Abstract
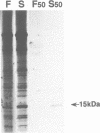
We have previously shown that cytoplasmic male sterility in sunflower is associated with the insertion into the mitochondrial DNA of a novel open reading frame (ORF) located 3' to the atpA gene. Here, we show that in mitochondria from the sterile line, this novel ORF (ORF522) is cotranscribed with atpA. We have identified the product of the ORF522 as being a 15 kDa protein previously observed in sterile plant mitochondria by in organello translation. Both Western blot analysis and in organello translation assays show reduced levels of the 15 kDa polypeptide upon restoration of fertility. Interestingly, this reduction is tissue specific since it is only observed in the male florets from restored hybrid plants. These results suggest that the 15 kDa novel polypeptide is probably responsible for the CMS phenotype. Northern blot analysis using RNA from both seedlings and male florets shows a flower-specific reduction in the level of the ORF522 transcript in the restored hybrid line. The reduction is not due to a reduced transcription rate as demonstrated by 'run-on' experiments using mitochondria isolated from male florets. This suggests that the product of the nuclear restorer gene acts at the post-transcriptional level to destabilize the novel mitochondrial transcript in a tissue-specific manner and restore male fertility.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bailey-Serres J., Hanson D. K., Fox T. D., Leaver C. J. Mitochondrial genome rearrangement leads to extension and relocation of the cytochrome c oxidase subunit I gene in sorghum. Cell. 1986 Nov 21;47(4):567–576. doi: 10.1016/0092-8674(86)90621-5. [DOI] [PubMed] [Google Scholar]
- Barkan A. Proteins encoded by a complex chloroplast transcription unit are each translated from both monocistronic and polycistronic mRNAs. EMBO J. 1988 Sep;7(9):2637–2644. doi: 10.1002/j.1460-2075.1988.tb03116.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castresana C., Garcia-Luque I., Alonso E., Malik V. S., Cashmore A. R. Both positive and negative regulatory elements mediate expression of a photoregulated CAB gene from Nicotiana plumbaginifolia. EMBO J. 1988 Jul;7(7):1929–1936. doi: 10.1002/j.1460-2075.1988.tb03030.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper P., Butler E., Newton K. J. Identification of a maize nuclear gene which influences the size and number of cox2 transcripts in mitochondria of perennial ++teosintes. Genetics. 1990 Oct;126(2):461–467. doi: 10.1093/genetics/126.2.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper P., Newton K. J. Maize nuclear background regulates the synthesis of a 22-kDa polypeptide in Zea luxurians mitochondria. Proc Natl Acad Sci U S A. 1989 Oct;86(19):7423–7426. doi: 10.1073/pnas.86.19.7423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewey R. E., Levings C. S., 3rd, Timothy D. H. Novel recombinations in the maize mitochondrial genome produce a unique transcriptional unit in the Texas male-sterile cytoplasm. Cell. 1986 Feb 14;44(3):439–449. doi: 10.1016/0092-8674(86)90465-4. [DOI] [PubMed] [Google Scholar]
