001), year of 2nd transplant (HR 0.57, 0.43–0.75, p = <0.001) for patients transplanted in later time periods, and functional status (HR1.96, 1.47–2.62, p = <0.001). In multivariate analyses (Table 3) allotransplants were associated with a higher risk of NRM (HR 7.14, 95% CI, 2.70–8.91; p = 0.001) and death (HR 2.38, 95% CI, 1.79 – 3.16; p < 0.001). Effect of therapy on treatment failure was only significant in subjects with Durie-Salmon stage III. In these patients, allotransplant is associated with a higher risk of relapse and treatment-failure compared to autotransplantation (HR 3.05, 95% CI, 2.20–4.22; p = 0.001).
Table 3.
Multivariate Analysis
| Factor | Level | N | HR | 95% CI | P-value |
|---|---|---|---|---|---|
| OS | |||||
| Main effect | Auto | 137 | 1 | ||
| NST/RIC | 152 | 2.38 | (1.79 – 3.16) | <0.001 | |
| Year of 2nd transplant | 1995–2004 | 148 | 1 | ||
| 2005–2008 | 141 | 0.57 | (0.43 – 0.75) | <0.001 | |
| Karnofsky score | ≥ 90 | 144 | 1 | 0.001* | |
| <90 | 118 | 1.96 | (1.47 – 2.62) | <0.001 | |
| Missing | 27 | 1.85 | (1.14 – 3.01) | 0.013 | |
| Treatment failure | |||||
| Interactional effect between main effect and Durie-Salmon Stage | DS stage I–II, Auto | 40 | 1 | ||
| DS stage I–II, NST/RIC | 54 | 1.33 | (0.87–2.02) | 0.189 | |
| DS stage III, Auto | 80 | 1 | |||
| DS stage III, NST/RIC | 88 | 3.05 | (2.20–4.22) | <0.001 | |
| DS missing, Auto | 17 | 1 | |||
| DS missing, NST/RIC | 10 | 1.53 | (0.63–3.70) | 0.344 | |
| Relapse | |||||
| Interactional effect between main effect and Durie-Salmon Stage | DS stage I–II, Auto | 40 | 1 | ||
| DS stage I–II, NST/RIC | 54 | 1.16 | (0.74–1.82) | 0.518 | |
| DS stage III, Auto | 80 | 1 | |||
| DS stage III, NST/RIC | 88 | 2.70 | (1.93–3.80) | <0.001 | |
| DS missing, Auto | 17 | 1 | |||
| DS missing, NST/RIC | 10 | 1.51 | (0.59–3.83) | 0.388 | |
| NRM | |||||
| Main effect | Auto | 137 | 1 | ||
| NST/RIC | 152 | 7.14 | (2.70–18.91) | <0.001 | |
overall p-value
Auto = autotransplant
NST/RIC = non-myeloablative stem cell transplant/reduced intensity conditioning
DS = Durie-Salmon
NRM = non-relapse mortality
Patients who underwent NST/RIC from related and unrelated donors had a similar outcome. The PFS and OS were similar at 1, 3 and 5 years (data not shown). The 3-year OS of patients who underwent NST/RIC from related donors was 19% (95% CI, 7–33) compared to patients whose donors were unrelated, 21% (95% CI, 14–28; p = 0.82). The TRM was also similar irrespective of donor type (HR 1.077, 95% CI 0.75–1.54, p = 0.68).
DISCUSSION
The optimal therapy for patients with resistant or relapsed MM after autotransplantation remains unknown. The immunomodulatory agents and proteosome inhibitors have greatly expanded the therapeutic armamentarium against MM and many patients can benefit from additional therapy after autotransplant relapse. However, the disease eventually progress or patients develop unacceptable toxicities that limit these therapies. Since autotransplantation induces durable remissions with acceptable toxicity, a second autotransplant is also a consideration. Several studies have documented that this approach is feasible and transplant centers frequently harvest enough stem cells for two transplants in preparation for a second autotransplant upon MM progression or relapse [11–17]. Other investigators prefer the use of allogeneic transplantation because the graft is free of tumor and has the potential to induce a graft-versus-MM effect [18–28]. Since the morbidity and mortality associated with myeloablative allogeneic transplantation is high, most centers have relied on low-intensity conditioning NST/RIC. The aim of our study was to have a better understanding whether one type of transplant was favored for patients with relapse/refractory MM requiring salvage transplantation.
