Abstract
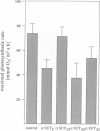
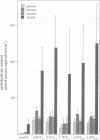
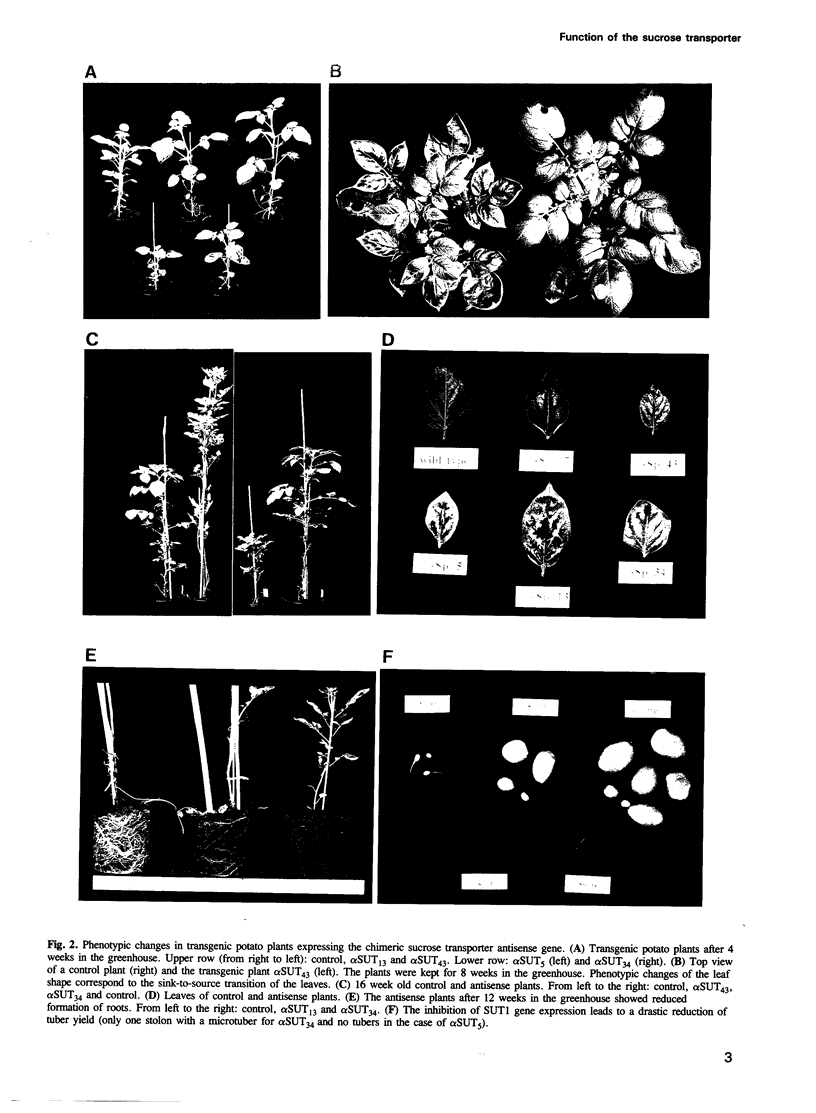
Sucrose is the principal transport form of assimilates in most plants. In many species, translocation of assimilates from the mesophyll into the phloem for long distance transport is assumed to be carrier mediated. A putative sucrose proton cotransporter cDNA has been isolated from potato and shown to be expressed mainly in the phloem of mature exporting leaves. To study the in vivo role and function of the protein, potato plants were transformed with an antisense construct of the sucrose transporter cDNA under control of the CaMV 35S promoter. Upon maturation of the leaves, five transformants that expressed reduced levels of sucrose transporter mRNA developed local bleaching and curling of leaves. These leaves contained > 20-fold higher concentrations of soluble carbohydrates and showed a 5-fold increase in starch content as compared with wild type plants, as expected from a block in export. Transgenic plants with a reduced amount of sucrose carrier mRNA show a dramatic reduction in root development and tuber yield. Maximal photosynthetic activity was reduced at least in the strongly affected transformants. The effects observed in the antisense plants strongly support an apoplastic model for phloem loading, in which the sucrose transporter located at the phloem plasma membrane represents the primary route for sugar uptake into the long distance distribution network.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bevan M. Binary Agrobacterium vectors for plant transformation. Nucleic Acids Res. 1984 Nov 26;12(22):8711–8721. doi: 10.1093/nar/12.22.8711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush D. R. Electrogenicity, pH-Dependence, and Stoichiometry of the Proton-Sucrose Symport. Plant Physiol. 1990 Aug;93(4):1590–1596. doi: 10.1104/pp.93.4.1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson C. D., Altabella T., Chrispeels M. J. Slow-growth phenotype of transgenic tomato expressing apoplastic invertase. Plant Physiol. 1991 Feb;95(2):420–425. doi: 10.1104/pp.95.2.420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frommer W. B., Hummel S., Riesmeier J. W. Expression cloning in yeast of a cDNA encoding a broad specificity amino acid permease from Arabidopsis thaliana. Proc Natl Acad Sci U S A. 1993 Jul 1;90(13):5944–5948. doi: 10.1073/pnas.90.13.5944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatz C., Frohberg C., Wendenburg R. Stringent repression and homogeneous de-repression by tetracycline of a modified CaMV 35S promoter in intact transgenic tobacco plants. Plant J. 1992 May;2(3):397–404. doi: 10.1111/j.1365-313x.1992.00397.x. [DOI] [PubMed] [Google Scholar]
- Gerhardt R., Stitt M., Heldt H. W. Subcellular Metabolite Levels in Spinach Leaves : Regulation of Sucrose Synthesis during Diurnal Alterations in Photosynthetic Partitioning. Plant Physiol. 1987 Feb;83(2):399–407. doi: 10.1104/pp.83.2.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heineke D., Sonnewald U., Büssis D., Günter G., Leidreiter K., Wilke I., Raschke K., Willmitzer L., Heldt H. W. Apoplastic expression of yeast-derived invertase in potato : effects on photosynthesis, leaf solute composition, water relations, and tuber composition. Plant Physiol. 1992 Sep;100(1):301–308. doi: 10.1104/pp.100.1.