- Dewey R. E., Schuster A. M., Levings C. S., Timothy D. H. Nucleotide sequence of F(0)-ATPase proteolipid (subunit 9) gene of maize mitochondria. Proc Natl Acad Sci U S A. 1985 Feb;82(4):1015–1019. doi: 10.1073/pnas.82.4.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewey R. E., Timothy D. H., Levings C. S., 3rd Chimeric mitochondrial genes expressed in the C male-sterile cytoplasm of maize. Curr Genet. 1991 Dec;20(6):475–482. doi: 10.1007/BF00334775. [DOI] [PubMed] [Google Scholar]
- Dewey R. E., Timothy D. H., Levings C. S. A mitochondrial protein associated with cytoplasmic male sterility in the T cytoplasm of maize. Proc Natl Acad Sci U S A. 1987 Aug;84(15):5374–5378. doi: 10.1073/pnas.84.15.5374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falconet D., Sevignac M., Quétier F. Nucleotide sequence and determination of the extremities of the 26S ribosomal RNA gene in wheat mitochondria: evidence for sequence rearrangements in the ribosomal genes of higher plants. Curr Genet. 1988;13(1):75–82. doi: 10.1007/BF00365760. [DOI] [PubMed] [Google Scholar]
- Forde B. G., Leaver C. J. Nuclear and cytoplasmic genes controlling synthesis of variant mitochondrial polypeptides in male-sterile maize. Proc Natl Acad Sci U S A. 1980 Jan;77(1):418–422. doi: 10.1073/pnas.77.1.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruissem W. Chloroplast gene expression: how plants turn their plastids on. Cell. 1989 Jan 27;56(2):161–170. doi: 10.1016/0092-8674(89)90889-1. [DOI] [PubMed] [Google Scholar]
- Hanson M. R. Plant mitochondrial mutations and male sterility. Annu Rev Genet. 1991;25:461–486. doi: 10.1146/annurev.ge.25.120191.002333. [DOI] [PubMed] [Google Scholar]
- Horn R., Köhler R. H., Zetsche K. A mitochondrial 16 kDa protein is associated with cytoplasmic male sterility in sunflower. Plant Mol Biol. 1991 Jul;17(1):29–36. doi: 10.1007/BF00036803. [DOI] [PubMed] [Google Scholar]
- Isaac P. G., Brennicke A., Dunbar S. M., Leaver C. J. The mitochondrial genome of fertile maize (Zea mays L.) contains two copies of the gene encoding the alpha-subunit of the F1-ATPase. Curr Genet. 1985;10(4):321–328. doi: 10.1007/BF00365628. [DOI] [PubMed] [Google Scholar]
- Isaac P. G., Jones V. P., Leaver C. J. The maize cytochrome c oxidase subunit I gene: sequence, expression and rearrangement in cytoplasmic male sterile plants. EMBO J. 1985 Jul;4(7):1617–1623. doi: 10.1002/j.1460-2075.1985.tb03828.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwabuchi M., Kyozuka J., Shimamoto K. Processing followed by complete editing of an altered mitochondrial atp6 RNA restores fertility of cytoplasmic male sterile rice. EMBO J. 1993 Apr;12(4):1437–1446. doi: 10.1002/j.1460-2075.1993.tb05787.