Our data demonstrate that patients who undergo autotransplantation rather than NST/RIC as their second transplant fare better across all measures including rates of progression. Major limitations of this study are the absence of cytogenetic data and a paucity of other prognostic factors available in the NST/RIC cohort. The autotransplant cohort was lower risk based on a longer time interval from 1st autotransplant to relapse.
If one compares these registry data to small series from individual institutions, our NRM is lower. The CIBMTR registry 1-year NRM for RIC/NST was 13% as compared to single institution reporting NRM varying from 11% to 26% [18–28]. An EBMTR analysis of large number of patients who underwent RIC/NST, most of them after autotransplant failure, reported a NRM of 22% [29]. Our results are remarkable when it is taken into consideration that 90% of the patients in this study underwent unrelated RIC/NST. Despite the lower NRM, both PFS and OS in the current study are lower than what has been reported in other studies: OS (24%–74%) and DFS (21%–61%) [18–28]. These differences may be in part a reflection of patient selection since most of these studies were from single institutions and had smaller numbers of patients.
The outcome of patients who underwent 2nd autotransplants in this study is similar to previously published reports despite the fact that we only included patients younger than 66 years of age [11–17].
In a biologic assignment trial comparing patients who underwent tandem autotransplants to patients who underwent autotransplants followed by NSC as their initial therapy for myeloma, the 3-year DFS and OS was similar. However, the NRM was higher in the autologous-NSC arm of the study, as observed in our study.
Our study has several strengths and limitations. Strengths include the large number of patients from multiple centers who contributed cases to this study over a long period of time, reflecting more accurately the practice of transplantation throughout this period. Limitations include the lack of information regarding prognostic factors including cytogenetics and International Staging System stage, information on maintenance therapy and the disease status at the time of alloHCT. With these caveats in mind, the conclusion from these data is that patients who undergo autotransplants as their second transplant fare better than NST/RIC across all measures including progression rates and NRM. Since disease status was not available for most allotransplant recipients, is not possible to determine which type of transplant is superior after autotransplant failure.
Supplementary Material
Acknowledgments
The CIBMTR is supported by Public Health Service Grant/Cooperative Agreement U24 CA076518 from the National Cancer Institute (NCI), the National Heart, Lung and Blood Institute (NHLBI) and the National Institute of Allergy and Infectious Diseases (NIAID); a Grant/Cooperative Agreement U10 HL069294 from NHLBI and NCI; a contract HHSH250201200016C with Health Resources and Services Administration (HRSA/DHHS); two Grants N00014-12-1-0142 and N00014-13-1-0039 from the Office of Naval Research; and grants from Allos Therapeutics, Inc.; Amgen, Inc.; Anonymous donation to the Medical College of Wisconsin; Ariad; Be the Match Foundation; Blue Cross and Blue Shield Association; Celgene Corporation; Fresenius-Biotech North America, Inc.; Gamida Cell Teva Joint Venture Ltd.; Genentech, Inc.;Gentium SpA; Genzyme Corporation; GlaxoSmithKline; HistoGenetics, Inc.; Kiadis Pharma; The Leukemia & Lymphoma Society; The Medical College of Wisconsin; Merck & Co, Inc.; Millennium: The Takeda Oncology Co.; Milliman USA, Inc.; Miltenyi Biotec, Inc.; National Marrow Donor Program; Onyx Pharmaceuticals; Optum Healthcare Solutions, Inc.; Osiris Therapeutics, Inc.; Otsuka America Pharmaceutical, Inc.; Remedy Informatics; Sanofi US; Seattle Genetics; Sigma-Tau Pharmaceuticals; Soligenix, Inc.; StemCyte, A Global Cord Blood Therapeutics Co.; Stemsoft Software, Inc.; Swedish Orphan Biovitrum; Tarix Pharmaceuticals; TerumoBCT; Teva Neuroscience, Inc.; THERAKOS, Inc.; and Wellpoint, Inc. The views expressed in this article do not reflect the official policy or position of the National Institute of Health, the Department of the Navy, the Department of Defense, or any other agency of the U.S. Government.
Footnotes
PREVIOUS PRESENTATIONS: Presented in part at the American Society of Hematology 53rd Annual Scientific Meeting held in San Diego, CA, December 10-13, 2011
CONFLICTS OF INTEREST: The authors declare no competing financial interests.