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King R. W., Zeevaart J. A. Enhancement of Phloem exudation from cut petioles by chelating agents. Plant Physiol. 1974 Jan;53(1):96–103. doi: 10.1104/pp.53.1.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemoine R., Gallet O., Gaillard C., Frommer W., Delrot S. Plasma membrane vesicles from source and sink leaves : changes in solute transport and polypeptide composition. Plant Physiol. 1992 Nov;100(3):1150–1156. doi: 10.1104/pp.100.3.1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin T. P., Caspar T., Somerville C., Preiss J. Isolation and Characterization of a Starchless Mutant of Arabidopsis thaliana (L.) Heynh Lacking ADPglucose Pyrophosphorylase Activity. Plant Physiol. 1988 Apr;86(4):1131–1135. doi: 10.1104/pp.86.4.1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maynard J. W., Lucas W. J. Sucrose and Glucose Uptake into Beta vulgaris Leaf Tissues : A Case for General (Apoplastic) Retrieval Systems. Plant Physiol. 1982 Nov;70(5):1436–1443. doi: 10.1104/pp.70.5.1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller-Röber B. T., Kossmann J., Hannah L. C., Willmitzer L., Sonnewald U. One of two different ADP-glucose pyrophosphorylase genes from potato responds strongly to elevated levels of sucrose. Mol Gen Genet. 1990 Oct;224(1):136–146. doi: 10.1007/BF00259460. [DOI] [PubMed] [Google Scholar]
- Riesmeier J. W., Flügge U. I., Schulz B., Heineke D., Heldt H. W., Willmitzer L., Frommer W. B. Antisense repression of the chloroplast triose phosphate translocator affects carbon partitioning in transgenic potato plants. Proc Natl Acad Sci U S A. 1993 Jul 1;90(13):6160–6164. doi: 10.1073/pnas.90.13.6160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riesmeier J. W., Willmitzer L., Frommer W. B. Isolation and characterization of a sucrose carrier cDNA from spinach by functional expression in yeast. EMBO J. 1992 Dec;11(13):4705–4713. doi: 10.1002/j.1460-2075.1992.tb05575.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocha-Sosa M., Sonnewald U., Frommer W., Stratmann M., Schell J., Willmitzer L. Both developmental and metabolic signals activate the promoter of a class I patatin gene. EMBO J. 1989 Jan;8(1):23–29. doi: 10.1002/j.1460-2075.1989.tb03344.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salanoubat M., Belliard G. The steady-state level of potato sucrose synthase mRNA is dependent on wounding, anaerobiosis and sucrose concentration. Gene. 1989 Dec 7;84(1):181–185. doi: 10.1016/0378-1119(89)90153-4. [DOI] [PubMed] [Google Scholar]
- Sonnewald U., Brauer M., von Schaewen A., Stitt M., Willmitzer L. Transgenic tobacco plants expressing yeast-derived invertase in either the cytosol, vacuole or apoplast: a powerful tool for studying sucrose metabolism and sink/source interactions. Plant J. 1991 Jul;1(1):95–106. doi: 10.1111/j.1365-313x.1991.00095.x. [DOI] [PubMed] [Google Scholar]
- von Schaewen A., Stitt M., Schmidt R., Sonnewald U., Willmitzer L. Expression of a yeast-derived invertase in the cell wall of tobacco and Arabidopsis plants leads to accumulation of carbohydrate and inhibition of photosynthesis and strongly influences growth and phenotype of transgenic tobacco plants. EMBO J. 1990 Oct;9(10):3033–3044. doi: 10.1002/j.1460-2075.1990.tb07499.x. [DOI] [PMC free article] [PubMed] [Google Scholar]