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johns C., Lu M., Lyznik A., Mackenzie S. A mitochondrial DNA sequence is associated with abnormal pollen development in cytoplasmic male sterile bean plants. Plant Cell. 1992 Apr;4(4):435–449. doi: 10.1105/tpc.4.4.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. Comparison of initiation of protein synthesis in procaryotes, eucaryotes, and organelles. Microbiol Rev. 1983 Mar;47(1):1–45. doi: 10.1128/mr.47.1.1-45.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnasamy S., Makaroff C. A. Characterization of the radish mitochondrial orfB locus: possible relationship with male sterility in Ogura radish. Curr Genet. 1993 Jul-Aug;24(1-2):156–163. doi: 10.1007/BF00324680. [DOI] [PubMed] [Google Scholar]
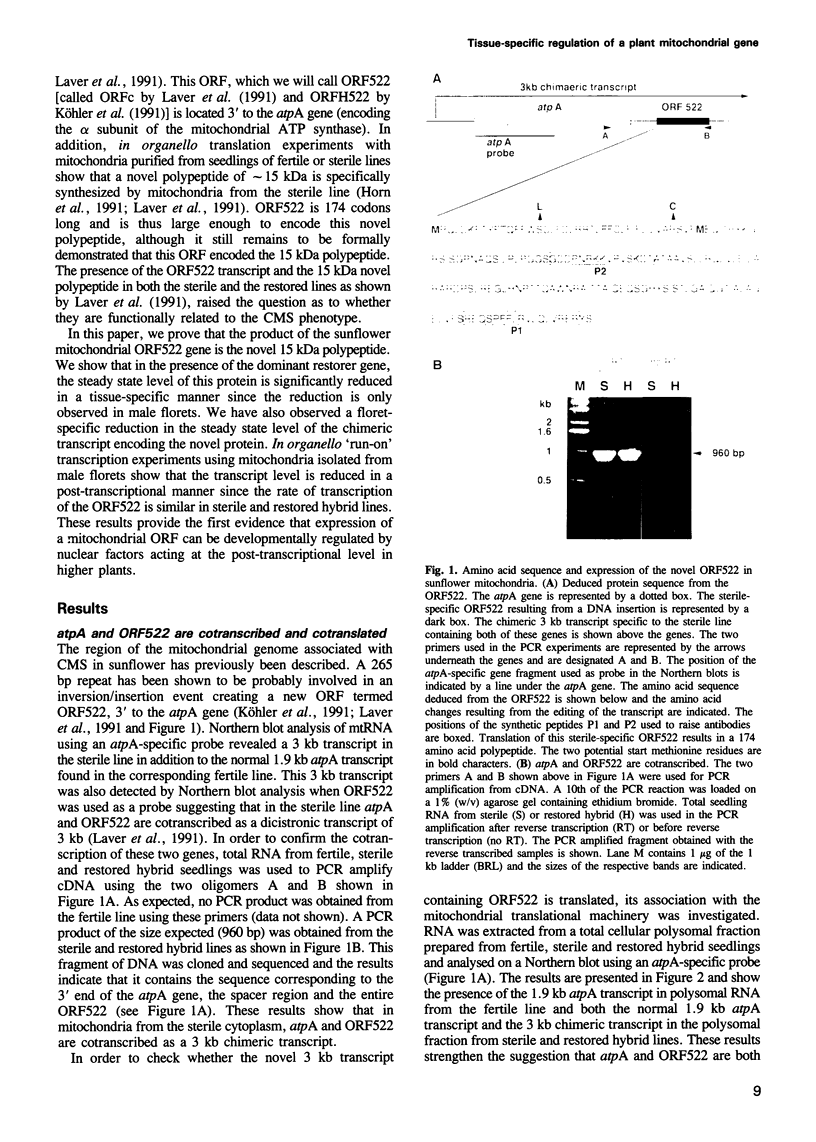
- Köhler R. H., Horn R., Lössl A., Zetsche K. Cytoplasmic male sterility in sunflower is correlated with the co-transcription of a new open reading frame with the atpA gene. Mol Gen Genet. 1991 Jul;227(3):369–376. doi: 10.1007/BF00273925. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laver H. K., Reynolds S. J., Moneger F., Leaver C. J. Mitochondrial genome organization and expression associated with cytoplasmic male sterility in sunflower (Helianthus annuus). Plant J. 1991 Sep;1(2):185–193. doi: 10.1111/j.1365-313x.1991.00185.x. [DOI] [PubMed] [Google Scholar]
- Lu B., Hanson M. R. A single nuclear gene specifies the abundance and extent of RNA editing of a plant mitochondrial transcript. Nucleic Acids Res. 1992 Nov 11;20(21):5699–5703. doi: 10.1093/nar/20.21.5699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makaroff C. A., Apel I. J., Palmer J. D. The atp6 coding region has been disrupted and a novel reading frame generated in the mitochondrial genome of cytoplasmic male-sterile radish. J Biol Chem. 1989 Jul 15;264(20):11706–11713. [PubMed] [Google Scholar]
- Martínez-Zapater J. M., Gil P., Capel J., Somerville C. R. Mutations at the Arabidopsis CHM locus promote rearrangements of the mitochondrial genome. Plant Cell. 1992 Aug;4(8):889–899. doi: 10.1105/tpc.4.8.889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monéger F., Mandaron P., Niogret M. F., Freyssinet G., Mache R. Expression of Chloroplast and Mitochondrial Genes during Microsporogenesis in Maize. Plant Physiol. 1992 Jun;99(2):396–400. doi: 10.1104/pp.99.2.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulligan R. M., Leon P., Walbot V. Transcriptional and posttranscriptional regulation of maize mitochondrial gene expression. Mol Cell Biol. 1991 Jan;11(1):533–543. doi: 10.1128/mcb.11.1.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton K. J., Walbot V. Maize mitochondria synthesize organ-specific polypeptides. Proc Natl Acad Sci U S A. 1985 Oct;82(20):6879–6883. doi: 10.1073/pnas.82.20.6879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nivison H. T., Hanson M. R. Identification of a mitochondrial protein associated with cytoplasmic male sterility in petunia. Plant Cell. 1989 Nov;1(11):1121–1130. doi: 10.1105/tpc.1.11.