References
- 1.Rajkumar SV. Treatment of multiple myeloma. Nat Rev Clin Oncol. 2011;8:479–491. doi: 10.1038/nrclinonc.2011.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jacubowiak J. Management strategies for relapsed/refractory multiple myeloma: current clinical perspectives. Semin Hematol. 2012;49 (Suppl 1):S16–32. doi: 10.1053/j.seminhematol.2012.05.003. [DOI] [PubMed] [Google Scholar]
- 3.Kumar SK, Lee JH, LaHuerta JJ, Morgan G, Richardson PG, Crowley J, et al. Risk of progression and survival in multiple myeloma relapsing after therapy with IMiDs and bortezomib: a multicenter international myeloma working group study. Leukemia. 2012;26:149–157. doi: 10.1038/leu.2011.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bensinger W, Rotta M, Storer B, Chauncey T, Holmberg L, Becker P, et al. Allo-SCT for multiple myeloma: a review of outcomes at a single transplant center. Bone Marrow Transplant. 2012;47:1312–1317. doi: 10.1038/bmt.2012.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bacigalupo A, Ballen K, Rizzo D, Giralt S, Lazarus H, Ho V, et al. Defining the intensity of conditioning regimens: working definitions. Biol Blood Marrow Transplant. 2009;15:1628–1633. doi: 10.1016/j.bbmt.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weisdorf D, Spellman S, Haagenson M, Horowitz M, Lee S, Anasetti C, et al. Classification of HLA-matching for retrospective analysis of unrelated donor transplantation: revised definitions to predict survival. Biol Blood Marrow Transplant. 2008;14:748–758. doi: 10.1016/j.bbmt.2008.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Przepiorka D, Weisdorf D, Martin P, Klingemann HG, Beatty P, Hows J, et al. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant. 1995;15:825–828. [PubMed] [Google Scholar]
- 8.Klein J, Moeschberger M. Survival Analysis: Techniques of Censored and Truncated Data. 2. New York: Springer-Verlag; 2003. [Google Scholar]
- 9.Kaplan E. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 10.Cox DR. Regression models and life tables. J R Stat Soc. 1972;34:187–220. [Google Scholar]
- 11.Burzynski JA, Toro JJ, Patel RC, et al. Toxicity of a second autologous peripheral blood transplant in patients with relapsed or recurrent multiple myeloma. Leuk Lymphoma. 2009;50:1442–1447. doi: 10.1080/10428190903085936. [DOI] [PubMed] [Google Scholar]
- 12.Olin RL, Vogl DT, Porter DL, Luger SM, Schuster SJ, Tsai DE, et al. Second auto-SCT is safe and effective salvage therapy for relapsed multiple myeloma. Bone Marrow Transplant. 2009;43:417–422. doi: 10.1038/bmt.2008.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fenk R, Liese V, Neubauer F, Bruns I, Kondakci M, Balleisen S, et al. Predictive factors for successful salvage high-dose therapy in patients with multiple myeloma relapsing after autologous blood stem cell transplantation. Leuk Lymphoma. 2011;52:1455–1462. doi: 10.3109/10428194.2011.575967. [DOI] [PubMed] [Google Scholar]
- 14.Jimenez-Zepeda VH, Mikhael J, Winter A, Franke N, Masih-Khan E, Trudel S, et al. Second autologous stem cell transplantation as salvage therapy for multiple myeloma: impact on progression-free and overall survival. Biol Blood Marrow Transplant. 2012;18:773–779. doi: 10.1016/j.bbmt.2011.10.044. [DOI] [PubMed] [Google Scholar]
- 15.Shah N, Ahmed F, Bashir Q, Qureshi S, Dinh Y, Rondon G, et al. Durable remissions with salvage second autotransplants in patients with multiple myeloma. Cancer. 2012;118:3549–3555. doi: 10.1002/cncr.26662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chow AW, Lee CH, Hiwase DK, To LB, Horvath N. Relapsed multiple myeloma: Who benefits from salvage autografts? Intern Med J. 2013;43:156–161. doi: 10.1111/j.1445-5994.2012.02867.x. [DOI] [PubMed] [Google Scholar]
- 17.Michaelis LC, Saad A, Zhong X, Le-Rademacher J, Freytes CO, Marks DI, et al. Salvage Second Hematopoietic Cell Transplantation in Myeloma. Biol Blood Marrow Transplant. 2013 Jan 5; doi: 10.1016/j.bbmt.2013.01.004. pii: S1083–8791(13)00017-7 Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Badros A, Barlogie B, Morris C, Desikan R, Martin SR, Munshi N, et al. High response rate in refractory and poor-risk multiple myeloma after allotransplantation using a nonmyeloablative conditioning regimen and donor lymphocyte infusions. Blood. 2001;97:2574–2579. doi: 10.1182/blood.v97.9.2574. [DOI] [PubMed] [Google Scholar]
- 19.Kroger N, Sayer HG, Schwerdtfeger R, Kiehl M, Nagler A, Renges H, et al. Unrelated stem cell transplantation in multiple myeloma after a reduced-intensity conditioning with pretransplantation antithymocyte globulin is highly effective with low transplantation-related mortality. Blood. 2002;100:3919–3924. doi: 10.1182/blood-2002-04-1150. [DOI] [PubMed] [Google Scholar]
- 20.Einsele H, Schafer HJ, Hebart H, Bader P, Meisner C, Plasswilm L, et al. Follow-up of patients with progressive multiple myeloma undergoing allografts after reduced-intensity conditioning. Br J Haematol. 2003;121:411–418. doi: 10.1046/j.1365-2141.2003.04299.x. [DOI] [PubMed] [Google Scholar]
- 21.Gerull S, Goerner M, Benner A, Hegenbart U, Klein U, Schaefer H, et al. Long-term outcome of nonmyeloablative allogeneic transplantation in patients with high-risk multiple myeloma. Bone Marrow Transplant. 2005;36:963–969. doi: 10.1038/sj.bmt.1705161. [DOI] [PubMed] [Google Scholar]
- 22.Pérez-Simón JA, Sureda A, Fernández-Aviles F, Sampol A, Cabrera JR, Caballero D, et al. Reduced-intensity conditioning allogeneic transplantation is associated with a high incidence of extramedullary relapses in multiple myeloma patients. Leukemia. 2006;20:542–545. doi: 10.1038/sj.leu.2404085. [DOI] [PubMed] [Google Scholar]
- 23.Qazilbach MH, Saliba R, De Lima M, Hosing C, Couriel D, Aleman A, et al. Second autologous or allogeneic transplantation after the failure of first autograft in patients with multiple myeloma. Cancer. 2006;106:1084–1089. doi: 10.1002/cncr.21700. [DOI] [PubMed] [Google Scholar]
- 24.Georges GE, Maris MB, Maloney DG, Sandmaier BM, Sorror ML, Shizuru JA, et al. Nonmyeloablative unrelated donor hematopoietic cell transplantation to treat patients with poor-risk, relapsed, or refractory multiple myeloma. Biol Blood Marrow Transplant. 2007;13:423–432. doi: 10.1016/j.bbmt.2006.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Majolino I, Davoli M, Carnevalli E, Locasciulli A, Di Bartolomeo P, Scime R, et al. Reduced intensity conditioning with thiotepa, fludarabine, and melphalan is effective in advanced multiple myeloma. Leuk Lymphoma. 2007;48:759–766. doi: 10.1080/10428190601186150. [DOI] [PubMed] [Google Scholar]
- 26.De Lavallade H, El-Cheikh J, Faucher C, Furst S, Stoppa AM, Coso D, et al. Reduced-intensity conditioning allogeneic SCT as salvage treatment for relapsed multiple myeloma. Bone Marrow Transplant. 2008;41:953–960. doi: 10.1038/bmt.2008.22. [DOI] [PubMed] [Google Scholar]
- 27.Osman K, Elliot B, Mandeli J, Scigliano E, Malone A, Isola L, et al. Non-myeloablative conditioning and allogeneic transplantation for multiple myeloma. Am J Hematol. 2010;85:249–254. doi: 10.1002/ajh.21633. [DOI] [PubMed] [Google Scholar]
- 28.Shimoni A, Hardan I, Ayuk F, Schilling G, Atanackovic D, Zeller W, et al. Allogenic hematopoietic stem cell transplantation with reduced-intensity conditioning in patients with refractory and recurrent multiple myeloma. Cancer. 2010;116:3621–3630. doi: 10.1002/cncr.25228. [DOI] [PubMed] [Google Scholar]
- 29.Crawley C, Lallancete M, Szydlo R, Gilleece M, Peggs K, Mackinnon S, et al. Outcomes for reduced-intensity allogeneic transplantation for multiple myeloma: an analysis of prognostic factors of the Leukemia Working Party of the EBMT. Blood. 2005;105:4532–4539. doi: 10.1182/blood-2004-06-2387. [DOI] [PubMed] [Google Scholar]
- 30.Krishnan A, Pasquini MC, Logan B, Stadtmauer EA, Vesole DH, Alyea E, III, et al. Tandem autologous versus single autologous transplantation followed by allogeneic hematopoietic cell transplantation for patients with multiple myeloma : Results from the Blood and Marrow Transplant Clinical Trials Network (BMT CTN) 0102 trial. Lancet Oncol. 2011;12 :1195–1203. doi: 10.1016/S1470-2045(11)70243-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