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pring D., Brennicke A., Schuster W. RNA editing gives a new meaning to the genetic information in mitochondria and chloroplasts. Plant Mol Biol. 1993 Mar;21(6):1163–1170. doi: 10.1007/BF00023611. [DOI] [PubMed] [Google Scholar]
- Pruitt K. D., Hanson M. R. Transcription of the Petunia mitochondrial CMS-associated Pcf locus in male sterile and fertility-restored lines. Mol Gen Genet. 1991 Jul;227(3):348–355. doi: 10.1007/BF00273922. [DOI] [PubMed] [Google Scholar]
- Quagliariello C., Saiardi A., Gallerani R. The cytochrome oxidase subunit III gene in sunflower mitochondria is cotranscribed with an open reading frame conserved in higher plants. Curr Genet. 1990 Nov;18(4):355–363. doi: 10.1007/BF00318217. [DOI] [PubMed] [Google Scholar]
- Rathburn H. B., Hedgcoth C. A chimeric open reading frame in the 5' flanking region of coxI mitochondrial DNA from cytoplasmic male-sterile wheat. Plant Mol Biol. 1991 May;16(5):909–912. doi: 10.1007/BF00015083. [DOI] [PubMed] [Google Scholar]
- Schuster W., Ternes R., Knoop V., Hiesel R., Wissinger B., Brennicke A. Distribution of RNA editing sites in Oenothera mitochondrial mRNAs and rRNAs. Curr Genet. 1991 Nov;20(5):397–404. doi: 10.1007/BF00317068. [DOI] [PubMed] [Google Scholar]
- Singh M., Brown G. G. Suppression of cytoplasmic male sterility by nuclear genes alters expression of a novel mitochondrial gene region. Plant Cell. 1991 Dec;3(12):1349–1362. doi: 10.1105/tpc.3.12.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small I. D., Isaac P. G., Leaver C. J. Stoichiometric differences in DNA molecules containing the atpA gene suggest mechanisms for the generation of mitochondrial genome diversity in maize. EMBO J. 1987 Apr;6(4):865–869. doi: 10.1002/j.1460-2075.1987.tb04832.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treinin M., Simchen G. Mitochondrial activity is required for the expression of IME1, a regulator of meiosis in yeast. Curr Genet. 1993 Mar;23(3):223–227. doi: 10.1007/BF00351500. [DOI] [PubMed] [Google Scholar]
- Ward G. C., Levings C. S., 3rd The protein-encoding gene T-urf13 is not edited in maize mitochondria. Plant Mol Biol. 1991 Nov;17(5):1083–1088. doi: 10.1007/BF00037148. [DOI] [PubMed] [Google Scholar]
- Warmke H. E., Lee S. L. Pollen Abortion in T Cytoplasmic Male-Sterile Corn (Zea mays): A Suggested Mechanism. Science. 1978 May 5;200(4341):561–563. doi: 10.1126/science.200.4341.561. [DOI] [PubMed] [Google Scholar]
- Young E. G., Hanson M. R. A fused mitochondrial gene associated with cytoplasmic male sterility is developmentally regulated. Cell. 1987 Jul 3;50(1):41–49. doi: 10.1016/0092-8674(87)90660-x. [DOI] [PubMed] [Google Scholar]